1. Introduction
The study of the ecology of birds of prey provides a variety of information about ecosystems because of their critical role in the functioning of ecosystems [
1]. Birds of prey may play different roles in ecosystems, such as flag-ships, surrogates, and umbrella species, as well as regulating prey populations and scavenging [
2,
3]. Due to their dependence on their prey, birds of prey may be considered as biodiversity indicators of their prey assemblage and overall biodiversity [
4,
5].
The large spatial requirements and specific environmental needs of birds of prey affect their low densities. Thus, the density of raptor populations depends mainly on the availability of good-quality habitats [
6]. The identification of the habitat preferences of rare species of birds of prey is essential for their adequate protection, especially in changing climate and landscape conditions. Although many European raptors nest in forests, they obtain most of their food in open areas, so they belong to dual-habitat species [
2]. Habitat protection should include breeding sites, as well as feeding areas, on the landscape scale. The relationship between habitat quality and diet composition is essential to understand the distribution and density of breeding pairs. To a great extent, nesting habitat parameters determine the diet composition of raptors during the breeding season [
6]. Birds are sensitive to limited food availability; therefore, food resources are one of the most critical factors to address in conservation research [
7].
The diet composition of birds of prey shows variability depending on specific habitat conditions and changes in primary prey availability. This variability is more pronounced in food generalists than in specialists. Food resources directly affect the fitness and survival of adults, breeding success, and the number of young raised, as well as their fitness [
8]. The study of feeding ecology shows how habitat and foraging patterns affect food composition and how the choice of nesting site affects where to find food [
9,
10]. On the other hand, the study of the raptor diet can explain how a predator’s exploitation of particular prey species affects their population dynamics. This is especially important for rare and endangered species of potential prey [
11].
The Golden Eagle
Aquila chrysaetos is one of the biggest European bird of prey species. It is a widely distributed raptor whose range covers the southern and northern parts of Europe and northern Africa, as well as a large part of Asia and North America. The European population has been strongly reduced during the last two centuries due to human persecution [
12,
13]. The GE is regarded as a habitat generalist inhabiting mountainous and lowland areas with a low stand density [
14]. It uses diverse environments: mountainous and upland areas, lowland forests, wetlands, and marginally even steppe and desert. It prefers inaccessible, low-density human population areas. This species nests on cliffs and trees [
12,
13]. In Poland, the GE inhabits only a limited area of the mountains. It selects vast forest areas, the highlands, and mountains with enormous meadows and river valleys. It establishes nests on rock shelves in high mountains and feeds above the upper forest border. In the lowlands, it lives in swamps and wetlands. Essential elements of the environment are dead-standing trees used for resting and perching [
15].
The GE is considered to be a top predator, mainly preying on medium-sized vertebrates and also feeding on carrion [
14]. The basis of its food is birds and mammals, and seldom reptilians, consumed in varying proportions. Numerous studies of food composition have documented the GE’s strong ecological plasticity and ability to successfully prey on a variety of species, usually at a biomass from 0.5 to 3 kg, e.g., [
16,
17,
18,
19,
20,
21]. Diet composition shows a high variability depending on the availability of suitable prey in local conditions. Usually, this raptor opportunistically preys on the most available game of the proper size in its feeding area [
12,
22].
The European population of the GE is estimated at 19,200–25,600 adult individuals with a status of Least Concern and increasing population trend [
23]. The largest populations of the GE inhabit Scandinavia, Russia, Spain, Italy, France, and Great Britain. Populations in central Europe are small, usually a few dozen pairs. The GE is listed in Annex I to the Directive 2009/147/EC of the European Parliament and the Council of 30 November 2009, on the conservation of wild birds. In Poland, the GE is a red-listed species with category EN [
24,
25], and is strictly protected. Its nests are protected by creation zones where entry and habitat alteration are prohibited. Almost the entire GE population occurs in the Polish part of the Carpathians, as a part of the cross-border population [
26]. Since 1993, the occupied nests or breeding territories of the GE have been monitored by members of the Eagle Conservation Committee [
27]. During the last 30 years, the Polish population was estimated at 30 breeding pairs, with some fluctuations. The previous two decades have seen a slight increase in numbers and expansion of range toward the west [
28,
29,
30].
Our work aimed to examine the relationship between the habitat preference and diet composition of the GE in the Polish Carpathians over 24 years. We hypothesized that the GE’s food composition would vary over its geographic range of occurrence. We assumed that the share of the main food groups, i.e., birds and mammals, would show no geographic variation. We expected that the proportion of raptors in the GE’s diet, whose higher densities are outside the mountains, would increase as latitude and altitude increased. The second hypothesis relates to habitat requirements and assumes that the GE, as a secretive and persecuted species in the past, chooses nesting sites tucked away deep in the forest, away from human settlements. We expected the results to allow us to formulate recommendations for protecting the GE in Poland.
2. Study Area
The study covered selected areas of the Polish Carpathians, with a total area of about 8860 km
2 lying in the following subprovinces: (i) Outer Western Carpathians (Beskid Niski, Beskid Sądecki, Gorce, Beskid Wyspowy, and Beskid Żywiecki), (ii) Central Carpathians Western Carpathians (Tatra Mountains, Pieniny, Spisko-Gubałówka Foothills, and Orava-Novotarska Basin), and (iii) Eastern Carpathians (Western Bieszczady and Sanocko-Turczańskie Mountains). The Carpathian Mountains are characterized by high forest cover (33–80%). This is dominated by the Norway Spruce
Picea abies, Beech
Fagus sylvaticus, and Silver Fir
Abies alba, which, together, form nearly 90% of forest stands. The Outer Western Carpathians are not very high mountains, with a few peaks exceeding 1000 m, while the central Western Carpathians have peaks reaching almost 2500 m above sea level [
31]. The Eastern Carpathians within the Polish borders are characterized by extensive complexes of forests, meadows, and pastures of former state farms, and at 1200 m above sea level, there is a floor of mountain meadows. Human density here is low—4–5 people/km
2. In the Polish Carpathians, only the Tatra Mts have a storied vegetation system [
29].
3. Methods
3.1. The Distribution of Nesting Territories and Nesting Trees
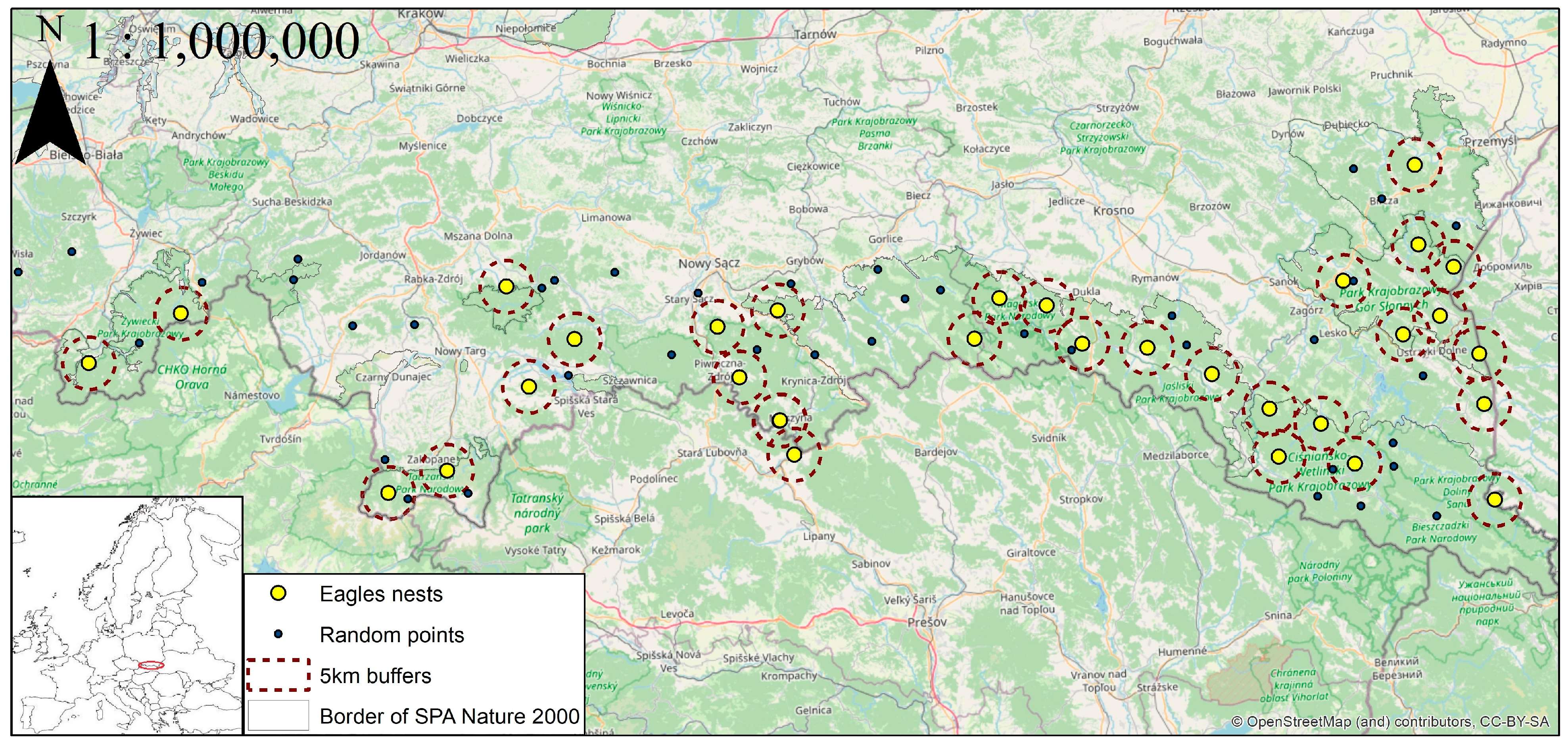
From 2000 to 2023, the field study was based on long-term monitoring of the GE’s breeding sites. During inspections in early spring (from 15.02 to 30.04), the occupation of known nests or territories was assessed. In addition, during peak mating activity, observations of the GE’s mating behavior from viewpoints were made. Field works were conducted on sunny days between 9 a.m. and 1 p.m. from points 3–4 km apart for 2 h. Then, on this basis, new nests were searched for. Inspections were conducted throughout the entire known range of the GE in Poland, considering places where only single individuals were encountered and at the area’s periphery, considering the potential for range expansion [
30]. The species of the nest tree was identified and its age was determined from Poland’s Forest Data Bank [
32].
3.2. The Structure and Size of Territory
The exact size and shape of the foraging area used by the eagles are not known in the study area, and the area used by the GE during breeding season shows substantial variation depending on the habitat structure and food abundance, as well as the assessment methods. For analysis, we assumed [
10] that the size of the territory was determined by 1/2 of the nearest neighbor distance (NND) between occupied nests, which, in our study, was 10.4 km. In order to assess the environmental structure of the potential territory, we evaluated the surface proportion of distinguished habitats in a 5 km buffer. We also measured the distance from the nest to the nearest forest edge, village, and river. Lastly, the length of rivers and hardened roads inside a 5 km buffer and the number of settlements were measured. Environmental data were taken from Poland’s Forest Data Bank, run by the State Forests [
32]. We used the Random point function in ArcMap to create 40 random points in the study area. We measured all habitat characteristics described above for the random points to compare the GEs’ habitat requirements with mean values in this part of Poland.
3.3. Dietary Data Collection and Prey Identification
Food remains and pellets were collected from the nests and the ground under the nests during inspections, combined with the ringing of chicks carried out in June or July. In total, food data came from 28 different GE territories. The guides [
33,
34,
35,
36] were used to identify some of the prey species remains. The number of prey was determined based on skeletal elements or feathers by identifying the individual prey. If the bones, feathers, or hair found belonged to one species and did not differ in size or degree of decomposition, it was assumed that they belonged to only one individual [
20]. About 80% of the identified prey were collected as remains from the nests (bones, beaks, furs, and feathers). The analysis of pellets provided information on small- and medium-sized birds, mainly pigeons based on rings and Passerifomes based on feathers. The hair and furs contained in the spits were not determined and were not included in the analyses. In the case of remains found in both pellets and food remains, the double counting of food items was avoided by assuming the lowest probable number of the eaten individuals. The minimum number of individuals (MNI) at each collection’s lowest possible taxonomic level was calculated from distinctive anatomical features by taking the minimum number derived from each source and combining them. If we found, e.g., the bill and feathers of the Ural Owl
Strix uralensis, we counted them as only one prey [
21]. For the identified prey species, the habitat in which the GE hunted them was assigned. Distinctions were made between forest species (F), open-area species (O), and anthropogenic species (A). Species were assigned to specific habitats based on characteristics in Polish regional monographs [
27,
33,
37]. The biomass of prey was not estimated; only its frequency was analyzed.
3.4. Statistical Analysis
To check how the GE diet changed across the area of this species range in Poland, we used a generalized linear model (GLM), using data for each nest, for which sufficient numbers of food items were collected. In the GLM models, we used latitude or longitude as the dependent variable and data about the share of most frequent prey in single nests in seasons as independent variables. The shares of the six most frequent bird food categories and five most frequent mammal categories of the GEs’ diet, each with a share above 2.5%, were used as independent variables. Only this threshold ensured the repeatability of food items across seasons in single nests. Nests with less than fifteen prey collected were deleted from detailed analyses (4 cases). After the preliminary data analysis, we decided to use as independent variables the proportions of Pigeons Colubmbidae, Corvids Corvidae, Buzzard Buteo buteo, Owls Strigiformes, Grouse Galliformes, Passerines, Roe Deer Capreolus capreolus, Hare Lepus europaeus, Red Fox Canis vulpes, and Martens Martes sp. Other predatory mammals were also used. The unit was a single nest from which at least 15 prey items were identified. The post hoc t-test was used to check the statistical significance of the analyzed parameters.
We used a logistic regression model (GLM) to compare nesting habitats and drew random points in the surrounding habitat. A layer including forests and mountains was created to draw potential nest points. We rejected water areas, villages, towns, and agricultural areas as habitats impossible for GE nesting. Nest or random points were used as a dependent variable. The data on non-forest area, distance to open area, distance to villages, distance to river, the length of rivers, the length of roads, and the number of villages were used as independent variables. The post hoc z-test was conducted in this model. Statistical analyses were performed in the R environment (ver. 4.1.3 with the R-studio overlay).