Spatial Distribution and Temporal Variation of Megafauna in Circalittoral and Bathyal Soft Bottoms of the Westernmost Biodiversity Hotspot of the Mediterranean Sea: The Alboran Ridge
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Statistical Analyses
3. Results
3.1. Biological Composition
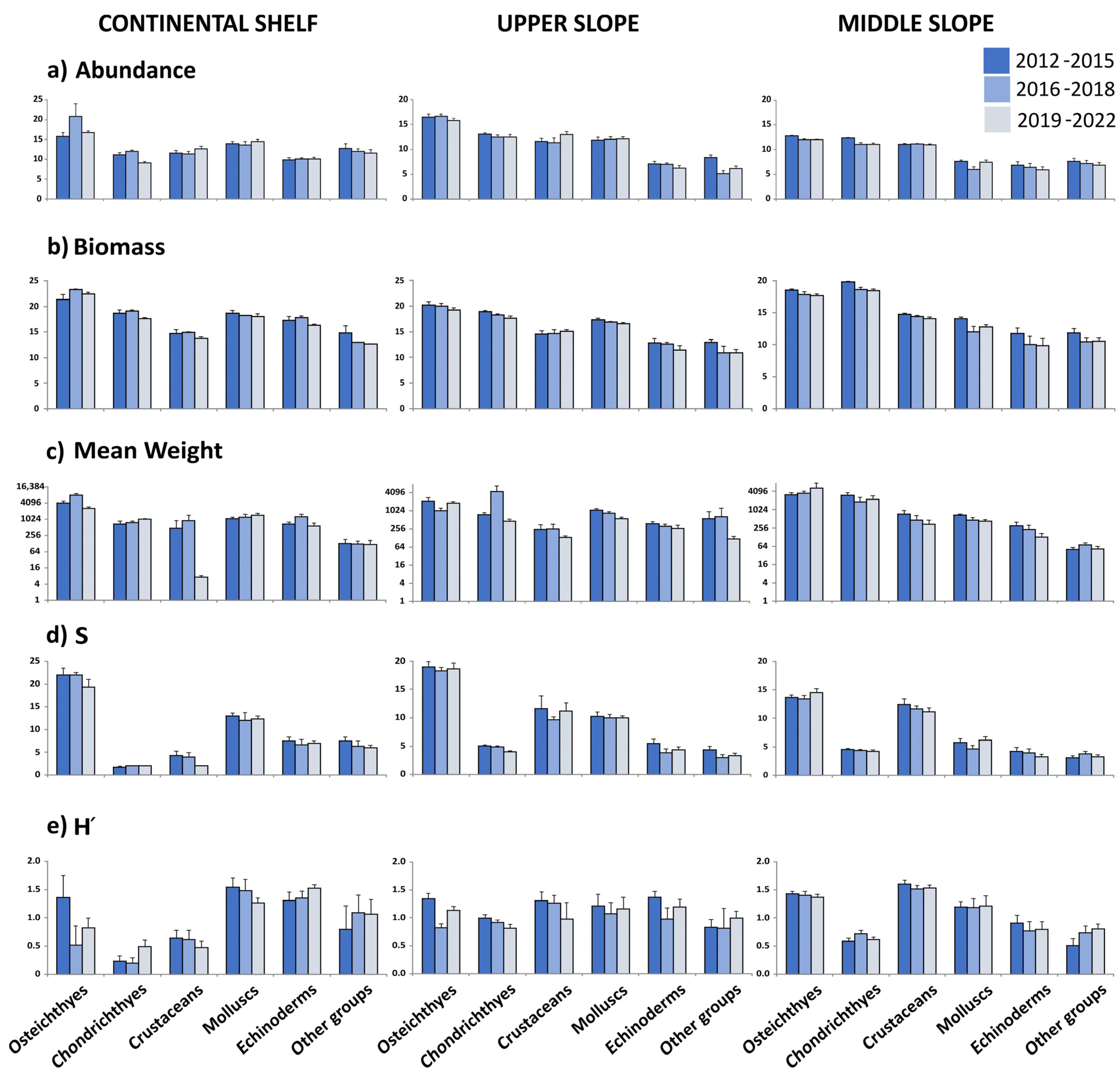
3.2. Community Metrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Templado, J. La diversidad marina en España. In Biodiversidad: Aproximación a la Diversidad Botánica y Zoológica en España; Viejo, J.L., Ed.; Memoria Real Sociedad Española de Historia Natural: Madrid, Spain, 2011; pp. 343–362. [Google Scholar]
- Templado, J.; Luque, A.A.; Moreno, D.; Tierno de Figueroa, J.M.; Sánchez-Tocino, L.; Aguilar, R.; De la Torriente, A. Invertebrates: The Realm of Diversity. In Alboran Sea–Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli, M., Eds.; Springer: Cham, Switzerland; Dordrecht, The Netherlands, 2021; pp. 359–430. [Google Scholar]
- Rueda, J.L.; Gofas, S.; Aguilar, R.; de la Torriente, A.; García Raso, J.E.; Lo Iacono, C.; Luque, A.A.; Marina, P.; Mateo-Ramírez, A.; Moya-Urbano, E.; et al. Benthic Fauna of Littoral and Deep-Sea Habitats of the Alboran Sea: A Hotspot of Biodiversity. In Alboran Sea–Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli, M., Eds.; Springer: Cham, Switzerland; Dordrecht, The Netherlands, 2021; pp. 285–358. [Google Scholar]
- Mateo-Ramírez, A.; Marina, P.; Moreno, D.; Alcántara, L.F.; Aguilar, R.; Báez, J.C.; Bárcenas, P.; Baro, J.; Caballero-Herrera, J.A.; Camiñas, J.A.; et al. Marine protected areas and key biodiversity areas of the Alboran Sea and adjacent areas. In Alboran Sea–Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli, M., Eds.; Springer: Cham, Switzerland; Dordrecht, The Netherlands, 2021; pp. 819–923. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Marine Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future Research. Mar. Pollut. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.L.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Maldonado, M.; Uriz, M.J. Biotic affinities in a transitional zone between the Mediterranean and the Atlantic: A biogeographical approach based on sponges. J. Biogeogr. 1995, 22, 89–110. [Google Scholar] [CrossRef]
- Gofas, S. Marine molluscs with a very restricted range in the Strait of Gibraltar. Divers. Distrib. 1999, 4, 255–266. [Google Scholar]
- Calvo, M.; Templado, J.; Moreno, D.; Ramos, M. La Reserva Marina de la isla de Alborán: Peculiaridades y estado actual de conocimiento sobre su flora y fauna bentónicas. In I Jornadas Internacionales sobre Reservas Marinas; Ministerio de Agricultura Pesca y Alimentación, Secretaría General de Pesca: Madrid, Spain, 2001; pp. 53–70. [Google Scholar]
- Moreno, D.; Capítulo, V. Tesoros sumergidos: La flora y fauna marinas. In Entre África y Europa. Historia Natural de las Isla de Alborán; Paracuellos, M., Nevado, J.C., Mota, J.F., Eds.; RENPA, Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2006; pp. 69–85. [Google Scholar]
- Templado, J.; Calvo, M.; Moreno, D.; Flores-Moya, A.; Conde, F.; Abad, R.; Rubio, J. Flora y Fauna de la Reserva Marina y Reserva de Pesca de la isla de Alborán; Secretaría General de Pesca Marítima (Ministerio de Agricultura, Pesca y Alimentación), Museo Nacional de Ciencias Naturales (Consejo Superior de Investigaciones Científicas): Madrid, Spain, 2006; 269p. [Google Scholar]
- Gofas, S.; Goutayer, J.; Luque, A.A.; Salas, C.; Templado, J. Espacio marino de Alborán, Proyecto LIFE+INDEMARES Fundación Biodiversidad del Ministerio de Agricultura; Alimentación y Medio Ambiente: Madrid, Spain, 2014. [Google Scholar]
- Templado, J.; Luque, A.A. Braquiópodos de los fondos de Corallium rubrum (L.) próximos a la isla de Alborán (SE de España). Bol. Inst. Español Oceanogr. 1986, 3, 111–114. [Google Scholar]
- Maldonado, M. Demosponges of the red coral bottoms from the Alboran Sea. J. Nat. Hist. 1992, 26, 1131–1161. [Google Scholar] [CrossRef]
- Maldonado, M. Demosponjas Litorales de Alborán. Faunística y Biogeografía. Ph.D. Thesis, Universidad Central de Barcelona, Barcelona, Spain, 1993. [Google Scholar]
- García-Raso, J.E. Resultados de la segunda campaña del IEO para la exploración de los fondos de coral rojo en el mar de Alborán. Bol. Inst. Español Oceanogr. 1989, 5, 27–36. [Google Scholar]
- Ciércoles, C.; García-Ruiz, C.; González, M.; Ortiz de Urbina, J.; López-González, N.; Urra, J.; Rueda, J.L. Molluscs collected with bottom otter trawl in the northern Alboran Sea: Main assemblages, spatial distribution and environmental linkage. Mediterr. Mar. Sci. 2018, 19, 209–222. [Google Scholar] [CrossRef]
- Moya-Urbano, E.; Urra, J.; Gofas, S.; Gallardo-Núñez, M.; Mateo-Ramírez, A.; Farias, C.; Ordinas, F.; Caballero-Herrera, J.A.; Bárcenas, P.; García-Ruiz, C.; et al. Molluscan assemblages in shelf and slope sedimentary habitats of the Northern Alboran Sea and their linkage to environmental variables. Reg. Stud. Mar. Sci. 2023, 65. [Google Scholar] [CrossRef]
- Ciércoles, C.; García-Ruíz, C.; Abelló, P.; Hidalgo, M.; Torres, P.; Gonzalez, M.; Mateo-Ramírez, A.; Rueda, J.L. Decapod crustacean assemblages on trawlable grounds in the northern Alboran Sea and Gulf of Vera. Sci. Mar. 2022, 86, e039. [Google Scholar] [CrossRef]
- Moya-Urbano, E.; Urra, J.; Majón-Cabeza, E.; Gallardo-Núñez, M.; Mateo-Ramínrez, A.; Farias, C.; Ordinas, F.; Moya, F.; Bárcenas, P.; García-Ruiz, C.; et al. Echinoderm assemblages from circalittoral and bathyal sedimentary habitats of the northern Alboran Sea (Western Mediterranean): Diversity, distribution and relationships with environmental variables. Thalasas in press.
- Bertrand, J.A.; Gil de Sola, L.; Papaconstantinou, C.; Relini, G.; Souplet, A. The general specifications of the MEDITS surveys. Sci. Mar. 2002, 66, 9–17. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2020, 83 (Suppl. 1), 9–20. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecological Methodology; Harper Collins: New York, NY, USA, 1989; pp. 1–645. [Google Scholar]
- WoRMS Editorial Board. World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 1 January 2024).
- Stamouli, C.; Zenetos, A.; Kalliniotis, A.; Voultsadou, E. Megabenthic invertebrate diversity in Mediterranean trawlable soft bottoms: A synthesis of current knowledge. Mediterr. Mar. Sci. 2022, 23, 447–459. [Google Scholar] [CrossRef]
- Serena, F.; Abella, A.J.; Bargnesi, F.; Barone, M.; Colloca, F.; Ferretti, F.; Fiorentino, F.; Jenrette, J.; Moro, S. Species diversity, taxonomy and distribution of Chondrichthyes in the Mediterranean and Black Sea. Eur. Zool. J. 2020, 87, 497–536. [Google Scholar] [CrossRef]
- Walker, P.; Cavanagh, R.D.; Ducrocq, M.; Fowler, S.L. Chapter 7–Regional Overviews: Northeast Atlantic (including Mediterranean and Black Sea). In Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes; Fowler, S.L., Cavanagh, R.D., Camhi, M., Burgess, G.H., Cailliet, G.M., Fordham, S.V., Simpfendorfer, C.A., Musick, J.A., Eds.; IUCN/SSC Shark Specialist Group IUCN Publications Services Unit: Gland, Switzerland; Cambridge, UK, 2005; pp. 71–95. [Google Scholar]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H. Loss of large predatory sharks from the Mediterranean Sea. Conserv. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef]
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Biagi, F.; Sartor, P.; Ardizzone, G.D.; Belcari, P.; Belluscio, A.; Serena, F. Analysis of demersal fish assemblages of the Tuscany and Latium coasts (north western Mediterranean). Sci. Mar. 2002, 66 (Suppl. 2), 233–242. [Google Scholar] [CrossRef]
- Ungaro, N.; Marano, C.A.; Marsan, R.; Martino, M.; Marzano, M.C.; Strippoli, G.; Vlora, A. Analysis of demersal species assemblages from trawl surveys in the South Adriatic Sea. Aquat. Living Resour. 1999, 12, 177–185. [Google Scholar] [CrossRef]
- Bertrand, J.; Gil De Sola, L.; Papaconstantinou, C.; Relini, G.; Souplet, A. Contribution on the distribution of elasmobranchs in the Mediterranean (from the MEDITS surveys). Biol. Mar. Mediterr. 2000, 7, 385–399. [Google Scholar]
- Dimech, M.; Camilleri, M.; Hiddink, J.G.; Kaiser, M.J.; Ragonese, S.; Schembri, P.J. Differences in demersal community structure and biomass size spectra within and outside the Maltese Fishery Management Zone (FMZ). Sci. Mar. 2008, 72, 669–682. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Ordines, F.; Terrasa, B.; Esteban, A.; García, C.; Guijarro, B.; Massutí, E. Demersal chondrichthyans in the western Mediterranean: Assemblages and biological parameters of their main species. Mar. Freshw. Res. 2015, 67, 636–652. [Google Scholar] [CrossRef]
- Templado, J.; Ballesteros, E.; García Raso, J.E.; San Martín, G.; López-García, E.; Salas, C.; Luque, A.A.; Sánchez-Lizaso, J.L.; Moreno, D. Las praderas de Posidonia oceanica. La comunidad posidonícola. In Praderas y Bosques Marinos de Andalucía; Luque, A.A., Templado, J.C., Eds.; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2004; pp. 89–116. [Google Scholar]
- Templado, J. Las especies del género Charonia (Mollusca: Gastropoda) en el Mediterráneo. In Les Espèces Marines à Protéger en Méditerranée; Bouderesque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1991; pp. 133–140. [Google Scholar]
- Barea-Azcón, J.M.; Ballesteros-Duperón, E.; Moreno, D. Libro Rojo de los Invertebrados de Andalucía; 4 tomos; Consejería de Medio Ambiente, Junta de Andalucía: Sevilla, Spain, 2008. [Google Scholar]
- Franco, J.N.; Tuya, F.; Bertocci, I.; Rodríguez, L.; Martínez, B.; Sousa-Pinto, I.; Arenas, F. The “golden kelp” Laminaria ochroleuca under global change: Integrating multiple eco-physiological responses with species distribution models. J. Ecol. 2018, 106, 47–58. [Google Scholar] [CrossRef]
- Flores-Moya, A. Warm temperate seaweed communities: A case study of deep-water kelp forests from the Alboran Sea (SW Mediterranean Sea) and the Strait of Gibraltar. In Seaweed Ecophysiology and Ecology. Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Ecological Studies; Springer: Berlin, Germany, 2012; Volume 219, pp. 315–327. [Google Scholar]
- García-Ruiz, C.; Lloris, D.; Rueda, J.L.; García-Martínez, M.C.; Gil de Sola, L. Spatial distribution of ichthyofauna in the northern Alboran Sea (western Mediterranean). J. Nat. Hist. 2015, 49, 19–20. [Google Scholar] [CrossRef]
- Abelló, P.; Valladares, F.J.; Castellón, A. Analysis of the structure of decapod crustacean assemblages off the Catalan coast (North-West Mediterranean). Mar. Biol. 1988, 98, 39–49. [Google Scholar] [CrossRef]
- González, M.; Sánchez, P. Cephalopod assemblages caught by trawling along the Iberian Peninsula Mediterranean coast. Sci. Mar. 2002, 66, 199–208. [Google Scholar] [CrossRef]
- Abad, E.; Preciado, I.; Serrano, A.; Baro, J. Demersal and epibenthic assemblages of trawlable grounds in the northern Alboran Sea (western Mediterranean). Sci. Mar. 2007, 71, 513–524. [Google Scholar] [CrossRef]
- Carney, R.S. Zonation of deep biota in continental margins. Oceanogr. Mar. Biol. Annu. Rev. 2005, 43, 211–278. [Google Scholar]
- Cartes, J.E.; Carrassón, M. Influence of trophic variables on the depth-range distributions and zonation rates of deep-sea megafauna: The case of the Western Mediterranean assemblages. Deep-Sea Res. I Oceanogr. Res. Pap. 2004, 51, 263–279. [Google Scholar] [CrossRef]
- Cartes, J.E.; Maynou, F.; Sardá, F.; Company, J.B.; Lloris, D.; Tudela, S. The Mediterranean deep-sea ecosystems. An overview of their diversity, structure, functioning and anthropogenic impacts. In The Mediterranean Deep-Sea Ecosystems. An Overview of Their Diversity, Structure, Functioning and Anthropogenic Impacts with a Proposal for Their Conservation; Tudela, S., Simard, F., Eds.; Coords; IUCN The World Conservation Union: Rome, Italy, 2004; pp. 9–38. [Google Scholar]
- Labropoulou, M.; Papaconstantinou, C. Community structure and diversity of demersal fish assemblages: The role of fishery. Sci. Mar. 2004, 68, 215–226. [Google Scholar] [CrossRef]
- Colloca, F.; Cardinale, M.; Belluscio, A.; Ardizzone, G. Pattern of distribution and diversity of demersal assemblages in the central Mediterranean Sea. Estuar. Coast. Shelf Sci. 2003, 56, 469–480. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Ciércoles, C.; González-Aguilar, M.; Torres, P.; Serna, J.M.; Rueda, J.L. Patrones de distribución espacio-temporal de especies ícticas demersales circalitorales y batiales en el mar de Alborán. In Proceedings of the XXV Bienal de la Real Sociedad Española de Historia Natural; Pérez de Rubín, J., Ramírez, T., Eds.; Real Sociedad Española de Historia Natural: Madrid, Spain, 2023; pp. 255–270. [Google Scholar]
- Cooper, L.H.N. The boar fish, Capros aper (L.), as a possible biological indicator of water movement. J. Mar. Biol. Assoc. UK 1952, 31, 351–362. [Google Scholar] [CrossRef]
- García-Ruiz, C. Estudio de la Distribución y Diversidad Ictiofaunística del Mar de Alborán. Ph.D. Thesis, University of Málaga, Málaga, Spain, 2012. [Google Scholar]
- García-Rodríguez, M.; Abelló, P.; Fernández, A.; Esteban, A. Demersal assemblages on the soft bottoms off the Catalan-Levante coast of the Spanish Mediterranean. J. Mar. Biol. 2011, 976396. [Google Scholar] [CrossRef][Green Version]
- Katsanevakis, S.; Maravelias, C.D.; Damalas, D.; Karageorgis, A.P.; Tsitsika, E.V.; Anagnostou, C.; Papaconstantinou, C. Spatio-temporal distribution and habitat use of commercial demersal species in the eastern Mediterranean Sea. Fish. Oceanogr. 2009, 18, 439–457. [Google Scholar] [CrossRef]
- Cartes, J.E.; Serrano, A.; Velasco, F.; Parra, S.; Sánchez, F. Community structure and dynamics of deep-water decapod assemblages from Le Danois Bank (Cantabrian Sea, NE Atlantic): Influence of environmental variables and food availability. Prog. Oceanogr. 2007, 75, 797–816. [Google Scholar] [CrossRef]
- Cartes, J.E.; Maynou, F.; Fanelli, E.; Romano, C.; Mamouridis, V.; Papiol, V. The distribution of megabenthic, invertebrate epifauna in the Balearic Basin (western Mediterranean) between 400 and 2300 m: Environmental gradients influencing assemblages composition and biomass trends. J. Sea Res. 2009, 61, 244–257. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Delgado, M.; Hidalgo, M. Patterns of spatial changes on demersal species in the Gulf of Cadiz and northern Alboran Sea. Mediterr. Mar. Sci. 2022, 23, 55–68. [Google Scholar] [CrossRef]
- Guijarro, B.; Fanelli, E.; Moranta, J.; Cartes, J.E.; Massutí, E. Small-scale differences in the distribution and population dynamics of pandalid shrimps in the western Mediterranean in relation to environmental factors. Fish. Res. 2012, 119–120, 33–47. [Google Scholar] [CrossRef]
- Carbonell, A.; Abelló, P. Distribution characteristics of pandalid shrimps (Decapoda: Caridea: Pandalidae) along the western Mediterranean Sea. J. Nat. Hist. 1998, 32, 1463–1474. [Google Scholar] [CrossRef]
- Carbonell, A.; Palmer, M.; Abelló, P.; Torres, P.; Alemany, R.; Gil de Sola, L. Mesoscale geographical patterns in the distribution of pandalid shrimps Plesionika spp. in the Western Mediterranean. Mar. Ecol. Prog. Ser. 2003, 247, 151–158. [Google Scholar] [CrossRef][Green Version]
- Abelló, P.; Carbonell, A.; Torres, P. Biogeography of epibenthic crustaceans on the shelf and upper slope off the Iberian Peninsula Mediterranean coasts: Implications for the establishment of natural management areas. Sci. Mar. 2002, 66, 183–198. [Google Scholar] [CrossRef]
- Colloca, F.; Mastrantonio, G.; Lasinio, G.J.; Ligas, A.; Sartor, P. Parapenaeus longirostris (Lucas, 1846) an early warning indicator species of global warming in the central Mediterranean Sea. J. Mar. Syst. 2014, 138, 29–39. [Google Scholar] [CrossRef]
- Pierce, G.J.; Sauer, W.; Allcock, A.L.; Smith, J.; Wangvoralak, S.; Jereb, P.; Hastie, L.; Lefkaditou, E. Advances in Squid Biology. Ecology and Fisheries Part I; Rosa, R., O’Dor, R., Pierce, G., Eds.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2013; pp. 73–108. ISBN 978-1-62808-33. [Google Scholar]
- Quetglas, A.; Ordines, F.; Gonzalez, M.; Zaragoza, N.; Mallol, S.; Valls, M.; De Mesa, A. Uncommon pelagic and deep-sea cephalopods in the Mediterranean: New data and literature review. Mediterr. Mar. Sci. 2013, 14, 69–85. [Google Scholar] [CrossRef][Green Version]
- Ordines, F.; Ferriol, P.; Moya, F.; Farias, C.; Rueda, J.L.; García Ruiz, C. First record of the sea cucumber Parastichopus tremulus (Gunnerus, 1767) (Echinodermata: Holothuroidea: Aspidochirotida) in the Mediterranean Sea (Alboran Sea, western Mediterranean). Cah. Biol. Mar. 2019, 60, 111–115. [Google Scholar]
- Tsagarakis, K.; Mytilineou, C.; Haralabous, J.; Lorance, P.; Politou, C.Y.; Dokos, J. Mesoscale spatio- temporal dynamics of demersal assemblages of the Eastern Ionian Sea in relationship with natural and fisheries factors. Aquat. Living Resour. 2013, 26, 381–397. [Google Scholar] [CrossRef]
- González Aguilar, M.; García-Ruiz, C.; García, T.; Serna, J.M.; Ciércoles, C.; Baro, J. Demersal Resources. In Alboran Sea—Ecosystems and Marine Resources; Báez, J.C., Vázquez, J.T., Camiñas, J.A., Malouli Idrissi, M., Eds.; Springer: Cham, Switzerland; Dordrecht, The Netherlands, 2021; pp. 285–358. ISBN 978-3-030-65515-0. [Google Scholar]
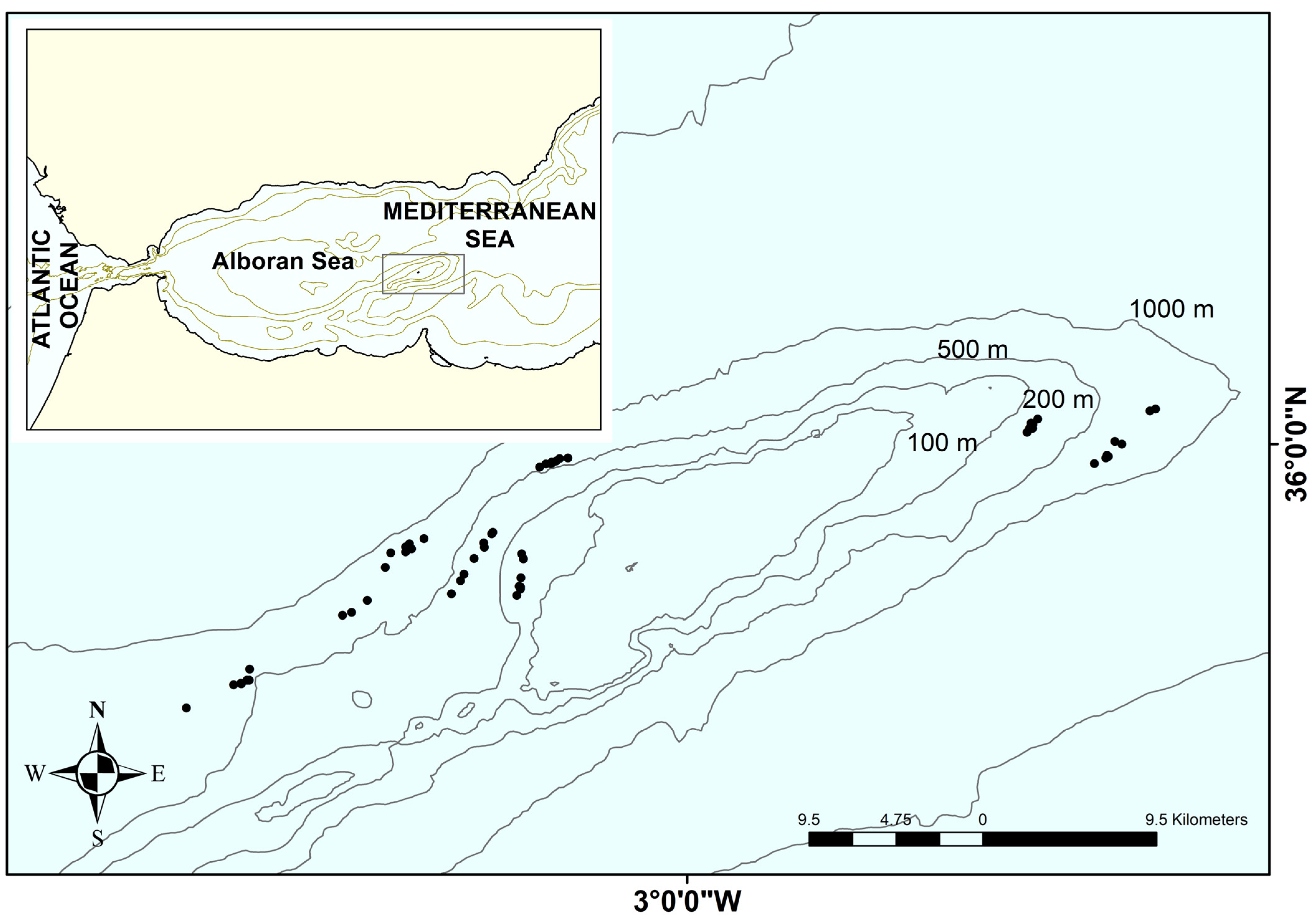

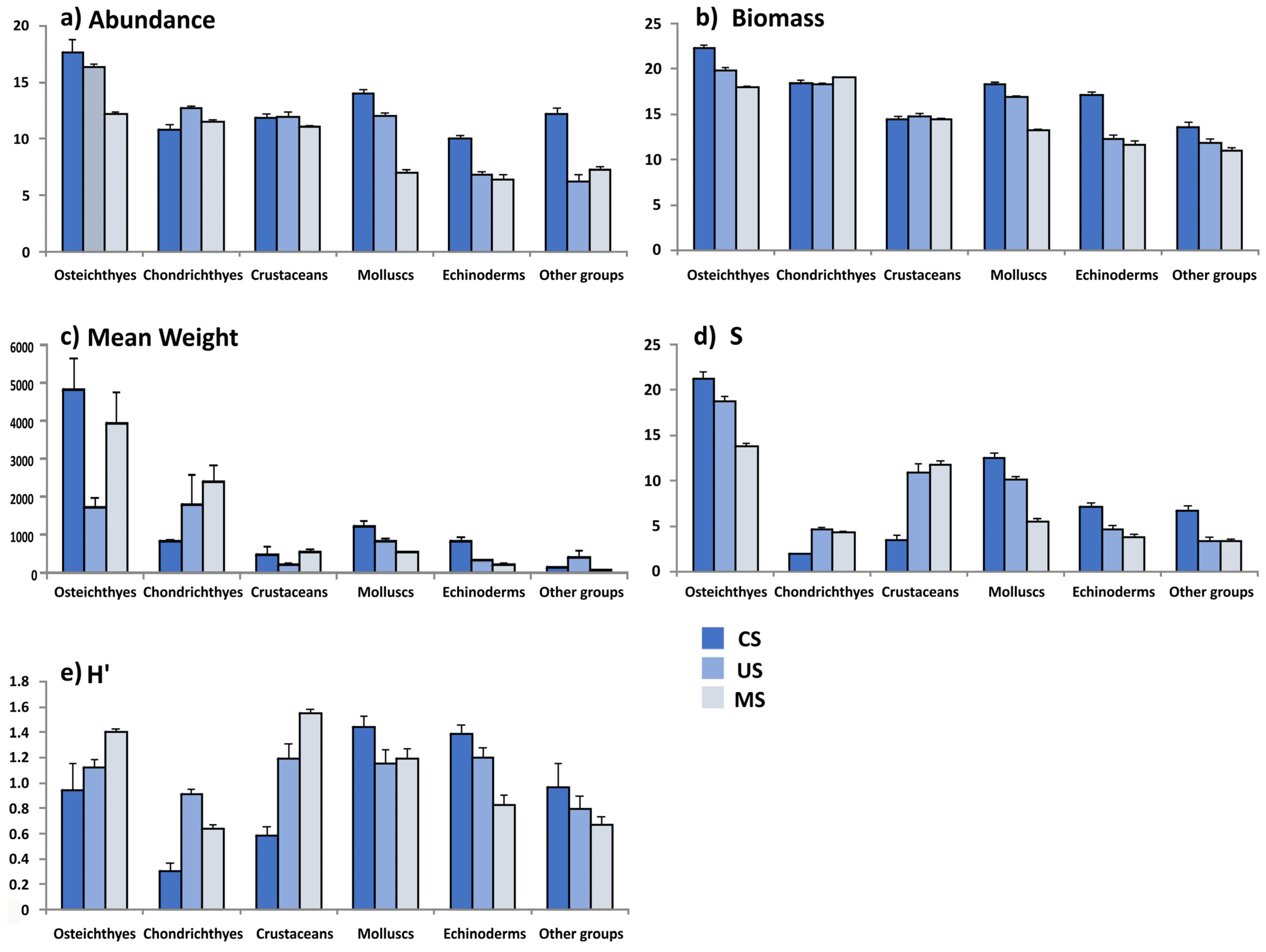
| Taxa | N | W | F Hauls | F Years | Min. Depth | Max. Depth |
|---|---|---|---|---|---|---|
| Osteichthyes | ||||||
| Alepocephalus rostratus Risso, 1820 | 2468 | 354,018 | 35.1 | 100 | 545 | 800 |
| Arctozenus risso (Bonaparte, 1840) | 2 | 26 | 2.7 | 10 | 739 | 800 |
| Argentina sphyraena Linnaeus, 1758 | 11 | 214 | 1.4 | 10 | 340 | 340 |
| Argyropelecus hemigymnus Cocco, 1829 | 260 | 196 | 64.9 | 100 | 328 | 800 |
| Arnoglossus imperialis (Rafinesque, 1810) | 640 | 12,107 | 16.2 | 100 | 115 | 360 |
| Arnoglossus laterna (Walbaum, 1792) | 6 | 60 | 2.7 | 20 | 117 | 352 |
| Arnoglossus thori Kyle, 1913 | 55 | 590 | 10.8 | 70 | 115 | 563 |
| Bathysolea profundicola (Vaillant, 1888) | 308 | 7434 | 27.0 | 100 | 328 | 545 |
| Benthosema glaciale (Reinhardt, 1837) | 353 | 329 | 27.0 | 70 | 332 | 800 |
| Benthosema suborbitale (Gilbert, 1913) | 11 | 9 | 1.4 | 10 | 751 | 751 |
| Blennius ocellaris Linnaeus, 1758 | 10 | 130 | 8.1 | 60 | 117 | 136 |
| Boops boops (Linnaeus, 1758) | 1958 | 174,822 | 16.2 | 100 | 115 | 363 |
| Callionymus maculatus Rafinesque, 1810 | 1 | 5 | 1.4 | 10 | 330 | 330 |
| Capros aper (Linnaeus, 1758) | 7361 | 74,426 | 47.3 | 100 | 115 | 686 |
| Carapus acus (Brünnich, 1768) | 2 | 10 | 2.7 | 20 | 117 | 130 |
| Centracanthus cirrus Rafinesque, 1810 | 4021 | 215,323 | 12.2 | 90 | 115 | 154 |
| Centrolophus niger (Gmelin, 1789) | 2 | 3474 | 2.7 | 20 | 558 | 791 |
| Cepola macrophthalma (Linnaeus, 1758) | 1 | 66 | 1.4 | 10 | 127 | 127 |
| Ceratoscopelus maderensis (Lowe, 1839) | 48 | 56 | 21.6 | 80 | 332 | 800 |
| Chauliodus sloani Bloch and Schneider, 1801 | 20 | 640 | 21.6 | 90 | 541 | 800 |
| Chelidonichthys cuculus (Linnaeus, 1758) | 117 | 13,602 | 12.2 | 90 | 115 | 154 |
| Chelidonichthys lastoviza (Bonnaterre, 1788) | 233 | 19,056 | 14.9 | 100 | 115 | 330 |
| Chelidonichthys lucerna (Linnaeus, 1758) | 1 | 2950 | 1.4 | 10 | 154 | 154 |
| Chlorophthalmus agassizi Bonaparte, 1840 | 1005 | 14,252 | 14.9 | 90 | 328 | 352 |
| Coelorinchus caelorhincus (Risso, 1810) | 85,251 | 776,586 | 52.7 | 100 | 136 | 793 |
| Coelorinchus mediterraneus Iwamoto and Ungaro, 2002 | 3 | 113 | 2.7 | 20 | 793 | 800 |
| Conger conger (Linnaeus, 1758) | 252 | 63,211 | 70.3 | 100 | 328 | 800 |
| Diaphus holti Tåning, 1918 | 3 | 6 | 2.7 | 20 | 739 | 793 |
| Diaphus sp. | 6 | 14 | 6.8 | 30 | 344 | 748 |
| Epigonus denticulatus Dieuzeide, 1950 | 19,818 | 247,369 | 52.7 | 100 | 328 | 800 |
| Epigonus telescopus (Risso, 1810) | 22 | 11,814 | 14.9 | 90 | 551 | 800 |
| Evermannella balbo (Risso, 1820) | 3 | 20 | 4.1 | 30 | 554 | 695 |
| Gadella maraldi (Risso, 1810) | 1 | 2 | 1.4 | 10 | 556 | 556 |
| Gadiculus argenteus Guichenot, 1850 | 92,970 | 517,419 | 35.1 | 100 | 117 | 764 |
| Gaidropsarus biscayensis (Collett, 1890) | 4 | 31 | 5.4 | 30 | 329 | 354 |
| Glossanodon leioglossus (Valenciennes, 1848) | 6375 | 100,310 | 4.1 | 20 | 117 | 351 |
| Helicolenus dactylopterus (Delaroche, 1809) | 3445 | 130,621 | 54.1 | 100 | 328 | 746 |
| Hoplostethus mediterraneus Cuvier, 1829 | 11,109 | 67,764 | 78.4 | 100 | 328 | 800 |
| Hygophum benoiti (Cocco, 1838) | 30 | 24 | 6.8 | 30 | 541 | 746 |
| Hygophum sp. | 1 | 3 | 1.4 | 10 | 689 | 689 |
| Hymenocephalus italicus Giglioli, 1884 | 1 | 10 | 1.4 | 10 | 329 | 329 |
| Lampanyctus crocodilus (Risso, 1810) | 3472 | 48,242 | 73.0 | 100 | 330 | 800 |
| Lepidopus caudatus (Euphrasen, 1788) | 41 | 876 | 14.9 | 60 | 115 | 526 |
| Lepidorhombus boscii (Risso, 1810) | 3 | 431 | 4.1 | 30 | 346 | 380 |
| Lepidotrigla cavillone (Lacepède, 1801) | 1 | 10 | 1.4 | 10 | 154 | 154 |
| Lepidotrigla dieuzeidei Blanc and Hureau, 1973 | 1403 | 19,440 | 13.5 | 100 | 115 | 154 |
| Lestidiops jayakari (Boulenger, 1889) | 4 | 31 | 2.7 | 20 | 332 | 800 |
| Lestidiops sphyrenoides (Risso, 1820) | 2 | 19 | 2.7 | 10 | 351 | 793 |
| Lobianchia dofleini (Zugmayer, 1911) | 22 | 18 | 16.2 | 40 | 344 | 751 |
| Lophius budegassa Spinola, 1807 | 83 | 139,641 | 52.7 | 100 | 115 | 800 |
| Lophius piscatorius Linnaeus, 1758 | 10 | 72,492 | 12.2 | 50 | 117 | 735 |
| Macroramphosus scolopax (Linnaeus, 1758) | 24 | 116 | 6.8 | 40 | 115 | 332 |
| Maurolicus muelleri (Gmelin, 1789) | 2 | 4 | 2.7 | 20 | 330 | 339 |
| Merluccius merluccius (Linnaeus, 1758) | 19 | 4274 | 8.1 | 50 | 115 | 374 |
| Microchirus variegatus (Donovan, 1808) | 36 | 916 | 8.1 | 50 | 115 | 330 |
| Micromesistius poutassou (Risso, 1827) | 33 | 2959 | 17.6 | 60 | 328 | 742 |
| Mullus surmuletus Linnaeus, 1758 | 3 | 298 | 4.1 | 30 | 128 | 154 |
| Myctophidae Gill, 1893 | 3 | 5 | 2.7 | 20 | 554 | 751 |
| Myctophum punctatum Rafinesque, 1810 | 15 | 27 | 14.9 | 70 | 115 | 642 |
| Nemichthys scolopaceus Richardson, 1848 | 1 | 50 | 1.4 | 10 | 773 | 773 |
| Nezumia aequalis (Günther, 1878) | 11,679 | 293,135 | 62.2 | 100 | 332 | 800 |
| Notacanthus bonaparte Risso, 1840 | 81 | 2713 | 28.4 | 90 | 545 | 800 |
| Notoscopelus bolini Nafpaktitis, 1975 | 1 | 4 | 1.4 | 10 | 739 | 739 |
| Notoscopelus elongatus (Costa, 1844) | 35 | 316 | 10.8 | 60 | 343 | 642 |
| Ophichthus rufus (Rafinesque, 1810) | 2 | 88 | 1.4 | 10 | 332 | 332 |
| Ophidion barbatum Linnaeus, 1758 | 3 | 18 | 4.1 | 30 | 117 | 154 |
| Ophisurus serpens (Linnaeus, 1758) | 2 | 1260 | 2.7 | 20 | 332 | 340 |
| Pagellus acarne (Risso, 1827) | 1331 | 102,381 | 24.3 | 100 | 115 | 380 |
| Pagellus bogaraveo (Brünnich, 1768) | 53 | 8535 | 28.4 | 90 | 329 | 800 |
| Pagellus erythrinus (Linnaeus, 1758) | 3 | 235 | 2.7 | 20 | 128 | 136 |
| Paralepis coregonoides Risso, 1820 | 5 | 38 | 4.1 | 10 | 328 | 558 |
| Peristedion cataphractum (Linnaeus, 1758) | 185 | 2572 | 16.2 | 70 | 330 | 374 |
| Phycis blennoides (Brünnich, 1768) | 900 | 134,402 | 82.4 | 100 | 328 | 800 |
| Sardina pilchardus (Walbaum, 1792) | 324 | 20,202 | 2.7 | 20 | 118 | 125 |
| Sardinella aurita Valenciennes, 1847 | 2 | 292 | 1.4 | 10 | 117 | 117 |
| Schedophilus medusophagus (Cocco, 1839) | 7 | 5951 | 6.8 | 40 | 642 | 800 |
| Scomber colias Gmelin, 1789 | 2 | 498 | 2.7 | 20 | 117 | 125 |
| Scomber scombrus Linnaeus, 1758 | 1 | 239 | 1.4 | 10 | 117 | 117 |
| Scorpaena elongata Cadenat, 1943 | 60 | 4873 | 20.3 | 100 | 115 | 380 |
| Scorpaena loppei Cadenat, 1943 | 60 | 766 | 12.2 | 90 | 115 | 154 |
| Serranus cabrilla (Linnaeus, 1758) | 325 | 10,661 | 13.5 | 100 | 115 | 154 |
| Serranus hepatus (Linnaeus, 1758) | 550 | 6694 | 13.5 | 100 | 115 | 154 |
| Sphoeroides pachygaster (Müller and Troschel, 1848) | 1 | 119 | 1.4 | 10 | 130 | 130 |
| Spicara smaris (Linnaeus, 1758) | 1 | 46 | 1.4 | 10 | 118 | 118 |
| Stomias boa (Risso, 1810) | 170 | 1069 | 56.8 | 100 | 329 | 800 |
| Symbolophorus veranyi (Moreau, 1888) | 1 | 5 | 1.4 | 10 | 593 | 593 |
| Symphurus ligulatus (Cocco, 1844) | 3 | 16 | 1.4 | 10 | 751 | 751 |
| Symphurus nigrescens Rafinesque, 1810 | 6 | 23 | 4.1 | 20 | 136 | 558 |
| Synchiropus phaeton (Günther, 1861) | 2181 | 41,666 | 40.5 | 100 | 136 | 800 |
| Trachinus draco (Linnaeus, 1758) | 13 | 1806 | 9.5 | 70 | 115 | 154 |
| Trachurus mediterraneus (Steindachner, 1868) | 43 | 1301 | 4.1 | 30 | 115 | 127 |
| Trachurus picturatus (Bowdich, 1825) | 117 | 10,712 | 9.5 | 70 | 115 | 564 |
| Trachurus trachurus (Linnaeus, 1758) | 8,075,908 | 4,063,083 | 35.1 | 100 | 115 | 695 |
| Trachyrincus scabrus (Rafinesque, 1810) | 4227 | 716,202 | 58.1 | 100 | 526 | 800 |
| Trachyscorpia cristulata (Goode and Bean, 1896) | 1 | 1950 | 1.4 | 10 | 746 | 746 |
| Trigla lyra Linnaeus, 1758 | 4 | 487 | 4.1 | 30 | 329 | 351 |
| Uranoscopus scaber Linnaeus, 1758 | 4 | 1072 | 5.4 | 40 | 117 | 130 |
| Vinciguerria sp. | 4 | 3 | 4.1 | 10 | 344 | 692 |
| Zeus faber Linnaeus, 1758 | 8 | 11,453 | 6.8 | 50 | 117 | 154 |
| Chondrichthyes | ||||||
| Centrophorus uyato (Rafinesque, 1810) (*) | 2 | 4550 | 1.4 | 10 | 558 | 558 |
| Chimaera monstrosa Linnaeus, 1758 | 97 | 40,101 | 44.6 | 100 | 380 | 800 |
| Dalatias licha (Bonnaterre, 1788) (*) | 9 | 22,012 | 10.8 | 70 | 526 | 793 |
| Dipturus nidarosiensis (Storm, 1881) | 24 | 8737.2 | 14.9 | 60 | 542 | 800 |
| Dipturus oxyrinchus (Linnaeus, 1758) | 4 | 9446 | 4.1 | 30 | 526 | 800 |
| Dipturus sp. | 1 | 1400 | 1.4 | 10 | 689 | 689 |
| Etmopterus spinax (Linnaeus, 1758) | 1947 | 196,400 | 64.9 | 100 | 329 | 800 |
| Galeorhinus galeus (Linnaeus, 1758) (*) | 1 | 9600 | 1.4 | 10 | 369 | 369 |
| Galeus atlanticus (Vaillant, 1888) | 5852 | 490,682 | 67.6 | 100 | 328 | 793 |
| Galeus melastomus Rafinesque, 1810 | 23,450 | 2,701,574 | 86.5 | 100 | 328 | 800 |
| Leucoraja circularis (Couch, 1838) (*) | 2 | 68 | 2.7 | 20 | 346 | 752 |
| Leucoraja melitensis (Clark, 1926) (*) | 128 | 32,284 | 6.8 | 10 | 117 | 764 |
| Leucoraja naevus (Müller and Henle, 1841) | 417 | 173,595 | 35.1 | 90 | 115 | 751 |
| Oxynotus centrina (Linnaeus, 1758) (*) | 2 | 1149 | 2.7 | 20 | 380 | 800 |
| Raja clavata Linnaeus, 1758 | 3 | 406 | 4.1 | 30 | 343 | 380 |
| Scyliorhinus canicula (Linnaeus, 1758) | 3593 | 439,875 | 47.3 | 100 | 115 | 582 |
| Tetronarce nobiliana (Bonaparte, 1835) | 10 | 46,583 | 10.8 | 40 | 332 | 791 |
| Torpedo marmorata Risso, 1810 | 3 | 1415 | 4.1 | 20 | 346 | 363 |
| Crustaceans | ||||||
| Acanthephyra pelagica (Risso, 1816) | 12 | 75 | 9.5 | 50 | 545 | 764 |
| Aegaeon lacazei (Gourret, 1887) | 4 | 6 | 5.4 | 30 | 360 | 569 |
| Alpheus glaber (Olivi, 1792) | 7 | 8 | 8.1 | 40 | 350 | 569 |
| Amalopenaeus elegans Smith, 1882 | 2 | 2 | 1.4 | 10 | 558 | 558 |
| Aristeus antennatus (Risso, 1816) | 1702 | 37,902 | 39.2 | 100 | 545 | 800 |
| Atelecyclus rotundatus (Olivi, 1792) | 5 | 40 | 5.4 | 20 | 117 | 330 |
| Bathynectes maravigna (Prestandrea, 1839) | 184 | 6238 | 45.9 | 100 | 329 | 800 |
| Calappa granulata (Linnaeus, 1758) | 17 | 1551 | 1.4 | 10 | 329 | 329 |
| Calocaris macandreae Bell, 1846 | 16 | 14 | 14.9 | 70 | 526 | 800 |
| Chlorotocus crassicornis (A. Costa, 1871) | 10 | 18 | 4.1 | 20 | 329 | 339 |
| Dardanus arrosor (Herbst, 1796) | 2602 | 11,857 | 68.9 | 100 | 115 | 800 |
| Deosergestes henseni (Ortmann, 1893) | 24 | 14 | 8.1 | 30 | 330 | 791 |
| Dorhynchus thomsoni Thomson, 1873 | 10 | 7 | 6.8 | 30 | 354 | 771 |
| Ebalia nux A. Milne-Edwards, 1883 | 1 | 1 | 1.4 | 10 | 564 | 564 |
| Ergasticus clouei A. Milne-Edwards, 1882 | 34 | 45 | 16.2 | 60 | 330 | 642 |
| Eurynome aspera (Pennant, 1777) | 1 | 1 | 1.4 | 10 | 117 | 117 |
| Eusergestes arcticus (Krøyer, 1855) | 3 | 3 | 4.1 | 20 | 554 | 743 |
| Geryon longipes A. Milne-Edwards, 1882 | 111 | 11,453 | 33.8 | 100 | 542 | 800 |
| Inachus aguiarii de Brito Capello, 1876 | 1 | 1 | 1.4 | 10 | 136 | 136 |
| Inachus dorsettensis (Pennant, 1777) | 6 | 9 | 6.8 | 40 | 117 | 541 |
| Inachus thoracicus Roux, 1830 | 2 | 2 | 1.4 | 10 | 125 | 125 |
| Iridonida speciosa (von Martens, 1878) | 650 | 1526 | 5.4 | 30 | 332 | 352 |
| Liocarcinus depurator (Linnaeus, 1758) | 64 | 1233 | 13.5 | 70 | 117 | 374 |
| Lophogaster typicus M. Sars, 1857 | 1 | 1 | 1.4 | 10 | 380 | 380 |
| Macropipus tuberculatus (Roux, 1830) | 452 | 8832 | 35.1 | 100 | 117 | 746 |
| Macropodia tenuirostris (Leach, 1814) | 34 | 56 | 17.6 | 90 | 115 | 692 |
| Medorippe lanata (Linnaeus, 1767) | 5 | 56 | 5.4 | 30 | 329 | 551 |
| Monodaeus couchii (Couch, 1851) | 32 | 149 | 31.1 | 100 | 117 | 800 |
| Munida intermedia A. Milne-Edwards & Bouvier, 1899 | 26 | 315 | 12.2 | 60 | 329 | 360 |
| Nephrops norvegicus (Linnaeus, 1758) | 136 | 12,207.4 | 43.2 | 100 | 329 | 793 |
| Pagurus alatus Fabricius, 1775 | 779 | 2871.4 | 59.5 | 100 | 344 | 800 |
| Pagurus excavatus (Herbst, 1791) | 1 | 7 | 1.4 | 10 | 330 | 330 |
| Pagurus prideaux Leach, 1815 | 3084 | 23,791 | 44.6 | 100 | 115 | 791 |
| Palinurus elephas (Fabricius, 1787) (*) | 3 | 4536 | 4.1 | 30 | 117 | 136 |
| Palinurus mauritanicus Gruvel, 1911 | 17 | 23,585 | 14.9 | 80 | 329 | 593 |
| Parapenaeus longirostris (Lucas, 1846) | 924 | 5961 | 21.6 | 100 | 329 | 551 |
| Parthenopoides massena (Roux, 1830) | 2 | 72 | 2.7 | 20 | 339 | 346 |
| Pasiphaea multidentata Esmark, 1866 | 462 | 2972 | 55.4 | 100 | 526 | 800 |
| Pasiphaea sivado (Risso, 1816) | 1 | 1 | 1.4 | 10 | 329 | 329 |
| Penaeopsis serrata Spence Bate, 1881 | 3 | 14 | 4.1 | 20 | 339 | 582 |
| Philocheras echinulatus (M. Sars, 1862) | 3 | 3 | 1.4 | 10 | 330 | 330 |
| Phronima sedentaria (Forskål, 1775) | 6 | 4 | 5.4 | 30 | 563 | 743 |
| Plesionika acanthonotus (Smith, 1882) | 1794 | 5131 | 58.1 | 100 | 526 | 800 |
| Plesionika antigai Zariquiey Álvarez, 1955 | 1169 | 1951 | 21.6 | 90 | 328 | 752 |
| Plesionika edwardsii (Brandt, 1851) | 523 | 22,068 | 17.6 | 80 | 329 | 593 |
| Plesionika gigliolii (Senna, 1902) | 8 | 19 | 6.8 | 50 | 526 | 556 |
| Plesionika heterocarpus (A. Costa, 1871) | 11,806 | 29,111 | 24.3 | 100 | 328 | 566 |
| Plesionika martia (A. Milne-Edwards, 1883) | 4257 | 26,267 | 67.6 | 100 | 330 | 800 |
| Plesionika narval (Fabricius, 1787) | 1 | 1 | 1.4 | 10 | 330 | 330 |
| Polybius henslowii Leach, 1820 | 1 | 4 | 1.4 | 10 | 136 | 136 |
| Polycheles typhlops Heller, 1862 | 180 | 1368 | 48.6 | 100 | 332 | 800 |
| Pontophilus spinosus (Leach, 1816) | 2 | 3 | 2.7 | 20 | 330 | 563 |
| Processa canaliculata Leach, 1815 | 12 | 30 | 10.8 | 50 | 329 | 569 |
| Processa nouveli Al-Adhub and Williamson, 1975 | 2 | 1 | 1.4 | 10 | 330 | 330 |
| Robustosergia robusta (Smith, 1882) | 1841 | 3263 | 60.8 | 100 | 339 | 800 |
| Scyramathia carpenteri (Thomson, 1873) | 33 | 243 | 28.4 | 90 | 354 | 800 |
| Solenocera membranacea (Risso, 1816) | 48 | 116 | 23.0 | 100 | 329 | 593 |
| Spinolambrus macrochelos (Herbst, 1790) | 1 | 46 | 1.4 | 10 | 329 | 329 |
| Squilla mantis (Linnaeus, 1758) | 2 | 5 | 1.4 | 10 | 551 | 551 |
| Molluscs | ||||||
| Abralia veranyi (Rüppell, 1844) | 5240 | 13,708 | 50.0 | 100 | 115 | 800 |
| Alloteuthis media (Linnaeus, 1758) | 44 | 185 | 9.5 | 50 | 117 | 563 |
| Alloteuthis sp. | 11 | 73 | 1.4 | 10 | 127 | 127 |
| Alloteuthis subulata (Lamarck, 1798) | 28 | 143 | 5.4 | 30 | 115 | 564 |
| Ancistrocheirus lesueurii (d’Orbigny, 1842) | 1 | 354 | 1.4 | 10 | 548 | 548 |
| Ancistroteuthis lichtensteinii (A. Férussac [in A. Férussac and d’Orbigny], 1835) | 13 | 326 | 16.2 | 70 | 352 | 800 |
| Aporrhais serresiana (Michaud, 1828) | 30 | 89 | 16.2 | 60 | 329 | 771 |
| Argonauta argo Linnaeus, 1758 | 1 | 5 | 1.4 | 10 | 593 | 593 |
| Babelomurex benoiti (Tiberi, 1855) (*) | 1 | 3 | 1.4 | 10 | 154 | 154 |
| Baptodoris cinnabarina Bergh, 1884 | 1 | 1 | 1.4 | 10 | 566 | 566 |
| Bathypolipus sp. | 77 | 1239 | 16.2 | 50 | 330 | 800 |
| Bathypolypus sponsalis (P. Fischer and H. Fischer, 1892) | 154 | 5608 | 51.4 | 90 | 328 | 800 |
| Berthellina edwardsii (Vayssière, 1897) | 5 | 16 | 1.4 | 10 | 125 | 125 |
| Brachioteuthis riisei (Steenstrup, 1882) | 6 | 35 | 5.4 | 40 | 541 | 800 |
| Buccinum humphreysianum Bennett, 1824 | 13 | 164 | 8.1 | 50 | 526 | 791 |
| Calliostoma granulatum (Born, 1778) | 2 | 4 | 2.7 | 20 | 350 | 360 |
| Callumbonella suturalis (R. A. Philippi, 1836) | 1 | 1 | 1.4 | 10 | 555 | 555 |
| Capulus ungaricus (Linnaeus, 1758) | 45 | 15 | 1.4 | 10 | 154 | 154 |
| Chama circinata Monterosato, 1878 | 230 | 421 | 5.4 | 40 | 115 | 154 |
| Chama gryphoides Linnaeus, 1758 | 5 | 16 | 1.4 | 10 | 125 | 125 |
| Charonia lampas (Linnaeus, 1758) (*) | 11 | 4395 | 9.5 | 70 | 117 | 136 |
| Chiton sp. | 30 | 30 | 1.4 | 10 | 154 | 154 |
| Clelandella miliaris (Brocchi, 1814) | 1 | 1 | 1.4 | 10 | 739 | 739 |
| Colus jeffreysianus (P. Fischer, 1868) | 8 | 54 | 4.1 | 10 | 542 | 739 |
| Colus sp. | 7 | 46 | 5.4 | 40 | 526 | 558 |
| Cymbulia peronii Blainville, 1818 | 159 | 395 | 18.9 | 40 | 130 | 793 |
| Eledone cirrhosa (Lamarck, 1798) | 2 | 587 | 2.7 | 20 | 343 | 354 |
| Eledone moschata (Lamarck, 1798) | 119 | 24,739 | 17.6 | 100 | 115 | 354 |
| Euspira fusca (Blainville, 1825) | 13 | 56 | 9.5 | 60 | 330 | 747 |
| Fusiturris similis (Bivona, 1838) | 16 | 56 | 5.4 | 30 | 350 | 773 |
| Galeodea rugosa (Linnaeus, 1771) | 61 | 3660 | 33.8 | 100 | 526 | 800 |
| Heteroteuthis dispar (Rüppell, 1844) | 1 | 5 | 1.4 | 10 | 556 | 556 |
| Histioteuthis bonnellii (A. Férussac, 1835) | 5 | 2050 | 5.4 | 30 | 526 | 582 |
| Histioteuthis reversa (A. E. Verrill, 1880) | 12 | 365 | 13.5 | 50 | 541 | 752 |
| Illex coindetii (Vérany, 1839) | 320 | 38,042 | 16.2 | 60 | 117 | 764 |
| Loligo forbesii Steenstrup, 1856 | 1332 | 109,041 | 32.4 | 100 | 115 | 380 |
| Loligo vulgaris Lamarck, 1798 | 778 | 9048 | 2.7 | 20 | 125 | 127 |
| Neopycnodonte cochlear (Poli, 1795) | 4283 | 27,131 | 25.7 | 100 | 115 | 800 |
| Neorossia caroli (Joubin, 1902) | 48 | 955 | 29.7 | 90 | 329 | 800 |
| Octopus salutii Vérany, 1839 | 2 | 747 | 2.7 | 20 | 329 | 369 |
| Octopus vulgaris Cuvier, 1797 | 168 | 66,470 | 14.9 | 100 | 115 | 360 |
| Onychoteuthis banksii (Leach, 1817) | 1 | 26 | 1.4 | 10 | 800 | 800 |
| Orania fusulus (Brocchi, 1814) | 9 | 9 | 1.4 | 10 | 130 | 130 |
| Patellogastropoda | 36 | 36 | 1.4 | 10 | 130 | 130 |
| Peltodoris atromaculata Bergh, 1880 | 4 | 5 | 2.7 | 20 | 136 | 154 |
| Pododesmus patelliformis (Linnaeus, 1761) | 150 | 150 | 1.4 | 10 | 115 | 115 |
| Pteria hirundo (Linnaeus, 1758) | 5 | 20 | 1.4 | 10 | 115 | 115 |
| Pteroctopus tetracirrhus (Delle Chiaje, 1830) | 6 | 2496 | 4.1 | 30 | 328 | 351 |
| Ranella olearium (Linnaeus, 1758) (*) | 66 | 9189 | 28.4 | 80 | 117 | 800 |
| Rhombosepion elegans (Blainville, 1827) | 147 | 1173 | 16.2 | 90 | 115 | 343 |
| Rhombosepion orbignyanum (Férussac, 1826) | 7670 | 134,792 | 40.5 | 100 | 115 | 380 |
| Rondeletiola minor (Naef, 1912) | 141 | 171 | 13.5 | 90 | 328 | 380 |
| Rossia macrosoma (Delle Chiaje, 1830) | 279 | 7155 | 29.7 | 100 | 115 | 569 |
| Scaeurgus unicirrhus (Delle Chiaje [in Férussac and d’Orbigny], 1841) | 4 | 785 | 4.1 | 20 | 128 | 339 |
| Scaphander lignarius (Linnaeus, 1758) | 5 | 85 | 5.4 | 40 | 526 | 592 |
| Semicassis saburon (Bruguière, 1792) | 1 | 23 | 1.4 | 10 | 526 | 526 |
| Sepietta oweniana (d’Orbigny, 1841) | 122 | 247 | 10.8 | 50 | 330 | 374 |
| Stoloteuthis leucoptera (A. E. Verrill, 1878) | 1 | 1 | 1.4 | 10 | 346 | 346 |
| Tetrarca tetragona (Poli, 1795) | 3385 | 3507 | 13.5 | 90 | 115 | 360 |
| Todarodes sagittatus (Lamarck, 1798) | 319 | 96,137 | 78.4 | 100 | 115 | 800 |
| Todaropsis eblanae (Ball, 1841) | 9 | 899 | 9.5 | 50 | 332 | 380 |
| Xenophora crispa (König, 1825) | 647 | 6429 | 44.6 | 100 | 115 | 773 |
| Echinoderms | ||||||
| Anseropoda placenta (Pennant, 1777) | 7 | 48 | 5.4 | 40 | 118 | 526 |
| Antedon mediterranea (Lamarck, 1816) | 1 | 1 | 1.4 | 10 | 735 | 735 |
| Asterina gibbosa (Pennant, 1777) (*) | 1 | 1 | 1.4 | 10 | 127 | 127 |
| Astropecten irregularis (Pennant, 1777) | 19 | 87 | 13.5 | 70 | 115 | 569 |
| Centrostephanus longispinus (Philippi, 1845) (*) | 2 | 89 | 2.7 | 20 | 117 | 125 |
| Chaetaster longipes (Bruzelius, 1805) | 85 | 661 | 31.1 | 100 | 115 | 739 |
| Cidaris cidaris (Linnaeus, 1758) | 179 | 4369 | 48.6 | 100 | 115 | 771 |
| Coscinasterias tenuispina (Lamarck, 1816) | 38 | 65 | 1.4 | 10 | 128 | 128 |
| Echinaster (Echinaster) sepositus (Retzius, 1783) | 1 | 70 | 1.4 | 10 | 130 | 130 |
| Echinus melo Lamarck, 1816 | 117 | 30,227 | 31.1 | 100 | 115 | 800 |
| Gracilechinus acutus (Lamarck, 1816) | 269 | 3249 | 44.6 | 100 | 115 | 800 |
| Hacelia attenuata Gray, 1840 (*) | 1 | 11 | 1.4 | 10 | 128 | 128 |
| Holothuria sp. | 23 | 6123 | 21.6 | 70 | 154 | 800 |
| Hymenodiscus coronata (Sars, 1871) | 554 | 18,229 | 18.9 | 90 | 136 | 771 |
| Laetmogonidae Ekman, 1926 | 85 | 2063 | 13.5 | 30 | 332 | 742 |
| Leptometra phalangium (Müller, 1841) | 15 | 7 | 8.1 | 60 | 526 | 771 |
| Luidia ciliaris (Philippi, 1837) | 9 | 222 | 5.4 | 30 | 117 | 773 |
| Luidia sarsii Düben and Koren in Düben, 1844 | 102 | 2504 | 32.4 | 100 | 330 | 791 |
| Luidia sp. | 3 | 42 | 4.1 | 30 | 344 | 742 |
| Marthasterias glacialis (Linnaeus, 1758) | 25 | 1352 | 6.8 | 40 | 115 | 800 |
| Mesothuria intestinalis (Ascanius, 1805) | 191 | 2946 | 18.9 | 40 | 380 | 800 |
| Molpadia musculus Risso, 1826 | 5 | 18 | 2.7 | 20 | 566 | 695 |
| Ophiocten abyssicolum (Forbes, 1843) | 1 | 1 | 1.4 | 10 | 551 | 551 |
| Ophioderma longicaudum (Bruzelius, 1805) | 66 | 349 | 16.2 | 50 | 115 | 541 |
| Ophiothrix fragilis (Abildgaard in O.F. Müller, 1789) | 45 | 176 | 10.8 | 60 | 118 | 773 |
| Ophiotrix sp. | 20 | 41 | 4.1 | 20 | 115 | 360 |
| Ophiura ophiura (Linnaeus, 1758) | 16 | 82 | 9.5 | 30 | 130 | 751 |
| Ophiura sp. | 31 | 16 | 2.7 | 20 | 128 | 346 |
| Parastichopus regalis (Cuvier, 1817) | 334 | 74,429 | 54.1 | 100 | 115 | 800 |
| Parastichopus tremulus (Gunnerus, 1767) | 1 | 277 | 1.4 | 10 | 689 | 689 |
| Sclerasterias sp. | 1 | 11 | 1.4 | 10 | 125 | 125 |
| Spatangus purpureus O.F. Müller, 1776 | 3 | 380 | 2.7 | 20 | 117 | 130 |
| Stylocidaris affinis (Philippi, 1845) | 1 | 210 | 1.4 | 10 | 354 | 354 |
| Tethyaster subinermis (Philippi, 1837) | 30 | 3651 | 29.7 | 100 | 125 | 800 |
| Ascidians | ||||||
| Ascidia mentula Müller, 1776 | 10 | 167 | 5.4 | 40 | 117 | 128 |
| Ascidia sp. | 3 | 9 | 1.4 | 10 | 593 | 593 |
| Ascidiidae Herdman, 1882 | 22 | 184 | 2.7 | 20 | 118 | 130 |
| Ciona intestinalis (Linnaeus, 1767) | 11 | 263 | 2.7 | 10 | 328 | 558 |
| Diazona violacea Savigny, 1816 | 20 | 3201 | 10.8 | 70 | 115 | 380 |
| Microcosmus sp. | 19 | 182 | 2.7 | 20 | 117 | 118 |
| Polycarpa pomaria (Savigny, 1816) | 1 | 7 | 1.4 | 10 | 127 | 127 |
| Polycitor sp. | 6 | 31 | 1.4 | 10 | 556 | 556 |
| Pyura sp. | 5 | 25 | 1.4 | 10 | 115 | 115 |
| Thaliacea | ||||||
| Pyrosoma atlanticum Péron, 1804 | 299 | 5810 | 52.7 | 90 | 115 | 800 |
| Salpa maxima Forskål, 1775 | 119 | 3282 | 23.0 | 70 | 330 | 771 |
| Salpa sp. | 356 | 415 | 8.1 | 20 | 374 | 773 |
| Cnidarians | ||||||
| Actinauge richardi (Marion, 1906) | 177 | 4233 | 39.2 | 90 | 343 | 800 |
| Actiniidae Rafinesque, 1815 | 56 | 2302 | 5.4 | 10 | 329 | 791 |
| Alcyonium palmatum Pallas, 1766 | 9 | 51 | 6.8 | 40 | 117 | 363 |
| Bebryce mollis Philippi, 1842 | 333 | 182 | 2.7 | 20 | 115 | 563 |
| Caryophyllia smithii Stokes and Broderip, 1828 | 150 | 75 | 1.4 | 10 | 115 | 115 |
| Cerianthus membranaceus (Gmelin, 1791) | 3 | 209 | 2.7 | 10 | 374 | 563 |
| Cerianthus sp. | 1 | 17 | 1.4 | 10 | 330 | 330 |
| Eunicella filiformis (Studer, 1878) (*) | 133 | 72 | 6.8 | 50 | 117 | 564 |
| Funiculina quadrangularis (Pallas, 1766) | 39 | 251 | 13.5 | 70 | 330 | 582 |
| Hormathia alba (Andres, 1881) | 7 | 7 | 2.7 | 20 | 592 | 743 |
| Isidella elongata (Esper, 1788)(*) | 143 | 19 | 4.1 | 30 | 689 | 771 |
| Kophobelemnon stelliferum (Müller, 1776) | 39 | 212 | 8.1 | 40 | 555 | 771 |
| Leptogorgia sarmentosa (Esper, 1791) | 1 | 1 | 1.4 | 10 | 592 | 592 |
| Lytocarpia myriophyllum (Linnaeus, 1758) | 5 | 3 | 1.4 | 10 | 526 | 526 |
| Nemertesia ramosa (Lamarck, 1816) | 1 | 1 | 1.4 | 10 | 380 | 380 |
| Paramuricea clavata (Risso, 1827) (*) | 1 | 2 | 1.4 | 10 | 115 | 115 |
| Pelagia noctiluca (Forsskål, 1775) | 2287 | 46,142 | 35.1 | 60 | 118 | 793 |
| Pennatula aculeata Danielssen, 1860 | 6 | 4 | 2.7 | 10 | 695 | 771 |
| Pennatula phosphorea Linnaeus, 1758 | 3 | 13 | 4.1 | 30 | 115 | 558 |
| Pennatula sp. | 2 | 12 | 1.4 | 10 | 548 | 548 |
| Spinimuricea atlantica (Johnson, 1862) (*) | 1 | 3 | 1.4 | 10 | 563 | 563 |
| Porifera | ||||||
| Asconema setubalense Kent, 1870 | 7 | 3574 | 8.1 | 60 | 339 | 380 |
| Axinella polypoides Schmidt, 1862 (*) | 1 | 2 | 1.4 | 10 | 569 | 569 |
| Phakellia ventilabrum (Linnaeus, 1767) | 1 | 87 | 1.4 | 10 | 346 | 346 |
| Poecillastra compressa (Bowerbank, 1866) | 1 | 220 | 1.4 | 10 | 380 | 380 |
| Porifera Grant, 1836 | 19 | 1265 | 16.2 | 70 | 117 | 566 |
| Suberites domuncula (Olivi, 1792) | 3 | 199 | 4.1 | 30 | 117 | 127 |
| Thenea muricata (Bowerbank, 1858) | 10 | 22 | 6.8 | 50 | 548 | 569 |
| Brachiopoda | ||||||
| Argyrotheca cuneata (Risso, 1826) | 1 | 1 | 1.4 | 10 | 127 | 127 |
| Megerlia sp. | 2 | 1 | 1.4 | 10 | 127 | 127 |
| Megerlia truncata (Linnaeus, 1767) | 532 | 158 | 8.1 | 60 | 115 | 136 |
| Novocrania anomala (Müller, 1776) | 1 | 1 | 1.4 | 10 | 136 | 136 |
| Terebratulina retusa (Linnaeus, 1758) | 181 | 106 | 8.1 | 60 | 118 | 564 |
| Terebratulina sp. | 32 | 32 | 1.4 | 10 | 117 | 117 |
| Annelida | ||||||
| Aphrodita aculeata Linnaeus, 1758 | 49 | 210 | 21.6 | 80 | 117 | 771 |
| Aphrodita sp. | 1 | 4 | 1.4 | 10 | 582 | 582 |
| Chloeia sp. | 1 | 1 | 1.4 | 10 | 592 | 592 |
| Chloeia venusta Quatrefages, 1866 | 1 | 1 | 1.4 | 10 | 695 | 695 |
| Hyalinoecia tubicola (O.F. Müller, 1776) | 1077 | 1109 | 13.5 | 70 | 117 | 564 |
| Laetmonice sp. | 6 | 16 | 1.4 | 10 | 125 | 125 |
| Polychaeta Grube, 1850 | 77 | 115 | 6.8 | 40 | 127 | 773 |
| Pontobdella muricata (Linnaeus, 1758) | 1 | 2 | 1.4 | 10 | 330 | 330 |
| Spiochaetopterus sp. | 145 | 121 | 5.4 | 30 | 125 | 771 |
| Sipuncula | ||||||
| Sipunculidae Rafinesque, 1814 | 15 | 76 | 5.4 | 40 | 551 | 752 |
| Algae | ||||||
| Laminaria ochroleuca Bachelot de la Pylaie, 1824 (*) | 3 | 456 | 1.4 | 10 | 117 | 117 |
| (a) Osteichthyes | (d) Molluscs | ||||
|---|---|---|---|---|---|
| CS—Average similarity: 70.68% | CS—Average similarity: 66.28% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Trachurus trachurus | 11.88 | 12.59 | Neopycnodonte cochlear | 8.69 | 17.08 |
| Pagellus acarne | 7.61 | 21.31 | Tetrarca tetragona | 7.38 | 29.61 |
| Lepidotrigla dieuzeidei | 7.66 | 29.88 | Xenophora crispa | 6.15 | 41.33 |
| Boops boops | 7.71 | 38.3 | Octopus vulgaris | 5.64 | 52.47 |
| Serranus hepatus | 6.81 | 45.97 | |||
| Arnoglossus imperialis | 6.88 | 53.57 | |||
| US—Average similarity: 62.99% | US—Average similarity: 54.38% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Coelorinchus caelorhincus | 10.18 | 16.22 | Rhombosepion orbignyanum | 6.58 | 23.4 |
| Gadiculus argenteus | 9.69 | 30.88 | Todarodes sagittatus | 4.23 | 39.02 |
| Capros aper | 6.6 | 40.07 | Rossia macrosoma | 4.31 | 53.78 |
| Synchiropus phaeton | 6.19 | 49.12 | |||
| Helicolenus dactylopterus | 5.24 | 56.19 | |||
| MS—Average similarity: 63.09% | MS—Average similarity: 33.48% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Nezumia aequalis | 7.54 | 19.28 | Todarodes sagittatus | 2.69 | 37.03 |
| Hoplostethus mediterraneus | 5.89 | 33.67 | Bathypolypus sponsalis | 2.11 | 58.28 |
| Lampanyctus crocodilus | 5.7 | 47.05 | |||
| Trachyrincus scabrus | 5.46 | 58.95 | |||
| (b) Chondrichthyes | (e) Echinoderms | ||||
| CS—Average similarity: 79.48% | CS—Average similarity: 50.91% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Scyliorhinus canicula | 7.39 | 71.05 | Parastichopus regalis | 5.78 | 32.27 |
| Echinus melo | 4.99 | 61.71 | |||
| US—Average similarity: 79.13% | US—Average similarity: 40.02% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Galeus melastomus | 8.1 | 33.17 | Parastichopus regalis | 2.78 | 33.42 |
| Scyliorhinus canicula | 6.87 | 62.39 | Cidaris cidaris | 2.75 | 63.83 |
| MS—Average similarity: 73.89% | MS—Average similarity: 19.13% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Galeus melastomus | 7.7 | 45.27 | Gracilechinus acutus | 2.01 | 27.6 |
| Etmopterus spinax | 5.67 | 77.8 | Cidaris cidaris | 1.25 | 43.61 |
| Luidia sarsi | 1.43 | 58.28 | |||
| (c) Crustaceans | (f) Other groups | ||||
| CS—Average similarity: 67.14% | CS—Average similarity: 26.26% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Pagurus prideaux | 6.91 | 50.07 | Megerlia truncata | 4.01 | 22.18 |
| Hyalinoecia tubicola | 3.78 | 42.61 | |||
| Diazona violacea | 2.7 | 60.54 | |||
| US—Average similarity: 53.45% | US—Average similarity: 22.14% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Macropipus tuberculatus | 4.97 | 18.44 | Pyrosoma atlanticum | 2.64 | 46 |
| Dardanus arrosor | 5.15 | 36.45 | Pelagia noctiluca | 1.42 | 61.51 |
| Pagurus prideaux | 5.38 | 54.34 | |||
| MS—Average similarity: 63.79% | MS—Average similarity: 24.01% | ||||
| Species | Av. Abund | Cum.% | Species | Av. Abund | Cum.% |
| Plesionika martia | 6.06 | 17.31 | Actinauge richardi | 2.21 | 41.38 |
| Plesionika acanthonotus | 5.52 | 33.61 | Pyrosoma atlanticum | 1.65 | 70.34 |
| Sergia robusta | 5.42 | 49.66 | |||
| Pagurus alatus | 4.18 | 60.77 | |||
| Osteichthyes | Chondrichthyes | Crustaceans | Molluscs | Echinoderms | Other Groups | |
|---|---|---|---|---|---|---|
| N | 51.8 *** | 17.6 *** | 5 | 54.1 *** | 19.4 *** | 21.7 *** |
| B | 34.2 *** | 8.5 ** | 0.2 | 55.1 *** | 26.6 *** | 11.7 *** |
| M.W. | 12.5 ** | 7.5 ** | 2.6 | 19.7 *** | 22.2 *** | 10.2 *** |
| S | 40 *** | 27.8 *** | 25.8 *** | 42.4 *** | 14.8 *** | 17.4 *** |
| H’ | 16.0 *** | 32.3 *** | 26.1 *** | 2.1 | 12.7 ** | 2.2 |
| CS (100–200 m) | Osteichthyes | Chondrichthyes | Crustaceans | Molluscs | Echinoderms | Other Groups |
| A | 4 | 5.9 | 1.1 | 0.9 | 0.2 | 1.8 |
| B | 3.1 | 2.9 | 2.9 | 0.6 | 2.2 | 3.1 |
| M.W. | 6.7 ** | 2.9 | 5.1 | 0.5 | 3 | 0.1 |
| S | 3.1 | 1.5 | 3.5 | 0.3 | 0.4 | 1.2 |
| H’ | 2.5 | 3.8 | 1.2 | 1.2 | 1.6 | 0.5 |
| US (201–500 m) | Osteichthyes | Chondrichthyes | Crustaceans | Molluscs | Echinoderms | Other Groups |
| A | 1.7 | 0.9 | 2.5 | 0.6 | 2.2 | 9.9 *** |
| B | 0.9 | 5.9 | 0.3 | 2 | 3.3 | 4.7 |
| M.W. | 3.7 | 2.9 | 0.6 | 6.2 ** | 1.5 | 2.5 |
| S | 0.2 | 4.2 | 0.5 | 0.4 | 2.9 | 2.4 |
| H’ | 10.3 *** | 3.1 | 0.8 | 0.5 | 3.2 | 2.7 |
| MS (501–800 m) | Osteichthyes | Chondrichthyes | Crustaceans | Molluscs | Echinoderms | Other Groups |
| A | 8 *** | 17.8 *** | 0.1 | 5.5 | 1.3 | 0.8 |
| B | 5 | 17.0 *** | 3.7 | 8.4 ** | 2.6 | 3.9 |
| M.W. | 1 | 5.7 | 4.8 | 5.5 | 1.8 | 1.5 |
| S | 1.8 | 0.6 | 0.7 | 2 | 0.7 | 0.7 |
| H’ | 0.9 | 2.5 | 0.5 | 0.2 | 0.7 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Ruiz, C.; Hidalgo, M.; Ciércoles, C.; González-Aguilar, M.; Torres, P.; Urra, J.; Rueda, J.L. Spatial Distribution and Temporal Variation of Megafauna in Circalittoral and Bathyal Soft Bottoms of the Westernmost Biodiversity Hotspot of the Mediterranean Sea: The Alboran Ridge. Diversity 2024, 16, 686. https://doi.org/10.3390/d16110686
García-Ruiz C, Hidalgo M, Ciércoles C, González-Aguilar M, Torres P, Urra J, Rueda JL. Spatial Distribution and Temporal Variation of Megafauna in Circalittoral and Bathyal Soft Bottoms of the Westernmost Biodiversity Hotspot of the Mediterranean Sea: The Alboran Ridge. Diversity. 2024; 16(11):686. https://doi.org/10.3390/d16110686
Chicago/Turabian StyleGarcía-Ruiz, Cristina, Manuel Hidalgo, Cristina Ciércoles, María González-Aguilar, Pedro Torres, Javier Urra, and José L. Rueda. 2024. "Spatial Distribution and Temporal Variation of Megafauna in Circalittoral and Bathyal Soft Bottoms of the Westernmost Biodiversity Hotspot of the Mediterranean Sea: The Alboran Ridge" Diversity 16, no. 11: 686. https://doi.org/10.3390/d16110686
APA StyleGarcía-Ruiz, C., Hidalgo, M., Ciércoles, C., González-Aguilar, M., Torres, P., Urra, J., & Rueda, J. L. (2024). Spatial Distribution and Temporal Variation of Megafauna in Circalittoral and Bathyal Soft Bottoms of the Westernmost Biodiversity Hotspot of the Mediterranean Sea: The Alboran Ridge. Diversity, 16(11), 686. https://doi.org/10.3390/d16110686






