Abstract
Understanding the mechanisms by which tropical forest fragmentation can affect the persistence of species and populations is of scientific and practical interest. However, nest survival has been one the least addressed of the potentially harmful effects associated with habitat fragmentation, and studies involving nest predator’s identification are still underdeveloped. The Pernambuco Endemism Center (PEC) is the part of the Atlantic Forest located north of the São Francisco River, in northeastern Brazil, where large forest tracts no longer exist and a wave of bird extinctions has occurred recently. Here, we investigated the nest survival of forest understory birds from three PEC fragments (690, 979, and 1036 ha), and we used infra-red camera traps for predators’ identification. Overall, the apparent nest survival was 15.5%, and nest-day-based survival probability for the four more representative species (including two endemic and threatened taxa) were 2.6, 4.4, 6.9, and 18.9%, being 2.7 to 8.5 times smaller than populations or related taxa from the Atlantic Forest of southeastern Brazil. Predators were marmosets (25%), opossums (25%), tegu (19.4%), coati (16.7%), snakes (8.3%), and hawks (5.5%). Jackknife2 model-predicted nest predator’s richness was 20.7 (SD = 1.6). We reinforce the evidence that nest predation associated with fragmentation can affect negatively the bird populations from tropical forests.
1. Introduction
Animal species and local populations have been extirpated worldwide because of the effects of habitat loss and fragmentation, and the impacts can be more dramatic in megadiverse regions in which the whole habitat is degraded [1,2,3]. Habitat fragmentation can affect organisms in many ways, including the habitat reduction for habitat-dependent species [1]; reduction in movements and gene flow [4]; exposure to border effects [5]; invasion of alien species [6]; impediment of certain organisms to change their geographic distributions in response to global climate changes [7]; and increase in conflicts between humans and wildlife [8]. Many bird species or local populations also have vanished because of high nest predation rates resulting from habitat disturbances. A commonly advocated theory to account for increased nest predation rates in fragmented habitats is the mesopredator release hypothesis [9,10], which predicts that trophic cascading effects caused by the loss of top predators permit small- and medium-sized animals (the main nest predators) to increase in density, culminating in elevated nest predation rates [11]. Furthermore, habitat deterioration can favor the invasion of exotic nest predators against which local bird communities have no evolutionary nest anti-predatory defenses, leading to increased bird reproductive failures [12,13,14]. Most of the reported cases of bird decline caused by nest predation are from shorebirds (e.g., [11,15]) or from experiments conducted in temperate regions [9], while for tropical forests, most of the data are derived from experiments using artificial nests, which provide only approximations of real nests’ survival [16]. Important data were generated for eight forest understory bird species studied in the tropical forests of Usambara Mountains, Tanzania, for which nest survival rates were higher in large than in small fragments [17]. On the other hand, nest survival of the chestnut-backed antbird Myrmeciza exsul was higher in fragments than in continuous tropical forests from Costa Rica [18], suggesting that the relationships between nesting birds and nest predators still need further investigations in tropical forests. In addition to the limited number of studies on natural nest predation in tropical forest fragments, studies involving nest predator’s identification are even more scarce, which precludes proper interpretations of the causes of habitat changes on nest predation rates (see [14,18]).
The Pernambuco Endemism Center (PEC) is the part of the Atlantic Forest located north of the São Francisco River, in northeastern Brazil, covering the coastal regions of the states of Alagoas, Pernambuco, Paraíba, and Rio Grande do Norte. This is the most disturbed of the three Atlantic Forest endemism centers, and due to the concentration of endemic organisms, it has been considered a hotspot within a hotspot [19]. Only 12.3% of the original 4.4 Mha of forested areas has remained (539,877 ha), and of this total, 29.5% is represented by fragments of 100–1000 ha; 24.5% is represented by fragments larger than 1000 ha; and the rest of the forest (46%) is distributed across fragments smaller than 100 ha [20]. For this reason, large mammals, including the top predators (jaguars and cougars), have been long extinct in the PEC, as well as about half of the medium-sized mammals [19]. Three bird species endemic to the PEC were recently extinct (the Pernambuco pygmy owl Glaucidium mooreorum, the cryptic treehunter Cichlocolaptes mazarbarnetti, and the Alagoas foliage-gleaner Philydor novaesi) [21,22], many are nearly extinct (e.g., the black-tailed leaftosser Sclerurus caudacutus caligineus, the Alagoas antwren Myrmotherula snowi, and the Alagoas black-throated trogon Trogon muriciensis) [22,23,24], and a great wave of extinctions will likely occur in the near future if intensive management plans are not implemented [24,25]. Paradoxically, none of the recently lost species were game birds or were targets of trapping. Instead, like many other threatened birds from the PEC, they are small forest birds that may have been victims to the harmful effects of habitat loss and fragmentation.
The scenario of intense habitat degradation and the loss of the top predators in the PEC suggest that nest predation intensity should be investigated as a potential cascading effect of habitat fragmentation contributing to the vulnerability of the bird communities. To give support to potential conservation management plans, nest predators also must be identified and their relative impacts to nesting success must be revealed, especially because, worldwide, the survival of a growing the number of bird species inhabiting disturbed ecosystems have relied on the population control of native or exotic nest predators [11]. Here, we investigated the survival of forest understory bird nests in three representative PEC fragments from Alagoas state, and we used infra-red camera traps for predators’ identification. An alpha diversity estimator was used to contrast observed and model-predicted numbers of nest predator species, and regarding the bird taxa with higher nest sample sizes, we estimated Mayfield’s nest survival probability [26]. Because large forested areas that could serve as a control no longer exist in the PEC, our goal was to answer the question of whether nest predation rates are higher in the PEC than for the same species or closely related congeners from the better preserved Atlantic Forest of southeastern Brazil.
2. Materials and Methods
2.1. Study Areas
Field work was conducted at three Atlantic Forest fragments from the state of Alagoas, northeastern Brazil: RPPN (Private Natural Heritage Reserves) Mata do Cedro (9°31′ S, 35°55′ W: 979 ha); a fragment belonging to Usina Coruripe (10°00′ S, 36°16′ W: 1036 ha); and RPPN Dubinha-Guimarães, previously called Mata do Matão (9°46′00″ S, 36°14′00″ W: 690 ha) (Figure 1). These areas are all isolated by sugar cane plantations, and the vegetation is classified as an open ombrophilous forest [27]. Selective logging was a common practice over the PEC fragments during the decades of 1970 and 1980, and today, these areas are fragments of forests in middle to late generation stages, with shadowed understories and emergent trees [21,27,28]. The climate is AS’ according to Köppen classification: tropical with a well-defined dry season (October through January) and the rainy season concentrated in the autumn and winter. Average annual precipitation is 1600–1700 mm, and average minimum and maximum temperatures are 21–22 °C and 30–31 °C, respectively [25,29].
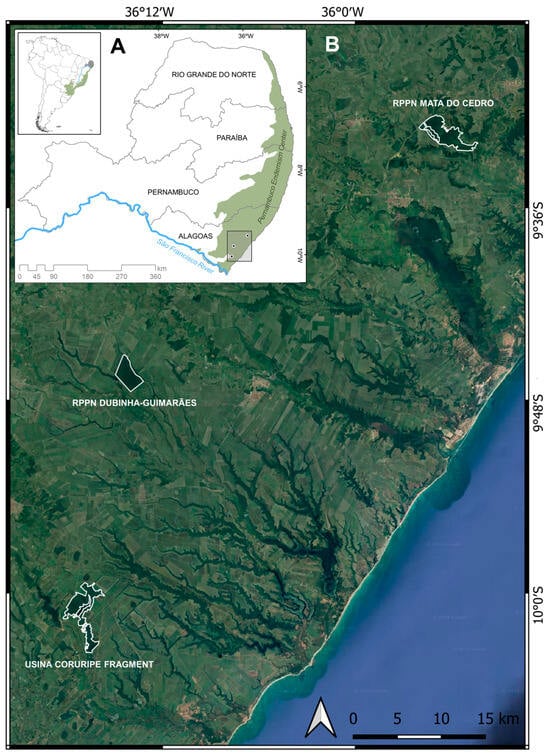
Figure 1.
Pernambuco Endemism Center (A) and study areas in the Pernambuco Endemism Center (B), northeastern Brazil. Dark green areas are forest remnants.
2.2. Nest Searches and Monitoring
Field work was conducted from September to May during 2021/2022 and 2022/2023 because these are the warmer months in which the days are longer, and some evidence from species-specific studies suggests that these months correspond to the seasons of higher bird reproductive activities in the region [30,31]. Nests were searched ad libitum by walking across the whole areas and were located based on evidence of parental individuals defending territories, carrying nest material, or delivering food to the nestlings and also by thoroughly inspecting the vegetation [32]. Programmed walks for nest searches occurred, on average, three days per month in each of the areas, but further nests were also found during the more frequent returns to these areas for nest and camera checking. Once found, nests were georeferenced for posterior re-sighting and were monitored for predator identification using digital camera traps Bushnell TrophyCam, model 119437C (Bushnell Outdoor Products, Kansas, MI, USA). The cameras were positioned 1–3 m from the nest, depending on the availability of tree branches to attach the camera. They were programmed to obtain 30 s videos, with low LED intensity (eight LEDs), “High” sensor level, intervals of 3 s between triggers, and recorded date and time, following the optimization procedures suggested by Ribeiro-Silva et al. [33]. Detections were stored on 14 GB memory cards. Nests and cameras were checked twice a week, but monitoring was intensified in nests containing nestlings near fledgling age to confirm nest fate in case of camera failures. Predation was assumed when nest contents disappeared before fledging age or when predation events were caught on cameras [33].
2.3. Nest Survival Estimates
For determining the magnitude of nest survival, estimating the simple percentage of nest losses is not enough because nests that were depredated in the early stages have lower chances of being found, leading to overestimated nest survival rates. Furthermore, most nests are found after the laying stage, meaning that the information obtained for each nest is often fragmented. For these reasons, we followed the broadly used nest-day-based method of Mayfield [26] to estimate nest survival, which accounts for the above sources of uncertainty by calculating daily survival rate (DSR). Nest days are the summation of the numbers of days that a number of nests of a target species are monitored. Then, the number of nest days subdivided by the number of nest losses results in the daily nest loss rate, and 1 minus the daily nest loss results in the daily nest survival rate. Here, we used the daily survival rate raised to the power of the nesting cycle length (incubation plus nestling period) to estimate the average probability of nest survival for a bird species [26], and we generated standard errors (SE) and 95% confidence limits (CL) using the method of [34]. We applied the method of Mayfield only for species for which nest samples were at least 10 (see also [17]), and for those species with lower nest sample sizes, only apparent nest survival was provided (the simple percentage of nests that survived to the fledging stage). We obtained nesting cycle lengths for the most representative bird species from the literature. Specifically, the nesting cycle duration for the plain antvireo Dysithamnus mentalis (Thamnophilidae) was obtained from [35] (25 days), the black-cheeked gnateater Conopophaga melanops (Conopophagidae) from [31] (32 days), and the blue-backed manakin Chiroxiphia pareola (Pipridae) from [36] (33 days). For the white-shouldered antshrike Thamnophilus aetiops (Thamnophilidae), due to the lack of specific information, we used the estimates available for the barred antshrike T. doliatus (26 days) [37].
2.4. Nest Predator’s Identification and Alpha Diversity
Among the animals recorded in this study by camera traps (see results below), small nocturnal marsupials are the most difficult to identify based solely on video recordings. However, the present work was part of a major project that aimed to perform faunal surveys in the same study areas, including small mammals and reptiles. Then, using the still unpublished species lists as references, we could identify even the small marsupials with satisfactory precision based on the diagnostic morphological characters of each species, i.e., the proportional sizes of tail, ears, and eyes. For taxonomic nomenclature, we followed the annotated Brazilian checklists of reptiles [38], birds [39], and mammals [40].
Because species richness (alpha diversity) is a proxy of sampling effort, uncovering all of the species in a target community can be a challenging task, especially in megadiverse habitats such as the Atlantic Forest [41]. Among the innumerous methods developed to estimate expected species richness based on collected data, the non-parametric estimators, e.g., Chao2, ICE, Jackknife1, and Jackknife2, have been widely used in studies involving camera trap data [42,43,44]. Here, we chose a Jackknife estimator because this class of estimator performed better than others in two comparative studies that used camera traps for faunal surveys in tropical forests [42,44]. Specifically, we used Jackknife 2, which is a model that takes into account the numbers of singletons (species represented by only one individual) and doubletons (species represented by only two individuals) to generate expected species richness [45,46], as both were present in our dataset. The 95% confidence interval and standard deviation (SD) were generated by the resampling method implemented in the R-package “vegan” [47], with 1000 permutations, and all of the analyses were performed in R Studio (version 2022.02.2). Because our study involved a number of endangered bird taxa in highly vulnerable habitats, nest sample sizes were moderate, and for this reason, we pooled the data from the three study areas together for the statistical analyses.
Although in some regular camera trap surveys the cameras can be arranged in the field across pre-defined grids or transects [42,48,49], in many other works, the cameras are distributed randomly across sites with animal traces, respecting only a minimum distance interval [43,44,50], and our study involving widespread nests may not differ from the latter. Although each camera–day can be treated as a sampling unit for species richness estimations, here, for graphical purposes, we partitioned our dataset into 50 camera–days subsets that were used as sampling unities for Jackknife2 calculations (see also [49]).
Nest density is low in such a way that the probability of capturing the same predator individuals across the different nests may be reduced. Because nest predations were unique events (partial nests predations were never observed) and often lasted only a few seconds, pseudoreplications were unlikely and all of the records were considered as independent detections, but when nests were depredated by groups of individuals, all of them were counted for modeling-expected species richness (see below). Records of animals that only approached the nests, without depredating the eggs or nestlings, were not considered.
3. Results
We monitored 84 nests of 15 bird species (Table 1), totaling 937 camera/days, with an average of 11.1 camera/days per nest. Of the 84 nests, 63 (75%) were depredated, 8 were lost due to other causes (abandonment, nest fall, or hatching failure) (9.5%), and 13 survived to the fledging stage (15.5%). The bird species with the highest nest sample sizes were D. mentalis (n = 23), T. aetiops (n = 10), C. melanops (n = 12), and C. pareola (n = 10) (Table 1). Nest survival probabilities estimated using the nest-day-based method of Mayfield for these four more representative species were 6.9% (SE = 2.0, CL = 2.8–11%) for D. mentalis (22 nest losses in 217 nest days), 18.9% (SE = 2.1, CL = 14.7–23.1%) for T. aetiops (eight nest losses in 129 nest days), 2.6% (SE = 3.0, CL = −3.5–8.6%) for C. melanops (11 nest losses in 103 nest days), and 4.4% (SE = 2.86, CL = −1.32–10.1%) for C. pareola (9 nest losses in 100 nest days).

Table 1.
Bird species that had their nests monitored with infra-red camera traps in three Atlantic Forest fragments from the Pernambuco Endemism Center. For each species, we provide the total number of nests monitored (Total), the number of depredated nests (Depredated), the number of nests that failed due to other causes, such as abandonment, nest fall, or due to the presence of infertile eggs (Failed), the number of successful nests (Successful), and apparent survival, i.e., the simple percentage of nests that survived to the fledging stage (Apparent Survival).
Predators were recorded by the camera traps in 36 (57%) of the 63 predation events. Of the 27 predation events not captured by the cameras, 2 were caused by camera malfunction, 4 were caused by memory cards malfunction, and 21 predation events were not caught, even with the cameras being in good conditions. Then, when we excluded the nests in which predators were not detected due to camera and memory cards malfunctions, the cameras were capable of recording predators in 63% of the predation events. The predators included 11 animal species: four reptiles, two birds, and five mammal species. Among the reptiles, we recorded the tegu Salvator merianae (Teiidae) and the snakes Chironius sp. (Colubridae), the indigo snake Drymarchon corais (Colubridae), and Philodryas sp. (Dipsadidae). Birds were represented by the gray-lined hawk Buteo nitidus (Accipitridae) and the collared forest falcon Micrastur semitorquatus (Falconidae). Among the mammals, we detected the big-eared opossum Didelphis aurita (Didelphidae), the Emilia’s gracile opossum Gracilinanus emiliae (Didelphidae), the woolly mouse opossum Marmosa demerarae (Didelphidae), the common marmoset Callithrix jacchus (Callitrichidae), and the South American coati Nasua nasua (Procyonidae) (Table 2; Figure S1; Videos S1–S5). Overall, 25% of the recorded predations were caused by marmosets, 25% by opossums, 19.4% by S. merianae, 16.7% by N. nasua, 8.3% by snakes, and 5.5% by hawks (Figure 2). Three of the nests depredated by the C. jacchus were depredated by groups of individuals, being one group of two and two groups of three individuals, while for the other nest predator species, only single individuals were observed depredating the nests. Then, when the numbers of individuals were considered, C. jacchus was the most representative nest predator, with 14 records (34% of the predator individuals). The Jackknife2 model-predicted nest predator’s richness estimate was 20.7 (95% CI = 18.4–23.9, SD = 1.6), without tendency to asymptotes. It suggested that at least 10 further rare nest predator species would be expected whether field effort was increased (Figure 3).

Table 2.
Nesting birds and numbers of nests depredated by each nest predator species across three Atlantic Forest fragments from the Pernambuco Endemism Center.
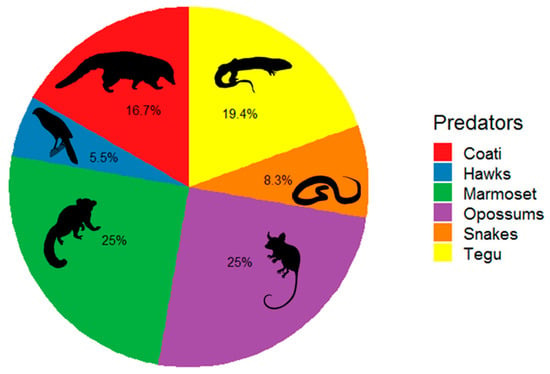
Figure 2.
Percentages of nests depredated by the main animal groups, including the tegu, snakes, the common marmoset, opossums, hawks, and the coati, in three Atlantic Forest fragments from the Pernambuco Endemism Center.
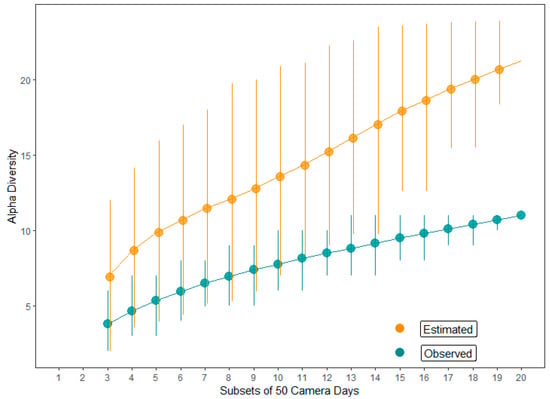
Figure 3.
Observed and estimated nest predator species richness derived from Jackknife2 estimator and 95% confidence intervals generated by resampling method.
4. Discussion
Testing whether forest fragmentation can affect predators’ community composition and nest survival rates should rely on comparisons between continuous and fragmented habitats [17,18]. However, continuous forests no longer exist in the PEC [20]. The only non-fragmented forest tracts of the whole Atlantic Forest are located in southeastern Brazil, especially at the coastal mountains of Serra do Mar, from the states of São Paulo, Paraná and Rio de Janeiro, where a complex of conservation units gather more than 1 million ha of continuous forests [33]. Despite the differences in species composition and physical parameters, Serra do Mar is the region that best preserves the original conditions of the Atlantic Forest and the only that could serve as a control. Within the scenario of extreme fragmentation of the PEC, some of the biggest and most important fragments are Pedra Talhada Biological Reserve (4500 ha), Engenho Coimbra, Usina Serra Grande (4300 ha), and Murici Ecological Station (6116 ha), which are also in the state of Alagoas and are considered as IBAs (Important Bird Areas) by BirdLife International [51]. For Pedra Talhada, valuable bird breeding biology information is available, including nest survival information for a few species [30,31], while Serra Grande and Murici Ecological Station should be in the scope of future works. Then, the best option to interpret the effects of forest fragmentation in the PEC is through comparisons with data from the continuous Atlantic Forest tracts from southeastern Brazil, with some larger fragments from the PEC, as well as with other tropical forest biomes, especially when information from the same bird species or congeners are available.
In one of the best preserved areas of the Serra do Mar continuum, the Carlos Botelho State Park, the apparent survival of 122 nests of 24 bird species was 49% [33], and in two Atlantic Forest fragments of 50 and 200 ha from the state of Minas Gerais, the apparent survival of 257 nests of 22 bird species was 44.5% [52], while in our study areas, this figure was only 15.5%. Although these are rough comparisons because they involve only apparent survival and bird communities with different compositions and breeding strategies, nest survival estimates based on daily nest survival for the taxa with higher sample sizes may provide better opportunities for comparisons. For D. mentalis, Mayfield’s nest survival probability in the fragments from Minas Gerais was 48% [52], and in our study, it was only 6.9%. For the Atlantic Forest congener, the blue manakin C. caudata, nest survival probability in the Serra do Mar continuum was 34% [53], and at the fragments from Minas Gerais, it was 60.4%, while the nest survival probability of C. pareola at the PEC fragments was only 4.4%. For C. melanops, the nest survival rate of the subspecies C. m. melanops, from the Serra do Mar continuum of the state of Paraná, was 22% [54], and for our study subspecies, C. melanops nigrifrons, it was 12% at Pedra Talhada Biological Reserve of the PEC [31] and only 2.6% in the fragments we studied. For another representative of the genus Thamnophilus that also breeds in Atlantic Forest tracts, the variable antshrike Thamnophilus caerulescens, the nest survival rate at the Atlantic Forest fragments from Minas Gerais was 50.6%, while for T. a. distans, the nest survival probability in the PEC fragments was 19%. Therefore, the values we found were 2.5 to 8.5 times lower than in other Atlantic Forest regions. At Pedra Talhada, nest survival probabilities were also estimated for the scalloped antbird Myrmoderus ruficauda and for the short-tailed antthrush Chamaeza camapanisona, and they were 22 and 32%, respectively [30,55]. Although nest survival rates seem to be a little higher at Pedra Talhada in comparison with our 690 to 1036 ha fragments, they are overall much smaller than the values found for continuous and fragmented areas from the Atlantic Forest of southeastern Brazil. In other tropical forest systems, the nest survival probability of the chestnut-backed antbird Myrmeciza exsul was 28 and 36% in two forest fragments of 41 and 92 ha from Costa Rica [18]. The values we found in the PEC fragments were closer to those found for eight forest understory bird species studied in the tropical forests of Usambara Mountains, Tanzania, where nest survival rates varied from less than 1% to 13.4%, but this study addressed much smaller fragments, with some presenting only around 0.2 ha [17]. It is worth noting, however, that in the two latter studies i.e., [17,18], nest survival rates were estimated using the likelihood-based model of [56], which is also based on nest days but may provide slightly different estimates when compared with the method of Mayfield. Despite the lack of adequate control areas for comparisons, this is evidence that the rates of nests losses are alarming in some of the important PEC fragments.
The model-estimated alpha diversity diverged in about 10 predator species in relation to the observed number of 11 species, which was likely caused by the occurrence of rare predator species. However, about 78% of the detected nest predations were caused by only four animal species, S. merianae, G. emiliae, C. jacchus, and N. nasua, suggesting that our sampling effort was enough to capture the main nest predators from our study areas. Marmosets and opossums are agile animals and excellent tree climbers. Some of our videos evidenced that not only the marmosets but also the opossums were capable of long jumps even between very thin bush branches and lianas. Although N. nasua is a larger ground mammal, it could climb the larger branches to access the nests, and when bushes or saplings were too thin to support its weights, it often grabbed and folded the whole saplings down to reach the nests and their contents. Salvator merianae is a large terrestrial lizard, and the observation of these animals climbing slender bushes and saplings to access nest contents was totally surprising. This capacity seemed to be attributed to juvenile individuals, but at least one larger adult was recorded standing its body in an upright position to reach the content of a nest that was about 60 cm aboveground. To our knowledge, all of these tactics used by these nest predators have been revealed here for the first time and may contribute to the understanding of the types of interactions between nesting birds and their predators.
Marmosets are plague species in southeastern Brazil, where they have become invasive animals mainly due to illegal faunal releases and because of the lack of predators and competitors [14]. Although C. jacchus is native to the forests of the PEC, some populations also may be experiencing the absence of predators (e.g., large hawks, owls, and felids), and like in southeastern Brazil, their population densities can be inflated in certain areas even within its original distribution, which deserves future studies. At least one of the nests was depredated by a C. jacchus female carrying a young on its back, suggesting that young marmosets can learn with their parents how to recognize nests as sources of food in their early life stages (see for instance [57] for foraging social learning in neotropical primates). Notably, the critically endangered white-collared kite Leptodon forbesi was filmed checking a nest that was already depredated by marmosets. Checking empty nests reveal a certain level of specialization, since the predator was capable of recognizing the nest per se as a source of food, even without eye contact with eggs or nestlings [58]. At the Carlos Botelho State Park, in southeastern Brazil, the white-necked hawk Amadonastur lacernulatus was an important nest predator [33], evidencing that the rare L. forbesi could have been a potential nest predator in the PEC that had its abundance drastically affected by habitat loss and fragmentation, as today, it is one of the most threatened birds of prey on earth [22].
The identity of nest predators has only recently being revealed for tropical forests, and the number of studies is still low [33,59,60]. In continuous Atlantic Forest tracts from São Paulo state, 28% of the nest predations were conducted by large birds of prey (white-necked hawk, barred-forest falcon Micrastur ruficollis, M. semitorquatus); 24% by large toucans (red-breasted toucan Ramphastos dicolorus); 24% by marsupials; 10% by primates; and 11% by other animals, including felids, mustelids, coati, and tegu, with snakes never detected [33]. Comparing predator diversity between the areas of the PEC and the Atlantic Forest of southeastern Brazil is not straightforward because they involve different endemism centers, and it is difficult to infer about how the original nest predator communities were in the PEC in the past. However, we cannot discard the possibility that alterations in nest predator communities can have contributed to the massive nest losses we observed for some bird species. While large birds of prey and large frugivore birds were the main nest predators at the Serra do Mar continuum, with marsupials and primates contributing only a little and reptiles being virtually absent in the samplings, at the PEC fragments, marsupials, marmosets, coatis, and reptiles were the main nest predators. These differences were somewhat expected because forest birds of prey and toucans, especially the large ones, may have been common in the past at the PEC, but today, they can be regularly found only at the largest fragments (e.g., Murici, Pedra Talhada, and Serra Grande). It is not only nest predator species’ composition but also their densities and behaviors that affect bird nest survival, and the relatively low nest survival rates we observed in the studied fragments can be evidence for increased incidences of certain animal groups acting as nest predators because their densities are inflated or because of the lack of other feeding resources, which must be in the scope of future investigations. In this pessimistic scenario, however, a positive finding was the fact that exotic and/or feral animals that can become the main nest predators in some disturbed ecosystems [14,61] were not detected depredating nests in our study areas, although potentially harmful species such as rats, cats, and domestic dogs are abundant in the farms and sugarcane plantations surrounding the fragments (personal observation).
Although D. mentalis and C. pareola are not endemic to the PEC and are not threatened, the subspecies of the black-cheeked gnateater C. m. nigrifrons and of the white-shouldered antshrike T. a. distans are endemic to the PEC and are listed as Vulnerable and Endangered, respectively, in the Brazilian red list, with T. a. distans occurring in only 25 fragments [22]. The fragments we analyzed are among the biggest for the pattern of spatial distribution of the PEC forests [20], and together with other fragments of similar size, they certainly hold significant portions of the total populations of many threatened and endemic taxa, so finding such small nest survival rates in fragments of these size classes was concerning. Because most of the vanishing bird species of the PEC are small insectivorous birds that are not subject to poaching and trapping, we suggest that nest survival rates should be better investigated in the few remaining fragments larger than 1000–2000 ha to see if they could provide better chances of nest survival, especially because top predators were long extinct in these areas too [19]. Although testing the mesopredator release hypothesis was far away from the scope of this work, we present evidence that nest loss rates were likely inflated in our study sites and predation was the main cause of nest failures. Our main conclusion is that low nest survival and the action of nest predators can be among the causes of bird population declines in the PEC, and these subjects must be in the scope of future conservation management plans aiming to minimize the unprecedented number of ongoing bird extinctions in this important hotspot. The survival of a growing number of endangered bird species worldwide has relied on nest predators management [62,63], and the identification of the nest predators’ community composition of the PEC fragments provided the first insights into the animal species that must be monitored and, if necessary, controlled as a way to improve the demographic aspects of the most threatened bird taxa endemic to the PEC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16040207/s1, Figure S1: A young common marmoset Callithrix jacchus (Callitrichidae) depredating a nest of the plain antvireo Dysithamnus mentalis (Thamnophilidae) together with its mother, just after leaving its back; Video S1: The tegu Salvator merianae (Teiidae) climbing a tree to depredate a nest of D. mentalis; Video S2: A snake Chironius sp. (Colubridae) depredating a nest of the threatened brown-winged mourner Schiffornis turdina intermedia (Tityridae); Video S3: Predation of a nest of the threatened black-cheeked gnateater Conopophaga melanops nigrifrons (Conopophagidae) by the Emilia’s gracile opossum Gracilinanus emiliae (Didelphidae); Video S4: Predation of a nest of the threatened S. t. intermedia by the coati Nasua nasua (Procyonidae); Video S5: Predation of a nest of D. mentalis by a group of the common marmoset Callithrix jacchus (Callitrichidae).
Author Contributions
Conceptualization, L.W.L.-A., M.C.C., M.R.F. and L.F.S.; methodology, all authors; validation, all authors; formal analysis, all authors; investigation, all authors; resources, L.F.S., M.R.F. and L.W.L.-A.; writing—original draft preparation, M.R.F. and L.W.L.-A.; writing—review and editing, all authors; visualization, all authors.; supervision, M.R.F.; project administration, L.F.S. and M.R.F.; funding acquisition, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was due to the ARCA project by the São Paulo Research Foundation—FAPESP (2017/23548-2). L.W.L.A. received a PhD fellowship from the National Council for Scientific and Technological Development—CNPq (Proc# 142308/2019-6), M.C.C. received a Post-Dctoral fellowship from FAPESP (2020/13489-1), and M.R.F. (Proc# 308702/2019-0 and Proc# 304213/2022-5) and L.F.S. (Proc.# 308337/2019-0) received Productivity Research Fellowships from CNPq.
Institutional Review Board Statement
The field work protocol of this study was approved by Sisbio/MMA (Process 66157-4) and by the Ethic Committee on Animal Use of the Federal University of São Carlos (CEUA/UFSCAR) (Process 1405291118).
Data Availability Statement
All data are presented in the manuscript.
Acknowledgments
The authors are grateful to Fernando Pinto, Sônia Roda, Marcela Daher, Carlos Monteiro, and Luzenilton Brito for providing logistical support during the field work. Flor Maria Guedes Las-Casas, Wallace Rodrigues Telino Junior, Guilherme Santos Toledo de Lima, Manoel Martins Dias Filho, and three anonymous referees provided important suggestions on the early versions of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.R.; Di Marco, M.; Rondinini, C.; Boitani, L. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef]
- Liu, J.; Wilson, M.; Hu, G.; Liu, J.; Wu, J.; Yu, M. How does habitat fragmentation affect the biodiversity and ecosystem functioning relationship? Landsc. Ecol. 2018, 33, 341–352. [Google Scholar] [CrossRef]
- Costa-Araújo, R.; Regolin, A.L.; Martello, F.; Souza-Alves, J.P.; Hrbek, T.; Ribeiro, M.C. Occurrence and conservation of the vulnerable titi monkey Callicebus melanochir in fragmented landscapes of the Atlantic Forest hotspot. Oryx 2021, 55, 916–923. [Google Scholar] [CrossRef]
- Schlaepfer, D.R.; Braschler, B.; Rusterholz, H.P.; Baur, B. Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: A meta-analysis. Ecosphere 2018, 9, e02488. [Google Scholar] [CrossRef]
- Slater, H.D.; Gillingham, P.K.; Pratt, V.; Eaton, B.; Fletcher, S.; Abdullah, A.; Supriadi; Korstjens, A.H. Living on the edge: Forest edge effects on microclimate and terrestrial mammal activity in disturbed lowland forest in Sumatra, Indonesia. Oryx 2024, 58, 228–239. [Google Scholar] [CrossRef]
- Didham, R.K.; Tylianakis, J.M.; Gemmell, N.J.; Rand, T.A.; Ewers, R.M. Interactive effects of habitat modification and species invasion on native species decline. Trends Ecol. Evol. 2007, 22, 489–496. [Google Scholar] [CrossRef]
- Krosby, M.; Tewksbury, J.; Haddad, N.M.; Hoekstra, J. Ecological connectivity for a changing climate. Conserv. Biol. 2010, 24, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.P.; Paudel, P.K.; Jnawali, S.R.; Neupane, P.R.; Köhl, M. Can forest fragmentation and configuration work as indicators of human–wildlife conflict? Evidences from human death and injury by wildlife attacks in Nepal. Ecol. Indic. 2017, 80, 74–83. [Google Scholar] [CrossRef]
- Rogers, C.; Caro, M. Song sparrows, top carnivores and nest predation: A test of the mesopredator release hypothesis. Oecologia 1998, 116, 227–233. [Google Scholar] [CrossRef]
- Robinson, W.D.; Sherry, T.W. Mechanisms of avian population decline and species loss in tropical forest fragments. J. Ornithol. 2012, 153, 141–152. [Google Scholar] [CrossRef]
- Stantial, M.L.; Cohen, J.B.; Darrah, A.J.; Farrell, S.L.; Maslo, B. The effect of top predator removal on the distribution of a mesocarnivore and nest survival of an endangered shorebird. Avian Conserv. Ecol. 2021, 16, 8. [Google Scholar] [CrossRef]
- Savidge, J.A. Extinction of an island forest avifauna by an introduced snake. Ecology 1987, 68, 660–668. [Google Scholar] [CrossRef]
- Bonnington, C.; Gaston, K.J.; Evans, K.L. Fearing the feline: Domestic cats reduce avian fecundity through trait-mediated indirect effects that increase nest predation by other species. J. Appl. Ecol. 2013, 50, 15–24. [Google Scholar] [CrossRef]
- Ballarini, Y.; Chaves, F.G.; Vecchi, M.B.; Alves, M.A.S. High rates of predation of the nests of two endemic antbirds of the Brazilian Atlantic Forest by invasive marmosets (Callithrix spp.). Ann. Zool. Fenn. 2021, 58, 31–40. [Google Scholar] [CrossRef]
- Dinsmore, S.J.; Gaines, E.P.; Pearson, S.F.; Lauten, D.J.; Castelein, K.A. Factors affecting Snowy Plover chick survival in a managed population. Condor 2017, 119, 34–43. [Google Scholar] [CrossRef][Green Version]
- Maina, G.G.; Jackson, W.M. Effects of fragmentation on artificial nest predation in a tropical forest in Kenya. Biol. Conserv. 2003, 111, 161–169. [Google Scholar] [CrossRef]
- Newmark, W.D.; Stanley, T.R. Habitat fragmentation reduces nest survival in an Afrotropical bird community in a biodiversity hotspot. Proc. Natl. Acad. Sci. USA 2011, 108, 1488–11493. [Google Scholar] [CrossRef] [PubMed]
- Visco, D.M.; Sherry, T.W. Increased abundance, but reduced nest predation in the chestnut-backed antbird in Costa Rican rainforest fragments: Surprising impacts of a pervasive snake species. Biol. Conserv. 2015, 188, 22–31. [Google Scholar] [CrossRef]
- Pontes, A.R.M.; Beltrão, A.C.M.; Normande, I.C.; Malta, A.D.J.R.; Silva Júnior, A.P.D.; Santos, A.M.M. Mass extinction and the disappearance of unknown mammal species: Scenario and perspectives of a biodiversity hotspot’s hotspot. PLoS ONE 2016, 11, e0150887. [Google Scholar]
- Dias, T.C.; Silveira, L.F.; Francisco, M.R. Spatiotemporal dynamics reveals forest rejuvenation, fragmentation, and edge effects in an Atlantic Forest hotspot, the Pernambuco Endemism Center, northeastern Brazil. PLoS ONE 2023, 18, e0291234. [Google Scholar] [CrossRef]
- Pereira, G.A.; Dantas, S.M.; Silveira, L.F.; Roda, A.S.; Albano, C.; Sonntag, F.A.; Leal, S.; Periquito, M.C.; Malacco, G.B.; Lees, A.C. Status of the globally threatened forest birds of northeast Brazil. Pap. Avulsos Zool. 2014, 54, 177–194. [Google Scholar] [CrossRef]
- Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume III—Aves; ICMBio/MMA: Brasília, Brazil, 2018.
- Dickens, J.K.; Bitton, P.P.; Bravo, A.; Silveira, L.F. Species limits, patterns of secondary contact and a new species in the Trogon rufus complex (Aves: Trogonidae). Zool. J. Linn. Soc. 2021, 193, 499–540. [Google Scholar] [CrossRef]
- Lima, R.D.; Silveira, L.F.; Lemos, R.C.A.; Lobo-Araujo, L.W.; Andrade, A.B.; Francisco, M.R.; Efe, M.A. An annotated avian inventory of the Brazilian state of Alagoas, one of the world’s most threatened avifauna. Pap. Avulsos Zool. 2022, 62, e202262034. [Google Scholar] [CrossRef]
- Develey, P.F.; Phalan, B. Bird Extinctions in Brazil’s Atlantic Forest and How They Can Be Prevented. Front. Ecol. Evol. 2021, 9, e624587. [Google Scholar] [CrossRef]
- Mayfield, H. Nesting success calculated from exposure. Wilson Bull. 1961, 73, 255–261. [Google Scholar]
- Roda, S.A.; Santos, A.M.M. Avaliação de Fragmentos Florestais Para uma Possível Reintrodução do Mutum-de-Alagoas em seu Ambiente natural; Centro de Pesquisas Ambientais do Nordeste CEPAN: Recife, Brazil, 2005. [Google Scholar]
- Pereira, G.A.; Araújo, H.F.P.; Azevedo-Júnior, S.M. Distribution and conservation of three important bird groups of the Atlantic Forest in north-east Brazil. Braz. J. Biol. 2016, 76, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.H.C.; Araújo-Filho, J.C.; Silva, A.B.; Santiago, G.A.C.F. Climatologia do Estado de Alagoas; Boletín de Pesquisa e Desenvolvimento: Embrapa, Brasil, 2012. [Google Scholar]
- Studer, A.; Sousa, M.C.; Barcena-Goyena, B. The breeding biology and nest success of the Short-tailed Antthrush Chamaeza campanisona (Aves: Formicariidae) in the Atlantic rainforest of northeastern Brazil. Zoologia 2018, 35, e12906. [Google Scholar] [CrossRef]
- Studer, A.; Sousa, M.C.; Barcena-Goyena, B. Breeding biology and nesting success of the endemic Black-cheeked Gnateater (Conopophaga melanops). Stud. Neotrop. Fauna Environ. 2019, 54, 157–162. [Google Scholar] [CrossRef]
- Martin, T.E.; Geupel, G.R. Nest-monitoring plots: Methods for locating nests and monitoring success. J. Field Ornithol. 1993, 64, 507–519. [Google Scholar]
- Ribeiro-Silva, L.; Perrella, D.F.; Biagolini, C.H., Jr.; Zima, P.V.Q.; Piratelli, A.J.; Schlindwein, M.N.; Galetti Junior, P.M.; Francisco, M.R. Testing camera traps as a potential tool for detecting nest predation of birds in a tropical rainforest environment. Zoologia 2018, 35, e14678. [Google Scholar] [CrossRef]
- Johnson, D.H. Estimating nest success: The Mayfield method and an alternative. Auk 1979, 96, 651–661. [Google Scholar]
- Zimmer, K.; Isler, M.L. Plain Antvireo (Dysithamnus mentalis). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Snow, D. Blue-backed Manakin (Chiroxiphia pareola). In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Koloff, J.; Mennill, D. Barred Antshrike (Thamnophilus doliatus). In Birds of the World; Schulenberg, T.S., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Costa, H.C.; Guedes, T.B.; Bérnils, R.S. Lista de répteis do Brasil: Padrões e tendências. Herpetol. Bras. 2021, 10, 109–279. [Google Scholar] [CrossRef]
- Pacheco, J.F.; Silveira, L.F.; Aleixo, A.; Agne, C.E.; Bencke, G.A.; Bravo, G.A.; Brito, G.R.R.; Cohn-Haft, M.; Maurício, G.N.; Naka, L.N.; et al. Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee—Second edition. Ornithol. Res. 2021, 29, 94–105. [Google Scholar] [CrossRef]
- Quintela, F.M.; Rosa, C.A.; Feijó, A. Updated and annotated checklist of recent mammals from Brazil. An. Acad. Bras. Cienc. 2020, 92, e20191004. [Google Scholar] [CrossRef] [PubMed]
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Pitman, R.L.; Mares, R.; Powell, G. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- Arévalo-Sandi, A.R.; Gonçalves, A.L.S.; Onizawa, K.; Yabe, T.; Spironello, W.R. Mammal diversity among vertical strata and the evaluation of a survey technique in a central Amazonian forest. Pap. Avulsos Zool. 2021, 61, e20216133. [Google Scholar] [CrossRef]
- Morales-Martinez, D.M.; Atuesta-Dimiani, N.; Martínez-Medina, D.; Gutiérrez-Sanabria, D.R.; Rodríguez-Posada, M.E. Completeness of rapid assessments of medium and large mammal diversity in the northwestern Amazon in Colombia. Acta Amazon. 2021, 51, 224–233. [Google Scholar] [CrossRef]
- Burnham, K.P.; Overton, W.S. Robust estimation of population size when capture probabilities vary among animals. Ecology 1979, 60, 927–936. [Google Scholar] [CrossRef]
- Palmer, M.W. Estimating species richness: The second-order Jackknife reconsidered. Ecology 1991, 72, 1512–1513. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 December 2023).
- Rosa, D.C.P.; Brocardo, C.R.; Rosa, C.; Castro, A.B.; Norris, D.; Fadini, R. Species-rich but defaunated: The case of medium and large-bodied mammals in a sustainable use protected area in the Amazon. Acta Amazon. 2021, 51, 323–333. [Google Scholar] [CrossRef]
- Ponce-Martins, M.; Lopes, C.K.M.; Carvalho-Jr, E.A.B.; Castro, F.M.R.; Paulac, M.J.; Pezzuti, J.C.B. Assessing the contribution of local experts in monitoring Neotropical vertebrates with camera traps, linear transects and track and sign surveys in the Amazon. Perspect. Ecol. Conserv. 2022, 20, 303–313. [Google Scholar] [CrossRef]
- Lim, S.J.; Han, S.H.; Kim, K.Y.; Hong, S.; Park, Y.C. Relative abundance of mammals and estimation of minimum trapping effort using camera traps in Jangsudae, Seoraksan National Park. Mamm. Study 2023, 48, 171–179. [Google Scholar] [CrossRef]
- Develey, P.F.; Goerck, J.M. Important Bird Areas Americas—Priority Sites for Biodiversity Conservation; BirdLife Conservation Series No. 16; BirdLife International: Cambridge, UK, 2009. [Google Scholar]
- Marini, M.A. Nesting success of birds from Atlantic Forest fragments. Rev. Bras. Ornitol. 2017, 25, 77–83. [Google Scholar] [CrossRef]
- Zima, P.V.Q.; Perrella, D.F.; Biagolini, C.H., Jr.; Ribeiro-Silva, L.; Francisco, M.R. Breeding behavior of the Atlantic Forest endemic Blue Manakin (Chiroxiphia caudata). Wilson J. Ornithol. 2017, 129, 53–61. [Google Scholar] [CrossRef]
- Lima, A.M.X.; Roper, J.J. Population dynamics of the black-cheeked gnateater (Conopophaga melanops, Conopophagidae) in southern Brazil. J. Trop. Ecol. 2009, 25, 605–613. [Google Scholar] [CrossRef]
- Studer, A.; Sousa, M.C.; Barcena-Goyena, B. Reproduction and nest success of the Scalloped Antbird, Myrmoderus ruficauda (Passeriformes: Thamnophilidae), in an Atlantic rainforest of northeastern Brazil. Atual. Ornitol. 2017; 199, 33–37. [Google Scholar]
- Dinsmore, S.J.; White, G.C.; Knopf, F.L. Advanced techniques for modeling avian nest survival. Ecology 2002, 83, 3476–3488. [Google Scholar] [CrossRef]
- Coelho, C.G.; Falótico, T.; Izar, P.; Mannu, M.; Resende, B.D.; Siqueira, J.O.; Ottoni, E.B. Social learning strategies for nut-cracking by tufted capuchin monkeys (Sapajus spp.). Anim. Cogn. 2015, 18, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Zima, P.V.Q.; Perrella, D.F.; Francisco, M.R. The influence of egg presence and eggshell colour in the attraction of visually oriented predators to nests of a tropical forest bird. Ibis 2021, 163, 1080–1086. [Google Scholar] [CrossRef]
- Cockle, K.L.; Bodrati, A.; Lammertink, M.; Bonaparte, E.B.; Ferreyra, C.; Di Sallo, F.G. Predators of bird nests in the Atlantic Forest of Argentina and Paraguay. Wilson J. Ornithol. 2016, 128, 120–131. [Google Scholar] [CrossRef]
- Londoño, G.A.; Gomez, J.P.; Sánchez-Martínez, M.A.; Levey, D.J.; Robinson, S.K. Changing patterns of nest predation and predator communities along a tropical elevation gradient. Ecol. Lett. 2022, 26, 609–620. [Google Scholar] [CrossRef]
- Duron, Q.; Bourguet, E.; Meringgo, H.D.; Millon, A.; Vidal, E. Invasive rats strengthen predation pressure on bird eggs in a South Pacific island rainforest. Curr. Zool. 2017, 63, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Vanderwerf, E.A.; Smith, D.G. Effects of alien rodent control on demography of the O’ahu ‘Elepaio, an endangered Hawaiian forest bird. Pac. Conserv. Biol. 2002, 8, 73–81. [Google Scholar] [CrossRef]
- Jansen, W.P. Rat Rattus control at nests of the endangered kakapo Strigops habroptilus on Codfish Island, New Zealand. Conserv. Evid. 2005, 2, 1–2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).