Bromeliad-Dwelling Frogs Revealed by Citizen Scientists
Abstract
1. Introduction
2. Materials and Methods
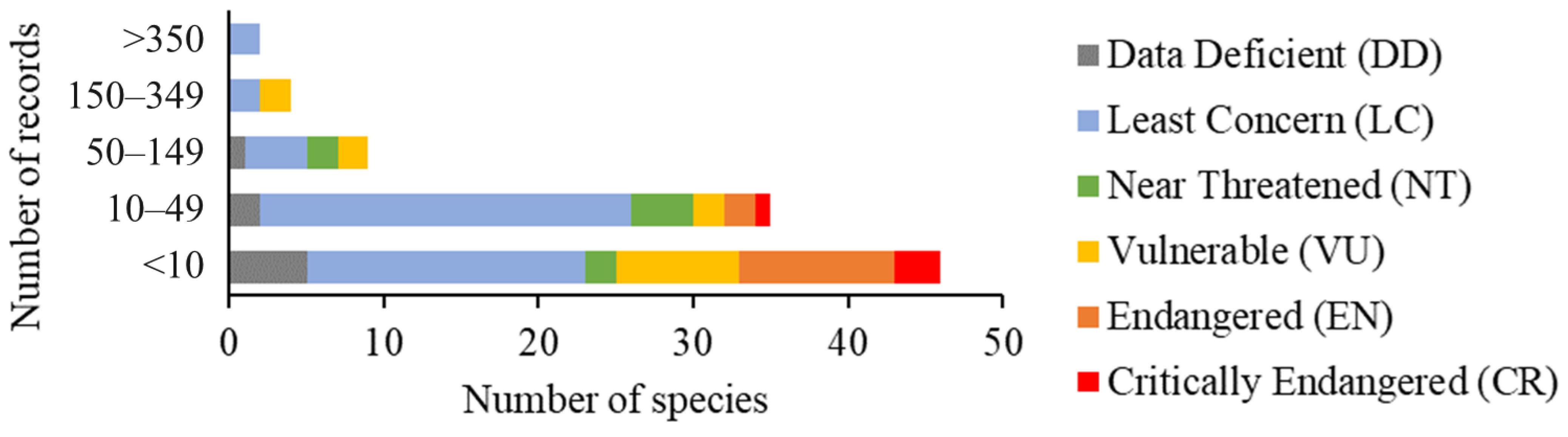
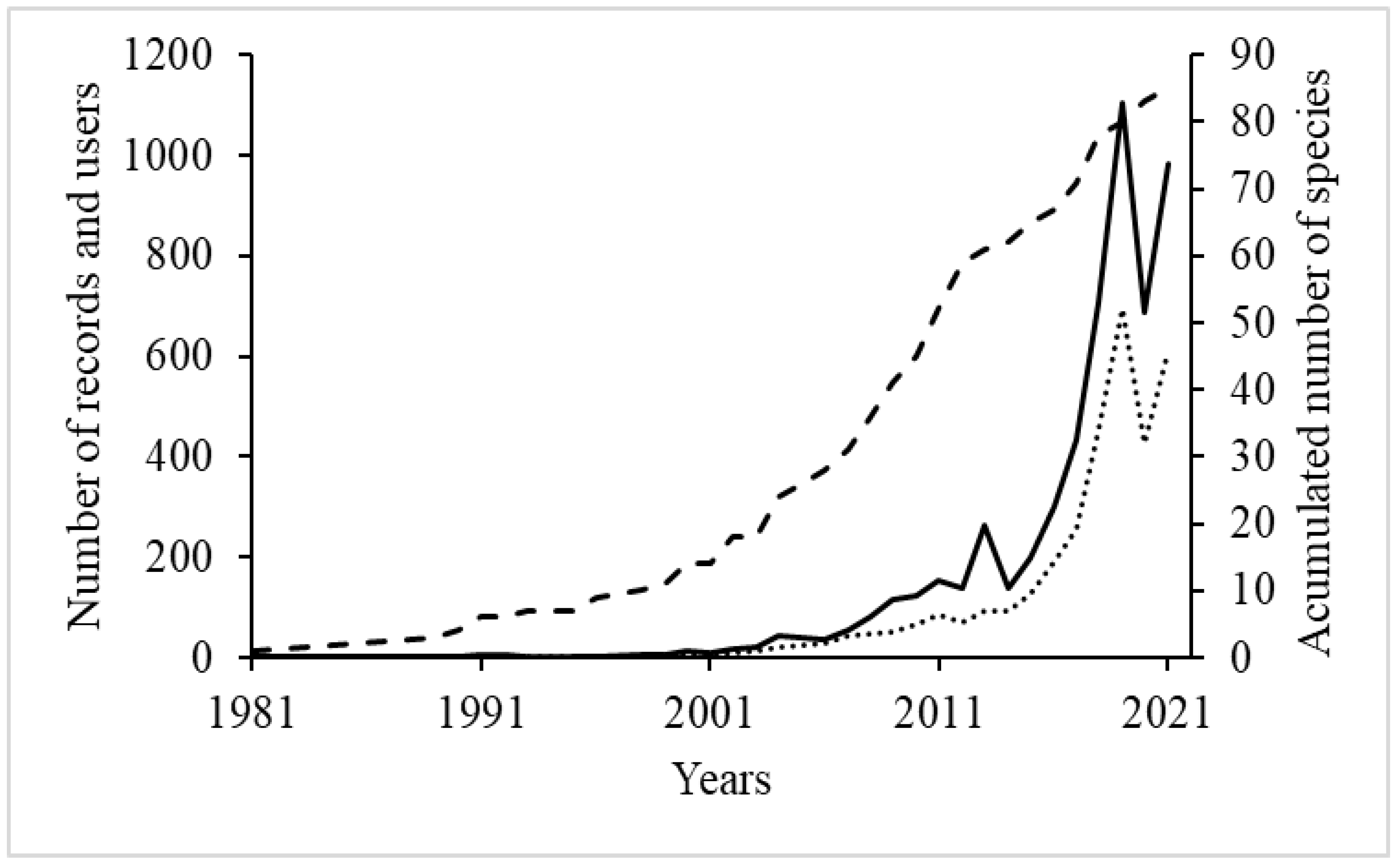
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoccoz, N.G.; Nichols, J.D.; Boulinier, T. Monitoring of biological diversity in space and time. Trends Ecol. Evol. 2001, 16, 446–453. [Google Scholar] [CrossRef]
- Paterson, J.S.; Araújo, M.B.; Berry, P.M.; Piper, J.M.; Rounsevell, M.D.A. Mitigation, adaptation, and the threat to biodiversity. Diversity 2008, 22, 1352–1355. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Gibbons, P.; Bourke, M.; Bugman, M.; Dickman, C.R.; Ferrier, S.; Fitzsimons, J.; Freudenberger, D.; Garnett, S.; Groves, C.; et al. Improving biodiversity monitoring. Austral Ecol. 2012, 37, 285–294. [Google Scholar] [CrossRef]
- Oliveira, U.; Paglia, A.P.; Brescovit, A.D.; Carvalho, C.J.B.; Silva, D.P.; Rezende, D.T.; Leite, F.S.F.; Batista, J.A.N.; Barbosa, J.P.P.P.; Stehmann, J.R.; et al. The strong influence of collection bias on biodiversity knowledge shortfalls of Brazilian terrestrial biodiversity. Divers. Distrib. 2016, 22, 1232–1244. [Google Scholar] [CrossRef]
- Britz, R.; Hundsdörfer, A.; Fritz, U. Funding, training, permits—The three big challenges of taxonomy. Megataxa 2020, 1, 49–52. [Google Scholar] [CrossRef]
- Engel, M.S.; Ceríaco, L.M.P.; Daniel, G.M.; Dellapé, P.M.; Löbl, I.; Marinov, M.; Reis, R.E.; Young, M.T.; Dubois, A.; Agarwal, I.; et al. The taxonomic impediment: A shortage of taxonomists, not the lack of technical approaches. Zool. J. Linn. Soc. 2021, 193, 381–387. [Google Scholar] [CrossRef]
- Pocock, M.J.; Chandler, M.; Bonney, R.; Thornhill, I.; Albin, A.; August, T.; Bachman, S.; Brown, P.M.J.; Gasparini, D.; Cunha, F.; et al. A vision for global biodiversity monitoring with citizen science. Adv. Ecol. Res. 2018, 59, 169–223. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Rowley, J.J.L.; Cornwell, W.K.; Poore, A.G.B.; Major, R.E. Improving big citizen science data: Moving beyond haphazard sampling. PLoS Biol. 2019, 17, e3000357. [Google Scholar] [CrossRef] [PubMed]
- iNaturalist. 2022. Available online: https://www.inaturalist.org (accessed on 11 December 2022).
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Marshall, N.J.; Kleine, D.A.; Dean, A.J. CoralWatch: Education, monitoring, and sustainability through citizen science. Front. Ecol. Environ. 2012, 10, 332–334. [Google Scholar] [CrossRef]
- Vianna, G.M.; Meekan, M.G.; Bornovski, T.H.; Meeuwig, J.J. Acoustic telemetry validates a citizen science approach for monitoring sharks on coral reefs. PLoS ONE 2014, 9, e95565. [Google Scholar] [CrossRef] [PubMed]
- Domroese, M.; Johnson, E. Why watch bees? Motivations of citizen science volunteers in the Great Pollinator Project. Biol. Conserv. 2017, 208, 40–47. [Google Scholar] [CrossRef]
- Rowley, J.J.L.; Callaghan, C.T.; Cutajar, T.; Portway, C.; Potter, K.; Mahony, S.; Trembath, D.F.; Flemons, P.; Woods, A. FrogID: Citizen scientists provide validated biodiversity data on frogs of Australia. Herpetol. Conserv. Biol. 2019, 14, 155–170. Available online: http://www.herpconbio.org/Volume_14/Issue_1/Rowley_etal_2019.pdf (accessed on 10 June 2024).
- Forti, L.R.; Szabo, J.K. The iNaturalist platform as a source of data to study amphibians in Brazil. An. Acad. Bras. Ciênc. 2023, 95, e20220828. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hoyos, D.; Méndez-Arrieta, R.; Méndez-Arrieta, A.; Seisdedos-de-Vergara, R.; Abarca, J.; Barrio-Amorós, C.; González-Maya, J. Anuran inventory in a locality of the buffer area of La Amistad International Park, Costa Rica: Pilot study for citizen science application. An. Biol. 2018, 40, 57–64. [Google Scholar] [CrossRef]
- Van Jaarsveld, A.S.; Freitag, S.; Chown, S.L.; Muller, C.; Koch, S.; Hull, H.; Bellamy, C.; Krüger, M.; Endrödy-Younga, S.; Mansell, M.W.; et al. Biodiversity assessment and conservation strategies. Science 1998, 279, 2106–2108. [Google Scholar] [CrossRef] [PubMed]
- Geijzendorffer, I.R.; Regan, E.C.; Pereira, H.M.; Brotons, L.; Brummitt, N.; Gavish, Y.; Haase, P.; Martin, C.S.; Mihoub, J.; Secades, C.; et al. Bridging the gap between biodiversity data and policy reporting needs: An essential biodiversity variables perspective. J. Appl. Ecol. 2016, 53, 1341–1350. [Google Scholar] [CrossRef]
- International Union for the Conservation of Nature (IUCN). IUCN Red List of Threatened Species. 2022. Available online: http://www.iucnredlist.org (accessed on 10 June 2024).
- Peixoto, O.L. Associação de anuros e bromeliáceas na Mata Atlântica. Rev. Univ. Rural 1995, 17, 75–83. [Google Scholar]
- Tonini, J.F.R.; Ferreira, R.B.; Pyron, R.A. Specialized breeding in plants affects diversification trajectories in Neotropical frogs. Evolution 2020, 74, 1815–1825. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Lourenço-de-Moraes, R.; Teixeira, R.L.; Beard, K.H. Frogs associations with bromeliads in an abandoned cacao plantation in northeastern Brazil. North-West. J. Zool. 2016, 12, 392–396. [Google Scholar]
- Sabagh, L.T.; Ferreira, R.B.; Rocha, C.F.D. Host bromeliads and their associated frog species: Further considerations on the importance of species interactions for conservation. Symbiosis 2017, 73, 201–211. [Google Scholar] [CrossRef]
- Gouda, E.J.; Butcher, D.; Dijkgraaf, L. Encyclopaedia of Bromeliads, Version 5. Utrecht University Botanic Gardens. Available online: http://bromeliad.nl/encyclopedia (accessed on 10 June 2024).
- Anacleto, A.; Negrelle, R.R.B. Extrativismo de rametes e propagação vegetativa de Aechmea nudicaulis (L.) Griseb. (Bromeliaceae). Sci. Agr. 2009, 10, 85–88. [Google Scholar] [CrossRef]
- Duran, S.; Monteiro, K. Jardim de Luxo Sustenta Tráfico de Plantas; Biodiversity Reporting Award; Folha de São Paulo: São Paulo, Brazil, 2001. [Google Scholar]
- Salles, R.; Silva-Soares, T. Phyllodytes luteolus (Anura: Hylidae) as an alien species in the Rio de Janeiro municipality, State of Rio de Janeiro, Southeastern Brazil. Herpetol. Notes 2010, 3, 257–258. [Google Scholar]
- Ferreira, R.B.; Beard, K.H.; Choi, R.T.; Pitt, W.C. Diet of the nonnative greenhouse frog (Eleutherodactylus planirostris) in Maui, Hawaii. J. Herpetol. 2015, 49, 586–593. [Google Scholar] [CrossRef]
- ICMBio. Sistema de Avaliação do Risco de Extinção da Biodiversidade—SALVE. 2024. Available online: https://salve.icmbio.gov.br/ (accessed on 12 June 2024).
- Lantyer-Silva, A.S.; Lirio, F.C.F.; Tonini, J.F.R.; Zocca, C.Z.; Fraga, C.N.; Waichert, C.; Kalnicky, E.A.; Marciano, E., Jr.; Lacerda, J.V.A.; Alves, J.; et al. Projeto Bromeligenous: Aliando pesquisa e educação em prol da conservação de anfíbios de bromélia. Herpetol. Bras. 2019, 8, 20–28. [Google Scholar]
- Frost, D.R. Amphibian Species of the World. 2022. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 4 April 2024).
- QGIS Development Team. QGIS Geographic Information System 2.2.0—Valmiera. Open Source Geospatial Foundation Project. 2014. Available online: http://qgis.osgeo.org/ (accessed on 25 August 2023).
- Westgate, M.J.; Scheele, B.C.; Ikin, K.; Hoefer, A.M.; Beaty, R.M.; Evans, M.; Osborne, W.; Hunter, D.; Rayner, L.; Driscoll, D.A. Citizen science program shows urban areas have lower occurrence of frog species, but not accelerated declines. PLoS ONE 2015, 10, e0140973. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.T.; Roberts, J.D.; Poore, A.G.B.; Alford, R.A.; Cogger, H.; Rowley, J.J.L. Citizen science data accurately predicts expert-derived species richness at a continental scale when sampling thresholds are met. Biodivers. Conserv. 2020, 29, 1323–1337. [Google Scholar] [CrossRef]
- Barata, I.M.; Santos, M.T.T.; Leite, F.S.F.; Garcia, P.C.A. A new species of Crossodactylodes (Anura: Leptodactylidae) from Minas Gerais, Brazil: First record of genus within the Espinhaço Mountain Range. Zootaxa 2013, 3731, 552–560. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Monico, A.T.; Zocca, C.; Santos, M.T.; Lirio, F.C.F.; Tonini, J.F.R.; Sabagh, L.; Cipriano, R.S.; Waichert, C.; Crump, M.L.; et al. Uncovering the natural history of the bromeligenous frog Crossodactylodes izecksohni (Leptodactylidae: Paratelmatobiinae). S. Am. J. Herpetol. 2019, 14, 136–145. [Google Scholar] [CrossRef]
- Heselhaus, R. Poison-Arrow Frogs: Their Natural History and Care in Captivity; Blandford: London, UK, 1992. [Google Scholar]
- Nori, J.; Loyola, R. On the worrying fate of data deficient amphibians. PLoS ONE 2015, 10, e0125055. [Google Scholar] [CrossRef]
- González-del-Pliego, P.; Freckleton, R.P.; Edwards, D.P.; Koo, M.S.; Scheffers, B.R.; Pyron, R.A.; Jetz, W. Phylogenetic and trait-based prediction of extinction risk for data-deficient amphibians. Curr. Biol. 2019, 29, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Jesus, M.D.; Zapelini, C.; Schiavetti, A. Can citizen science help delimit the geographical distribution of a species? The case of the Callistoctopus sp. (“eastern octopus”) on the Brazilian coast. Ethnobiol. Conserv. 2021, 10, 3. [Google Scholar] [CrossRef]
- Lever, C. The Cane Toad. The History and Ecology of a Successful Colonist; Westbury Academic and Scientic Publishing: Otley, UK, 2001. [Google Scholar]
- Kalnicky, E.A.; Brunson, M.W.; Beard, K.H. A social-ecological systems approach to non-native species: Habituation and its effect on management of coqui frogs in Hawaii. Biol. Conserv. 2014, 180, 187–195. [Google Scholar] [CrossRef]
- Byers, J.E.; Reichard, S.H.; Randall, J.M.; Parker, I.M.; Smith, C.S.; Lonsdale, W.M.; Atkinson, I.A.E.; Seastedt, T.R.; Williamson, M.; Chornesky, E.; et al. Directing research to reduce the impacts of nonindigenous species. Conserv. Biol. 2002, 16, 630–640. [Google Scholar] [CrossRef]
- Kraus, F. Alien Reptiles and Amphibians—A Scientific Compendium and Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Mehta, S.V.; Haight, R.G.; Homan, F.R.; Polasky, S.; Venette, R.C. Optimal detection and control strategies for invasive species management. Ecol. Econ. 2007, 61, 237–245. [Google Scholar] [CrossRef]
- Rosa, R.M.; Cavallari, D.C.; Salvador, R.B. iNaturalist as a tool in the study of tropical molluscs. PLoS ONE 2022, 17, e0268048. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.F. Understanding sampling and taxonomic biases recorded by citizen scientists. J. Insect Conserv. 2014, 18, 753–756. [Google Scholar] [CrossRef]
- Dennis, E.B.; Morgan, B.J.T.; Brereton, T.M.; Roy, D.B.; Fox, R. Using citizen science butterfly counts to predict species population trends. Conserv. Biol. 2017, 31, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Savage, M. The Amphibians and Reptiles of Costa Rica: A Herpetofauna between Two Continents, between Two Seas; The University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Miranda, Z.J.G. Bromélias do Cerrado: Populações Vulneráveis. 2004. Available online: http://www.zoonews.com.br/noticias2/noticia.php?idnoticia=45354 (accessed on 10 June 2024).
- Duellman, W.E. The Hylid Frogs of Middle America 1; Monograph of the Museum of Natural History, The University of Kansas; Museum of Natural History: New York, NY, USA, 1970; Volume 1, pp. 1–428. [Google Scholar]
- Estrada, A.R. Las puestas de Eleutherodactylus varians (Gundlach and Peters). Rev. Biol. 1990, 4, 163–167. [Google Scholar]
- Faivovich, J.; Haddad, C.F.B.; Garcia, P.C.A.; Frost, D.R.; Campbell, J.A.; Wheeler, W.C. Systematic review of the frog family Hylidae, with special reference to hylinae: Phylogenetic analysis and taxonomic revision. Bull. Am. Mus. Nat. Hist. 2005, 294, 1–240. [Google Scholar] [CrossRef]
- Hedges, S.B.; Duellman, W.E.; Heinicke, M.P. New world direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa 2008, 1737, 1–182. [Google Scholar] [CrossRef]
- Mendelson, J.R.; Eichenbaum, A.; Campbell, J.A. Taxonomic review of the populations of the fringe-limbed treefrogs (Hylidae: Ecnomiohyla) in Mexico and nuclear Central America. South Am. J. Herpetol. 2015, 10, 187–194. [Google Scholar] [CrossRef]
- Rowley, J.J.L.; Callaghan, C.T. The FrogID dataset: Expert-validated occurrence records of Australia’s frogs collected by citizen scientists. ZooKeys 2020, 912, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, A.; Simard, A.; Brunet, N.; Elliott, K.H. The scientific contributions of citizen science applied to rare or threatened animals. Conserv. Biol. 2022, 36, e13976. [Google Scholar] [CrossRef] [PubMed]


| Species (Organized by Families) | Common Names | N (N%) | Conservation Status |
|---|---|---|---|
| Aromobatidae | |||
| Anomaloglossus beebei (Noble, 1923) | Golden Rocket Frog | 24 (<1%) | EN |
| Brachycephalidae | |||
| Ischnocnema nasuta (Lutz, 1925) | Pointy-Nosed Robber Frog | 3 (<1%) | LC |
| Ischnocnema venancioi (Lutz, 1958) | Venancio’s Robber Frog | 2 (<1%) | LC |
| Bufonidae | |||
| Dendrophryniscus berthalutzae Izecksohn, 1994 | NA | 3 (<1%) | LC |
| Dendrophryniscus brevipollicatus Jiménez de la Espada, 1870 | Coastal Tree Toad | 14 (<1%) | LC |
| Frostius pernambucensis (Bokermann, 1962) | Frost’s Toad | 1 (<1%) | LC |
| Melanophryniscus alipioi Langone, Segalla, Bornschein and de Sá, 2008 | NA | 3 (<1%) | DD |
| Melanophryniscus milanoi Baldo, Bornschein, Pie, Firkowski, Ribeiro and Belmonte-Lopes, 2015 | NA | 1 (<1%) | NE |
| Dendrobatidae | |||
| Adelphobates quinquevittatus (Steindachner, 1864) | Rio Madeira Poison Frog | 10 (<1%) | LC |
| Andinobates bombetes (Myers and Daly, 1980) | Cauca Poison Frog | 55 (1%) | VU |
| Andinobates daleswansoni (Rueda-Almonacid, Rada, Sánchez-Pacheco, Velásquez-Álvarez and Quevedo-Gil, 2006) | NA | 3 (<1%) | EN |
| Andinobates dorisswansonae (Rueda-Almonacid, Rada, Sánchez-Pacheco, Velásquez-Álvarez and Quevedo-Gil, 2006) | NA | 6 (<1%) | VU |
| Andinobates fulguritus (Silverstone, 1975) | Yellowbelly Poison Frog | 27 (<1%) | LC |
| Andinobates minutus (Shreve, 1935) | Bluebelly Poison Frog | 27 (<1%) | LC |
| Andinobates opisthomelas (Boulenger, 1899) | Andean Poison Frog | 56 (1%) | VU |
| Andinobates tolimensis (Bernal-Bautista, Luna-Mora, Gallego and Quevedo-Gil, 2007) | NA | 1 (<1%) | VU |
| Andinobates victimatus Márquez, Mejía-Vargas, Palacios-Rodríguez, Ramírez-Castañeda and Amézquita, 2017 | NA | 11 (<1%) | EN |
| Andinobates virolinensis (Ruiz-Carranza and Ramírez-Pinilla, 1992) | Santander Poison Frog | 7 (<1%) | VU |
| Colostethus ruthveni Kaplan, 1997 | NA | 14 (<1%) | NT |
| Dendrobates auratus (Girard, 1855) | Green and Black Poison Frog | 1672 (29%) | LC |
| Dendrobates leucomelas Steindachner, 1864 | Yellow-headed Poison Frog | 27 (<1%) | LC |
| Dendrobates tinctorius (Cuvier, 1797) | Dyeing Poison Frog | 81 (1%) | LC |
| Excidobates condor Almendáriz, Ron and Brito M., 2012 | NA | 1 (<1%) | EN |
| Excidobates mysteriosus (Myers, 1982) | Maranon Poison Frog | 2 (<1%) | EN |
| Oophaga granulifera (Taylor, 1958) | Granular Poison Frog | 171 (3%) | VU |
| Oophaga histrionica (Berthold, 1845) | Harlequin Poison Frog | 44 (<1%) | CR |
| Oophaga lehmanni (Myers and Daly, 1976) | Lehmann’s Poison Frog | 9 (<1%) | CR |
| Oophaga pumilio (Schmidt, 1857) | Strawberry Poison Frog | 1992 (35%) | LC |
| Oophaga sylvatica (Funkhouser, 1956) | NA | 116 (2%) | NT |
| Oophaga vicentei (Jungfer, Weygoldt and Juraske, 1996) | NA | 8 (<1%) | EN |
| Phyllobates lugubris (Schmidt, 1857) | Lovely Poison Frog | 56 (1%) | LC |
| Phyllobates vittatus (Cope, 1893) | Golfodulcean Poison Frog | 197 (3%) | VU |
| Ranitomeya amazonica (Schulte, 1999) | NA | 29 (<1%) | DD |
| Ranitomeya benedicta Brown, Twomey, Pepper and Sanchez-Rodriguez, 2008 | NA | 1 (<1%) | VU |
| Ranitomeya fantastica (Boulenger, 1884) | Red-headed Poison Frog | 21 (<1%) | VU |
| Ranitomeya flavovittata (Schulte, 1999) | NA | 7 (<1%) | LC |
| Ranitomeya reticulata (Boulenger, 1884) | Redback Poison Frog | 55 (1%) | LC |
| Ranitomeya toraro Brown, Caldwell, Twomey, Melo-Sampaio and Souza, 2011 | NA | 8 (<1%) | NE |
| Ranitomeya uakarii (Brown, Schulte and Summers, 2006) | NA | 14 (<1%) | LC |
| Ranitomeya vanzolinii (Myers, 1982) | Brazilian Poison Frog | 5 (<1%) | LC |
| Ranitomeya variabilis (Zimmermann and Zimmermann, 1988) | Zimmermann’s Poison Frog | 100 (2%) | DD |
| Ranitomeya ventrimaculata (Shreve, 1935) | Amazonian Poison Frog | 44 (<1%) | LC |
| Ranitomeya yavaricola Pérez-Peña, Chávez, Twomey and Brown, 2010 | NA | 2 (<1%) | DD |
| Eleutherodactylidae | |||
| Adelophryne maranguapensis Hoogmoed, Borges and Cascon, 1994 | NA | 3 (<1%) | EN |
| Diasporus diastema (Cope, 1875) | Caretta Robber Frog | 247 (4%) | LC |
| Diasporus vocator (Taylor, 1955) | Agua Buena Robber Frog | 11 (<1%) | LC |
| Eleutherodactylus auriculatoides Noble, 1923 | Northern Hammer Frog | 3 (<1%) | EN |
| Eleutherodactylus flavescens Noble, 1923 | Yellow Split-toed Frog | 14 (<1%) | NT |
| Eleutherodactylus wetmorei Cochran, 1932 | Tiburon Whistling Frog | 4 (<1%) | VU |
| Hemiphractidae | |||
| Flectonotus fitzgeraldi (Parker, 1934) | Dwarf Marsupial Frog | 28 (<1%) | LC |
| Flectonotus pygmaeus (Boettger, 1893) | Puerto Cabello Treefrog | 4 (<1%) | LC |
| Fritziana goeldii (Boulenger, 1895) | Colonia Alpina Treefrog | 29 (<1%) | LC |
| Fritziana mitus Walker, Wachlevski, Nogueira da Costa, Nogueira-Costa, Garcia and Haddad, 2018 | NA | 7 (<1%) | NE |
| Fritziana tonimi Walker, Gasparini and Haddad, 2016 | NA | 1 (<1%) | NE |
| Fritziana ulei (Miranda-Ribeiro, 1926) | NA | 1 (<1%) | NE |
| Gastrotheca fissipes (Boulenger, 1888) | Igaracu Marsupial Frog | 2 (<1%) | LC |
| Hylidae | |||
| Bokermannohyla astartea (Bokermann, 1967) | Paranapiacaba Treefrog | 2 (<1%) | LC |
| Bromeliohyla bromeliacia (Schmidt, 1933) | Bromeliad Treefrog | 12 (<1%) | LC |
| Bromeliohyla dendroscarta (Taylor, 1940) | Greater Bromeliad Treefrog | 1 (<1%) | EN |
| Bromeliohyla melacaena (McCranie and Castañeda, 2006) | NA | 4 (<1%) | EN |
| Ecnomiohyla minera (Wilson, McCranie and Williams, 1985) | Guatemala Treefrog | 1 (<1%) | VU |
| Isthmohyla picadoi (Dunn, 1937) | Volcan Barba Treefrog | 3 (<1%) | LC |
| Ololygon alcatraz (Lutz, 1973) | Alcatraz Snouted Treefrog | 1 (<1%) | CR |
| Ololygon arduoa (Peixoto, 2002) | NA | 6 (<1%) | DD |
| Ololygon perpusilla (Lutz and Lutz, 1939) | Bandeirantes Snouted Treefrog | 25 (<1%) | LC |
| Osteocephalus deridens Jungfer, Ron, Seipp and Almendáriz, 2000 | NA | 11 (<1%) | LC |
| Osteocephalus fuscifacies Jungfer, Ron, Seipp and Almendáriz, 2000 | NA | 16 (<1%) | LC |
| Osteocephalus oophagus Jungfer and Schiesari, 1995 | Oophagous Slender-legged Treefrog | 45 (<1%) | LC |
| Osteocephalus planiceps Cope, 1874 | NA | 108 (2%) | LC |
| Osteopilus crucialis (Harlan, 1826) | NA | 1 (<1%) | VU |
| Osteopilus marianae (Dunn, 1926) | Jamaican Yellow Treefrog | 1 (<1%) | EN |
| Osteopilus ocellatus (Linnaeus, 1758) | Jamaican Laughing Treefrog | 12 (<1%) | NT |
| Osteopilus wilderi (Dunn, 1925) | Jamaican Green Treefrog | 2 (<1%) | VU |
| Phyllodytes edelmoi Peixoto, Caramaschi and Freire, 2003 | NA | 20 (<1%) | DD |
| Phyllodytes gyrinaethes Peixoto, Caramaschi and Freire, 2003 | NA | 1 (<1%) | DD |
| Phyllodytes luteolus (Wied-Neuwied, 1821) | Yellow Heart-Tongued Frogs | 19 (<1%) | LC |
| Phyllodytes melanomystax Caramaschi, Silva and Britto-Pereira, 1992 | Bahia Heart-Tongued Frogs | 1 (<1%) | LC |
| Phytotriades auratus (Boulenger, 1917) | Trinidad Golden Treefrog | 1 (<1%) | EN |
| Triprion spinosus (Steindachner, 1864) | Coronated Treefrog | 67 (1%) | NT |
| Leptodactylidae | |||
| Crossodactylodes izecksohni Peixoto, 1983 | Izecksohn’s Bromeliad Frogs | 1 (<1%) | NT |
| Physalaemus spiniger (Miranda-Ribeiro, 1926) | Iguape Dwarf Frog | 11 (<1%) | LC |
| Microhylidae | |||
| Chiasmocleis antenori (Walker, 1973) | Ecuador Silent Frog | 5 (<1%) | LC |
| Strabomantidae | |||
| Pristimantis acuminatus (Shreve, 1935) | Canelos Robber Frog | 24 (<1%) | LC |
| Pristimantis aureolineatus (Guayasamin, Ron, Cisneros-Heredia, Lamar and McCracken, 2006) | NA | 4 (<1%) | LC |
| Pristimantis waoranii (McCracken, Forstner and Dixon, 2007) | NA | 3 (<1%) | DD |
| Total | 5692 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zocca, C.; Ghilardi-Lopes, N.P.; Ferreira, R.B. Bromeliad-Dwelling Frogs Revealed by Citizen Scientists. Diversity 2024, 16, 363. https://doi.org/10.3390/d16070363
Zocca C, Ghilardi-Lopes NP, Ferreira RB. Bromeliad-Dwelling Frogs Revealed by Citizen Scientists. Diversity. 2024; 16(7):363. https://doi.org/10.3390/d16070363
Chicago/Turabian StyleZocca, Cássio, Natalia Pirani Ghilardi-Lopes, and Rodrigo Barbosa Ferreira. 2024. "Bromeliad-Dwelling Frogs Revealed by Citizen Scientists" Diversity 16, no. 7: 363. https://doi.org/10.3390/d16070363
APA StyleZocca, C., Ghilardi-Lopes, N. P., & Ferreira, R. B. (2024). Bromeliad-Dwelling Frogs Revealed by Citizen Scientists. Diversity, 16(7), 363. https://doi.org/10.3390/d16070363







