First Report on Three Lesser-Known Magelona Species from Korean Waters: Details of All Thoracic Chaetigers and Methyl Green Staining Patterns
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Systematics
3.2. Magelona sachalinensis Buzhinskaja, 1985 [19]
3.2.1. Material Examined
3.2.2. Species Diagnosis
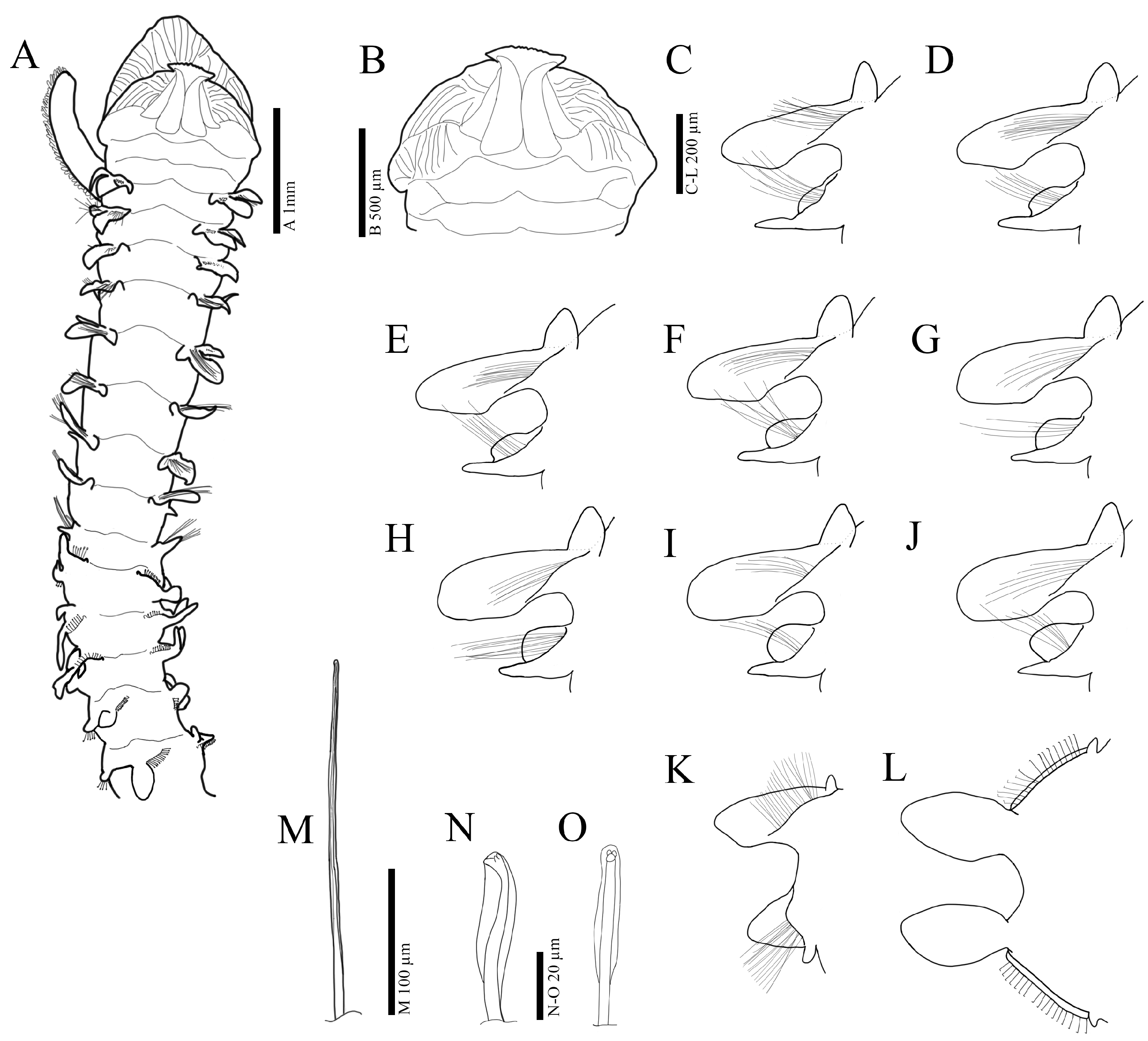
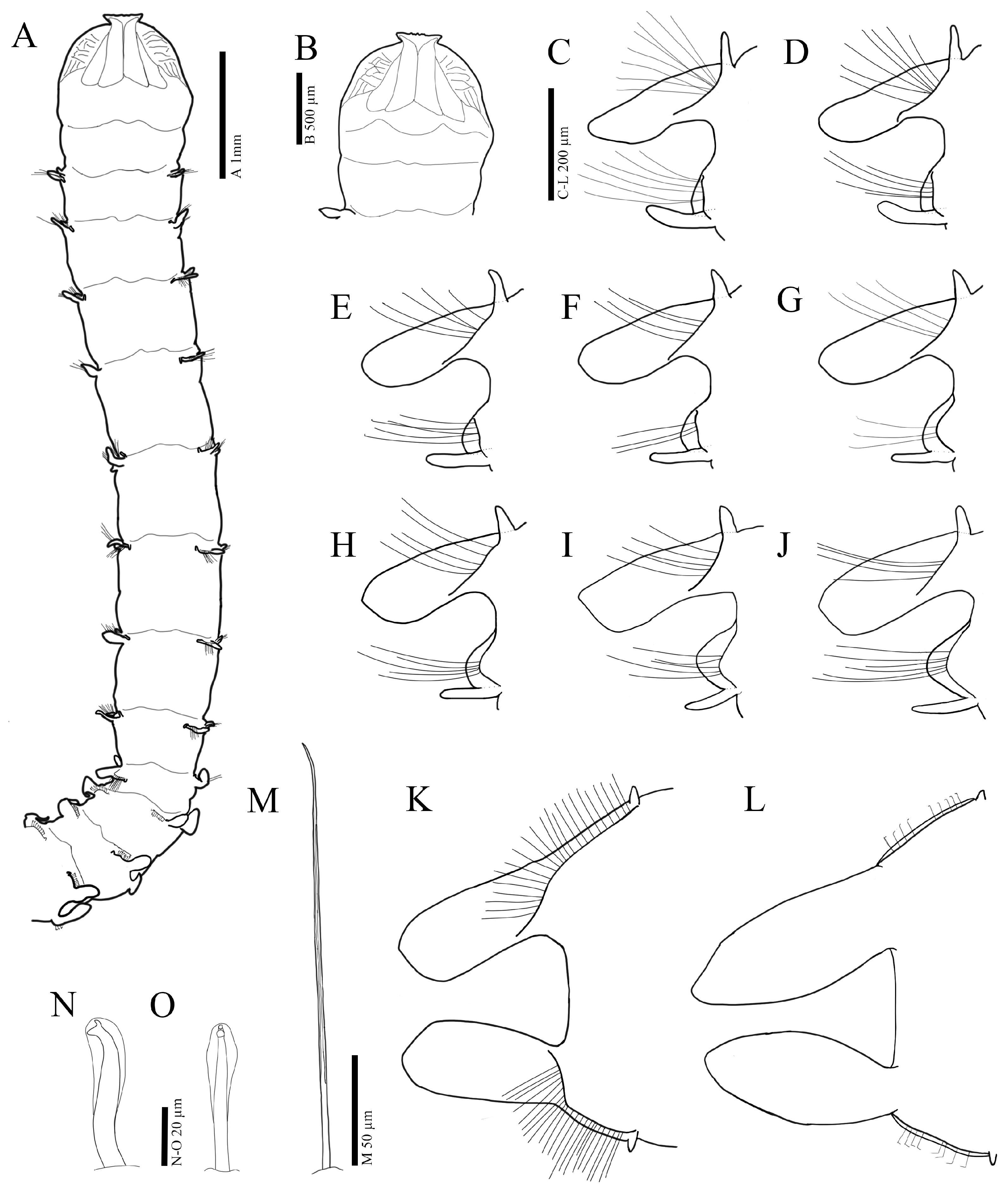
3.2.3. Description
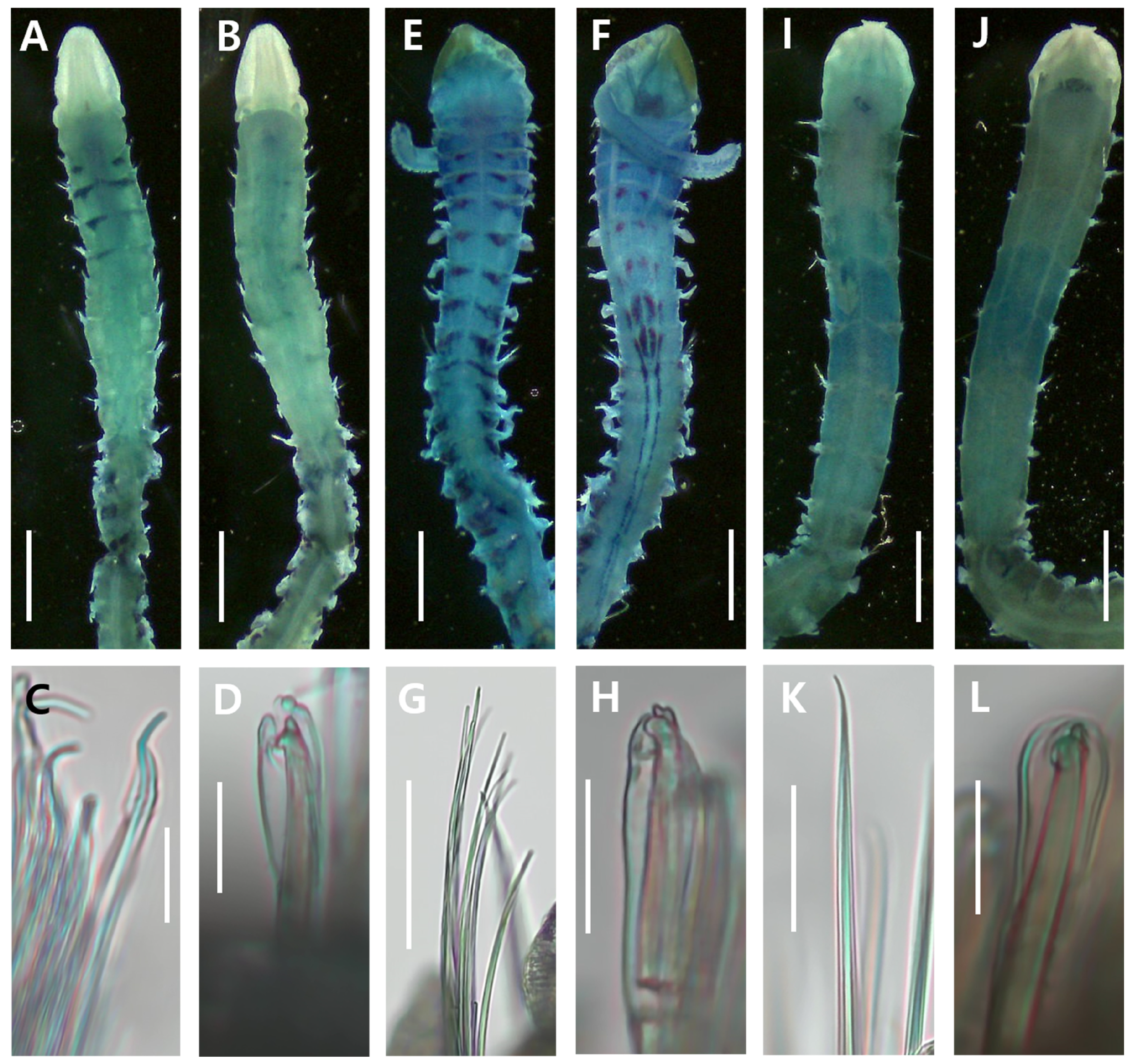
3.2.4. Methyl Green Staining Patterns
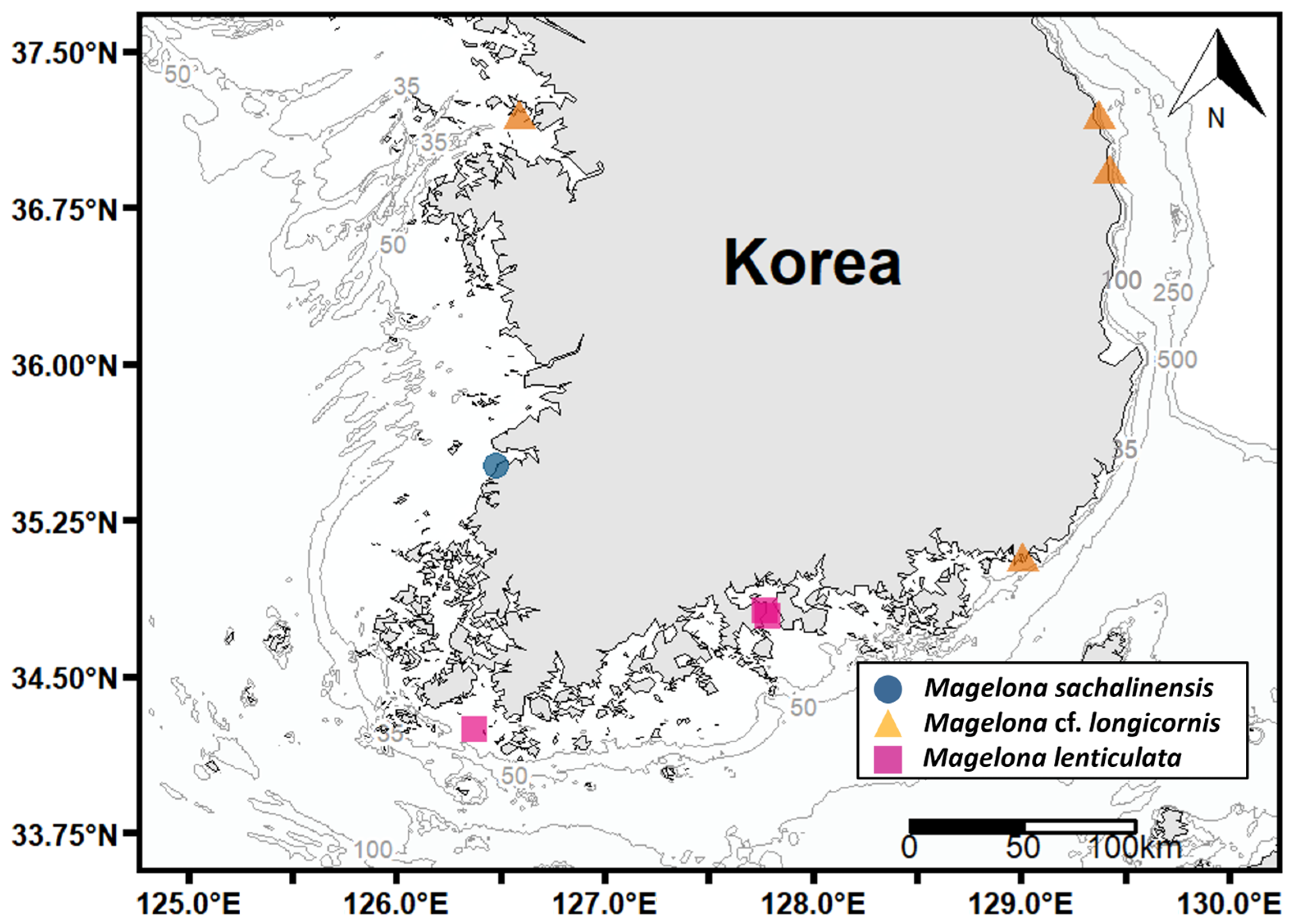
3.2.5. Distribution and Ecology
3.2.6. Remarks
3.3. Magelona lenticulata Gallardo, 1968 [11]
3.3.1. Material Examined
3.3.2. Species Diagnosis
3.3.3. Description
3.3.4. Methyl Green Staining Patterns
3.3.5. Distribution and Ecology
3.3.6. Remarks
3.4. Magelona cf. longicornis Johnson, 1901 [22]
3.4.1. Material Examined
3.4.2. Species Diagnosis
3.4.3. Description
3.4.4. Methyl Green Staining Patterns
3.4.5. Distribution and Ecology
3.4.6. Remarks
4. Discussion
| Key to North-Western Pacific species of Magelona (modified from Mortimer et al. [29]) | |
| 1. Prostomium with distinct prostomial horns | 2 |
| - Prostomium without prostomial horns or with rudimentary horns | 11 |
| 2. Prostomial anterior margin appearing smooth | 3 |
| - Prostomial anterior margin appearing crenulate | 5 |
| 3. Dark purple pigmented band located between chaetigers 5–8 | M. japonica |
| - No distinct pigmentation of the thoracic region | 4 |
| 4. Prostomium wider than long, thoracic chaetigers without superior dorsal lobes | M. koreana |
| - Prostomium longer than wide, thoracic chaetigers 1–8 with superior dorsal lobes | M. boninensis |
| 5. Prostomium width approximately similar to length | 6 |
| - Prostomium wider than long | 7 |
| 6. Abdominal hooded hooks bidentate | M. crenulifrons |
| - Abdominal hooded hooks tridentate | M. cf. cornuta |
| 7. Superior dorsal lobes present in the thorax | 8 |
| - No superior dorsal lobes present in thorax; prostomial anterior margin only minutely crenulate | M. petersenae |
| 8. Abdominal hooded hooks bidentate | 9 |
| - Abdominal hooded hooks tridentate | M. lenticulata |
| 9. Small superior dorsal lobes present on chaetiger 9, parapodial postchaetal lamellae of chaetiger 9 rectangle | M. cf. longicornis |
| - No superior dorsal lobes present on chaetiger 9 | M. methae |
| 10. Chaetiger 9 with special chaetae | 11 |
| - Chaetiger 9 bearing capillary chaetae only, as in chaetigers 1–8 | 14 |
| 11. Abdominal hooded hooks tridentate | 12 |
| - Abdominal hooded hooks bidentate | M. sachalinensis |
| 12. Anterior thoracic notopodial lamellae with smooth or lightly crenulated upper edges | 13 |
| - Anterior thoracic notopodial lamellae with pectinate upper edges | M. pectinata |
| 13. Abdominal parapodia without triangular processes at the inner margin of chaetal rows | M. parochilis |
| - Notopodial lamellae of chaetiger 8 distinctly bilobed, abdominal parapodia with triangular processes at the inner margin of chaetal rows | M. tinae |
| 14. Abdominal hooded hooks polydentate | M. agoensis |
| - Abdominal hooded hooks bi- or tridentate | 15 |
| 15. Thoracic notopodia with superior dorsal lobes | 16 |
| - Thoracic notopodia without superior dorsal lobes | 19 |
| 16. Prostomium wider than long | 17 |
| - Prostomium longer than wide | 18 |
| 17. Thoracic notopodial lamellae slender, no superior dorsal lobe on chaetiger 9 | M. kamala |
| - Stout species, with distinct armor-like appearance, thoracic lamellae foliaceous, superior dorsal lobe on chaetiger 9 present | M. armatis |
| 18. Prostomium only marginally longer than wide, anterior margin squared | M. noppi |
| - Prostomium length greater than width, rudimentary horns with a triangular anterior margin | M. alba |
| 19. Anterior thoracic neuropodial lamellae distinctly scoop-shaped | M. cf. cincta |
| - Anterior thoracic neuropodial lamellae slender triangular | 20 |
| 20. Moderate to large species with rudimentary horns, broad palps with several rows of papillae | M. mickminni |
| - Prostomial margin rounded to straight, minute species with slender palps carrying two rows of papillae | M. pygmaea |
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, J.; Ramage, G. The Polychaeta Sedentaria of the Firth of Forth. Earth Environ. Sci. Trans. R. Soc. Edinb. 1888, 33, 635–684. [Google Scholar] [CrossRef]
- Parapar, J.; Mortimer, K.; Capa, M.; Moreira, J. On the systematics and biodiversity of the Palaeoannelida. Diversity 2021, 13, 41. [Google Scholar] [CrossRef]
- Rouse, G.; Pleijel, F.; Tilic, E. Annelida; Oxford University Press: Oxford, UK, 2022; p. 432. [Google Scholar]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 173–284. [Google Scholar]
- Kükenthal, W. Annelida Basal Groups and Pleistoannelida, Sedentaria I. Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2019; Volume 1, p. 480. [Google Scholar]
- Meißner, K.; Alexander, D. Distribution of Magelona species (Polychaeta: Magelonidae) in the German Bight (North Sea): A modeling approach. Zoosymposia 2009, 2, 567–586. [Google Scholar] [CrossRef]
- Borja, A.; Franco, J.; Pérez, V. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar. Pollut. Bull. 2000, 40, 1100–1114. [Google Scholar] [CrossRef]
- Hiscock, K.; Langmead, O.; Warwick, R.; Smith, A. Identification of Seabed Indicator Species to Support Implementation of the EU Habitats and Water Framework Directives; Report to the Joint Nature Conservation Committee and the Environment Agency from the Marine Biological Association; MarLIN: Plymouth, UK, 2005; pp. 1–77. [Google Scholar]
- Taylor, A.; Mortimer, K.; Jimi, N. Unearthing the diversity of Japanese Magelona (Annelida: Magelonidae); three species new to science, and a redescription of Magelona japonica. Zootaxa 2022, 5196, 451–491. [Google Scholar] [CrossRef] [PubMed]
- Nateewathana, A.; Hylleberg, J. Magelonid polychaetes from Thailand, the Andaman Sea, with descriptions of eight new species. In Systematics, Biology and Morphology of World Polychaeta; Brill: Leiden, The Netherlands, 1991; pp. 169–184. [Google Scholar]
- Gallardo, V.A. Polychaeta from the Bay of Nhatrang, South Vietnam. Naga Rep. 1967, 4, 101–102. [Google Scholar]
- Müller, F. Einiges über die Annelidenfauna der Insel Santa Catharina an der brasilianischen Küste. Arch. Naturgeschichte 1858, 24, 211–220. [Google Scholar]
- Mortimer, K.; Fitzhugh, K.; Dos Brasil, A.C.; Lana, P. Who’s who in Magelona: Phylogenetic hypotheses under Magelonidae Cunningham & Ramage, 1888 (Annelida: Polychaeta). PeerJ 2021, 9, e11993. [Google Scholar]
- Brasil, A.d.S. Filogenia de Magelonidae Cunningham & Ramage, 1888 (Annelida–Polychaeta) com base na morfologia externa. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2003. [Google Scholar]
- Okuda, S. Spioniform polychaetes from Japan. J. Fac. Sci. Hokkaido Imp. Univ. 1937, 5, 217–254. [Google Scholar]
- Zhou, J.; Mortimer, K. A new species of Magelona (Polychaeta: Magelonidae) from Chinese coastal waters. J. Mar. Biol. Assoc. UK 2013, 93, 1503–1510. [Google Scholar] [CrossRef]
- Lee, H.-E.; Lee, G.H.; Min, G.-S. First Record of Magelona parochilis (Annelida: Magelonidae) in South Korea. Anim. Syst. Evol. Divers. 2022, 38, 149–156. [Google Scholar]
- Paik, E.I. Illustrated Encyclopedia of Fauna and Flora of Korea; Ministry of Education: Seoul, Republic of Korea, 1989; p. 764.
- Buzhinskaja, G. Polychaeta of the shelf off south Sakhalin and their ecology. Issled. Fauny Morei 1985, 30, 72–224. [Google Scholar]
- Müller, J.; Bartolomaeus, T. Chaetal arrangement and type diversity in two Magelona species (Magelonidae, Annelida) with ultrastructural details of the internal support chaetae. J. Morphol. 2022, 283, 852–866. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.L. Three new species of Magelona (Annelida, Polychaeta) and a redescription of Magelona pitelkai Hartman. Proc. Biol. Soc. Wash. 1978, 91, 336–363. [Google Scholar]
- Johnson, H.P. The polychaeta of the Puget Sound region. Proc. Boston Soc. Nat. Hist. 1901, 29, 381–437. [Google Scholar]
- Jones, M.L. Magelona berkeleyi n. sp. from Puget Sound (Annelida: Polychaeta), with a further redescription of Magelona longicornis Johnson and a consideration of recently described species of Magelona. J. Fish. Board Can. 1971, 28, 1445–1454. [Google Scholar] [CrossRef]
- Mortimer, K.; Blake, J.A.; Harrendence, K. A review of the Magelonidae (Annelida: Polychaeta) of Northeast America, including a description of a new species and re-descriptions of Magelona riojai and Magelona sacculata. Proc. Biol. Soc. Wash. 2021, 134, 209–242. [Google Scholar] [CrossRef]
- Mortimer, K.; Kongsrud, J.A.; Willassen, E. Integrative taxonomy of West African Magelona (Annelida: Magelonidae): Species with thoracic pigmentation. Zool. J. Linn. Soc. 2022, 194, 1134–1176. [Google Scholar] [CrossRef]
- Kitamori, R. Magelonidae (Polychaetous annelids) from Japan, including the description of a new species. Bull. Tokai Reg. Fish. Res. Lab. 1967, 50, 49–54. [Google Scholar]
- Hartman, O. Deep-Water Benthic Polychaetous Annelids off New England to Bermuda and Other North Atlantic Areas; Occasional Papers of the Allan Hancock Foundation: Los Angeles, CA, USA, 1965; Volume 28, p. 384. [Google Scholar]
- Hartman, O.; Reish, D.J. The marine annelids of Oregon. Or. State Monogr. Stud. Zool. 1950, 6, 29–33. [Google Scholar]
- Mortimer, K.; Mills, K.; Gil, J. Re-evaluation of Rostraria bierii Tokioka, 1970 (Annelida) from Seto, Japan as a magelonid, with a review of the Magelonidae of the western Pacific. Acta Oceanol. Sin. 2022, 41, 61–69. [Google Scholar]

| Prostomial Shape | Prostomial Horn | Prostomial Anterior Margin | SDL of ch 1–8 | SDL/VNL on Chaetiger 9 | Dentition of Hooded Hook | |
|---|---|---|---|---|---|---|
| M. japonica | Wider than long | Present | Smooth | Absent | Both absent | Tridentate |
| M. koreana | Wider than long | Present | Smooth | Absent | VNL present | Tridentate |
| M. parochilis | Longer than wide | Absent | Smooth | Tapering conical | VNL present | Tridentate |
| M. sachalinensis | Longer than wide | Absent | Smooth | Tiny in ch 1–4, cirriform in ch 5–8 | Both present | Bidentate |
| M. cf. longicornis | Wider than long | Present | Crenulate | Tapering conical | Both present | Bidentate |
| M. lenticulata | Wider than long | Present | Crenulate | Triangular, foliaceous | Both present | Tridentate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Kwon, I.-Y.; Soh, H.-Y.; Jeong, M.-K. First Report on Three Lesser-Known Magelona Species from Korean Waters: Details of All Thoracic Chaetigers and Methyl Green Staining Patterns. Diversity 2024, 16, 112. https://doi.org/10.3390/d16020112
Kim D-H, Kwon I-Y, Soh H-Y, Jeong M-K. First Report on Three Lesser-Known Magelona Species from Korean Waters: Details of All Thoracic Chaetigers and Methyl Green Staining Patterns. Diversity. 2024; 16(2):112. https://doi.org/10.3390/d16020112
Chicago/Turabian StyleKim, Dae-Hun, In-Yeong Kwon, Ho-Young Soh, and Man-Ki Jeong. 2024. "First Report on Three Lesser-Known Magelona Species from Korean Waters: Details of All Thoracic Chaetigers and Methyl Green Staining Patterns" Diversity 16, no. 2: 112. https://doi.org/10.3390/d16020112
APA StyleKim, D.-H., Kwon, I.-Y., Soh, H.-Y., & Jeong, M.-K. (2024). First Report on Three Lesser-Known Magelona Species from Korean Waters: Details of All Thoracic Chaetigers and Methyl Green Staining Patterns. Diversity, 16(2), 112. https://doi.org/10.3390/d16020112







