Does Size Matter? Two Subterranean Biodiversity Hotspots in the Lessini Mountains in the Veneto Prealps in Northern Italy
Abstract
1. Introduction
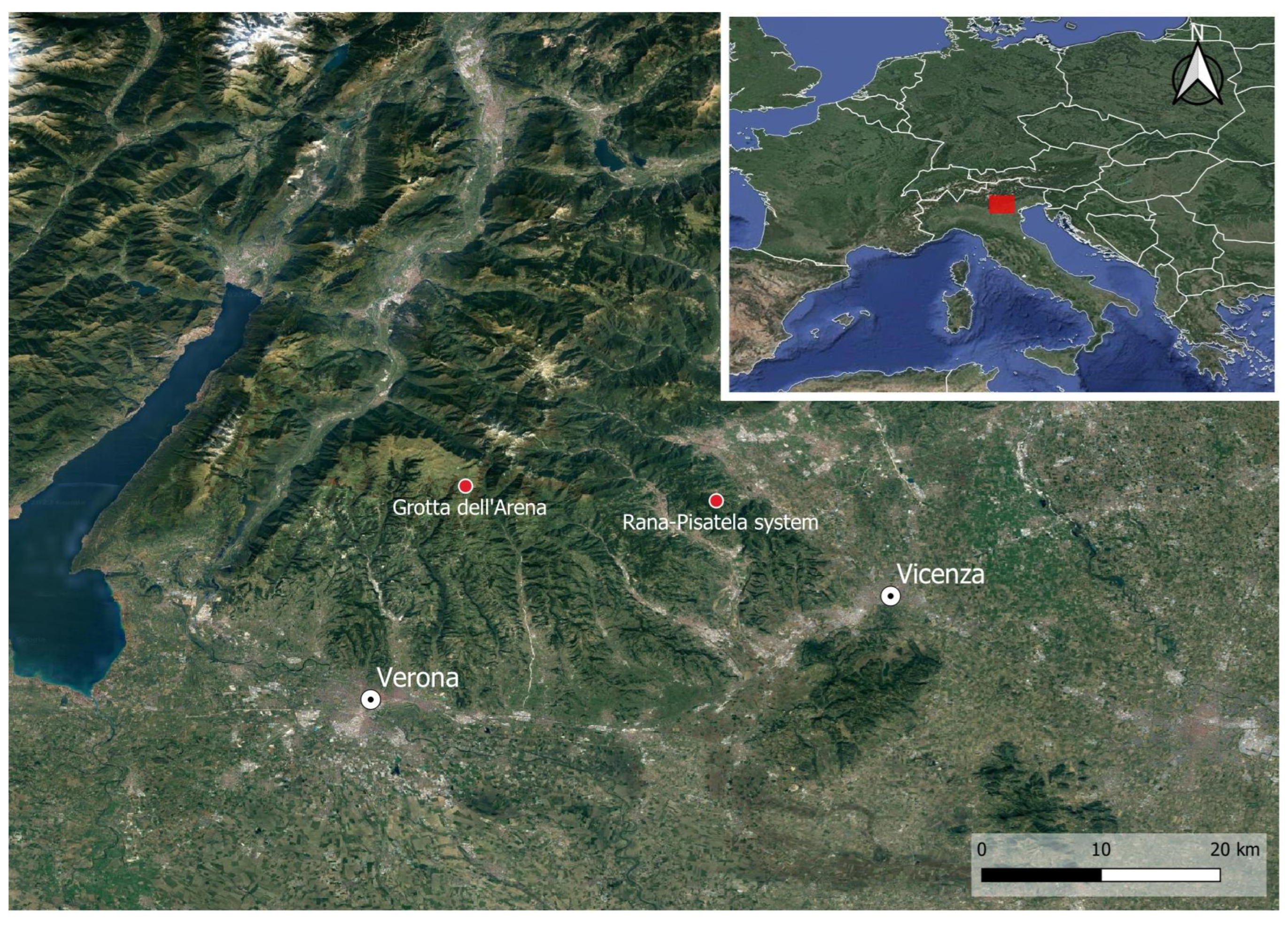
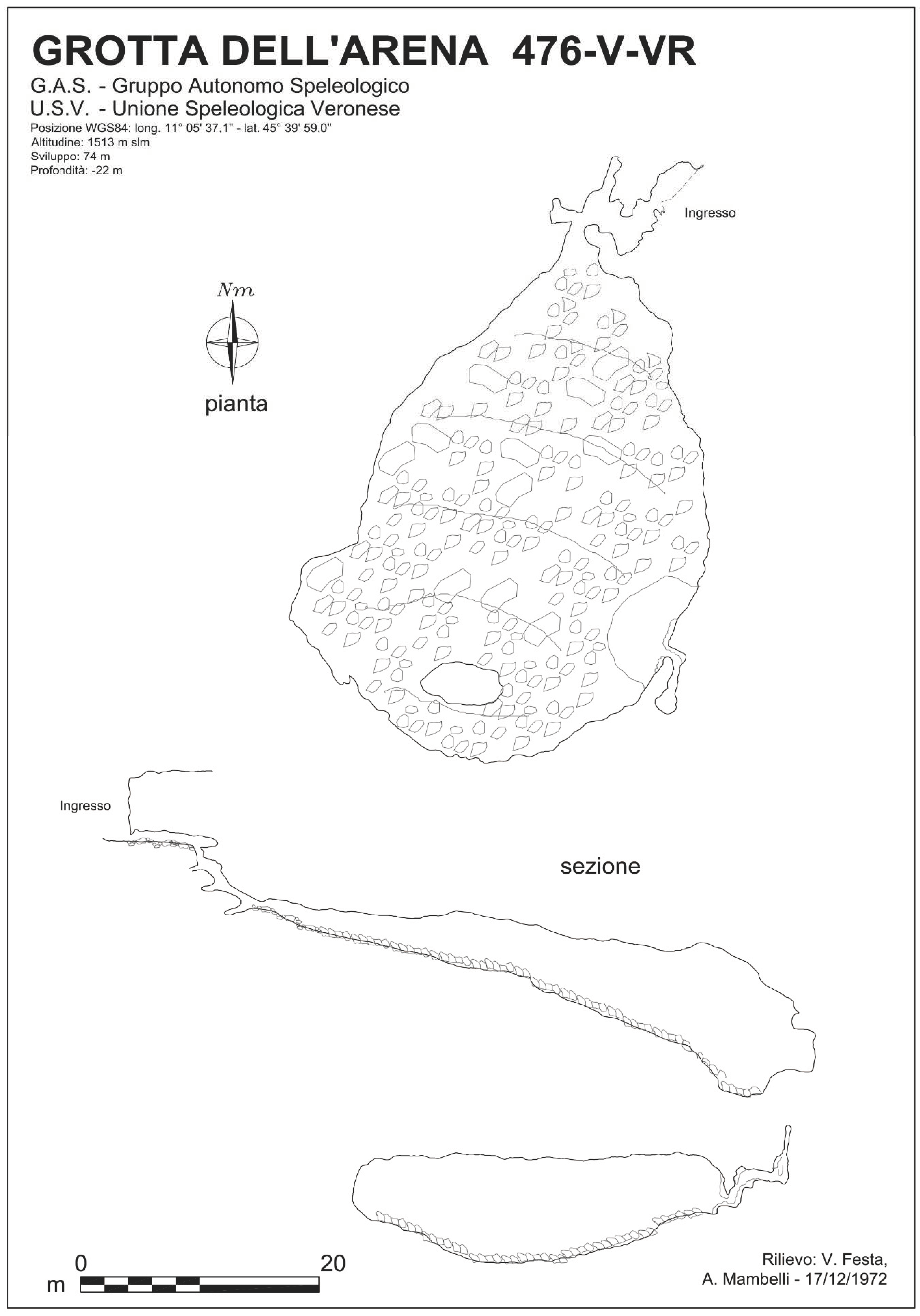
1.1. Arena Cave (476 V/VR)
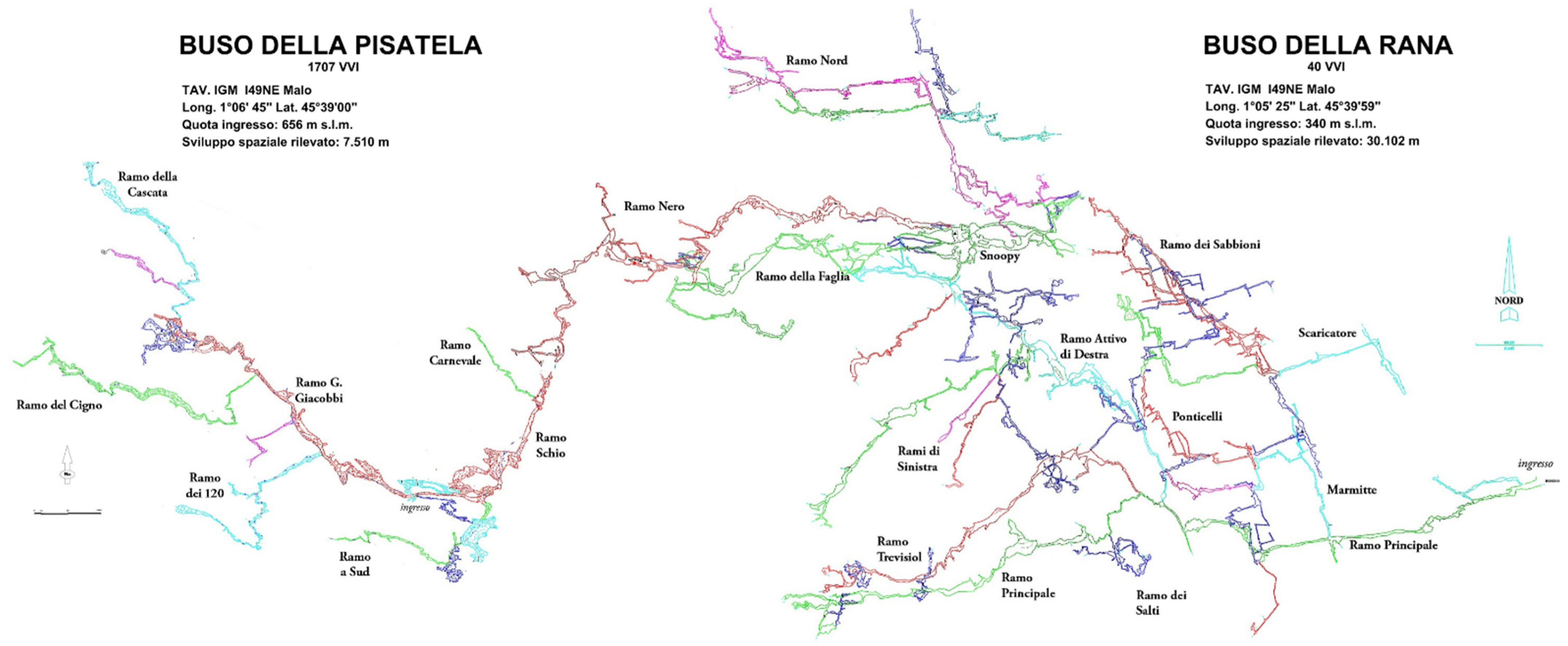
1.2. Buso della Rana-Pisatela Cave System (40 V–VI/1707 V–VI)
2. Materials and Methods
2.1. Sampling and Museum Collections
2.2. Bibliographic Research
3. Results
3.1. Terrestrial Fauna
3.2. Aquatic Fauna
4. Discussion
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marazzi, S. Atlante Orografico Delle Alpi—SOIUSA; Priuli and Verlucca: Scarmagno, Italy, 2005. [Google Scholar]
- Sauro, U. Il Paesaggio Degli Alti Lessini: Studio Geomorfologico; Museo Civico di Storia Naturale di Verona: Verona, Italy, 1973; Volume 6, 161p. [Google Scholar]
- Caoduro, G.; Ruffo, S. La Grotta dell’Arena, un biotopo di eccezionale interesse negli alti Lessini. In La Lessinia Ieri Oggi Domani: Quaderno Culturale; Editrice La Grafica: Lavagno, Italy, 1998; pp. 39–44. [Google Scholar]
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The mid-latitude biodiversity ridge in terrestrial cave fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Sauro, U. Aspects of contact karst in the Venetian Fore-Alps. Acta Carsologica 2001, 30, 89–102. [Google Scholar]
- Latella, L.; Sauro, U. Aspects of the Evolution of an Important Geo-Ecosystem in the Lessinian Mountain (Venetian Prealps, Italy). Acta Carsologica 2007, 36, 69–75. [Google Scholar] [CrossRef]
- Pasa, A. Carsismo ed idrografia carsica del Gruppo del Monte Baldo e dei Lessini Veronesi. CNR Cent. Studi Geogr. Fis. Ric. Sulla Morfol. Idrogr. Carsismo 1954, 5, 1–150. [Google Scholar]
- Sauro, U. Aspetti dell’evoluzione carsica legata a particolari condizioni litologiche e tettoniche negli Alti Lessini. Boll. Soc. Geol. Ital. 1974, 93, 945–969. [Google Scholar]
- Tisato, N.; Sauro, F.; Bernasconi, S.M.; Bruijn, R.H.; De Waele, J. Hypogenic contribution to speleogenesis in a predominant epigenic karst system: A case study from the Venetian Alps, Italy. Geomorphology 2012, 151, 156–163. [Google Scholar] [CrossRef]
- Sistema carsico Rana-Pisatella. Available online: www.busodellarana.it (accessed on 1 August 2023).
- Latella, L.; Verdari, N.; Gobbi, M. Distribution of terrestrial cave-dwelling arthropods in two adjacent Prealpine Italian areas with different glacial histories. Zool. Stud. 2012, 51, 1113–1121. [Google Scholar]
- Avesani, D.; Latella, L. Spatio-temporal distribution of the genus Chionea (Diptera, Limoniidae) in the Buso del Valon ice cave and other caves in the Lessini Mountains (Northern Italy). Boll. Mus. Civ. St. Nat. Verona 2016, 40, 11–16. [Google Scholar]
- Bruno, M.C.; Cottarelli, V.; Grasso, R.; Latella, L.; Zaupa, S.; Spena, M.T. Epikarst crustaceans from some Italian caves: Endemisms and spatial scales. Biogeographia 2018, 33, 1–18. [Google Scholar]
- Pipan, T. Epikarst—A Promising Habitat; ZRC Publishing: Ljubljana, Slovenia, 2005; pp. 1–101. [Google Scholar]
- Pipan, T.; Christman, M.C.; Culver, D.C. Dynamics of epikarst communities: Microgeographic pattern and environmental determinants of epikarst copepods in Organ Cave, West Virginia. Am. Midl. Nat. 2006, 156, 75–87. [Google Scholar] [CrossRef]
- Allegranzi, A.; Bartolomei, G.; Broglio, A.; Pasa, A.; Rigobello, A.; Ruffo, S. Il Buso della Rana (40 V-VI). Ras. Speleol. Ital. 1960, 12, 99–163. [Google Scholar]
- Caoduro, G.; Osella, G.; Ruffo, S. La fauna cavernicola della regione veronese. Memorie del Museo Civico di Storia Naturale di Verona. Sez. Biol. 1994, 6, 1–144. [Google Scholar]
- Latella, L.; Sauro, U. Note di Storia Naturale del sottosuolo dei Monti Lessini e del suo popolamento. Quaderno Culturale Lessinia Ieri Oggi Domani 2006, 34, 57–64. [Google Scholar]
- Latella, L. Il contributo del Museo Civico di Storia Naturale di Verona allo sviluppo della biospeleologia. Studi Trentini Di Sci. Nat. Acta Biol. 2004, 81, 15–22. [Google Scholar]
- Ruffo, S. Le attuali conoscenze sulla fauna cavernicola della Regione Pugliese. Mem. Biogeogr. Adriat. 1957, 3, 1–143. [Google Scholar]
- Sket, B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Pezzoli, E. Il genere Zospeum Bourguignat, 1856 in Italia (Gastropoda Polmonata Basommatophora). Censimento delle stazioni ad oggi segnalate. Nat. Brescia. 1992, 27, 123–169. [Google Scholar]
- Petri, I.; Ballarin, F.; Latella, L. Seasonal abundance and spatio-temporal distribution of the troglophylic harvestman Ischyropsalis ravasinii (Arachnida, Opiliones, Ischyropsalididae) in the Buso del Valon ice cave, Eastern Italian Prealps. Subterr. Biol. 2022, 42, 151–164. [Google Scholar] [CrossRef]
- Gardini, G. The species of the Chthonius heterodactylus group (Arachnida, Pseudoscorpiones, Chthoniidae) from the eastern Alps and the Carpathians. Zootaxa 2014, 3887, 101–137. [Google Scholar] [CrossRef][Green Version]
- Casale, A.; Vigna Taglianti, A. Note su Italaphaenops dimaioi Ghidini (Coleoptera, Carabidae). Boll. del Mus. Civ. Stor. Nat. di Verona 1976, 2, 293–314. [Google Scholar][Green Version]
- Sciaky, R.; Vigna Taglianti, A. The genus Lessinodytes (Coleoptera, Carabidae, Trechinae). Mém. Biospéol. 1990, 17, 169–173. [Google Scholar][Green Version]
- Monguzzi, R. Nuovi Dati Per La Conoscenza Del Genere Lessinodytes Vigna Taglianti, 1982 (Coleoptera Carabidae Trechinae). Nat. Brescia. 1993, 29, 179–183. [Google Scholar][Green Version]
- Faille, A.; Casale, A.; Balke, M.; Ribera, I. A molecular phylogeny of Alpine subterranean Trechini (Coleoptera: Carabidae). BMC Evol. Biol. 2013, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Latella, L. The Subterranean Cholevinae of Italy. In Cave Biodiversity: Speciation and Diversity of Subterranean Fauna; Johns Hopkins University Press: Baltimore, MD, USA, 2022; p. 164. [Google Scholar]
- Giachino, P.M.; Vailati, D. I Cholevidae delle Alpi e Prealpi italiane: Inventario, analisi faunistica e origine del popolamento del settore compreso fra i corsi dei fiumi Ticino e Tagliamento (Coleoptera). Biogeogr. J. Integr. Biogeogr. 2005, 26, 229–378. [Google Scholar] [CrossRef]
- Deharveng, L.; Stoch, F.; Gibert, J.; Bedos, A.; Galassi, D.; Zagmajster, M.; Brancelj, A.; Camacho, A.; Fiers, F.; Martin, P.; et al. Groundwater biodiversity in Europe. Freshw. Biol. 2009, 54, 709–726. [Google Scholar] [CrossRef]
- Papi, F.; Pipan, T. Ecological studies of an epikarst community in Snežna jama na planini Arto an ice cave in north central Slovenia. Acta Carsologica 2011, 40, 3. [Google Scholar] [CrossRef]
- Chappuis, P.A. Nouveaux Copépodes cavernicoles des genres Cyclops et Canthocamptus (Note préliminaire). Bul. Soc. De Stiint. Din Cluj 1923, 1, 584–590. [Google Scholar]
- Chappuis, P.A. Nouveaux Copépodes cavernicoles. Bul. Soc. De Stiint. Din Cluj 1928, 2, 20–34. [Google Scholar]
- Chappuis, P.A. Biospeologica LIX. Copepodes (premiere serie), avec l’énumeration de tous les copepodes cavernicoles connus en 1931. Arch. De Zool. Expérimentale Et Générale 1933, 76, 1–56. [Google Scholar]
- Galassi, D.M.P. Groundwater copepods: Diversity patterns over ecological and evolutionary scales. Hydrobiologia 2001, 453, 227–253. [Google Scholar] [CrossRef]
- Ruffo, S.; Stoch, F. Checklist and Distribution of the Italian Fauna. Memorie del Museo Civico di Storia Naturale di Verona, II Serie, Sezione Scienze Della Vita 17; Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2006; with CD-ROM. [Google Scholar]
- Mori, N.; Brancelj, A. Differences in aquatic microcrustacean assemblages between temporary and perennial springs of an alpine karstic aquifer. Int. J. Speleol. 2013, 42, 3–9. [Google Scholar] [CrossRef]
- Stoch, F. A new genus and two new species of Canthocamptidae (Copepoda, Harpacticoida) from caves in northern Italy. Hydrobiologia 1997, 350, 49–61. [Google Scholar] [CrossRef]
- Stoch, F. New and little known Parastenocaris (Copepoda, Harpacticoida, Parastenocarididae) from cave waters in Northeastern Italy. Boll. Del Mus. Civ. Di Stor. Nat. Di Verona 2000, 24, 195–206. [Google Scholar]
- Hughes, P.D. Quaternary glacial history of the Mediterranean mountains. Progr. Phys. Geogr. 2006, 30, 334–364. [Google Scholar] [CrossRef]
- Bassetti, M.; Borsato, A. Evoluzione geomorfologica e vegetazionale della bassa valle dell’Adige dall’ultimo massimo glaciale: Sintesi delle conoscenze e riferimenti ad aree limitrofe. Studi Trentini Sci. Nat. Acta Geol. 2007, 82, 31–42. [Google Scholar]
- Sommaruga, M.; Zorzin, R. Evidences of Morphologies and Glacial Deposits into High Valle del Chiampo (Northern Italy, Vicenza Province): First Results. Boll. Del Mus. Civ. Di Stor. Nat. Di Verona Geol. Paleontol. Preist. 2018, 42, 43–71. [Google Scholar]
- Sbordoni, V. Advances in speciation of cave animals. In Mechanisms of Speciation; Barigozzi, C., Ed.; Liss: New York, NY, USA, 1982; pp. 219–224. [Google Scholar]
- Humphreys, W.F. Relict fauna and their derivation. In Ecosystems of the World; Wilkens, H., Culver, D.C., Humphreys, W.F., Eds.; Subterranean Ecosystems; Elsevier: Amsterdam, The Netherlands, 2000; Volume 30, pp. 417–432. [Google Scholar]
- Assmann, T.; Casale, A.; Drees, C.; Habel, J.C.; Matern, A.; Schuldt, A. Review: The dark side of relict species biology: Cave animals as ancient lineages. In Relict Species: Phylogeography and Conservation Biology; Assmann, T., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 91–103. [Google Scholar]
- Hampe, A.; Jump, A.S. Climate relicts: Past, present, future. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 313–333. [Google Scholar] [CrossRef]
- Galassi, D.M.; Stoch, F.; Fiasca, B.; Di Lorenzo, T.; Gattone, E. Groundwater biodiversity patterns in the Lessinian Massif of northern Italy. Freshw. Biol. 2009, 54, 830–847. [Google Scholar] [CrossRef]









| Class | Order | Family | Genus/Species/Subspecies | Status | Arena | Rana-Pisatella |
|---|---|---|---|---|---|---|
| Gastropoda | Ellobiida | Ellobiidae | Zospeum globosum Kuščer, 1928 | Tb | 1 | 1 |
| Arachnida | Opiliones | Ischyropsalididae | Ischyropsalis strandti Kratochvíl, 1936 | Tb | 1 | 1 |
| Arachnida | Pseudoscorpionida | Neobisiidae | Neobisium (Blothrus) torrei (Simon, 1881) | Tb | 1 | 1 |
| Arachnida | Pseudoscorpionida | Neobisiidae | Balkanoroncus boldorii (Beier, 1931) | Tb | 1 | 0 |
| Arachnida | Pseudoscorpionida | Chthoniidae | Chthonius lessiniensis Schawaller, 1982 | Tb | 1 | 0 |
| Malacostraca | Isopoda | Trichoniscidae | Androniscus (Dentigeroniscus) degener Brian, 1926 | Tb | 1 | 1 |
| Diplopoda | Chordeumatida | Craspedosomatidae | Lessinosoma paolettii Strasser, 1967 | Tb | 1 Tl | 0 |
| Diplopoda | Chordeumatida | Iulidae | Trogloiulus boldorii Manfredi, 1940 | Tb | 1 | 0 |
| Collembola | Poduromorpha | Onychiuridae | Onychiurus hauseri Dallai, 1975 | Tb | 1 | 0 |
| Collembola | Entomobryomorpha | Entomobryidae | Pseudosinella concii Gisin, 1950 | Tb | 1 | 0 |
| Collembola | Entomobryomorpha | Entomobryidae | Pseudosinella sp. | Tb | 1 | 0 |
| Insecta | Coleoptera | Carabidae | Italaphaenops dimaioi Ghidini, 1964 | Tb | 1 | 0 |
| Insecta | Coleoptera | Carabidae | Lessynodytes pivai Vigna Taglianti & Sciaky, 1988 | Tb | 1 Tl | 0 |
| Insecta | Coleoptera | Carabidae | Orotrechus pominii Tamanini, 1953 | Tb | 1 | 1 |
| Insecta | Coleoptera | Carabidae | Orotrechus vicentinus juccii Pomini, 1940 | Tb | 1 | 0 |
| Insecta | Coleoptera | Leiodidae | Halberria zorzii (Ruffo, 1950) | Tb | 1 Tl | 0 |
| Insecta | Coleoptera | Leiodidae | Lessiniella trevisioli Pavan, 1941 | Tb | 0 | 1 Tl |
| Insecta | Coleoptera | Leiodidae | Neobathyscia fabianii (Dodero, 1904) | Tb | 0 | 1 |
| Copepoda | Cyclopoida | Cyclopidae | Speocyclops infernus Kiefer, 1930 | Stb | 1 | 1 |
| Copepoda | Harpactoida | Camptocamptidae | Elaphoidella phreatica (Chappuis, 1925) | Stb | 0 | 1 |
| Copepoda | Harpactoida | Camptocamptidae | Elaphoidella ruffoi Chappuis, 1953 | Stb | 0 | 1 |
| Copepoda | Harpactoida | Camptocamptidae | Elaphoidella sp. A1 | Stb | 1 | 0 |
| Copepoda | Harpactoida | Camptocamptidae | Elaphoidella sp. A | Stb | 0 | 1 |
| Copepoda | Harpactoida | Camptocamptidae | Ceuthonectes serbicus Chappuis, 1924 | Stb | 0 | 1 |
| Copepoda | Harpactoida | Camptocamptidae | Lessinocamptus insoletus (Chappuis, 1928) | Stb | 0 | 1 Tl |
| Copepoda | Harpactoida | Camptocamptidae | Lessinocamptus pivai Stoch, 1997 | Stb | 0 | 1 Tl |
| Copepoda | Harpactoida | Camptocamptidae | Lessinocamptus caoduroi Stoch, 1997 | Stb | 1 Tl | 0 |
| Copepoda | Harpactoida | Camptocamptidae | Bryocamptus (Limocamptus) echinatus (Mrazek, 1893) | Stb | 1 | 0 |
| Copepoda | Harpactoida | Camptocamptidae | Moraria (M.) sp. A1 | Stb | 1 | 0 |
| Copepoda | Harpactoida | Parastenocaridiidae | Parastenocaris ranae Stoch, 2000 | Stb | 0 | 1 Tl |
| Copepoda | Harpactoida | Amaeridae | Nitocrella psammophila Chappuis, 1955 | Stb | 1 | 1 |
| Malacostraca | Amphipoda | Niphargidae | Niphargus costozzae Schellenberg, 1935 | Stb | 0 | 1 |
| Malacostraca | Amphipoda | Niphargidae | Niphargus similis Karaman & Ruffo, 1989 | Stb | 1 | 0 |
| Malacostraca | Isopoda | Sphaeromatidae | Monolistra (Typhlosphaeroma) bericum bericum Fabiani, 1901 | Stb | 0 | 1 |
| Malacostraca | Bathynellacea | Bathynellidae | Bathynella sp. | Stb | 1 | 0 |
| Tot. | 24 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latella, L. Does Size Matter? Two Subterranean Biodiversity Hotspots in the Lessini Mountains in the Veneto Prealps in Northern Italy. Diversity 2024, 16, 25. https://doi.org/10.3390/d16010025
Latella L. Does Size Matter? Two Subterranean Biodiversity Hotspots in the Lessini Mountains in the Veneto Prealps in Northern Italy. Diversity. 2024; 16(1):25. https://doi.org/10.3390/d16010025
Chicago/Turabian StyleLatella, Leonardo. 2024. "Does Size Matter? Two Subterranean Biodiversity Hotspots in the Lessini Mountains in the Veneto Prealps in Northern Italy" Diversity 16, no. 1: 25. https://doi.org/10.3390/d16010025
APA StyleLatella, L. (2024). Does Size Matter? Two Subterranean Biodiversity Hotspots in the Lessini Mountains in the Veneto Prealps in Northern Italy. Diversity, 16(1), 25. https://doi.org/10.3390/d16010025








