Large-Scale Re-Implantation Efforts for Posidonia oceanica Restoration in the Ligurian Sea: Progress and Challenges
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Protection and Conservation of Posidonia oceanica Meadows; RAMOGE and RAC/SPA: Tunis, Tunisia, 2012; pp. 1–202. [Google Scholar]
- Campagne, C.S.; Salles, J.M.; Boissery, P.; Deter, J. The seagrass Posidonia oceanica: Ecosystem services identification and economic evaluation of goods and benefits. Mar. Pollut. Bull. 2014, 97, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Picone, F.; Buonocore, E.; D’Agostaro, R.; Donati, S.; Chemello, R.; Franzese, P.P. Integrating natural capital assessment and marine spatial planning: A case study in the Mediterranean Sea. Ecol. Modell. 2017, 361, 1–13. [Google Scholar] [CrossRef]
- Vassallo, P.; Paoli, C.; Rovere, A.; Montefalcone, M.; Morri, C.; Bianchi, C.N. The value of the seagrass Posidonia oceanica: A natural capital assessment. Mar. Pollut. Bull. 2013, 75, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Monnier, B.; Pergent, G.; Valette-Sansevin, A.; Boudouresque, C.F.; Mateo, M.A.; Pergent-Martini, C. The Posidonia oceanica matte: A unique coastal carbon sink for climate change mitigation and implications for management. Vie Milieu 2020, 70, 17–24. [Google Scholar]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000; p. 298. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Bio. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, R.; Kendrick, G.A.; Kenworthy, J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Turschwell, M.P.; Connolly, R.M.; Dunic, J.C.; Sievers, M.; Buelow, C.A.; Pearson, R.M.; Tulloch, V.J.D.; Côté, I.M.; Unsworth, R.K.F.; Collier, C.J.; et al. Anthropogenic pressures and life history predict trajectories of seagrass meadow extent at a global scale. Proc. Natl. Acad. Sci. USA 2021, 118, e2110802118. [Google Scholar] [CrossRef]
- Abadie, A.; Lejeune, P.; Pergent, G.; Gobert, S. From mechanical to chemical impact of anchoring in seagrasses: The premises of anthropogenic patch generation in Posidonia oceanica meadows. Mar. Pollut. Bull. 2016, 109, 61–71. [Google Scholar] [CrossRef]
- Abadie, A.; Richir, J.; Lejeune, P.; Leduc, M.; Gobert, S. Structural changes of seagrass seascapes driven by natural and anthropogenic factors: A multidisciplinary approach. Front. Ecol. Evol. 2019, 7, 190. [Google Scholar] [CrossRef]
- Garrido, M.; Lafabrie, C.; Torre, F.; Fernandez, C.; Pasqualini, V. Resilience and stability of Cymodocea nodosa seagrass meadows over the last four decades in a Mediterranean lagoon. Estuar. Coast. Shelf Sci. 2013, 130, 89–98. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M. Rhizome elongation and seagrass clonal growth. Mar. Ecol. Prog. Ser. 1998, 174, 269–280. [Google Scholar] [CrossRef]
- Alcoverro, T.; Duarte, C.M.; Romero, J. Annual growth dynamics of Posidonia oceanica: Contribution of large-scale versus local factors to seasonality. Mar. Ecol. Prog. Ser. 1995, 120, 203–210. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Cristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed]
- Montefalcone, M.; Albertelli, G.; Morri, C.; Bianchi, C.N. Urban seagrass: Status of Posidonia oceanica facing the Genoa city waterfront (Italy) and implications for management. Mar. Pollut. Bull. 2007, 54, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Burgos, E.; Montefalcone, M.; Ferrari, M.; Paoli, C.; Vassallo, P.; Morri, C.; Bianchi, C.N. Eco-system functions and economic wealth: Trajectories of change in seagrass meadows. J. Clean. Prod. 2017, 168, 1108–1119. [Google Scholar] [CrossRef]
- Oprandi, A.; Bianchi, C.N.; Karayali, O.; Morri, C.; Rigo, I.; Montefalcone, M. RESQUE: A novel comprehensive approach to compare the performance of different indices in evaluating seagrass health. Ecol. Indic. 2021, 131, 108118. [Google Scholar] [CrossRef]
- De Los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; Van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 3356. [Google Scholar] [CrossRef] [PubMed]
- Calumpong, H.P.; Fonseca, M.S. Seagrass transplantation and other seagrass restoration methods. In Global Seagrass Research Methods; Short, F.T., Coles, R.G., Eds.; Elsevier Science Publ.: Amsterdam, The Netherland, 2001; Volume 22, pp. 425–442. [Google Scholar]
- Maggi, P. Les herbiers à Posidonies et la pollution urbaine dans le Golfe de Giens (Var). Ann. Inst. Michel Pacha 1972, 5, 1–11. [Google Scholar]
- Maggi, P. Le problème de la disparition des herbiers à posidonies dans le golfe de Giens (Var). Sci. Pêche Bull. Inst. Sci. Technol. Pêches Marit. 1973, 221, 7–20. [Google Scholar]
- Cooper, G.F. Jardinier de la Mer. Association-Fondation, G. Cooper pour la reconquête des milieux naturels détruits. Cahier 1976, 1, 1–57. [Google Scholar]
- Cooper, G. Réimplantation de Posidonia oceanica: Protection des implants. Bull. Ecol. 1982, 13, 65–73. [Google Scholar]
- Cinelli, F. La fanerogame marine: Problemi di trapianto e di riforestazione. Mem. Biol. Mar. Ocean. Suppl. 1980, 10, 17–25. [Google Scholar]
- Giaccone, G.; Calvo, S. Restaurazione del manto vegetale mediante trapianto di Posidonia oceanica (Linneo) Delile. Risultati preliminari. Mem. Biol. Mar. 1980, 10, 207–211. [Google Scholar]
- Sandulli, R. Relazione Finale—Reimpianto Della Fanerogama Marina Posidonia oceanica Nel Golfo Paradiso; Castalia S.P.A.: Genova, Italy, 1994; p. 31. [Google Scholar]
- Bavestrello, G.; Cattaneo-Vietti, R. Relazione Finale—Trapianto Sperimentale di Posidonia Oceanica Nel Golfo di Rapallo; Castalia S.P.A.: Genova, Italy, 1997; p. 37. [Google Scholar]
- Sandulli, R. Posidonia in regresso? Riforestiamo! Sperimentata con successo anche sui fondali liguri la tecnica di reimpianto delle talee. Aqua 1994, 95, 27–29. [Google Scholar]
- Meinesz, A.; Molenaar, H.; Bellone, E.; Loques, F. Vegetative reproduction in Posidonia oceanica (L.) Delile. I—Effects of rhizome length and transplantation season in orthotropic shoots. PSZN I Mar. Ecol. 1992, 13, 163–174. [Google Scholar] [CrossRef]
- Molenaar, H.; Meinesz, A. Vegetative reproduction in Posidonia oceanica. II—Effects of depth changes on transplanted orthotropic shoots. PSZN I Mar. Ecol. 1992, 13, 175–185. [Google Scholar] [CrossRef]
- Sánchez-Lizaso, J.L.; Fernández-Torquemada, Y.; González-Correa, J.M. Evaluation of the viability of Posidonia oceanica transplants associated with a marina expansion. Bot. Mar. 2009, 52, 471–476. [Google Scholar] [CrossRef]
- Robello, C.; Oprandi, A.; Mancini, I.; Bavestrello, G.; Montefalcone, M. Effectiveness of a Posidonia oceanica (L.) Delile transplantation in the Gulf of Tigullio (Ligurian sea) 23 years later. Biol. Mar. Med. 2024; in press. [Google Scholar]
- Pansini, A.; Bosch-Belmar, M.; Berlino, M.; Sarà, G.; Ceccherelli, G. Collating evidence on the restoration efforts of the seagrass Posidonia oceanica: Current knowledge and gaps. Sci. Total Environ. 2022, 851, 158320. [Google Scholar] [CrossRef]
- Piazzi, L.; Acunto, S.; Frau, F.; Atzori, F.; Cinti, M.F.; Leone, L.M.; Ceccherelli, G. Environmental engineering techniques to restore degraded Posidonia oceanica meadows. Water 2021, 13, 661. [Google Scholar] [CrossRef]
- Fraschetti, S.; McOwen, C.; Papa, L.; Papadopoulou, N.; Bilan, M.; Boström, C.; Capdevila, P.; Carreiro-Silva, M.; Carugati, L.; Cebrian, E.; et al. Where Is More Important than How in Coastal and Marine Ecosystems Restoration. Front. Mar. Sci. 2021, 8, 626843. [Google Scholar] [CrossRef]
- Oprandi, A.; Mucerino, L.; de Leo, F.; Bianchi, C.N.; Morri, C.; Azzola, A.; Benelli, F.; Besio, G.; Ferrari, M.; Montefalcone, M. Effects of a severe storm on seagrass meadows. Sci. Total Environ. 2020, 748, 141373. [Google Scholar] [CrossRef] [PubMed]
- Rigo, I.; Dapueto, G.; Paoli, C.; Massa, F.; Oprandi, A.; Venturini, S.; Merotto, L.; Fanciulli, G.; Cappanera, V.; Montefalcone, M.; et al. Changes in the ecological status and natural capital of Posidonia oceanica meadows due to human pressure and extreme events. Vie Milieu 2020, 70, 137–148. [Google Scholar]
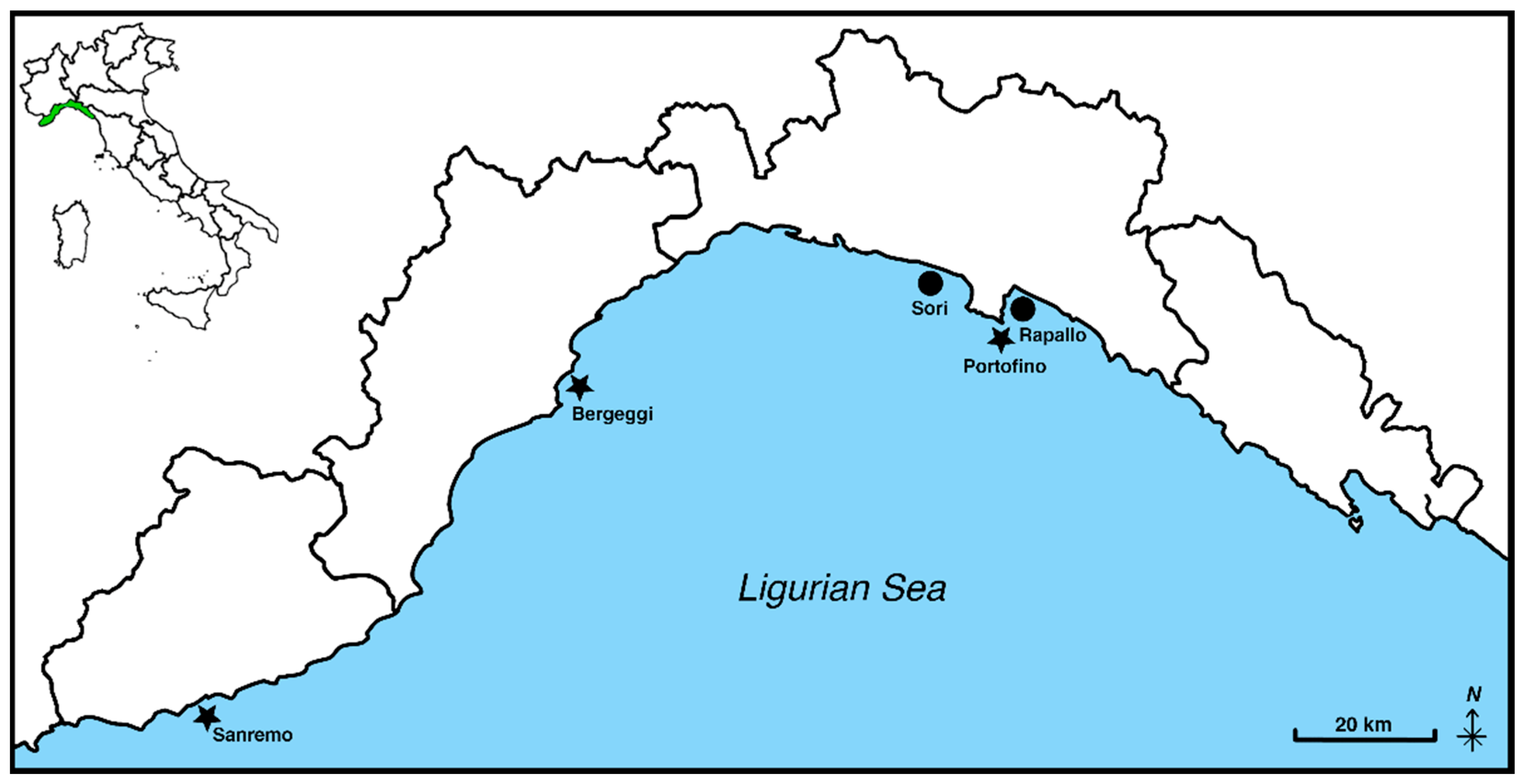

| Survival Rate (%) | |||||
|---|---|---|---|---|---|
| Sites | Starting Date | 1st Monitoring | 2nd Monitoring | 3rd Monitoring | 4rd Monitoring |
| Punta Pedale (Portofino) | July 2022 | 88 | 64 | 64 | 40 |
| Bergeggi | June 2023 | 90 | 70 | ||
| Pian di Poma (Sanremo) | September 2023 | n.a. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robello, C.; Acunto, S.; Leone, L.M.; Mancini, I.; Oprandi, A.; Montefalcone, M. Large-Scale Re-Implantation Efforts for Posidonia oceanica Restoration in the Ligurian Sea: Progress and Challenges. Diversity 2024, 16, 226. https://doi.org/10.3390/d16040226
Robello C, Acunto S, Leone LM, Mancini I, Oprandi A, Montefalcone M. Large-Scale Re-Implantation Efforts for Posidonia oceanica Restoration in the Ligurian Sea: Progress and Challenges. Diversity. 2024; 16(4):226. https://doi.org/10.3390/d16040226
Chicago/Turabian StyleRobello, Chiara, Stefano Acunto, Laura Marianna Leone, Ilaria Mancini, Alice Oprandi, and Monica Montefalcone. 2024. "Large-Scale Re-Implantation Efforts for Posidonia oceanica Restoration in the Ligurian Sea: Progress and Challenges" Diversity 16, no. 4: 226. https://doi.org/10.3390/d16040226
APA StyleRobello, C., Acunto, S., Leone, L. M., Mancini, I., Oprandi, A., & Montefalcone, M. (2024). Large-Scale Re-Implantation Efforts for Posidonia oceanica Restoration in the Ligurian Sea: Progress and Challenges. Diversity, 16(4), 226. https://doi.org/10.3390/d16040226







