Detritus from Ice and Plankton Algae as an Important Food Source for Macroinfaunal Communities in the Canadian Arctic
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Culture of Labeled Phytodetritus
2.3. Incubations Set-Up
2.4. Sample Processing
2.5. Calculations of Carbon and Nitrogen Uptake
2.6. Statistical Analyses
3. Results
3.1. Macroinfaunal Community Composition
3.2. Ice Algae and Phytoplankton Total C and N Uptake Rates
3.3. Biomass-Specific Uptake of Ice Algae and Phytoplankton C and N
4. Discussion
4.1. Macroinfaunal Community
4.2. Ice Algae and Phytoplankton C and N Uptakes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billen, G.; Joiris, C.; Meyer-Reil, L.; Linderboom, H. Role of bacteria in the North Sea ecosystem. Neth. J. Sea Res. 1990, 26, 265–293. [Google Scholar] [CrossRef]
- Gooday, A.J.; Turley, C.M.; Allen, J.A. Responses by benthic organisms to inputs of organic material to the ocean floor: A review. Philos. Trans. R. Soc. Lond. 1990, 331, 119–138. [Google Scholar]
- Pfannkuche, O. Benthic response to the sedimentation of particulate organic matter at the BIOTRANS station, 47° N, 20° W. Deep Sea Res. Part II 1993, 40, 135–149. [Google Scholar] [CrossRef]
- Smith, C.R.; Hoover, D.J.; Doan, S.E.; Pope, R.H.; Demaster, D.J.; Dobbs, F.C.; Altabet, M.A. Phytodetritus at the abyssal seafloor across 10° of latitude in the central equatorial Pacific. Deep Sea Res. Part II Top. Stud. Oceanogr. 1996, 43, 1309–1338. [Google Scholar] [CrossRef]
- Olivier, F.; Gaillard, B.; Thébault, J.; Meziane, T.; Tremblay, R.; Dumont, D.; Bélanger, S.; Gosselin, M.; Jolivet, A.; Chauvaud, L.; et al. Shells of the bivalve Astarte moerchi give new evidence of a strong pelagic-benthic coupling shift occurring since the late 1970s in the North Water polynya. Philos. Trans. R. Soc. A 2020, 378, 20190353. [Google Scholar] [CrossRef]
- Leu, E.; Søreide, J.E.; Hessen, D.O.; Falk-Petersen, S.; Berge, J. Consequences of changing sea-ice cover for primary and secondary producers in the European Arctic shelf seas: Timing, quantity, and quality. Prog. Oceanogr. 2011, 90, 18–32. [Google Scholar] [CrossRef]
- Cota, G.; Home, E. Physical control of arctic ice algal production. Mar. Ecol. Prog. Ser. 1989, 52, 111–121. [Google Scholar] [CrossRef]
- Hsiao, S.I.C. Dynamics of ice algae and phytoplankton in Frobisher Bay. Pol. Biol. 1992, 12, 645–651. [Google Scholar] [CrossRef]
- Leu, E.; Mundy, C.J.; Assmy, P.; Campbell, K.; Gabrielsen, T.M.; Gosselin, M.; Juul-Pedersen, T.; Gradinger, R. Arctic spring awakening—Steering principles behind the phenology of vernal ice algal blooms. Prog. Oceanogr. 2015, 139, 151–170. [Google Scholar] [CrossRef]
- Schewe, I.; Soltwedel, T. Benthic response to ice-edge-induced particle flux in the Arctic Ocean. Pol. Biol. 2003, 26, 610–620. [Google Scholar] [CrossRef]
- Gosselin, M.; Levasseur, M.; Wheeler, P.A.; Horner, R.A.; Booth, B.C. New measurements of phytoplankton and ice algal production in the Arctic Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1623–1644. [Google Scholar] [CrossRef]
- Forest, A.; Tremblay, J.-É.; Gratton, Y.; Martin, J.; Gagnon, J.; Darnis, G.; Sampei, M.; Fortier, L.; Ardyna, M.; Gosselin, M.; et al. Biogenic carbon flows through the planktonic food web of the Amundsen Gulf (Arctic Ocean): A synthesis of field measurements and inverse modeling analyses. Prog. Oceanogr. 2011, 91, 410–436. [Google Scholar] [CrossRef]
- Hegseth, E.N. Primary production of the northern Barents Sea. Pol. Res. 1998, 17, 113–123. [Google Scholar] [CrossRef]
- Berge, J.; Renaud, P.E.; Darnis, G.; Cottier, F.; Last, K.; Gabrielsen, T.M.; Johnsen, G.; Seuthe, L.; Weslawski, J.M.; Leu, E.; et al. In the dark: A review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 2015, 139, 258–271. [Google Scholar] [CrossRef]
- Apollonio, S. Chlorophyll in Arctic sea ice. Arctic 1965, 18, 118–122. [Google Scholar] [CrossRef]
- North, C.A.; Lovvorn, J.R.; Kolts, J.M.; Brooks, M.L.; Cooper, L.W.; Grebmeier, J.M. Deposit-feeder diets in the Bering Sea: Potential effects of climatic loss of sea ice-related microalgal blooms. Ecol. Appl. 2014, 24, 1525–1542. [Google Scholar] [CrossRef] [PubMed]
- Anning, J. The Development and Decline of the Epontic Algal Community in Barrow Strait. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 1989. [Google Scholar]
- Ambrose, W.; Clough, L.; Tilney, P.; Beer, L. Role of echinoderms in benthic remineralization in the Chukchi Sea. Mar. Biol. 2001, 139, 937–949. [Google Scholar]
- Lovvorn, J.R.; Cooper, L.W.; Brooks, M.L.; De Ruyck, C.C.; Bump, J.K.; Grebmeier, J.M. Organic matter pathways to zooplankton and benthos under pack ice in late winter and open water in late summer in the north-central Bering Sea. Mar. Ecol. Prog. Ser. 2005, 291, 135–150. [Google Scholar] [CrossRef]
- Arrigo, K.R. Sea Ice Ecosystems. Annu. Rev. Mar. Sci. 2014, 6, 439–467. [Google Scholar] [CrossRef]
- Legendre, L.; Ackley, S.F.; Dieckmann, G.S.; Gulliksen, B.; Horner, R.; Hoshiai, T.; Melnikov, I.A.; Reeburgh, W.S.; Spindler, M.; Sullivan, C.W. Ecology of Sea Ice Biota, 2. Global Significance. Pol. Biol. 1992, 12, 429–444. [Google Scholar] [CrossRef]
- Tamelander, T.; Reigstad, M.; Hop, H.; Ratkova, T. Ice algal assemblages and vertical export of organic matter from sea ice in the Barents Sea and Nansen Basin (Arctic Ocean). Pol. Biol. 2009, 32, 1261–1273. [Google Scholar] [CrossRef]
- Morata, N.; Michaud, E.; Włodarska-Kowalczuk, M. Impact of early food input on the Arctic benthos activities during the polar night. Pol. Biol. 2013, 38, 99–114. [Google Scholar] [CrossRef]
- Yunda-Guarin, G.; Brown, T.A.; Michel, L.N.; Saint-Béat, B.; Amiraux, R.; Nozais, C.; Archambault, P. Reliance of deep-sea benthic macrofauna on ice-derived organic matter highlighted by multiple trophic markers during spring in Baffin Bay, Canadian Arctic. Elem. Sci. Anthr. 2020, 8, 47. [Google Scholar] [CrossRef]
- Kwok, R. Arctic sea ice thickness, volume, and multiyear ice coverage: Losses and coupled variability (1958–2018). Environ. Res. Lett. 2018, 13, 105005. [Google Scholar] [CrossRef]
- Lang, A.; Yang, S.; Kaas, E. Sea ice thickness and recent Arctic warming. Geophys. Res. Lett. 2017, 44, 409–418. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.; Pabi, S. Impact of a shrinking Arctic ice cover on marine primary production. Geophys. Res. Lett. 2008, 35, L19603. [Google Scholar] [CrossRef]
- Wassmann, P. Arctic marine ecosystems in an era of rapid climate change. Prog. Oceanogr. 2011, 90, 1–17. [Google Scholar] [CrossRef]
- Bélanger, S.; Babin, M.; Tremblay, J.-É. Increasing cloudiness in Arctic damps the increase in phytoplankton primary production due to sea ice receding. Biogeosciences 2013, 10, 4087–4101. [Google Scholar] [CrossRef]
- Falk-Petersen, S.; Sargent, J.; Henderson, J.; Hegseth, E.; Hop, H.; Okolodkov, Y. Lipids and fatty acids in ice algae and phytoplankton from the Marginal Ice Zone in the Barents Sea. Pol. Biol. 1998, 20, 41–47. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Carroll, M.L.; Ambrose, W.G.; Clough, L.M.; Zou, L.; Lopez, G.R. Rapid consumption of phytoplankton and ice algae by Arctic soft-sediment benthic communities: Evidence using natural and 13C-labeled food materials. J. Mar. Res. 2007, 65, 561–588. [Google Scholar] [CrossRef]
- Sun, M.-Y.; Clough, L.M.; Carroll, M.L.; Dai, J.; Ambrose, W.G.; Lopez, G.R. Different responses of two common Arctic macrobenthic species (Macoma balthica and Monoporeia affinis) to phytoplankton and ice algae: Will climate change impacts be species specific? J. Exp. Mar. Biol. Ecol. 2009, 376, 110–121. [Google Scholar] [CrossRef]
- Olsen, R.E.; Henderson, R.J.; Ringø, E. Lipids of Arctic Charr, Salvelinus alpinus (L.) I. Dietary Induced Changes in Lipid Class and Fatty Acid Composition. Fish Physiol. Biochem. 1991, 9, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Kainz, M.; Arts, M.T.; Mazumder, A. Essential fatty acids in the planktonic food web and their ecological role for higher trophic levels. Limnol. Oceanogr. 2004, 49, 1784–1793. [Google Scholar] [CrossRef]
- Müller-Navarra, D. Evidence that a highly unsaturated fatty acid limits Daphnia growth in nature. Arch. Hydrobiol. 1995, 132, 297. [Google Scholar] [CrossRef]
- McMahon, K.; Ambrose WG, J.; Johnson, B.; Sun, M.; Lopez, G.; Clough, L.; Carroll, M. Benthic community response to ice algae and phytoplankton in Ny Ålesund, Svalbard. Mar. Ecol. Prog. Ser. 2006, 310, 1–14. [Google Scholar] [CrossRef]
- Søreide, J.E.; Leu, E.; Berge, J.; Graeve, M.; Falk-Petersen, S. Timing of blooms, algal food quality and Calanus glacialis reproduction and growth in a changing Arctic. Glob. Chang. Biol. 2010, 16, 3154–3163. [Google Scholar] [CrossRef]
- Brown, T.A.; Belt, S.T.; Ferguson, S.H.; Yurkowski, D.J.; Davison, N.J.; Barnett, J.E.F.; Jepson, P.D. Identification of the sea ice diatom biomarker IP25 and related lipids in marine mammals: A potential method for investigating regional variations in dietary sources within higher trophic level marine systems. J. Exp. Mar. Biol. Ecol. 2013, 441, 99–104. [Google Scholar] [CrossRef]
- Søreide, J.E.; Hop, H.; Carroll, M.L.; Falk-Petersen, S.; Hegseth, E.N. Seasonal food web structures and sympagic–pelagic coupling in the European Arctic revealed by stable isotopes and a two-source food web model. Prog. Oceanogr. 2006, 71, 59–87. [Google Scholar] [CrossRef]
- Tamelander, T.; Renaud, P.; Hop, H.; Carroll, M.; Ambrose WG, J.; Hobson, K. Trophic relationships and pelagic-benthic coupling during summer in the Barents Sea Marginal Ice Zone, revealed by stable carbon and nitrogen isotope measurements. Mar. Ecol. Prog. Ser. 2006, 310, 33–46. [Google Scholar] [CrossRef]
- Hobson, K.A.; Fisk, A.; Karnovsky, N.; Holst, M.; Gagnon, J.-M.; Fortier, M. A stable isotope (δ13C, δ15N) model for the North Water food web: Implications for evaluating trophodynamics and the flow of energy and contaminants. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5131–5150. [Google Scholar] [CrossRef]
- Gradinger, R. Sea-ice algae: Major contributors to primary production and algal biomass in the Chukchi and Beaufort Seas during May/June 2002. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1201–1212. [Google Scholar] [CrossRef]
- Iken, K.; Bluhm, B.; Gradinger, R. Food web structure in the high Arctic Canada Basin: Evidence from δ13C and δ15N analysis. Pol. Biol. 2005, 28, 238–249. [Google Scholar] [CrossRef]
- Aberle, N.; Witte, U. Deep-sea macrofauna exposed to a simulated sedimentation event in the abyssal NE Atlantic: In situ pulse-chase experiments using 13C-labelled phytodetritus. Mar. Ecol. Prog. Ser. 2003, 251, 37–47. [Google Scholar] [CrossRef]
- Witte, U.; Aberle, N.; Sand, M.; Wenzhöfer, F. Rapid response of a deep-sea benthic community to POM enrichment: An in situ experimental study. Mar. Ecol. Prog. Ser. 2003, 251, 27–36. [Google Scholar] [CrossRef]
- Witte, U.; Wenzhöfer, F.; Sommer, S.; Boetius, A.; Heinz, P.; Aberle, N.; Sand, M.; Cremer, A.; Abraham, W.-R.; Jørgensen, B.B.; et al. In situ experimental evidence of the fate of a phytodetritus pulse at the abyssal sea floor. Nature 2003, 424, 763–766. [Google Scholar] [CrossRef]
- Woulds, C.; Bell, J.B.; Glover, A.G.; Bouillon, S.; Brown, L.S. Benthic carbon fixation and cycling in diffuse hydrothermal and background sediments in the Bransfield Strait, Antarctica. Biogeosciences 2020, 17, 1–12. [Google Scholar] [CrossRef]
- Mäkelä, A.; Witte, U.; Archambault, P. Ice algae versus phytoplankton: Resource utilization by Arctic deep sea macroinfauna revealed through isotope labelling experiments. Mar. Ecol. Prog. Ser. 2017, 572, 1–18. [Google Scholar] [CrossRef]
- Roy, V.; Iken, K.; Gosselin, M.; Tremblay, J.-É.; Bélanger, S.; Archambault, P. Benthic faunal assimilation pathways and depth-related changes in food-web structure across the Canadian Arctic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 102, 55–71. [Google Scholar] [CrossRef]
- Forest, A.; Sampei, M.; Hattori, H.; Makabe, R.; Sasaki, H.; Fukuchi, M.; Wassmann, P.; Fortier, L. Particulate organic carbon fluxes on the slope of the Mackenzie Shelf (Beaufort Sea): Physical and biological forcing of shelf-basin exchanges. J. Mar. Syst. 2007, 68, 39–54. [Google Scholar] [CrossRef]
- Barber, D.G.; Hanesiak, J.M. Meteorological forcing of sea ice concentrations in the southern Beaufort Sea over the period 1979 to 2000. J. Geophys. Res. 2004, 109, 2003JC002027. [Google Scholar] [CrossRef]
- Sakshaug, E. Primary and secondary production in the Arctic Seas. In The Organic Carbon Cycle in the Arctic Ocean; Springer: Berlin/Heidelberg, Germany, 2004; pp. 57–81. [Google Scholar]
- Carmack, E.C.; Macdonald, R.W.; Jasper, S. Phytoplankton productivity on the Canadian Shelf of the Beaufort Sea. Mar. Ecol. Prog. Ser. 2004, 277, 37–50. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Annual cycles of sea ice and phytoplankton in Cape Bathurst polynya, southeastern Beaufort Sea, Canadian Arctic. Geophys. Res. Lett. 2004, 31, L08304. [Google Scholar] [CrossRef]
- Tang, C.C.L.; Ross, C.K.; Yao, T.; Petrie, B.; DeTracey, B.M.; Dunlap, E. The circulation, water masses and sea-ice of Baffin Bay. Prog. Oceanogr. 2004, 63, 183–228. [Google Scholar] [CrossRef]
- Fox, A.; Walker, B.D. Sources and Cycling of Particulate Organic Matter in Baffin Bay: A Multi-Isotope δ13C, δ15N, and Δ14C Approach. Front. Mar. Sci. 2022, 9, 846025. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 3-527-61399-4. [Google Scholar]
- Lovejoy, C.; Legendre, L.; Martineau, M.-J.; Bâcle, J.; von Quillfeldt, C.H. Distribution of phytoplankton and other protists in the North Water. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5027–5047. [Google Scholar] [CrossRef]
- Caron, G.; Michel, C.; Gosselin, M. Seasonal contributions of phytoplankton and fecal pellets to the organic carbon sinking flux in the North Water (northern Baffin Bay). Mar. Ecol. Prog. Ser. 2004, 283, 1–13. [Google Scholar] [CrossRef]
- Lafond, A.; Leblanc, K.; Quéguiner, B.; Moriceau, B.; Leynaert, A.; Cornet, V.; Legras, J.; Ras, J.; Parenteau, M.; Garcia, N.; et al. Late spring bloom development of pelagic diatoms in Baffin Bay. Elem. Sci. Anthr. 2019, 7, 44. [Google Scholar] [CrossRef]
- Rozanska, M.; Gosselin, M.; Poulin, M.; Wiktor, J.; Michel, C. Influence of Environmental Factors on the Development of Bottom Ice Protist Communities during the Winter–Spring Transition. Mar. Ecol. Prog. Ser. 2009, 386, 43–59. [Google Scholar] [CrossRef]
- Horner, R.; Schrader, G.C. Relative contributions of ice algae, phytoplankton, and benthic microalgae to primary production in nearshore regions of the Beaufort Sea. Arctic 1982, 35, 485–503. [Google Scholar] [CrossRef]
- Ambrose, W.G.; Quillfeldt, C.V.; Clough, L.M.; Tilney, P.V.R.; Tucker, T. The sub-ice algal community in the Chukchi sea: Large- and small-scale patterns of abundance based on images from a remotely operated vehicle. Pol. Biol. 2005, 28, 784–795. [Google Scholar] [CrossRef]
- Poulin, M.; Underwood, G.J.C.; Michel, C. Sub-ice colonial Melosira arctica in Arctic first-year ice. Diatom. Res. 2014, 29, 213–221. [Google Scholar] [CrossRef]
- Hasle, G.R.; Medlin, L.K.; Syvertsen, E.E. Synedropsis gen. nov., a genus of araphid diatoms associated with sea ice. Phycologia 1994, 33, 248–270. [Google Scholar] [CrossRef]
- Lalande, C.; Forest, A.; Barber, D.G.; Gratton, Y.; Fortier, L. Variability in the annual cycle of vertical particulate organic carbon export on Arctic shelves: Contrasting the Laptev Sea, Northern Baffin Bay and the Beaufort Sea. Cont. Shelf Res. 2009, 29, 2157–2165. [Google Scholar] [CrossRef]
- Link, H.; Piepenburg, D.; Archambault, P. Are hotspots always hotspots? The relationship between diversity, resource and ecosystem functions in the Arctic. PLoS ONE 2013, 8, e74077. [Google Scholar] [CrossRef] [PubMed]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- Ahyong, S.; Boyko, C.B.; Bailly, N.; Bernot, J.; Bieler, R.; Brandão, S.N.; Daly, M.; De Grave, S.; Gofas, S.; Hernandez, F.; et al. World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org (accessed on 10 December 2022).
- Yokoyama, H.; Tamaki, A.; Harada, K.; Shimoda, K.; Koyama, K.; Ishihi, Y. Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar. Ecol. Prog. Ser. 2005, 296, 115–128. [Google Scholar] [CrossRef]
- Kazanidis, G.; Bourgeois, S.; Witte, U.F.M. On the effects of acid pre-treatment on the elemental and isotopic composition of lightly- and heavily-calcified marine invertebrates. Ocean Sci. J. 2019, 54, 257–270. [Google Scholar] [CrossRef]
- Martinez Arbizu, P. PairwiseAdonis: Pairwise multilevel comparison using adonis. R Package Version 0.4 2020, 1. [Google Scholar]
- Oksanen, J. Multivariate analysis of ecological communities in R: Vegan tutorial. R Package Version 2011, 1, 1–43. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Gooday, A.J.; Levin, L.A.; Linke, P.; Heeger, T. The role of benthic foraminifera in deep-sea food webs and carbon cycling. In Deep-Sea Food Chains and the Global Carbon Cycle; Rowe, G.T., Pariente, V., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 63–91. ISBN 978-94-010-5082-1. [Google Scholar]
- Hensen, C.; Zabel, M.; Schulz, H.N. Benthic cycling of oxygen, nitrogen and phosphorus. Mar. Geochem. 2006, 207–240. [Google Scholar]
- Ouellette, D.; Desrosiers, G.; Gagne, J.; Gilbert, F.; Poggiale, J.; Blier, P.; Stora, G. Effects of temperature on in vitro sediment reworking processes by a gallery biodiffusor, the polychaete Neanthes virens. Mar. Ecol. Prog. Ser. 2004, 266, 185–193. [Google Scholar] [CrossRef]
- Dunton, K. Arctic biogeography: The paradox of the marine benthic fauna and flora. Trends Ecol. Evol. 1992, 7, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Dumais, P.-O.; Grant, C.; Bluhm, B.A.; De Montety, L.; De Coeli, L.T.; Tremblay, J.-É.; Archambault, P. Description and spatial modelling of benthic communities distribution in the Canadian Arctic Archipelago. Front. Mar. Sci. 2022, 9, 898852. [Google Scholar] [CrossRef]
- Conlan, K.; Aitken, A.; Hendrycks, E.; McClelland, C.; Melling, H. Distribution patterns of Canadian Beaufort Shelf macrobenthos. J. Mar. Syst. 2008, 74, 864–886. [Google Scholar] [CrossRef]
- Grebmeier, J.; Feder, H.; McRoy, C. Pelagic-benthic coupling on the shelf of the northern Bering and Chukchi Seas. II. Benthic community structure. Mar. Ecol. Prog. Ser. 1989, 51, 253–268. [Google Scholar] [CrossRef]
- Cochrane, S.K.J.; Denisenko, S.G.; Renaud, P.E.; Emblow, C.S.; Ambrose, W.G.; Ellingsen, I.H.; Skarðhamar, J. Benthic macrofauna and productivity regimes in the Barents Sea—Ecological implications in a changing Arctic. J. Sea Res. 2009, 61, 222–233. [Google Scholar] [CrossRef]
- Carroll, M.L.; Ambrose, W.G. Benthic infaunal community variability on the northern Svalbard shelf. Pol. Biol. 2012, 35, 1259–1272. [Google Scholar] [CrossRef]
- Pearson, T.H.; Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Ann. Rev. 1978, 16, 229–311. [Google Scholar]
- Ruhl, H.A.; Ellena, J.A.; Smith, K.L. Connections between climate, food limitation, and carbon cycling in abyssal sediment communities. Proc. Natl. Acad. Sci. USA 2008, 105, 17006–17011. [Google Scholar] [CrossRef]
- Flach, E.; Heip, C. Vertical distribution of macrozoobenthos within the sediment on the continental slope of the Goban Spur area (NE Atlantic). Mar. Ecol. Prog. Ser. 1996, 141, 55–66. [Google Scholar] [CrossRef]
- Dauwe, B.; Herman, P.M.J.; Heip, C.H.R. Communitv structure and bioturbation potential of macrofauna at four North Sea stations with contrasting food supply. Mar. Ecol. Prog. Ser. 1998, 173, 67–83. [Google Scholar] [CrossRef]
- Jumars, P.; Mayer, L.; Deming, J.; Baross, J.; Wheatcroft, R. Deep-sea deposit-feeding strategies suggested by environmental and feeding constraints. Philos. Trans. R. Soc. Lond. 1990, 331, 85–101. [Google Scholar]
- Bühring, S.I.; Lampadariou, N.; Moodley, L.; Tselepides, A.; Witte, U. Benthic microbial and whole-community responses to different amounts of 13C-enriched algae: In situ experiments in the deep Cretan Sea (Eastern Mediterranean). Limnol. Oceanogr. 2006, 51, 157–165. [Google Scholar] [CrossRef][Green Version]
- Cunha, M.R.; Paterson, G.L.J.; Amaro, T.; Blackbird, S.; de Stigter, H.C.; Ferreira, C.; Glover, A.; Hilário, A.; Kiriakoulakis, K.; Neal, L.; et al. Biodiversity of macrofaunal assemblages from three Portuguese submarine canyons (NE Atlantic). Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 2433–2447. [Google Scholar] [CrossRef]
- Paterson, G.L.J.; Glover, A.G.; Cunha, M.R.; Neal, L.; de Stigter, H.C.; Kiriakoulakis, K.; Billett, D.S.M.; Wolff, G.A.; Tiago, A.; Ravara, A.; et al. Disturbance, productivity and diversity in deep-sea canyons: A worm’s eye view. Deep Sea Res. Part II Top. Stud. Oceanogr. 2011, 58, 2448–2460. [Google Scholar] [CrossRef]
- Gooday, A.J.; Hughes, J.A. Foraminifera associated with phytodetritus deposits at a bathyal site in the northern Rockall Trough (NE Atlantic): Seasonal contrasts and a comparison of stained and dead assemblages. Mar. Micropaleontol. 2002, 46, 83–110. [Google Scholar] [CrossRef]
- Fontanier, C.; Jorissen, F.J.; Chaillou, G.; David, C.; Anschutz, P.; Lafon, V. Seasonal and interannual variability of benthic foraminiferal faunas at 550m depth in the Bay of Biscay. Deep Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 457–494. [Google Scholar] [CrossRef]
- Mohan, K.; Gupta, A.K.; Bhaumik, A.K. Distribution of deep-sea benthic foraminifera in the Neogene of Blake Ridge, NW Atlantic Ocean. J. Micropalaeontol. 2011, 30, 33–74. [Google Scholar] [CrossRef]
- Smart, C.W.; Gooday, A.J. Recent benthic foraminifera in the abyssal Northeast Atlantic Ocean; relation to phytodetrital inputs. J. Foraminifer. Res. 1997, 27, 85–92. [Google Scholar] [CrossRef]
- Wollenburg, J.E.; Kuhnt, W. The response of benthic foraminifers to carbon flux and primary production in the Arctic Ocean. Mar. Micropaleontol. 2000, 40, 189–231. [Google Scholar] [CrossRef]
- Gooday, A.J. Deep-sea benthic foraminiferal species which exploit phytodetritus: Characteristic features and controls on distribution. Mar. Micropaleontol. 1993, 22, 187–205. [Google Scholar] [CrossRef]
- Sampei, M.; Sasaki, H.; Hattori, H.; Fukuchi, M.; Hargrave, B. Fate of sinking particles, especially fecal pellets, within the epipelagic zone in the North Water (NOW) polynya of northern Baffin Bay. Mar. Ecol. Prog. Ser. 2004, 278, 17–25. [Google Scholar] [CrossRef]
- Wlodarska-Kowalczuk, M.; Pearson, T.H. Soft-bottom macrobenthic faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Pol. Biol. 2004, 27, 155–167. [Google Scholar] [CrossRef]
- Thomson, D.H. Marine benthos in the Eastern Canadian high Arctic: Multivariate analyses of standing crop and community structure. Arctic 1982, 35, 61–67. [Google Scholar] [CrossRef]
- Conlan, K.; Hendrycks, E.; Aitken, A.; Williams, B.; Blasco, S.; Crawford, E. Macrofaunal biomass distribution on the Canadian Beaufort Shelf. J. Mar. Syst. 2013, 127, 76–87. [Google Scholar] [CrossRef]
- Renaud, P.E.; Riedel, A.; Michel, C.; Morata, N.; Gosselin, M.; Juul-Pedersen, T.; Chiuchiolo, A. Seasonal variation in benthic community oxygen demand: A response to an ice algal bloom in the Beaufort Sea, Canadian Arctic? J. Mar. Syst. 2007, 67, 1–12. [Google Scholar] [CrossRef]
- McConnaughey, T.; McRoy, C.P. Food-Web structure and the fractionation of Carbon isotopes in the bering sea. Mar. Biol. 1979, 53, 257–262. [Google Scholar] [CrossRef]
- Hunter, W.R.; Jamieson, A.; Huvenne, V.A.I.; Witte, U. Food quality determines sediment community responses to marine vs. terrigenous organic matter in a submarine canyon. Biogeosci. Discuss. 2012, 9, 11331–11374. [Google Scholar] [CrossRef]
- Mäkelä, A.; Witte, U.; Archambault, P. Short-term processing of ice algal- and phytoplankton-derived carbon by Arctic benthic communities revealed through isotope labelling experiments. Mar. Ecol. Prog. Ser. 2018, 600, 21–39. [Google Scholar] [CrossRef]
- Mayor, D.J.; Thornton, B.; Hay, S.; Zuur, A.F.; Nicol, G.W.; McWilliam, J.M.; Witte, U.F.M. Resource quality affects carbon cycling in deep-sea sediments. ISME J. 2012, 6, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Gooday, A.J. Biological responses to seasonally varying fluxes of organic matter to the ocean floor: A review. J. Oceanogr. 2002, 58, 305–332. [Google Scholar] [CrossRef]
- Van Oevelen, D.; Soetaert, K.; Middelburg, J.J.; Herman, P.M.J.; Moodley, L.; Hamels, I.; Moens, T.; Heip, C.H.R. Carbon flows through a benthic food web: Integrating biomass, isotope and tracer data. J. Mar. Res. 2006, 64, 453–482. [Google Scholar] [CrossRef]
- Woulds, C.; Cowie, G.L.; Levin, L.A.; Andersson, J.H.; Middelburg, J.J.; Vandewiele, S.; Lamont, P.A.; Larkin, K.E.; Gooday, A.J.; Schumacher, S.; et al. Oxygen as a control on sea floor biological communities and their roles in sedimentary carbon cycling. Limnol. Oceanogr. 2007, 52, 1698–1709. [Google Scholar] [CrossRef]
- Sweetman, A.; Witte, U. Response of an abyssal macrofaunal community to a phytodetrital pulse. Mar. Ecol. Prog. Ser. 2008, 355, 73–84. [Google Scholar] [CrossRef]
- Sweetman, A.K.; Witte, U. Macrofaunal response to phytodetritus in a bathyal Norwegian fjord. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 1503–1514. [Google Scholar] [CrossRef]
- Bender, K.; Davis, W.R. The effect of feeding by Yoldia limatula on bioturbation. Ophelia 1984, 23, 91–100. [Google Scholar] [CrossRef]
- Levin, L.; Blair, N.; Martin, C.; DeMaster, D.; Plaia, G.; Thomas, C. Macrofaunal processing of phytodetritus at two sites on the Carolina margin: In situ experiments using 13C-labeled diatoms. Mar. Ecol. Prog. Ser. 1999, 182, 37–54. [Google Scholar] [CrossRef]
- Macdonald, T.; Burd, B.; van Roodselaar, A. Facultative feeding and consistency of trophic structure in marine soft-bottom macrobenthic communities. Mar. Ecol. Prog. Ser. 2012, 445, 129–140. [Google Scholar] [CrossRef]
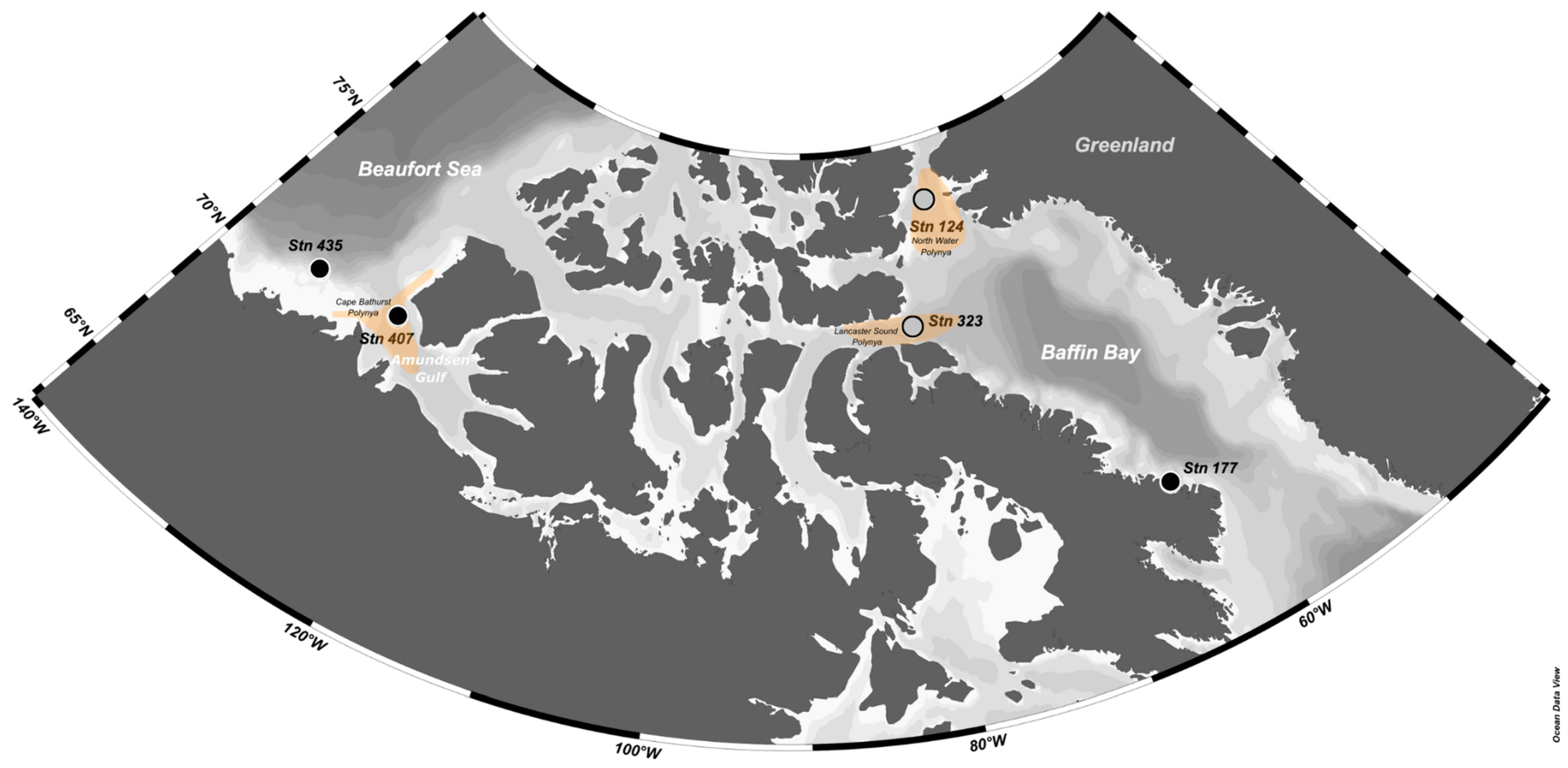

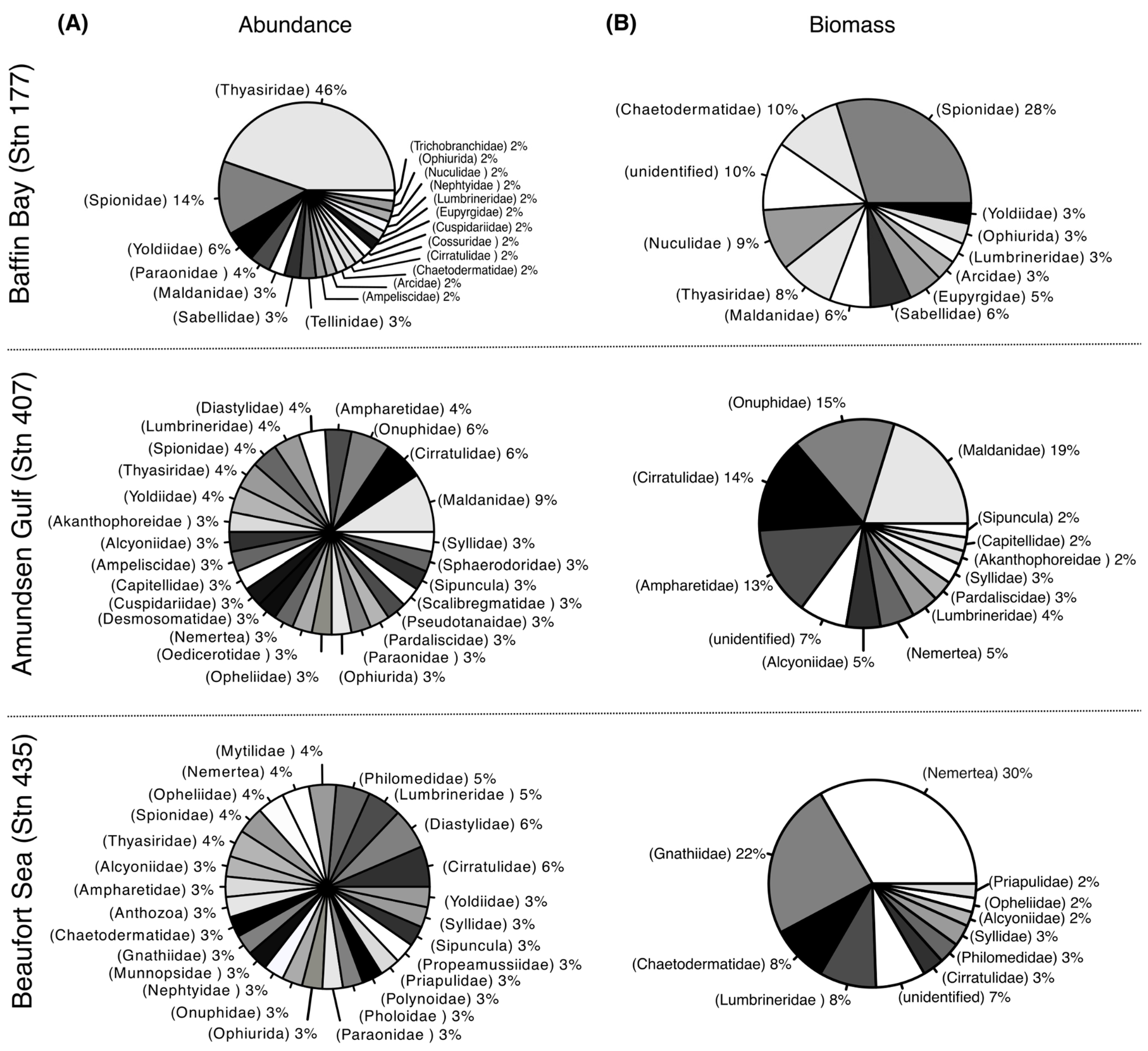
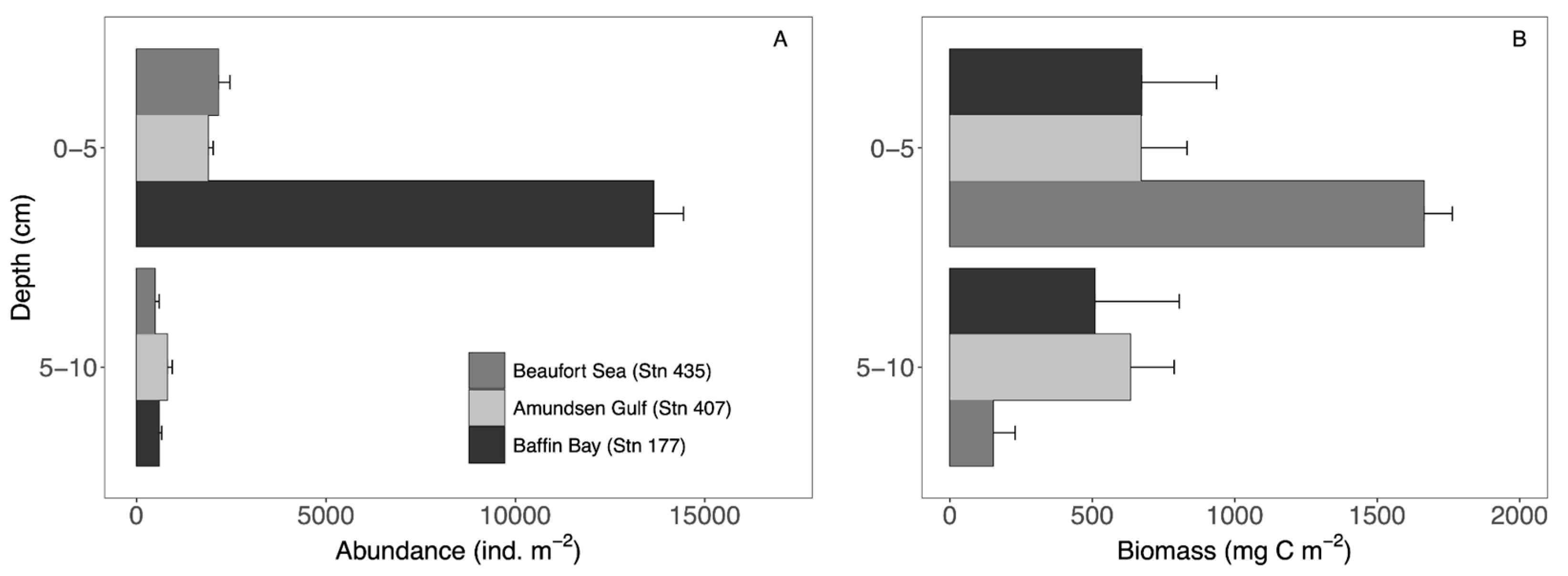
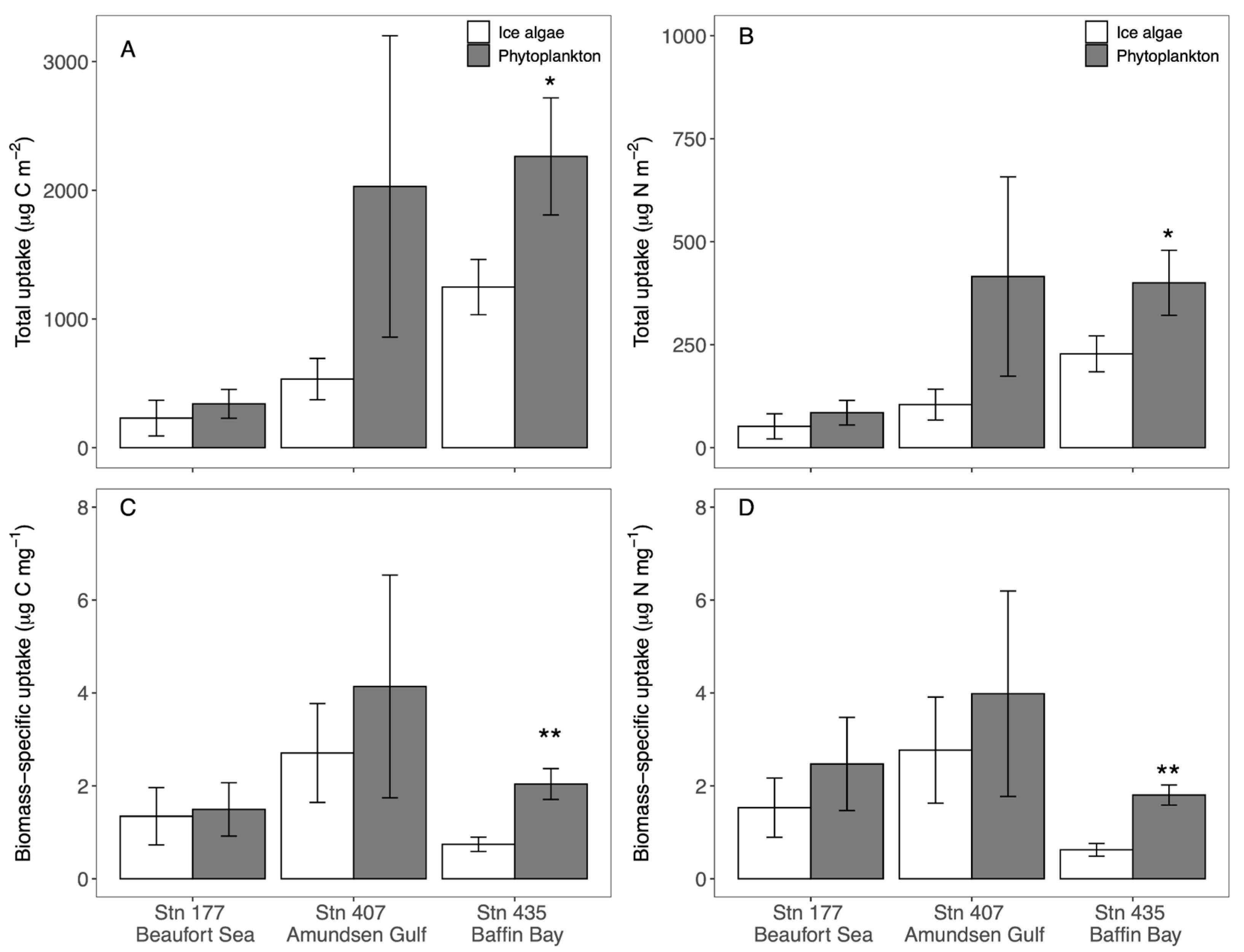

| Beaufort Sea | Amundsen Gulf | Baffin Bay | |
|---|---|---|---|
| ArcticNet 2015 station number | 435 | 407 | 177 |
| Latitude | 71°04.74′ N | 70°59.62′ N | 67°28.430′ N |
| Longitude | 133°37.96′ W | 126°03.39′ W | 63°41.526′ W |
| Date sampled | 27 August | 23 August | 25 October |
| Depth (m) | 300 | 382 | 376 |
| Bottom T (°C) | 0.49 | 0.37 | 0.78 |
| Bottom dissolved O2 (mL/L) | 6.3 | 6.4 | 5.2 |
| Bottom salinity (psu) | 34.08 | 34.83 | 34.11 |
| MAX Chl a in column water (mg/m3) | 0.7 | 3.34 | 0.53 |
| Chl a in sediments (mg/m3) | 0.96 | 0.27 | 0.48 |
| Sediment OM content (% DW) | 8.53 | 10.60 | 5.57 |
| Surface sediment δ13C (n = 3) | −25.20 ± 0.09 | −23.78 ± 0.20 | −22.20 ± 0.28 |
| Surface sediment δ15N (n = 3) | 6.17 ± 1.39 | 7.09. ± 0.49 | 7.88. ± 1.19 |
| C:N (w/w, n = 3) | 7.56 ± 0.12 | 6.96 ± 0.08 | 7.03 ± 0.84 |
| Median grain size (µm) | 15.488 | 7.299 | 10.417 |
| Pairs | F.Model | R2 | p.Value | p.Adjusted |
|---|---|---|---|---|
| Baffin Bay vs. Amundsen Gulf | 34.621470 | 0.5528690 | 0.001 | 0.003 |
| Baffin Bay vs. Beaufort Sea | 26.253965 | 0.4839087 | 0.001 | 0.003 |
| Amundsen Gulf vs. Beaufort Sea | 6.047447 | 0.1776182 | 0.001 | 0.003 |
| Total C Uptake | Total N Uptake | |||||
|---|---|---|---|---|---|---|
| Beaufort Sea Station | t | df | p | t | df | p |
| Whole community | −0.269 | 5.640 | 0.798 | −0.446 | 6.348 | 0.671 |
| Polychaetes | 0.683 | 3.784 | 0.534 a | 0.375 | 3.776 | 0.728 a |
| Bivalves | −3.038 | 2.009 | 0.092 | −2.932 | 2.01 | 0.099 |
| Amundsen Gulf station | t | df | p | t | df | p |
| Whole community | 0.103 | 4.889 | 0.922 a | 0.042 | 5.186 | 0.968 a |
| Polychaetes | 0.233 | 5.261 | 0.825 a | 0.151 | 5.353 | 0.885 a |
| Bivalves | U = 14 | 0.110 | U = 15 | 0.059 | ||
| Baffin Bay station | t | df | p | t | df | p |
| Whole community | −2.020 | 5.697 | 0.092 | −1.913 | 6.23 | 0.100 |
| Polychaetes | −2.392 | 5.658 | 0.056 | −2.11 | 6.127 | 0.078 |
| Bivalves | 0.248 | 5.122 | 0.814 | −0.425 | 5.736 | 0.686 |
| Biomass-Specific C Uptake | Biomass-Specific N Uptake | |||||
|---|---|---|---|---|---|---|
| Beaufort Sea station | t | df | p | t | df | p |
| Whole community | 0.372 | 5.603 | 0.724 | −0.357 | 6.906 | 0.732 |
| Polychaetes | 0.451 | 4.459 | 0.673 a | 0.582 | 4.762 | 0.587 a |
| Bivalves | −1.761 | 3.320 | 0.168 | −1.744 | 3.111 | 0.176 |
| Amundsen Gulf station | t | df | p | t | df | p |
| Whole community | −0.003 | 7.242 | 0.997 a | U = 14 | 0.841 | |
| Polychaetes | 0.087 | 5.679 | 0.933 a | 0.112 | 5.787 | 0.914 a |
| Bivalves | 0.636 | 3.144 | 0.568 | 0.712 | 3.078 | 0.526 |
| Baffin Bay station | t | df | p | t | df | p |
| Whole community | −3.457 | 5.241 | 0.017 | −4.719 | 7.401 | 0.002 |
| Polychaetes | −2.191 | 5.881 | 0.071 a | −2.415 | 4.391 | 0.067 |
| Bivalves | −2.3924 | 7.343 | 0.046 | −2.330 | 6.812 | 0.054 |
| REGION (Station) | Abundance (Ind. m−2) | Biomass (mg C m−2) | Depth (m) | C Added (mg C m−2) | IA C Uptake (mg C m−2) | % of the Total C Added | PP C Uptake (mg C m−2) | % of the Total C Added |
|---|---|---|---|---|---|---|---|---|
| North Baffin Bay (Stn 124) a | 10,952 | 3190 ± 432 | 709 | 1475 | 7.9 ± 1.8 | 0.5 ± 0.1 | 4.3 ± 0.9 | 0.3 ± 0.1 |
| Lancaster Sound (Stn 323) a | 8355 | 2110 ± 345 | 794 | 600 | 3.1 ± 1.0 | 0.5 ± 0.2 | 3.3 ± 0.4 | 0.6 ± 0.1 |
| Baffin Bay (Stn 177) b | 14,000 ± 795 | 2208 ± 549 | 376 | 545 | 1.25 ± 0.21 | 0.2 ± 0.03 | 2.27 ± 0.45 | 0.4 ± 0.08 |
| Baffin Bay c | 1482 | 251/500 | - | - | - | - | - | |
| Amundsen Gulf (Stn 407) b | 2644 ± 191 | 1890 ± 1087 | 382 | 600 | 0.53 ± 0.16 | 0.1 ± 0.03 | 2.02 ± 1.17 | 0.3 ± 0.2 |
| Amundsen Gulf d,e | 2578 ± 730 | 100 to 49,000 | 259 | - | - | - | - | - |
| Beaufort Sea (Stn 435) b | 2444 ± 297 | 1234 ± 1297 | 300 | 425 | 0.29 ± 0.16 | 0.1 ± 0.04 | 0.34 ± 0.11 | 0.1 ± 0.03 |
| Beaufort Sea d,e | 828 to 2041 | 100 to 49,000 | 70/440 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, G.; Archambault, P.; Witte, U.; Mäkelä, A.; Kazanidis, G.; Ciancio, J.E.; Bourgeois, S.; Nozais, C. Detritus from Ice and Plankton Algae as an Important Food Source for Macroinfaunal Communities in the Canadian Arctic. Diversity 2024, 16, 605. https://doi.org/10.3390/d16100605
Bravo G, Archambault P, Witte U, Mäkelä A, Kazanidis G, Ciancio JE, Bourgeois S, Nozais C. Detritus from Ice and Plankton Algae as an Important Food Source for Macroinfaunal Communities in the Canadian Arctic. Diversity. 2024; 16(10):605. https://doi.org/10.3390/d16100605
Chicago/Turabian StyleBravo, Gonzalo, Philippe Archambault, Ursula Witte, Anni Mäkelä, Georgios Kazanidis, Javier E. Ciancio, Solveig Bourgeois, and Christian Nozais. 2024. "Detritus from Ice and Plankton Algae as an Important Food Source for Macroinfaunal Communities in the Canadian Arctic" Diversity 16, no. 10: 605. https://doi.org/10.3390/d16100605
APA StyleBravo, G., Archambault, P., Witte, U., Mäkelä, A., Kazanidis, G., Ciancio, J. E., Bourgeois, S., & Nozais, C. (2024). Detritus from Ice and Plankton Algae as an Important Food Source for Macroinfaunal Communities in the Canadian Arctic. Diversity, 16(10), 605. https://doi.org/10.3390/d16100605







