Effects of Land Use Change on Avian Diversity in the Semi-Arid Area of Longxi Loess Plateau
Abstract
1. Introduction
2. Materials and Methods
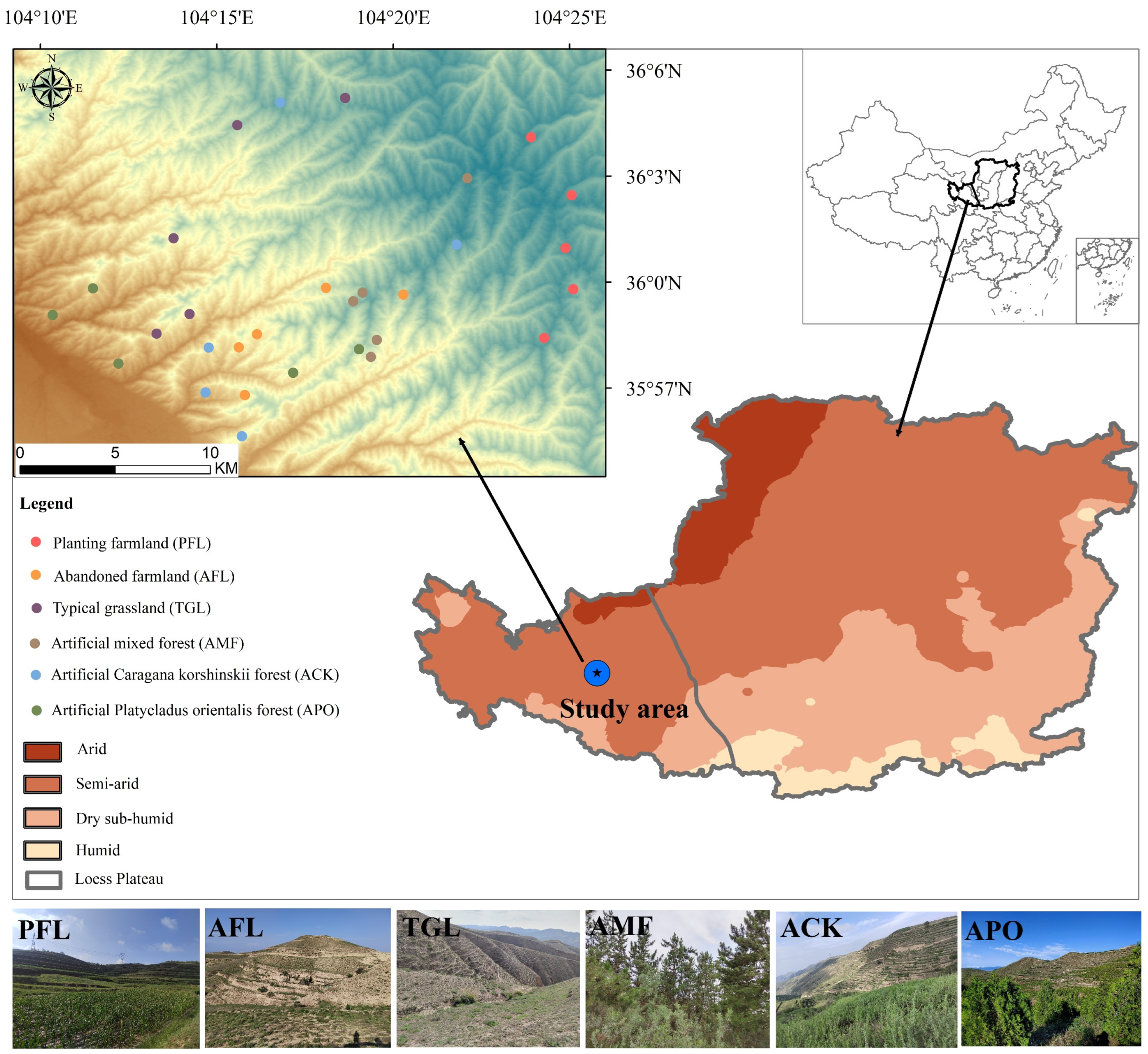
2.1. Study Area
2.2. Study Design
2.3. Avifaunal Sampling
2.4. Functional Traits
2.5. Phylogenetic Data
2.6. Statistical Analysis
3. Results
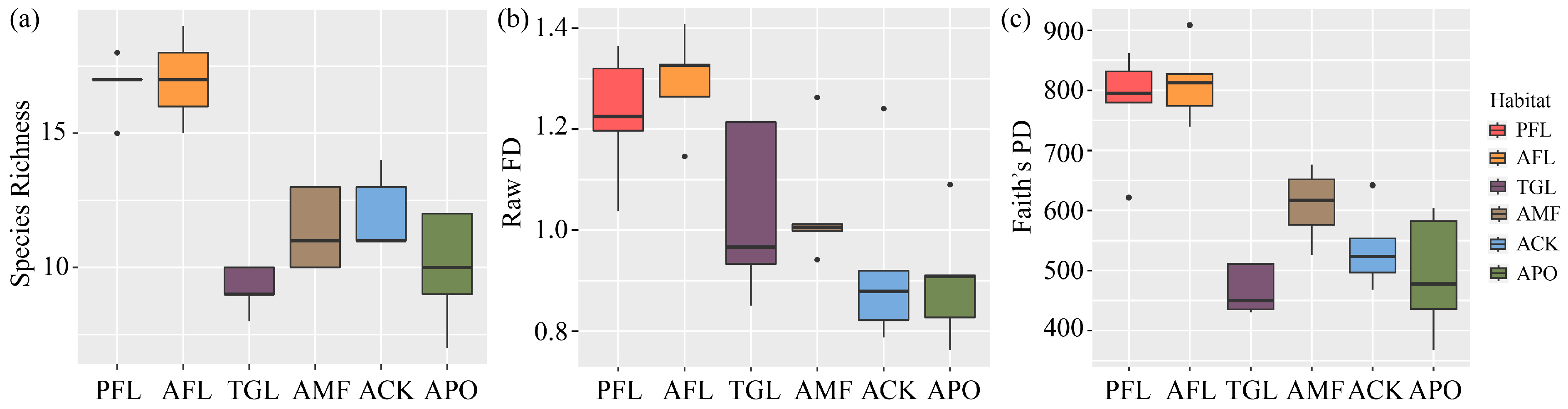
3.1. Species Composition
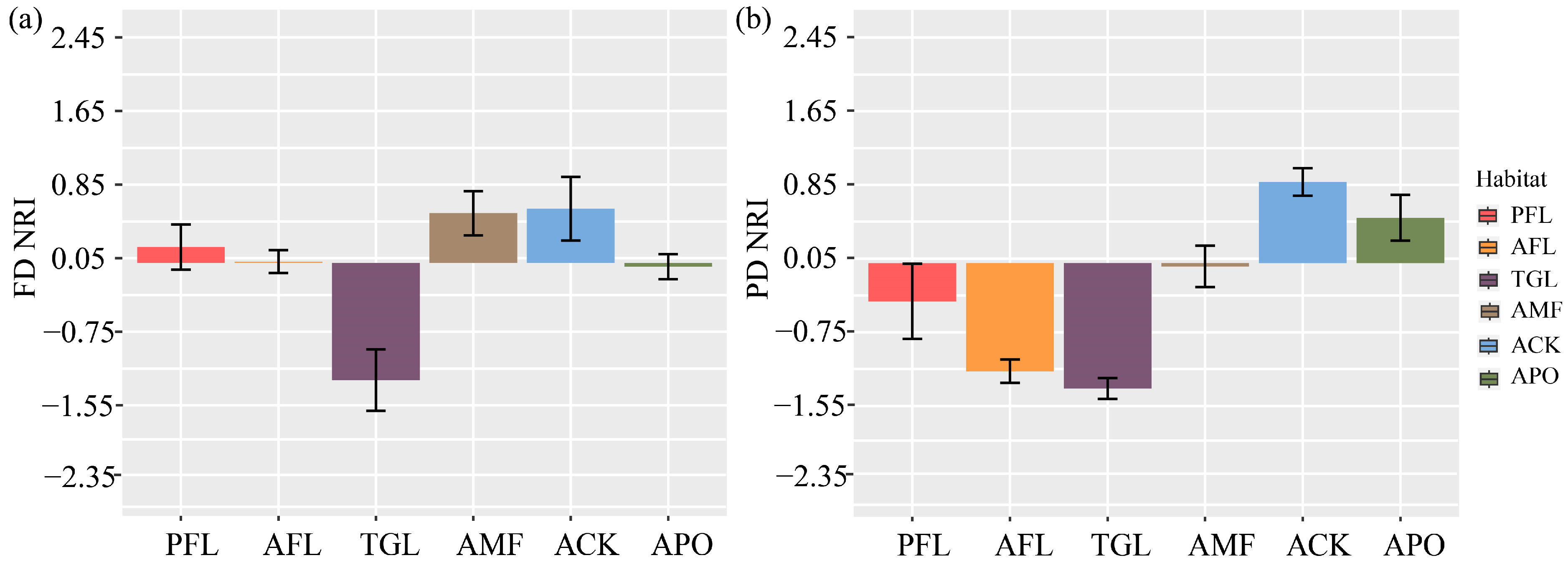
3.2. Functional Diversity
3.3. Phylogenetic Diversity
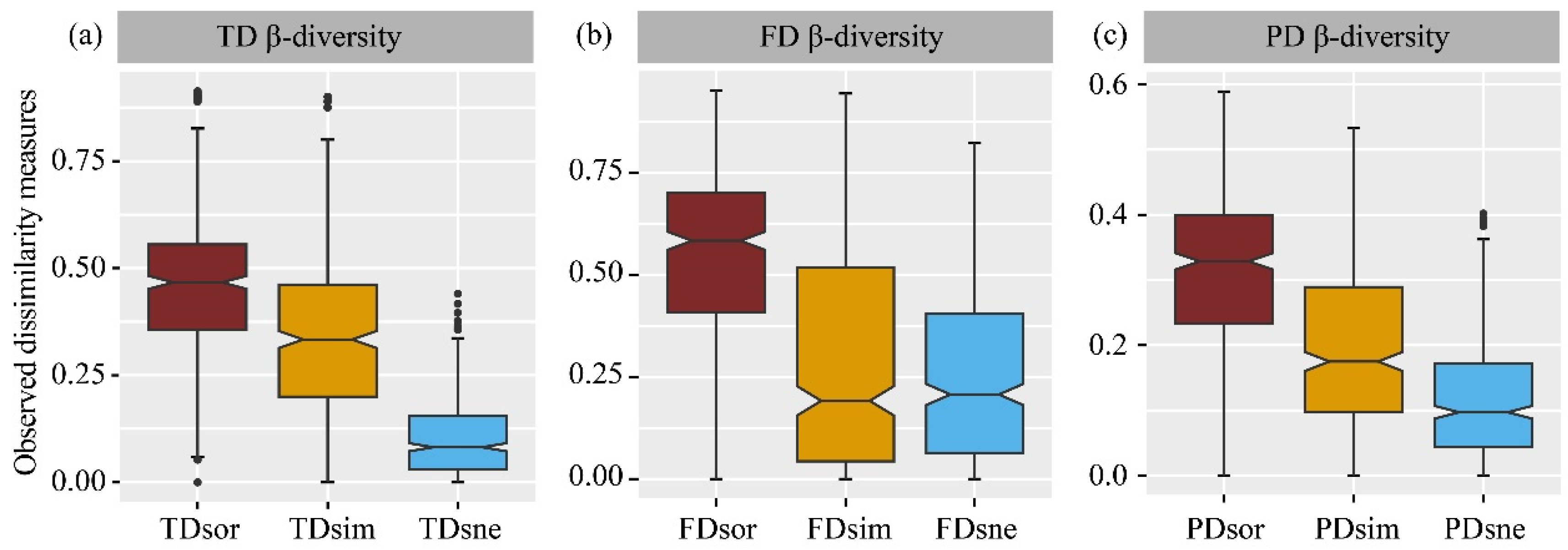
3.4. β–Diversity
4. Discussion
4.1. Different Responses of Taxonomic Diversity to Land Use Change
4.2. Different Responses of Functional Diversity to Land Use Change
4.3. Different Responses of Phylogenetic Diversity to Land Use Change
4.4. Different Responses of β-Diversity to Land Use Change
4.5. Implications for Biodiversity Conservation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; BörgeR, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Rurangwa, M.L.; Aguirre-Gutiérrez, J.; Matthews, T.J.; Niyigaba, P.; Wayman, J.P.; Tobias, J.A.; Whittaker, R.J. Effects of land-use change on avian taxonomic, functional and phylogenetic diversity in a tropical montane rainforest. Divers. Distrib. 2021, 27, 1732–1746. [Google Scholar] [CrossRef]
- Si, X.F.; Cadotte, M.W.; Zeng, D.; Baselga, A.; Zhao, Y.H.; Li, J.Q.; Wu, Y.R.; Wang, S.Y.; Ding, P. Functional and phylogenetic structure of island bird communities. J. Anim. Ecol. 2017, 86, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Semenchuk, P.; Plutzar, C.; Kastner, T.; Matej, S.; Bidoglio, G.; Erb, K.-H.; Essl, F.; Haberl, H.; Wessely, J.; Krausmann, F.; et al. Relative effects of land conversion and land-use intensity on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Ehlers Smith, D.A.; Ehlers Smith, Y.C.; Downs, C. Drivers of fine-scale avian functional diversity with changing land use: An assessment of the effects of eco-estate housing development and management. Landsc. Ecol. 2019, 234, 537–549. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Etard, A.; Pigot, A.L.; Newbold, T. Intensive human land uses negatively affect vertebrate functional diversity. Ecol. Lett. 2022, 25, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Allan, E.; Manning, P.; Alt, F.; Binkenstein, J.; Blaser, S.; Blüthgen, N.; Böhm, S.; Grassein, F.; Hölzel, N.; Klaus, V.H.; et al. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 2015, 18, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Mouquet, N.; Devictor, V.; Meynard, C.N.; Munoz, F.; Bersier, L.-F.; Chave, J.; Couteron, P.; Dalecky, P.; Fontaine, C.; Gravel, D.; et al. Ecophylogenetics: Advances and perspectives. Biol. Rev. 2012, 87, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Dinnage, R.; Tilman, D. Phylogenetic diversity promotes ecosystem stability. Ecology 2012, 93, 223–233. [Google Scholar] [CrossRef]
- Davies, J.; Poulsen, L.; Schulte-Herbrüggen, B.; Mackinnon, K.; Crawhall, N.; Henwood, W.D.; Dudley, N.; Smith, J.; Gudka, M. Conserving Dryland Biodiversity; IUCN (International Union for the Conservation of Nature): Nairobi, Kenya, 2012. [Google Scholar]
- Wang, Y.Q.; Shao, M.A.; Liu, Z.P.; Warrington, D.N. Investigation of factors controlling the regional-scale distribution of dried soil layers under forestland on the Loess Plateau, China. Surv. Geophys. 2012, 33, 311–330. [Google Scholar] [CrossRef]
- Gao, H.D.; Pang, G.W.; Li, Z.B.; Cheng, S.D. Evaluating the potential of vegetation restoration in the Loess Plateau. Acta Geogr. Sin. 2017, 72, 863–874. [Google Scholar]
- Zhu, Y.; Wang, Y.F.; Chen, L.D. Responses of ground-active arthropods to black locust (Robinia pseudoacacia L.) afforestation in the Loess Plateau of China. Catena 2019, 183, 104233. [Google Scholar] [CrossRef]
- Yang, X.; Shao, M.A.; Li, T.C.; Gan, M.; Chen, M.Y. Community characteristics and distribution patterns of soil fauna after vegetation restoration in the northern Loess Plateau. Ecol. Indic. 2021, 122, 107236. [Google Scholar] [CrossRef]
- Guan, C.; Chen, N.; Dong, X.X.; Wei, X.L.; Zhao, C.M. Responses of biological soil crusts to different types of vegetation restoration on the Chinese Loess Plateau: A case study. Restor. Ecol. 2022, 31, e13805. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, N.; Yu, K.L.; Zhao, C.M. The effects of fine roots and arbuscular mycorrhizal fungi on soil macropores. Soil Tillage Res. 2023, 225, 105528. [Google Scholar] [CrossRef]
- Hutto, R.L.; Pletschet, S.M.; Hendricks, P. A fixed-radius point count method for nonbreeding and breeding season use. Auk 1986, 103, 593–602. [Google Scholar] [CrossRef]
- Ralph, C.J.; Sauer, J.R.; Droege, S. Monitoring Bird Populations by Point Counts; Pacific Southwest Research Station: Placerville, CA, USA, 1995.
- Miles, D.B.; Ricklefs, R.E.; Travis, J. Concordance of ecomorphological relationships in three assemblages of passerine birds. Am. Nat. 1987, 129, 347–364. [Google Scholar] [CrossRef]
- Freeman, B.G.; Weeks, T.; Schluter, D.; Tobias, J.A. The latitudinal gradient in rates of evolution for bird beaks, a species interaction trait. Ecol. Lett. 2022, 25, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.A.; Cornwallis, C.K.; Derryberry, E.P.; Claramunt, S.; Brumfield, R.T.; Seddon, N. Species coexistence and the dynamics of phenotypic evolution in adaptive radiation. Nature 2014, 506, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Dawideit, B.A.; Phillimore, A.B.; Laube, I.; Leisler, B.; Böhning-Gaese, K. Ecomorphological predictors of natal dispersal distances in birds. J. Anim. Ecol. 2009, 78, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Claramunt, S.; Derryberry, E.P.; Remsen, J.V., Jr.; Brunfield, R.T. High dispersal ability inhibits speciation in a continental radiation of passerine birds. Proc. R. Soc. B 2012, 279, 1567–1574. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [PubMed]
- Bregman, T.P.; Lees, A.C.; MacGregor, H.E.A.; Darski, B.; de Moura, N.G.; Aleixo, A.; Barlow, J.; Tobias, J.A. Using avian functional traits to assess the impact of land-cover change on ecosystem processes linked to resilience in tropical forests. Proc. R. Soc. B 2016, 283, 20161289. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12. 2014. Available online: https://cran.r-project.org/web/packages/FD/index.html (accessed on 10 April 2024).
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: Rtools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; Kembel, S.W. Phylocom: Software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics 2008, 24, 2098–2100. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Kamp, J.; Reinhard, A.; Frenzel, M.; Kämpfer, S.; Trappe, J.; Hölzel, N. Farmland bird responses to land abandonment in Western Siberia. Agric. Ecosyst. Environ. 2018, 268, 61–69. [Google Scholar] [CrossRef]
- Hua, F.Y.; Wang, X.Y.; Zheng, X.L.; Fisher, B.; Wang, L.; Zhu, J.G.; Tang, Y.; Yu, D.W.; Wilcove, D.S. Opportunities for biodiversity gains under the world’s largest reforestation programme. Nat. Commun. 2016, 7, 12717. [Google Scholar] [CrossRef] [PubMed]
- Potapov, A.M.; Dupérré, N.; Jochum, M.; Dreczko, K.; Klarner, B.; Barnes, A.D.; Krashevska, V.; Rembold, K.; Kreft, H.; Brose, U.; et al. Functional losses in ground spider communities due to habitat structure degradation under tropical land-use change. Ecology 2020, 101, e02957. [Google Scholar] [CrossRef]
- Rocha, J.; Laps, R.R.; Machado, C.G.; Campiolo, S. The conservation value of cacao agroforestry for bird functional diversity in tropical agricultural landscapes. Ecol. Evol. 2019, 9, 7903–7913. [Google Scholar] [CrossRef] [PubMed]
- Birkhofer, K.; Smith, H.G.; Weisser, W.W.; Wolters, V.; Gossner, M.M. Land-use effects on the functional distinctness of arthropod communities. Ecography 2015, 38, 889–900. [Google Scholar] [CrossRef]
- Mace, G.M.; Gittleman, J.L.; Purvis, A. Preserving the tree of life. Science 2003, 300, 1707–1709. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Devictor, V.; Schweiger, O. Phylogenetic diversity and nature conservation: Where are we? Trends Ecol. Evol. 2013, 28, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Frishkoff, L.O.; Karp, D.S.; M’Gonigle, L.K.; Mendenhall, C.D.; Zook, J.; Kremen, C.; Hadly, E.A.; Daily, G.C. Loss of avian phylogenetic diversity in neotropical agricultural systems. Science 2014, 345, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Cosset, C.C.P.; Edwards, D.P. The effects of restoring logged tropical forests on avian phylogenetic and functional diversity. Ecol. Appl. 2017, 27, 1932–1945. [Google Scholar] [CrossRef] [PubMed]
- Sobral, F.L.; Cianciaruso, M.V. Functional and phylogenetic structure of forest and savanna bird assemblages across spatial scales. Ecography 2016, 39, 533–541. [Google Scholar] [CrossRef]
- Chapman, P.M.; Tobias, J.A.; Edwards, D.P.; Davies, R.G. Contrasting impacts of land-use change on phylogenetic and functional diversity of tropical forest birds. J. Appl. Ecol. 2018, 55, 1604–1614. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Chase, J.M.; Kraft, N.J.B.; Smith, K.G.; Vellend, M.; Inouye, B. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2011, 2, 1–11. [Google Scholar] [CrossRef]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Weinstein, B.G.; Tinoco, B.; Parra, J.L.; Brown, L.M.; McGuire, J.A.; Gary Stiles, F.; Graham, C.H. Taxonomic, phylogenetic, and trait beta diversity in South American hummingbirds. Am. Nat. 2014, 184, 211–224. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; Chapin, F.S.; Tecco, P.A.; Gurvich, D.E.; Grigulis, K. Functional diversity—At the crossroads between ecosystem functioning and environmental filters. In Terrestrial Ecosystems in a Changing World; Springer: Berlin/Heidelberg, Germany, 2007; Chapter 7; pp. 81–91. [Google Scholar] [CrossRef]
- Mitra, S.S.; Sheldon, F.H. Use of an exotic tree plantation by Bornean lowland forest birds. Auk 1993, 110, 529–540. [Google Scholar] [CrossRef]
- Barlow, J.; Mestre, L.A.M.; Gardner, T.A.; Peres, C.A. The value of primary, secondary and plantation forests for Amazonian birds. Biol. Conserv. 2007, 136, 212–231. [Google Scholar] [CrossRef]
- Hua, F.Y.; Bruijnzeel, L.A.; Meli, P.; Martin, P.A.; Zhang, J.; Nakagawa, S.; Miao, X.R.; Wang, W.Y.; Mcevor, C.; Pena-Arancibia, J.L. The biodiversity and ecosystem service contributions and trade-offs of forest restoration approaches. Science 2022, 376, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.A.; Sheard, C.; Pigot, A.L.; Devenish, A.J.; Yang, J.; Sayol, F.; Neate-Clegg, M.H.C.; Alioravainen, N.; Weeks, T.L.; Barber, R.A.; et al. AVONET: Morphological, ecological and geographical data for all birds. Ecol. Lett. 2022, 25, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T. Vertebrate Fauna of Gansu Province; Gansu Science and Technology Press: Lanzhou, China, 1991. [Google Scholar]
- Wang, Y.P.; Song, Y.F.; Zhong, Y.Q.; Chen, C.W.; Zhao, Y.H.; Zeng, D.; Wu, Y.R.; Ding, P. A dataset on the life-history and ecological traits of Chinese birds. Biodivers. Sci. 2021, 29, 1149–1153. [Google Scholar] [CrossRef]
- Wilman, H.; Belmaker, J.; Simpson, J. EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals: Ecological Archives E095-178. Ecology 2014, 95, 2027. [Google Scholar] [CrossRef]


| Comparison | FRic | FEve | FDiv | |||
|---|---|---|---|---|---|---|
| Z | p | t | p | t | p | |
| PFL-AFL | −0.503 | 0.615 | −0.902 | 0.393 | −1.258 | 0.244 |
| PFL-TGL | 0.754 | 0.451 | −1.851 | 0.101 | −0.154 | 0.881 |
| PFL-AMF | 1.186 | 0.236 | −0.700 | 0.504 | 4.778 | 0.001 ** |
| PFL-ACK | 2.658 | 0.008 ** | −2.049 | 0.075 | 3.458 | 0.009 ** |
| PFL-APO | 2.479 | 0.013 * | 1.728 | 0.122 | 3.178 | 0.013 * |
| AFL-TGL | 1.257 | 0.209 | −0.804 | 0.445 | 1.019 | 0.338 |
| AFL-AMF | 1.688 | 0.091 | 0.079 | 0.939 | 6.511 | <0.001 *** |
| AFL-ACK | 3.161 | 0.002 ** | −0.723 | 0.490 | 5.472 | <0.001 *** |
| AFL-APO | 2.982 | 0.003 ** | 1.825 | 0.105 | 5.383 | <0.001 *** |
| TGL-AMF | −1.257 | 0.666 | 0.827 | 0.432 | 4.846 | 0.001 ** |
| TGL-ACK | 1.904 | 0.057 | 0.179 | 0.862 | 3.548 | 0.008 ** |
| TGL-APO | 1.724 | 0.085 | 2.699 | 0.027 * | 3.274 | 0.011 * |
| AMF-ACK | 1.473 | 0.141 | −0.750 | 0.476 | −1.714 | 0.125 |
| AMF-APO | 1.293 | 0.196 | 1.518 | 0.167 | −2.175 | 0.061 |
| ACK-APO | −0.120 | 0.857 | 3.107 | 0.015 * | −0.455 | 0.661 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, R.; Zhang, D.; Zhou, Q.; Wang, Y.; Zhang, L. Effects of Land Use Change on Avian Diversity in the Semi-Arid Area of Longxi Loess Plateau. Diversity 2024, 16, 235. https://doi.org/10.3390/d16040235
Mao R, Zhang D, Zhou Q, Wang Y, Zhang L. Effects of Land Use Change on Avian Diversity in the Semi-Arid Area of Longxi Loess Plateau. Diversity. 2024; 16(4):235. https://doi.org/10.3390/d16040235
Chicago/Turabian StyleMao, Ruirui, Dexi Zhang, Qian Zhou, Yizhu Wang, and Lixun Zhang. 2024. "Effects of Land Use Change on Avian Diversity in the Semi-Arid Area of Longxi Loess Plateau" Diversity 16, no. 4: 235. https://doi.org/10.3390/d16040235
APA StyleMao, R., Zhang, D., Zhou, Q., Wang, Y., & Zhang, L. (2024). Effects of Land Use Change on Avian Diversity in the Semi-Arid Area of Longxi Loess Plateau. Diversity, 16(4), 235. https://doi.org/10.3390/d16040235






