Effects of Hemiparasites in Grassland Restorations Are Not Universal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Field Sites
2.3. Vegetation Surveys
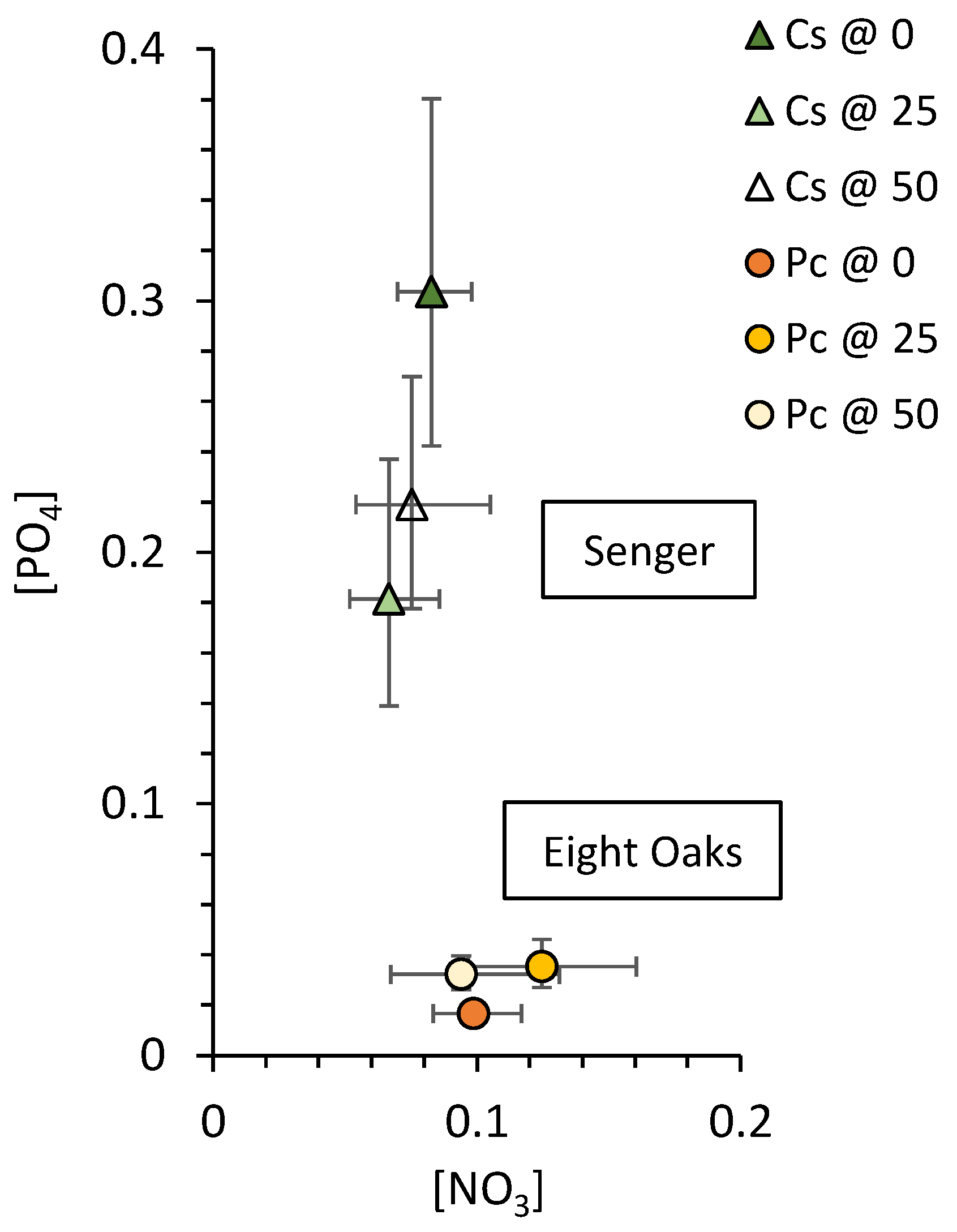
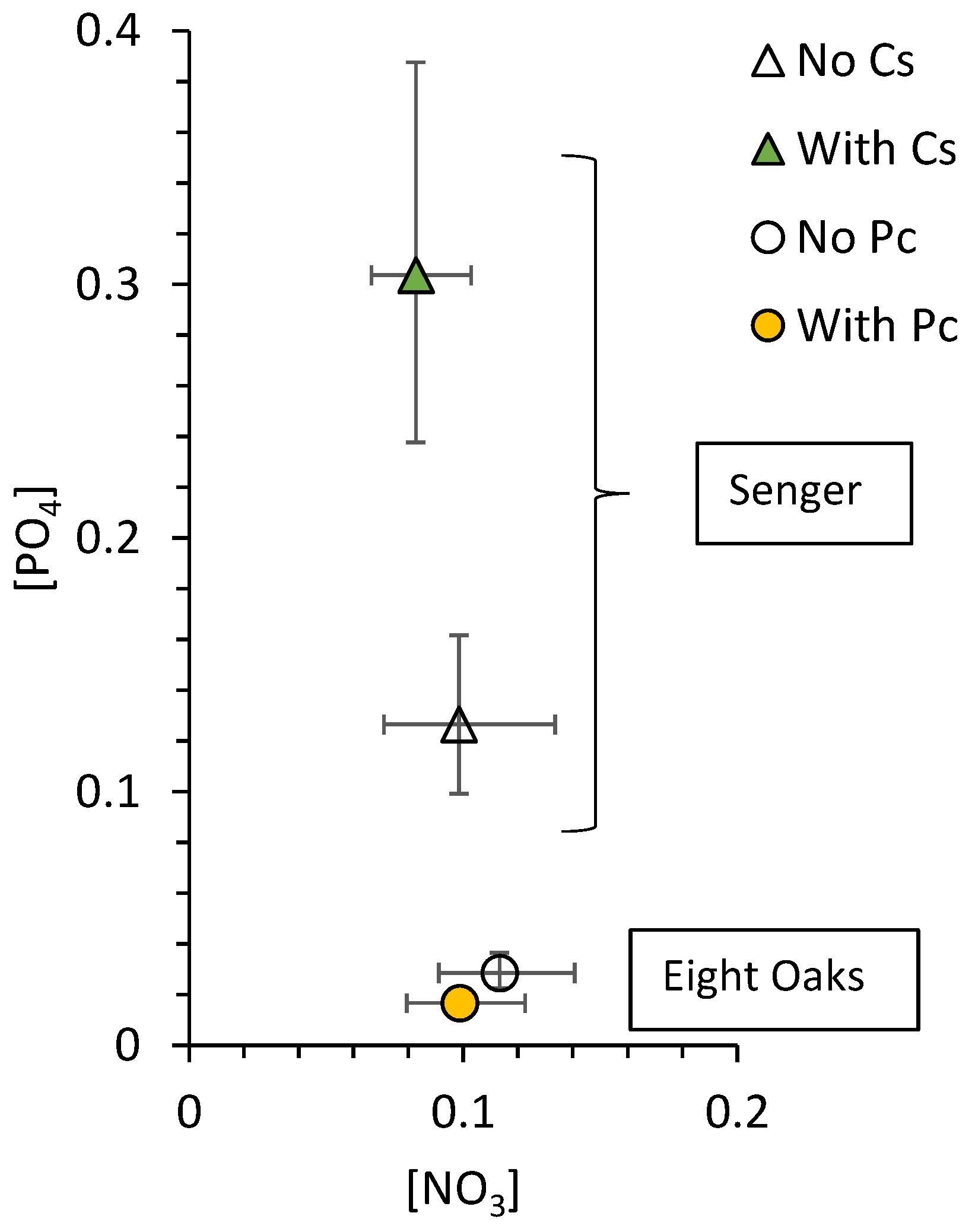
2.4. Soil Nutrients
3. Results
3.1. Vegetation Surveys
3.2. Soil Nutrients
4. Discussion
4.1. Keystone Species Hypothesis
4.2. Ecosystem Engineer Hypothesis
4.3. Prairie Management
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Noss, R.F.; La Roe, E.T., III; Scott, J.M. Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation. Biological Report 28; U.S. Department of Interior, National Biological Service: Washington, DC, USA 1995.
- Samson, F.B.; Knopf, F.; Ostlie, W.R. Great Plains Ecosystems: Past, present, and future. Wildlife Soc. B 2004, 32, 6–15. [Google Scholar] [CrossRef]
- Anderson, R.C. Evolution and origin of the Central Grassland of North America: Climate, fire, and mammalian grazers. J. Torrey Bot. Soc. 2006, 133, 626–647. [Google Scholar] [CrossRef]
- Samson, F.B.; Knopf, F.L. Prairie conservation in North America. BioScience 1994, 44, 418–421. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland ecosystem services: A systematic review of research advances and future directions. Landscape Ecol. 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Fuhlendorf, S.D.; Engle, D.M. Application of the fire-grazing interaction to restore a shifting mosaic on tallgrass prairie. J. Appl. Ecol. 2004, 41, 604–614. [Google Scholar] [CrossRef]
- Towne, E.G.; Hartnett, D.C.; Cochran, R.C. Vegetation trends in tallgrass prairie from bison and cattle grazing. Ecol. Appl. 2005, 15, 1550–1559. [Google Scholar] [CrossRef]
- Hong, P.; Schmid, B.; De Laender, F.; Eisenhauer, N.; Craven, D.; De Boeck, H.J.; Hautier, Y.; Petchey, O.L.; Reich, P.B.; Steudel, B.; et al. Biodiversity promotes ecosystem functioning despite environmental change. Ecol. Lett. 2022, 25, 555–569. [Google Scholar] [CrossRef]
- Cameron, D.D.; Hwangbo, J.; Keith, A.M.; Geniez, J.; Kraushaar, D.; Rowntree, J.; Seel, W.E. Interactions between the hemiparasitic angiosperm Rhinanthus minor and its hosts: From the cell to the ecosystem. Folia Geobot. 2005, 40, 217–229. [Google Scholar] [CrossRef]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Davic, R. Linking keystone species and functional groups: A new operational definition of the keystone species concept. Conserv. Ecol. 2003, 7, r11. [Google Scholar] [CrossRef]
- Bullock, J.M.; Pywell, R.F. Rhinanthus: A tool for restoring diverse grassland? Folia Geobot. 2005, 40, 273–288. [Google Scholar] [CrossRef]
- Decleer, K.; Bonte, D.; Van Diggelen, R. The hemiparasite Pedicularis palustris: ‘Ecosystem engineer’ for fen-meadow restoration. J. Nat. Conserv. 2013, 21, 65–71. [Google Scholar] [CrossRef]
- Bao, G.; Suetsugu, K.; Wang, H.; Yao, X.; Liu, L.; Ou, J.; Li, C. Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res. 2015, 30, 507–515. [Google Scholar] [CrossRef]
- Fibich, P.; Lepš, J.; Chytrý, M.; Těšitel, J. Root hemiparasitic plants are associated with high diversity in temperate grasslands. J. Veg. Sci. 2017, 28, 184–191. [Google Scholar] [CrossRef]
- Tĕšitel, J.; Mládek, J.; Horník, J.; Tĕšitelová, T.; Adamec, V.; Tichý, L. Suppressing competitive dominants and community restoration with native parasitic plants using the hemiparasitic Rhinanthus alectorolophus and the dominant grass Calamagrostis epigejos. J. Appl. Ecol. 2017, 54, 1487–1495. [Google Scholar] [CrossRef]
- Gibson, C.C.; Watkinson, A.R. The role of the hemiparasitic annual Rhinanthus minor in determining grassland community structure. Oecologia 1992, 89, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Chaudron, C.; Mazalová, M.; Kuras, T.; Malenovský, I.; Mládek, J. Introducing ecosystem engineers for grassland biodiversity conservation: A review of the effects of hemiparasitic Rhinanthus species on plant and animal communities at multiple trophic levels. Perspect. Plant Ecol. 2021, 52, 125633. [Google Scholar] [CrossRef]
- Quested, H.M. Parasitic plants—Impacts on nutrient cycling. Plant Soil 2008, 311, 269–272. [Google Scholar] [CrossRef]
- Fisher, J.B.; Phoenix, G.K.; Childs, D.Z.; Press, M.C.; Smith, S.W.; Pilkington, M.G.; Cameron, D.D. Parasitic plant liter input: A novel indirect mechanism influencing plant community structure. New Phytol. 2013, 198, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Smith, R.S.; Shiel, R.S.; Peacock, S.; Simkin, J.M.; Quirk, H.; Hobbs, P.J. Parasitic plants indirectly regulate below-ground properties in grassland communities. Nature 2006, 439, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Quested, H.M.; Press, M.C.; Callaghan, T.V.; Cornelissen, J.H.C. The hemiparasitic angiosperm Bartsia alpina has the potential to accelerate decomposition in sub-arctic communities. Oecologia 2002, 130, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Quested, H.M.; Press, M.C.; Callaghan, T.V. Litter of the hemiparasite Bartsia alpina enhances plant growth: Evidence for a functional role in nutrient cycling. Oecologia 2003, 135, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Berendse, F.; Jonasson, S. Nutrient use and nutrient cycling in northern ecosystems. In Arctic Ecosystems in a Changing Climate, an Ecophysiological Perspective; Chapin, F.S., Jefferies, R.L., Reynolds, J.F., Shaver, G.S., Svoboda, J., Eds.; Academic Press: San Diego, CA, USA, 1992; pp. 337–356. [Google Scholar]
- Callaghan, T.V.; Jonasson, S. Arctic terrestrial ecosystems and environmental change. Philos. Trans. R. Soc. Lond. 1995, 352, 259–276. [Google Scholar] [CrossRef]
- Hedberg, A.M.; Borowicz, V.A.; Armstrong, J.E. Interactions between a hemiparasitic plant, Pedicularis canadensis L. (Orobanchaceae), and members of a tallgrass prairie community. J. Torrey Bot. Soc. 2005, 132, 401–410. [Google Scholar] [CrossRef]
- Borowicz, V.A.; Armstrong, J.E. Resource limitation and the role of a hemiparasite on a restored prairie. Oecologia 2012, 169, 783–792. [Google Scholar] [CrossRef]
- DiGiovanni, J.P.; Wysocki, W.P.; Burke, S.V.; Duvall, M.R.; Barber, N.A. The role of hemiparasitic plants: Influencing tallgrass prairie quality, diversity, and structure. Restor. Ecol. 2017, 25, 405–413. [Google Scholar] [CrossRef]
- Borowicz, V.A.; Walder, M.R.; Armstrong, J.E. Coming undone: Hemiparasite presence and effects in a prairie grassland diminish over time. Oecologia 2019, 190, 679–688. [Google Scholar] [CrossRef]
- Wilhelm, G.; Rericha, L. Flora of the Chicago Region: A Floristic and Ecological Synthesis; Indiana Academy of Science: Indianapolis, IN, USA, 2017. [Google Scholar]
- USDA, NRCS. The PLANTS Database. Available online: http://plants.usda.gov (accessed on 14 June 2021).
- Schneider, M.J.; Stermitz, F.R. Uptake of host plant alkaloids by root parasitic Pedicularis species. Phytochemistry 1990, 29, 1811–1814. [Google Scholar] [CrossRef]
- Piehl, M.A. The parasitic behavior of Dasistoma macrophylla. Rhodora 1962, 64, 331–336. [Google Scholar]
- Bach, E.M.; Kleiman, B.P. Twenty years of tallgrass prairie restoration in northern Illinois, USA. Ecol. Solut. Evid. 2021, 2, e12101. [Google Scholar] [CrossRef]
- Daubenmire, R. A canopy coverage method of vegetation analysis. Northwest Sci. 1959, 33, 43–64. [Google Scholar]
- Bauer, J.T.; Koziol, L.; Bever, J.D. Ecology of Floristic Quality Assessment: Testing for correlations between coefficients of conservatism, species traits and mycorrhizal responsiveness. AoB Plants 2018, 10, plx073. [Google Scholar] [CrossRef] [PubMed]
- Taft, J.B.; Wilhelm, G.S.; Ladd, D.M.; Masters, L.A. Floristic quality assessment for vegetation in Illinois, a method for assessing vegetation integrity. Erigenia 1997, 15, 3–95. [Google Scholar]
- Spyreas, G. Floristic Quality Assessment: A critique, a defense, and a primer. Ecosphere 2019, 10, e02825. [Google Scholar] [CrossRef]
- Sims, G.K.; Ellsworth, T.R.; Mulvaney, R.L. Microscale determination of inorganic nitrogen in water and soil extracts. Commun. Soil Sci. Plant Anal. 1995, 26, 303–316. [Google Scholar] [CrossRef]
- Hood-Nowotny, R.; Hinko-Najera Umana, N.; Inselbacher, E.; Oswald-Lachouani, P.; Wanek, W. Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Nutr. Manag. Soil Plant Anal. 2010, 74, 1018–1027. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.; Chauhan, B. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Matthies, D. Parasitic and competitive interactions between the hemiparasites Rhinanthus serotinus and Odontities rubra and their host Medicago sativa. J. Ecol. 1995, 83, 245–251. [Google Scholar] [CrossRef]
- Watson, D.M. Parasitic plants as facilitators: More dryad than Dracula? J. Ecol. 2009, 97, 1151–1159. [Google Scholar] [CrossRef]
- Davies, D.M.; Graves, J.D.; Elias, C.O.; Williams, P.J. The impact of Rhinanthus spp. on sward productivity and composition: Implications for the restoration of species-rich grasslands. Biol. Conserv. 1997, 82, 87–93. [Google Scholar] [CrossRef]
- Van Hoveln, M.D.; Evans, B.A.; Borowicz, V.A. Hemiparasite-host plant interactions and the impact of herbivory: A field experiment. Botany 2001, 89, 537–544. [Google Scholar] [CrossRef]
- Ameloot, E.; Verheyen, K.; Hermy, H. Meta-analysis of standing crop production by Rhinanthus spp. and its effect on vegetation structure. Folia Geobot. 2005, 40, 289–310. [Google Scholar] [CrossRef]
- Spasojevic, M.; Suding, K. Contrasting effects of hemiparasites on ecosystem processes: Can positive litter effects offset the negative effects of parasitism? Oecologia 2011, 165, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Demey, A.; Ameloot, E.; Staelens, J.; De Schrijver, A.; Verstraeten, G.; Boeckx, P.; Hermy, M.; Verheyen, K. Effects of two contrasting hemiparasitic plant species on biomass production and nitrogen availability. Oecologia 2013, 173, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil. Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Baer, S.G.; Meyer, C.K.; Bach, E.M.; Klopf, R.P.; Six, J. Contrasting ecosystem recovery on two soil textures: Implications for carbon mitigation and grassland conservation. Ecosphere 2010, 1, 1–22. [Google Scholar] [CrossRef]
- Heinze, J.; Simons, N.K.; Seibold, S.; Wacker, A.; Weithoff, G.; Gossner, M.M.; Prati, D.; Bezemer, T.M.; Joshi, J. The relative importance of plant-soil feedbacks for plant-species performance increases with decreasing intensity of herbivory. Oecologia 2019, 190, 651–664. [Google Scholar] [CrossRef]
- Hamman, S.T.; Dunwiddie, P.W.; Nuckols, J.L.; McKinley, M. Fire as a restoration tool in Pacific Northwest prairies and oak woodlands: Challenges, successes, and future directions. Northwest Sci. 2011, 85, 317–328. [Google Scholar] [CrossRef]
- Rook, E.J.; Fischer, D.G.; Seyferth, R.D.; Kirsch, J.L.; LeRoy, C.J.; Hamman, S. Responses of prairie vegetation to fire, herbicide, and invasive species legacy. Northwest Sci. 2011, 85, 288–302. [Google Scholar] [CrossRef]
- Henderson, R.A. Are there keystone plant species driving diversity in Midwest prairies. In Promoting prairie, Proceedings of the 18th North American Prairie Conference; Fore, S., Ed.; Truman State University: Kirksville, MO, USA, 2003; pp. 63–66. [Google Scholar]
- Pywell, R.F.; Bullock, J.M.; Walker, K.J.; Coulson, S.J.; Gregory, S.J.; Stevenson, M.J. Facilitating grassland diversification using the hemiparasitic plant Rhinanthus minor. J. Appl. Ecol. 2004, 41, 880–887. [Google Scholar] [CrossRef]
- Stein, C.; Rißmann, C.; Hempel, S.; Renker, C.; Buscot, F.; Prati, D.; Auge, H. Interactive effects of mycorrhizae and a root hemiparasite on plant community productivity and diversity. Oecologia 2009, 159, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Matthies, D.; Schmid, B. Root hemiparasites and plant diversity in experimental grassland communities. J. Ecol. 2000, 88, 634–644. [Google Scholar] [CrossRef]
- Walder, M.; Armstrong, J.E.; Borowicz, V.A. Limiting similarity, biotic resistance, nutrient supply, or enemies? What accounts for the invasion success of an exotic legume? Biol. Invasions 2019, 21, 435–449. [Google Scholar] [CrossRef]
- Perring, M.P.; Standish, R.J.; Price, J.N.; Craig, M.D.; Erickson, T.E.; Ruthrof, K.X.; Whiteley, A.S.; Valentine, L.E.; Hobbs, R.J. Advances in restoration ecology: Rising to the challenges of the coming decades. Ecosphere 2015, 6, 131. [Google Scholar] [CrossRef]


| (a) C. sessiliflora | Median (µg/cm2) | Range | n |
| Absent | 0.073 | 0.068–0.107 | 9 |
| Present | 0.083 | 0.069–0.148 | 6 |
| Χ2 = 1.690, df = 1, p = 0.184 | |||
| (b) P. canadensis | Median (µg/cm2) | Range | n |
| Absent | 0.070 | 0.068–0.084 | 10 |
| Present | 0.069 | 0.051–0.073 | 10 |
| Χ2 = 3.451, df = 1, p = 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheidel, A.; Borowicz, V. Effects of Hemiparasites in Grassland Restorations Are Not Universal. Diversity 2024, 16, 102. https://doi.org/10.3390/d16020102
Scheidel A, Borowicz V. Effects of Hemiparasites in Grassland Restorations Are Not Universal. Diversity. 2024; 16(2):102. https://doi.org/10.3390/d16020102
Chicago/Turabian StyleScheidel, Anna, and Victoria Borowicz. 2024. "Effects of Hemiparasites in Grassland Restorations Are Not Universal" Diversity 16, no. 2: 102. https://doi.org/10.3390/d16020102
APA StyleScheidel, A., & Borowicz, V. (2024). Effects of Hemiparasites in Grassland Restorations Are Not Universal. Diversity, 16(2), 102. https://doi.org/10.3390/d16020102





