Brief Review of Morphological Characters in the Identification of Muscomorpha (Diptera) of Sanitary and Forensic Importance
Abstract
1. Introduction
2. Phylogeny
3. Medical–Veterinary Importance as Pathogen Transmitters
4. Medical–Veterinary Importance as Causes of Myiasis
5. Forensic Importance
6. Problems in Identification
7. Identification Using Scanning Electron Microscopy
8. Discussion
8.1. Eggs
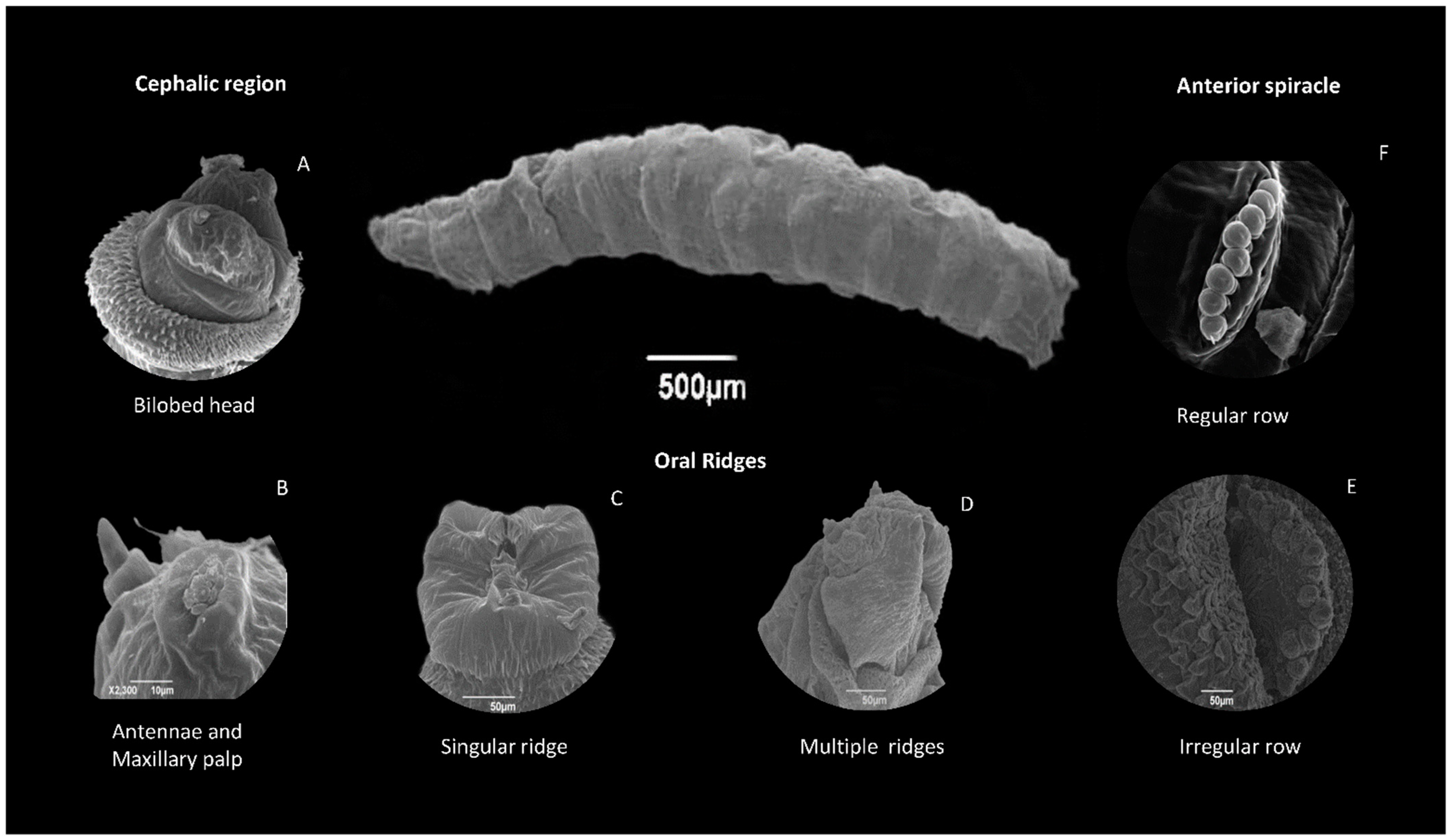
8.2. Larvae
8.2.1. Head
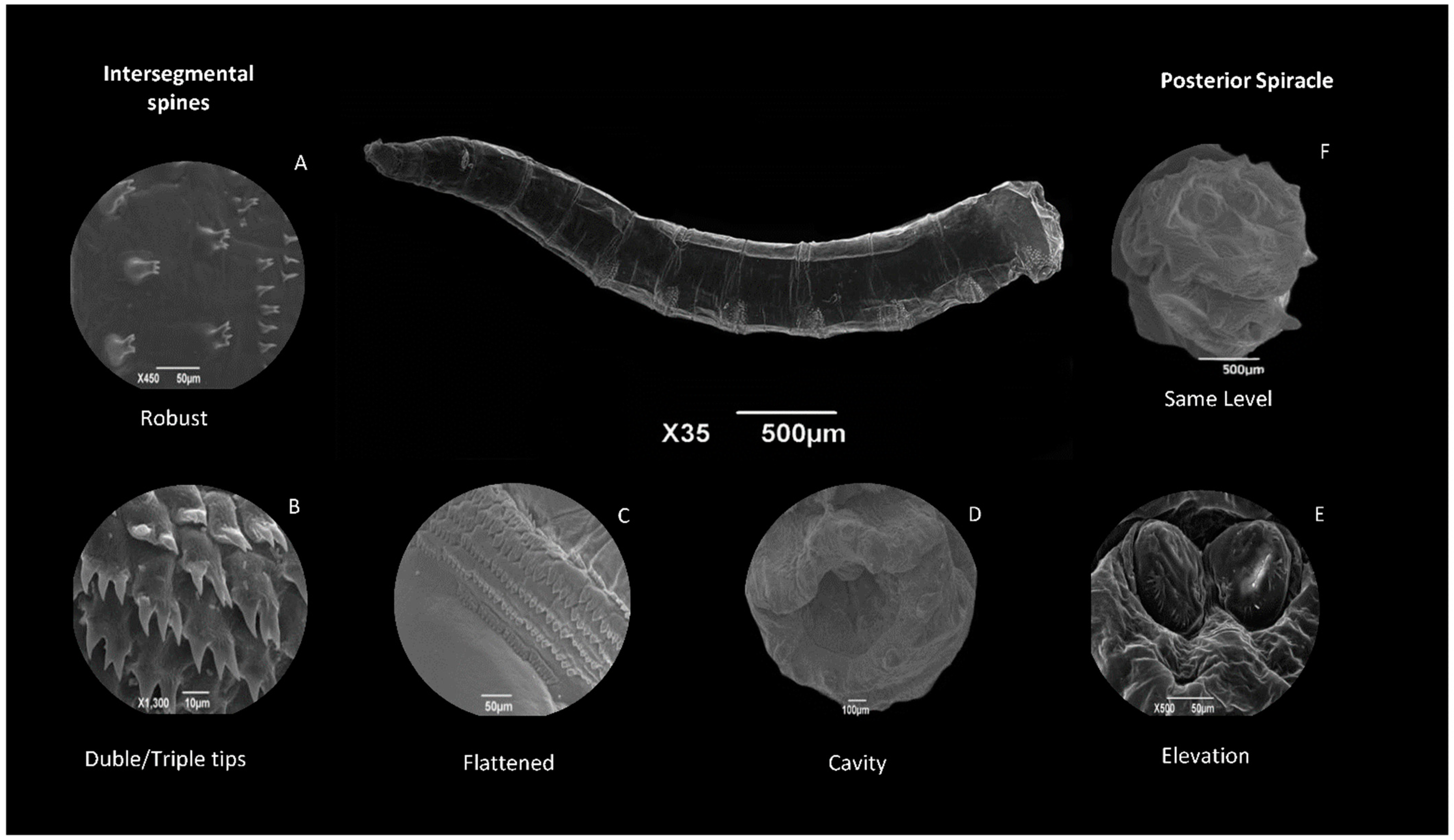
8.2.2. Body Segments
8.2.3. Anal Segment
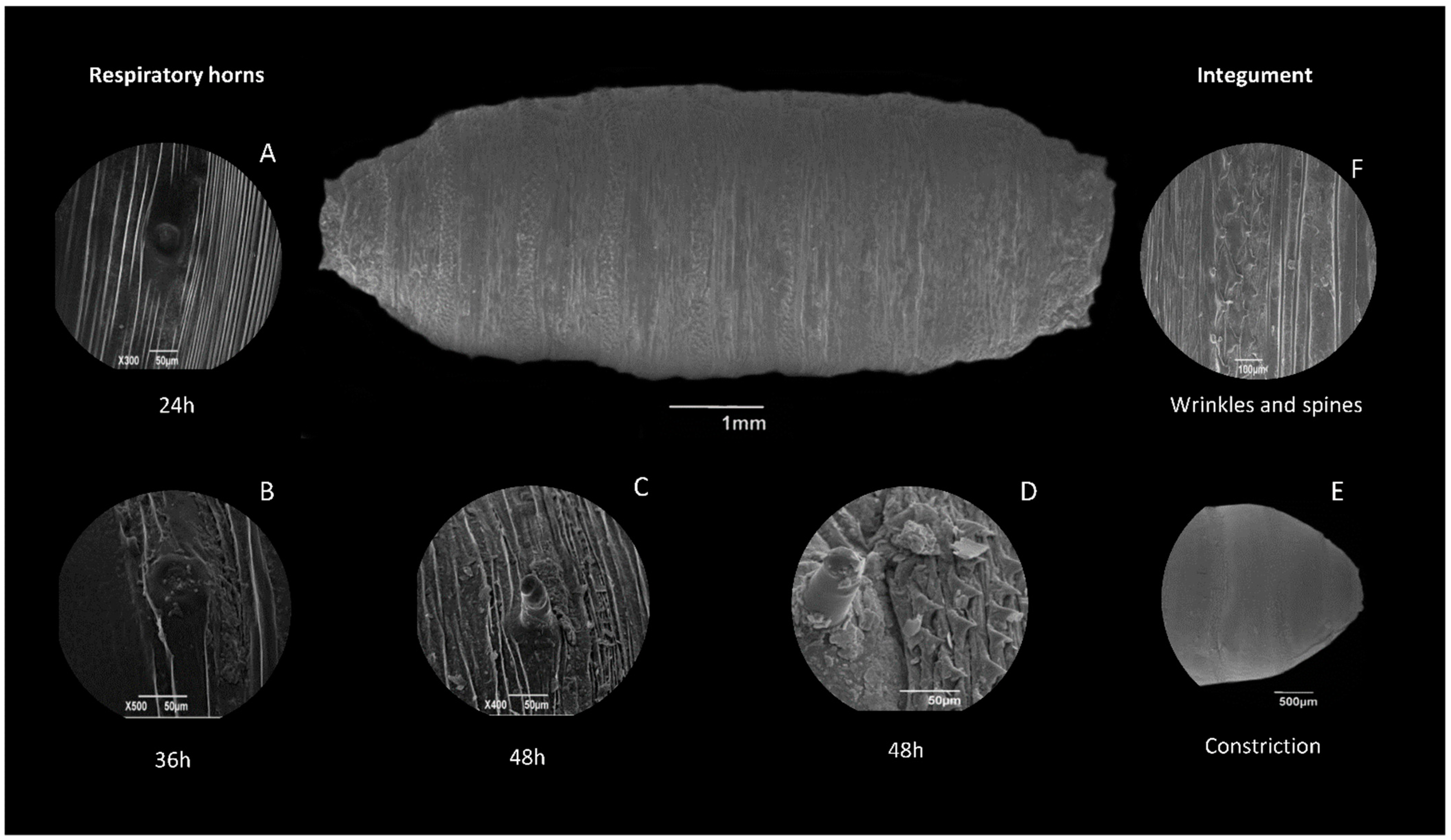
8.3. Puparia
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D.; Engel, M.S. Evolution of the Insects, 1st ed.; Cambridge University Press: New York, NY, USA, 2005; 755p. [Google Scholar]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Ann. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef]
- Rafael, J.A.; Aguiar, A.P.; Amorim, D.S. Knowledge of insect diversity in Brazil: Challenges and Advances. Neotr. Entomol. 2009, 38, 565–570. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thompson, F.C. The Diptera Site. The biosystematic Database of World Diptera. Nomenclator Status Statistics. Version 10.5. Available online: www.sl.barc.usda.gov/diptera/names/Status/bdwdstat.htm (accessed on 6 October 2023).
- Wiegmann, B.M.; Trautwein, M.D.; Winkler, I.S.; Barr, N.B.; Kim, J.W.; Lambkin, C.; Bertone, M.A.; Cassel, B.K.; Bayless, K.M.; Heimberg, A.M.; et al. Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. USA 2011, 108, 5690–5695. [Google Scholar] [CrossRef]
- Carvalho, C.J.B.; Moura, M.O.; Ribeiro, P.B. Chave para adultos de dípteros (Muscidae, Fanniidae, Anthomyiidae) associados ao ambiente humano no Brasil. Rev. Bras. Entomol. 2002, 46, 107–114. [Google Scholar] [CrossRef]
- Amorim, D.S. Neotropical Diptera Diversity: Richness, patterns and perspectives. In Diptera Diversity: Status, Challenges and Tools; Pape, T., Bickel, D., Meier, R., Eds.; Koninklije Brill NV: Leiden, The Netherlands, 2009; pp. 71–97. [Google Scholar]
- Yeates, D.K.; Wiegmann, B.M.; Courtney, G.W.; Meier, R.; Lambkin, C.; Pape, T. Phylogeny and systematics of Diptera: Two decades of progress and prospects. Zootaxa 2007, 1668, 565–590. [Google Scholar] [CrossRef]
- Amorim, D.S.; Yeates, D.K. Pesky gnats: Ridding dipteran classification of the Nematocera. Stud. Dipterol. 2006, 13, 3–9. [Google Scholar]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Serra-Freire, N.M.; Mello, R.P. Entomologia & Acarologia na Medicina Veterinária; Ed. LF Livros: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. Insect Systematics: Phylogeny and Classification. In The Insects: An outline of Entomology; Gullan, P.J., Cranston, P.S., Eds.; Wiley Blackwell: London, UK, 2008; pp. 189–222. [Google Scholar]
- Nuorteva, P. Synanthropy of blowflies (Dipt. Calliphoridae) in Finland. Ann. Zool. Fenn. 1963, 29, 1–49. [Google Scholar]
- Maldonado, M.A.; Centeno, N. Quantifying the potential pathogens transmission of the blowflies (Diptera: Calliphoridae). Mem. Inst. Oswaldo Cruz. 2003, 98, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.H.; Prado, A.P.; Buralli, G.M. Dispersal and distribution of three newly introduced species of Chrysomya R.D. (Diptera: Calliphoridae) in Brazil. Rev. Bras. Entomol. 1979, 23, 245–255. [Google Scholar]
- Olsen, A.R. Regulatory action criteria for filth and other extraneous materials. III. Review of flies and foodborne enteric disease. Regul. Toxicol. Pharmacol. 1998, 28, 199–211. [Google Scholar] [CrossRef]
- Braack, L.E.; Retief, P.F. Dispersal, density and habitat preference of the blow-flies Chrysomyia albiceps (Wd.) and Chrysomyia marginalis (Wd.) (Diptera: Calliphoridae). Onderstepoort J. Vet. Res. 1986, 53, 13–18. [Google Scholar] [PubMed]
- Greenberg, B. Flies and Diseases. Volume I: Ecology, Classification and Biotic Associations; Princeton University: Princeton, NJ, USA, 1971. [Google Scholar]
- Butler, J.F.; Garcia-Maruniak, A.; Meek, F.; Maruniak, J.E. Wild Florida House Flies (Musca domestica) as Carriers of Pathogenic Bacteria. Fla. Entomol. 2010, 93, 218–223. [Google Scholar] [CrossRef]
- Paraluppi, N.D.; Vasconcelos, J.C.; Aquino, J.S.; Castellón, E.G.; Silva, M.S.B.C. Calliphoridae (Diptera) in Manaus: IV Bacteria isolated from blowflies collected in street markets. Acta Amazon. 1996, 26, 93–96. [Google Scholar] [CrossRef][Green Version]
- Oliveira, V.C.; d’Almeida, J.M.; Abalém-de-Sá, I.V.; Mandarino, J.R.; Solari, C.A. Enterobactérias associadas a adultos de Musca domestica (Linnaeus, 1758) (Diptera: Muscidae) e Chrysomya megacephala (Fabricius, 1754) (Diptera: Calliphoridae) no Jardim Zoológico, Rio de Janeiro. Arq. Bras. Med. Veterinária E Zootec. 2006, 58, 556–561. [Google Scholar] [CrossRef][Green Version]
- Chandrakar, C.; Shakya, S.; Patyal, A.; Jain, A.; Ali, S.L.; Mishra, O.P. ERIC-PCR-based molecular typing of multidrug-resistant Escherichia coli isolated from houseflies (Musca domestica) in the environment of milk and meat shops. Lett. Appl. Microbiol. 2022, 75, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Bakry, N.; Awad, W.; Ahmed, S.; Kamel, M. The role of Musca domestica and milk in transmitting pathogenic multidrug-resistant Escherichia coli and associated phylogroups to neonatal calves. Environ. Sci. Pollut. Res. Int. 2022, 29, 39593–39609. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Nagaraja, T.G.; Zurek, L. Transmission of Escherichia coli O157:H7 to cattle by house flies. Prev. Vet. Med. 2007, 80, 74–81. [Google Scholar] [CrossRef]
- Guimarães, J.H.; Papavaro, N. Myiasis in Man and Animals in the Neotropical Region; Plêiade: São Paulo, Brazil, 1999; 308p. [Google Scholar]
- Rodríguez-Ruiz, M.T.; Acosta, A.M.; Cifuentes-Cardozo, E.; Chirveches, M.A.; Rosselli, D. Otomyiasis: Systematic Review. Int. Arch. Otorhinolaryngol. 2019, 23, 104–109. [Google Scholar] [CrossRef]
- Rey, L. Bases da Parasitologia Médica, 3rd ed.; Guanabara-Koogan: Rio de Janeiro, Brazil, 2010; 420p. [Google Scholar]
- Siqueira, J.A.A.; Ubirajara-Filho, C.R.C.; Silva, T.R.M.; Lima, T.A.R.F.; Costa-Junior, L.M.; Alves, L.C.; Carvalho, G.A.; Ramos, R.A.N. Occurrence and anatomical distribution of myiasis caused by Cochliomyia hominivorax (Diptera: Calliphoridae) in swine. Vet. Parasitol. Reg. Stud. Rep. 2020, 22, 100481. [Google Scholar] [CrossRef]
- Barros, A.T.M.; Vazquez, S.A.S. Recomendações para Prevenção e Controle de Bicheiras em Bezerros no Pantanal. Com. Tec. Embrapa 2004, 35, 1–9. [Google Scholar]
- Duarte, E.R.; Rocha, F.T.; Teixeira, L.M.; Silva, R.B.; Nogueira, F.A.; Silva, N.O.; Almeida, A.C. Occurrence and treatment of cutaneous myiasis in sheep reared in semi-arid conditions in northern Minas Gerais. Pesq. Vet. Bras. 2012, 32, 490–494. [Google Scholar] [CrossRef]
- Smith, K.G.V. A Manual of Forensic Entomology; Cornell University: London, UK, 1986. [Google Scholar]
- Carvalho, C.J.B.; Ribeiro, P.B. Chave de identificação das espécies de Calliphoridae (Diptera) do sul do Brasil. Rev. Bras. Parasitol. Vet. 2000, 9, 169–173. [Google Scholar]
- Oliveira-Costa, J. Entomologia Forense—Quando os Insetos são Vestígios, 1st ed.; Millenium: Campinas, Brazil, 2003. [Google Scholar]
- Garção-Neto, C.H.; Cortinhas, L.B.; Mendonça, P.M.; Duarte, M.L.; Barbosa, T.A.N.; Queiroz, M.M.C. Dípteros Necrófagos do Brasil: Contribuições à Entomologia Forense; Autografia: Rio de Janeiro, Brazil, 2023; 298p. [Google Scholar]
- Payne, J.A. A summer carrion of the baby pig Sus scrofa Linnaeus. Ecology 1965, 46, 592–602. [Google Scholar] [CrossRef]
- Jirón, L.F.; Cartín, V.M. Insect succession in the decomposition of a mammal in Costa Rica. J. N. Y. Entomol. Soc. 1981, 89, 158–165. [Google Scholar]
- Bourel, B.; Callet, B.; Hédouin, V.; Gosset, D. Flies Eggs: A New Method for the Estimation of Short-term Post-mortem Interval? For. Sci. Int. 2003, 135, 27–34. [Google Scholar] [CrossRef]
- Barbosa, R.R.; Mello-Patiu, C.A.; Ururahy-Rodrigues, A.; Barbosa, C.G.; Queiroz, M.M.C. Temporal distribution of ten calyptrate dipteran species of medicolegal importance in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz. 2010, 105, 191–198. [Google Scholar] [CrossRef]
- Vasconcelos, S.D.; Cruz, T.M.; Salgado, R.L.; Thyssen, P.J. Dipterans Associated with a Decomposing Animal Carcass in a Rainforest Fragment in Brazil: Notes on the Early Arrival and Colonization by Necrophagous Species. J. Ins. Sci. 2013, 13, 1–11. [Google Scholar] [CrossRef]
- Schroeder, H.; Klotzbach, H.; Elias, S.; Augustin, C.; Pueschel, K. Use of PCR-RFLP for differentiation of Calliphoridae larvae (Diptera: Calliphoridae) on human corpses. For. Sci. Int. 2003, 132, 76–78. [Google Scholar] [CrossRef]
- Mendonça, P.M.; Barbosa, R.R.; Carriço, C.; Cortinhas, L.B.; Santos-Mallet, J.R.; Queiroz, M.M.C. Ultrastructure of immature stages of Lucilia cuprina (Diptera: Calliphoridae) using scanning electron microscopy. Acta Trop. 2014, 136, 123–128. [Google Scholar] [CrossRef]
- Cortinhas, L.B.; Mendonça, P.M.; Braga, M.V.; Queiroz, M.M.C. Ultrastructure of the immature stages of Musca domestica (Diptera: Muscidae: Muscinae). J. Med. Entomol. 2020, 57, 1712–1721. [Google Scholar] [CrossRef]
- Albuquerque, D.O.; Pamplona, D.; Carvalho, C.B.J. Contribuição ao conhecimento dos Fannia R. D., 1830 da Região Neotropical (Diptera, Fanniidae). Arch. Mus. Nac. R. J. 1981, 56, 9–34. [Google Scholar]
- Carvalho, C.J.B. Muscidae (Diptera) of the Neotropical Region: Taxonomy; Editora da UFPR: Curitiba, Brazil, 2002. [Google Scholar]
- Mello, R.P. Chave para identificação das formas adultas das espécies da família Calliphoridae (Diptera, Brachycera, Cyclorrapha) Encontradas no Brasil. Entomol. Vect. 2003, 10, 255–268. [Google Scholar]
- Carvalho, C.J.B.; Mello-Patiu, C.A. Key to the adults of the most common forensic species of Diptera in South America. Rev. Bras. Entomol. 2008, 52, 390–406. [Google Scholar] [CrossRef]
- Florez, E.; Wolff, M. Descripción y Clave de los Estadios Inmaduros de las Principales Especies de Calliphoridae (Diptera) de Importancia Forense en Colombia. Neotr. Entomol. 2009, 38, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.M.C.; Mello, R.P.; Lima, M.M. Morphological aspects of the larval instars of Chrysomya albiceps (Diptera: Calliphoridae) reared in the laboratory. Mem. Inst. Oswaldo Cruz. 1997, 92, 187–196. [Google Scholar] [CrossRef]
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; 705p. [Google Scholar]
- Greenberg, B.; Singh, D. Species identification of Calliphorid (Diptera) eggs. J. Med. Entomol. 1995, 32, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Erzinclioglu, Y.Z. The value of chorionic structure and size in the diagnosis of blowfly eggs. Med. Vet. Entomol. 1989, 3, 281–285. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Narongchai, P.; Kanchai, C.; Vichairat, K.; Piangjai, S.; Boonsriwong, W.; Sripakdee, D.; Chaiwong, T.; Kuntalue, B.; Siriwattanarungsee, S.; et al. Morphological comparison between Chrysomya rufifacies (Macquart) and Chrysomya villeneuvi Patton (Diptera: Calliphoridae) puparia, forensically important blow flies. Forensic Sci. Int. 2006, 164, 230–234. [Google Scholar] [CrossRef]
- Carvalho, L.S. Redescrição das Larvas de Terceiro Instar de Cinco Espécies de Dípteros Califorídeos (Insecta: Diptera) de Importância para a Entomologia Forense; Magister Science; Brasília University: Brasília, Brazil, 2006. [Google Scholar]
- Mendonça, P.M.; Santos-Mallet, J.R.; Queiroz, M.M.C. Ultrastructure of immature stages of the blowfly Chrysomya putoria (Wiedemann, 1818) (Diptera: Calliphoridae). Microsc. Res. Tech. 2012, 75, 206–211. [Google Scholar] [CrossRef]
- Mendonça, P.M.; Barbosa, R.R.; Cortinhas, L.B.; Santos-Mallet, J.R.; Queiroz, M.M.C. Ultrastructure of immature stages of Peckia (Euboettcheria) collusor (Diptera: Sarcophagidae). Acta Trop. 2013, 128, 522–527. [Google Scholar] [CrossRef]
- Mendonça, P.M.; Barbosa, R.R.; Cortinhas, L.B.; Santos-Mallet, J.R.; Queiroz, M.M.C. Ultrastructure of immature stages of Cochliomyia macellaria (Diptera: Calliphoridae), a fly of medical and veterinary importance. Parasitol. Res. 2014, 113, 3675–3683. [Google Scholar] [CrossRef]
- Cortinhas, L.B.; Mendonça, P.M.; Barbosa, R.R.; Queiroz, M.M.C. Ultrastructure of immature stages of the black dump fly: Ophyra aenescens (Wiedemann, 1830) (Diptera: Muscidae: Azeliinae). Acta Trop. 2016, 158, 125–129. [Google Scholar] [CrossRef]
- Hinton, H.E. A fly larva that tolerates dehydration and temperatures of −270 °C to +102 °C. Nature 1960, 188, 336–337. [Google Scholar] [CrossRef]
- Mendonça, P.M.; Santos-Mallet, J.R.; Mello, R.P.; Gomes, L.; Queiroz, M.M.C. Identification of fly eggs using scanning electron microscopy for forensic investigations. Micron 2008, 39, 802–807. [Google Scholar] [CrossRef]
- Liu, D.; Greenberg, B. Immature stages of some flies of forensic importance. Ann. Entomol. Soc. Am. 1989, 82, 80–93. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Bunchu, N.; Chaiwong, T.; Kuntalue, B.; Sukontason, K. Fine structure of the eggshell of the blow fly, Lucilia cuprina. J. Insect. Sci. 2007, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.D.; Newman Junior, S.M. Chorionic structure of the egg of the screwworm Cochliomyia hominivorax (Diptera: Calliphoridae). J. Med. Entomol. 1991, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Kunich, J.C. Entomology and the Law: Flies as Forensic Indicators; Cambridge University Press: Cambridge, UK, 2002; 330p. [Google Scholar]
- Teskey, H.J. Morphology and Terminology—Larvae. In Manual of Nearctic Diptera, Volume 1; McAlpine, J.F., Peterson, B.V., Shewell, G.E., Teskey, J.H., Vockeroth, J.R., Wood, D.M., Eds.; Research Branch, Supply & Services Canada: Ottawa, ON, Canada, 1981. [Google Scholar]
- Guimarães, J.H.; Amorim, D.S. Diptera. In Insetos Imaturos: Metamorfose e Identificação; Costa, C., Ide, S., Simonka, C.E., Eds.; Holos: São Paulo, Brazil, 2006; 249p. [Google Scholar]
- Chu, I.W.; Axtell, R.C. Fine structure of the dorsal organ of the house fly larva, Musca domestica. Z. Zellforsch. Mikrosk. Anat. 1971, 117, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.R.; Guevara, J.D.E. Scanning electron microscopy of the larval instars of Cochliomyia hominivorax. Med. Vet. Entomol. 1993, 7, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Szpila, K.; Hall, M.J.R.; Wardhana, A.H.; Pape, T. Morphology of the first instar larva of obligatory traumatic myiasis agents (Diptera: Calliphoridae, Sarcophagidae). Parasitol. Res. 2014, 113, 1629–1640. [Google Scholar] [CrossRef]
- Singh, D.; Garg, R.; Wadhawan, B. Ultramorphological characteristics of immature stages of a forensically important fly Parasarcophaga ruficornis (Fabricius) (Diptera: Sarcophagidae). Parasitol. Res. 2012, 110, 821–831. [Google Scholar] [CrossRef]
- Lakes-Harlan, R.; Bailley, W.J.; Schikorski, T. The auditory system of an atympanate buchcricket Phasmodes ranatriformes (Westwood) (Tettigoniidae, Orthoptera). J. Exp. Biol. 1991, 158, 307–324. [Google Scholar] [CrossRef]
- Sukontason, K.; Sribanditmongkol, P.; Ngoen-klan, R.; Klong-klaew, T.; Moophayak, K.; Sukontason, K.L. Differentiation between Lucilia cuprina and Hemipyrellia ligurriens (Diptera: Calliphoridae) larvae for use in forensic entomology applications. Parasitol. Res. 2010, 106, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Szyska, M.L. Immature stages and biology of fifteen species of Peruvian Calliphoridae (Diptera). Ann. Entomol. Soc. Am. 1984, 77, 488–517. [Google Scholar] [CrossRef]
- Sukontason, K.; Sukontason, K.L.; Piangjai, S.; Chaiwong, T.; Boonchu, N.; Kurahashi, H.; Vogtsberger, R.C. Larval ultrastructure of Parasarcophaga dux (Thomson) (Diptera: Sarcophagidae). Micron. 2003, 34, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Lopes, H.S.; Leite, A.C.R. Third contribution to the knowledge of the Raviniini (Diptera: Sarcophagidae), based on observations of the larvae, using scanning electron microscopy. Mem. Inst. Oswaldo Cruz. 1987, 82, 407–413. [Google Scholar] [CrossRef]
- Silva-Xavier, A.; Queiroz, M.M.C. Ultrastructure analysis of the immature stages of Ravinia belforti (Diptera: Sarcophagidae), a species of medical-veterinary and forensic importance, by scanning electron microscopy. Acta Trop. 2016, 159, 192–199. [Google Scholar] [CrossRef]
- Ruiz-Martinez, I.; Soler-Cruz, M.D.; Benitez-Rodriguez, R.; Perez-Jimenez, J.M.; Diaz-Lopez, M. Postembryonic development of Wohlfahrtia magnifica (Schiner, 1862) (Diptera: Sarcophagidae). J. Parasitol. 1989, 75, 531–539. [Google Scholar] [CrossRef]
- Uni, S.; Shinonaga, S.; Nishio, Y.; Fukunaga, A.; Iseki, M.; Okamoto, T.; Ueda, N.; Miki, T. Ophthalmomyiasis caused by Sarcophaga crassipalpis (Diptera: Sarcophagidae) in a hospital patient. J. Med. Entomol. 1999, 36, 906–908. [Google Scholar] [CrossRef]
- Vairo, K.P. Sarcophagidae (Diptera) de Potencial Interesse Forense de Curitiba, Paraná: Chave Pictórica para as Espécies e Morfologia dos Estágios Imaturos de Sarcodexia lambens (Wiedemann). Magister Thesis, Federal University from Paraná, Paraná, Brazil, 2011. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendonça, P.M.; Cortinhas, L.B.; Garção-Neto, C.H.; Queiroz, M.M.d.C. Brief Review of Morphological Characters in the Identification of Muscomorpha (Diptera) of Sanitary and Forensic Importance. Diversity 2024, 16, 599. https://doi.org/10.3390/d16100599
Mendonça PM, Cortinhas LB, Garção-Neto CH, Queiroz MMdC. Brief Review of Morphological Characters in the Identification of Muscomorpha (Diptera) of Sanitary and Forensic Importance. Diversity. 2024; 16(10):599. https://doi.org/10.3390/d16100599
Chicago/Turabian StyleMendonça, Paloma Martins, Lucas Barbosa Cortinhas, Carlos Henrique Garção-Neto, and Margareth Maria de Carvalho Queiroz. 2024. "Brief Review of Morphological Characters in the Identification of Muscomorpha (Diptera) of Sanitary and Forensic Importance" Diversity 16, no. 10: 599. https://doi.org/10.3390/d16100599
APA StyleMendonça, P. M., Cortinhas, L. B., Garção-Neto, C. H., & Queiroz, M. M. d. C. (2024). Brief Review of Morphological Characters in the Identification of Muscomorpha (Diptera) of Sanitary and Forensic Importance. Diversity, 16(10), 599. https://doi.org/10.3390/d16100599





