Abstract
The identification of patterns and mechanisms of wildfire effects on biodiversity is of significant conservation importance. The research was conducted in a zone of mixed and broad-leaved forests. Carabid beetles were studied in eight sample plots that varied in relation to the 2010 and 2021 fires through 2022 and 2023. A total of 8667 specimens of 108 species of carabid beetles were counted. In 2022, plots were clearly differentiated by the pyrogenic disturbance in terms of the carabid species structure. As the pyrogenic disturbance increases, the total abundance of brachypterous as well as herbivore species decreases sharply. The carabids that were the most prevalent in an undisturbed forest (Carabus arcensis, Carabus glabratus, Pterostichus oblongopunctatus, and Amara brunnea) demonstrated sensitivity to the 2010 fire. The pyrophilous species Pterostichus quadrifoveolatus and Sericoda quadripunctata were attracted to a moderately burned area. Poecilus lepidus is confined to areas burned in 2010 and not affected by the 2021 fire. A moderately burned area was characterized by increased abundance and number of species. In 2023, the degree of similarity of carabid populations between sample plots did not generally increase, but only the 2010 fire was identified as a significant factor in population differentiation. The abundance of carabids decreased in a moderately burned area and an unburned area near the fire edge. The abundance and number of species increased markedly in heavily burned areas due to open habitat species, while the abundance of Carabus arcensis and Amara brunnea decreased in unburned areas. Forest megafires threaten the biodiversity of carabid beetles in pine and secondary forests.
1. Introduction
The study of wildfires occupies a prominent position within the field of ecological science and practice [1,2,3,4,5,6]. From a local perspective and in the short term, fire is perceived as a catastrophic disturbance to the forest ecosystem. At the scale of large areas and over the long term, fire is an important tool for maintaining biodiversity in ecosystems [7,8]. Fires of moderate intensity and frequency are considered favorable for maintaining biodiversity [9,10,11,12,13]. However, the specific levels of pyrogenic disturbances remain poorly studied. Traditionally, wildfires in boreal forests have been considered to be a rare event, occurring less than once per century. Wildfires have recently become more frequent in numerous regions. An important conservation issue is how this will affect biodiversity [9,14,15].
Carabidae (Coleoptera) have been a common object of studies on the effects of fire on biodiversity. The high species diversity and abundance of Carabidae, combined with the relative simplicity of study techniques, make this family well suited for ecological studies. A large number of papers have been published on this topic, all of which do not allow citation of the article format [16,17,18,19,20]. The existence of pyrophilous species among carabids is generally recognized. Under the influence of fire, the proportion of macropterous and open habitat species in forests increases [21]. However, several aspects of the effects of wildfire on carabid taxocenes remain controversial. Species diversity and abundance of carabids are generally reduced in wildfire-affected areas, but some studies have reported the opposite effect see [20,21,22,23,24,25]. How does post-fire colonization of an area occur by flying individuals from distant habitats, by walking migrants from adjacent unburned areas, or by individuals that have survived the fire in locally less disturbed or undisturbed areas within a disturbed ecosystem called perfugia by Gongalski [26]? How does the succession of carabid complexes occur after fire: how fast, what are the patterns, and what are the mechanisms? In coniferous forests, the post-fire cycle of forest regeneration and the carabid communities within them is long, lasting many decades [27]. What is the effect of repeated fires before the regeneration cycle is complete? These issues indicate the need for further research on the effects of megafires on Carabidae fauna and abundance.
The geographical aspect, which determines the structural and functional characteristics of forest ecosystems and the species composition of Carabidae, is also of great interest. Most of the studies that have revealed these regularities have been conducted in the boreal forests of Northern Europe and North America [28,29]. Opposite tendencies are often observed in the forest-steppe zone [30,31]. The zone of mixed and broad-leaved forests is of particular interest. Studies in the zone of mixed and broad-leaved forests are few [20,32]. In Mordovia, the effects of the 2010 fire were studied five years later [33]. The number of carabid species increased after the fire, but the abundance decreased, especially of the dominant species Carabus arcensis and Pterostichus oblongopucntatus. The relatively short period of material collection did not allow us to identify all the patterns of forest fire effects on this group of animals, and the results obtained showed the need to continue research in this direction. Also immediately after the 2021 fires, flying insect forms were examined in several plots, including control plots that had not burned for a long time [34].
The present paper analyzes the results of a natural experiment set up by nature in a large number of pine and secondary forests in the center of the East European Plain, in the zone of mixed and broad-leaved forests. Wildfires covered a significant area in 2010 and 2021, and the fire contours were not identical, so there are areas with different combinations, different intensities, and different distances from the fire edge. A complex of carabids was studied for two years, the first and second year after the 2021 fire. The objectives were 1) to compare plots that burned and did not burn in 2010 and 2021, as well as plots that differed in burn intensity and were located at different distances from the fire edge, in the first year after the 2021 fire in terms of total abundance and species diversity of carabids, species traits, and abundance of individual species; 2) to identify differentiation of carabid complexes depending on characteristics of attitude to fire; 3) to identify changes in studied traits and similarities of carabid complexes in the second year after the 2021 fire.
2. Materials and Methods
2.1. Study Area
The research was conducted on the territory of the Mordovia State Nature Reserve (Central European Russia) (Figure 1). The total area of the Mordovia State Nature Reserve is 321.62 km2. The reserve is located on the southern border of the taiga zone and on the border with forest-steppe ecosystems. Most of the protected area is covered by forest ecosystems, some of which were affected by fires in July and August 2010. These fires in many areas have contributed to large debris of dry trees, deadwood, and the appearance of dead trees. Then new megafires were observed in August 2021. In the past, Pinus sylvestris L. was the main forest forming tree species in this area. Pine trees formed communities of pure or mixed forests. However, after the fires of 2010, many pine forests were damaged and replaced by thickets of Betula pendula Roth. Tilia cordata Mill. forms communities of pure forests in the northern part of the protected area. Oak forests occupy relatively small areas and are widespread in the floodplain of the Moksha River in the western part of the protected area. Soils are classified as predominantly sandy with varying degrees of podzolization in most of the territory. The average annual precipitation is 406.6–681.3 mm. The average annual air temperature is 4.7 °C. Maximum values are recorded in July and minimal values in February [35].
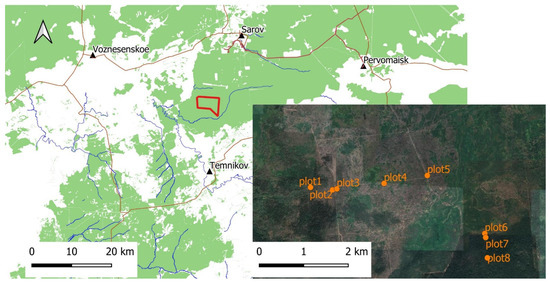
Figure 1.
Maps of the plots: location of the study area on the map (cartographic base is Open Street Map) and location of the plot on Google Satellite Image.
Studies were conducted in eight plots, which are briefly described in Table 1.

Table 1.
Brief description of the plots.
2.2. Sampling Procedures
Plastic cups were used as pitfall traps. Pitfall traps were placed in a line of 10 traps in a single row. The distance between the traps was 1.5 m. The upper diameter of such a cup is 93 mm, the lower diameter is 55 mm, and the height is 142 mm. The fixative was 4% formalin. The traps were placed from April to October in 2022 and 2023. Intervals between sampling of material varied from 15 to 20 days. A total of 8667 adult carabids were counted.
2.3. Data Analysis
We analyze two species traits: wing morphology (brachypterous, macropterous, dimorphic) and feeding (zoophagous [carnivore], herbivore [granivore], omnivore) according to some references [36,37,38,39]. As zoophagous we consider all species assigned to the corresponding class of adult life forms in Sharova’s system [40]. Within the class of myxophytophagous, we consider as herbivores (seed-eating, granivorous) those species for which there is evidence of preferential feeding on plant food [40,41,42], other representatives of myxophytophagous are considered as omnivores. Forest specialist, forest generalist, and open habitat species were distinguished by habitat preference; their abundance was not summarized, but the information was used to discuss the distribution of numerous species. Life cycles were characterized according to the work of A.V. Matalin [43].
As an indicator of abundance, the activity density of carabids, measured in ex per 100 trap days, was estimated. Diversity indices (Shannon index H, Simpson index in form 1-D, Berger–Parker index) were calculated in the program Past version 4.04. The statistical significance of Shannon index differences was evaluated by t-test [44]. The R software environment version 4.1.3 [45] was applied for all other statistical processing. The analysis of variance (ANOVA) function was applied to evaluate the influence of the factors. The multivariate analysis was conducted using the vegan 2.6–4 package [46].
3. Results
3.1. Species Composition and Structure
A total of 108 species were identified in the study area over the two-year period (Table 2). However, most species were represented by single specimens. In total for both years, only 50 species were observed with an abundance of 10 or more specimens. Nineteen species can be considered relatively abundant (Figure 2), and their distribution is discussed below. The most abundant and frequently occurring species was Harpalus rufipes (De Geer, 1774). Then five species—Pterostichus quadrifoveolatus Letzner, 1852, Carabus arcensis baschkiricus Breuning, 1932, Carabus glabratus Paykull, 1790, Amara brunnea (Gyllenhal, 1810), and Poecilus lepidus (Leske, 1785)—followed with a large gap to other carabids. The species Pterostichus niger (Schaller, 1783), Pterostichus oblongopunctatus (Fabricius, 1787), and Calathus micropterus (Duftschmid, 1812) were locally abundant, although their occurrence was not particularly high. The species Amara bifrons (Gyllenhal, 1810), Harpalus latus (Linnaeus, 1758), and Harpalus rubripes (Duftschmid, 1812) were observed with high occurrence but moderate abundance.

Table 2.
Species composition of carabids collected during two years. “Specimens” means total number of specimens collected during each year from all plots. “Number of Plots” is number of sample plots which a species was found during each year (max = 8). Sign * means species considered as omnivorous, **—species considered as herbivorous, other species are considered as zoophagous.
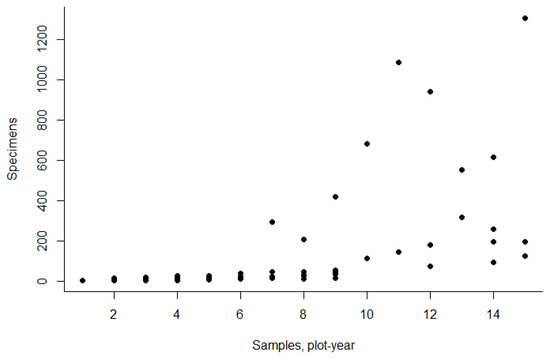
Figure 2.
Occurrence (number of samples, plot per year) and abundance (number of specimens) of carabid species.
The carabid population in the control plot should be characterized (plot 8). Although the total number of species varies between years (28 and 37), the set of dominant species is stable. All of these species are typical of pine forests [37,47,48,49]. They belong to different groups of life forms. Carabus arcensis and C. glabratus are zoophagous walking epigeobionts, Pterostichus oblongopunctatus is a zoophagous soil-litter stratobiont, and Amara brunnea is a myxophytophagous geochorthobiont. The first two species are differentiated by size and life cycle (breeding and overwintering stages). Thus, a well-structured complex of carabids was formed in the control plot.
3.2. Changes in the First Year after the Megafire
The minimal species number was obtained in the heavily burned plots (plots 3–5) (Table 3). The maximal species number was obtained in the moderately burned plot (plot 6) and in the unburned plot close to the burned plot (plot 7). Belonging to a burned or unburned plot of 2010 or 2021 is not a statistically significant factor (Table 4).

Table 3.
Summary of characteristics of the carabid assemblages in the studied plots. Upper line—2022, lower line—2023.

Table 4.
Effect of 2010 and 2021 fires on some characteristics of carabid complexes (ANOVA results). All variables excluding of species number are dynamic density.
Abundance also showed no statistically significant differences between burned and unburned plots in 2010, and the same was true for the 2021 fire (Table 4). However, the lowest abundance was found in heavily burned plots (plot 4 and plot 5), while the highest abundance was found in moderately burned and unburned plots (plot 6, plot 7, plot 8).
The highest Shannon index diversity was observed in the unburned plot near the edge of the 2021 fire (plot 7), and in the plots affected only by the 2010 fire (plot 1 and plot 2). In both the heavily burned plots and the control plot, diversity is considerably lower (differences are statistically significant). The Simpson index is similarly distributed; conversely, the Berger-Parker index (Table 3).
The most pronounced differences in the fauna between plots are observed in the abundance of brachypterous carabids, which are two orders of magnitude higher in unburned and moderately burned plots than in heavily burned plots (Table 3). Accordingly, brachypterous carabids are absolutely dominant in the control plot, accounting for 74%, and only 2–8% in plots that were heavily burned in 2010 or 2021 (plots 1–5). Ownership of burned or unburned plots was not statistically significant (Table 4). However, a significant correlation with the distance from the edge of the fire was revealed: brachypterous carabids are practically absent in the depth of the burned massif, far from the fire they are very abundant, and near the edge of the fire different variants are realized (Figure 3). The differences in abundance of macropterous species are not so great; they are most abundant in the moderately burned area (plot 6).
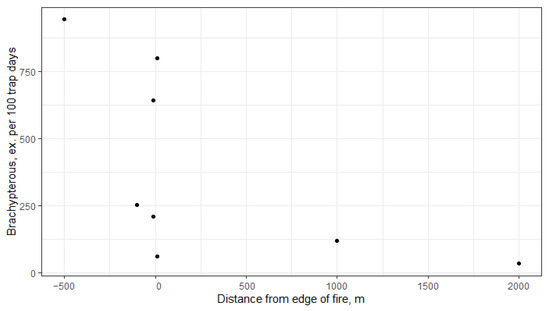
Figure 3.
Brachypterous carabid abundance depends on distance from the edge of fire. Spearman’s rank correlation rho = −0.735, p-value = 0.0378.
Herbivorous carabids reduced their abundance very strongly from the control plot to the center of the fire, with the effect of belonging to the area burned in 2010 being statistically significant (Table 4). There is also a significant non-linear correlation with distance from the edge of the fire (Figure 4). Omnivorous carabids are most abundant in the intermediate disturbance zone—plot 6 and plot 7, and belonging to a burned area is not significant (Table 4). The proportion of omnivorous carabids was greater in all fire-affected plots than in the control, although this was not proportional to burn intensity and distance. The greatest proportion was observed in plots 3 and 4.
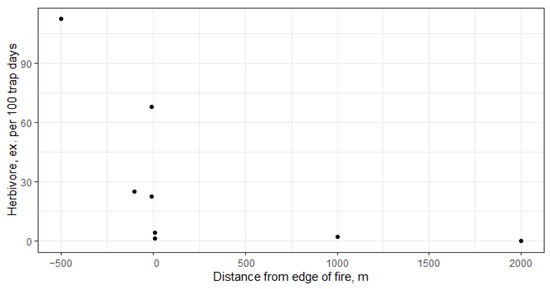
Figure 4.
Herbivore carabid abundance depends on distance from the edge of fire. Spearman’s rank correlation rho = −0.916, p-value = 0.0014.
With regard to species structure, carabid complexes are clearly arranged along a gradient of pyrogenic disturbance, from undisturbed (plot 8) to heavily burned (plot 5, plot 4, plot 3) (Figure 5). Significant differences in species structure are characteristic of plots burned in both 2010 and 2021, with the 2010 fire accounting for more variability (Table 5). The initial two dimensions of NMDS, which describe the differentiation of carabid complexes, are significantly correlated with the abundance of herbivorous carabids and the abundance of brachypterous species (Table 6). The distance from the edge of the fire did not appear to be a significant factor in differentiating carabid complexes. This is presumably due to a non-linear relationship.
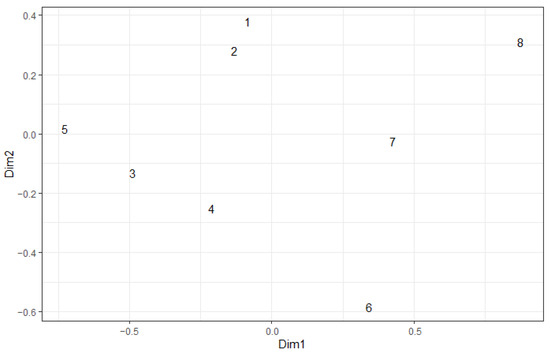
Figure 5.
NMDS ordination plot of carabid complexes in 2022. Jaccard index for non-binary data. 1–8 are numbers of plots.

Table 5.
Effect of 2010 and 2021 fires on carabid assemblages structure: Permutational Multivariate Analysis of Variance Using Distance Matrices’.

Table 6.
Correlation of selected variables with the first two NMDS dimensions.
The dominant carabids in the undisturbed forest (plot 8) were C. arcensis, C. glabratus, P. oblongopunctatus, and A. brunnea. These species were sensitive to the 2010 fire, while the 2021 repeated fire had no significant effect on their abundance (Table 4). Both pyrophilous species showed no significant relationship with area burned in 2010 or 2021 (Table 4). Pterostichus quadrifoveolatus was common, but was most abundant in the partially burned plot (plot 6) where it dominated (47%) among the carabids. Sericoda quadripunctata was found only in the partially burned plot and only as a single specimen.
Harpalus rufipes was the superdominant species in the completely burned plots (plot 3, plot 4 and plot 5). It was most abundant in moderately burned and unburned plots near the edge of the fire (plots 6 and 7), although it was more abundant in a heavily burned plot at a moderate distance from the edge of the fire (plot 4) than in similar plots near the edge of the fire and in the deepest part of the fire. Pterostichus niger was most abundant in the moderately burned plot (plot 6), while it was virtually absent from the heavily burned plots. Poecilus lepidus preferred plots that burned in 2010 and plots that did not burn in 2021 (plot 1 and plot 2). Both fires were therefore significant.
3.3. Changes in the Second Year after the Megafire
In the second year of the study, the number of species increased in all plots except plot 7. However, in heavily burned plots 4 and 5, the increase in species is much higher (2.5 times). But all the species that appeared in 2023 were single species. Shannon and Simpson index values increased significantly in all sample plots, but most significantly in the control plot and plots exposed to the 2021 fire.
Dynamic density increased most (3.2 times) in the heavily burned plot close to the unburned plot (plot 3). In the heavily burned plot at the epicenter of the fire (plot 5), there was a significant increase (1.8 times). However, it remained significantly lower than in the other plots. In the control plot, the dynamic density was virtually unchanged. The moderately burned plot (plot 6) and the unburned plot close to the edge of the fire (plot 7) had a 30% decrease in dynamic density.
The abundance of brachypterous decreased in 2023. The abundance of macropterous increased except in plot 7. The dynamic density of herbivorous decreased. The dynamic density of omnivorous became highest in plot 2 at the expense of Harpalus, increased significantly in the control plot (plot 8) at the expense of Amara. In 2023 compared to 2022, the dynamic density of the forest species C. arcensis and A. brunnea decreased and the abundance of Amara communis (Panzer, 1797) increased in both unburned plots (Table 3). The pyrophilous species P. quadrifoveolatus decreased in abundance in both plots where it was relatively abundant (plot 6 and plot 7). Therefore, the abundance of several myxophytophagous, open habitat species has increased in the study area: Amara bifrons (Gyllenhal, 1810), Amara consularis (Duftschmid, 1812), Harpalus latus (Linnaeus, 1758), Harpalus smaragdinus (Duftschmid, 1812), Harpalus tardus (Panzer, 1796). Harpalus rufipes became more evenly distributed across the study area, decreasing in abundance in plots 6 and 7 and increasing in most of the other plots. P. niger became less abundant in plot 6, while its abundance doubled in plot 8. In plot 7, the abundance of the forest species P. oblongopunctatus and the meadow species Poecilus versicolor decreased significantly.
In general, species structure differentiation from unburned to heavily burned was maintained. However, plots affected by fire only in 2010 (plots 1 and 2) now are not contrast to plots affected by both 2010 and 2021 fires (Figure 6). That is, the 2021 fire effect was no longer statistically significant by MANOVA (Table 5). The study year has no significant effect on the degree of similarity between sample plots (Table 7), i.e., differences between sample plots have not smoothed out in the second year after the fire.
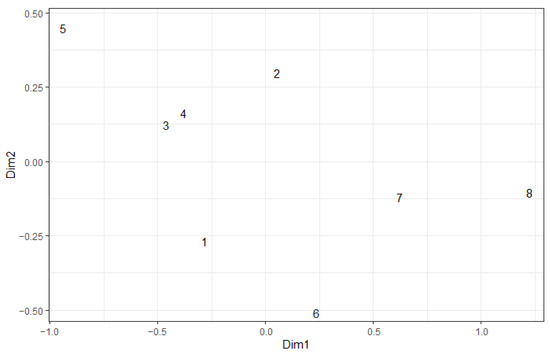
Figure 6.
NMDS ordination plot of carabid complexes in 2023. Jaccard index for non-binary data. 1–8 are numbers of plots.

Table 7.
Effect of year on similarity matrix (Jaccard index for non-binary data) of carabids: Multivariate homogeneity of groups dispersions.
4. Discussion
The negative effect of fire on total abundance and the number of carabid species is consistent with a central tendency observed in boreal forests [19,21,23], which distinguishes them from forest-steppe and steppe ecosystems [30,31,50]. While the opposite tendency of positive response is also observed in the mixed and broadleaved forest zone [20], the increase in the number of species is often due to migrants and sporadic species [32]. At the same time, the increase in dynamic density may be the effect of an increase in carabid activity after fire, rather than an increase in abundance [51,52]. The mechanisms of the negative effects of fire are diverse; in addition to the direct effects, there is the degradation of shelter and foraging habitat. Fire-affected plots had fewer insects in beer traps [53]. An increase in species richness and dynamic density of carabids in the intermediate disturbance plot (partially burned) is also an expected effect [54]. The effect of fire on this plot is confirmed by a decrease in the dynamic density of carabids the following year. The effect of fire on individual carabid groups was more unexpected.
Pyrophilous species should be most abundant in heavily burned plots [55,56,57,58]. In our case, their attraction to moderately burned plots was found with almost complete absence in heavily burned plots. Although the abundance of pyrophilous carabids decreases each year after fire, their dominance persists for more than a year [32,59]. Therefore, pyrophilous species could not have disappeared before traps were set in the burned plots. The most likely reason for this effect is the extremely low amount of wood burned in 2021, as most of it was burned in 2010, and pyrophilous species are attracted by gases, the products of wood combustion [55]. In any case, the previous wildfire is, in many ways, similar to logging, which also negatively affects pyrophilous species in Northern Europe [23,60]. In the case of logging, the presence of previously invaded open habitat species is also assumed as a hindering mechanism. However, we have no basis for such an assumption, as open habitat species are also rare in heavily burned plots. For Sericoda quadripunctata we can also assume the initial low abundance in the studied landscape, which does not allow to occupy a large-scale fire area [20]. In some models, the existence of pyrophilous species has been shown to be influenced by the carrying capacity of the unburned matrix rather than fire frequency [61].
The herbaceous vegetation in the studied pine and secondary forests explains the negative effect on the herbivore Carabidae. This is the difference between the study area and boreal forests with moss and lichen cover and poor herbaceous cover, and herbaceous ecosystems in hotter and drier conditions where, in the absence of fire, the ground cover is poorly fodder for herbivores or granivores [31]. At the same time, it is possible that the observed effect is due to individual traits of the dominant species, Amara brunnea, a species characteristic of old coniferous forests [62].
The expected effect in the second year after megafires is a reduction in the abundance of pyrophilous species [59]. The increase in the number of species in the landscape as a whole is apparently the result of random processes. The detection of more species may have been influenced by the longer exposure time of the traps overlapping the spring migration. The weather conditions of the year, which determine migration activity, may also have had an effect. It is not possible to speak about the occurrence of new species in the sample plots because of the small number of individuals. The situation is different for plots that burned heavily in 2021. Although all new species are also sporadic compared to 2022, there is a notable increase in the abundance of already named meadow-field species (genera Harpalus and Amara) in 2023, which suggests that these plots are inhabited. The “first settler” of burned areas, H. rufipes, as well as the species of the named genera that settled in the second year, is typically united with the latter in the group of open habitat species. However, it is primarily a field species that inhabits cultivated and disturbed lands with moderate projective cover [39,63].
It is of greater interest to note the effect of a decrease in the abundance of stenotopic forest species in unburned plots, while an increase in the abundance of some meadow-field carabids is observed. For a plot close to the edge of the fire, this may be due to attenuating the effects of the intermediate disturbance as well as the edge effect. For a plot far from the fire, it can be explained by both weather effects and delayed effects of the fire. It is possible that the landscape as a whole has become more attractive to meadow species as a result of the recovery of herbaceous vegetation after the fire. Different species life cycles explain the uneven dynamics of abundance see [43]. Specifically, the fire-intolerant C. arcensis and P. oblongopunctatus have an annual life cycle, breeding in the spring and overwintering as adults. A. brunnea is likely to develop within one year in this natural zone, although it overwinters at the larval stage (in the north, it has a biennial life cycle). C. glabratus, in which some individuals develop for two years, demonstrated resistance to changes. As for the uneven changes in the abundance of P. niger and H. rufipes in different biotopes, they may be the result of both the high mobility (good migratory abilities) of these species [64,65] and the presence of different generations in their populations due to the polyvariant life cycles [42,66].
The results obtained suggest that in the first years after fire, carabid populations in burned areas with very sparse or no vegetation sensu [67] as well as in unburned forest habitats show signs of meadow communities. The plots that burned in 2010, about a dozen years after the fire, now have communities dominated by meadow species. The plots in the second year after the 2021 megafire are still far from the structure of the carabid complex of the plots formed by the 2010 fire, although they are converging with them compared to the previous year. Thus, the results confirm the long time of community recovery in pine forests [27,68], in contrast to the rapid recovery in herbaceous ecosystems [30,49].
Several publications [23,26] show that the fauna and structure of carabid complexes in burned boreal forests are not determined by distance from the edge of the fire, but by the intensity of burnout. The recovery of carabid communities is not due to walking migrants from neighboring unburned areas, but to individuals that survived the fire in perfugia, as well as flying species from distant habitats. A similar situation is observed in the tallgrass prairie [49]. Our results do not contradict these ideas: heavily burned plots have a low abundance of brachypterous species, and colonization in the third year is by macropterous species. Therefore, the most likely source of colonization of heavily burned plots away from the edge is long-distance migrations.
5. Conclusions
Thus, the severe burnout of pine forests in the 2010 fire resulted in the degradation of the forest complex of carabids and its replacement by a complex close to the meadow complex. The latter is distinguished by a reduction in the dynamic density of carabids and the impoverishment of a number of life forms (e.g., zoophagous walking epigeobionts). A second fire, occurring 11 years after the initial major fire with severe burnout, resulted in the destruction of a secondary complex dominated by meadow species. The year after the fire, the area was a virtual pyrogenic desert, inhabited or visited by only a few generalist species. It is not until the second year after the fire that these plots are colonized by meadow species and some convergence with meadow complexes undisturbed by the megafire occurs, but the dominant species composition is still very different. The moderately burned plot was characterized by increased species number and dynamic density of some species in the year after the fire. However, the effect of increased dynamic density disappeared by the second year after the fire. At the same time, pyrophilous species were quite abundant only in the plot that was moderately burned. Our data suggest that, at present, fires negatively affect the carabid complex of pine and secondary forests of Central European Russia. The occurrence of fires in dry pine forests represents a significant threat to the region’s biodiversity, as these forests provide optimal habitat for numerous species.
Author Contributions
Conceptualization, A.B.R. and V.V.A.; methodology, S.K.A., A.B.R. and M.N.E.; software, A.B.R.; validation, A.B.R. and V.V.A.; formal analysis, A.B.R. and V.V.A.; investigation, M.N.E.; resources, M.N.E., S.K.A. and A.B.R.; data curation, V.V.A.; writing—original draft preparation, V.V.A. and A.B.R.; writing—review and editing, V.V.A.; visualization, V.V.A.; supervision, A.B.R.; project administration, A.B.R.; funding acquisition, A.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 22-14-00026.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Granström, A. Fire management for biodiversity in the European boreal forest. Scand. J. For. Res. 2001, 16 (Suppl. 3), 62–69. [Google Scholar] [CrossRef]
- Driscoll, D.A.; Lindenmayer, D.B.; Bennett, A.F.; Bode, M.; Bradstock, R.A.; Cary, G.J.; York, A. Fire management for biodiversity conservation: Key research questions and our capacity to answer them. Biol. Conserv. 2010, 143, 1928–1939. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef] [PubMed]
- Kharitonova, A.O.; Kharitonova, T.I. The effect of landscape pattern on the 2010 wildfire spread in the Mordovia State Nature Reserve, Russia. Nat. Conserv. Res. 2021, 6, 29–41. [Google Scholar] [CrossRef]
- Tiberio, F.C.S.; Xavier, R.O.; Dodonov, P.; Silva Matos, D.M. Fire has short-term negative effects on a super-dominant native fern, Pteridium arachnoideum (Dennstaedtiaceae), in a Brazilian savanna. Nat. Conserv. Res. 2022, 7, 15–25. [Google Scholar] [CrossRef]
- Atutova, Z.V. Post-fire restoration of pine forests in the Badary area, Tunkinskiy National Park, Russia. Nat. Conserv. Res. 2023, 8, 22–32. [Google Scholar] [CrossRef]
- Viljur, M.L.; Abella, S.R.; Adámek, M.; Alencar, J.B.R.; Barber, N.A.; Beudert, B.; Burkle, L.A.; Cagnolo, L.; Campos, B.R.; Chao, A.; et al. The effect of natural disturbances on forest biodiversity: An ecological synthesis. Biol. Rev. 2022, 97, 1930–1947. [Google Scholar] [CrossRef]
- Mahmoodzadeh, A.; Eisapoor, S.S.; Mirghiasy, S.A. Biodiversity in the third millennium. Sci. Rep. Life Sci. 2022, 3, 1–14. [Google Scholar] [CrossRef]
- Certini, G.; Moya, D.; Lucas-Borja, M.E.; Mastrolonardo, G. The impact of fire on soil-dwelling biota: A review. For. Ecol. Manag. 2021, 488, 118989. [Google Scholar] [CrossRef]
- El Khayati, M.; Chergui, B.; Barranco, P.; Fahd, S.; Ruiz, J.L.; Taheri, A.; Santos, X. Assessing the Response of Different Soil Arthropod Communities to Fire: A Case Study from Northwestern Africa. Fire 2023, 6, 206. [Google Scholar] [CrossRef]
- Vilkova, V.V.; Kazeev, K.S.; Privizentseva, D.A.; Nizhelsky, M.S.; Kolesnikov, S.I. Activity in post-pyrogenic soils in the Utrish State Nature Reserve (Russia) in the early succession stages. Nat. Conserv. Res. 2023, 8, 10–23. [Google Scholar] [CrossRef]
- Vilkova, V.V.; Kazeev, K.S.; Nizhelskiy, M.S.; Kolesnikov, S.I.; Kozun, Y.S. Changes in soil properties of xerophytic forests in Southern Russia after anthropogenic impact. Nat. Conserv. Res. 2024, 9, 61–72. [Google Scholar] [CrossRef]
- Bouderbala, I.; Labadie, G.; Béland, J.M.; Tremblay, J.A.; Boulanger, Y.; Hébert, C.; Desrosiers, P.; Allard, A.; Fortin, D. Long-term effect of forest harvesting on boreal species assemblages under climate change. PLoS Clim. 2023, 2, e0000179. [Google Scholar] [CrossRef]
- Mansoor, S.; Farooq, I.; Kachroo, M.M.; Mahmoud, A.E.D.; Fawzy, M.; Popescu, S.M.; Ahmad, P. Elevation in wildfire frequencies with respect to the climate change. J. Environ. Manag. 2022, 301, 113769. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, M.P.; Bobrovsky, M.V.; Shanin, V.N.; Khanina, L.G.; Grabarnik, P.Y.; Stamenov, M.N.; Ivanova, N.V. Data on 30-year stand dynamics in an old-growth broad-leaved forest in the Kaluzhskie Zaseki State Nature Reserve, Russia. Nat. Conserv. Res. 2022, 7 (Suppl. 1), 24–37. [Google Scholar] [CrossRef]
- Gandhi, K.J.; Spence, J.R.; Langor, D.W.; Morgantini, L.E. Fire residuals as habitat reserves for epigaeic beetles (Coleoptera: Carabidae and Staphylinidae). Biol. Conserv. 2001, 102, 131–141. [Google Scholar] [CrossRef]
- Saint-Germain, M.; Larrivée, M.; Drapeau, P.; Fahrig, L.; Buddle, C.M. Short-term response of ground beetles (Coleoptera: Carabidae) to fire and logging in a spruce-dominated boreal landscape. For. Ecol. Manag. 2005, 212, 118–126. [Google Scholar] [CrossRef]
- Gongalsky, K.; Midtgaard, F.; Overgaard, H. Effects of prescribed forest burning on carabid beetles (Coleoptera: Carabidae): A case study in south-eastern Norway. Entomol. Fenn. 2006, 17, 325–333. [Google Scholar] [CrossRef]
- Mason, S.C., Jr.; Shirey, V.; Waite, E.S.; Gallagher, M.R.; Skowronski, N.S. Exploring Prescribed Fire Severity Effects on Ground Beetle (Coleoptera: Carabidae) Taxonomic and Functional Community Composition. Fire 2023, 6, 366. [Google Scholar] [CrossRef]
- Zumr, V.; Remeš, J.; Nakládal, O. Short-Term Response of Ground Beetles (Coleoptera: Carabidae) to Fire in Formerly Managed Coniferous Forest in Central Europe. Fire 2024, 7, 76. [Google Scholar] [CrossRef]
- Holliday, N.J. Species responses of carabid beetles (Coleoptera: Carabidae) during post-fire regeneration of boreal forest. Can. Entomol. 1991, 123, 1369–1389. [Google Scholar] [CrossRef]
- Fernández, M.F.; Costas, J.S. Recolonization of a burnt pine forest (Pinus pinaster) by Carabidae (Coleoptera). Eur. J. Soil Biol. 2004, 40, 47–53. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Wikars, L.-O.; Persson, T. Ground beetle (Coleoptera: Carabidae) responses to a forest wildfire in northern Europe. Russ. Entomol. J. 2008, 17, 273–282. [Google Scholar]
- Mason, S.C., Jr.; Shirey, V.; Ponisio, L.C.; Gelhaus, J.K. Responses from bees, butterflies, and ground beetles to different fire and site characteristics: A global meta-analysis. Biol. Conserv. 2021, 261, 109265. [Google Scholar] [CrossRef]
- Belluz, V.; Langor, D.W.; Niemelä, J.K.; He, F.; Spence, J.R. Long-term responses of ground beetles (Coleoptera: Carabidae) to clear-cutting and wildfire in lodgepole pine stands of western Alberta, Canada. Can. Entomol. 2022, 154, e41. [Google Scholar] [CrossRef]
- Gongalsky, K.B. Perfugia as a mechanism for the recovery of soil fauna after ecosystem disturbances. Russ. J. Ecosyst. Ecol. 2017, 2. [Google Scholar] [CrossRef]
- Paquin, P. Carabid beetle (Coleoptera: Carabidae) diversity in the black spruce succession of eastern Canada. Biol. Conserv. 2008, 141, 261–275. [Google Scholar] [CrossRef]
- Lassau, S.A.; Hochuli, D.F.; Cassis, G.; Reid, C.A. Effects of habitat complexity on forest beetle diversity: Do functional groups respond consistently? Divers. Distrib. 2005, 11, 73–82. [Google Scholar] [CrossRef]
- Toïgo, M.; Paillet, Y.; Noblecourt, T.; Soldati, F.; Gosselin, F.; Dauffy-Richard, E. Does forest management abandonment matter more than habitat characteristics for ground beetles? Biol. Conserv. 2013, 157, 215–224. [Google Scholar] [CrossRef]
- Mordkovich, V.G.; Berezina, O.G. Effect of fire on the pedobiont communities of a birch-aspen grove in the southern forest-steppe of West Siberia. Euroasian Entomol. J. 2009, 8, 279–283. [Google Scholar]
- Samu, F.; Kádár, F.; Ónodi, G.; Kertész, M.; Szirányi, A.; Szita, É.; Fetykó, K.; Neidert, D.; Botos, E.; Altbäcker, V. Differential ecological responses of two generalist arthropod groups, spiders and carabid beetles (Araneae, Carabidae), to the effects of wildfire. Community Ecol. 2010, 11, 129–139. [Google Scholar] [CrossRef]
- Matalin, A.V.; Trushitsina, O.S.; Makarov, K.V. Influence of different types of wildfire on the community structure of ground beetles (Coleoptera, Carabidae) in pine forests of the Meshchera Lowlands. In Proceedings of the 18th European Carabidologist Meeting, Rennes, France, 25–29 September 2017; p. 87. [Google Scholar]
- Ruchin, A.B.; Alekseev, S.K.; Khapugin, A.A. Post-fire fauna of carabid beetles (Coleoptera, Carabidae) in forests of the Mordovia State Nature Reserve (Russia). Nat. Conserv. Res. 2019, 4 (Suppl. 1), 11–20. [Google Scholar] [CrossRef]
- Ruchin, A.B. The selected insect families and their seasonal dynamics in the Mordovia State nature reserve in the burned areas of 2021. J. Wildl. Biodivers. 2024, 8, 17–38. [Google Scholar] [CrossRef]
- Khapugin, A.A.; Vargot, E.V.; Chugunov, G.G. Vegetation recovery in fire-damaged forests: A case study at the southern boundary of the taiga zone. For. Stud. 2016, 64, 39–50. [Google Scholar] [CrossRef]
- Koch, K. Die Käfer Mitteleuropas: Ökologie. Bd. 1; Goecke & Evers: Keltern, Germany, 1989; 440p. [Google Scholar]
- Lindroth, C.H. Ground Beetles (Carabidae) of Fennoscandia: A Zoogeographic Study: Part 1. Specific Knowledge regarding the Species; Intercept Ltd.: Andover, UK, 1992; pp. xxviii + 630. [Google Scholar]
- Ribera, I.; Foster, G.N.; Downie, I.S.; McCracken, D.I.; Abernethy, V.J. A comparative study of the morphology and life traits of Scottish ground beetles (Coleoptera, Carabidae). Ann. Zool. Fenn. 1999, 36, 21–37. [Google Scholar]
- Aleksanov, V.V.; Alekseev, S.K. Inventory of the Ground Beetles (Coleoptera, Carabidae) of Kaluga Urban Okrug; Ministry of Natural Resources and Ecology of the Kaluga Oblast: Izhevsk, Russia, 2019; 278p.
- Sharova, I.K. Life Forms of Ground Beetles (Coleoptera, Carabidae); Nauka: Moscow, Russia, 1981; 24p. [Google Scholar]
- Hengeveld, R. Polyphagy, oligophagy and food specialization in ground beetles (Coleoptera: Carabidae). Neth. J. Zool. 1980, 30, 564–584. [Google Scholar] [CrossRef]
- Talarico, F.; Giglio, A.; Pizzolotto, R.; Brandmayr, P. A synthesis of feeding habits and reproduction rhythm in Italian seed-feeding ground beetles (Coleoptera: Carabidae). EJE 2016, 113, 325–336. [Google Scholar] [CrossRef]
- Matalin, A.V. The life cycles of grounds beetles (Coleoptera, Carabidae) in West Palaearctic. Ph.D. Thesis, Moscow State Pedagogical University, Moscow, Russia, 2011; 549p. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 June 2024).
- Oksanen, J. Vegan: Ecological Diversity. Available online: http://cran.r-project.org/web/packages/vegan/vignettes/diversity-vegan.pdf (accessed on 1 October 2022).
- Fedorenko, D.N. The fauna of ground-beetles (Coleoptera, Carabidae) of Moscow Province. In Insects of the Moscow Region: Problems of Cadastre and Protection; Nauka: Moscow, Russia, 1988; pp. 20–46. [Google Scholar]
- Skłodowski, J.; Garbalińska, P. Ground beetle (Coleoptera, Carabidae) assemblages inhabiting Scots pine stands of Puszcza Piska Forest: Six-year responses to a tornado impact. In ZooKeys, Carabid Beetles as Bioindicators: Biogeographical, Ecological and Environmental Studies; Kotze, D.J., Assmann, T., Noordijk, J., Turin, H., Vermeulen, R., Eds.; Pensoft Publishers: Moscow, Russia, 2011; Volume 100, pp. 371–392. [Google Scholar] [CrossRef]
- Alekseev, S.K.; Ruchin, A.B. Fauna and abundance of ground beetle (Coleoptera, Carabidae) in pine forests. Entomol. Appl. Sci. Lett. 2020, 7, 1–9. [Google Scholar]
- Cook, W.M.; Holt, R.D. Fire frequency and mosaic burning effects on a tallgrass prairie ground beetle assemblage. Biodivers. Conserv. 2006, 15, 2301–2323. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Bergeron, J.A.C.; Work, T.T.; Spence, J.R. Low intensity surface fire instigates movement by adults of Calosoma frigidum (Coleoptera, Carabidae). ZooKeys 2011, 147, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Ariza, G.M.; Jácome, J.; Kotze, D.J. Carabid beetles of tropical dry forests display traits that cope with a harsh environment. Int. J. Trop. Insect Sci. 2021, 41, 3011–3021. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Egorov, L.V.; MacGowan, I.; Makarkin, V.N.; Antropov, A.V.; Gornostaev, N.G.; Khapugin, A.A.; Dvořák, L.; Esin, M.N. Post-fire insect fauna explored by crown fermental traps in forests of the European Russia. Sci. Rep. 2021, 11, 21334. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Duelli, P.; Obrist, M.K. Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 2006, 149, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Gonganlsky, K.B.; Wikars, L.-O.; Persson, T. Dynamics of pyrophilous carabids in a burned pine forest in Central Sweden. Baltic, J. Coleopterol. 2003, 3, 107–111. [Google Scholar]
- Pradella, C.; Wermelinger, B.; Obrist, M.K.; Duelli, P.; Moretti, M. On the occurrence of five pyrophilous beetle species in the Swiss Central Alps (Leuk, Canton Valais). Mitteilungen Schweiz. Entomol. Ges. 2010, 83, 187–197. [Google Scholar]
- Bell, A.J.; Calladine, K.S.; Wardle, D.A.; Phillips, I.D. Rapid colonization of the post-burn environment improves egg survival in pyrophilic ground beetles. Ecosphere 2022, 13, e4213. [Google Scholar] [CrossRef]
- Bell, A.J. Like moths to a flame: A review of what we know about pyrophilic insects. For. Ecol. Manag. 2023, 528, 120629. [Google Scholar] [CrossRef]
- Süda, I.; Voolma, K.; Õunap, H. Short-term monitoring of fire-adapted Coleoptera in burnt pine forest of northern Estonia. Acta Biol. Univ. Daugavp. 2009, 9, 43–48. [Google Scholar]
- Wikars, L.O. Clear-cutting before burning prevents establishment of the fire-adapted Agonum quadripunctatum (Coleoptera: Carabidae). Ann. Zool. Fenn. 1995, 32, 375–384. [Google Scholar]
- Saint-Germain, M.; Drapeau, P.; Buddle, C.M. Persistence of pyrophilous insects in fire-driven boreal forests: Population dynamics in burned and unburned habitats. Divers. Distrib. 2008, 14, 713–720. [Google Scholar] [CrossRef]
- Niemelä, J.; Haila, Y.; Halme, E.; Lahti, T.; Pajunen, T.; Punttila, P. The distribution of carabid beetles in fragments of old coniferous taiga and adjacent managed forest. Ann. Zool. Fenn. 1988, 25, 107–119. [Google Scholar]
- Wallin, H. Spatial and temporal distribution of some abundance carabid beetles (Col., Carabidae) in cereal fields and adjacent habitats. Pedobiologia 1985, 28, 19–34. [Google Scholar]
- Wallin, H.; Ekbom, B.S. Movements of carabid beetles (Coleoptera: Carabidae) inhabiting cereal fields: A field tracing study. Oecologia 1988, 77, 39–43. [Google Scholar] [CrossRef]
- Zhang, J.; Drummond, F.A.; Liebman, M.; Hartke, A. Phenology and dispersal of Harpalus rufipes De Geer (Coleoptera: Carabidae) in agroecosystems in Maine. J. Agric. Entomol. 1997, 14, 171–186. [Google Scholar]
- Matalin, A.V. Evolution of biennial life cycles in ground beetles (Coleoptera, Carabidae) of the Western Palaearctic. In Proceedings of the XIII European Carabidologist Meeting, Blagoevgrad, Bulgaria, 20–24 August 2008; Back to the roots and back to the future. pp. 259–284. [Google Scholar]
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS habitat classification revised 2004. Report to: European Environment Agency-European Topic Centre on Nature Protection and Biodiversity. Estuar. Coast. Shelf Sci. 2004, 62, 127–143. [Google Scholar]
- Skłodowski, J. Multi-phase recovery of carabid assemblages during 19 years of secondary succession in forest stands disturbed by windstorm without salvage logging in northern Poland. Sci. Total Environ. 2023, 862, 160763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).