Frost Cracks Show a Slight Effect on Fungal Richness in Stem Wood of Hybrid Aspen Trees in Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Isolation and Identification
2.3. Data Analysis
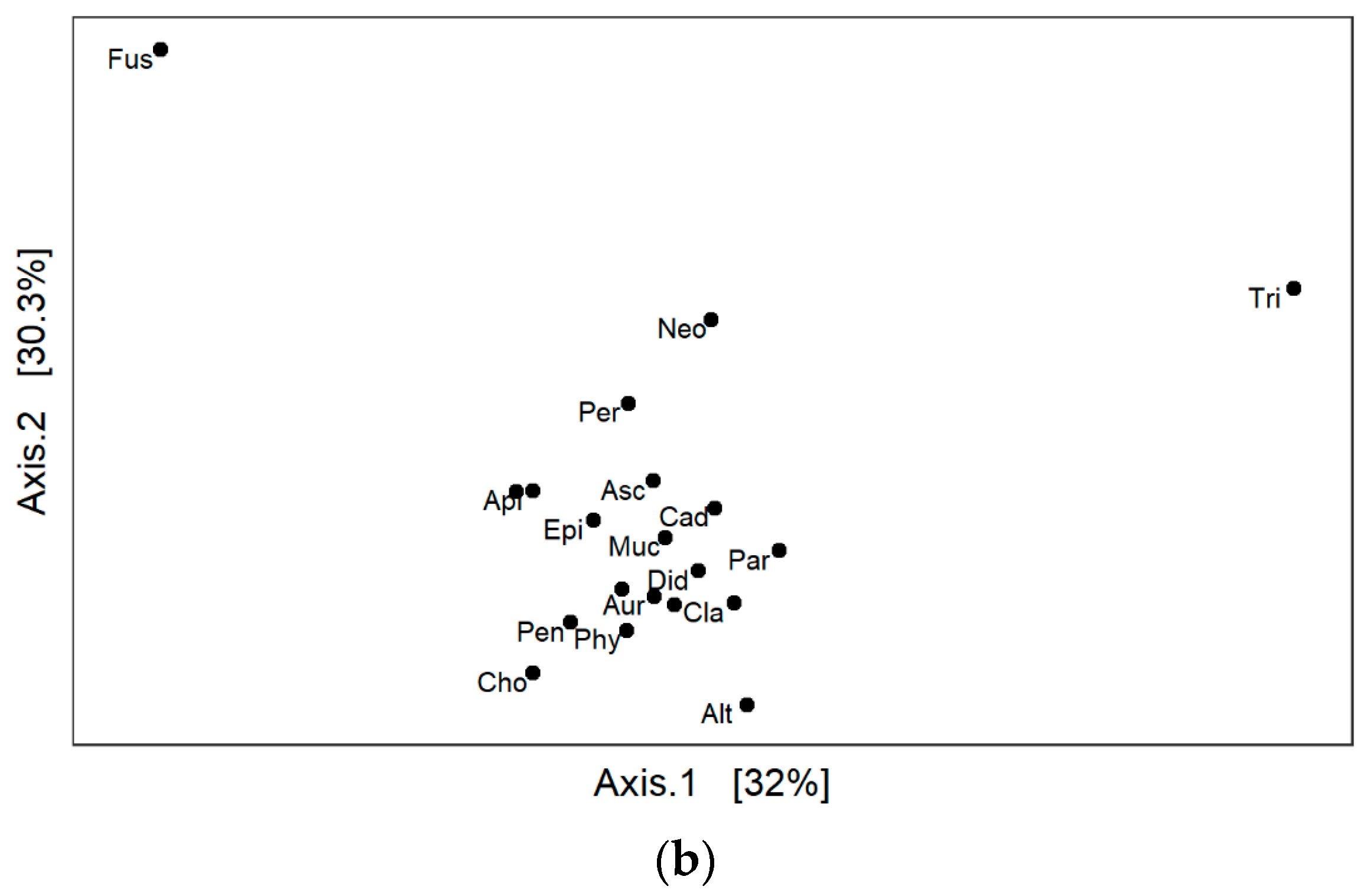
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutter, R.; Stål, G.; Arnesson Ceder, L.; Lim, H.; Padari, A.; Tullus, H.; Nordin, A.; Lundmark, T. Climate Benefit of Different Tree Species on Former Agricultural Land in Northern Europe. Forests 2021, 12, 1810. [Google Scholar] [CrossRef]
- Tullus, A.; Rytter, L.; Tullus, T.; Weih, M.; Tullus, H. Short-rotation forestry with hybrid aspen (Populus tremula L.× P. tremuloides Michx.) in Northern Europe. Scand. J. For. Res. 2012, 27, 10–29. [Google Scholar] [CrossRef]
- Rytter, L.; Ingerslev, M.; Kilpeläinen, A.; Torssonen, P.; Lazdina, D.; Löf, M.; Madsen, P.; Muiste, P.; Stener, L.G. Increased forest biomass production in the Nordic and Baltic countries—A review on current and future opportunities. Silva Fenn. 2016, 50, 1660. [Google Scholar] [CrossRef]
- Šēnhofa, S.; Zeps, M.; Gailis, A.; Kāpostiņš, R.; Jansons, Ā. Development of stem cracks in young hybrid aspen plantations. For. Stud. 2016, 65, 16–23. [Google Scholar] [CrossRef]
- Čakšs, R.; Zeltinš, P.; Čakša, L.; Zeps, M.; Jansons, A. The Effects of Frost Cracks and Large Poplar Borer Damage on Stem Rot in Hybrid Aspen (Populus tremula L. × Populus tremuloides Michx.) Clones. Forests 2022, 13, 593. [Google Scholar] [CrossRef]
- DeBell, D.S.; Singleton, R.; Harrington, C.A.; Gartner, B.L. Wood density and fiber length in young Populus stems: Relation to clone, age, growth rate, and pruning. Wood Fiber Sci. 2002, 4, 529–539. [Google Scholar]
- Zeps, M.; Gailis, A.; Smilga, S.; Miezite, O.; Sisenis, L.; Zariņa, I. Hybrid aspen clone wood mechanical properties. Agron. Res. 2016, 14, 1147–1152. [Google Scholar]
- Vasaitis, R.; Bakys, R.; Vasiliauskas, A. Discoloration and associated fungi in stems of silver birch (Betula pendula Roth.) following logging damage. For. Pathol. 2012, 42, 387–392. [Google Scholar] [CrossRef]
- Arhipova, N.; Jansons, A.; Zaluma, A.; Gaitnieks, T.; Vasaitis, R. Bark stripping of Pinus contorta caused by moose and deer: Wounding patterns, discoloration of wood, and associated fungi. Can. J. For. Res. 2015, 45, 1434–1438. [Google Scholar] [CrossRef]
- Burņeviča, N.; Jansons, Ā.; Zaļuma, A.; Kļaviņa, D.; Jansons, J.; Gaitnieks, T. Fungi inhabiting bark stripping wounds made by large game on stems of Picea abies (L.) Karst. in Latvia. Baltic For. 2016, 22, 2–7. [Google Scholar]
- Linnakoski, R.; Kasanen, R.; Lasarov, I.; Marttinen, T.; Oghenekaro, A.O.; Sun, H.; Asiegbu, F.O.; Wingfield, M.J.; Hantula, J.; Heliövaara, K. Cadophora margaritata sp. nov. and other fungi associated with the longhorn beetles Anoplophora glabripennis and Saperda carcharias in Finland. Antonie Leeuwenhoek 2018, 111, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Zaluma, A.; Strike, Z.; Rieksts-Riekstiņš, R.; Gaitnieks, T.; Vasaitis, R. Long-term pathological consequences of resin tapping wounds on stems of Scots pine (Pinus sylvestris L.). Trees 2022, 36, 1507–1514. [Google Scholar] [CrossRef]
- Basham, J.T. Decay of trembling aspen. Can. J. Bot. 1958, 36, 491–505. [Google Scholar] [CrossRef]
- Thomas, G.P.; Etheridge, D.E.; Paul, G. Fungi and decay in aspen and balsam poplar in the boreal forest region, Alberta. Can. J. Bot. 1960, 38, 459–466. [Google Scholar] [CrossRef]
- Kasanen, R.; Hantula, J.; Vuorinen, M.; Stenlid, J.; Solheim, H.; Kurkela, T. Migrational capacity of Fennoscandian populations of Venturia tremulae. Mycol. Res. 2004, 108, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lutter, R.; Drenkhan, R.; Tullus, A.; Jürimaa, K.; Tullus, T.; Tullus, H. First record of Entoleuca mammata in hybrid aspen plantations in hemiboreal Estonia and stand–environmental factors affecting its prevalence. Eur. J. For. Res. 2019, 138, 263–274. [Google Scholar] [CrossRef]
- Ilstedt, B.; Gullberg, U. Genetic variation in a 26-year old hybrid aspen trial in southern Sweden. Scand. J. For. Res. 1993, 8, 185–192. [Google Scholar] [CrossRef]
- Kasanen, R.; Hantula, J.; Kurkela, T. Neofabraea populi in hybrid aspen stands in southern Finland. Scand. J. For. Res. 2002, 17, 391–397. [Google Scholar] [CrossRef]
- Striganavičiūtė, G.; Žiauka, J.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Impact of Plant-Associated Bacteria on the In Vitro Growth and Pathogenic Resistance against Phellinus tremulae of Different Aspen (Populus) Genotypes. Microorganisms 2021, 9, 1901. [Google Scholar] [CrossRef]
- Ottosson, E.; Nordén, J.; Dahlberg, A.; Edman, M.; Jönsson, M.; Larsson, K.-H.; Olsson, J.; Penttilä, R.; Stenlid, J.; Ovaskainen, O. Species associations during the succession of wood-inhabiting fungal communities. Fungal Ecol. 2014, 11, 17–28. [Google Scholar] [CrossRef]
- Abrego, N. Wood-inhabiting fungal communities: Opportunities for integration of empirical and theoretical community ecology. Fungal Ecol. 2022, 59, 101112. [Google Scholar] [CrossRef]
- Klavina, D.; Tedersoo, L.; Agan, A.; Zaluma, A.; Bitenieks, K.; Polmanis, K.; Daugaviete, M.; Gaitnieks, T.; Drenkhan, R. Effect of stand thinning, former land use and individual tree parameters on wood inhabiting fungal community composition in young living Norway spruce. Fungal Ecol. 2023, 65, 101281. [Google Scholar] [CrossRef]
- Tullus, A.; Tullus, H.; Vares, A.; Kanal, A. Early growth of hybrid aspen (Populus × wettsteinii Hämet-Ahti) plantations on former agricultural lands in Estonia. For. Ecol. Manag. 2007, 245, 118–129. [Google Scholar] [CrossRef]
- Zeps, M. Potential of Hybrid Aspen (Populus tremuloides Michx. × Populus tremula L.) Production in Latvia. Ph.D. Thesis, Latvia University of Agriculture, Jelgava, Latvia, 2017. [Google Scholar]
- Kärki, T.; Vainikainen, V. Determining the quality of aspen (Populus tremula) logs for mechanical wood processing in Finland. For. Prod. J. 2004, 54, 64–71. [Google Scholar]
- Hinds, T.E. Diseases. Aspen: Ecology and Management in the Western United States. In General Technical Report RM-119; DeByle, N.V., Winokur, R.P., Eds.; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1985; pp. 87–106. [Google Scholar] [CrossRef]
- Arhipova, N. Heart Rot of Spruce and Alder Forests in Latvia—Impact and Possibilities for Silvicultural Control. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990; pp. 315–322. ISBN 9780080886718. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2018, 47, D259–D264. [Google Scholar] [CrossRef]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.D.; Engelbrecht Clemmensen, K.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. Fungal Traits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2019; ISBN 9781544336473. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, G.; Legendre, P.; Minchinm, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-2. Available online: https://CRAN.R-project.org/package=vegan (accessed on 22 November 2023).
- Martinez Arbizu, P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.4. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 22 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 22 November 2023).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data, R Package Version 2.4.1. 2009. Available online: https://CRAN.R-project.org/package=gplots (accessed on 22 November 2023).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Christiansen, E.; Krokene, P.; Berryman, A.A.; Franceschi, V.R.; Krekling, T.; Lieutier, F.; Lönneborg, A.; Solheim, H. Mechanical injury and fungal infection induce acquired resistance in Norway spruce. Tree Physiol. 1999, 19, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol. Ecol. 2000, 31, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hafner, P.; Gričar, J.; Skudnik, M.; Levanič, T. Variations in Environmental Signals in Tree-Ring Indices in Trees with Different Growth Potential. PLoS ONE 2015, 10, e0143918. [Google Scholar] [CrossRef][Green Version]
- Terhonen, E.; Blumenstein, K.; Kovalchuk, A.; Asiegbu, F.O. Forest Tree Microbiomes and Associated Fungal Endophytes: Functional Roles and Impact on Forest Health. Forests 2019, 10, 42. [Google Scholar] [CrossRef]
- Kukor, J.J. Effects of Bark and Sapwood Microorganisms on Development of Hypoxylon Canker of Aspen. Ph.D. Thesis, University of Michigan, Michigan, MI, USA, 1979. [Google Scholar]
- Smith, J.A.; Blanchette, R.A.; Ostry, M.E.; Anderson, N.A. Etiology of bronze leaf Disease of Populus. Plant Dis. 2002, 86, 462–469. [Google Scholar] [CrossRef]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Boavida Ferreira, R.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 2019, 14, 0213273. [Google Scholar] [CrossRef]
- Kwaśna, H.; Szewczyk, W.; Baranowska, M.; Gallas, E.; Wiśniewska, M.; Behnke-Borowczyk, J. Mycobiota associated with the vascular wilt of poplar. Plants 2021, 10, 892. [Google Scholar] [CrossRef]
- Taguiam, J.D.; Evallo, E.; Balendres, M.A. Epicoccum species: Ubiquitous plant pathogens and effective biological control agents. Eur. J. Plant Pathol. 2021, 159, 713–725. [Google Scholar] [CrossRef]
- Schneider, R.; Riopel, M.; Pothier, D.; Côté, L. Predicting decay and round-wood end use volume in trembling aspen (Populus tremuloides Michx.). Ann. For. Sci. 2008, 65, 608. [Google Scholar] [CrossRef]
- Trifonov, L.S.; Chakravarty, P.; Hiratsuka, Y.; Ayer, W.A. Antifungal activity of metabolites of Peniophora polygonia against the aspen decay fungus Phellinus tremulae. Eur. J. For. Pathol. 1992, 22, 441–448. [Google Scholar] [CrossRef]
- Churakov, B.P.; Kornilina, V.V.; Zamaldinov, I.T. The influence of heartwood rot on industrial wood yield in aspen stands. Lesovedenie 2011, 2, 19–24. [Google Scholar]
- Cellerino, G.P. Review of Fungal Diseases in Poplar; (FAO) Food and Agriculture Organization of the United Nations: Rome, Italy, 1999; AC492/E; Available online: https://www.fao.org/3/AC492E/AC492E00.htm (accessed on 22 November 2023).
- Dhillon, G.P.S.; Sandhu, J.S.; Singh, P. Variation among Poplar (Populus deltoides Bartr.) Clones for Growth, Wood Traits and Tolerance to Leaf Spot Diseases. Curr. Agric. Res. J. 2020, 8, 128–136. [Google Scholar] [CrossRef]
- Matić, S.; Tabone, G.; Garibaldi, A.; Gullino, M.A. Alternaria Leaf Spot Caused by Alternaria Species: An Emerging Problem on Ornamental Plants in Italy. Plant Dis. 2020, 104, 2275–2287. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, H.; Jankowiak, R.; Bilański, P.; Hausner, G. Structure and abundance of Fusarium communities inhabiting the litter of beech forests in central Europe. Forests 2021, 12, 811. [Google Scholar] [CrossRef]
- Vasić, T.; Jevremović, D.; Milenković, S.; Vujović, T.; Leposavić, A. Morphological and pathogenic characteristics of Alternaria alternata isolates from plum (Prunus domestica L.). Acta Hortic. 2021, 1322, 313–318. [Google Scholar] [CrossRef]
- Brotman, Y.; Kapuganti, J.G.; Viterbo, A. Trichoderma. Curr. Biol. 2010, 20, 390–391. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The threat of pests and pathogens and the potential for biological control in forest ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Bensch, K.; Groenewald, J.Z.; Dijksterhuis, J.; Starink-Willemse, M.; Andersen, B.; Summerell, B.A.; Shin, H.D.; Dugan, F.M.; Schroers, H.J.; Braun, U.; et al. Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud. Mycol. 2010, 67, 1–94. [Google Scholar] [CrossRef]
- Moricca, S.; Ragazzi, A.; Assante, G. Biocontrol of Rust Fungi by Cladosporium tenuissimum. In Rust Diseases of Willow and Poplar; Pei, M.H., McCracken, A.R., Eds.; CABI Publishing: Oxfordshire, UK, 2005; Chapter 19; ISBN 978-0-85199-999-9. [Google Scholar]
- Tyagi, K.; Kumar, P.; Pandey, A.; Ginwal, H.S.; Barthwal, S.; Nautiyal, R.; Meena, R.K. First record of Cladosporium oxysporum as a potential novel fungal hyperparasite of Melampsora medusae f. sp. deltoidae and screening of Populus deltoides clones against leaf rust. 3 Biotech. 2023, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Lazdiņa, D.; Šēnhofa, S.; Zeps, M.; Makovskis, K.; Bebre, I.; Jansons, A. The early growth and fall frost damage of poplar clones in Latvia. Agron. Res. 2016, 14, 109–122. [Google Scholar]
- Chung, K.R. Stress response and pathogenicity of the necrotrophic fungal pathogen Alternaria alternata. Scientifica 2012, 2012, 635431. [Google Scholar] [CrossRef] [PubMed]
- Åström, B.; Ramstedt, M. Stem cankers on Swedish biomass willows caused by Cryptodiaporthe salicella and other fungi. Eur. J. Forest Pathol. 1994, 24, 264–276. [Google Scholar] [CrossRef]
- Sieber, T.N.; Kowalski, T.; Holdenrieder, O. Fungal assemblages in stem and twig lesions of Quercus robur in Switzerland. Mycol. Res. 1995, 99, 534–538. [Google Scholar] [CrossRef]
- Hashemi, H.; Mohammadi, H.; Abdollahzadeh, J. Symptoms and fungi associated with elm trees decline in Iran. Eur. J. For. Res. 2017, 136, 857–879. [Google Scholar] [CrossRef]
- Ostry, M.E.; Wilson, L.F.; McNabb, H.S., Jr.; Moore, L.M. A Guide to Insect, Disease, and Animal Pests of Poplars, Agriculture Handbook (No. 677); US Department of Agriculture, Forest Service: Washington, DC, USA, 1988.
- Hiratsuka, Y.; Chakravarty, P. Role of Phialemonium curvatum as a potential biological control agent against a blue stain fungus on aspen. Eur. J. Forest Pathol. 1999, 29, 305–310. [Google Scholar] [CrossRef]
- Hutchison, L.J. Wood-inhabiting microfungi isolated from Populus tremuloides from Alberta and northeastern British Columbia. Can. J. Bot. 1999, 77, 898–905. [Google Scholar] [CrossRef]
- Przybyl, K. Fungi and minerals occurring in heartwood discolorations in Quercus robur trees. Acta Soc. Bot. Pol. 2007, 76, 55–60. [Google Scholar] [CrossRef]
- Olembo, T.W. Phoma herbarum Westend.: A pathogen of Acacia mearnsii De Wild. in Kenya. East Afr. Agric. For. J. 1972, 38, 201–206. [Google Scholar] [CrossRef]
- Hamberg, L.; Lemola, J.; Hantula, J. The potential of the decay fungus Chondrostereum purpureum in the biocontrol of broadleaved tree species. Fungal Ecol. 2017, 30, 67–75. [Google Scholar] [CrossRef]
- Vartiamäki, H.; Hantula, J.; Uotila, A. Susceptibility of silver birch pruning wounds to infection by white-rot fungus (Chondrostereum purpureum), a potential bioherbicide. Silva Fenn. 2009, 43, 537–547. [Google Scholar] [CrossRef][Green Version]

| Genus | Primary Lifestyle * | Relative Occurrence (%) and Number of Trees (in Brackets) |
|---|---|---|
| Trichoderma | mycoparasite | 49 (101) |
| Penicillium | unspecified saprotroph | 44 (91) |
| Alternaria | plant pathogen | 40 (81) |
| Cladosporium | litter saprotroph | 39 (80) |
| Fusarium | plant pathogen | 35 (72) |
| Cadophora | litter saprotroph | 22 (46) |
| Didymella | plant pathogen | 20 (41) |
| Aureobasidium | sooty mold | 19 (39) |
| Phialocephala | soil saprotroph | 14 (30) |
| Epicoccum | plant pathogen | 11 (24) |
| Ascocoryne | wood saprotroph | 9 (18) |
| Ophiostoma | plant pathogen | 9 (18) |
| Paraconiothyrium | saprotroph | 7 (15) |
| Apiospora | plant pathogen | 7 (14) |
| Physalospora | wood saprotroph | 6 (12) |
| Mucor | soil saprotroph | 5 (11) |
| Hypoxylon | wood saprotroph | 3 (7) |
| Neobulgaria | wood saprotroph | 3 (6) |
| Chondrostereum | plant pathogen | 3 (6) |
| Periconia | plant pathogen | 2 (5) |
| Primary Lifestyle | Relative Occurrence (%) and Number of Trees (in Brackets) |
|---|---|
| Plant pathogens | 66 (135) |
| Litter saprotrophs | 56 (115) |
| Unspecified saprotrophs | 46 (95) |
| Mycoparasites | 34 (69) |
| Soil saprotrophs | 20 (42) |
| Wood saprotrophs | 20 (40) |
| Number of All Fungal Taxa | Number of Plant Pathogen Taxa | Number of Saprotroph Taxa | |
|---|---|---|---|
| Fixed effect, χ2 | |||
| Sampling height | 229.87 ** | 38.29 ** | 142.59 ** |
| Presence/absence of frost cracks | 4.41 * | 5.38 * | 1.92 |
| Clone | 4.32 | 0.61 | 5.14 |
| Tree height | 0.03 | 0.01 | 0.18 |
| Stem diameter | 0.63 | 0.00 | 0.05 |
| Random effect, variance | |||
| Tree:(block:trial) | 6.00 × 10−10 | 6.54 × 10−9 | 0.00 × 100 |
| Block: trial | 0.00 × 100 | 0.00 × 100 | 0.00 × 100 |
| Trial | 3.29 × 10−3 | 2.06 × 10−2 | 0.05 |
| Alternaria | Fusarium | Cladosporium | Trichoderma | Penicillium | |
|---|---|---|---|---|---|
| Fixed effect, χ2 | |||||
| Sampling height | 20.54 ** | 14.97 ** | 0.00 | 5.86 * | 25.77 ** |
| Presence/absence of frost cracks | 2.39 | 0.83 | 0.71 | 0.05 | 0.83 |
| Clone | 2.19 | 1.65 | 9.77 * | 0.85 | 7.86 |
| Tree height | 0.80 | 0.03 | 0.00 | 0.41 | 0.00 |
| Tree diameter | 0.06 | 0.48 | 0.05 | 0.20 | 2.85 |
| Random effect, variance | |||||
| Tree: (block:trial) | 5.06 × 10−5 | 4.24 × 10−7 | 3.01 × 10−7 | 2.74 × 10−6 | 1.11 × 10−1 |
| Block: trial | 4.12 × 10−1 | 3.70 × 10−1 | 2.85 × 10−3 | 3.94 × 10−1 | 2.95 × 10−5 |
| Trial | 3.47 × 10−2 | 1.02 × 10−1 | 2.80 × 10−1 | 3.22 × 10−2 | 2.87 × 10−7 |
| Factor | Degree of Freedom | Sequential Sums of Squares | F Statistics | Partial R-Square Values | Partial p-Values |
|---|---|---|---|---|---|
| Sampling height | 1 | 2.474 | 23.626 | 0.054 | 0.001 ** |
| Clone | 4 | 0.311 | 0.742 | 0.007 | 0.714 |
| Trial | 2 | 0.505 | 2.412 | 0.011 | 0.025 * |
| Residuals | 402 | 42.104 | 0.925 | ||
| Total | 409 | 45.514 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kļaviņa, D.; Matisons, R.; Auniņa, A.; Striķe, Z.; Ciseļonoka, L.; Krastiņa, K.; Zeps, M.; Jansons, Ā.; Bitenieks, K.; Ruņģis, D.E.; et al. Frost Cracks Show a Slight Effect on Fungal Richness in Stem Wood of Hybrid Aspen Trees in Latvia. Diversity 2024, 16, 14. https://doi.org/10.3390/d16010014
Kļaviņa D, Matisons R, Auniņa A, Striķe Z, Ciseļonoka L, Krastiņa K, Zeps M, Jansons Ā, Bitenieks K, Ruņģis DE, et al. Frost Cracks Show a Slight Effect on Fungal Richness in Stem Wood of Hybrid Aspen Trees in Latvia. Diversity. 2024; 16(1):14. https://doi.org/10.3390/d16010014
Chicago/Turabian StyleKļaviņa, Dārta, Roberts Matisons, Annija Auniņa, Zane Striķe, Laima Ciseļonoka, Keitlīna Krastiņa, Mārtiņš Zeps, Āris Jansons, Krišs Bitenieks, Dainis Edgars Ruņģis, and et al. 2024. "Frost Cracks Show a Slight Effect on Fungal Richness in Stem Wood of Hybrid Aspen Trees in Latvia" Diversity 16, no. 1: 14. https://doi.org/10.3390/d16010014
APA StyleKļaviņa, D., Matisons, R., Auniņa, A., Striķe, Z., Ciseļonoka, L., Krastiņa, K., Zeps, M., Jansons, Ā., Bitenieks, K., Ruņģis, D. E., & Gaitnieks, T. (2024). Frost Cracks Show a Slight Effect on Fungal Richness in Stem Wood of Hybrid Aspen Trees in Latvia. Diversity, 16(1), 14. https://doi.org/10.3390/d16010014








