The Geographical Differences in the Bird Prey of the Great Evening Bat (Ia io)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Fecal Collection
2.3. DNA Extraction and PCR Amplification
2.4. Sequence Analysis and Taxonomic Identification
2.5. Statistical Analysis
3. Result
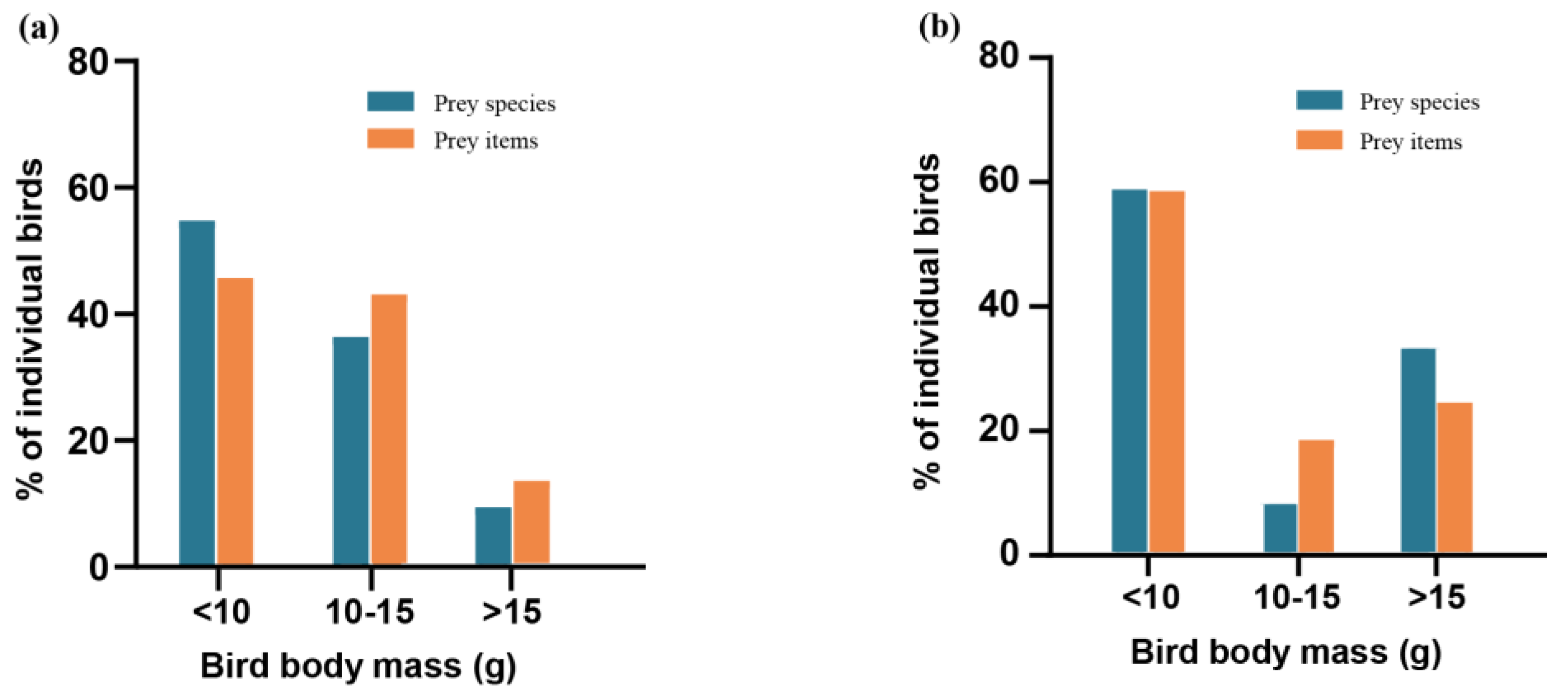
3.1. Differences in Bird Species Diversity among Different Populations of the Great Evening Bat
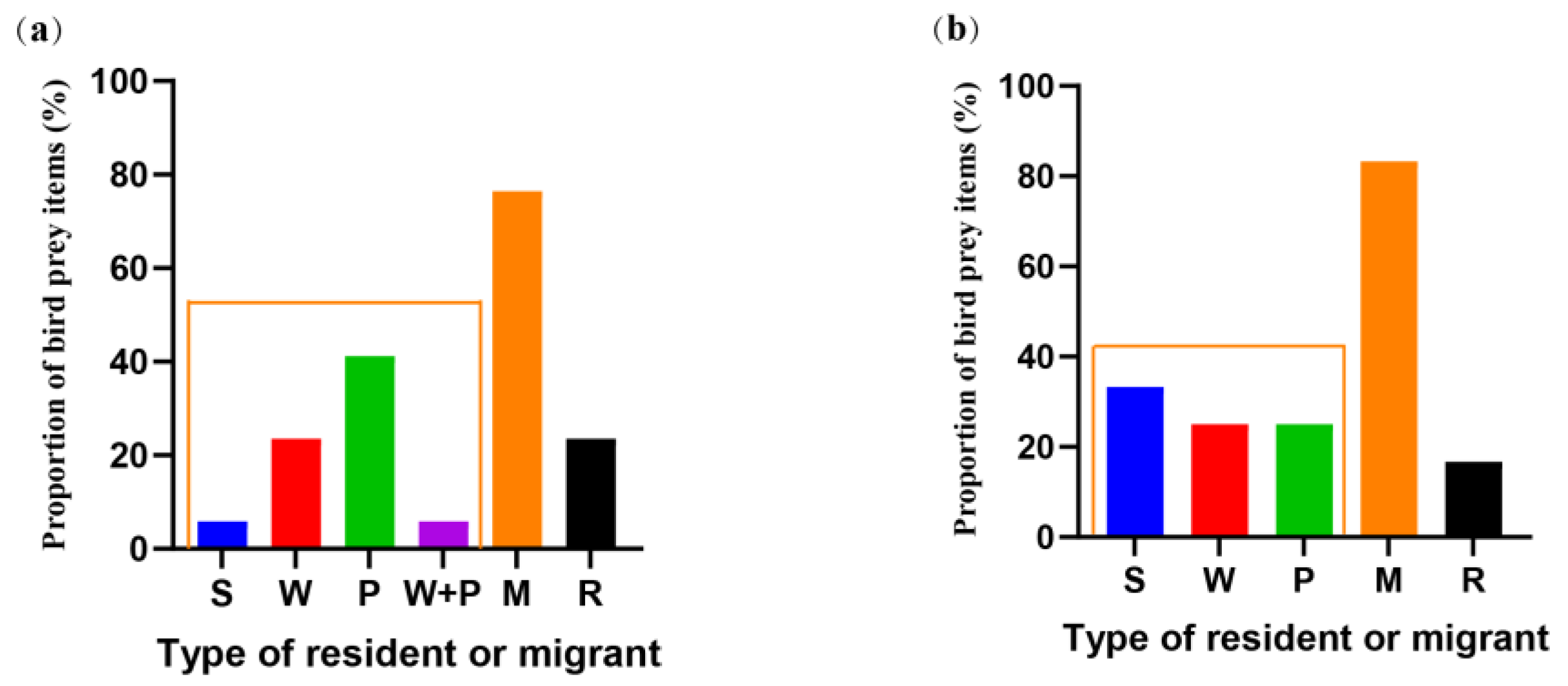
3.2. Selection of Bird Prey Types by Different Populations of the Great Evening Bat
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986; Volume 1. [Google Scholar]
- Maucieri, D.; Barclay, R.M. Consumption of spiders by the little brown bat (Myotis lucifugus) and the long-eared myotis (Myotis evotis) in the Rocky Mountains of Alberta, Canada. Can. J. Zool. 2021, 99, 221–226. [Google Scholar] [CrossRef]
- Kaupas, L.A.; Barclay, R.M. Temperature-dependent consumption of spiders by little brown bats (Myotis lucifugus), but not northern long-eared bats (Myotis septentrionalis), in northern Canada. Can. J. Zool. 2018, 96, 261–268. [Google Scholar] [CrossRef]
- Aspetsberger, F.; Brandsen, D.; Jacobs, D.S. Geographic variation in the morphology, echolocation and diet of the little free-tailed bat, Chaerephon pumilus(Molossidae). Afr. Zool. 2003, 38, 245–254. [Google Scholar] [CrossRef]
- Clare, E.L.; Symondson, W.O.; Broders, H.; Fabianek, F.; Fraser, E.E.; MacKenzie, A.; Boughen, A.; Hamilton, R.; Willis, C.K.; Martinez-Nuñez, F. The diet of Myotis lucifugus across Canada: Assessing foraging quality and diet variability. Mol. Ecol. 2014, 23, 3618–3632. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr. Food of the big brown bat Eptesicus fuscus from maternity colonies in Indiana and Illinois. Am. Midl. Nat. 1995, 134, 346–360. [Google Scholar] [CrossRef]
- Leite, T.; Batista, A.; Lima, F.; Barbosa, J.; Mather, J. Geographic variability of Octopus insularis diet: From oceanic island to continental populations. Aquat. Biol. 2016, 25, 17–27. [Google Scholar] [CrossRef]
- Popa-Lisseanu, A.G.; Delgado-Huertas, A.; Forero, M.G.; Rodríguez, A.; Arlettaz, R.; Ibáñez, C. Bats’ Conquest of a Formidable Foraging Niche: The Myriads of Nocturnally Migrating Songbirds. PLoS ONE 2007, 2, e205. [Google Scholar] [CrossRef] [PubMed]
- Norberg, U.M.; Rayner, J.M.V. Ecological morphology and flight in bats (Mammalia; Chiroptera): Wing adaptations, flight performance, foraging strategy and echolocation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1987, 316, 335–427. [Google Scholar] [CrossRef]
- Thabah, A.; Li, G.; Wang, Y.; Liang, B.; Hu, K.; Zhang, S.; Jones, G. Diet, Echolocation Calls, and Phylogenetic Affinities of the Great Evening Bat (Ia io; Vespertilionidae): Another Carnivorous Bat. J. Mammal 2007, 88, 728–735. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Tan, L.; Shen, Q.; Chen, Z.; Gong, Y.; Xiang, Z.; Zhang, L. Ia io found in Guangdong Province. Chin. J. Zool. 2013, 2, 287–291. [Google Scholar]
- Zukal, J. Handbook of the Mammals of the World. J. Vertebr. Biol. 2020, 69, E2003. [Google Scholar] [CrossRef]
- Ingala, M.R.; Simmons, N.B.; Wultsch, C.; Krampis, K.; Speer, K.A.; Perkins, S.L. Comparing Microbiome Sampling Methods in a Wild Mammal: Fecal and Intestinal Samples Record Different Signals of Host Ecology, Evolution. Front. Microbiol. 2018, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, C.; Juste, J.; García-Mudarra, J.L.; Agirre-Mendi, P.T. Bat predation on nocturnally migrating birds. Proc. Natl. Acad. Sci. USA 2001, 98, 9700–9702. [Google Scholar] [CrossRef] [PubMed]
- Gual-Suárez, F.; Medellín, R.A. We eat meat: A review of carnivory in bats. Mammal Rev. 2021, 51, 540–558. [Google Scholar] [CrossRef]
- Fukui, D.; Dewa, H.; Katsuta, S.; Sato, A. Bird predation by the birdlike noctule in Japan. J. Mammal 2013, 94, 657–661. [Google Scholar] [CrossRef][Green Version]
- Norberg, U.M.; Fenton, M.B. Carnivorous bats? Biol. J. Linn. Soc. 1988, 33, 383–394. [Google Scholar] [CrossRef]
- Osgood, W.H.; Allen, G.M. The Mammals of China and Mongolia. Part 2 of Vol. XI of Natural History of Central Asia. J. Mammal 1941, 22, 206–208. [Google Scholar] [CrossRef]
- Csorba, G. The distribution of the great evening bat Ia io in the Indo-Malayan region. Myotis 1998, 36, 197–201. [Google Scholar]
- Shi, B.; Wang, Y.; Gong, L.; Chang, Y.; Liu, T.; Zhao, X.; Lin, A.; Feng, J.; Jiang, T. Correlation of skull morphology and bite force in a bird-eating bat (Ia io; Vespertilionidae). Front. Zool. 2020, 17, 1–14. [Google Scholar] [CrossRef]
- Davis, N.E.; Forsyth, D.M.; Triggs, B.; Pascoe, C.; Benshemesh, J.; Robley, A.; Lawrence, J.; Ritchie, E.G.; Nimmo, D.G.; Lumsden, L.F. Interspecific and geographic variation in the diets of sympatric carnivores: Dingoes/wild dogs and red foxes in south-eastern Australia. PLoS ONE 2015, 10, e0120975. [Google Scholar] [CrossRef]
- Scherer, R.; Doll, A.; Rea, L.; Christ, A.; Stricker, C.; Witteveen, B.; Kline, T.; Kurle, C.; Wunder, M. Stable isotope values in pup vibrissae reveal geographic variation in diets of gestating Steller sea lions Eumetopias jubatus. Mar. Ecol. Prog. Ser. 2015, 527, 261–274. [Google Scholar] [CrossRef]
- Law, B.; Gonsalves, L.; Chidel, M.; McConville, A. When bat eats bat: Diet and roosts of the greater broad-nosed bat (Scoteanax rueppellii) across different regions and habitats. Aust. Mammal. 2023. [Google Scholar] [CrossRef]
- Xie, J.; He, G.; He, T. Influence of climatic factors on soil types and distribution in Guizhou. J. Zhejiang Agric. Sci. 2015, 56, 510–514. [Google Scholar]
- An, G.; Guo, Z.; Ye, P. Climatic Changes and Impacts on Water Quality of Erhai Lake in Dali Area, Yunnan Province over the Period from 1989 to 2019. Geoscience 2022, 36, 406. [Google Scholar]
- Pastor-Beviá, D.; Ibáñez, C.; García-Mudarra, J.L.; Juste, J. A Molecular Approach to the Study of Avian DNA in Bat Faeces. Acta Chiropterolog. 2014, 16, 451–460. [Google Scholar] [CrossRef][Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Aizpurua, O.; Budinski, I.; Georgiakakis, P.; Gopalakrishnan, S.; Ibañez, C.; Mata, V.; Rebelo, H.; Russo, D.; Szodoray-Parádi, F.; Zhelyazkova, V.; et al. Agriculture shapes the trophic niche of a bat preying on multiple pest arthropods across Europe: Evidence from DNA metabarcoding. Mol. Ecol. 2018, 27, 815–825. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, K. The Avifauna of Guizhou; Guizhou People’s Publishing House: Guiyang, China, 1986. [Google Scholar]
- Cheng, T.; Long, Z.; Lu, T. Fauna Sinica: Aves. Volume 10 Passeriformes, Muscicapidae I. Turdinae; Science Press: Beijing, China, 1995. [Google Scholar]
- Fu, T.; Song, Y.; Gao, W. Fauna Sinica, Aves Volume 14 Passeriformes, Ploceidae and Fringillidae; Science Press: Beijing, China, 1998. [Google Scholar]
- Cheng, T.; Long, Z.; Zheng, B. Fauna Sinica: Aves, Volume 13, Passeriformes, Paridae and Zosteropidae; Science Press: Beijing, China, 1982. [Google Scholar]
- Zheng, G. A Checklist on the Classification and Distribution of the Birds of China; Geological Publishing House: Bath, UK, 2005. [Google Scholar]
- Gong, L.; Shi, B.; Wu, H.; Feng, J.; Jiang, T. Who’s for dinner? Bird prey diversity and choice in the great evening bat, Ia io. Ecol. Evol. 2021, 11, 8400–8409. [Google Scholar] [CrossRef]
- Ibáñez, C.; Popa-Lisseanu, A.G.; Pastor-Beviá, D.; García-Mudarra, J.L.; Juste, J. Concealed by darkness: Interactions between predatory bats and nocturnally migrating songbirds illuminated by DNA sequencing. Mol. Ecol. 2016, 25, 5254–5263. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Simpson, G. Package ‘vegan’ J. Community Ecol. 2019, 2. [Google Scholar]
- Allen, B.; Kon, M.; Bar-Yam, Y. A New Phylogenetic Diversity Measure Generalizing the Shannon Index and Its Application to Phyllostomid Bats. Am. Nat. 2009, 174, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Razgour, O.; Clare, E.L.; Zeale, M.R.K.; Hanmer, J.; Schnell, I.B.; Rasmussen, M.; Gilbert, T.P.; Jones, G. High-throughput sequencing offers insight into mechanisms of resource partitioning in cryptic bat species. Ecol. Evol. 2011, 1, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.W.; Brown, J.S.; Ydenberg, R.C. Foraging: Behavior and Ecology; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Fenton, M.B.; Fleming, T.H. Ecological Interactions between Bats and Nocturnal Birds. Biotropica 1976, 8, 104–110. [Google Scholar] [CrossRef]
- Bingxue, Z. The Study on Nocturnal Migrating Birds in Yunnan Province; Yunnan University: Kunming, China, 2015. [Google Scholar]
- Wang, H.; Wang, Y.; Xiang, Z.; Li, Q.; Li, R. The present condition of the bird resource in Guizhou. Environ. Prot. Technol. 2011, 17, 5–8+13. [Google Scholar]
- Schmaljohann, H. Proximate mechanisms affecting seasonal differences in migration speed of avian species. Sci. Rep. 2018, 8, 4106. [Google Scholar] [CrossRef]
- Clough, Y.; Putra, D.D.; Pitopang, R.; Tscharntke, T. Local and landscape factors determine functional bird diversity in Indonesian cacao agroforestry. Biol. Conserv: England. 2009, 142, 1032–1041. [Google Scholar] [CrossRef]
- Czenze, Z.J.; Tucker, J.L.; Clare, E.L.; Littlefair, J.E.; Hemprich-Bennett, D.; Oliveira, H.F.M.; Brigham, R.M.; Hickey, A.J.R.; Parsons, S. Spatiotemporal and demographic variation in the diet of New Zealand lesser short-tailed bats (Mystacina tuberculata). Ecol. Evol. 2018, 8, 7599–7610. [Google Scholar] [CrossRef]
- Krebs, J.R.; Stephens, D.W. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Wang, Z.; Gong, L.; Huang, Z.; Geng, Y.; Zhang, W.; Si, M.; Wu, H.; Feng, J.; Jiang, T. Linking changes in individual specialization and population niche of space use across seasons in the great evening bat (Ia io). Mov. Ecol. 2023, 11, 32. [Google Scholar] [CrossRef]
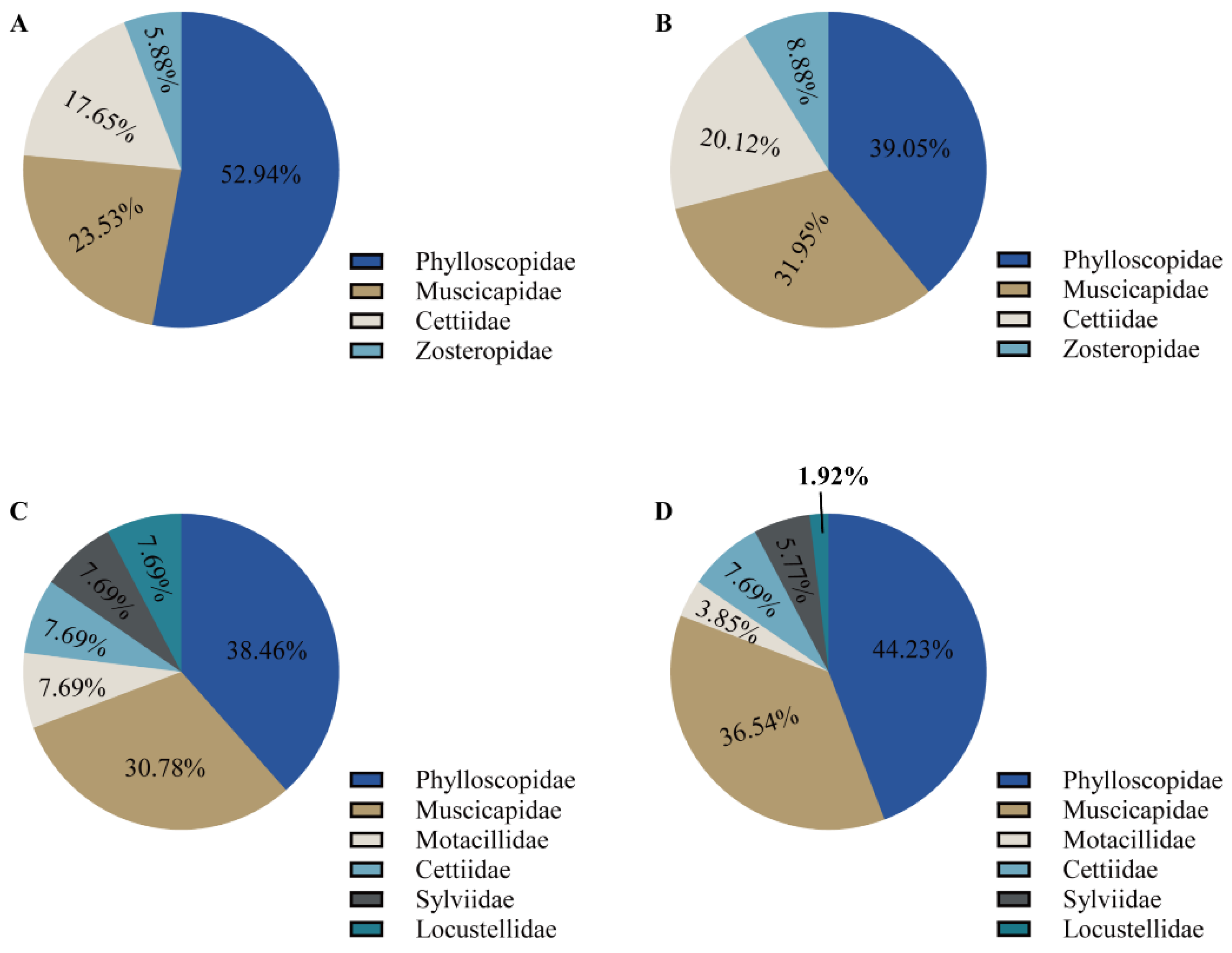
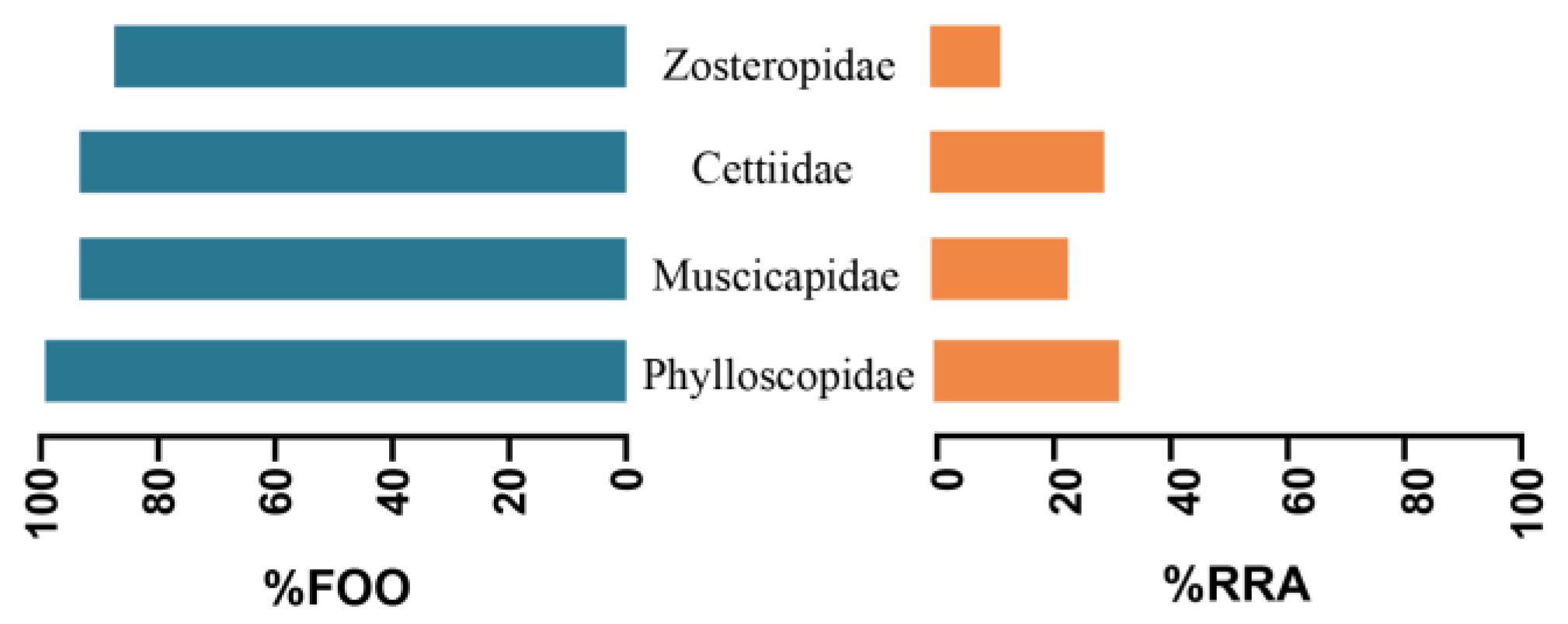
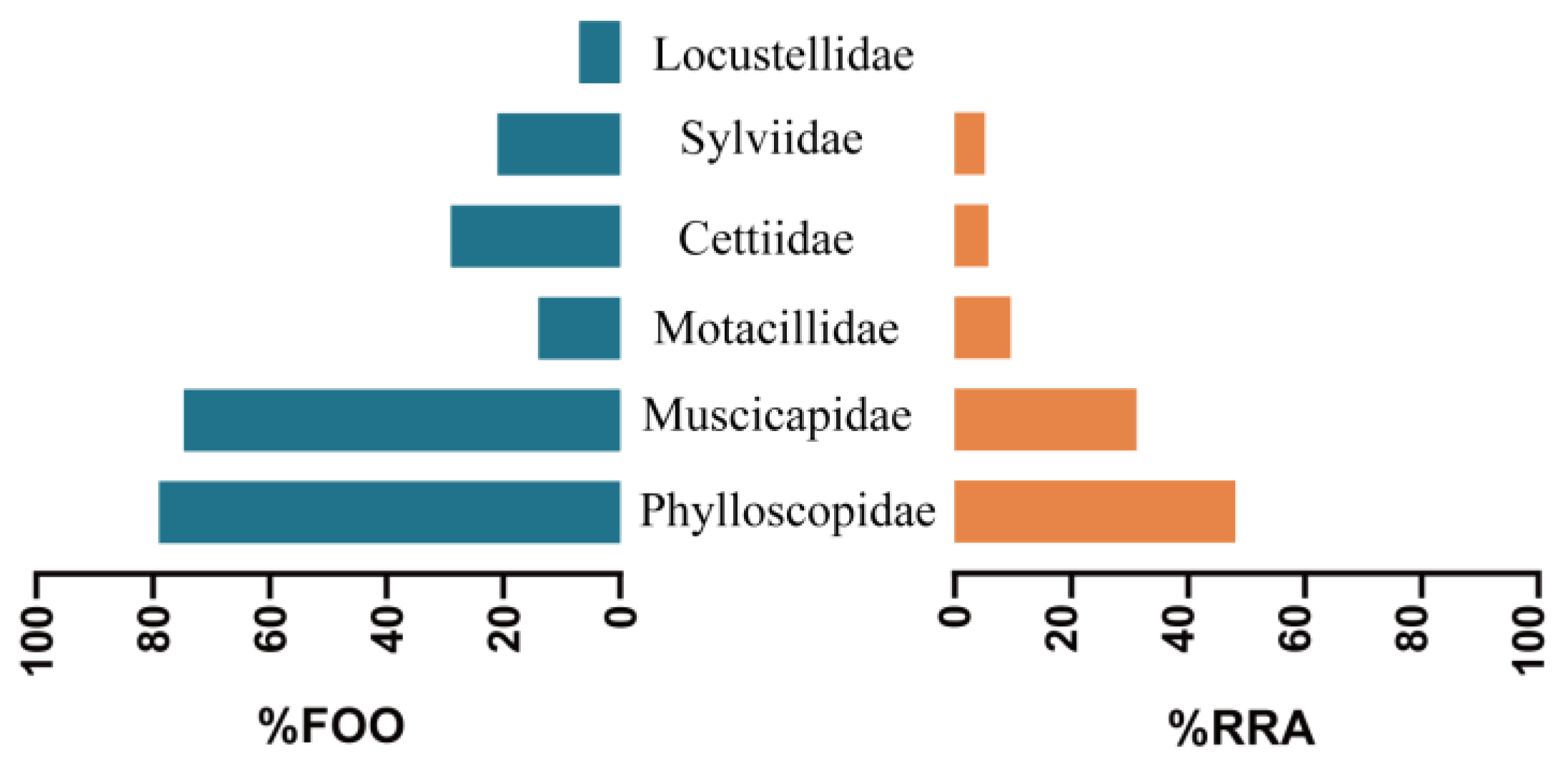
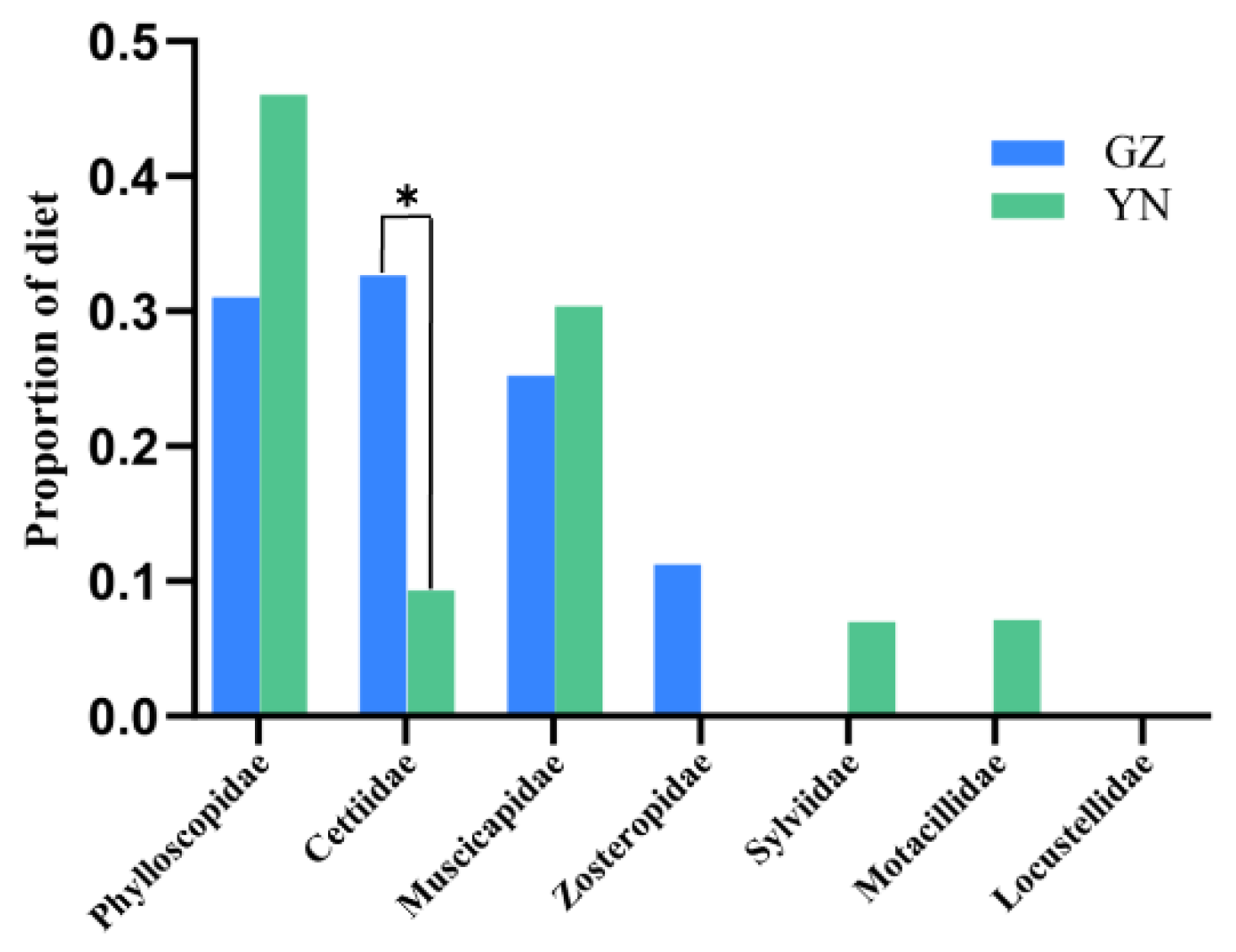

| Family | Genus and Species | Similarity % | Frequency | Migration Pattern | Body Mass (g) |
|---|---|---|---|---|---|
| Phylloscopidae | Phylloscopus proregulus | 100 | 6 | W | 6.3 |
| Phylloscopus kansuensis | 100 | 1 | P | - | |
| Phylloscopus trochiloides | 100 | 13 | P | 8.46 | |
| Phylloscopus burkii | 99.73 | 7 | P | - | |
| Phylloscopus fuscatus | 100 | 10 | P | 8.7 | |
| Phylloscopus hainanus | 98.91 | 7 | P | - | |
| Phylloscopus castaniceps | 100 | 1 | W | ||
| Phylloscopus yunnanensis | 100 | 15 | R | 3.5 | |
| Phylloscopus inornatus | 100 | 6 | W | 7.55 | |
| Muscicapidae | Myiomela leucura | 100 | 14 | R | - |
| Tarsiger cyanurus | 100 | 14 | W | 14.1 | |
| Calliope calliope | 100 | 15 | P | 18.5 | |
| Ficedula strophiata | 100 | 11 | S | 12.75 | |
| Cettiidae | Horornis flavolivaceus | 100 | 2 | P | 9.25 |
| Horornis fortipes | 100 | 16 | R | 10.2 | |
| Abroscopus albogularis | 99.45 | 16 | R | - | |
| Zosteropidae | Zosterops erythropleurus | 100 | 15 | P, W | 10.7 |
| Family | Genus and Species | Similarity (%) | Frequency | Migration Pattern | Body Mass (g) |
|---|---|---|---|---|---|
| Phylloscopidae | Phylloscopus trochiloides | 100 | 2 | W | 8.46 |
| Phylloscopus fuscatus | 99.73 | 3 | S | 8.7 | |
| Phylloscopus inornatus | 99.73 | 7 | p | 5.95 | |
| Phylloscopus reguloides | 100 | 6 | S | 7.65 | |
| Phylloscopus armandii | 100 | 5 | S | 8.63 | |
| Muscicapidae | Saxicola ferreus | 98.91 | 2 | R | 16.05 |
| Saxicola torquatus | 100 | 3 | - | 17.46 | |
| Ficedula albicilla | 100 | 9 | P | - | |
| Phoenicurus auroreus | 100 | 5 | W | 15.5 | |
| Motacillidae | Anthus hodgsoni | 100 | 2 | W | 21.5 |
| Locustellidae | Locustella tacsanowskia | 100 | 1 | S | - |
| Cettiidae | Phyllergates cucullatus | 99.73 | 3 | R | 7 |
| Sylviidae | Urosphena squameiceps | 100 | 3 | P | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wu, H.; Gong, L.; Liu, Y.; Jiang, T.; Feng, J. The Geographical Differences in the Bird Prey of the Great Evening Bat (Ia io). Diversity 2023, 15, 982. https://doi.org/10.3390/d15090982
Liu Y, Wu H, Gong L, Liu Y, Jiang T, Feng J. The Geographical Differences in the Bird Prey of the Great Evening Bat (Ia io). Diversity. 2023; 15(9):982. https://doi.org/10.3390/d15090982
Chicago/Turabian StyleLiu, Yu, Hui Wu, Lixin Gong, Yingying Liu, Tinglei Jiang, and Jiang Feng. 2023. "The Geographical Differences in the Bird Prey of the Great Evening Bat (Ia io)" Diversity 15, no. 9: 982. https://doi.org/10.3390/d15090982
APA StyleLiu, Y., Wu, H., Gong, L., Liu, Y., Jiang, T., & Feng, J. (2023). The Geographical Differences in the Bird Prey of the Great Evening Bat (Ia io). Diversity, 15(9), 982. https://doi.org/10.3390/d15090982






