Orchid Diversity at Three Elevations in the Mountain Sandstone Plateaus of the Cordillera del CóndorEcuador
Abstract
1. Introduction
2. Materials and Methods
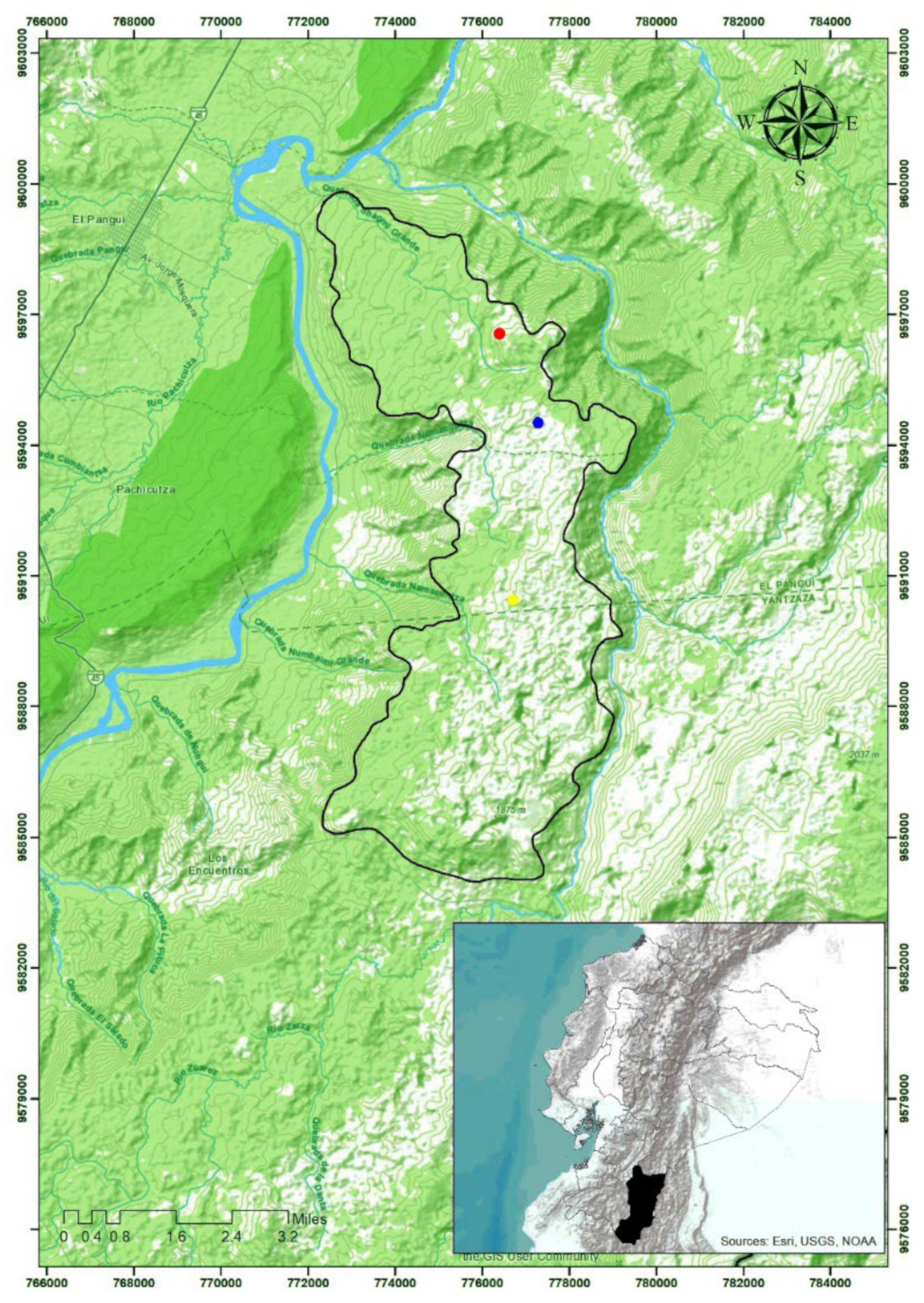
2.1. Study Area
2.2. Plot Demarcation and Sampling
2.3. Statistical Data
3. Results
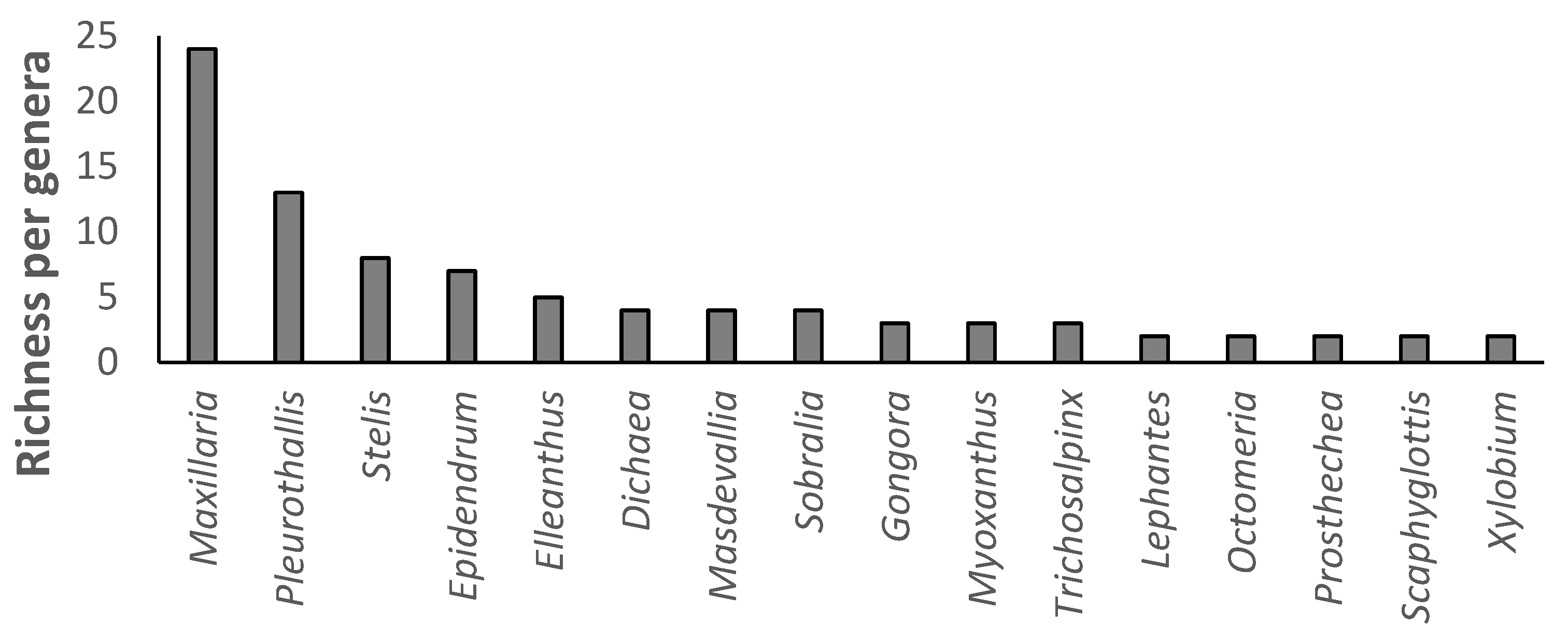
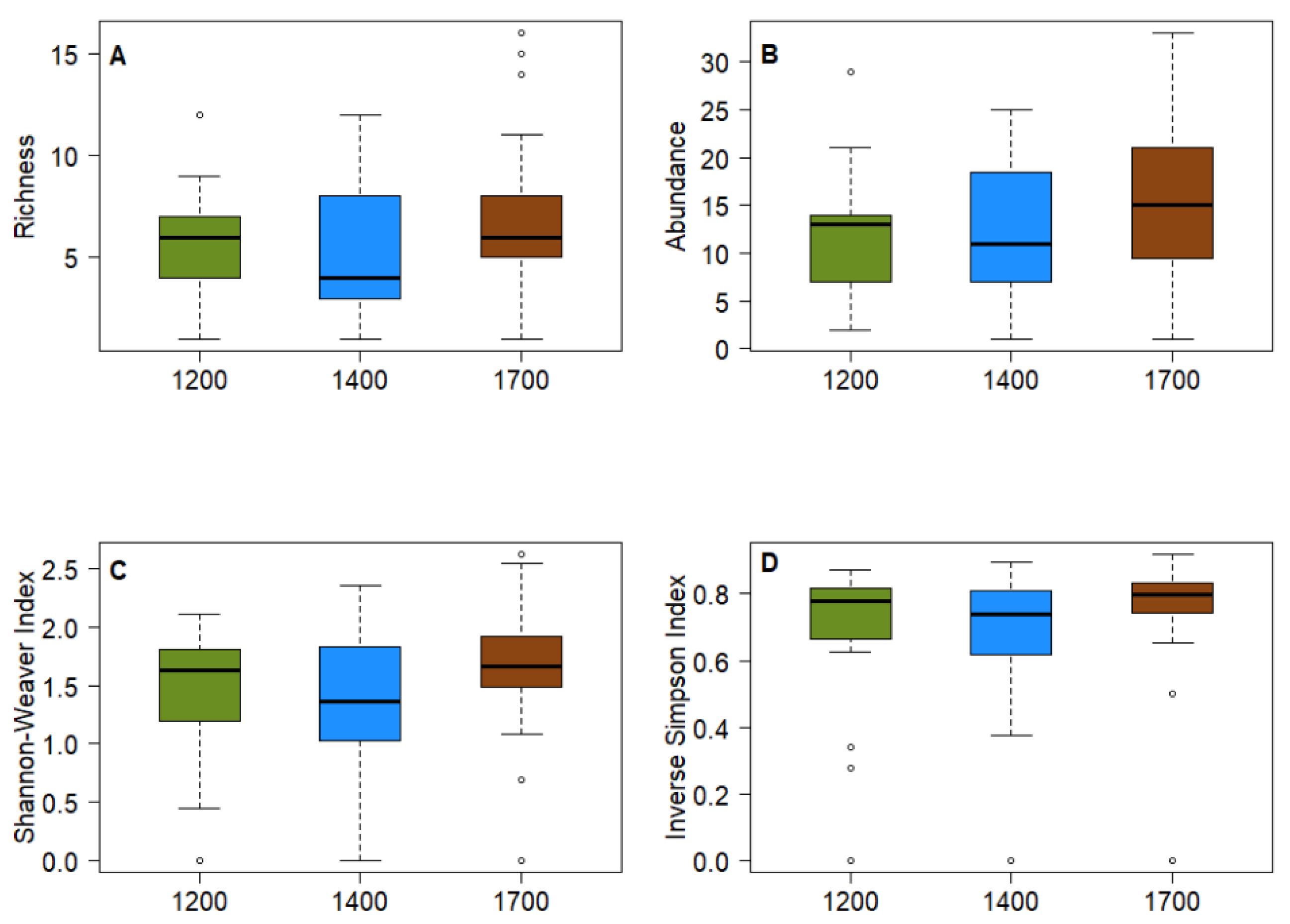
3.1. Alpha Diversity
3.2. Density
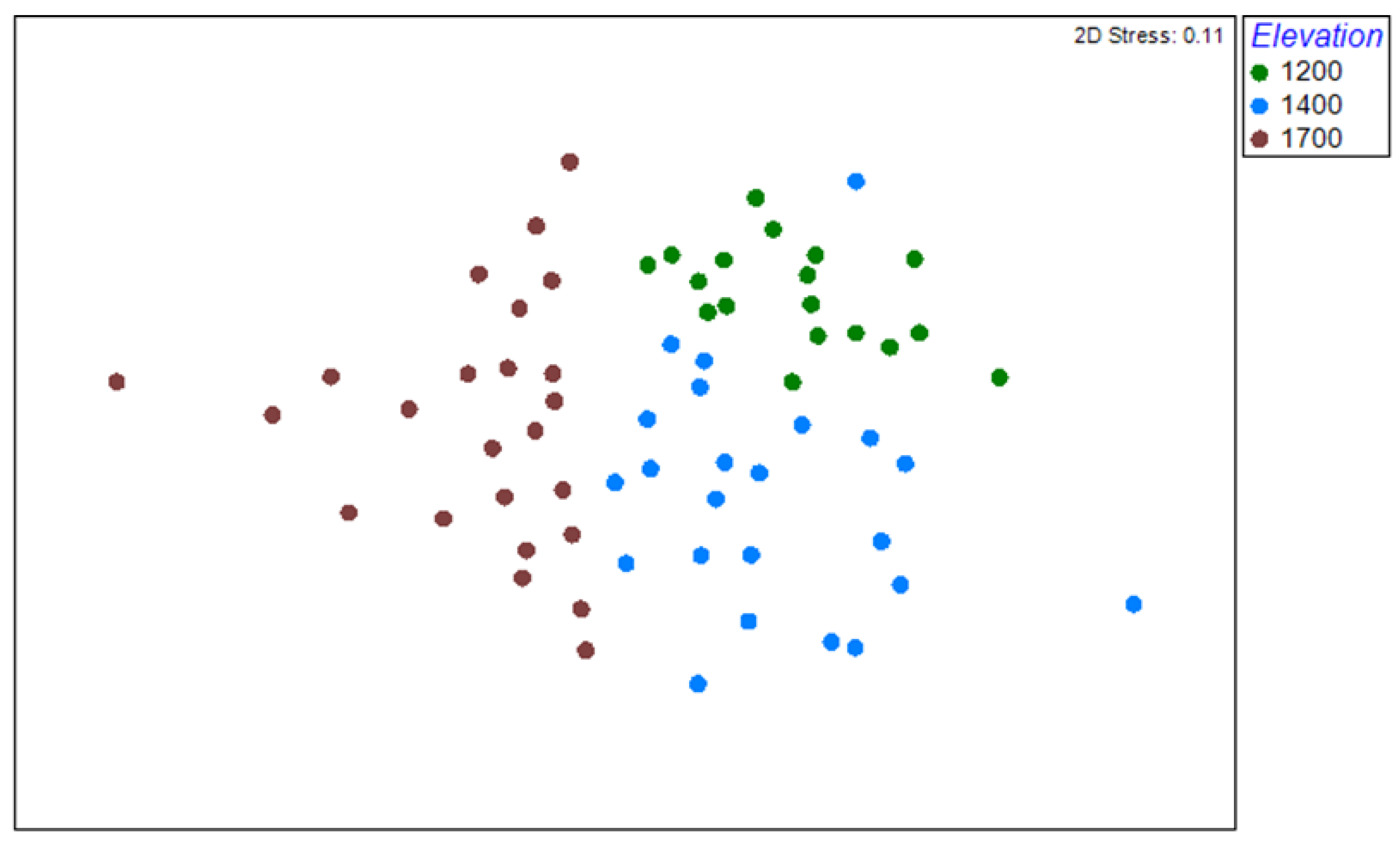
3.3. Beta Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | 1200 | 1400 | 1700 |
|---|---|---|---|
| Brachionidium ballatrix Luer y Hirtz | 0 | 0 | 2 |
| Chondroscaphe merana (Dodson y Neudecker) Dressler | 0 | 0 | 12 |
| Cleistes moritzii (Rchb. f.) Garay y Dunst. | 2 | 2 | 0 |
| Cryptarrhena lunata R. Br. | 0 | 0 | 4 |
| Dichaea galeata Dodson | 0 | 0 | 5 |
| Dichaea histrio Rchb. f. | 0 | 0 | 10 |
| Dichaea trulla Rchb. f. | 0 | 5 | 0 |
| Dichaea venezuelensis Carnevali & I. Ramírez | 10 | 0 | 0 |
| Dracula rezequiana Luer y R. Hawley | 0 | 0 | 1 |
| Dryadella aurea Luer y Hirtz | 0 | 2 | 6 |
| Echinosepala lappiformis (A.H. Heller y L.O. Williams) Pridgeon y M.W. Chase | 0 | 1 | 0 |
| Elleanthus blatteus Garay | 11 | 1 | 14 |
| Elleanthus discolor (Rchb. f. & Warsz.) Rchb. f. | 9 | 2 | 0 |
| Elleanthus fractiflexus Schltr. | 0 | 13 | 10 |
| Elleanthus oliganthus (Poepp. & Endl.) Rchb. f. | 0 | 0 | 24 |
| Elleanthus hymenophorus (Rchb. f.) Rchb. f. | 0 | 8 | 0 |
| Eloyella jostii Dodson y Dalström | 0 | 0 | 1 |
| Epidendrum aff. nocturnum | 2 | 5 | 0 |
| Epidendrum jasminosmun Hágsater y Dodson | 0 | 2 | 0 |
| Epidendrum lanipes Lindl. | 0 | 0 | 4 |
| Epidendrum moronense Dodson y Hágsater | 0 | 0 | 4 |
| Epidendrum sp.1 | 0 | 0 | 6 |
| Epidendrum sp.2 | 10 | 0 | 0 |
| Epidendrum tridens Poepp. y Endl. | 0 | 7 | 0 |
| Epilyna embreei Dodson | 0 | 0 | 18 |
| Erycina pusilla (L.) N.H. Williams M.W. Chase | 0 | 3 | 0 |
| Eurystyles sp. | 0 | 0 | 16 |
| Gongora portentosa Rchb. f. | 0 | 0 | 1 |
| Gongora scaphephorus Rchb. f. & Warsz. | 0 | 0 | 6 |
| Gongora sp. | 0 | 0 | 2 |
| Huntleya wallisii (Rchb. f.) Rolfe | 0 | 2 | 0 |
| Lepanthes uxoria Luer y Hirtz | 0 | 0 | 12 |
| Lepanthes dictydion Luer y Hirtz | 0 | 3 | 0 |
| Lepanthopsis floripecten (Rchb. f.) Ames | 0 | 3 | 2 |
| Liparis nervosa (Thunb.) Lindl. | 2 | 2 | 0 |
| Lycomormium fiskei Dulce | 0 | 0 | 10 |
| Masdevallia angulata Rchb. f. | 0 | 0 | 4 |
| Masdevallia brachyura F. Lehm. y Kraenzl. | 0 | 0 | 5 |
| Masdevallia Hirtzii Luer y Andreetta | 0 | 0 | 1 |
| Masdevallia lintricula Königer | 0 | 0 | 5 |
| Maxillaria thurstoniorum Dodson | 0 | 0 | 4 |
| Maxillaria aurea (Poepp. & Endl.) L.O. Williams | 3 | 5 | 0 |
| Maxillaria aurorae D.E. Benn. y Christenson | 1 | 0 | 0 |
| Maxillaria buchtienii Schltr. | 7 | 0 | 0 |
| Maxillaria burtonii D.E. Benn. y Christenson | 0 | 2 | 0 |
| Maxillaria chicana Dodson | 1 | 0 | 0 |
| Maxillaria chlorantha Lindl. | 0 | 0 | 4 |
| Maxillaria discolor (G. Lodd. ex Lindl.) Rchb. f. | 0 | 4 | 0 |
| Maxillaria disticha (Lindl.) C. Schweinf. | 0 | 0 | 8 |
| Maxillaria fletcheriana J.G. Fowler | 2 | 0 | 0 |
| Maxillaria foetida D.E. Benn. y Christenson | 0 | 0 | 7 |
| Maxillaria grayi Dodson | 39 | 10 | 0 |
| Maxillaria longibracteata (Lindl.) Rchb. f. | 5 | 0 | 0 |
| Maxillaria mapiriensis (Kraenzl.) L.O. Williams | 28 | 14 | 0 |
| Maxillaria nasuta Rchb. f. | 0 | 11 | 16 |
| Maxillaria notylioglossa Rchb. f. | 0 | 10 | 8 |
| Maxillaria pendens Pabst | 5 | 0 | 0 |
| Maxillaria pendula (Poepp. & Endl.) C. Schweinf. | 0 | 0 | 4 |
| Maxillaria porrecta Lindl. | 0 | 0 | 3 |
| Maxillaria quitensis (Rchb. f.) C. Schweinf. | 0 | 0 | 12 |
| Maxillaria rufescens Lindl. | 0 | 2 | 1 |
| Maxillaria villosa (Barb. Rodr.) Cogn. | 0 | 6 | 1 |
| Maxillaria virguncula Rchb. f. | 0 | 0 | 9 |
| Maxillaria xantholeuca Schltr. | 0 | 7 | 0 |
| Microchillus sp. | 0 | 0 | 1 |
| Miltoniopsis bismarkii Dodson y D.E. Benn. | 0 | 0 | 5 |
| Mormodes rolfeana Tilo | 0 | 0 | 1 |
| Mormolyca polyphylla Garay y M. Wirth | 0 | 1 | 8 |
| Muscarella sp. | 0 | 7 | 0 |
| Myoxanthus ceratothallis (Rchb. f.) Luer | 0 | 1 | 0 |
| Myoxanthus georgei (Luer) Luer | 2 | 1 | 0 |
| Myoxanthus sp. | 0 | 2 | 0 |
| Octomeria panguiensis Vélez-Abarca, M.M. Jiménez & Baquero | 0 | 0 | 2 |
| Octomeria candidae Vélez-Abarca, M.M. Jiménez & Baquero | 1 | 0 | 0 |
| Otoglossum globuliferum (Kunth) N.H. Williams y M.W. Chase | 9 | 8 | 0 |
| Ophidion pleurothallopsis (Kraenzl.) Luer | 0 | 0 | 10 |
| Pleurothallis acestrophylla Luer | 0 | 1 | 0 |
| Pleurothallis erythrium Luer | 0 | 3 | 0 |
| Pleurothallis adeleae Luer | 4 | 0 | 0 |
| Pleurothallis cordata (Ruiz & Pav.) Lindl. | 3 | 1 | 8 |
| Pleurothallis cordifolia Rchb. f. & Wagener | 1 | 0 | 0 |
| Pleurothallis litotes Luer | 13 | 0 | 2 |
| Pleurothallis niveoglobula Luer | 0 | 0 | 2 |
| Pleurothallis revoluta (Ruiz & Pav.) Garay | 0 | 2 | 7 |
| Pleurothallis floribunda Poepp. y Endl. | 14 | 3 | 0 |
| Pleurothallis ruscifolia (Jacq.) R. Br. | 0 | 15 | 0 |
| Pleurothallis ariana-dayanarum Vélez-Abarca, M.M. Jiménez & D. Gut. del Pozo | 0 | 5 | 0 |
| Pleurothallis valvola Luer y Hirtz | 0 | 4 | 0 |
| Pleurothallis kashi-menkakarai Mash., Vélez-Abarca & M.M. Jiménez | 0 | 0 | 4 |
| Polycycnis escobariana G. Gerlach | 2 | 0 | 0 |
| Prosthechea grammatoglossa (Rchb. f.) W.E. Higgins | 0 | 0 | 12 |
| Prosthechea vespa (Vell.) W.E. Higgins | 1 | 1 | 0 |
| Prosthechea venezuelana (Schltr.) W.E. Higgins | 2 | 0 | 0 |
| Scaphyglottis punctulata (Rchb. f.) C. Schweinf. | 0 | 0 | 2 |
| Scaphyglottis summersii L.O. Williams | 0 | 0 | 12 |
| Sievekingia cristata Garay | 0 | 0 | 1 |
| Sigmatostalix picta Rchb. f. | 0 | 0 | 2 |
| Sobralia fimbriata Poepp. & Endl. | 1 | 0 | 0 |
| Sobralia fragrans Lindl. | 4 | 3 | 0 |
| Sobralia powelli Schltr. | 1 | 0 | 0 |
| Sobralia setigera Poepp. & Endl. | 2 | 2 | 0 |
| Stanhopea anfracta Rolfe | 0 | 0 | 2 |
| Stelis kefersteiniana (Rchb. f.) Pridgeon & M.W. Chase | 8 | 0 | 2 |
| Stelis imraei (Lindl.) Pridgeon & M.W.Chase. | 0 | 10 | 19 |
| Stelis maloi Luer | 0 | 0 | 2 |
| Stelis sp1. | 0 | 15 | 0 |
| Stelis ortegae Luer & Hirtz | 8 | 14 | 0 |
| Stelis floribunda Kunth | 13 | 2 | 0 |
| Stelis sp2. | 0 | 31 | 0 |
| Stelis sp3. | 0 | 1 | 0 |
| Telipogon sp. | 1 | 0 | 0 |
| Trichosalpinx aff. dura | 2 | 4 | 0 |
| Trichosalpinx memor (Rchb. f.) Luer | 0 | 1 | 0 |
| Trichosalpinx dura (Lindl.) Luer | 0 | 0 | 1 |
| Trigonidium grande Garay | 0 | 7 | 0 |
| Vanilla sp. | 2 | 0 | 0 |
| Xylobium pallidiflorum (Hook.) G. Nicholson | 0 | 0 | 4 |
| Xylobium squalens (Lindl.) Lindl. | 0 | 0 | 9 |
| Zootrophion hypodiscus (Rchb. f.) Luer | 0 | 6 | 0 |
References
- Sánchez Recuay, M.; Calderón Rodríguez, A. Evaluación preliminar de orquídeas en el Parque Nacional Cutervo, Cajamarca-Perú. Ecol. Apl. 2010, 9, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Chen, W.-Y.; Huang, J.-L.; Bi, Y.-F.; Yang, X.-F. Orchid species richness along elevational and environmental gradients in Yunnan, China. PLoS ONE 2015, 10, e0142621. [Google Scholar] [CrossRef] [PubMed]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Cambridge University Press: Cambridge, MA, USA, 1993; p. 314. [Google Scholar]
- Fay, M.F.; Chase, M.W. Orchid biology: From Linnaeus via Darwin to the 21st century. Ann. Bot. 2009, 104, 359–364. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Endara, L.; Williams, N.; León-Yánez, S. Explorando los patrones de endemismo de las orquídeas ecuatorianas: Implicaciones para su conservacíon. In Proceedings of the X Congreso Latinoamericano de Botánica, La Serena, Chile, 4–10 October 2010. [Google Scholar]
- BirdLife. Important Bird Areas Factsheet. Cordillera del Cóndor. Ecuador. Available online: http://www.birdlife.org/worldwide/partnership/our-history (accessed on 18 February 2021).
- Schulenberg, T.S.; Awbrey, K. The Cordillera del Cóndor region of Ecuador and Perú: A biological assessment. In Rapid Assessment Papers; Conservation International: Washington, DC, USA, 1997. [Google Scholar]
- Luer, C.A. Two new pleurothallids from the Cordillera del Condor. Am. Orchid Soc. Bull. 1989, 58, 133. [Google Scholar]
- Renner, S.S. A history of botanical exploration in Amazonian Ecuador, 1739–1988. Smithson. Contrib. Bot. 1993, 82, 1–39. [Google Scholar] [CrossRef]
- Teague, W. Collecting orchids for the sake of science. Am. Orchid Soc. Bull. 1989, 58, 126–132. [Google Scholar]
- Dodson, C.H. A new Maxillaria from Ecuador. Harvard Pap. Bot. 2003, 7, 437–438. [Google Scholar]
- Jost, L. New pleurothallids orchids from the Cordillera del Condor of Ecuador. Selbyana 2004, 25, 11. [Google Scholar]
- Doucette, A.; Portilla, J.; Cameron, K.M. Ten new taxa in the orchid subtribe Pleurothallidinae (Epidendroideae, Epidendreae) from Ecuador. Phytotaxa 2016, 257, 230–248. [Google Scholar] [CrossRef]
- Wilson, M.; Baquero, L.; Dupree, K.; Jiménez, M.M.; LeBlanc, C.M.; Merino, G.; Werner, J.D. Three new species of Pleurothallis (Orchidaceae: Pleurothallidinae) in subsection Macrophyllae-Fasciculatae from Northern South America. Lankesteriana 2016, 16, 349–366. [Google Scholar] [CrossRef]
- Hágsater Gartenberg, E.; Santiago Ayala, E. Icones Orchidacearum 16(1). The Genus Epidendrum. Part 12. “Species New & Old in Epidendrum”; Herbario AMO: Ciudad de México, México, 2018; pp. 1601–1667. [Google Scholar]
- Salazar, G.A.; Tobar, F.; Jiménez-Machorro, R.; Freire, E.; Peñafiel Cevallos, M. Sarcoglottis neillii (Orchidaceae: Spiranthinae), a new species from the Andean Tepui Region of Ecuador and Peru. Phytotaxa 2019, 427, 1–8. [Google Scholar] [CrossRef]
- Baquero, L.E.; Donoso, J.J.; Jiménez, M.M. A new gold-colored Lepanthes (Pleurothallidinae: Orchidaceae) from Southeast Ecuador. Lankesteriana 2020, 20, 257–262. [Google Scholar] [CrossRef]
- Dalström, S.; Higgins, W. A new small-flowered Cyrtochilum species (Orchidaceae: Oncidiinae) from the Condor mountains in Ecuador. Lankesteriana 2020, 20, 159–166. [Google Scholar] [CrossRef]
- Vélez-Abarca, L.; Jiménez, M.M.; Baquero, L.E. Octomeria candidae (Orchidaceae: Pleurothallidinae), a new species from the Cordillera del Cóndor, Ecuador. Lankesteriana 2020, 20, 345–351. [Google Scholar] [CrossRef]
- Jadán, O.; Aguirre, Z. Flora de los Tepuyes de la Cuenca Alta del Río Nangaritza, Cordillera del Cóndor. In Evaluación Ecológica Rápida de la Biodiversidad de los Tepuyes de la Cuenca Alta del Río Nangaritza, Cordillera del Cóndor; Conservación Internacional: Quito, Ecuador, 2011; pp. 41–48. [Google Scholar]
- Oswaldo, J.; Hugo, C.; Wilmer, T.; Ismael, P.; Wilson, Q.; Omar, C. Successional forests stages influence the composition and diversity of vascular epiphytes communities from Andean Montane Forests. Ecol. Indic. 2022, 143, 109366. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S. The Role of Ecological Factors in Distribution and Abundance of Terrestrial Orchids. In Orchids Phytochemistry, Biology and Horticulture; Reference Series in Phytochemistry; Mérillon, J.-M., Kodja, H., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 3–72. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of Distribution, Abundance and Composition of Forest Terrestrial Orchids. Biodivers. Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Štípková, Z.; Kindlmann, P. Role of Way of Life, Latitude, Elevation and Climate on the Richness and Distribution of Orchid Species. Biodivers. Conserv. 2019, 28, 75–96. [Google Scholar] [CrossRef]
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Pollination Mechanisms Are Driving Orchid Distribution in Space. Sci. Rep. 2020, 10, 850. [Google Scholar] [CrossRef]
- Hrivnák, M.; Slezák, M.; Galvánek, D.; Vlčko, J.; Belanová, E.; Rízová, V.; Senko, D.; Hrivnák, R. Species Richness, Ecology, and Prediction of Orchids in Central Europe: Local-Scale Study. Diversity 2020, 12, 154. [Google Scholar] [CrossRef]
- Hietz, P.; Buchberger, G.; Winkler, M. Effect of forest disturbance on abundance and distribution of epiphytic bromeliads and orchids. Ecotropica 2006, 12, 103–112. [Google Scholar]
- Gradstein, S.R. Epiphytes of tropical montane forests—Impact of deforestation and climate change. In The Tropical Mountain Forest. Patterns and Processes in a Biodiversity Hotspot; Gradstein, S.R., Homeier, J., Gansert, D., Eds.; University Press: Göttingen, Germany, 2008; pp. 51–65. [Google Scholar]
- Zhang, S.; Yang, Y.; Li, J.; Qin, J.; Zhang, W.; Huang, W.; Hu, H. Physiological diversity of orchids. Plant Divers. 2018, 40, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Larrea, M.L.; Werner, F.A. Response of vascular epiphyte diversity to different land-use intensities in a neotropical montane wet forest. For. Ecol. Manag. 2010, 260, 1950–1955. [Google Scholar] [CrossRef]
- Alzate-Q, N.F.; García-Franco, J.G.; Flores-Palacios, A.; Krömer, T.; Laborde, J. Influence of land use types on the composition and diversity of orchids and their phorophytes in cloud forest fragments. Flora 2019, 260, 151463. [Google Scholar] [CrossRef]
- Nöske, N.M.; Hilt, N.; Werner, F.A.; Brehm, G.; Fiedler, K.; Sipman, H.J.; Gradstein, S.R. Disturbance effects on diversity of epiphytes and moths in a montane forest in Ecuador. Basic Appl. Ecol. 2008, 9, 4–12. [Google Scholar] [CrossRef]
- Krömer, T.; Kessler, M.; Robbert Gradstein, S.; Acebey, A. Diversity patterns of vascular epiphytes along an elevational gradient in the Andes. J. Biogeogr. 2005, 32, 1799–1809. [Google Scholar] [CrossRef]
- Acharya, K.P.; Vetaas, O.R.; Birks, H.J.B. Orchid species richness along Himalayan elevational gradients. J. Biogeogr. 2011, 38, 1821–1833. [Google Scholar] [CrossRef]
- Tian, H.; Xing, F. Elevational diversity patterns of orchids in Nanling National Nature Reserve, northern Guangdong Province. Biodivers. Sci. 2008, 16, 75–82. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Kindlmann, P.; Stevanović, V. Orchid diversity along an altitudinal gradient in the central Balkans. Front. Ecol. Evol. 2022, 10, 929266. [Google Scholar]
- Küper, W.; Kreft, H.; Nieder, J.; Köster, N.; Barthlott, W. Large-scale diversity patterns of vascular epiphytes in Neotropical montane rain forests. J. Biogeogr. 2004, 31, 1477–1487. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Micheneau, C.; Roberts, D.L.; Pailler, T. Elevational gradients of species diversity, breeding system and floral traits of orchid species on Réunion Island. J. Biogeogr. 2005, 32, 1751–1761. [Google Scholar] [CrossRef]
- Ai, Y.Y.; Liu, Q.; Hu, H.X.; Shen, T.; Mo, Y.X.; Wu, X.F.; Li, J.L.; Dossa, G.G.; Song, L. Terrestrial and epiphytic orchids exhibit different diversity and distribution patterns along an elevation gradient of Mt. Victoria, Myanmar. Glob. Ecol. Conserv. 2023, 42, e02408. [Google Scholar] [CrossRef]
- Richter, M.; Diertl, K.H.; Emck, P.; Peters, T.; Beck, E. Reasons for an outstanding plant diversity in the tropical Andes of Southern Ecuador. Landsc. Online 2009, 12, 1–35. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G., Jr.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef]
- Stephenson, N.L.; Mantgem, P.J. Forest turnover rates follow global and regional patterns of productivity: Patterns in forest turnover rates. Ecol. Lett. 2005, 8, 524–531. [Google Scholar] [CrossRef]
- Homeier, J.; Breckle, S.W.; Günter, S.; Rollenbeck, R.T.; Leuschner, C. Tree Diversity, Forest Structure and Productivity along Altitudinal and Topographical Gradients in a Species-Rich Ecuadorian Montane Rain Forest: Ecuadorian Montane Forest Diversity and Structure. Biotropica 2010, 42, 140–148. [Google Scholar] [CrossRef]
- Girardin, C.A.J.; Malhi, Y.; Aragao, L.E.O.C.; Mamani, M.; Huaraca Huasco, W.; Durand, L.; Feeley, K.J.; Rapp, J.; Silva-Espejo, J.E.; Silman, M.; et al. Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Glob. Chang. Biol. 2010, 16, 3176–3192. [Google Scholar] [CrossRef]
- Blundo, C.; Malizia, L.R.; Blake, J.G.; Brown, A.D. Tree species distribution in Andean forests: Influence of regional and local factors. J. Trop. Ecol. 2012, 28, 83–95. [Google Scholar] [CrossRef]
- Anderson-Teixeira, K.J.; Miller, A.D.; Mohan, J.E.; Hudiburg, T.W.; Duval, B.D.; DeLucia, E.H. Altered dynamics of forest recovery under a changing climate. Glob. Chang. Biol. 2013, 19, 2001–2021. [Google Scholar] [CrossRef]
- Ministerio del Ambiente del Ecuador. Sistema de Clasificación de los Ecosistemas del Ecuador Continental; Subsecretaría de Patrimonio Natural: Quito, Ecuador, 2013. [Google Scholar]
- INAMHI. Servicios Meteorológicos. 2019. Available online: http://www.serviciometeorologico.gob.ec/ (accessed on 18 February 2021).
- Neill, D. Inventario Botánico de la Región de la Cordillera del Cóndor, Ecuador y Perú: Actividades y Resultados Científicos del Proyecto 2004–2007; Missouri Botanical Garden: Saint Louis, MO, USA, 2007; p. 47. Available online: http://www.mobot.org/MOBOT/Research/ecuador/cordillera/pdf/EntireSpanishReport.pdf (accessed on 18 February 2021).
- Stadtmüller, T. Los Bosques Nublados en el Trópico Húmedo: Distribución e importancia hidrológica. In Curso Corto de Bases Hidrológicas para el Manejo de Cuencas; CATIE (Centro Agronómico Tropical de Investigación y Educación): Cartago, Costa Rica, 1987. [Google Scholar]
- Hammer, Ø.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, IV, 1–9. 2001. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 25 October 2019).
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Package ‘Vegan’: Community Ecology Package Version 2.5-7; The Comprehensive R Archive Network: Vienna, Austria, 2018. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D.W. Package: “Labdsv”: Ordination and Multivariate Analysis for Ecology Package Version 2.0-1; The Comprehensive R Archive Network: Vienna, Austria, 2019. [Google Scholar]
- Ingram, S.W.; Ferrel-Ingram, K.; Nadkarni, N.M. Floristic composition of vascular epiphytes in a Neotropical cloud forest, Monteverde, Costa Rica. Selbyana 1996, 17, 88–103. [Google Scholar]
- Kreft, H.; Köster, N.; Küper, W.; Nieder, J.; Barthlott, W. Diversity and biogeography of vascular epiphytes in Western Amazonia, Yasuní, Ecuador. J. Biogeogr. 2004, 31, 1463–1476. [Google Scholar] [CrossRef]
- Ibisch, P.; Boegner, A.; Nieder, J.; Barthlott, W. How diverse are neotropical epiphytes? An analysis based on the ‘Catalogue of the flowering plants and gymnosperms of Peru’. Ecotropica 1996, 2, 13–28. [Google Scholar]
- Mites, M.; García-Mozo, H.; Galán, C.; Oña, E. Analysis of the Orchidaceae Diversity in the Pululahua Reserve, Ecuador: Opportunities and Constraints as Regards the Biodiversity Conservation of the Cloud Mountain Forest. Plants 2022, 11, 698. [Google Scholar] [CrossRef]
- Krömer, T.; Gradstein, S.R.; Acebey, A. Diversidad y ecología de epífitas vasculares en bosques montanos primarios y secundarios de Bolivia. Ecol. Boliv. 2007, 42, 23–33. [Google Scholar]
- Kersten, R.A.; Silva, S.M. The floristic compositions of vascular epiphytes of a seasonally inundated forest on the coastal plain of Ilha do Mel Island, Brazil. Rev. Biol. Trop. 2006, 54, 935–942. [Google Scholar] [CrossRef][Green Version]
- Mejía, H.; Pino, N. Diversidad de orquídeas epífitas en un bosque húmedo tropical (bh-t) del departamento del Chocó, Colombia. Acta Biol. Colomb. 2010, 15, 37–46. [Google Scholar]
- Hurtado Alza, H.A.; Orozco Ávila, J.; Pérez Betancur, J.F. Caracterización y distribución vertical de epífitas vasculares -orquídeas y bromelias- y hospederos en ecosistema de selva en sur de Perú. Rev. Investig. Agrar. Ambient. 2017, 8, 91–106. [Google Scholar] [CrossRef][Green Version]
- Vélez-Abarca, L.; Jiménez, M.M.; Baquero, L.E. Two new autogamous species of Octomeria (Orchidaceae: Pleurothallidinae) from the Cordillera del Cóndor, Ecuador. Lankesteriana 2021, 21, 33–44. [Google Scholar]
- Jiménez, M.M.; Vélez-Abarca, L.; Baquero, L.E.; Naranjo, C. A taxonomic revision of genus Phloeophila (Orchidaceae, Pleurothallidinae) in Ecuador. Plant Fungal Syst. 2021, 66, 37–45. [Google Scholar] [CrossRef]
- Jimenez, M.M.; Ocupa Horna, L.; Vélez-Abarca, L. A new species of Pleurothallis (Orchidaceae: Pleurothallidinae) from Zamora in the Province of Zamora Chinchipe, Ecuador. Phytotaxa 2021, 518, 79–86. [Google Scholar] [CrossRef]
- Jiménez, M.M.; Ocupa Horna, L.; Vélez-Abarca, L. Comparettia bennettii (Orchidaceae: Oncidiinae), a new record for Ecuador. Lankesteriana 2020, 20, 353–357. [Google Scholar] [CrossRef]
- Rahbek, C. The elevational gradient of species richness: A uniform pattern. Ecography 1995, 18, 200–205. [Google Scholar] [CrossRef]
- Sanders, N.J. Elevational gradients in ant species richness: Area, geometry, and Rapoport’s rule. Ecography 2002, 25, 25–32. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, G.; Zang, R.; Zhang, J.; Lu, X.; Huang, J. Distribution of vascular epiphytes along a tropical elevational gradient: Disentangling abiotic and biotic determinants. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Herrera, P.; Suárez, J.P.; Sánchez-Rodríguez, A.; Molina, M.C.; Prieto, M.; Méndez, M. Many broadly-shared mycobionts characterize mycorrhizal interactions of two coexisting epiphytic orchids in a high elevation tropical forest. Fungal Ecol. 2019, 39, 26–36. [Google Scholar] [CrossRef]
- Zarate-García, A.M.; Noguera-Savelli, E.; Andrade-Canto, S.B.; Zavaleta-Mancera, H.A.; Gauthier, A.; Alatorre-Cobos, F. Bark water storage capacity influences epiphytic orchid preference for host trees. Am. J. Bot. 2020, 107, 726–734. [Google Scholar] [CrossRef]
- Rocha, F.; Waechter, J. Ecological distribution of terrestrial orchids in a south Brazilian Atlantic region. Nord. J. Bot. 2010, 28, 112–118. [Google Scholar] [CrossRef]

| Factor | df | MS | Pseudo-F | p | CV (%) |
|---|---|---|---|---|---|
| Elevation | 2 | 20,990 | 2.8416 | 0.005 | 25.155 |
| Plot (elevation) | 6 | 7369.6 | 2.0756 | 0.001 | 23.033 |
| Error | 56 | 3550.5 | 59.586 |
| Species | Elevation | Indicator Value | p-Value |
|---|---|---|---|
| Maxillaria grayi Dodson | 1200 | 63.2 | 0.0002 |
| Elleanthus oliganthus (Poepp. & Endl.) Rchb. f. | 1700 | 41.7 | 0.0002 |
| Maxillaria mapiriensis (Kraenzl.) L.O. Williams | 1200 | 35.2 | 0.0018 |
| Stelis pittieri (Schltr.) Rojas-Alv. & Karremans | 1200 | 33.8 | 0.0016 |
| Stelis ortegae Luer & Hirtz | 1400 | 33.3 | 0.0006 |
| Euristyles sp. | 1700 | 25 | 0.0088 |
| Epidendrum sp. | 1200 | 23.5 | 0.006 |
| Stelis imraei (Lindl.) Pridgeon M.W. Chase | 1700 | 21.8 | 0.0408 |
| Maxillaria aurea (Poepp. & Endl.) L.O. Williams | 1400 | 20.8 | 0.0102 |
| Maxillaria aurorae D.E. Benn. & Christenson | 1400 | 20.8 | 0.0102 |
| Maxillaria villosa (Barb. Rodr.) Cogn. | 1400 | 20.8 | 0.0156 |
| Chondroscaphe merana (Dodson & Neudecker) Dressler | 1700 | 20.8 | 0.0186 |
| Ophidion pleurothallopsis (Kraenzl.) Luer | 1700 | 20.8 | 0.0182 |
| Dichaea venezuelensis Carnevali & I. Ramírez | 1200 | 17.6 | 0.0152 |
| Maxillaria buchtienii Schltr. | 1200 | 17.6 | 0.0132 |
| Elleanthus hymenophorus (Rchb. f.) Rchb. f. | 1400 | 16.7 | 0.0356 |
| Epidendrum tridens Poepp. & Endl. | 1400 | 16,7 | 0.0372 |
| Trichosalpinx aff. dura | 1400 | 16.7 | 0.0378 |
| Masdevallia brachyura F. Lehm. & Kraenzl. | 1700 | 16.7 | 0.0348 |
| Lepanthes uxoria Luer & Hirtz | 1700 | 16.7 | 0.0354 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Abarca, L.; Jiménez, M.M.; Ramírez-Iglesias, E.; Parra-Suarez, S.; Torracchi-Carrasco, E.; Benítez, Á. Orchid Diversity at Three Elevations in the Mountain Sandstone Plateaus of the Cordillera del CóndorEcuador. Diversity 2023, 15, 979. https://doi.org/10.3390/d15090979
Vélez-Abarca L, Jiménez MM, Ramírez-Iglesias E, Parra-Suarez S, Torracchi-Carrasco E, Benítez Á. Orchid Diversity at Three Elevations in the Mountain Sandstone Plateaus of the Cordillera del CóndorEcuador. Diversity. 2023; 15(9):979. https://doi.org/10.3390/d15090979
Chicago/Turabian StyleVélez-Abarca, Leisberth, Marco M. Jiménez, Elizabeth Ramírez-Iglesias, Silvia Parra-Suarez, Esteban Torracchi-Carrasco, and Ángel Benítez. 2023. "Orchid Diversity at Three Elevations in the Mountain Sandstone Plateaus of the Cordillera del CóndorEcuador" Diversity 15, no. 9: 979. https://doi.org/10.3390/d15090979
APA StyleVélez-Abarca, L., Jiménez, M. M., Ramírez-Iglesias, E., Parra-Suarez, S., Torracchi-Carrasco, E., & Benítez, Á. (2023). Orchid Diversity at Three Elevations in the Mountain Sandstone Plateaus of the Cordillera del CóndorEcuador. Diversity, 15(9), 979. https://doi.org/10.3390/d15090979







