Updated List of Bryophytes from Cape Verde Archipelago
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Field Sampling and Herbarium Material
2.3. Data Analysis
3. Results
3.1. Updated List
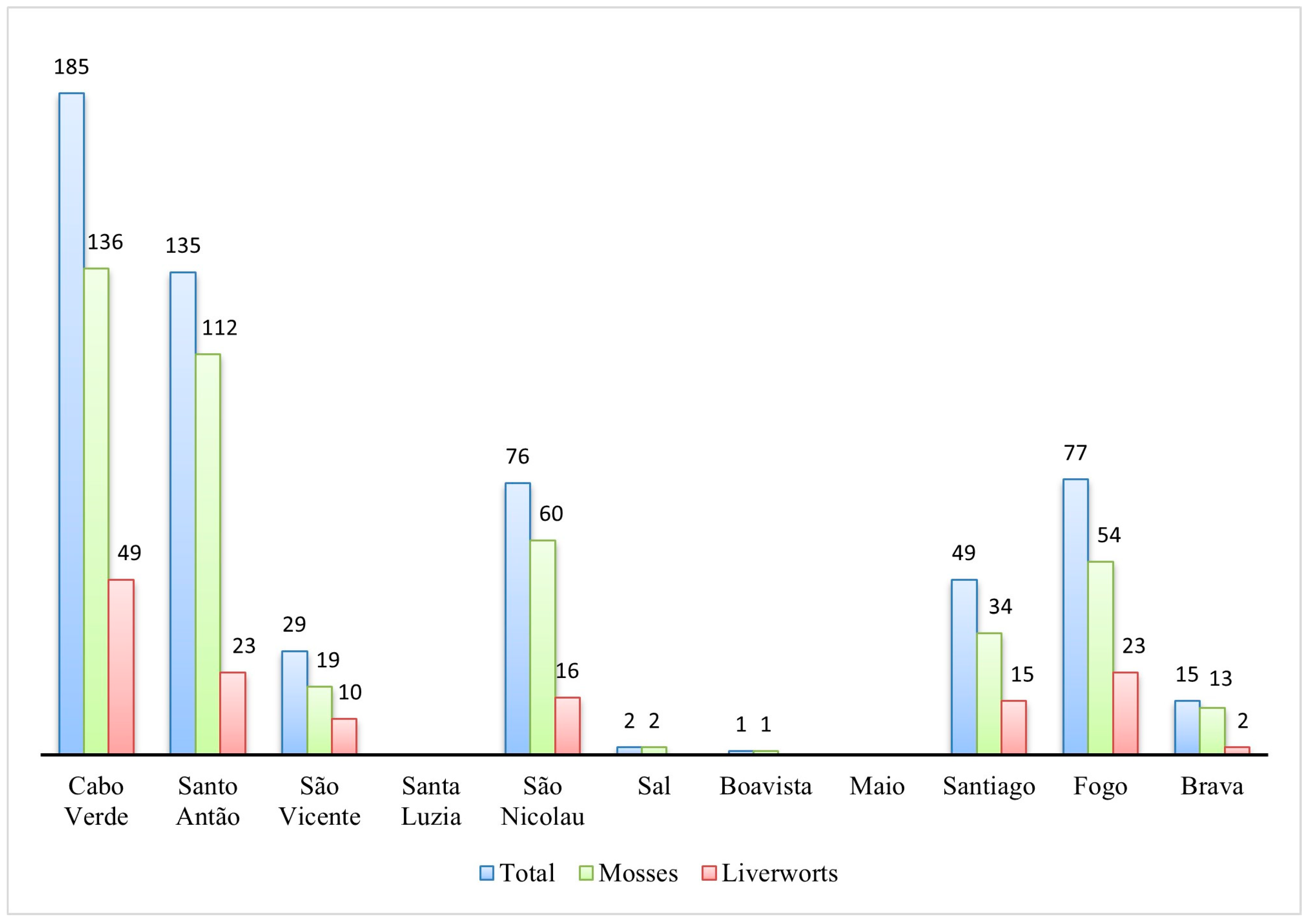
3.2. Bryophyte Diversity
3.3. Comparing Bryophyte Diversity between Islands
3.4. Endemism
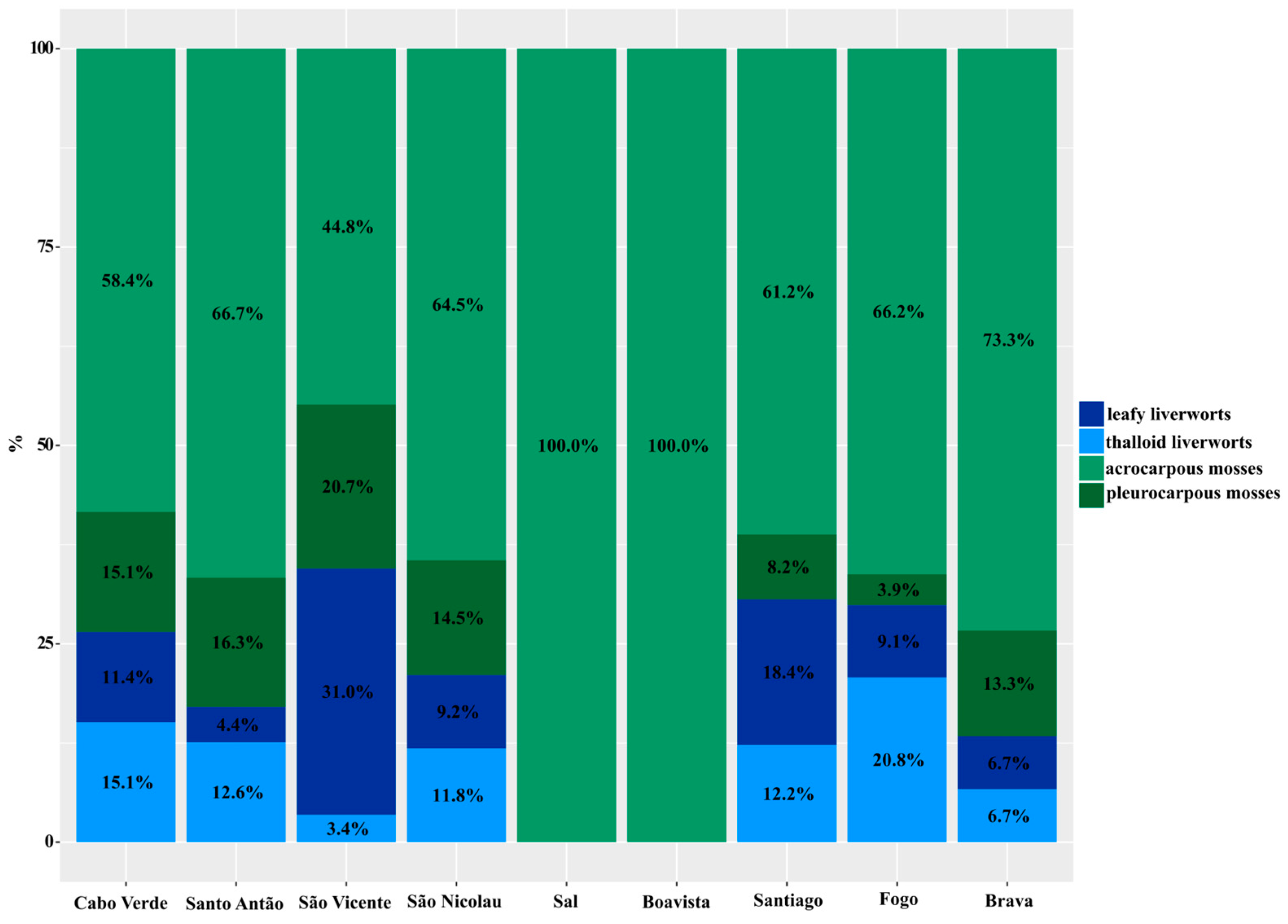
3.5. Bryophyte Growth Forms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Updated List of Bryophytes from Cape Verde Archipelago
- List of Taxa
| Family | Genus | Taxon | Santo Antão | São Vicente | Santa Luzia | São Nicolau | Sal | Boavista | Maio | Santiago | Fogo | Brava | Endemism | Growth Form |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthocerotaceae | Anthoceros | Anthoceros punctatus L. | 1 | non | tha | |||||||||
| Notothyladaceae | Phaeoceros | Phaeoceros carolinianus (Michx.) Prosk. | 1 | 1 | 1 | non | tha | |||||||
| Adelanthaceae | Syzygiella | Syzygiella manca (Mont.) Steph. (1*) | non | fol | ||||||||||
| Aneuraceae | Aneura | Aneura pinguis (L.) Dumort. (2*) | 1 | non | tha | |||||||||
| Aytoniaceae | Mannia | Mannia androgyna (L.) A.Evans | 1 | 1 | 1 | 1 | non | tha | ||||||
| Aytoniaceae | Plagiochasma | Plagiochasma eximium (Schiffn.) Steph. | 1 | 1 | 1 | non | tha | |||||||
| Aytoniaceae | Plagiochasma | Plagiochasma rupestre (J.R.Forst. et G.Forst.) Steph. var. rupestre | 1 | 1 | 1 | 1 | 1 | 1 | non | tha | ||||
| Aytoniaceae | Reboulia | Reboulia hemisphaerica (L.) Raddi | 1 | 1 | 1 | 1 | non | tha | ||||||
| Cyathodiaceae | Cyathodium | Cyathodium cavernarum Kunze | 1 | 1 | non | tha | ||||||||
| Exormothecaceae | Exormotheca | Exormotheca martins-loussaoae Sim-Sim, A.Martins, J.Patiño & C.A.Garcia (3*) | 1 | 1 | 1 | end | tha | |||||||
| Fossombroniaceae | Fossombronia | Fossombronia angulosa (Dicks.) Raddi | 1 | 1 | non | tha | ||||||||
| Fossombroniaceae | Fossombronia | Fossombronia pusilla (L.) Nees | 1 | 1 | non | tha | ||||||||
| Frullaniaceae | Frullania | Frullania dilatata (L.) Dumort. | 1 | 1 | 1 | non | fol | |||||||
| Frullaniaceae | Frullania | Frullania ericoides (Nees) Mont. | 1 | 1 | 1 | 1 | 1 | non | fol | |||||
| Frullaniaceae | Frullania | Frullania socotrana Mitt. | 1 | 1 | 1 | 1 | 1 | non | fol | |||||
| Frullaniaceae | Frullania | Frullania spongiosa Steph. (4*) | 1 | 1 | 1 | 1 | non | fol | ||||||
| Frullaniaceae | Frullania | Frullania tamarisci (L.) Dumort. | 1 | 1 | 1 | 1 | 1 | non | fol | |||||
| Lejeuneaceae | Acrolejeunea | Acrolejeunea emergens (Mitt.) Steph. | non | fol | ||||||||||
| Lejeuneaceae | Cheilolejeunea | Cheilolejeunea rigidula (Nees ex Mont.) R.M.Schust. (5*) | 1 | non | fol | |||||||||
| Lejeuneaceae | Cheilolejeunea | Cheilolejeunea xanthocarpa (Lehm. & Lindenb.) Malombe (6*) | non | fol | ||||||||||
| Lejeuneaceae | Lejeunea | Lejeunea capensis Gottsche | 1 | non | fol | |||||||||
| Lejeuneaceae | Lejeunea | Lejeunea eckloniana Lindenb. | 1 | non | fol | |||||||||
| Lejeuneaceae | Lejeunea | Lejeunea flava (Sw.) Nees | 1 | non | fol | |||||||||
| Lejeuneaceae | Lejeunea | Lejeunea lamacerina (Steph.) Schiffn. | 1 | non | fol | |||||||||
| Lejeuneaceae | Marchesinia | Marchesinia mackaii (Hook.) Gray | non | fol | ||||||||||
| Lejeuneaceae | Microlejeunea | Microlejeunea ulicina (Taylor) Steph. | 1 | non | fol | |||||||||
| Lejeuneaceae | Myriocoleopsis | Myriocoleopsis minutissima (Sm.) R.L.Zhu, Y.Yu & Pócs | 1 | 1 | 1 | non | fol | |||||||
| Lophocoleaceae | Lophocolea | Lophocolea bidentata (L.) Dumort. | 1 | non | fol | |||||||||
| Lunulariaceae | Lunularia | Lunularia cruciata (L.) Dumort. ex Lindb. | 1 | 1 | non | tha | ||||||||
| Marchantiaceae | Marchantia | Marchantia paleacea Bertol. | 1 | 1 | non | tha | ||||||||
| Marchantiaceae | Marchantia | Marchantia pappeana Lehm. subsp. pappeana | non | tha | ||||||||||
| Pleuroziaceae | Pleurozia | Pleurozia gigantea (F.Weber) Lindb. (7*) | non | fol | ||||||||||
| Porellaceae | Porella | Porella arboris-vitae (With.) Grolle | 1 | 1 | non | fol | ||||||||
| Porellaceae | Porella | Porella canariensis (F.Weber) Underw. (8*) | 1 | 1 | non | fol | ||||||||
| Radulaceae | Radula | Radula lindenbergiana Gottsche ex C.Hartm. | 1 | 1 | 1 | non | fol | |||||||
| Ricciaceae | Riccia | Riccia atromarginata Levier (9*) | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia atropurpurea Sim (10*) | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia cavernosa Hoffm. | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia ciliata Hoffm. | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia congoana Stephani (11*) | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia crinita Taylor (12*) | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia crystallina L. (13*) | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia frostii Austin | non | tha | ||||||||||
| Ricciaceae | Riccia | Riccia gougetiana Durieu & Mont. | non | tha | ||||||||||
| Ricciaceae | Riccia | Riccia macrocarpa Levier (14*) | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia nigrella DC. (15*) | 1 | 1 | 1 | non | tha | |||||||
| Ricciaceae | Riccia | Riccia sorocarpa Bisch. subsp. sorocarpa | 1 | non | tha | |||||||||
| Ricciaceae | Riccia | Riccia trabutiana Steph. (16*) | 1 | non | tha | |||||||||
| Targioniaceae | Targionia | Targionia hypophylla L. | 1 | 1 | non | tha | ||||||||
| Amblystegiaceae | Leptodictyum | Leptodictyum riparium (Hedw.) Warns | 1 | non | ple | |||||||||
| Bartramiaceae | Bartramia | Bartramia laevisphaera (Taylor) Müll.Hal. | 1 | 1 | 1 | non | acr | |||||||
| Bartramiaceae | Bartramia | Bartramia aprica Müll. Hal. Brid. (17*) | 1 | non | acr | |||||||||
| Bartramiaceae | Philonotis | Philonotis calcarea (Bruch & Schimp.) Schimp. | non | acr | ||||||||||
| Bartramiaceae | Philonotis | Philonotis fontana (Hedw.) Brid. | 1 | non | acr | |||||||||
| Bartramiaceae | Philonotis | Philonotis hastata (Duby) Wijk & Margad. | 1 | 1 | non | acr | ||||||||
| Bartramiaceae | Philonotis | Philonotis marchica (Hedw.) Brid. | 1 | 1 | non | acr | ||||||||
| Bartramiaceae | Philonotis | Philonotis nanothecioidea Paris & Broth. | 1 | non | acr | |||||||||
| Bartramiaceae | Philonotis | Philonotis rigida Brid. | 1 | 1 | 1 | non | acr | |||||||
| Brachytheciaceae | Homalothecium | Homalothecium aureum (Spruce) H.Rob. (18*) | 1 | non | ple | |||||||||
| Brachytheciaceae | Homalothecium | Homalothecium sericeum (Hedw.) Schimp. | 1 | 1 | 1 | 1 | 1 | non | ple | |||||
| Brachytheciaceae | Kindbergia | Kindbergia praelonga (Hedw.) Ochyra | 1 | non | ple | |||||||||
| Brachytheciaceae | Oxyrrhynchium | Oxyrrhynchium speciosum (Brid.) Warnst. | 1 | non | ple | |||||||||
| Brachytheciaceae | Palamocladium | Palamocladium leskeoides (Hook.) E.Britton | 1 | 1 | 1 | 1 | non | ple | ||||||
| Brachytheciaceae | Plasteurhynchium | Plasteurhynchium meridionale (Schimp.) M.Fleisch. | 1 | non | ple | |||||||||
| Brachytheciaceae | Rhynchostegium | Rhynchostegium megapolitanum (Blandow ex F.Weber & D.Mohr) Schimp. | 1 | non | ple | |||||||||
| Brachytheciaceae | Rhynchostegium | Rhynchostegium riparioides (Hedw.) Cardot | 1 | non | ple | |||||||||
| Brachytheciaceae | Scleropodium | Scleropodium touretii (Brid.) L.F.Koch | 1 | non | ple | |||||||||
| Brachytheciaceae | Scorpiurium | Scorpiurium circinatum (Bruch) M.Fleisch. & Loeske | 1 | 1 | 1 | 1 | non | ple | ||||||
| Bryaceae | Anomobryum | Anomobryum apiculatum (Schwägr.) D.Bell & Holyoak | 1 | non | acr | |||||||||
| Bryaceae | Anomobryum | Anomobryum julaceum (Schrad. ex P.Gaertn., E.Mey & Scherb.) Schimp. | 1 | 1 | non | acr | ||||||||
| Bryaceae | Anomobryum | Anomobryum notarisii (Mitt.) D.Bell. & Holyoak | 1 | non | acr | |||||||||
| Bryaceae | Brachymenium | Brachymenium acuminatum Harv. | 1 | 1 | 1 | 1 | non | acr | ||||||
| Bryaceae | Brachymenium | Brachymenium exile (Dozy & Molk.) Bosch. & Sande Lac. | 1 | 1 | 1 | 1 | 1 | non | acr | |||||
| Bryaceae | Bryum | Bryum anomodon Mont. | 1 | 1 | 1 | end | acr | |||||||
| Bryaceae | Bryum | Bryum argenteum Hedw. | 1 | 1 | 1 | non | acr | |||||||
| Bryaceae | Bryum | Bryum canariense Brid. | 1 | 1 | 1 | non | acr | |||||||
| Bryaceae | Bryum | Bryum dichotomum Hedw. (19*) | 1 | 1 | non | acr | ||||||||
| Bryaceae | Bryum | Bryum kikuyuense (Broth. & Thér.) N.Pedersen | 1 | 1 | 1 | 1 | non | acr | ||||||
| Bryaceae | Perssonia | Perssonia sanguinea Bizot | 1 | 1 | 1 | end | acr | |||||||
| Bryaceae | Ptychostomum | Ptychostomum capillare (Hedw.) Holyoak & N.Pedersen | 1 | 1 | non | acr | ||||||||
| Bryaceae | Ptychostomum | Ptychostomum cellulare (Hook.) D.Bell & Holyoak | 1 | non | acr | |||||||||
| Cryphaeaceae | Cryphaea | Cryphaea heteromalla (Hedw.) D.Mohr (20*) | 1 | 1 | non | ple | ||||||||
| Entodontaceae | Entodon | Entodon pseudoseductrix (Müll.Hal.) A.Jaeger | 1 | 1 | 1 | end | ple | |||||||
| Entodontaceae | Entodon | Entodon schleicheri (Schimp.) Demet. | 1 | non | ple | |||||||||
| Erpodiaceae | Erpodium | Erpodium grossirete Müll.Hal. | non | ple | ||||||||||
| Erpodiaceae | Erpodium | Erpodium perrottetii (Mont.) A.Jaeger & Sauerb. | 1 | non | ple | |||||||||
| Fabroniaceae | Fabronia | Fabronia leikipiae Müll.Hal. | 1 | 1 | non | ple | ||||||||
| Fissidentaceae | Fissidens | Fissidens allorgei P.de la Varde | 1 | 1 | 1 | end | acr | |||||||
| Fissidentaceae | Fissidens | Fissidens androgynus Bruch ex C.Krauss | non | acr | ||||||||||
| Fissidentaceae | Fissidens | Fissidens bogosicus Müll.Hal. | 1 | 1 | non | acr | ||||||||
| Fissidentaceae | Fissidens | Fissidens crispus Mont. | 1 | non | acr | |||||||||
| Fissidentaceae | Fissidens | Fissidens danckelmannii Müll.Hal. (21*) | 1 | 1 | 1 | non | acr | |||||||
| Fissidentaceae | Fissidens | Fissidens flaccidus Mitt. (22*) | 1 | 1 | 1 | non | acr | |||||||
| Fissidentaceae | Fissidens | Fissidens megalotis subsp. helictocaulos (Müll.Hal.) Brugg.-Nann. | 1 | 1 | 1 | non | acr | |||||||
| Fissidentaceae | Fissidens | Fissidens sciophyllus Mitt. (21*) | 1 | 1 | 1 | non | acr | |||||||
| Fissidentaceae | Fissidens | Fissidens usambaricus Broth. | non | acr | ||||||||||
| Funariaceae | Entosthodon | Entosthodon kroonkurk Dirkse & Brugués (23*) | 1 | non | acr | |||||||||
| Funariaceae | Funaria | Funaria chevalieri P.de la Varde | 1 | end | acr | |||||||||
| Funariaceae | Funaria | Funaria hygrometrica Hedw. | 1 | 1 | non | acr | ||||||||
| Grimmiaceae | Grimmia | Grimmia incurva Schwägr. (24*) | 1 | non | acr | |||||||||
| Grimmiaceae | Grimmia | Grimmia laevigata (Brid.) Brid. | 1 | 1 | non | acr | ||||||||
| Grimmiaceae | Grimmia | Grimmia lisae De Not. | 1 | 1 | non | acr | ||||||||
| Grimmiaceae | Grimmia | Grimmia trichophylla Grev. | 1 | 1 | non | acr | ||||||||
| Hedwigiaceae | Braunia | Braunia alopecura (Brid.) Limpr. | 1 | 1 | non | acr | ||||||||
| Hedwigiaceae | Hedwigia | Hedwigia ciliata (Hedw.) P.Beauv. | 1 | 1 | non | acr | ||||||||
| Hedwigiaceae | Hedwigidium | Hedwigidium integrifolium (P. Beauv.) C.E.O. Jensen (25*) | non | acr | ||||||||||
| Hypnaceae | Hypnum | Hypnum cupressiforme Hedw. var. cupressiforme | 1 | non | ple | |||||||||
| Hypnaceae | Platygyriella | Platygyriella densa (Hook.) W.R.Buck | 1 | 1 | non | ple | ||||||||
| Leskeaceae | Lindbergia | Lindbergia patentifolia Dixon (26*) | 1 | non | ple | |||||||||
| Leskeaceae | Pseudoleskeopsis | Pseudoleskeopsis pseudoattenuata (Müll.Hal.) Thér. | 1 | non | ple | |||||||||
| Leskeaceae | Pseudoleskeopsis | Pseudoleskeopsis bollei (Broth. & Geh.) P.Rao | 1 | 1 | 1 | end | ple | |||||||
| Leucobryaceae | Campylopus | Campylopus pilifer Brid. | 1 | 1 | 1 | 1 | 1 | non | acr | |||||
| Leucobryaceae | Campylopus | Campylopus pyriformis (Schultz) Brid. | non | acr | ||||||||||
| Leucodontaceae | Leucodon | Leucodon sciuroides (Hedw.) Schwägr. | 1 | 1 | non | ple | ||||||||
| Leucodontaceae | Nogopterium | Nogopterium gracile (Hedw.) Crosby & W.R.Buck | 1 | 1 | non | ple | ||||||||
| Meesiaceae | Leptobryum | Leptobryum pyriforme (Hedw.) Wilson | 1 | 1 | non | acr | ||||||||
| Mniaceae | Epipterygium | Epipterygium tozeri (Grev.) Lindb. | 1 | non | acr | |||||||||
| Neckeraceae | Leptodon | Leptodon longisetus Mont. (27*) | 1 | 1 | 1 | non | ple | |||||||
| Neckeraceae | Exsertotheca | Exsertotheca intermedia (Brid.) S.Olsson, Enroth & D.Quandt | 1 | non | ple | |||||||||
| Orthotrichaceae | Groutiella | Groutiella tomentosa (Hornsch.) Wijk & Marg. | 1 | 1 | 1 | non | acr | |||||||
| Orthotrichaceae | Lewinskya | Lewinskya acuminata (H.Philib.) F.Lara, Garilleti & Goffinet (28*) | 1 | non | acr | |||||||||
| Orthotrichaceae | Orthotrichum | Orthotrichum diaphanum Brid. (29*) | 1 | 1 | 1 | non | acr | |||||||
| Orthotrichaceae | Orthotrichum | Orthotrichum pumilum Sw. ex anon. | 1 | non | acr | |||||||||
| Orthotrichaceae | Zygodon | Zygodon conoideus (Dicks.) Hook. & Taylor | 1 | non | acr | |||||||||
| Pottiaceae | Aloina | Aloina ambigua (Bruch & Schimp.) Limpr. (30*) | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Aloina | Aloina rigida (Hedw.) Limpr. | 1 | non | acr | |||||||||
| Pottiaceae | Anoectangium | Anoectangium aestivum (Hedw.) Mitt. | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Barbula | Barbula unguiculata Hedw. | 1 | non | acr | |||||||||
| Pottiaceae | Bryoceuthospora | Bryoceuthospora aethiopica (Welw. & Duby) R.H.Zander (31*) | 1 | non | acr | |||||||||
| Pottiaceae | Bryoerythrophyllum | Bryoerythrophyllum campylocarpum (Müll.Hal.) H.A.Crum (32*) | 1 | non | acr | |||||||||
| Pottiaceae | Bryoerythrophyllum | Bryoerythrophyllum ferruginascens (Stirt.) Giacom. | 1 | non | acr | |||||||||
| Pottiaceae | Bryoerythrophyllum | Bryoerythrophyllum inaequalifolium (Taylor) R.H.Zander (33*) | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Chenia | Chenia leptophylla (Müll.Hal.) R.H.Zander (34*) | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Chionoloma | Chionoloma tenuirostre (Hook. & Taylor) M.Alonso, M.J.Cano & J.A.Jiménez var. tenuirostre | 1 | non | acr | |||||||||
| Pottiaceae | Crossidium | Crossidium crassinervium (De Not.) Jur. | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Crossidium | Crossidium geheebii (Broth.) Broth. (35*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Crossidium | Crossidium squamiferum (Viv.) Jur. | non | acr | ||||||||||
| Pottiaceae | Didymodon | Didymodon australasiae (Hook. & Grev.) R.H.Zander | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Didymodon | Didymodon caboverdeanus J.A.Jiménez & M.J.Cano (36*) | 1 | 1 | 1 | end | acr | |||||||
| Pottiaceae | Didymodon | Didymodon fallax (Hedw.) R.H.Zander | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Didymodon | Didymodon hastatus (Mitt.) R.H. Zander (37*) | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Didymodon | Didymodon insulanus (De Not.) M.O.Hill | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Didymodon | Didymodon revolutus (Cardot) R.S.Williams (38*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Didymodon | Didymodon rigidulus Hedw. | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Didymodon | Didymodon tophaceus (Brid.) Lisa subsp. tophaceus | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Didymodon | Didymodon tophaceus subsp. sicculus (M.J.Cano, Ros, García Zam. & J.Guerra) Jan Kučera (39*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Didymodon | Didymodon vinealis (Brid.) R.H.Zander (40*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Eucladium | Eucladium verticillatum (With.) Bruch & Schimp. | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Gymnostomiella | Gymnostomiella erosula (Müll.Hal. ex Dusén) Arts | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Gymnostomiella | Gymnostomiella vernicosa (Hook. ex Harv.) M.Fleisch. | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Gymnostomum | Gymnostomum aeruginosum Sm. | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Gymnostomum | Gymnostomum calcareum Nees & Hornsch. | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Hydrogonium | Hydrogonium arcuatum (Griff.) Wijk & Margad. (41*) | 1 | non | acr | |||||||||
| Pottiaceae | Hydrogonium | Hydrogonium bolleanum (Müll.Hal.) A.Jaeger | 1 | 1 | 1 | 1 | 1 | non | acr | |||||
| Pottiaceae | Hydrogonium | Hydrogonium consanguineum (Thwaites & Mitt.) Hilp. | 1 | non | acr | |||||||||
| Pottiaceae | Hydrogonium | Hydrogonium orientale (F.Weber) Jan Kučera (42*) | 1 | 1 | 1 | 1 | 1 | 1 | non | acr | ||||
| Pottiaceae | Hymenostylium | Hymenostylium congoanum Dixon & Naveau | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Hyophila | Hyophila involuta (Hook.) A.Jaeger (43*) | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Microbryum | Microbryum davallianum (Sm.) R.H.Zander (44*) | 1 | non | acr | |||||||||
| Pottiaceae | Microbryum | Microbryum starckeanum (Hedw.) R.H.Zanderm (45*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Streblotrichum | Streblotrichum convolutum (Hedw.) P.Beauv. var. convolutum | 1 | 1 | 1 | 1 | 1 | 1 | non | acr | ||||
| Pottiaceae | Syntrichia | Syntrichia amphidiacea (Müll.Hal.) R.H.Zander (46*) | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Syntrichia | Syntrichia fragilis (Taylor) Ochyra (47*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Syntrichia | Syntrichia laevipila Brid. | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Timmiella | Timmiella barbuloides (Brid.) Mönk. | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Timmiella | Timmiella cameruniae Broth. (48*) | 1 | non | acr | |||||||||
| Pottiaceae | Tortella | Tortella nitida (Lindb.) Broth. (49*) | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Tortella | Tortella squarrosa (Brid.) Limpr. | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Tortula | Tortula atrovirens (Sm.) Lindb. (50*) | 1 | 1 | 1 | non | acr | |||||||
| Pottiaceae | Tortula | Tortula bogosica (Müll.Hal.) R.H.Zander (51*) | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Tortula | Tortula bolanderi (Lesq. & James) M.Howe (52*) | 1 | non | acr | |||||||||
| Pottiaceae | Tortula | Tortula canescens Mont. (53*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Tortula | Tortula cuneifolia (Dicks.) Turner | 1 | non | acr | |||||||||
| Pottiaceae | Tortula | Tortula muralis Hedw. (54*) | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Tortula | Tortula revolvens (Schimp.) G.Roth (55*) | 1 | non | acr | |||||||||
| Pottiaceae | Tortula | Tortula solmsii (Schimp.) Limpr. (56*) | 1 | 1 | 1 | 1 | non | acr | ||||||
| Pottiaceae | Tortula | Tortula vahliana (Schultz) Mont. (57*) | 1 | non | acr | |||||||||
| Pottiaceae | Trichostomum | Trichostomum brachydontium Bruch | 1 | 1 | 1 | 1 | 1 | 1 | non | acr | ||||
| Pottiaceae | Trichostomum | Trichostomum crispulum Bruch | 1 | 1 | 1 | 1 | 1 | 1 | non | acr | ||||
| Pottiaceae | Weissia | Weissia brachycarpa (Nees & Hornsch.) Jur. | 1 | non | acr | |||||||||
| Pottiaceae | Weissia | Weissia condensa (Voit) Lindb. | 1 | 1 | non | acr | ||||||||
| Pottiaceae | Weissia | Weissia controversa Hedw. var. controversa (58*) | 1 | 1 | 1 | 1 | 1 | non | acr | |||||
| Ptychomitriaceae | Ptychomitrium | Ptychomitrium nigrescens (Kunze) Wijk & Margad. | 1 | 1 | 1 | 1 | non | acr | ||||||
| Stereophyllaceae | Entodontopsis | Entodontopsis leucostega (Brid.) W.R.Buck & Ireland | 1 | non | ple | |||||||||
| Thuidiaceae | Herpetineuron | Herpetineuron toccoae (Sull. & Lesq.) Cardot | 1 | non | ple | |||||||||
| Excluded taxa | ||||||||||||||
| Exormothecaceae | Exormotheca | Exormotheca pustulosa Mitt. (59*) | ||||||||||||
| Ptychomitriaceae | Ptychomitrium | Ptychomitrium subcrispatum Thér. & P.de la Varde (60*) | ||||||||||||
| Pottiaceae | Barbula javanica | Barbula javanica Dozy & Molk. (61*) | ||||||||||||
| Doubtful taxa | ||||||||||||||
| Ditrichaceae | Ditrichum | Ditrichum sp. (62*) | ||||||||||||
| Ditrichaceae | Pleuridium | Pleuridium pappeanum (Müll.Hal.) A.Jaeger (63*) | ||||||||||||
- List of Synonyms
| Acrolejeunea emergens (Mitt.) Steph. var. emergens = Acrolejeunea emergens (Mitt.) Steph. |
| Amblystegium riparium (Hedw.) Bruch, Schimp. & W.Gümbel = Leptodictyum riparium (Hedw.) Warnst |
| Anacolia laevisphaera (Taylor) Flowers = Bartramia laevisphaera (Taylor) Müll.Hal. |
| Anoectangium euchloron (Schwägr.) Mitt. = Anoectangium aestivum (Hedw.) Mitt. |
| Anomobryum julaceum (P.Gaertn., B.Mey. & Scherb.) Schimp. var. julaceum = Anomobryum julaceum (Schrad. ex P.Gaertn., E.Mey & Scherb.) Schimp. |
| Anomobryum juliforme Solms. = Anomobryum julaceum (Schrad. ex P.Gaertn., E.Mey & Scherb.) Schimp. |
| Barbula acuta (Brid.) Brid. = Didymodon rigidulus Hedw. |
| Barbula arcuata Griff. = Hydrogonium arcuatum (Griff.) Wijk & Margad. |
| Barbula bolleana (Müll.Hal.) Broth. = Hydrogonium bolleanum (Müll.Hal.) A.Jaeger |
| Barbula consanguinea (Thwaites & Mitt.) A.Jaeger = Hydrogonium consanguineum (Thwaites & Mitt.) Hilp. |
| Barbula convoluta Hedw. = Streblotrichum convolutum (Hedw.) P.Beauv. var. convolutum |
| Barbula convoluta Hedw. var. convoluta = Streblotrichum convolutum (Hedw.) P.Beauv. var. convolutum |
| Barbula cylindrica (Taylor) Schimp. = Streblotrichum convolutum (Hedw.) P.Beauv. var. convolutum |
| Barbula fallax Hedw. = Didymodon fallax (Hedw.) R.H.Zander |
| Barbula indica (Hook.) Spreng. = Hydrogonium orientale (F.Weber) Jan Kučera |
| Barbula indica (Hook.) Spreng. var. indica = Hydrogonium orientale (F.Weber) Jan Kučera |
| Barbula lambarenensis P. de la Varde = Hydrogonium orientale (F.Weber) Jan Kučera |
| Barbula sulcata Geh. = Streblotrichum convolutum (Hedw.) P.Beauv. var. convolutum |
| Bartramia stricta auct. eur., non Brid. = Bartramia aprica Müll. Hal. Brid. |
| Brachymenium borgenianum Hampe = Brachymenium acuminatum Harv. |
| Brachymenium notarisii (Mitt.) A.J.Shaw = Anomobryum notarisii (Mitt.) D.Bell. & Holyoak |
| Brachymenium philonotula Broth. = Bryum kikuyuense (Broth. & Thér.) N.Pedersen |
| Bryosedgwickia densa (Hook.) Bizot & P. de la Varde = Platygyriella densa (Hook.) W.R.Buck |
| Bryum apiculatum Schwägr. = Anomobryum apiculatum (Schwägr.) D.Bell & Holyoak |
| Bryum argenteum Hedw. var. argenteum = Bryum argenteum Hedw. |
| Bryum canariense Brid. var. canariense = Bryum canariense Brid. |
| Bryum capillare Hedw. = Ptychostomum capillare (Hedw.) Holyoak & N.Pedersen |
| Bryum capillare Hedw. var. capillare = Ptychostomum capillare (Hedw.) Holyoak & N.Pedersen |
| Bryum cellulare Hook. = Ptychostomum cellulare (Hook.) D.Bell & Holyoak |
| Campylopus pilifer Brid. subsp. Pilifer = Campylopus pilifer Brid. |
| Campylopus pyriformis (Schultz) Brid. var. pyriformis = Campylopus pyriformis (Schultz) Brid. |
| Cololejeunea minutissima (Sm.) Schiffn. = Myriocoleopsis minutissima (Sm.) R.L.Zhu, Y.Yu & Pócs |
| Crossidium chloronotus (Brid.) Limpr. = Crossidium squamiferum (Viv.) Jur. |
| Cryphaea bollei Broth. & Geh. = Pseudoleskeopsis bollei (Broth. & Geh.) P.Rao |
| Cryptoleptodon longisetus (Mont.) Enroth = Leptodon longisetus Mont. |
| Cyathodium africanum Mitt. = Cyathodium cavernarum Kunze |
| Desmatodon bogosicus Müll.Hal. = Tortula bogosica (Müll.Hal.) R.H.Zander |
| Desmatodon convolutus (Brid.) Grout = Tortula atrovirens (Sm.) Lindb. |
| Didymodon australasiae (Hook. & Grev.) R.H. Zander var. australasiae = Didymodon australasiae (Hook. & Grev.) R.H.Zander |
| Didymodon fallax (Hedw.) R.H.Zander var. fallax = Didymodon fallax (Hedw.) R.H.Zander |
| Didymodon maschalogena (Renauld & Cardot) Broth. = Didymodon hastatus (Mitt.) R.H.Zander |
| Didymodon rigidulus Hedw. var. gracilis (Schleich. ex Hook. & Grev.) R.H.Zander = Didymodon rigidulus Hedw. |
| Didymodon rigidulus Hedw. var. rigidulus = Didymodon rigidulus Hedw. |
| Didymodon sicculus M.J.Cano, Ros, García-Zam. & J.Guerra = Didymodon tophaceus (Brid.) Lisa subsp. sicculus (M.J.Cano, Ros, García Zam. & J.Guerra) Jan Kučera |
| Didymodon vinealis (Brid.) R.H.Zander var. vinealis = Didymodon vinealis (Brid.) R.H.Zander |
| Didymodon vinealis (Brid.) Zander var. flaccidus (B.S.G.) Zander = Streblotrichum convolutum (Hedw.) P.Beauv. var. convolutum |
| Eurhynchium meridionale Bruch, Schimp. & W.Gümbel = Plasteurhynchium meridionale (Schimp.) M.Fleisch. |
| Eurhynchium praelongum (Hedw.) Schimp. = Kindbergia praelonga (Hedw.) Ochyra |
| Eurhynchium speciosum (Brid.) Jur. = Oxyrrhynchium speciosum (Brid.) Warnst. |
| Fissidens alatus P. de la Varde = Fissidens megalotis subsp. helictocaulos (Müll.Hal.) Brugg.-Nann. |
| Fissidens bocarangensis P. de la Varde = Fissidens flaccidus Mitt. |
| Fissidens helictocaulos Müll.Hal. = Fissidens megalotis subsp. helictocaulos (Müll.Hal.) Brugg.-Nann. |
| Fissidens minutulus Sull. = Fissidens crispus Mont. |
| Fissidens sciophyllus Mitt. fo. Sciophyllus = Fissidens sciophyllus Mitt. |
| Frullania bystroemii S.W.Arnell = Frullania spongiosa Steph. |
| Frullania nervosa Mont. = Frullania tamarisci (L.) Dumort. |
| Funaria calvescens Schwägr. = Funaria hygrometrica Hedw. |
| Funaria hygrometrica Hedw. var. calvescens (Schwägr.) Kindb. = Funaria hygrometrica Hedw. |
| Funaria hygrometrica Hedw. var. hygrometrica = Funaria hygrometrica Hedw. |
| Grimaldia dichotoma Raddi = Mannia androgyna (L.) A.Evans |
| Grimmia leucophaea Grev. = Grimmia laevigata (Brid.) Brid. |
| Grimmia trichophylla De Not. subsp. lisae (De Not.) Boulay = Grimmia lisae De Not. |
| Grimmia trichophylla Grev. var. trichophylla = Grimmia trichophylla Grev. |
| Groutiella laxotorquata (Besch.) Wijk & Margad. = Groutiella tomentosa (Hornsch.) Wijk & Marg. |
| Groutiella sarcotricha (Müll.Hal. ex Broth.) Wijk & Margad. = Groutiella tomentosa (Hornsch.) Wijk & Marg. |
| Gymnostomiella vernicosa (Hook. ex Harv.) M.Fleisch. var. monodii (P. de la Varde) Sérgio = Gymnostomiella vernicosa (Hook. ex Harv.) M.Fleisch. |
| Gymnostomiella vernicosa (Hook. ex Harv.) M.Fleisch. var. vernicosa = Gymnostomiella vernicosa (Hook. ex Harv.) M.Fleisch. |
| Gymnostomum rupestre Schleich. ex Schwägr. = Gymnostomum aeruginosum Sm. |
| Haplodontium notarisii (Mitt.) Broth. = Anomobryum notarisii (Mitt.) D.Bell. & Holyoak |
| Hedwigia albicans Lindb. = Hedwigia ciliata (Hedw.) P.Beauv. |
| Hedwigia ciliata (Hedw.) Ehrh. ex P.Beauv. var. ciliata = Hedwigia ciliata (Hedw.) P.Beauv. |
| Hedwigia integrifolia P.Beauv. = Hedwigidium integrifolium (P. Beauv.) C.E.O. Jensen |
| Homalothecium nilgheriense (Mont.) H.Rob. = Palamocladium leskeoides (Hook.) E.Britton |
| Hymenostomum tortile (Schwägr.) Bruch, Schimp. & W.Gümbel = Weissia condensa (Voit) Lindb. |
| Hyophila crenulata Müll. Hal. ex Dusén var. brevifolia Bizot = Hyophila involuta (Hook.) A.Jaeger |
| Hyophila crenulata Müll.Hal. ex Paris = Hyophila involuta (Hook.) A.Jaeger |
| Hyophila excurrentinervis Paris & Broth. = Trichostomum brachydontium Bruch |
| Hyophila machadoana Sérgio = Bryoerythrophyllum campylocarpum (Müll.Hal.) H.A.Crum |
| Kindbergia praelonga (Bruch, Schimp. & W.Gümbel) Ochyra var. praelonga = Kindbergia praelonga (Hedw.) Ochyra |
| Lejeunea caespitosa Lindenb. = Lejeunea capensis Gottsche |
| Lejeunea ulicina (Taylor) Gottsche, Lindenb. & Nees = Microlejeunea ulicina (Taylor) Steph. |
| Leptophascum leptophyllum (Müll.Hal.) J.Guerra & M.J.Cano = Chenia leptophylla (Müll.Hal.) R.H.Zander |
| Leucodon sciuroides (Hedw.) Schwägr. var. morensis (Schwägr.) De Not. = Leucodon sciuroides (Hedw.) Schwägr. |
| Lophocolea cuspidata (Nees) Limpr. = Lophocolea bidentata (L.) Dumort. |
| Meesia bolleana Müll.Hal. = Hydrogonium bolleanum (Müll.Hal.) A.Jaeger |
| Neckera cladorrhizans Hedw. = Entodon schleicheri (Schimp.) Demet. |
| Neckera intermedia Brid. = Exsertotheca intermedia (Brid.) S.Olsson, Enroth & D.Quandt |
| Neckera pseudoseductrix Müll.Hal = Entodon pseudoseductrix (Müll.Hal.) A.Jaeger |
| Orthotrichum diaphanum Schrad. ex Brid. var. diaphanum = Orthotrichum diaphanum Brid. |
| Orthotrichum schimperi Hammar = Orthotrichum pumilum Sw. ex anon. |
| Oxyrrhynchium praelongum (Hedw.) Warnst. = Kindbergia praelonga (Hedw.) Ochyra |
| Oxystegus cylindricus (Brid.) Hilp. = Chionoloma tenuirostre (Hook. & Taylor) M.Alonso, M.J.Cano & J.A.Jiménez var. tenuirostre |
| Oxystegus tenuirostris (Hook. & Taylor) A.J.E.Smith = Chionoloma tenuirostre (Hook. & Taylor) M.Alonso, M.J.Cano & J.A.Jiménez var. tenuirostre |
| Palamocladium nilgheriense (Mont.) Müll.Hal. = Palamocladium leskeoides (Hook.) E.Britton |
| Philonotis hastata (Duby) Wijk & Margad. var. gemmiclada P.de la Varde, nom. nud. = Philonotis hastata (Duby) Wijk & Margad. |
| Philonotis marchica (Hedw.) Brid. var. marchica = Philonotis marchica (Hedw.) Brid. |
| Philonotis obtusata Müll.Hal. ex Renauld & Cardot = Philonotis hastata (Duby) Wijk & Margad. |
| Pinnatella revoluta Bizot = Leptodon longisetus Mont. |
| Plagiochasma aitonia Lindenb. et Nees = Plagiochasma rupestre (J.R.Forst. et G.Forst.) Steph. var. rupestre |
| Platyhypnidium riparioides (Hedw.) M.Fleisch. = Rhynchostegium riparioides (Hedw.) Cardot |
| Platyhypnidium rusciforme (Hedw.) Dixon = Rhynchostegium riparioides (Hedw.) Cardot |
| Pleurochaete squarrosa (Brid.) Lindb. = Tortella squarrosa (Brid.) Limpr. |
| Pleurochaete squarrosa (Brid.) Lindb. var. squarrosa = Tortella squarrosa (Brid.) Limpr. |
| Pseudoleskea pseudoattenuata (Müll.Hal.) Broth. = Pseudoleskeopsis pseudoattenuata (Müll.Hal.) Thér. |
| Pterogonium gracile (Hedw.) Sm. = Nogopterium gracile (Hedw.) Crosby & W.R.Buck |
| Ptychomitrium subcrispatum Thér. & P. de la Varde var. obscurum Bizot = Ptychomitrium nigrescens (Kunze) Wijk & Margad. |
| Rhynchostegium megapolitanum Bruch, Schimp. & W.Gümbel var. megapolitanum = Rhynchostegium megapolitanum (Blandow ex F.Weber & D.Mohr) Schimp. |
| Rhynchostegium megapolitanum Bruch, Schimp. & W.Gümbel var. meridionale Schimp. = Rhynchostegium megapolitanum (Blandow ex F.Weber & D.Mohr) Schimp. |
| Riccia minima L. = Riccia nigrella DC. |
| Riccia sorocarpa Bisch. var. heegii Schiffn. = Riccia sorocarpa Bisch. subsp. sorocarpa |
| Scleropodium touretii (Brid.) L.Koch var. touretii = Scleropodium touretii (Brid.) L.F.Koch |
| Semibarbula lambarenensis (P.de la Varde) Hilp. = Hydrogonium orientale (F.Weber) Jan Kučera |
| Semibarbula orientalis (F.Weber) Wijk & Margad. = Hydrogonium orientale (F.Weber) Jan Kučera |
| Splachnobryum erosulum Müll.Hal. ex Dusén = Gymnostomiella erosula (Müll.Hal. ex Dusén) Arts |
| Stereophyllum auriculatum A.Gepp = Entodontopsis leucostega (Brid.) W.R.Buck & Ireland |
| Syntrichia laevipila Brid. var. laevipila = Syntrichia laevipila Brid. |
| Timmiella barbula Limpr. = Timmiella barbuloides (Brid.) Mönk. |
| Tortula amphidiacea (Müll.Hal.) Broth. = Syntrichia amphidiacea (Müll.Hal.) R.H.Zander |
| Tortula erubescens (Müll.Hal.) Broth., hom.illeg. = Syntrichia fragilis (Taylor) Ochyra |
| Tortula fragilis Taylor = Syntrichia fragilis (Taylor) Ochyra |
| Tortula hildebrandtii (Müll.Hal.) Broth. = Syntrichia fragilis (Taylor) Ochyra |
| Tortula laevipila (Brid.) Schwägr. var. laevipila = Syntrichia laevipila Brid. |
| Tortula marginata (Bruch & Schimp.) Spruce subsp. limbata (Lindb.) Podp. = Tortula solmsii (Schimp.) Limpr. |
| Tortula solmsii var. minor G.Roth = Tortula solmsii (Schimp.) Limpr. |
| Tortula squarrosa (Brid.) De Not. = Tortella squarrosa (Brid.) Limpr. |
| Tortula subcaroliniana Bizot = Syntrichia amphidiacea (Müll.Hal.) R.H.Zander |
| Trichostomum barbula Schwägr. = Timmiella barbuloides (Brid.) Mönk. |
| Trichostomum bolleanum (Müll.Hal.) Müll.Hal. = Hydrogonium bolleanum (Müll.Hal.) A.Jaeger |
| Trichostomum brachydontium Bruch var. brachydontium = Trichostomum brachydontium Bruch |
| Trichostomum crispulum Bruch var. angustifolium Bruch & Schimp. = Trichostomum crispulum Bruch |
| Trichostomum crispulum Bruch var. crispulum = Trichostomum crispulum Bruch |
| Trichostum tenuirostris (Hook. & Taylor) Lindb. = Chionoloma tenuirostre (Hook. & Taylor) M.Alonso, M.J.Cano & J.A.Jiménez var. tenuirostre |
| Trichostum tenuirostris (Hook. & Taylor) Lindb. var. tenuirostre = Chionoloma tenuirostre (Hook. & Taylor) M.Alonso, M.J.Cano & J.A.Jiménez var. tenuirostre |
| Weissia brachycarpa (Nees & Hornsch.) M.O.Hill var. brachycarpa = Weissia brachycarpa (Nees & Hornsch.) Jur. |
| Weissia microstoma (Hedw.) C. Müll. = Weissia brachycarpa (Nees & Hornsch.) Jur. |
| Weissia tortilis (Schwaegr.) C. Mull., hom. Illeg = Weissia condensa (Voit) Lindb. |
| Weissia vardei Bizot = Trichostomum crispulum Bruch |
| Zygodon bolleanus Müll.Hal. = Anoectangium aestivum (Hedw.) Mitt. |
References
- Patiño, J.; Mateo, R.G.; Zanatta, F.; Marquet, A.; Aranda, S.C.; Borges, P.A.V.; Dirkse, G.; Gabriel, R.; Gonzalez-Mancebo, M.; Guisan, A.; et al. Climate threat on the Macaronesian endemic bryophyte flora. Sci. Rep. 2016, 6, 29156. [Google Scholar] [CrossRef] [PubMed]
- Sim-Sim, M.; Ruas, S.; Fontinha, S.; Hedenäs, L.; Sérgio, C.; Lobo, C. Bryophyte conservation on a North Atlantic hotspot: Threatened bryophytes in Madeira and Selvagens Archipelagos (Portugal). Syst. Biodivers. 2014, 12, 315–330. [Google Scholar] [CrossRef]
- Hodgetts, N.; Cálix, M.; Englefield, E.; Fettes, N.; García Criado, M.; Patin, L.; Nieto, A.; Bergamini, A.; Bisang, I.; Baisheva, E.; et al. A Miniature World in Decline: European Red List of Mosses, Liverworts and Hornworts; IUCN: Brussels, Belgium, 2019. [Google Scholar]
- Caujape-Castells, J.; Tye, A.; Crawford, D.J.; Santos-Guerra, A.; Sakai, A.; Beaver, K.; Lobin, W.; Florens, F.B.V.; Moura, M.; Jardim, R.; et al. Conservation of oceanic island floras: Present and future global challenges. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 107–129. [Google Scholar] [CrossRef]
- Brochmann, C.; Rustan, Ø.; Lobin, W.; Kilian, N. The endemic vascular plants of the Cape Verde Islands, W. Africa. Sommerfeltia 1997, 24, 1–356. [Google Scholar] [CrossRef]
- O’Shea, B.J. Check-list of the mosses of sub–Saharan Africa (version 5, 12/06). Trop. Bryol. Res. 2006, 6, 1–252. [Google Scholar]
- Vanderpoorten, A.; Laenen, B.; Rumsey, F.; Gonzalez-Mancebo, J.M.; Gabriel, R.; Carine, M.A. Dispersal, diversity and evolution of the Macaronesian cryptogamic floras. In Plants and Islands, 2nd ed.; Bramwell, D., Caujape-Castells, J., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 338–364. [Google Scholar]
- Patiño, J.; González-Mancebo, J.M. Bryophyta. In Lista Preliminar de Espécies Silvestres de Cabo Verde (Fungos, Plantas e Animais Terrestres); Arechavaleta, M., Zurita, N., Marrero, M.C., Martín, J.L., Eds.; Consejería de Medio Ambiente y Ordenación Territorial Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 2005; pp. 34–37. [Google Scholar]
- Garcia, C.A.; Sérgio, C.; Martins, A.; Rodrigues, A.S.; Sim-Sim, M. A contribution to the knowledge of the bryophytes of the Cape Verde Islands, with an emphasis on Santo Antão and São Vicente. J. Bryol. 2021, 43, 122–128. [Google Scholar] [CrossRef]
- Martins, A.; Garcia, C.A.; Patiño, J.; Sim-Sim, M. Exormotheca martins-loussaoae (Exormothecaceae, Hepaticae), a new species from Cape Verde. Plant Biosyst. 2023, 157, 294–300. [Google Scholar] [CrossRef]
- Frahm, J.P.; Lindlar, A.; Sollman, P.; Fischer, E. Bryophytes from the Cape Verde islands. Trop. Bryol. 1996, 12, 123–153. [Google Scholar]
- González-Mancebo, J.M.; Draper, I.; Lara, F.; Marrero, J.D.; Munoz, J.; Patino, J.; Romaguera, F.; Vanderpoorten, A. Amendments to the bryophyte flora of the Cape Verde and Canary Islands. Cryptogam. Bryol. 2009, 30, 433. [Google Scholar]
- Ellis, L.T.; Alegro, A.; Šegota, V.; Bakalin, V.A.; Barone, R.; Borovichev, E.A.; Hugonnot, V.; Lebouvier, M.; Nobis, M.; Nowak, A.; et al. New national and regional bryophyte records, 44. J. Bryol. 2015, 37, 233–234. [Google Scholar] [CrossRef]
- Ellis, L.T.; Ah-Peng, C.; Aranda, S.C.; Bednarek-Ochyra, H.; Borovichev, E.A.; Cykowska-Marzencka, B.; Duarte, M.C.; Enroth, J.; Erzberger, P.; Fedosov, V.; et al. New national and regional bryophyte records, 45. J. Bryol. 2015, 37, 314. [Google Scholar] [CrossRef]
- Cano, M.J. New records of Pottiaceae (Bryophyta) from Cape Verde. Nova Hedwig. 2016, 103, 373–383. [Google Scholar] [CrossRef]
- Jiménez, J.A.; Cano, M.J. Didymodon caboverdeanus JA Jiménez & MJ Cano (Pottiaceae, Musci), a new species from the Cape Verde archipelago. J. Bryol. 2017, 39, 171–176. [Google Scholar] [CrossRef]
- Sérgio, C.; Stow, S. Identity of the endemic African species Gymnostomiella monodii P. de la Varde (Pottiaceae, Musci) and new records for Macaronesia (Cape Verde). J. Bryol. 2017, 39, 304–308. [Google Scholar] [CrossRef]
- Dirkse, G.M.; Nieuwkoop, J.A.; Vanderpoorten, A.; Losada-Lima, A.; González-Mancebo, J.M.; Patiño, J.; Sotiaux, A.; Hernández-Hernández, R.; Rodríguez-Romero, A. New bryophyte records from Macaronesia. Cryptogam. Bryol. 2018, 39, 61–76. [Google Scholar] [CrossRef]
- Sérgio, C.; Melo, I. Two remarkable new Riccia (Marchantiales, Ricciaceae) records for the bryophyte flora of Cape Verde Islands, an important biogeographical refuge area. Bryophyt. Divers. Evol. 2019, 41, 71–76. [Google Scholar] [CrossRef]
- Sim-Sim, M.; Martins, A.; Rodrigues, A.S.B.; Garcia, C.A.; Sérgio, C.; Van Rooy, J.; González-Mancebo, J.M.; Vanderpoorten, A.; Patiño, J.; Hedenäs, L. Ptychomitrium subcrispatum Thér. & P. de la Varde, an East Southern African species excluded from the Cape Verde bryoflora. J. Bryol. 2019, 41, 281–284. [Google Scholar] [CrossRef]
- Ellis, L.T.; Afonina, O.M.; Atwood, J.J.; Bednarek-Ochyra, H.; Burghardt, M.; Dragićević, S.; Vuksanović, S.; Espinoza-Prieto, B.; Opisso, J.; Goga, M.; et al. New national and regional bryophyte records, 62. J. Bryol. 2020, 42, 200. [Google Scholar] [CrossRef]
- Hodgetts, N.G.; Söderström, L.; Blockeel, T.L.; Caspari, S.; Ignatov, M.S.; Konstantinova, N.A.; Lockhart, N.; Papp, B.; Schröck, C.; Sim-Sim, M.; et al. An annotated checklist of bryophytes of Europe, Macaronesia and Cyprus. J. Bryol. 2020, 42, 1–116. [Google Scholar] [CrossRef]
- Wigginton, M.J. Checklist and distribution of the liverworts and hornworts of sub-Saharan Africa, including the East African Islands. Edition 4. Trop. Bryol. Res. 2018, 9, 1–138. [Google Scholar]
- Luna, E.D. Seta length variation and the refutation of Hedwigidium=Braunia (Hedwigiaceae, Bryopsida). Acta Bot. Mex. 2021, 128, e1810. [Google Scholar] [CrossRef]
- Boch, S.; Martins, A.; Ruas, S.; Fontinha, S.; Carvalho, P.; Reis, F.; Bergamini, A.; Sim-Sim, M. Bryophyte and macrolichen diversity show contrasting elevation relationships and are negatively affected by disturbances in laurel forests of Madeira island. J. Veg. Sci. 2019, 30, 1122–1133. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Catarino, S.; Gomes, I.; Fernandes, C.; Costa, J.C.; Caujapé-Castells, J.; Duarte, M.C. IUCN Red List assessment of the Cape Verde endemic flora: Towards a global strategy for plant conservation in Macaronesia. Bot. J. Linn. Soc. 2016, 180, 413–425. [Google Scholar] [CrossRef]
- Kürschner, H.; Frey, W.; Parolly, G. Patterns and adaptive trends of life forms, life strategies and ecomorphological structures in tropical epiphytic bryophytes a pantropical synopsis. Nova Hedwig. 1999, 69, 73–99. [Google Scholar] [CrossRef]
- Proctor, M.C.F.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Hodgetts, N.; Lockhart, N. Checklist and country status of European bryophytes–update 2020. In Irish Wildlife Manuals, No. 123; National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht: Dublin, Ireland, 2020. [Google Scholar]
- van Zuijlen, K.; Nobis, M.P.; Hedenäs, L.; Hodgetts, N.; Calleja Alarcón, J.A.; Albertos, B.; Bernhardt-Römermann, M.; Gabriel, R.; Garilleti, R.; Lara, F.; et al. Bryophytes of Europe Traits (BET) data set: A fundamental tool for ecological studies. J. Veg. Sci. 2023, 34, e13179. [Google Scholar] [CrossRef]
- Coelho, M.C.; Gabriel, R.; Hespanhol, H.; Borges, P.A.; Ah-Peng, C. Bryophyte diversity along an elevational gradient on Pico Island (Azores, Portugal). Diversity 2021, 13, 162. [Google Scholar] [CrossRef]
- Lloret, F.; González-Mancebo, J.M. Altitudinal distribution patterns of bryophytes in the Canary Islands and vulnerability to climate change. Flora 2011, 206, 769–781. [Google Scholar] [CrossRef]
- Diniz, A.C.; Matos, G.C. Carta de Zonagem Agro-Ecológica e da Vegetação de Cabo Verde. IV: Ilha de São Vicente. Garcia De Orta Ser. Bot. 1994, 12, 69–100. [Google Scholar]
- Patiño, J.; Vanderpoorten, A. Bryophyte Biogeography. Crit. Rev. Plant Sci. 2018, 37, 175–209. [Google Scholar] [CrossRef]
- Rodrigues, A.S.B.; Martins, A.; Garcia, C.A.; Sérgio, C.; Porley, R.; Fontinha, S.; González-Mancebo, J.M.; Gabriel, R.; Phephu, N.; Van Rooy, J.; et al. Climate-driven vicariance and long-distance dispersal explain the Rand Flora pattern in the liverwort Exormotheca pustulosa (Marchantiophyta). Biol. J. Linn. Soc. 2020, 130, 480–496. [Google Scholar] [CrossRef]
- Mairal, M.; Sanmartín, I.; Pellissier, L. Lineage-specific climatic niche drives the tempo of vicariance in the Rand Flora. J. Biogeogr. 2017, 44, 911–923. [Google Scholar] [CrossRef]
- Sollman, P. Some new synonyms in tropical pottiaceous mosses. Lindbergia 1990, 16, 22–24. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim-Sim, M.; Martins, A.; Garcia, C.A. Updated List of Bryophytes from Cape Verde Archipelago. Diversity 2024, 16, 217. https://doi.org/10.3390/d16040217
Sim-Sim M, Martins A, Garcia CA. Updated List of Bryophytes from Cape Verde Archipelago. Diversity. 2024; 16(4):217. https://doi.org/10.3390/d16040217
Chicago/Turabian StyleSim-Sim, Manuela, Anabela Martins, and Cesár Augusto Garcia. 2024. "Updated List of Bryophytes from Cape Verde Archipelago" Diversity 16, no. 4: 217. https://doi.org/10.3390/d16040217
APA StyleSim-Sim, M., Martins, A., & Garcia, C. A. (2024). Updated List of Bryophytes from Cape Verde Archipelago. Diversity, 16(4), 217. https://doi.org/10.3390/d16040217






