Multi-Scale Habitat Selection by the Wintering Whooper Swan (Cygnus cygnus) in Manas National Wetland Park, Northwestern China
Abstract
1. Introduction
2. Materials and Methods
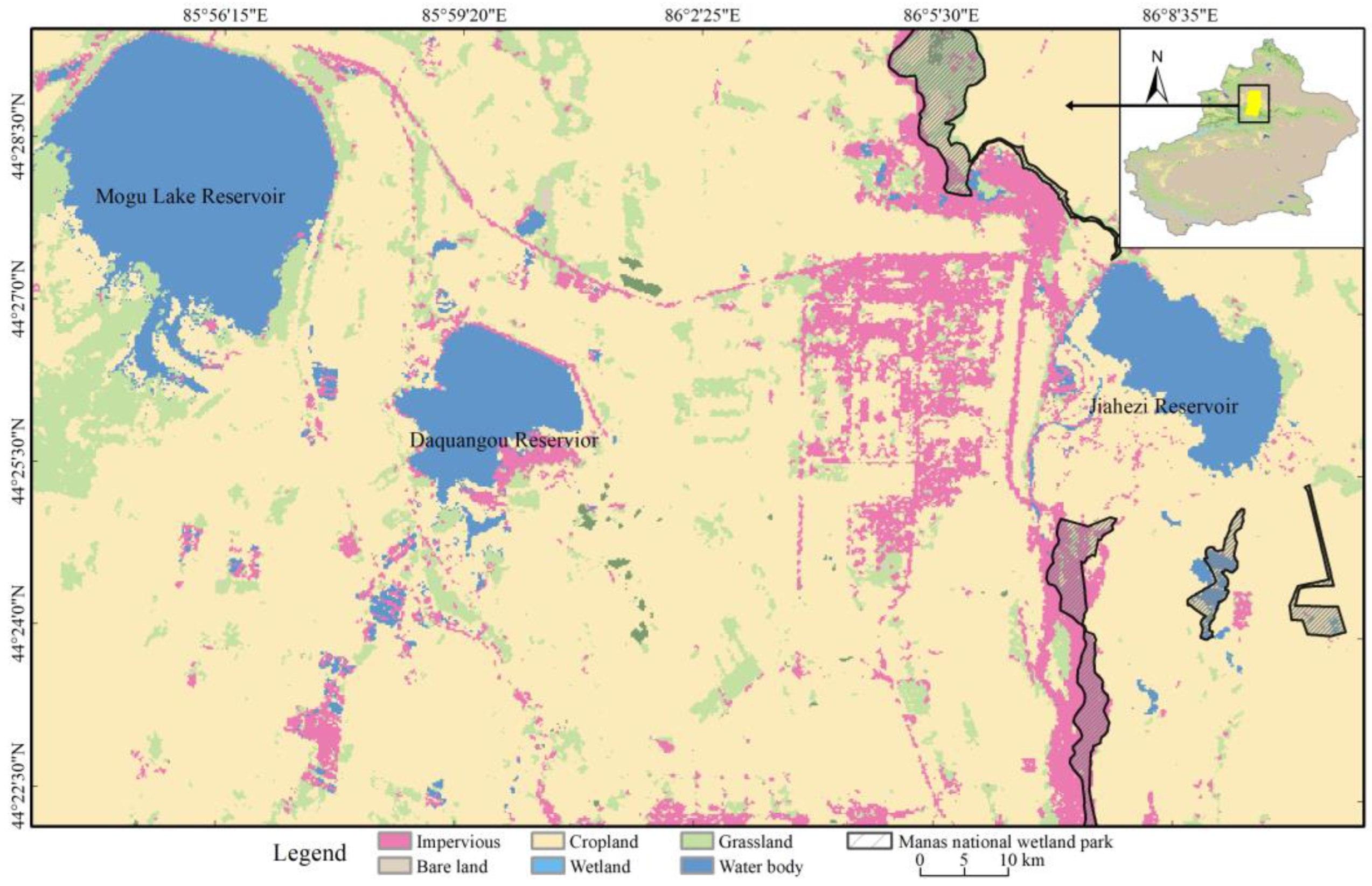
2.1. Study Area
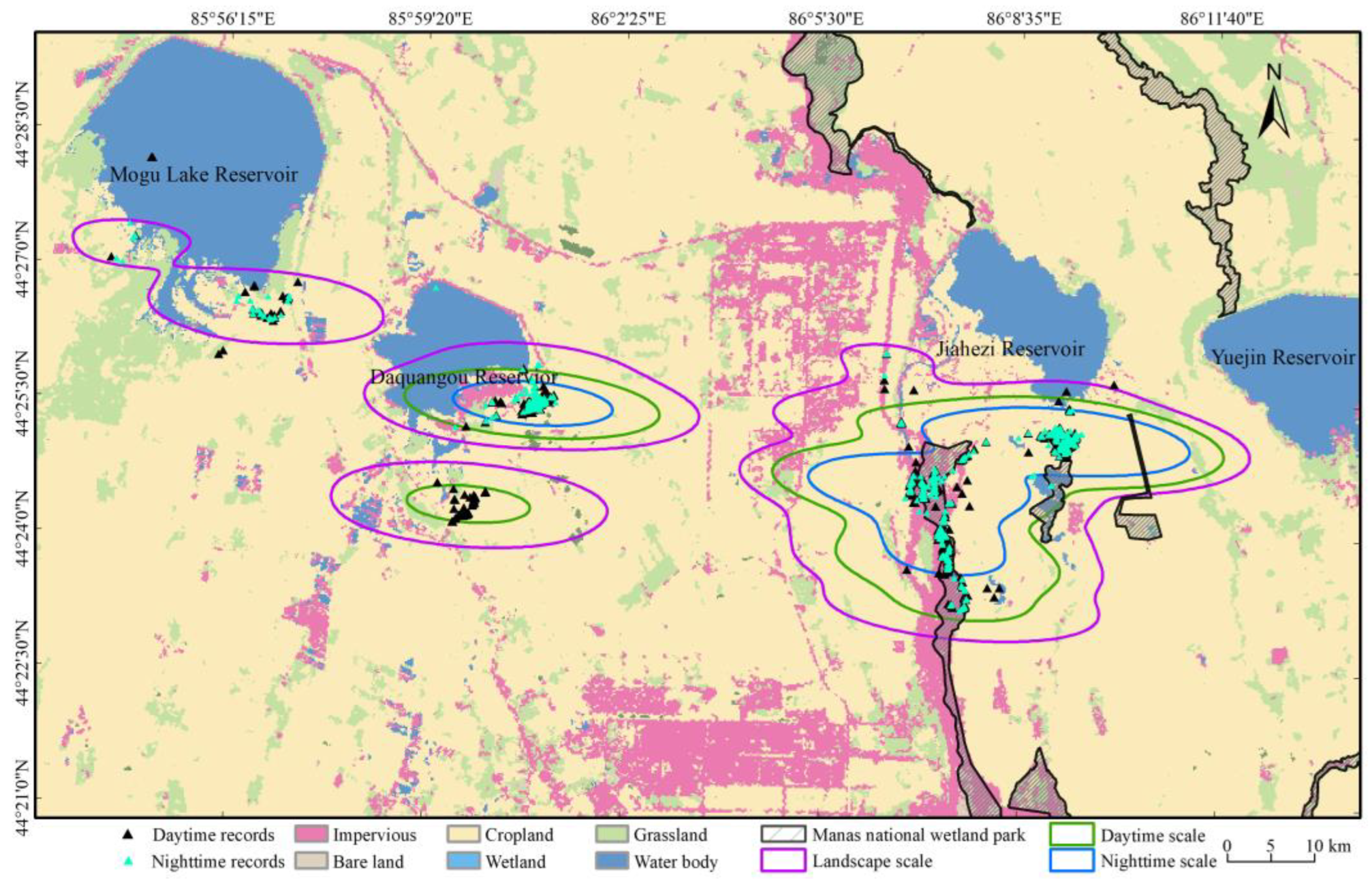
2.2. Bird Tracking Data
2.3. Radius of the Landscape, Daytime, and Nighttime Scales
2.4. Environmental Data
2.5. Landscape Metrics
2.6. Habitat Selection Modeling
3. Results
3.1. Partitioning of Different Scale Radii
3.2. Habitat Selection of Wintering Whooper Swans at Different Scales
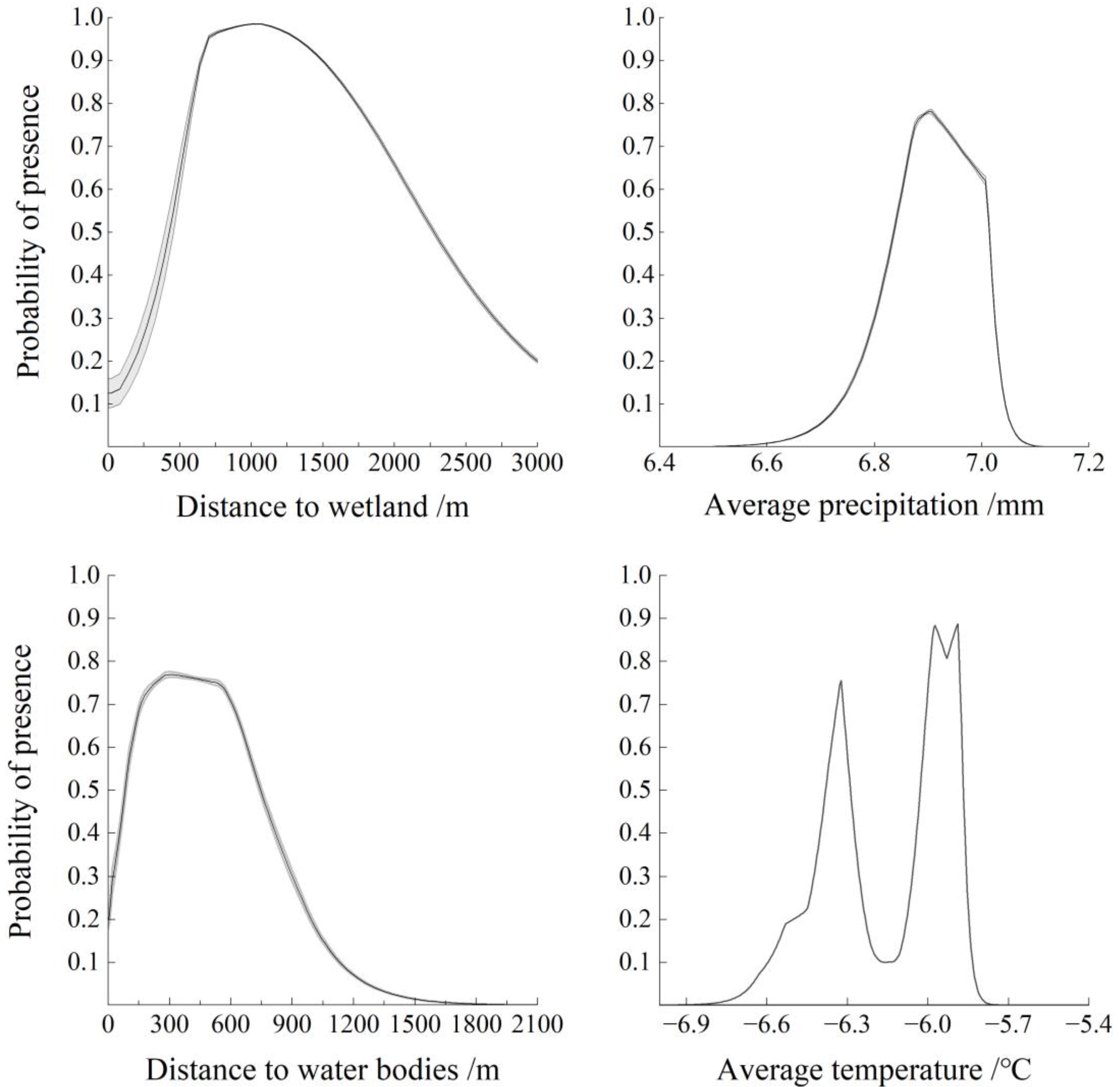
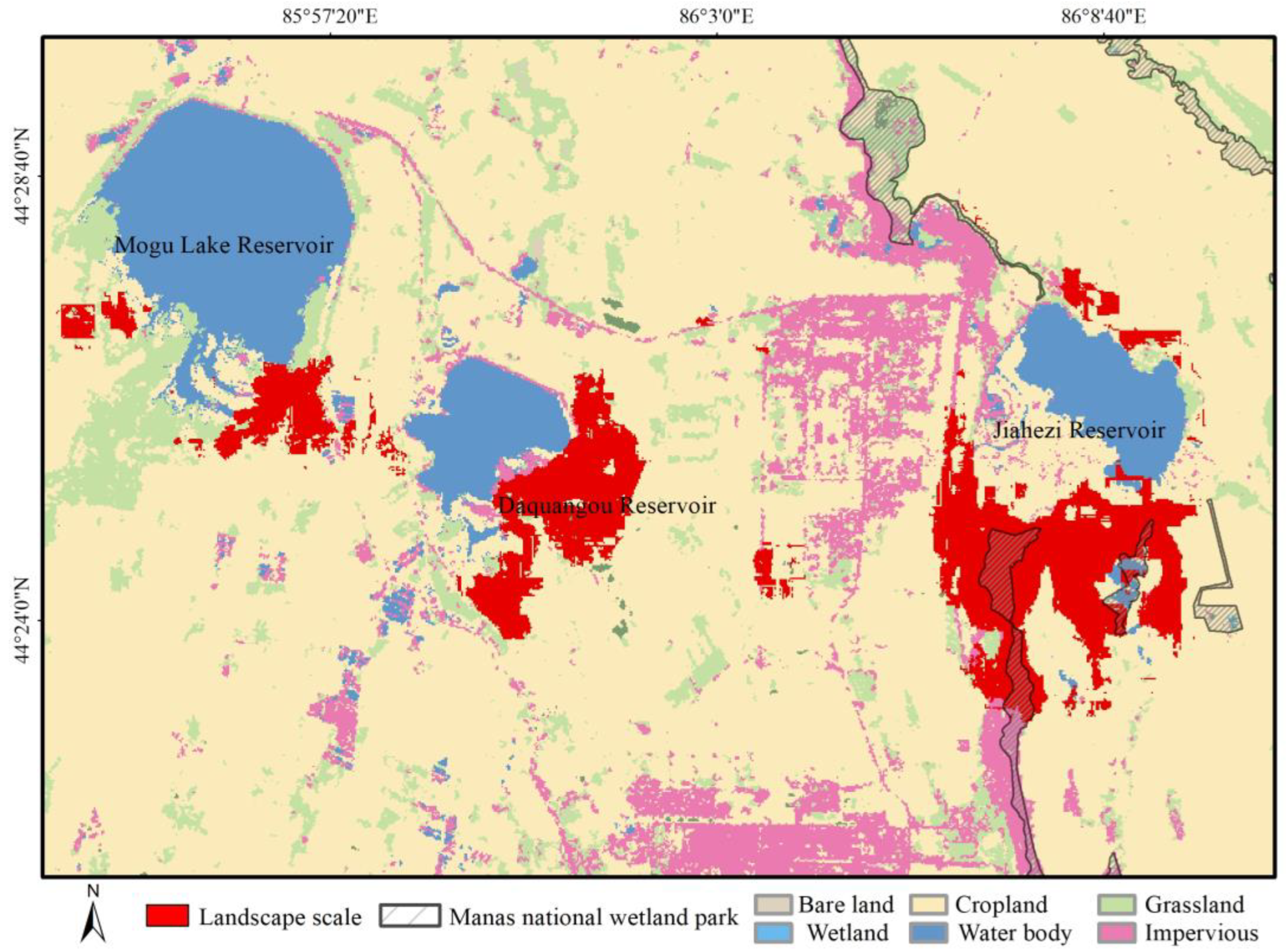
3.2.1. Habitat Selection of Wintering Whooper Swans at the Landscape Scale
3.2.2. Habitat Selection of Wintering Whooper Swans at the Daytime Scale
3.2.3. Habitat Selection of Wintering Whooper Swans at the Nighttime Scale
4. Discussion
4.1. Wintering Whooper Swan Habitat Selection at the Landscape Scale
4.2. Wintering Whooper Swan Habitat Selection at the Daytime Scale
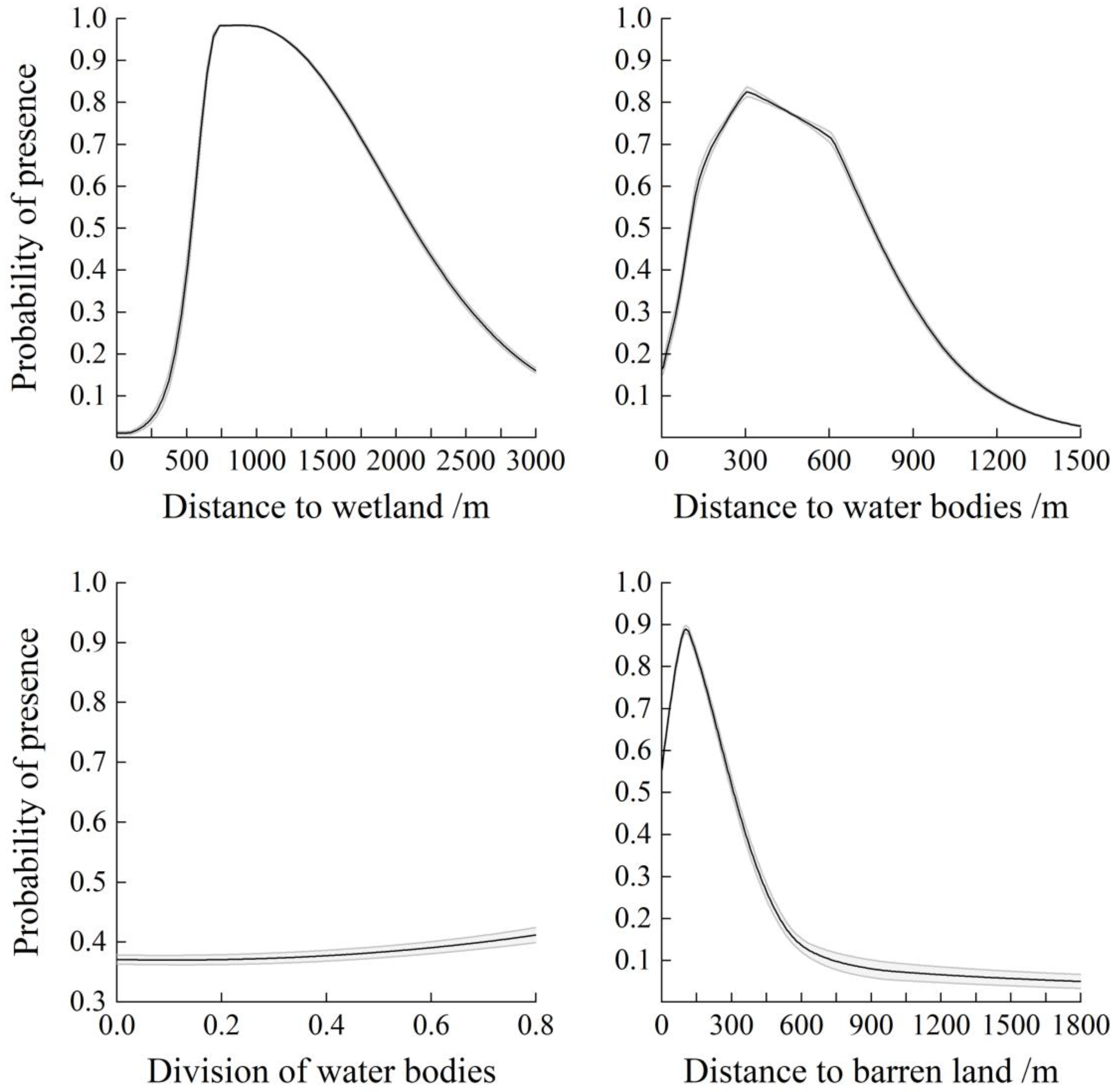
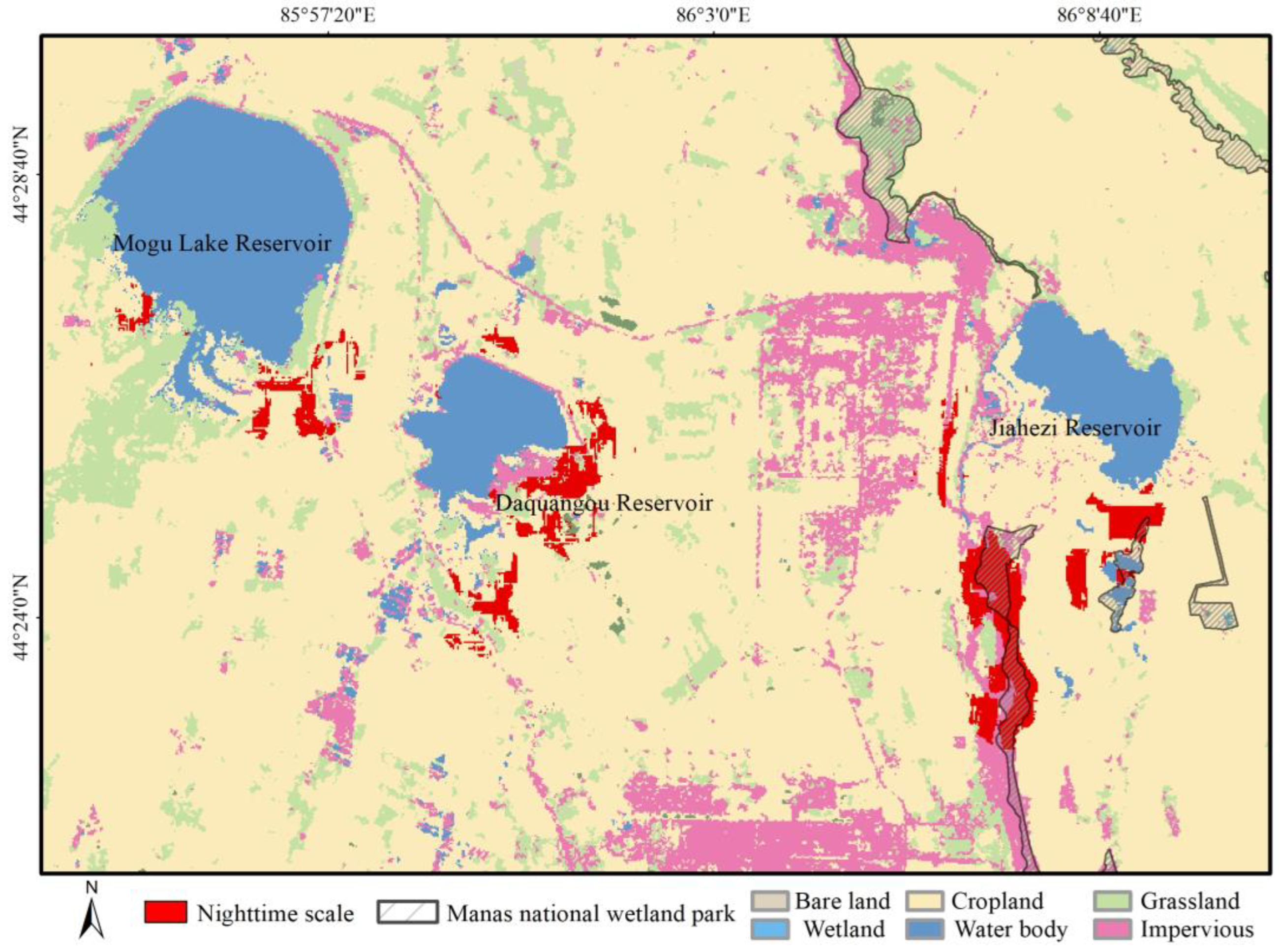
4.3. Wintering Whooper Swan Habitat Selection at the Nighttime Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reunanen, P.; Monkkonen, M.; Nikula, A. Habitat requirements of the Siberian flying squirrel in northern Finland: Comparing field survey and remote sensing data. Ann. Zool. Fenn. 2002, 1, 7–20. [Google Scholar]
- Yang, W.; Zhong, W.; Gao, X. A review of studies on avain habitat selection. Arid Zone Res. 2000, 3, 71–78. [Google Scholar]
- McGarigal, K.; Wan, H.Y.; Zeller, K.A.; Timm, B.C.; Cushman, S.A. Multi–scale habitat selection modeling: A review and outlook. Landsc. Ecol. 2016, 6, 1161–1175. [Google Scholar] [CrossRef]
- Sun, H.; Yu, H.; Li, Y.; Yu, W.; Gao, R.; Zhang, C.; Zhang, Y.; Hong, J. Habitat selection for the wintering of whooper swan in Sanmenxia. Wetl. Sci. Manag. 2018, 3, 50–53. [Google Scholar]
- Kotliar, N.B.; Wiens, J.A. Multiple scales of patchiness and patch structure-a hierarchical framework for the study of heterogeneity. Oikos 1990, 2, 253–260. [Google Scholar] [CrossRef]
- Marcolin, F.; Lakatos, T.; Galle, R.; Batary, P. Fragment connectivity shapes bird communities through functional trait filtering in two types of grasslands. Glob. Ecol. Conserv. 2021, 28, e01687. [Google Scholar] [CrossRef]
- Neuschulz, E.L.; Brown, M.; Farwig, N. Frequent bird movements across a highly fragmented landscape: The role of species traits and forest matrix. Anim. Conserv. 2013, 2, 170–179. [Google Scholar] [CrossRef]
- Jourdan, C.; Fort, J.; Pinaud, D.; Delaporte, P.; Herault, T.; Jankovic, M.; Jomat, L.; Lachaussee, N.; Pineau, P.; Robin, F.; et al. Daytime, tidal amplitude and protected areas influence movements and habitat use on mudflats of wintering black–tailed godwits. Estuar. Coast. Shelf Sci. 2022, 268, 107782. [Google Scholar] [CrossRef]
- Joo, S.; Choi, Y.-S.; Lee, S.-Y. Home range and habitat use of the swan goose (Anser cygnoides L. 1758) during wintering in the seocheon tidal flat, south Korea, using gps–based telemetry. Animals 2022, 12, 3048. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, L.; Si, Y. Multi–scale habitat selection by two declining east Asian waterfowl species at their core spring stopover area. Ecol. Indic. 2018, 87, 127–135. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Cao, L.; Xiong, H.; Zhao, Q. Characteristics of stopover site selection during the migration of swan goose. Acta Ecol. Sin. 2024, 2, 570–578. [Google Scholar]
- Chen, H.; Wang, G.; Wang, C.; Xue, F.; Cheng, H.; Zhang, Y.N. Activity rhythms, home range characteristics, and habitat selection of reintroduced red–crowned crane. Acta Ecol. Sin. 2024, 4, 1526–1538. [Google Scholar]
- Jia, R.; Gao, R.; Ru, W.; Kong, D.; Ji, Z.; Zhang, G. Using satellite tracking to identify the factors affecting the spring migration timing of whooper swans (Cygnus cygnus). Acta Ecol. Sin. 2021, 41, 6075–6082. [Google Scholar]
- Hoodless, A.N.; Hirons, G.J.M. Habitat selection and foraging behaviour of breeding Eurasian woodcock (Scolopax rusticola): A comparison between contrasting landscapes. Ibis 2007, 149, 234–249. [Google Scholar] [CrossRef]
- Yang, X.T.; Duan, Z.Z.; Li, S.S.; Zhang, C.X.; Qu, M.; Hua, G.D.; Niu, X.A.; Hu, H.J.; Yu, D.M. Factors driving the abundance of wintering waterbirds in coastal areas of Guangdong province, China. Front. Ecol. Evol. 2022, 9, 808105. [Google Scholar] [CrossRef]
- Booth, T.H. Using biodiversity databases to verify and improve descriptions of tree species climatic requirements. For. Ecol. Manag. 2014, 315, 95–102. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 2, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G. Classification and Distribution of Birds in China; Science Publishers: Beijing, China, 2023; pp. 102–106. [Google Scholar]
- Soriano-Redondo, A.; Inger, R.; Sherley, R.B.; Rees, E.C.; Abadi, F.; McElwaine, G.; Colhoun, K.; Einarsson, O.; Thorstensen, S.; Newth, J.; et al. Demographic rates reveal the benefits of protected areas in a long–lived migratory bird. Proc. Natl. Acad. Sci. USA 2023, 12, e2212035120. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Nam, H.K.; Park, J.Y.; Kang, S.G.; Batbayar, N.; Kim, D.W.; Hwang, J.W.; Tsend, O.; Natsagdorj, T.; Nergui, J.; et al. Migration routes and differences in migration strategies of whooper swans between spring and autumn. Avian Res. 2023, 14, 100113. [Google Scholar] [CrossRef]
- Yang, L. Status of biodiversity in Manas national wetland park and countermeasures for its conservation. Xinjiang For. 2021, 4, 10–12. [Google Scholar]
- Yang, L.; Xie, F.B. Monitoring and analysis of common crane migration patterns in Manas national wetland park. Xinjiang For. 2022, 3, 28–30. [Google Scholar]
- Yang, L.; Xie, F.B.; Wang, L. Observation and analysis of the daily behavior of whooper swans during the overwintering period in Manas national wetland park. Xinjiang For. 2022, 6, 22–25. [Google Scholar]
- Noonan, M.J.; Martinez-Garcia, R.; Davis, G.H.; Crofoot, M.C.; Kays, R.; Hirsch, B.; Caillaud, D.; Payne, E.; Sih, A.; Sinn, D.L.; et al. Estimating encounter location distributions from animal tracking data. Methods Ecol. Evol. 2021, 7, 1158–1173. [Google Scholar] [CrossRef]
- Fleming, C.H.; Fagan, W.F.; Mueller, T.; Olson, K.A.; Leimgruber, P.; Calabrese, J.M. Rigorous home range estimation with movement data: A new autocorrelated kernel density estimator. Ecology 2015, 5, 1182–1188. [Google Scholar] [CrossRef]
- Signer, J.; Fieberg, J.; Avgar, T. Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol. Evol. 2019, 2, 880–890. [Google Scholar] [CrossRef]
- Li, J.Y.; Zhang, Y.; Zhao, L.; Deng, W.Q.; Qian, F.W.; Ma, K.M. Scale and landscape features matter for understanding waterbird habitat selection. Remote Sens. 2021, 13, 4397. [Google Scholar] [CrossRef]
- Miguet, P.; Jackson, H.B.; Jackson, N.D.; Martin, A.E.; Fahrig, L. What determines the spatial extent of landscape effects on species? Landsc. Ecol. 2016, 6, 1177–1194. [Google Scholar] [CrossRef]
- Bellamy, C.; Scott, C.; Altringham, J. Multiscale, presence-only habitat suitability models: Fine-resolution maps for eight bat species. J. Appl. Ecol. 2013, 4, 892–901. [Google Scholar] [CrossRef]
- Byrne, M.E.; McCoy, J.C.; Hinton, J.W.; Chamberlain, M.J.; Collier, B.A. Using dynamic Brownian bridge movement modelling to measure temporal patterns of habitat selection. J. Anim. Ecol. 2014, 5, 1234–1243. [Google Scholar] [CrossRef]
- Palm, E.C.; Newman, S.H.; Prosser, D.J.; Xiao, X.M.; Ze, L.; Batbayar, N.; Balachandran, S.; Takekawa, J.Y. Mapping migratory flyways in Asia using dynamic Brownian bridge movement models. Mov. Ecol. 2015, 3, 3. [Google Scholar] [CrossRef]
- Jia, R.; Li, S.-H.; Meng, W.-Y.; Gao, R.-Y.; Ru, W.-D.; Li, Y.-F.; Ji, Z.-H.; Zhang, G.-G.; Liu, D.-P.; Lu, J. Wintering home range and habitat use of the whooper swans (Cygnus cygnus) in Sanmenxia wetland, China. Ecol. Res. 2019, 5, 637–643. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high-resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 15, 1965–1978. [Google Scholar] [CrossRef]
- Price, D.T.; McKenney, D.W.; Nalder, I.A.; Hutchinson, M.F.; Kesteven, J.L. A comparison of two statistical methods for spatial interpolation of Canadian monthly mean climate data. Agric. For. Meteorol. 2000, 101, 81–94. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X. The 30 m annual land cover dataset and its dynamics in China from 1990 to 2019. Earth Syst. Sci. Data 2021, 8, 3907–3925. [Google Scholar] [CrossRef]
- Fardila, D.; Kelly, L.T.; Moore, J.L.; McCarthy, M.A. A systematic review reveals changes in where and how we have studied habitat loss and fragmentation over 20 years. Biol. Conserv. 2017, 212, 130–138. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 3, 247–260. [Google Scholar] [CrossRef]
- Ethier, K.; Fahrig, L. Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landsc. Ecol. 2011, 6, 865–876. [Google Scholar] [CrossRef]
- Soifer, L.G.; Donovan, S.K.; Brentjens, E.T.; Bratt, A.R. Piecing together cities to support bird diversity: Development and forest edge density affect bird richness in urban environments. Landsc. Urban Plan. 2021, 213, 104122. [Google Scholar] [CrossRef]
- Hinsley, S.A.; Bellamy, P.E. The influence of hedge structure, management and landscape context on the value of hedgerows to birds: A review. J. Environ. Manag. 2000, 1, 33–49. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 2, 191–203. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. Spthin: An r package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 5, 541–545. [Google Scholar] [CrossRef]
- Masto, N.M.; Hsiung, A.C.; Kaminski, R.M.; Ross, B.E.; Kneece, M.R.; Wilkerson, G.L.; Baldwin, R.F.; Hanks, R.D.; Wiggers, E.P.; Folk, T.H.; et al. Waterbird-habitat relationships in South Carolina: Implications for protection, restoration, and management of coastal and inland wetlands. Restor. Ecol. 2023, 31, e13956. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, X.; Zhang, Y.; Cao, L.; Fox, A.D. Drivers of waterbird communities and their declines on Yangtze River floodplain lakes. Biol. Conserv. 2018, 218, 240–246. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, C.; Lu, J.; Hou, Y. Surveys on the population dynamic of whooper swans Cygnus cygnus at important wintering areas in China. Sichuan J. Zool. 2014, 03, 456–459. [Google Scholar]
- Huang, Y.J.; Wang, P.; Yang, Z.Y.; Yu, P.; Ye, T.T.; Guo, Y.M.; Huang, L. Spatiotemporal characteristics and influencing factors for joint events of air pollution wave and cold wave in China. Environ. Int. 2024, 184, 108475. [Google Scholar] [CrossRef]
- Brunbjerg, A.K.; Hoye, T.T.; Eskildsen, A.; Nygaard, B.; Damgaard, C.F.; Ejrnæs, R. The collapse of marsh fritillary (Euphydryas aurinia) populations associated with declining host plant abundance. Biol. Conserv. 2017, 211, 117–124. [Google Scholar] [CrossRef]
- Duan, H.L.; Pan, Y.W.; Yu, X.B.; Xia, S.X. Effects of habitat change on the wintering waterbird community in China’s largest freshwater lake. Remote Sens. 2023, 18, 4582. [Google Scholar] [CrossRef]
- Van den Broeke, M.S. Radar indications of altered foraging behavior during the February 2021 severe north American cold wave. Avain Biol. Res. 2023, 1, 14–20. [Google Scholar] [CrossRef]
- Li, S.; Meng, W.; Chen, L.; Li, Y.; Gao, R.; Ru, W.; Sun, M.; Dai, Q.; Zhang, G.; Lu, J. The spring waterbird community and home range of the whooper swan Cygnus cygnus at the upper and middle reaches of yellow river in inner Mongolia, China. Chin. J. Ecol. 2017, 7, 1910–1916. [Google Scholar]
- Cotza, A.; Tomassini, O.; Corlatti, L.; Ferretti, F.; Davoli, M.; Bassano, B.; Lovari, S. Reproductive payoffs of territoriality are snow–dependent in a mountain ungulate, the Alpine chamois. J. Zool. 2023, 3, 225–236. [Google Scholar] [CrossRef]
- Cohen, J.M.; Fink, D.; Zuckerberg, B. Extreme winter weather disrupts bird occurrence and abundance patterns at geographic scales. Ecography 2021, 8, 1143–1155. [Google Scholar] [CrossRef]
- Delgado, M.D.; Arlettaz, R.; Bettega, C.; Brambilla, M.; Hernando, M.D.; España, A.; Fernández–González, A.; Fernandez–Martín, A.; Gil, J.A.; Hernández–Gómez, S.; et al. Spatio–temporal variation in the wintering associations of an alpine bird. Proc. R. Soc. B–Biol. Sci. 2021, 288, 20210690. [Google Scholar] [CrossRef] [PubMed]
- Arias, L.N.C.; Wilson, S.; Bayly, N.J. Community modeling reveals the importance of elevation and land cover in shaping migratory bird abundance in the Andes. Ecol. Appl. 2022, 1, e02481. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, L.Z.; Yu, C.; Wei, Z.H.; Li, C.H. Nest habitat distribution and spatio-temporal dynamics based on multi-scale modeling: Implications for the endangered oriental storks (Ciconia boyciana) conservation in China. Glob. Ecol. Conserv. 2023, 43, e02439. [Google Scholar] [CrossRef]
- Siriwardena, G.M.; Calbrade, N.A.; Vickery, J.A.; Sutherland, W.J. The effect of the spatial distribution of winter seed food resources on their use by farmland birds. J. Appl. Ecol. 2006, 4, 628–639. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lu, Y.; Chen, C.; Zhang, G.W.; Wang, Q.Z.; Xu, Z.; Wang, Z.S. Behavioural responses of the whooper swans Cygnus cygnus to human disturbance and their adaptability to the different habitats in the Rongcheng lagoon of China. Ecohydrology 2018, 7, e2013. [Google Scholar] [CrossRef]
- Tian, X.; Lu, Y.; Chen, C.; Liu, Y. A study on distribution and behaviors of whooper swans during the pre-winter period in the swan lake of Rongcheng. Wetl. Sci. Manag. 2017, 13, 29–34. [Google Scholar]
- Liu, L.; Gao, L.; Liu, X.; Li, W.; Zhang, J.; Cao, L.; Du, C. Diet and feeding ecology of whooper swan (Cygnus cygnus) and tundra swan (C. columbianus) at the yellow river wetland of Baotou in spring season. Russ. J. Ecol. 2022, 5, 419–425. [Google Scholar] [CrossRef]





| No. | Age | Tracking Period | Tracking Sites | Tracking Interval (hours) | Tracking Days |
|---|---|---|---|---|---|
| 2199 | Adult | 2021/1/28–2021/2/21 | 240 | 2 | 24 |
| 2196 | Adult | 2021/1/28–2021/2/10 | 135 | 2 | 14 |
| 2239 | Adult | 2021/1/28–2021/2/14 | 187 | 2 | 17 |
| 2123 | Adult | 2021/1/28–2021/3/10 | 214 | 2 | 40 |
| 2108 | Adult | 2021/1/28–2021/3/30 | 679 | 2 | 60 |
| 2193 | Juvenile | 2021/1/29–2021/3/22 | 511 | 2 | 51 |
| 2186 | Juvenile | 2021/1/28–2021/3/18 | 436 | 2 | 48 |
| 2581 | Juvenile | 2021/1/28–2021/3/19 | 544 | 2 | 49 |
| V40 | Juvenile | 2021/1/28–2021/3/25 | 711 | 2 | 55 |
| Scale | Variable | Description |
|---|---|---|
| Landscape, daytime, and nighttime | Percentage of specific land cover type (%) | Percentage of focal patch type coverage by landscape type |
| Shannon’s diversity index (-) | Heterogeneity of landscape | |
| Patch density of specific land types (km2) | Divides the total area of the specific landscape by the total number of patches | |
| Edge density of specific land types (m/km2) | Sum of edge lengths for focal patch type divided by total area | |
| Landscape shape index of focal land cover types (-) | Standardized calculation method for focal land type using total landscape area | |
| Mean patch shape index (-) | Average shape index for each patch of the focal type | |
| Mean specific patch area (m2) | Average area of each patch of focal type | |
| Aggregation index (%) | Distribution of focal patch types | |
| Landscape division index (-) | Measure of dispersion for specific patch types | |
| Patch cohesion index (-) | Connectivity measurement between patches | |
| Landscape | Mean temperature (°C) | Mean temperature of 1–3 months |
| Landscape | Mean precipitation (mm) | Mean precipitation of 1–3 months |
| Landscape, daytime, and nighttime | Distance to each land cover type (m) | Minimum distance to specified land cover type |
| Scale | Radius km | AUC | Standard Error |
|---|---|---|---|
| Landscape scale | 20 | 0.974 | 0.0006 |
| 15 | 0.976 | 0.0007 | |
| 10 | 0.975 | 0.001 | |
| Daytime scale | 8 | 0.978 | 0.001 |
| 7 | 0.977 | 0.001 | |
| 6 | 0.975 | 0.002 | |
| Nighttime scale | 3 | 0.950 | 0.002 |
| 2 | 0.945 | 0.004 | |
| 1 | 0.935 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Ma, X.; Yang, W.; Xu, F. Multi-Scale Habitat Selection by the Wintering Whooper Swan (Cygnus cygnus) in Manas National Wetland Park, Northwestern China. Diversity 2024, 16, 306. https://doi.org/10.3390/d16050306
Yan H, Ma X, Yang W, Xu F. Multi-Scale Habitat Selection by the Wintering Whooper Swan (Cygnus cygnus) in Manas National Wetland Park, Northwestern China. Diversity. 2024; 16(5):306. https://doi.org/10.3390/d16050306
Chicago/Turabian StyleYan, Han, Xuejun Ma, Weikang Yang, and Feng Xu. 2024. "Multi-Scale Habitat Selection by the Wintering Whooper Swan (Cygnus cygnus) in Manas National Wetland Park, Northwestern China" Diversity 16, no. 5: 306. https://doi.org/10.3390/d16050306
APA StyleYan, H., Ma, X., Yang, W., & Xu, F. (2024). Multi-Scale Habitat Selection by the Wintering Whooper Swan (Cygnus cygnus) in Manas National Wetland Park, Northwestern China. Diversity, 16(5), 306. https://doi.org/10.3390/d16050306






