A New Species of Diacyclops (Copepoda, Cyclopoida) from the D. crassicaudis (Sars, 1863) Species Group with Critical Taxonomy Remarks †
Abstract
1. Introduction
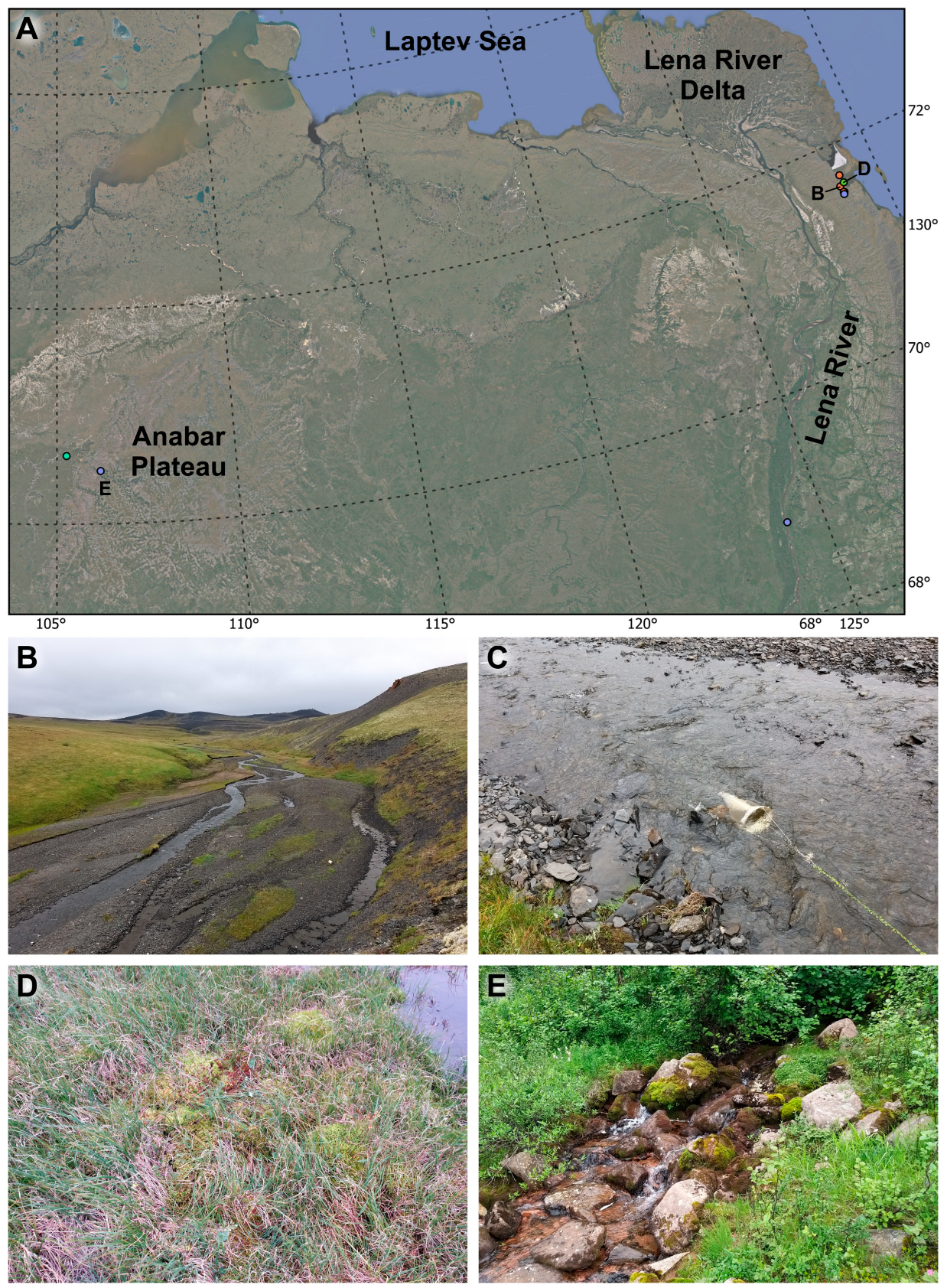
2. Materials and Methods
- Ratio of the length of the coxal seta to the distance between the base of this seta and the end of the inner spine of the P4 basis;
- Ratio of the length to the width of the distal segment of the P4 endopod;
- Ratio of the length of the outer seta to the length of the distal segment of the P4 endopod;
- Ratio of the length of the inner apical spine to the length of the outer spine of the distal segment of the P4 endopod;
- Ratio of the length of the distal inner seta to the length of the proximal inner seta of the distal segment of the P4 endopod;
- Ratio of the length of the inner apical spine to the length of the distal segment of the P4 endopod;
- Ratio of the sum of the lengths of the inner setae of the distal segment of the P4 endopod to the length of the distal segment of the P4 endopod;
- Ratio of the length to the width of caudal rami;
- Ratio of the distance from the base of the caudal ramus to seta II to the length of the caudal ramus;
- Ratio of the length of the outer spine (seta III) to the length of the caudal ramus;
- Ratio of the length of the inner seta (seta VI) to the length of the outer spine (seta III);
- Ratio of the length of the inner seta (seta VI) to the width of the caudal ramus;
- Ratio of the length of the dorsal seta (seta VII) to the length of the caudal ramus.
| Specimen | Species | Sample |
|---|---|---|
| d_Holotype d_Paratype1 d_Paratype2 | D. dyabdar sp. nov. | type locality |
| d_Y1, d_Y2 | D. dyabdar sp. nov. | North Yakutia, Tiksi area 71.529281° N, 128.770956° E |
| d_A1 | D. dyabdar sp. nov. | Anabar plateau 70.635118° N, 105.266465° E |
| c_Y1, c_Y2 | D. crassicaudis | North Yakutia, Tiksi area 71.529281° N, 128.770956° E |
| c_T1, c_T2, c_T3, c_T4, c_T5 | D. crassicaudis | Tatarstan Republic 55.767969° N, 48.846781° E |
| specimen | species/subspecies | reference |
| c_c_R4 | D. crassicaudis s. str. | [22] |
| c_c_R1 | D. crassicaudis s. str. | [23] |
| c_c_R3 | D. crassicaudis s. str. | [24] |
| c_c_R5 | D. crassicaudis s. str. | [25] |
| c_c_R2 | D. crassicaudis s. str. | [26] |
| c_brachycer._R1 | D. crassicaudis brachycercus | [22] |
| c_cretensis_R3 | D. crassicaudis cretensis | [27] |
| c_cretensis_R1, | D. crassicaudis cretensis | [28] |
| c_cretensis_R2 | D. crassicaudis cf. cretensis | [28] |
| c_lagrecai_R1 | D. crassicaudis lagrecai | [13] |
| c_taipehen._R1 | D. crassicaudis taipehensis | [29] |
| c_trinacriae_R1 | D. crassicaudis trinacriae | [13] |
| fontinalis_R1 | D. fontinalis | [30] |
| iranicus_R1 | D. iranicus | [31] |
| antrincola_R1 | D. antrincola | [32] |
| antrincola_R3 | D. antrincola | [33] |
| antrincola_R2 | D. antrincola | [34] |
| c_cosana_R1 | D. crassicaudis var. cosana | [35] |
| ruffoi_R1 | D. ruffoi | [12] |
| skopljensis_R1 | D. skopljensis | [36] |
| c_s.lat._R1 | D. crassicaudis s. lat. | [37] |
| karamani_R1 | D. karamani | [36] |
3. Molecular Analysis
4. Results
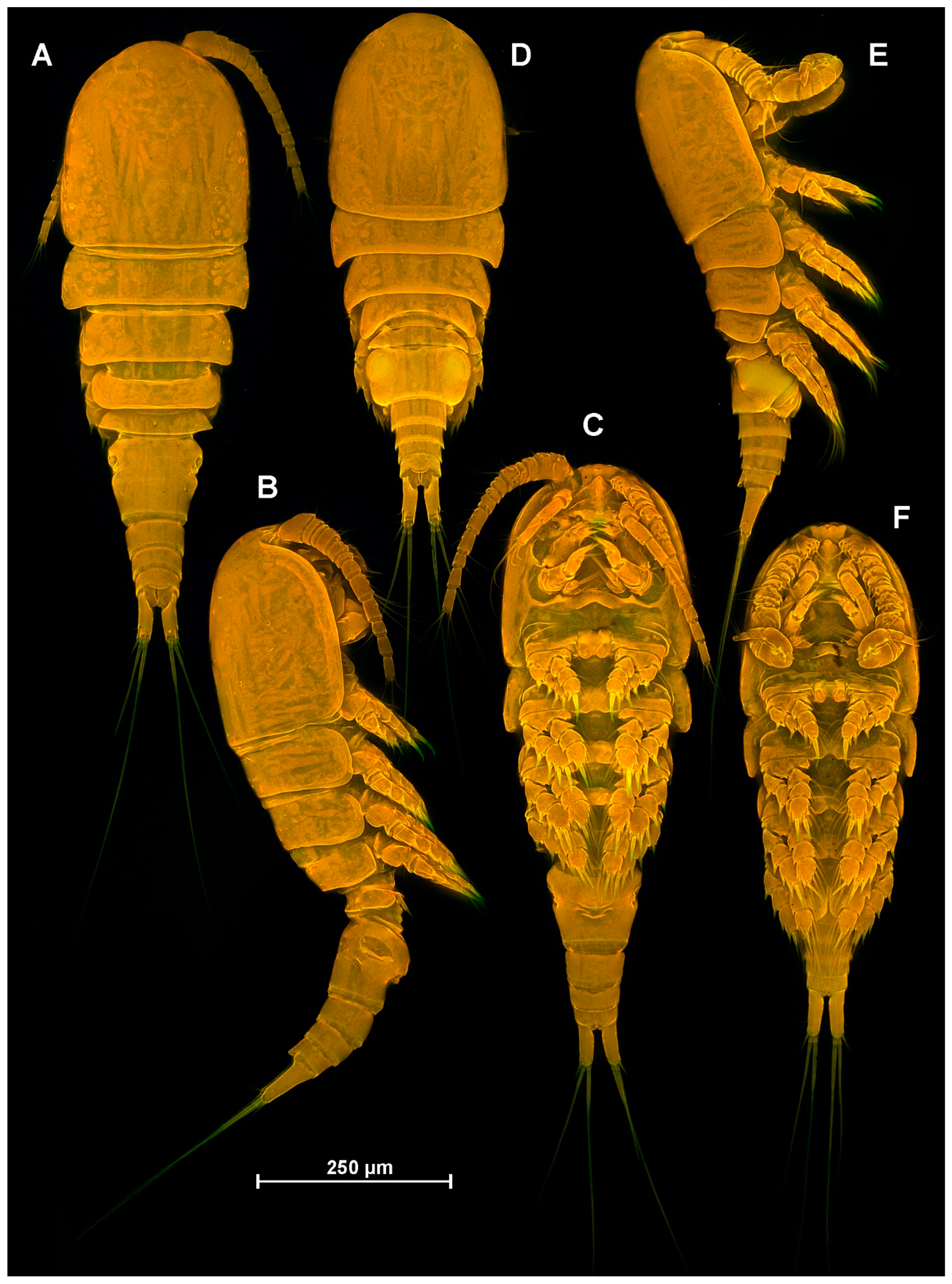
4.1. Species Diacyclops dyabdar sp. nov.
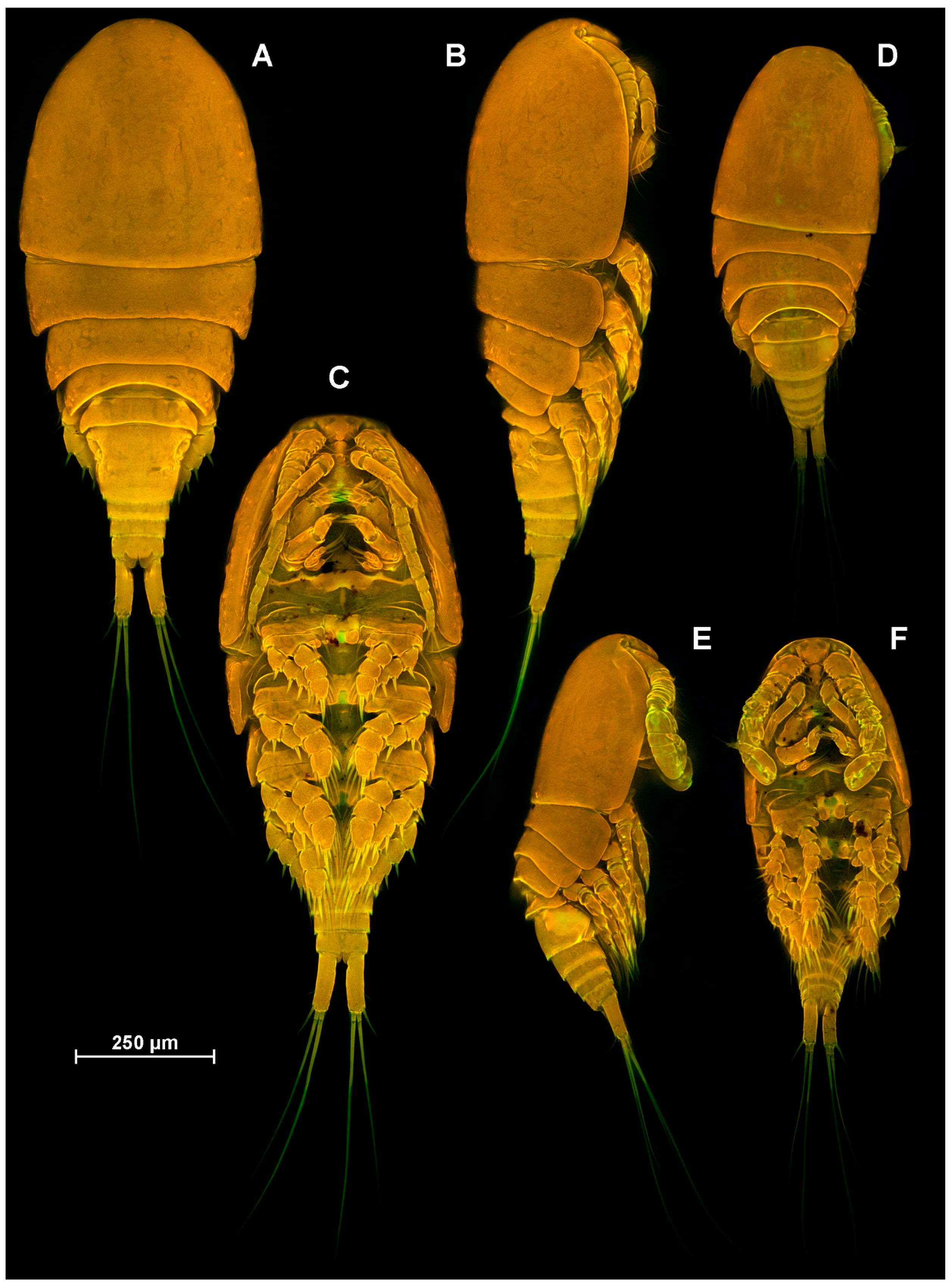
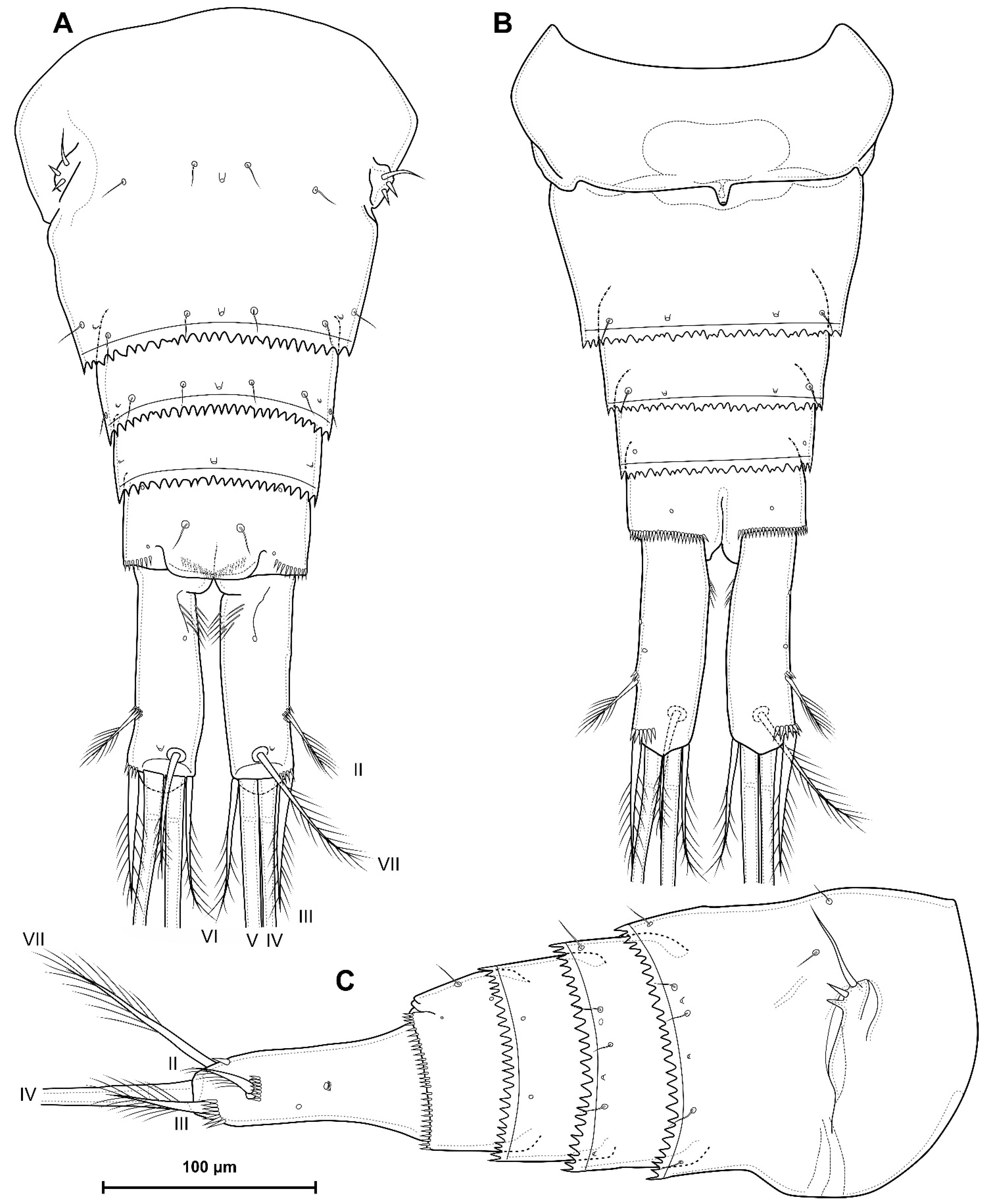
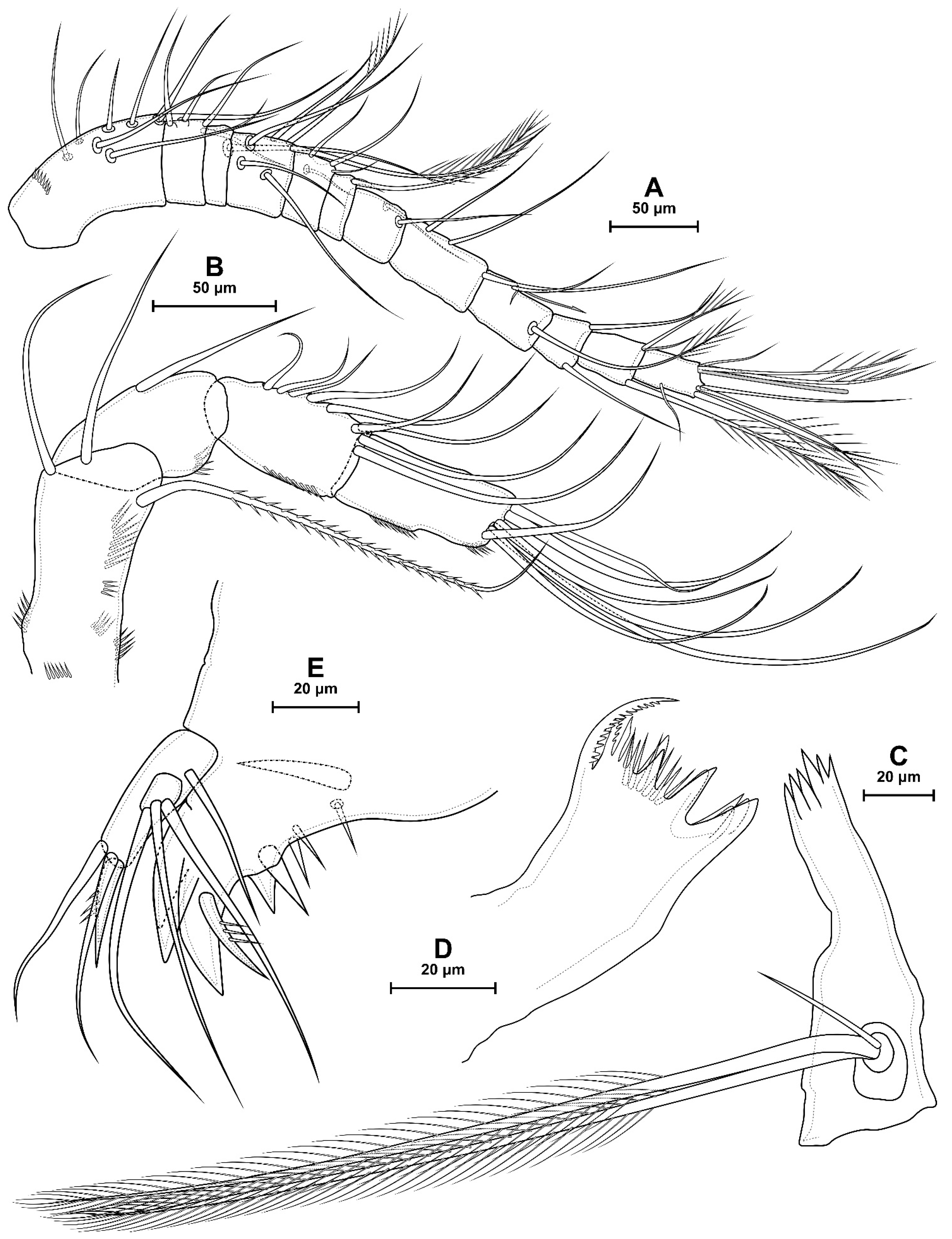
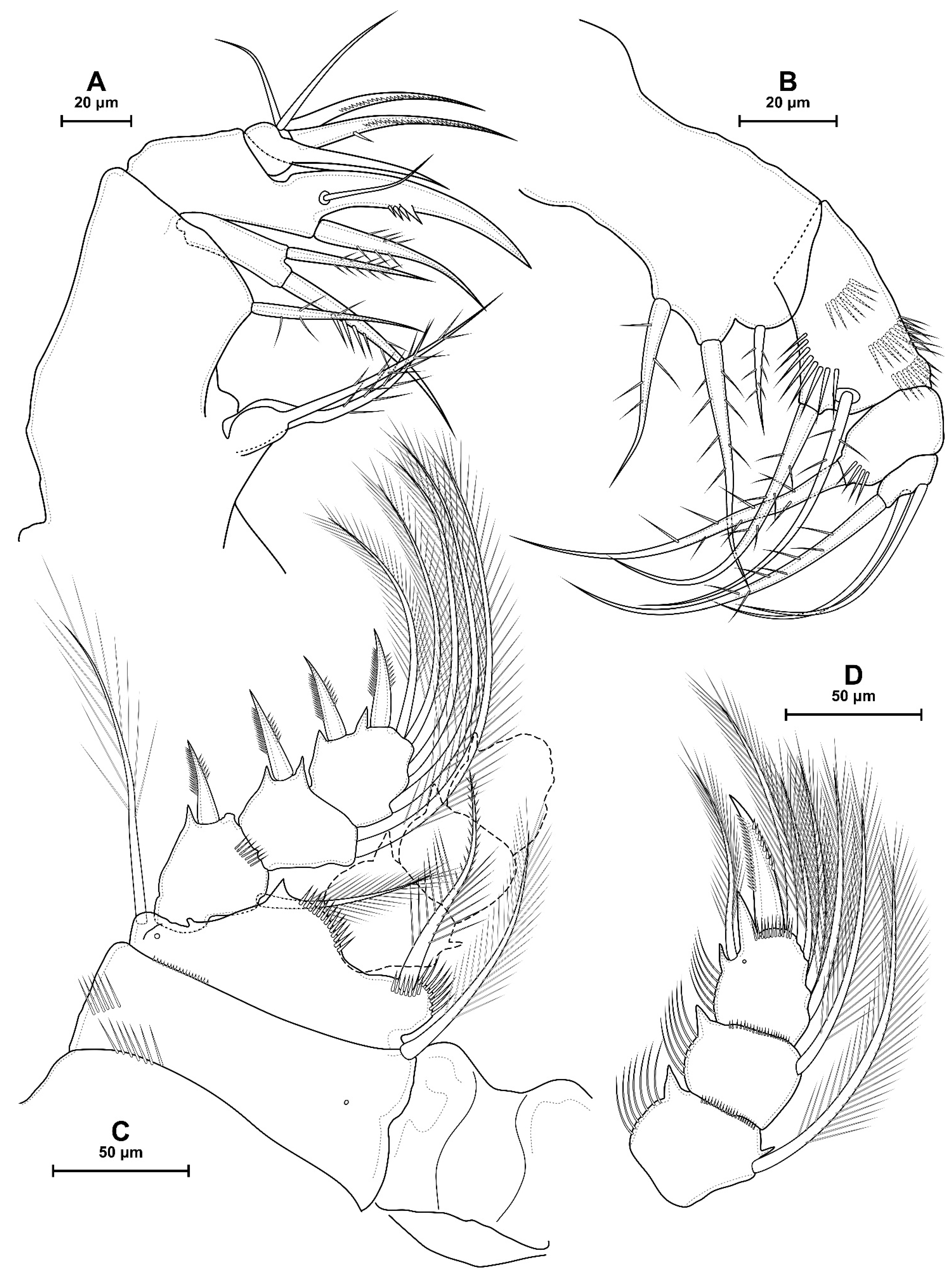

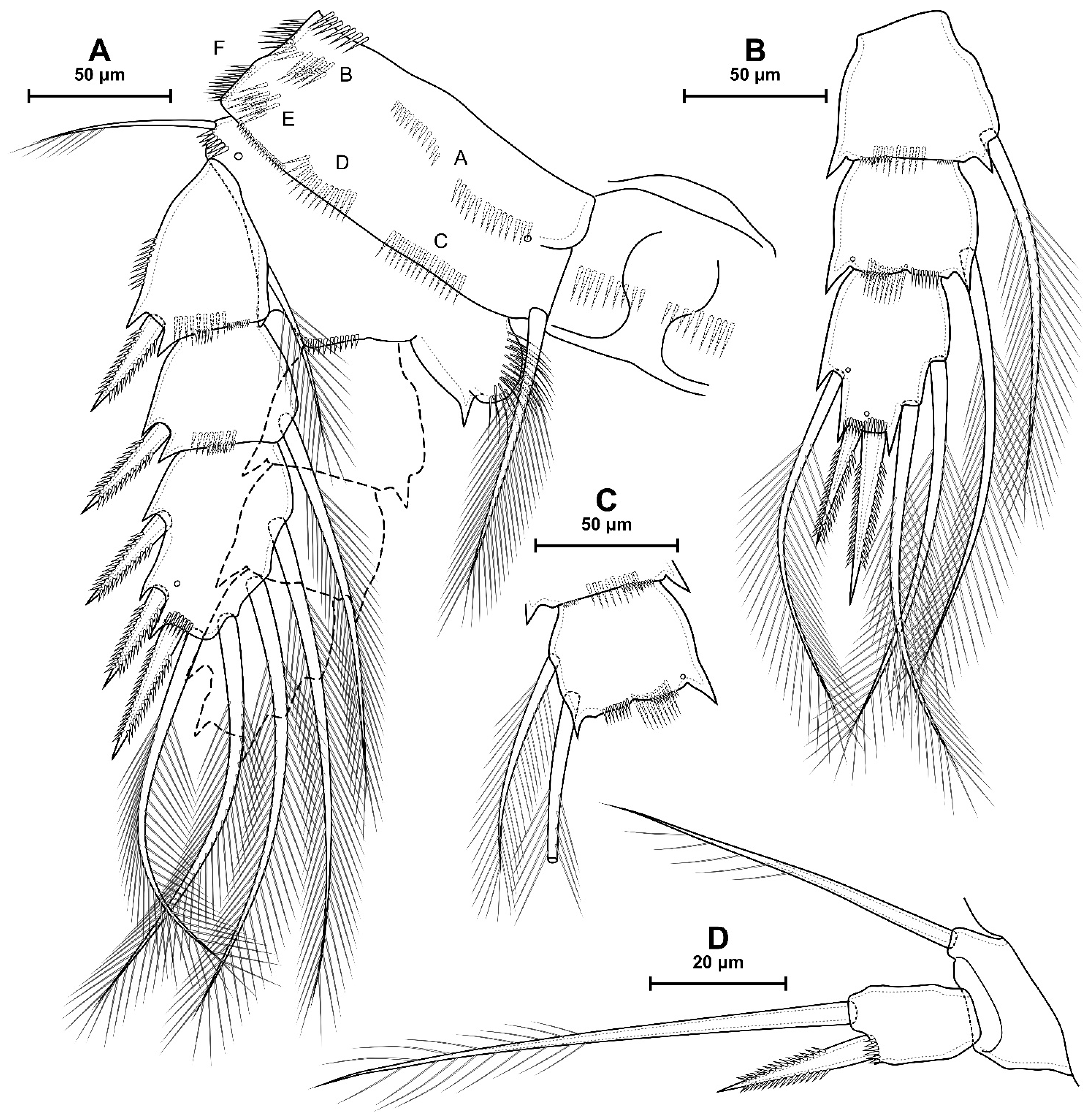
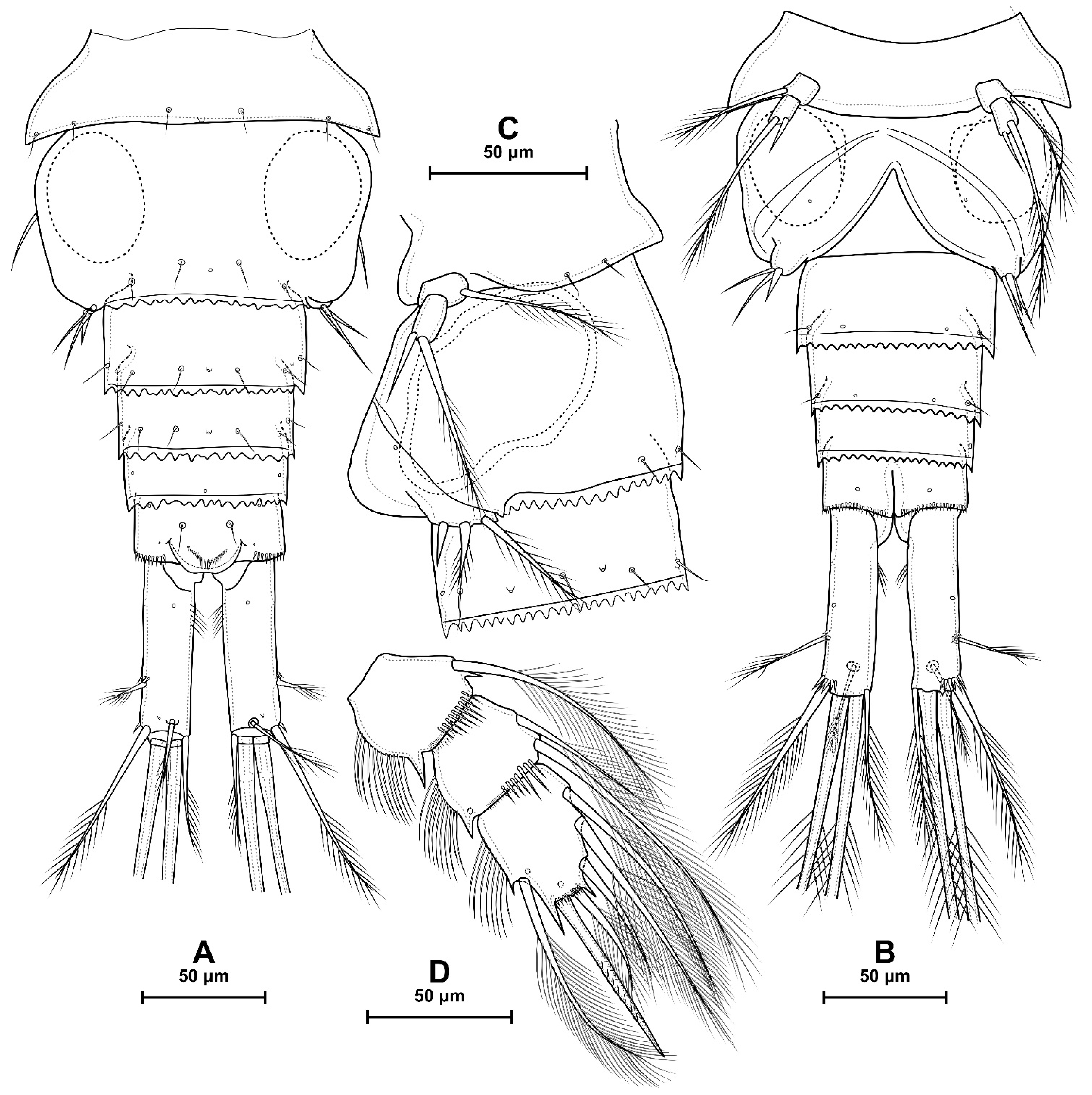

4.2. Morphometric Analysis
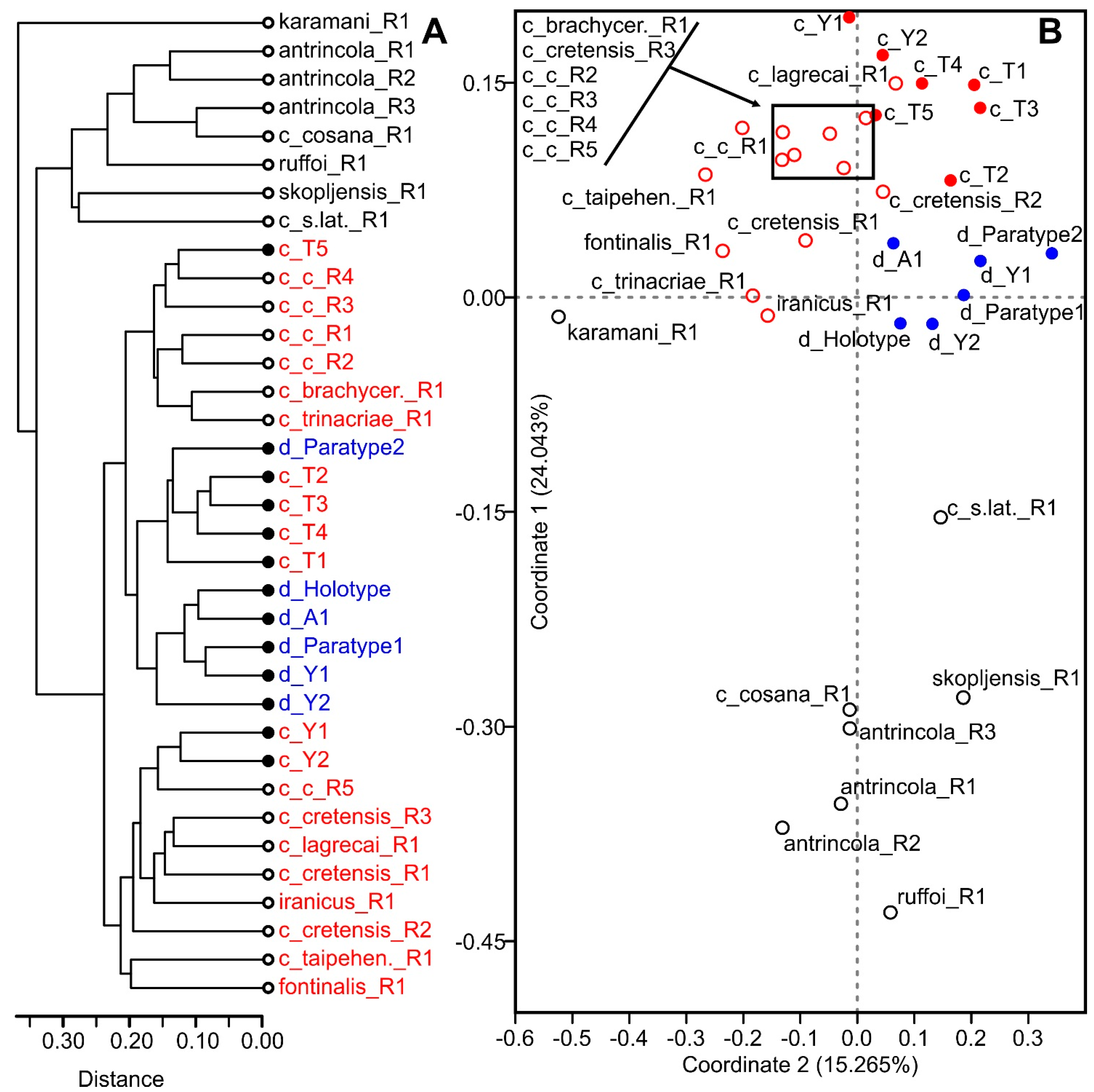
4.3. Molecular Analysis
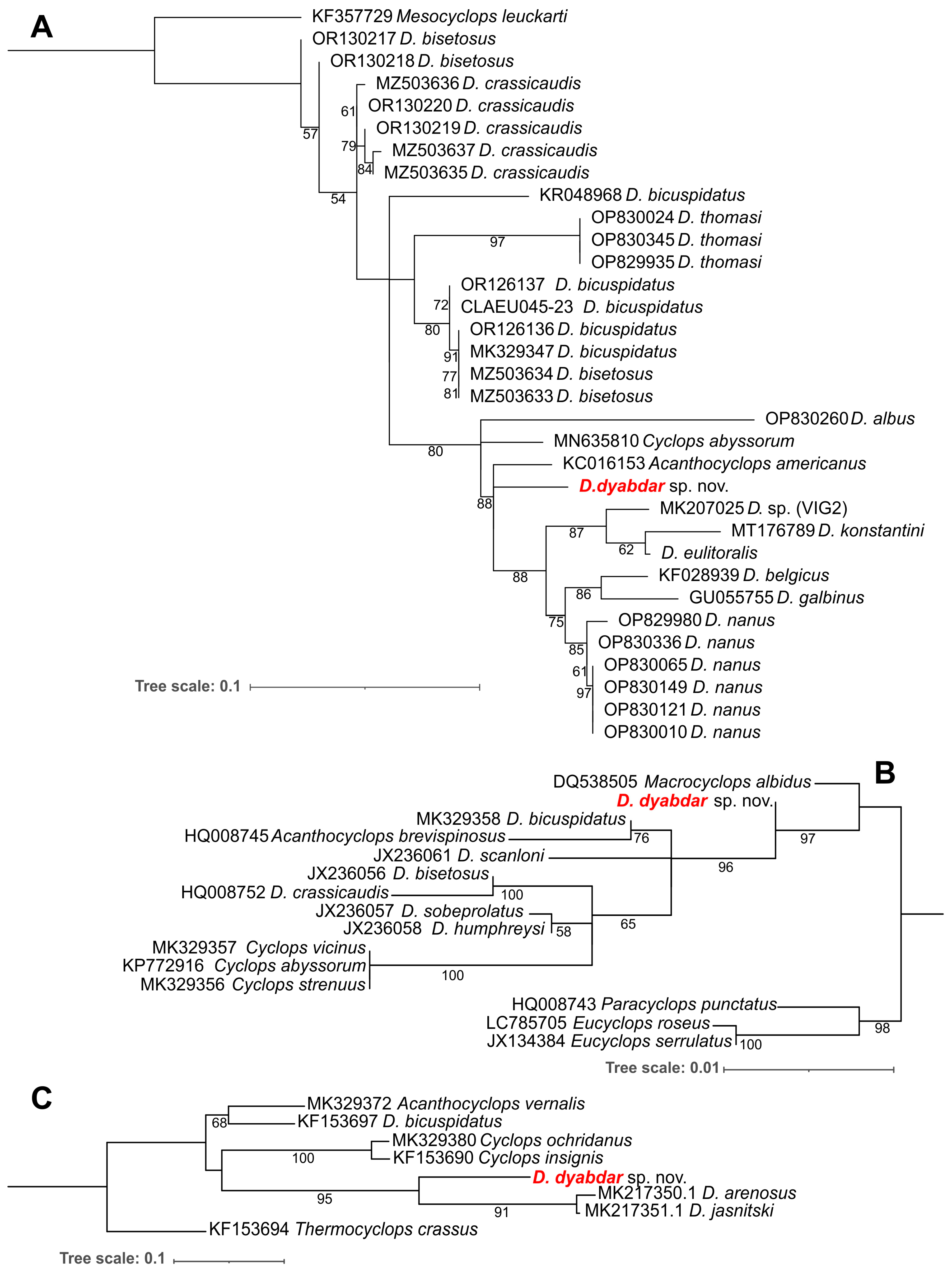
4.4. Diacyclops crassicaudis Morphological Species Group
4.5. Diacyclops crassicaudis (Sars, 1863)
4.6. Diacyclops cf. crassicaudis (Sars, 1863)

5. Discussion
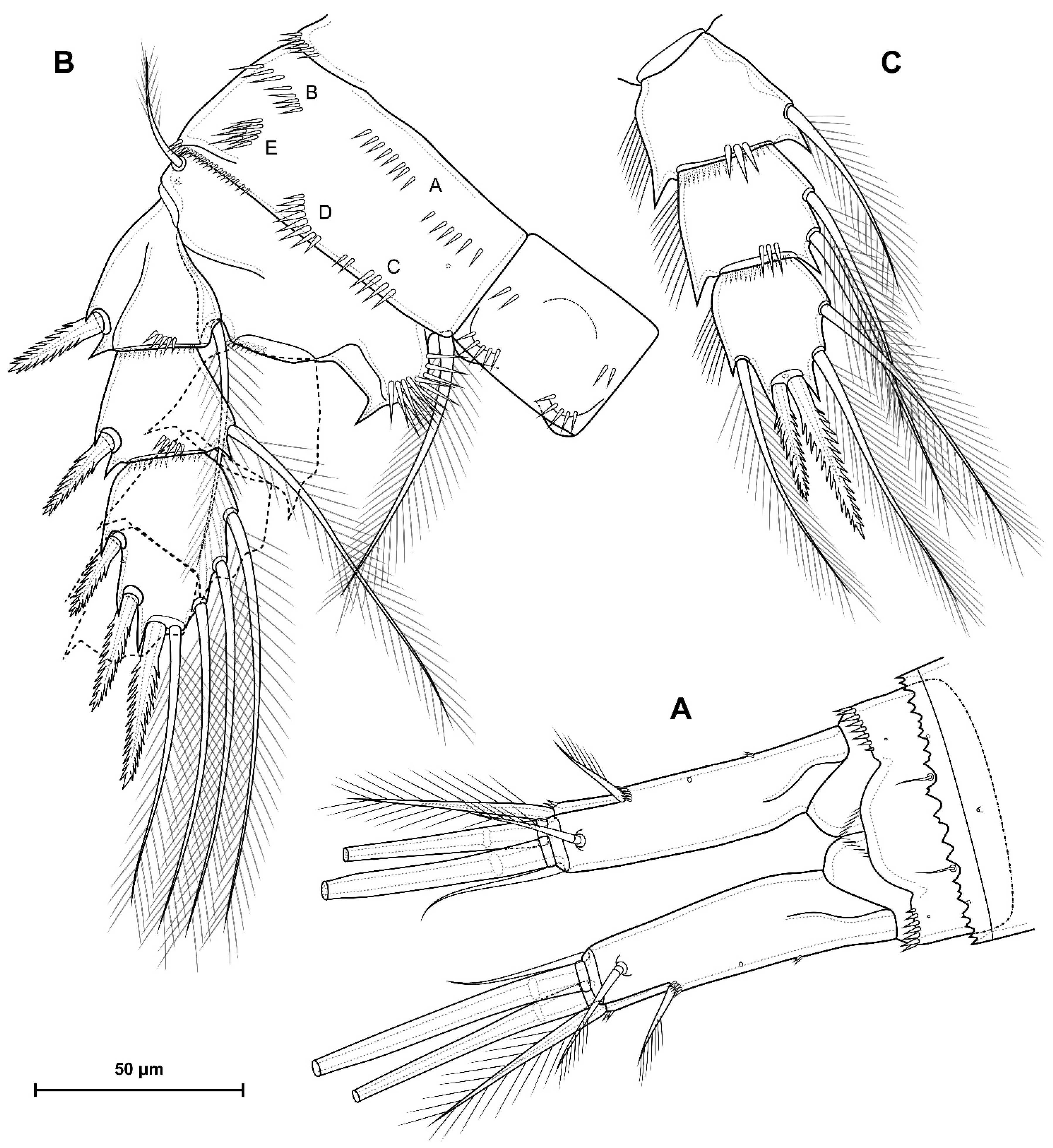
5.1. Difference between D. crassicaudis and D. dyabdar sp. nov.
- Females of D. dyabdar sp. nov. are significantly larger than females of D. crassicaudis.
- The caudal rami of D. dyabdar sp. nov. have a group of thin inner setules at the base.
- The caudal rami of D. crassicaudis distally after seta II have a well-marked ridge, which is absent in D. dyabdar sp. nov.
- P4 coxa in D. dyabdar sp. nov. have spinular group F.
- The first segments of exopods P3 and P4 in D. dyabdar sp. nov. have outer groups of well-marked spinules.
- The intercoxal sclerite of P4 on the posterior side has one row of spinules in D. dyabdar sp. nov. and two rows of spinules in D. crassicaudis.
- The intercoxal sclerite of P4 in D. crassicaudis has outstanding humps and D. dyabdar sp. nov. are without them.
- P4 basis has a group of outer spinules in D. dyabdar sp. nov. but not in D. crassicaudis.
- The inner lobe of the P4 basis is strongly protruded in D. dyabdar sp. nov., but in D. crassicaudis, this lobe forms only a semicircle.
- The pore on P4 Exp 3 of D. crassicaudis is displaced to the outer side.
- P4 Enp3 has two pores in D. dyabdar sp. nov. and one in D. crassicaudis.
5.2. Diacyclops crassicaudis Species Group
5.3. Morphometric Indices in Taxonomy of Diacyclops crassicaudis Species Group
5.4. About Dubious Taxa within the Genus Diacyclops
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chertoprud, E.S.; Novichkova, A.A. Crustaceans in the Meiobenthos and Plankton of the Thermokarst Lakes and Polygonal Ponds in the Lena River Delta (Northern Yakutia, Russia): Species Composition and Factors Regulating Assemblage Structures. Water 2021, 13, 1936. [Google Scholar] [CrossRef]
- Novikov, A.; Abramova, E.; Novichkova, A.; Chertoprud, E. Unveiling Copepod Diversity and Faunal Patterns in Middle Siberia: Insights from Tiksi Settlement Vicinity. Acta Biol. Sib. 2023, 9, 683–708. [Google Scholar]
- Novikov, A.; Sharafutdinova, D.; Chertoprud, E. Two New Species of Bryocamptus (Copepoda, Harpacticoida, Canthocamptidae) from the Russian Arctic and Comparison with Bryocamptus Minutus (Claus, 1863). ZooKeys 2023, 1138, 89–141. [Google Scholar] [CrossRef]
- Chertoprud, E.S.; Novichkova, A.A.; Novikov, A.A.; Fefilova, E.B.; Vorobjeva, L.V.; Pechenkin, D.S.; Glubokov, A.I. Assemblages of Meiobenthic and Planktonic Microcrustaceans (Cladocera and Copepoda) from Small Water Bodies of Mountain Subarctic (Putorana Plateau, Middle Siberia). Diversity 2022, 14, 492. [Google Scholar] [CrossRef]
- Walter, T.C.; Boxshall, G.A. World of Copepods Database. Diacyclops Kiefer, 1927. Available online: https://www.Marinespecies.Org/Copepoda/Aphia.Php?P=taxdetails&id=149783 (accessed on 4 February 2024).
- Boxshall, G.A.; Evstigneeva, T.D. The Evolution of Species Flocks of Copepods in Lake Baikal: A Preliminary Analysis. Ergeb. Limnol. 1994, 44, 235–246. [Google Scholar]
- Mazepova, G.F. A new species of cyclops (Crustacea, Copepoda) from the deep zone of Baikal. Zool. Zhurnal 1970, 49, 354–361. [Google Scholar]
- Stoch, F. How Many Species of Diacyclops? New Taxonomic Characters and Species Richness in a Freshwater Cyclopid Genus (Copepoda, Cyclopoida). Hydrobiologia 2001, 453, 525–531. [Google Scholar] [CrossRef]
- Karanovic, T.; Grygier, M.J.; Lee, W. Endemism of Subterranean Diacyclops in Korea and Japan, with Descriptions of Seven New Species of the Languidoides-Group and Redescriptions of D. Brevifurcus Ishida, 2006 and D. Suoensis Ito, 1954 (Crustacea, Copepoda, Cyclopoida). ZooKeys 2013, 267, 1–76. [Google Scholar] [CrossRef][Green Version]
- Karanovic, T. Subterranean Copepods (Crustacea, Copepoda) from the Pilbara Region in Western Australia. Rec. West. Aust. Mus. Suppl. 2006, 70, 1. [Google Scholar] [CrossRef]
- Pesce, G.L.; De Laurentiis, P. Copepods from Ground Waters of Western Australia. III. Diacyclops Humphreysi n. Sp., and Comments on the Diacyclops Crassicaudis-Complex (Copepoda, Cyclopidae). Crustaceana 1996, 69, 524–531. [Google Scholar]
- Kiefer, F. Ruderfusskrebse (Crustacea Copepoda) Aus Dem Interstitial Einiger Norditalienischen Flusse. Boll. Mus. Civ. Stor. Nat. Verona 1981, 8, 275–285. [Google Scholar]
- Pesce, G.L.; Galassi, D.P. Copepodi Di Acque Sotterranee Della Sicilia. Animalia 1987, 14, 193–235. [Google Scholar]
- Rylov, V.M. Fauna USSR. Crustaceana. Cyclopoida of Freshwater; Moscow-Leningrad: Moscow, Russia, 1948. [Google Scholar]
- Chappuis, P.A. Eine Neue Methode Zur Untersuchung Der Grundwasserfauna. Acta Scientifica Mathematisch. Naturwissenschaftlichen Univ. Fr. Josephinae Kolozsvar. 1942, 6, 1–7. [Google Scholar]
- Karaman, S. Die Fauna Der Unterirdischen Gewässer Jugoslaviens: Mit 5 Abbildungen. Int. Ver. Für Theor. Und Angew. Limnol. Verhandlungen 1935, 7, 46–73. [Google Scholar] [CrossRef]
- Gower, J.C. A General Coefficient of Similarity and Some of Its Properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution; The Ray Society: London, UK, 1991. [Google Scholar]
- Sewell, R.B.S. The Littoral and Semi-Parasitic Cyclopoida, Monstrilloida and Notodelphyoida. John Murray Exped 1933–34 Sci. Rep. 1949, 9, 17–199. [Google Scholar]
- Ferrari, F.D.; Ivanenko, V.N. The Identity of Protopodal Segments and the Ramus of Maxilla 2 of Copepods (Copepoda). Crustaceana 2008, 81, 823–835. [Google Scholar] [CrossRef]
- Einsle, U. A Further Criterion for the Identification of Species in the Genus Cyclops s. Str.(Copepoda, Cyclopoida). Crustaceana 1985, 49, 299–309. [Google Scholar] [CrossRef]
- Monchenko, V.I. Fauna of Ukraine. Cyclopidae; Naukova Dumka: Kiev, Ukraine, 1974. [Google Scholar]
- Sars, G.O. An Account of the Crustacea of Norway Vol. VI. Copepoda. Cyclopoida. Parts III and IV. Cyclopidae; Bergen Museum: Bergen, Norway, 1913. [Google Scholar]
- Ito, T. Groundwater Copepods from South-Western Japan. Hydrobiologia 1957, 11, 1–28. [Google Scholar] [CrossRef]
- Reid, J.W. Redescription of Diacyclops Nearcticus (Kiefer, 1934) and Description of Four Similar New Congeners from North America, with Comments on D. Crassicaudis (G. 0. Sars, 1863) and D. Crassicaudis Var. Brachycercus (Kiefer, 1927) (Crustacea: Copepoda). Can. J. Zool. 1992, 70, 1445–1469. [Google Scholar] [CrossRef]
- Gurney, R. British Fresh-Water Copepoda. Volume III; The Ray Society: London, UK, 1933. [Google Scholar]
- Kim, H.S.; Chang, C.Y. Freshwater Cyclopoid Copepods (Cyclopoida, Cyclopidae) of Korea. Korean J. Syst. Zool. 1989, 5, 225–256. [Google Scholar]
- Pesce, G.L.; Maggi, D. Cyclopides et Calanoïdes Des Eaux Phréatiques de La Grèce Méridionale et Insulaire (Crutacea: Copepoda). Ecol. Mediterr. 1981, 7, 163–182. [Google Scholar] [CrossRef]
- Harada, I. Studien Über Die Süsswasserfauna Formosas, IV. Susswasser-Cyclopiden Aus Formosa. Süsswasser-Cyclopiden Formosa. Annot. Zool. Jpn. 1931, 13, 149–168. [Google Scholar]
- Naidenow, W. Eine Neue Acanthocyclops (Diacyclops)-Art Aus Bulgarischen Grundgewassern. Comptes Rendus L’académie Bulg. Sci. 1969, 22, 1063–1066. [Google Scholar]
- Pesce, G.; Maggi, D. Diacyclops Iranicus n. Sp., a Phreatic Cyclopoid from Subterranean Waters of Iran (Crustacea: Copepoda). Rev. Suisse Zool. 1982, 89, 177–181. [Google Scholar] [CrossRef]
- Kiefer, F. Ein Neuer Cyclopide (Crustacea-Copepoda) Aus Einer Hohle in Mittelitalien. Riv. Idroniologia 1967, 6, 133–138. [Google Scholar]
- Pesce, G.L. The Occurrence of Diacyclops Antrincola Kiefer (Crustaces, Copepoda) in Subterranean Waters of Turkey, and Remarks on Its Variability and Distribution. Commun. Fac. Sci. L’université D’ankara 1980, 24, 1–6. [Google Scholar]
- Pesce, G.L.; Fusacchia, G.; Maggi, D.; Tete, P. Ricerche Faunistiche in Acque Freatiche Del Salento. Thalass. Salentina 1978, 8, 3–51. [Google Scholar]
- Stella, E.; Salvadori, F.B. La Fauna Acquatica Della Grotta “Di Punta Degli Stretti” (Monte Argentario). Arch. Zool. Ital. 1953, 38, 441–483. [Google Scholar]
- Kiefer, F. Neue Süsswassercopepoden Aus Jugoslawien. I. Cyclopiden. Zool. Anz. 1932, 101, 49–60. [Google Scholar]
- Pesce, G.L. Cyclopids from Ground Waters of Turkey and Secription of Diacyclops Languidoides Anatolicus n. Ssp. Fragm. Entomol. 1992, 24, 1–12. [Google Scholar]
- Mayor, T.Y.; Sheveleva, N.G.; Sukhanova, L.V.; Timoshkin, O.A.; Kiril’chik, S.V. Molecular-Phylogenetic Analysis of Cyclopoids (Copepoda: Cyclopoida) from Lake Baikal and Its Water Catchment Basin. Russ. J. Genet. 2010, 46, 1373–1380. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Phillips, R.B.; Matsuoka, M.P.; Konon, I.; Reed, K.M. Phylogenetic Analysis of Mitochondrial and Nuclear Sequences Supports Inclusion of Acantholingua Ohridana in the Genus Salmo. Copeia 2000, 2000, 546–550. [Google Scholar] [CrossRef]
- White, T.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Spears, T.; Abele, L.G.; Kim, W. The Monophyly of Brachyuran Crabs: A Phylogenetic Study Based on 18S rRNÅ. Syst. Biol. 1992, 41, 446–461. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Team, U. Unipro UGENE: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Steel, M.A.; Lockhart, P.J.; Penny, D. Confidence in Evolutionary Trees from Biological Sequence Data. Nature 1993, 364, 440–442. [Google Scholar] [CrossRef]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An Index of Substitution Saturation and Its Application. Mol. Phylogenetics Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Sars, G.O. Oversigt Af de Indenlandske Ferskvandscopepoder. Forh. Vidensk.-Selsk. Christ. 1863, 1862, 212–262. [Google Scholar]
- Coker, R.E. Anomalies of Crustacean Distribution in the Carolinas with List of Cyclopoids of the General Region of Chapel Hill, N.C. J. Elisha Mitchell Sci. Soc. 1938, 54, 76–87. [Google Scholar]
- Karpowicz, M.; Smolska, S.; Świsłocka, M.; Moroz, J. First Insight into Groundwater Copepods of the Polish Lowland. Water 2021, 13, 2086. [Google Scholar] [CrossRef]
- Scott, T. Report on the Marine and Freshwater Crustacea from Franz-Josef Land, Collected by Mr. William S. Bruce, of the Jackson-Harmsworth Expedition. J. Linn. Soc. Zool. 1899, 27, 60–126. [Google Scholar] [CrossRef]
- Willey, A. Northern Cyclopidae and Canthocamptidae. Trans. R. Soc. Can. 1925, 5, 137–158. [Google Scholar]
- Kiefer, F. Freilebende Susswasser-Copepoden Aus Nordamerika. I. Cyclopiden. Zool. Anz. 1927, 72, 262–268. [Google Scholar]
- Yeatman, H.C. American Cyclopoid Copepods of the Viridis-Vernalis Group, (Including a Description of Cyclops Carolinianus n. Sp.). Am. Midl. Nat. 1944, 32, 1–90. [Google Scholar] [CrossRef]
- Kiefer, F. Beiträge Zur Copepodenkunde (XI). 27. Eine Neue Unterart Des Cyclops Crassicaudis Sars. 28. Ein Paracyclops Fimbriatus Mit Aussergewöhnlich Gebautem Fünften Fusspaar. 29. Die Gattungsnamen Paracyclops Claus, Ectocyclops Brady Und Platycyclops Sars. Zool. Anz. 1928, 79, 244–250. [Google Scholar]
- Reid, J.W. New Records and New Species of the Genus Diacyclops (Crustacea: Copepoda) from Subterranean Habitats in Southern Indiana, USA. Jeffersoniana. 2004, 12, 1–65. [Google Scholar]
- Boxshall, G.A.; Evstigneeva, T.D.; Clark, P.F. A New Interstitial Cyclopoid Copepod from a Sandy Beach on the Western Shore of Lake Baikal, Siberia. Hydrobiologia 1993, 268, 99–107. [Google Scholar] [CrossRef]
- Mirabdullayev, I.; Rustamova, N. Redescription of Diacyclops Alticola Kiefer, 1935 (Copepoda, Cyclopoida) from the Pamirs (Tajikistan). Turk. J. Zool. 2007, 31, 411–417. [Google Scholar]
- Shen, C.J.; Sung, T.H. Notes on Copepoda Collected from Shigatze and Gyangtse Regions in Tibet, China. Acta Zool. Sin. 1963, 15, 79–97. [Google Scholar]
- Mahoon, M.S.; Zia, Z. Taxonomic Studies in Copepoda (Calanoida and Cyclopoida). Biologia 1985, 31, 251–292. [Google Scholar]
- Schutze, M.L.M.; da Rocha, C.E.F.; Boxshall, G.A. Antennulary Development during the Copepodid Phase in the Family Cyclopidae (Copepoda, Cyclopoida). Zoosystema 2000, 22, 749–806. [Google Scholar]
- Parveen, K.; Mahoon, M.S.; Saleem, P.M. An Addition to the Copepod Fauna of the Punjab. Biologia 1988, 34, 49–78. [Google Scholar]
- Najam-Un-Nisa; Mahoon, M.S.; Khan, M.I. Copepods (Cyclopoida) of the Punjab (Pakistan). Biologia 1987, 33, 121–138. [Google Scholar]
| Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
| P1 | 0-1 | 1-1 | I-1; I-1: II, 2, 2 | 0-1; 0-1; 1, I + 1; 3 |
| P2 | 0-1 | 1-0 | I-1; I-1: II, I + 1, 3 | 0-1; 0-2; 1, I + 1; 3 |
| P3 | 0-1 | 1-0 | I-1; I-1: II, I + 1, 3 | 0-1; 0-2; 1, I + 1; 3 |
| P4 | 0-1 | 1-0 | I-1; I-1: II, I + 1, 3 | 0-1; 0-2; 1, II; 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikov, A.A.; Sharafutdinova, D.N.; Mayor, T.Y.; Chertoprud, E.S. A New Species of Diacyclops (Copepoda, Cyclopoida) from the D. crassicaudis (Sars, 1863) Species Group with Critical Taxonomy Remarks. Diversity 2024, 16, 208. https://doi.org/10.3390/d16040208
Novikov AA, Sharafutdinova DN, Mayor TY, Chertoprud ES. A New Species of Diacyclops (Copepoda, Cyclopoida) from the D. crassicaudis (Sars, 1863) Species Group with Critical Taxonomy Remarks. Diversity. 2024; 16(4):208. https://doi.org/10.3390/d16040208
Chicago/Turabian StyleNovikov, Aleksandr A., Dayana N. Sharafutdinova, Tatyana Yu. Mayor, and Elena S. Chertoprud. 2024. "A New Species of Diacyclops (Copepoda, Cyclopoida) from the D. crassicaudis (Sars, 1863) Species Group with Critical Taxonomy Remarks" Diversity 16, no. 4: 208. https://doi.org/10.3390/d16040208
APA StyleNovikov, A. A., Sharafutdinova, D. N., Mayor, T. Y., & Chertoprud, E. S. (2024). A New Species of Diacyclops (Copepoda, Cyclopoida) from the D. crassicaudis (Sars, 1863) Species Group with Critical Taxonomy Remarks. Diversity, 16(4), 208. https://doi.org/10.3390/d16040208








