Blackfordia virginica in Non-Native Distribution Range: A Potential Food Source for Humans?
Abstract
1. Introduction
2. Materials and Methods
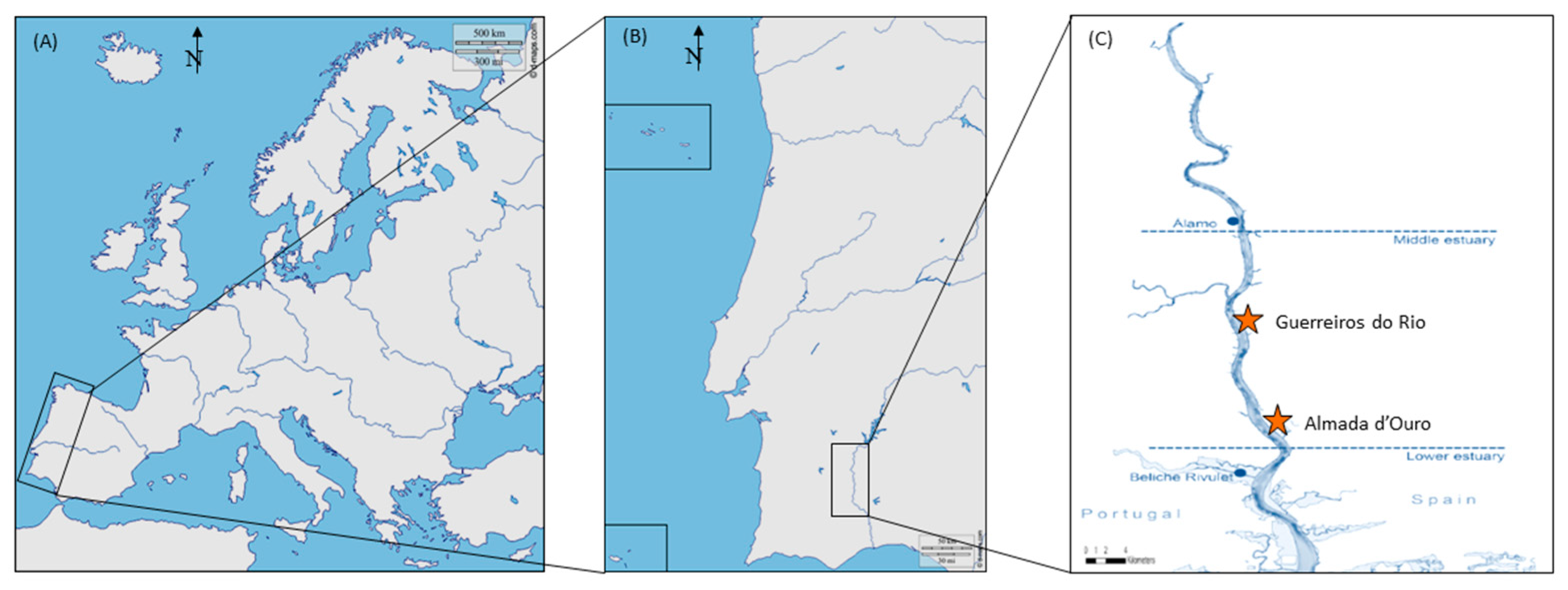
2.1. Study Site
2.2. Sample Collection
2.3. Nutritional Profile
2.3.1. Proximate Composition
2.3.2. Amino Acids
2.3.3. Fatty Acid Methyl Esters (FAMEs)
2.3.4. Minerals
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallardo, B.; Clavero, M.; Sánchez, M.I.; Vilà, M. Global ecological impacts of invasive species in aquatic ecosystems. Glob. Chang. Biol. 2016, 22, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Env. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Connelly, N.A.; O’Neill, C.R.; Knuth, B.A.; Brown, T.L. Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environ. Manag. 2007, 40, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pejchar, L.; Mooney, H.A. Invasive species, ecosystem services and human well-being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef]
- Keller, R.P.; Geist, J.; Jeschke, J.M.; Kühn, I. Invasive species in Europe: Ecology, status, and policy. Environ. Sci. Eur. 2011, 23, 23. [Google Scholar] [CrossRef]
- Fantle-Lepczyk, J.E.; Haubrock, P.J.; Kramer, A.M.; Cuthbert, R.N.; Turbelin, A.J.; Crystal-Ornelas, R.; Diagne, C.; Courchamp, F. Economic costs of biological invasions in the United States. Sci. Total Environ. 2022, 806, 151318. [Google Scholar] [CrossRef]
- Hulme, P.E. Invasive species challenge the global response to emerging diseases. Trends Parasitol. 2014, 30, 267–270. [Google Scholar] [CrossRef]
- Chinchio, E.; Crotta, M.; Romeo, C.; Drewe, J.A.; Guitian, J.; Ferrari, N. Invasive alien species and disease risk: An open challenge in public and animal health. PLoS Pathog. 2020, 16, e1008922. [Google Scholar] [CrossRef]
- Bédry, R.; de Haro, L.; Bentur, Y.; Senechal, N.; Galil, B. Toxicological risks on the human health of populations living around the Mediterranean Sea linked to the invasion of non-indigenous marine species from the Red Sea: A review. Toxicon 2021, 191, 69–82. [Google Scholar] [CrossRef]
- Morais, P.; Gaspar, M.; Garel, E.; Baptista, V.; Cruz, J.; Cerveira, I.; Leitão, F.; Teodósio, M.A. The Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its non-native distribution into the Ria Formosa Lagoon and the Guadiana Estuary (SW-Iberian Peninsula, Europe). BioInvasions Rec. 2019, 8, 123–133. [Google Scholar] [CrossRef]
- Cerveira, I.; Dias, E.; Baptista, V.; Teodósio, M.A.; Morais, P. Invasive fish keeps native feeding strategy despite high niche overlap with a congener species. Reg. Stud. Mar. Sci. 2021, 47, 101969. [Google Scholar] [CrossRef]
- Cerveira, I.; Baptista, V.; Teodósio, M.A.; Morais, P. What’s for dinner? Assessing the value of an edible invasive species and outreach actions to promote its consumption. Biol. Invasions 2022, 24, 815–829. [Google Scholar] [CrossRef]
- Chícharo, L.; Morais, P.; Chícharo, M.A. Inter-annual differences of ichthyofauna structure of the Guadiana Estuary and adjacent coastal area (SE Portugal/SW Spain): Before and after Alqueva dam construction. Estuar. Coast. Shelf Sci. 2006, 70, 39–51. [Google Scholar] [CrossRef]
- Morais, P. The Life Cycle of Engraulis encrasicolus Sensu Lato in the Guadiana Estuary: Ecology, Ecohydrology and Biology. Ph.D. Thesis, University of Algarve, Faro, Portugal, 2007; p. 238. [Google Scholar]
- Morais, P. Review on the major ecosystem impacts caused by damming and watershed development in an Iberian basin (SW-Europe)—focus on the Guadiana Estuary. Ann. Limnol. Int. J. Lim. 2008, 44, 69–81. [Google Scholar] [CrossRef]
- Chícharo, M.A.; Leitão, T.; Range, P.; Gutierrez, C.; Morales, J.; Morais, P.; Chícharo, L. Alien species in the Guadiana Estuary (SE-Portugal/SW-Spain): Blackfordia virginica (Cnidaria, Hydrozoa) and Palaemon macrodactylus (Crustacea, Decapoda): Potential impacts and mitigation measures. Estuar. Coast. Shelf Sci. 2009, 4, 501–506. [Google Scholar] [CrossRef]
- Morais, P.; Teodósio, J.; Reis, J.; Chícharo, M.A.; Chícharo, L. The Asian clam Corbicula fluminea (Müller, 1774) in the Guadiana River Basin (southwestern Iberian Peninsula): Setting the record straight. Aquat. Invasions 2009, 4, 681–684. [Google Scholar] [CrossRef]
- Morais, P.; Teodósio, M.A. The transatlantic introduction of weakfish Cynoscion regalis (Bloch and Schneider, 1801) (Sciaenidae, Pisces) into Europe. BioInvasions Rec. 2016, 5, 259–265. [Google Scholar] [CrossRef]
- Seyer, T.; Morais, P.; Amorim, K.; Leitão, F.; Martins, F.; Teodósio, M.A. On the presence of the Ponto-Caspian hydrozoan Cordylophora caspia (Pallas, 1771) in an Iberian estuary: Highlights on the introduction vectors and invasion routes. BioInvasions Rec. 2017, 6, 331–337. [Google Scholar] [CrossRef]
- Encarnação, J.; Teodósio, M.A.; Morais, P. The arrival of a non-indigenous ecosystem engineer to a heavily invaded and flow-regulated estuary in Europe. BioInvasions Rec. 2024, 13, 83–95. [Google Scholar] [CrossRef]
- Freire, M.; Genzano, G.N.; Neumann-Leitão, S.; Pérez, C.D. The non-indigenous medusa Blackfordia virginica (Hydrozoa, Leptothecata) in tropical Brazil: 50 years of unnoticed presence. Biol. Invasions 2014, 16, 1–5. [Google Scholar] [CrossRef]
- Jaspers, C.; Huwer, B.; Weiland-Bräuer, N.; Clemmesen, C. First record of the nonindigenous jellyfish Blackfordia virginica (Mayer, 1910) in the Baltic Sea. Helgol. Mar. Res. 2018, 72, 13. [Google Scholar] [CrossRef]
- Carman, M.R.; Grunden, D.W.; Govindarajan, A.F. Species-specific crab predation on the hydrozoan clinging jellyfish Gonionemus sp. (Cnidaria, Hydrozoa), subsequent crab mortality, and possible ecological consequences. PeerJ 2017, 5, e3966. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torri, L.; Tuccillo, F.; Bonelli, S.; Piraino, S.; Antonella Leone, A. The attitudes of Italian consumers towards jellyfish as novel food. Food Qual. Pref. 2020, 79, 103782. [Google Scholar] [CrossRef]
- Yuferova, A.A. The impact of different drying modes of Scyphozoan jellyfish Rhopilema esculentum and Aurelia aurita on the protein and carbohydrate components in their composition and the possibility of their use as dried prepared food. J. Food Process Eng. 2015, 40, 12326. [Google Scholar] [CrossRef]
- Zhu, S.; Ye, M.; Xu, J.; Guo, C.; Zheng, H.; Hu, J.; Chen, J. Lipid profile in different parts of edible jellyfish Rhopilema esculentum. J. Agric. Food Chem. 2015, 63, 8283–8291. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Wijesekara, W.L.I.; Perera, P.R.D.; Senanayake, S.A.M.; Pathmalal, M.; Marapana, R.A.U.J. Nutritional value and potential applications of jellyfish. J. Aquat. Food Prod. Technol. 2022, 31, 445–482. [Google Scholar] [CrossRef]
- Mollo, E. Chasing chances in a changing sea. Mar. Drugs 2022, 20, 311. [Google Scholar] [CrossRef]
- Marques, R.; Bouvier, C.; Darnaude, M.A.; Molinero, J.C.; Przybyla, C.; Soriano, S.; Tomasini, J.A.; Bonnet, D. Jellyfish as an alternative source of food for opportunistic fishes. J. Exp. Mar. Biol. Ecol. 2016, 485, 1–7. [Google Scholar] [CrossRef]
- Hays, G.C.; Doyle, T.K.; Houghton, J.D.R. A paradigm shift in the trophic importance of jellyfish? Trends Ecol. Evol. 2018, 33, 874–884. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. AOAC Official Method 990.03, Protein (Crude) in Animal Feed, Combustion Method. In Official Methods of Analysis of AOAC International, 18th ed., Revision 1; AOAC International: Gaithersburg, MD, USA, 2006; Chapter 4; pp. 30–31. [Google Scholar]
- Pereira, H.; Barreira, L.; Custódio, L.; Alrokayan, S.; Mouffouk, F.; Varela, J.; Ben-Hamadou, R. Isolation and fatty acid profile of selected microalgae strains from the Red Sea for biofuel production. Energies 2013, 6, 2773–2783. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- FAO. Food and Nutrition Paper 77. Food Energy—Methods of Analysis and Conversion Factors; Report of a Technical Workshop; FAO: Rome, Italy, 2022. [Google Scholar]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Sustainable Food and Agriculture. Available online: http://www.fao.org/sustainability/en/ (accessed on 9 July 2022).
- Organisation for Economic Co-Operation and Development. The Future of Food. Available online: https://web-archive.oecd.org/2012-06-15/149007-35391719.pdf (accessed on 9 July 2022).
- United Nations Educational, Scientific and Cultural Organization. What Is Sustainable Consumption? Available online: https://www.unep.org/explore-topics/resource-efficiency/what-we-do/sustainable-consumption-and-production-policies (accessed on 9 July 2022).
- Raposo, A.; Alasqah, I.; Alfheeaid, H.A.; Alsharari, Z.D.; Alturki, H.A.; Raheem, D. Jellyfish as Food: A narrative review. Foods 2022, 11, 2773. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Mazna, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Sato, H.; Yoshie-Stark, Y.; Ogushi, M.; Tanaka, Y. Differences in the biochemical compositions of two dietary jellyfish species and their effects on the growth and survival of Ibacus novemdentatus phyllosomas. Aquac. Nutr. 2016, 22, 25–33. [Google Scholar] [CrossRef]
- Prieto, L.; Enrique-Navarro, A.; Li Volsi, R.; Ortega, M. The large jellyfish Rhizostoma luteum as sustainable a resource for antioxidant properties, nutraceutical value and biomedical applications. Mar. Drugs 2018, 16, 396. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef]
- Stenvers, V.; Chi, X.; Javidpour, J. Seasonal variability of the fatty acid composition in Aurelia aurita (Cnidaria: Scyphozoa): Implications for gelativore food web studies. J. Plankton Res. 2020, 42, 440–452. [Google Scholar] [CrossRef]
- Doyle, T.K.; Houghton, J.D.; McDevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-composition. J. Exp. Mar. Biol. Ecol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Huang, Y.A.-W. Cannonball jellyfish (Stomolophus meleagris) as a food resource. J. Food Sci. 1988, 53, 341–343. [Google Scholar] [CrossRef]
- Morais, Z.B.; Pintao, A.M.; Costa, I.M.; Calejo, M.T.; Bandarra, N.M.; Abreu, P. Composition and in vitro antioxidant effects of jellyfish Catostylus tagi from Sado Estuary (SW Portugal). J. Aquat. Food Prod. Tech. 2009, 18, 90–107. [Google Scholar] [CrossRef]
- Costa, R.; Capillo, G.; Albergamo, A.; Volsi, L.R.; Bartolomeo, G.; Bua, G.; Ferracane, A.; Savoca, S.; Gervasi, T.; Rando, R.; et al. A multi-screening evaluation of the nutritional and nutraceutical potential of the Mediterranean jellyfish Pelagia noctiluca. Mar. Drugs 2019, 17, 172. [Google Scholar] [CrossRef]
- Moura, D.; Gomes, A.; Mendes, I.; Aníbal, J. Guadiana River Estuary. Investigating the Past, Present and Future; University of Algarve, Centre for Marine and Environmental Research (CIMA): Faro, Portugal, 2017; p. 140. [Google Scholar]
- Muñoz-Vera, A.; García, G.; García-Sánchez, A. Metal bioaccumulation pattern by Cotylorhiza tuberculata (Cnidaria, Scyphozoa) in the Mar Menor coastal lagoon (SE Spain). Environ. Sci. Pollut. Res. 2015, 22, 19157–19169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Shao, Z.; Li, C.; Yu, L.; Raja, M.A.; Liu, C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum kishinouye for use in hemostatic applications. PLoS ONE 2017, 12, e0169731. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, R.; Srinivasan, K.; Singh, S. Effect of arginineand glycine intake ratios on dyslipidemia and selected biomarkers implicated in cardiovascular disease: A study with hypercholesterolemic rats. Biomed. Pharmacother. 2017, 91, 408–414. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods—A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- German, J.B.; Dillard, C.J. Saturated fats: A perspective from lactation and milk composition. Lipids 2010, 45, 915–923. [Google Scholar] [CrossRef]
- Reglinski, K.; Steinfort-Effelsberg, L.; Sezgin, E.; Klose, C.; Platta, H.; Girzalsky, W.; Eggeling, C.; Erdmann, R. Fluidity and lipid composition of membranes of peroxisomes, mitochondria and the ER from oleic acid-induced Saccharomyces cerevisiae. Front. Cell Dev. Biol. 2020, 8, 574363. [Google Scholar] [CrossRef]
- Teodósio, M.A.; Baptista, V.; Dias, L.; Encarnação, J.; Cruz, J. Tracking the invasion: Long-term trends in density, size, and sex ratio of Blackfordia virginica in a non-indigenous environment [Conference presentation abstract]. In Proceedings of the ICES/PICES 7th International Zooplankton Production Symposium, Hobart, Tasmania, Australia, 17–22 March 2024. [Google Scholar]
- Nowaczyk, A.; David, V.; Lepage, M.; Goarant, A.; De Oliveira, É.; Sautour, B. Spatial and temporal patterns of occurrence of three alien hydromedusae, Blackfordia virginica (Mayer, 1910), Nemopsis bachei (Agassiz, 1849) and Maeotias marginata (Modeer, 1791), in the Gironde Estuary (France). Aquat. Invasions 2016, 11, 397–409. [Google Scholar] [CrossRef]
- Marques, F.; Angélico, M.M.; Costa, J.L.; Teodósio, M.A.; Presado, P.; Fernandes, A.; Chaínho, A.; Domingos, I. Ecological aspects and potential impacts of the non-native hydromedusa Blackfordia virginica in a temperate estuary. Estuar. Coast. Shelf Sci. 2017, 197, 69–79. [Google Scholar] [CrossRef]
- Huang, X.; Liu, B.; Guo, D.; Zhong, Y.; Li, S.; Liu, X.; Laws, E.A.; Huang, B. Blackfordia virginica blooms shift the trophic structure to smaller size plankton in subtropical shallow waters. Mar. Pollut. Bull. 2021, 163, 111990. [Google Scholar] [CrossRef]
| Proximate Composition | Fresh Weight (F) | Dry Weight (DW) |
|---|---|---|
| Moisture | 98.6 ± 0.06 | 1.30 ± 0.01 |
| Ash | 0.40 ± 0.02 | 30.5 ± 1.55 |
| Crude protein | 0.10 ± 0.08 | 7.62 ± 0.62 |
| Total fat | 0.00 ± 0.00 | 0.01 ± 0.00 |
| Carbohydrates | 0.81 ± 0.18 | 60.5 ± 9.03 |
| Energetic value (Kcal) | 4.12 ± 0.73 | 281 ± 43.1 |
| Minerals | Symbol | Contents |
|---|---|---|
| Essential macro elements (mg/100 g DW) | ||
| Sodium | Na | 728 ± 5.35 |
| Magnesium | Mg | 76.1 ± 3.29 |
| Potassium | K | 56.9 ± 8.87 |
| Calcium | Ca | 17.4 ± 0.23 |
| Essential trace elements (μg/100 g DW) | ||
| Iron | Fe | 1208 ± 212 |
| Zink | Zn | 110 ± 16.3 |
| Manganese | Mn | 45.4 ± 4.33 |
| Copper | Cu | 26.3 ± 1.53 |
| Selenium | Se | n.d. |
| Nonessential trace elements (μg/100 g DW) | ||
| Cadmium | Cd | 337.71 ± 9.16 |
| Nickel | Ni | 68.56 ± 6.55 |
| Chromium | Cr | 4.08 ± 0.58 |
| Arsenic | As | 2.31 ± 0.04 |
| Lead | Pb | n.d. |
| Amino Acids | Symbol | Contents | |
|---|---|---|---|
| mg/100 g | % | ||
| Glutamic acid + Glutamine | Glx | 821 ± 8.40 | 16.8 |
| Glycine | Gly | 471 ± 10.20 | 9.66 |
| Alanine | Ala | 469 ± 4.80 | 9.63 |
| Aspartic acid + Asparagine | Asx | 432 ± 11.30 | 8.86 |
| Arginine | Arg | 353 ± 8.50 | 7.25 |
| Proline | Pro | 326 ± 6.00 | 6.70 |
| Tyrosine | Tyr | 300 ± 2.80 | 6.15 |
| Serine | Ser | 245 ± 4.30 | 5.04 |
| Taurine | Tau | 48.0 ± 0.10 | 0.98 |
| Cystine | Cys | 3.6 ± 0.10 | 0.07 |
| Total non-essential AA | Σ NEAA | 3471 ± 0.56 | 71.1 |
| Lysine | Lys | 281 ± 3.00 | 5.77 |
| Leucine | Leu | 265 ± 11.60 | 5.43 |
| Valine | Val | 265 ± 11.60 | 5.43 |
| Threonine | Thr | 227 ± 7.00 | 4.67 |
| Isoleucine | Ile | 158 ± 1.20 | 3.24 |
| Phenylalanine | Phe | 129 ± 0.80 | 2.66 |
| Methionine | Met | 68 ± 2.10 | 1.40 |
| Histidine | His | 10.2 ± 0.10 | 0.21 |
| Total essential AA | Σ EAA | 1405 ± 0.32 | 28.8 |
| EAA/NEAA EAA/TAA | 0.41 0.30 | ||
| LYS/ARG | 0.79 | ||
| FAME | Synonyms * | Chemical Formula | Fatty Acid | μg/100 g DW | % |
|---|---|---|---|---|---|
| Saturated Fatty Acids (SFAs) | |||||
| Methyl decanoate | Capric acid methyl ester | C11H22O2 | C11:0 | 1198 ± 2.53 | 19.9 ± 0.02 |
| Dodecamethylcyclohexasiloxane | Cyclohexasiloxane, dodecamethyl | C12H36O6Si6 | C12:0 | 40.5 ± 5.54 | 0.68 ± 0.05 |
| Methyl dodecanoate | Lauric acid methyl ester | C13H26O2 | C13:0 | 899 ± 0.78 | 14.9 ± 0.01 |
| Methyl tetradecanoate | Myristic acid methyl ester | C15H30O2 | C15:0 | 998 ± 0.73 | 16.6 ± 0.01 |
| Methyl palmitate | Palmitic acid methyl ester | C17H34O2 | C17:0 | 887 ± 0.76 | 14.8 ± 0.01 |
| Methyl heptadecanoate | Margaric acid methyl ester | C18H36O2 | C18:0 | 19.5 ± 0.38 | 0.32 ± 0.01 |
| Methyl stearate | Octadecanoic acid, methyl ester | C19H38O2 | C19:0 | 421 ± 12.3 | 7.03 ± 0.12 |
| Methyl arachidate | Arachidic acid methyl ester | C21H42O2 | C21:0 | 99.8 ± 0.18 | 1.66 ± 0.00 |
| Tricosanoic acid | Tricosylic acid | C23H46O2 | C23:0 | 50.5 ± 0.37 | 0.84 ± 0.01 |
| Total SFA | 4616 | 76.9 | |||
| Monounsaturated fatty acids (MUFAs) | |||||
| Methyl Cis-9-Tetradecenoate | Myristoleic acid methyl ester | C15H28O2 | C15:1 n-9 | 393 ± 10.1 | 6.56 ± 0.10 |
| Methyl palmitoleate | Methyl (Z)-hexadec-9-enoate | C17H32O2 | C36:1 n-9 | 200 ± 0.01 | 3.34 ± 0.00 |
| Methyl oleate | Oleic acid methyl ester | C19H36O2 | C19:1 n-9 | 666 ± 0.02 | 11.1 ± 0.00 |
| Total MUFA | 1260 | 21.0 | |||
| Polyunsaturated fatty acids (PUFAs) | |||||
| Methyl linolenate | Linolenic acid methyl ester | C19H32O2 | C19:3 n-3 | 100 ± 0.21 | 1.67 ± 0.00 |
| Squalene | Spinacene | C30H50 | C30:6 n-2 | 19.4 ± 22.3 | 0.32 ± 0.22 |
| Total PUFA | 119 | 1.99 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, M.; Dias, E.; Custódio, L.; Encarnação, J.; Cruz, J.; Baptista, V.; Teodósio, M.A. Blackfordia virginica in Non-Native Distribution Range: A Potential Food Source for Humans? Diversity 2024, 16, 729. https://doi.org/10.3390/d16120729
Cruz M, Dias E, Custódio L, Encarnação J, Cruz J, Baptista V, Teodósio MA. Blackfordia virginica in Non-Native Distribution Range: A Potential Food Source for Humans? Diversity. 2024; 16(12):729. https://doi.org/10.3390/d16120729
Chicago/Turabian StyleCruz, Mariana, Ester Dias, Luísa Custódio, João Encarnação, Joana Cruz, Vânia Baptista, and Maria Alexandra Teodósio. 2024. "Blackfordia virginica in Non-Native Distribution Range: A Potential Food Source for Humans?" Diversity 16, no. 12: 729. https://doi.org/10.3390/d16120729
APA StyleCruz, M., Dias, E., Custódio, L., Encarnação, J., Cruz, J., Baptista, V., & Teodósio, M. A. (2024). Blackfordia virginica in Non-Native Distribution Range: A Potential Food Source for Humans? Diversity, 16(12), 729. https://doi.org/10.3390/d16120729










