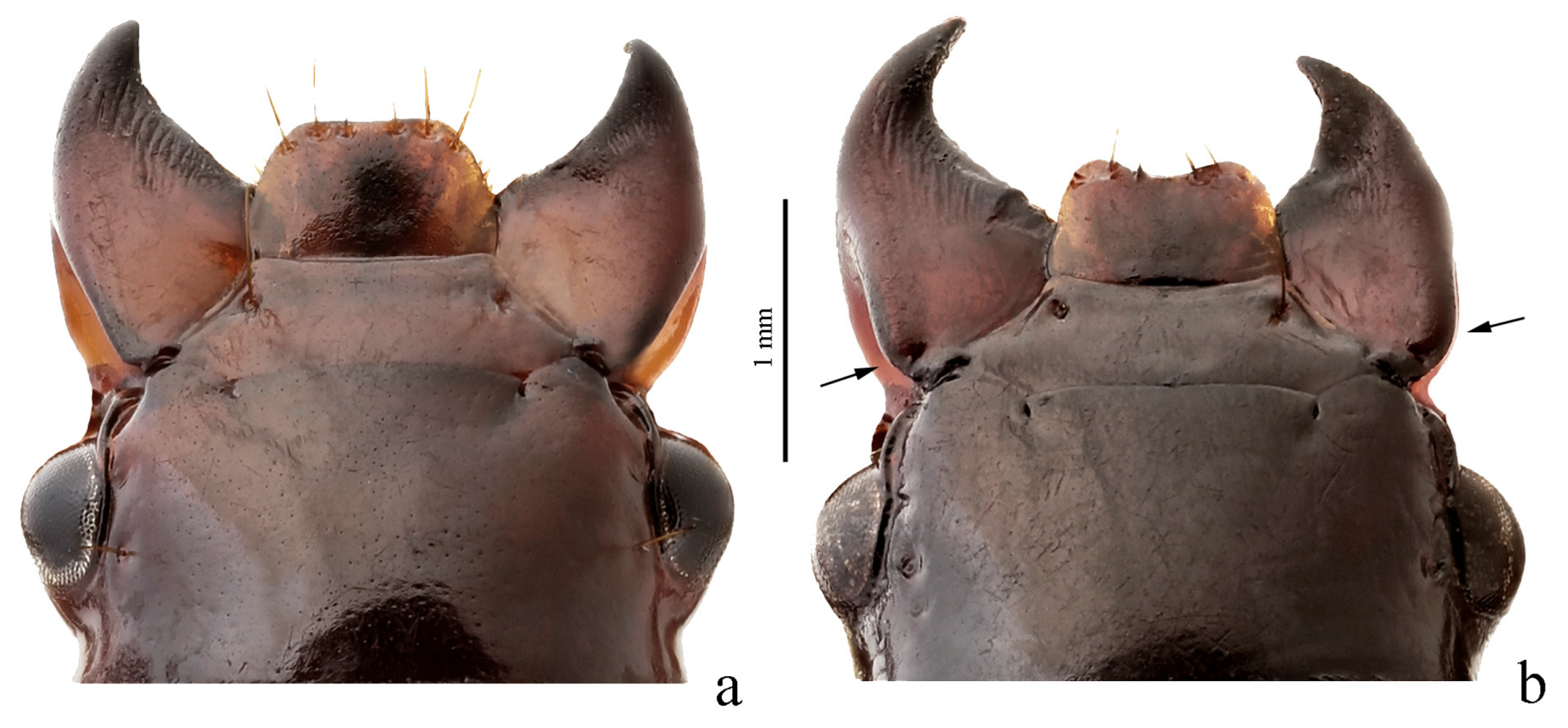
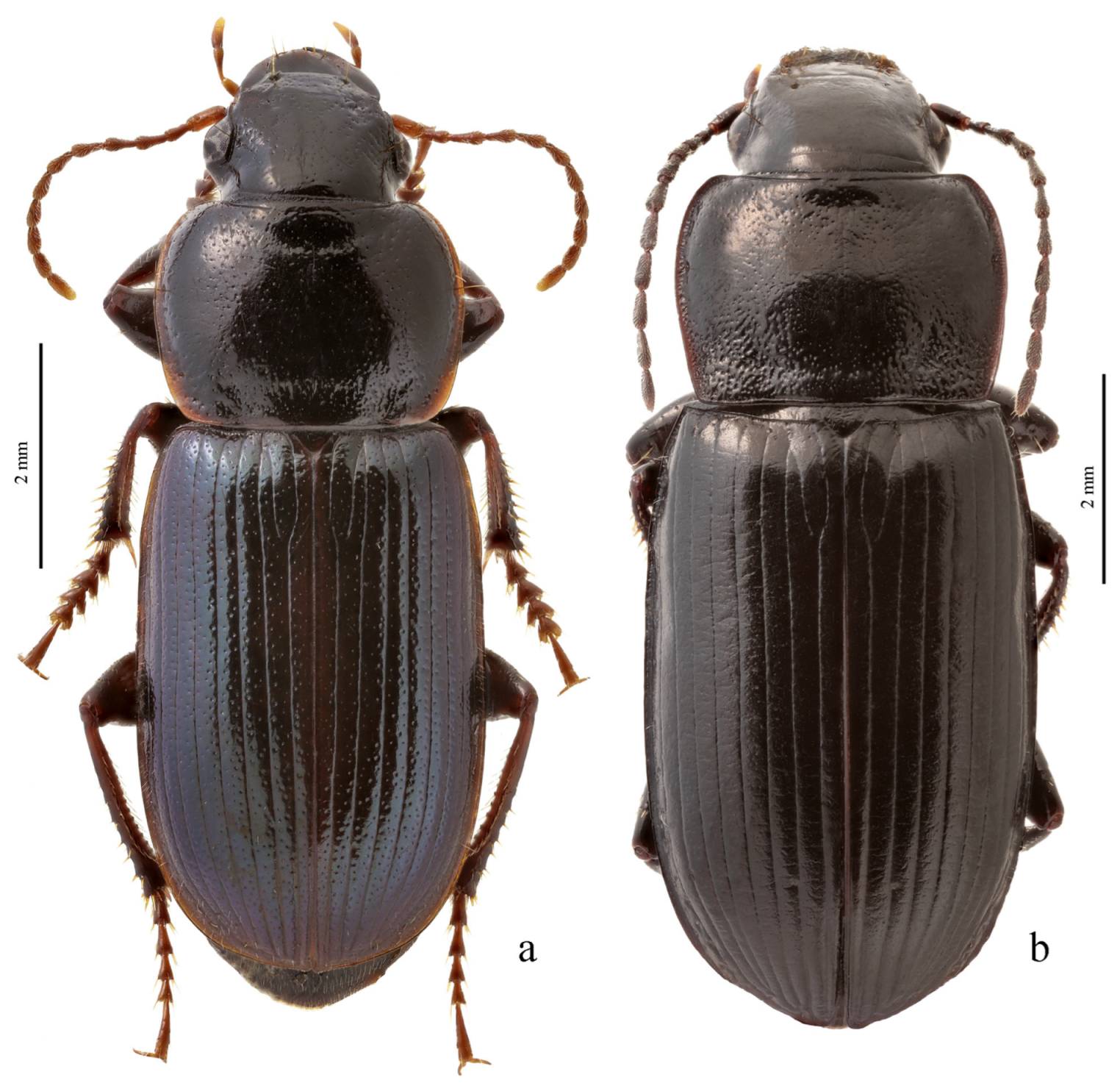
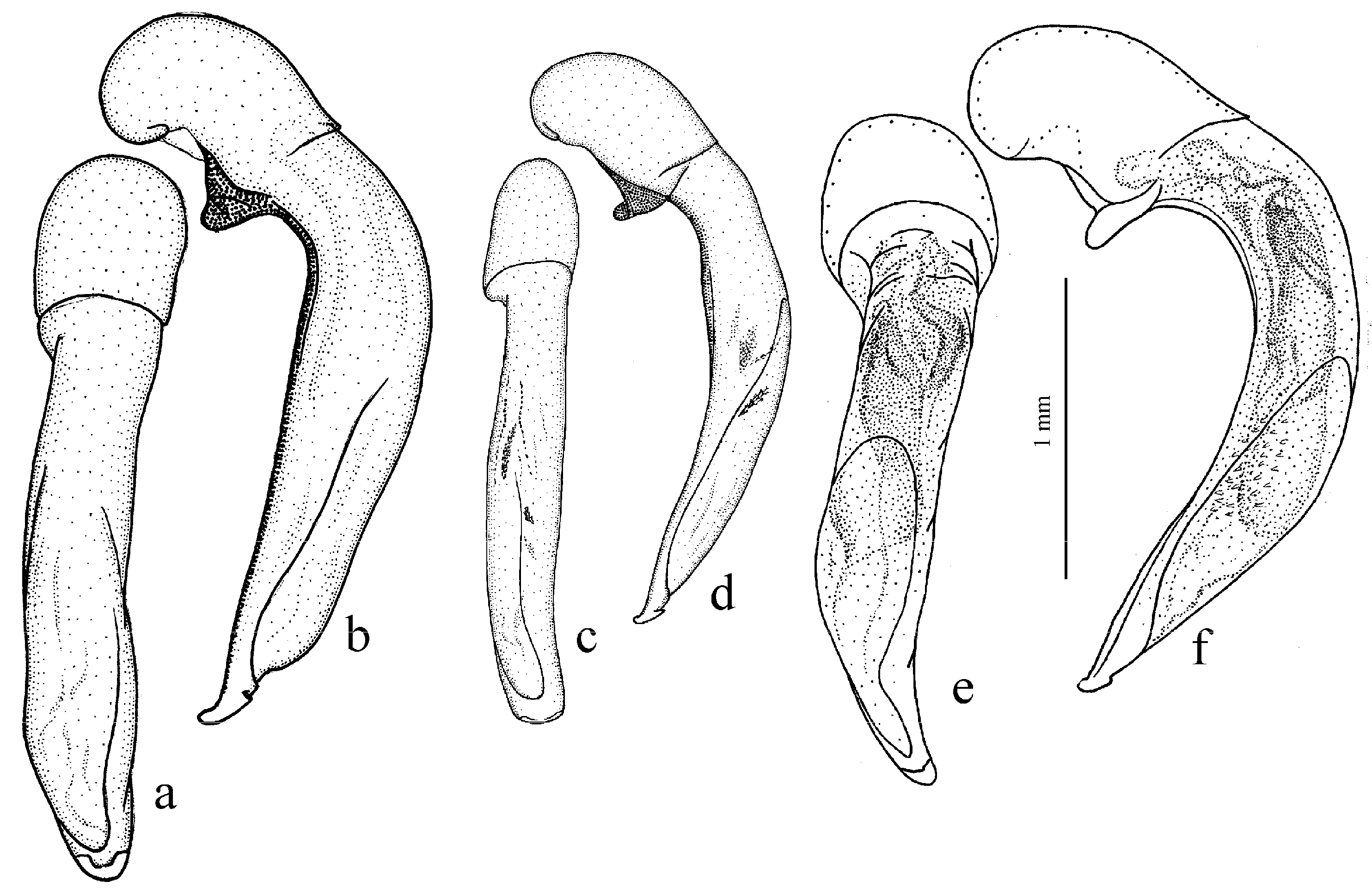
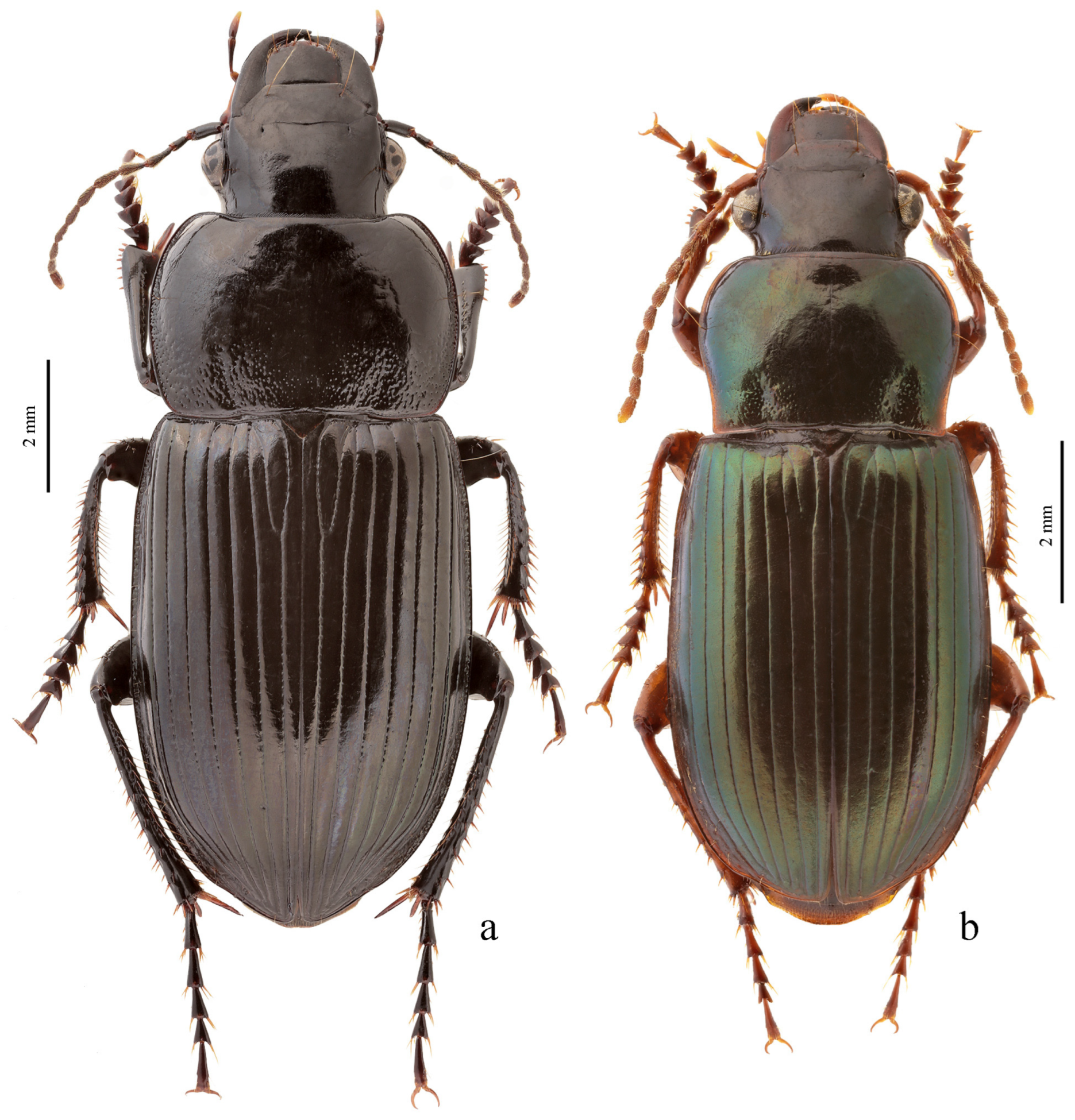
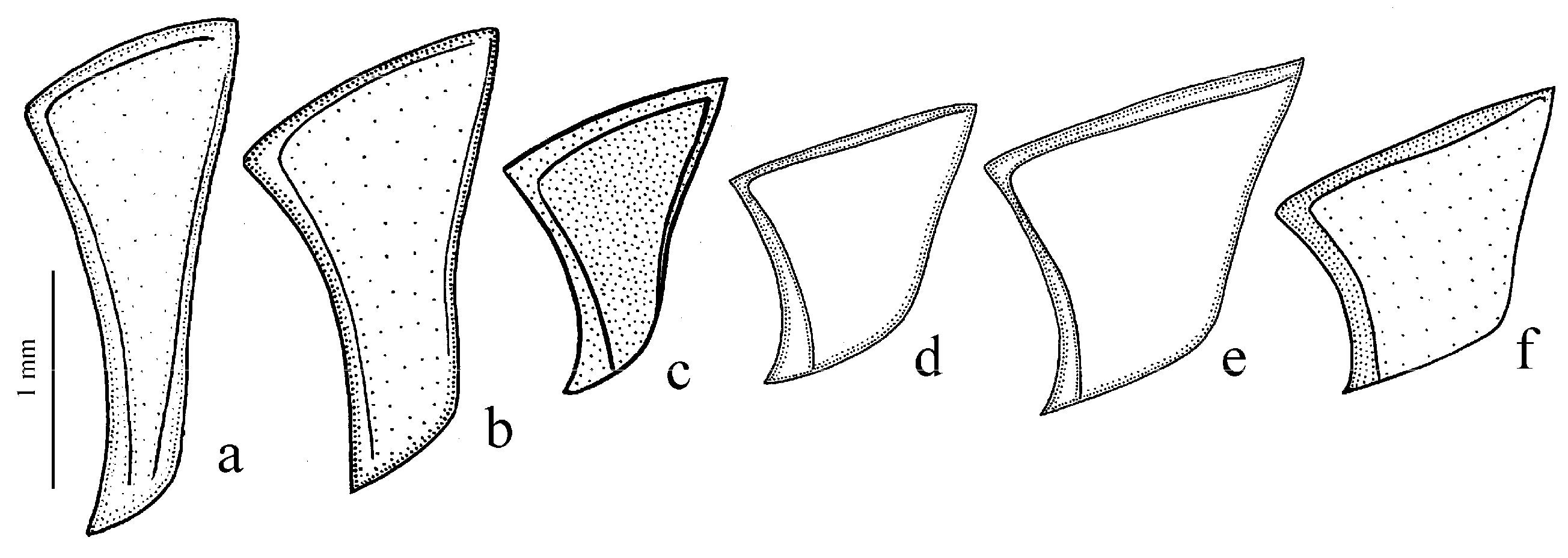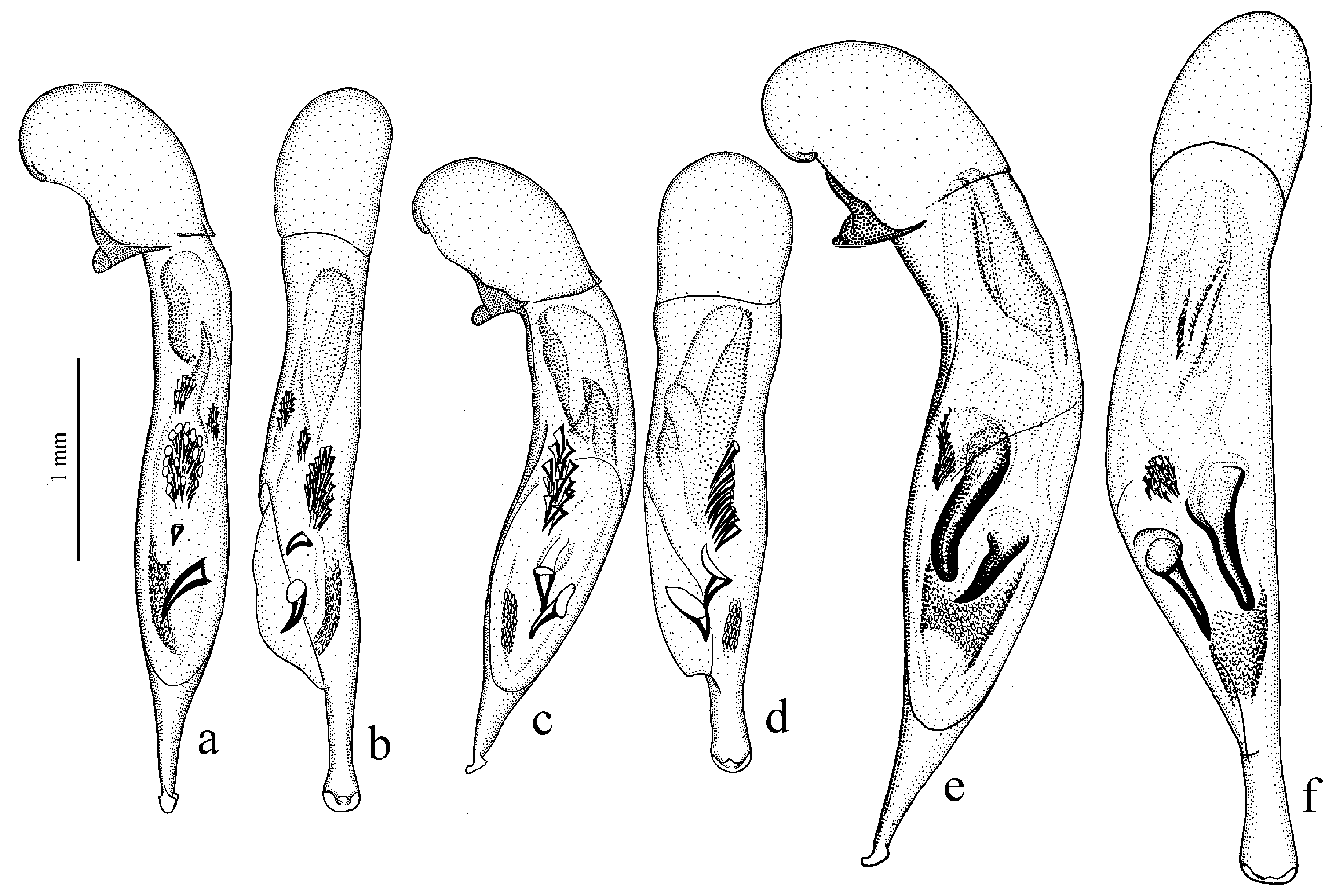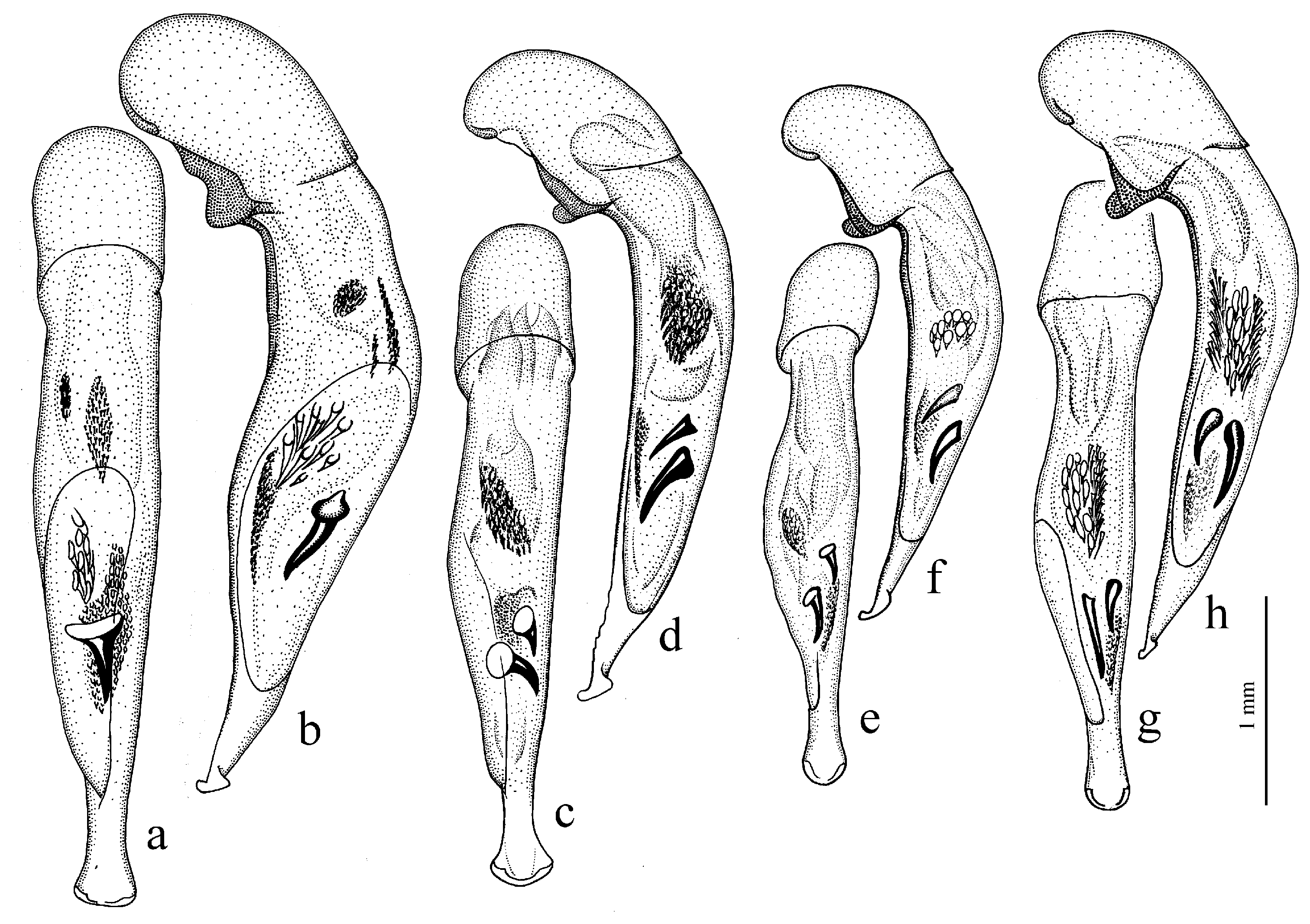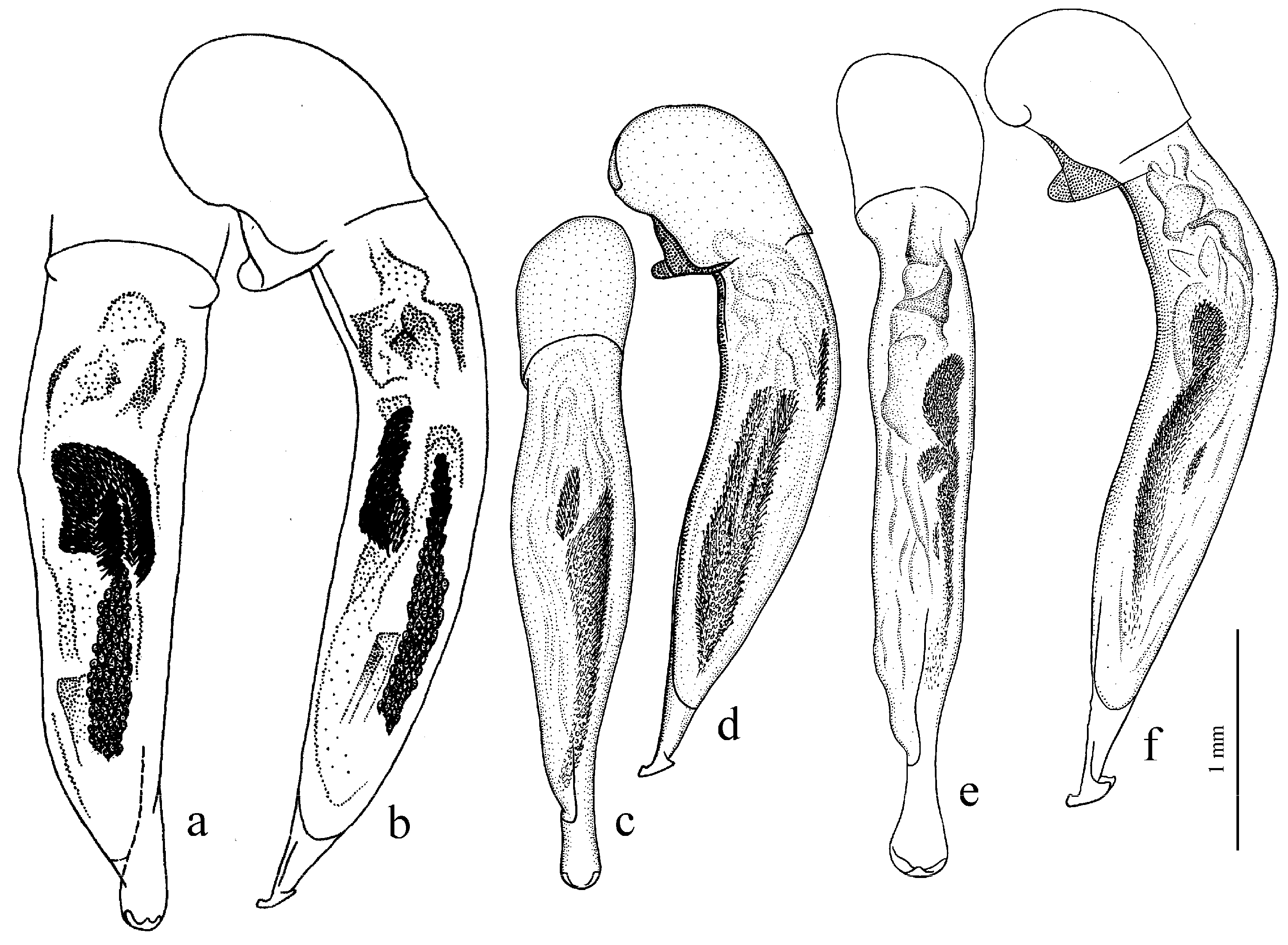3.3.9. Harpalus Group
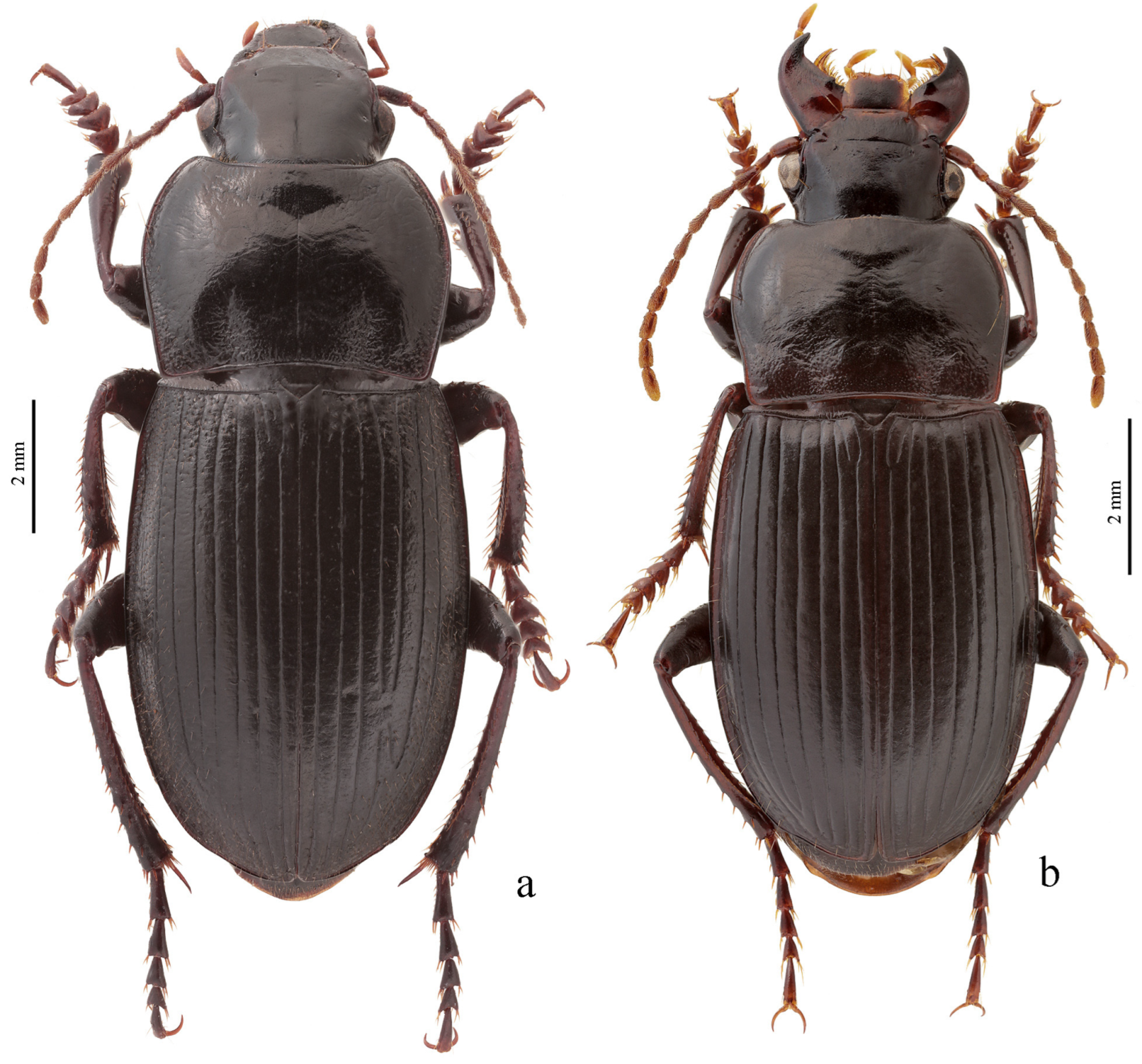
Diagnosis. Body glabrous or more or less densely pubescent. Head generally impunctate and glabrous, rarely finely punctate and setose dorsally; temporae in most members glabrous, in some species setose. Antennae pubescent from antennomere 3. Mentum and submentum separated by complete transverse suture (occasionally fused in some Afroharpalus subg. n.); labial basal palpomere more or less cylindrical, without carina on ventral side. Pronotum with one or several lateral setae on each side, bordered along basal margin, with glabrous or setose basal edge. Elytra with one or several setigerous discal pores on interval 3, in some members also on intervals 5 and 7; in some members, occasionally or constantly, discal pores absent; intervals 5 and 7, more rarely also 3, in many members with short row of preapical setigerous pores; subapical sinuation variable from rather deep to very shallow or indistinct; basal border glabrous or setose. Metacoxa in most members without posteromedial setigerous pore, very rarely this pore present. Protibia with one to three (very rarely four) ventroapical spines arranged in a transverse row and generally with at least three (very rarely two) preapical spines on outer margin of tibia; preapical spines either isolated from spines on ventral surface or arranged with them in a single row; ventroapical tubercle in male absent or more or less prominent; apical spur simple, lanceolate. Dorsal side of tarsi glabrous or more or less densely pubescent; tarsomere 5 with thin setae ventrally; male mesotarsomere 1 generally with adhesive scales ventrally, rarely without them. Abdominal sternites with or without additional setae; last visible abdominal sternite and tergite of both sexes either similar in both sexes or with more or less pronounced sexual dimorphism (apex of last visible sternite truncate or emarginate in male, and narrowed and swollen in female). Median lobe of aedeagus in most species with distinct, oblique or transverse, apical capitulum, rarely without it; internal sac either without any sclerotic elements or with more or less developed armament consisting of spiny patches, groups of spines and one or two separate spines.
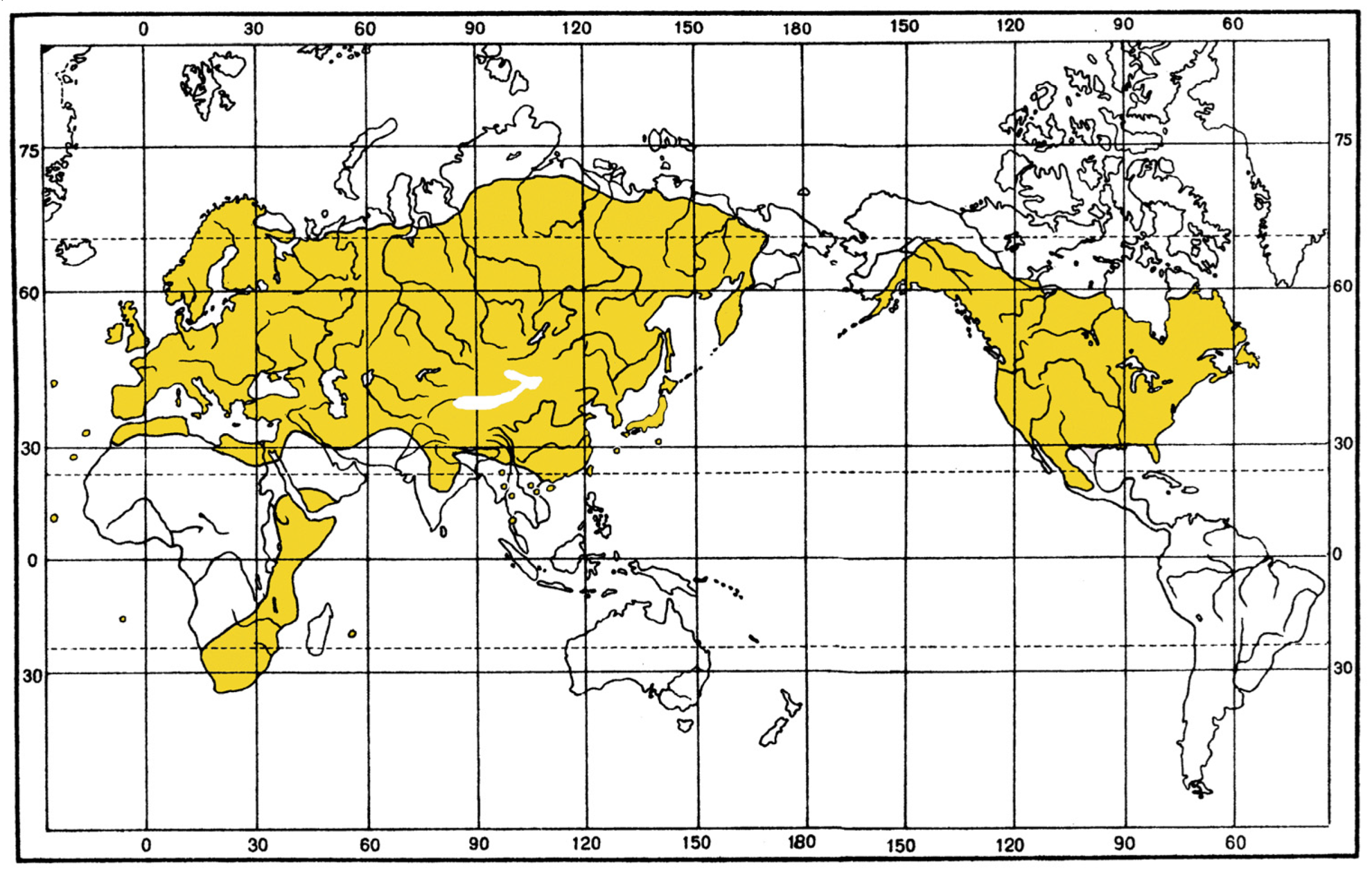
Composition and distribution. This group comprises 58 subgenera combined into 19 subgroups, which are distributed in the Holarctic and Afrotropical regions. The range of this subgeneric group, which includes most species of the genus, almost completely coincides with the generic range, but its representatives do not spread in the Cabo Verde Islands and the Oriental region.
Remarks. This group corresponds to the subgenus
Harpalus sensu stricto in the understanding of many recent authors (e.g., [
1,
7,
8,
29,
51], etc.) and comprises most species of the genus.
An analysis of the distribution of characters in this very diverse group shows that all its members can be divided into three subgeneric complexes or phyletic stocks: one Afrotropical stock, corresponding to the modern subgenus
Afroharpalus subg. n., and two Holarctic stocks, the
latus stock and the
affinis stock, respectively [
8]. The
latus stock comprises the
Hyloharpalus,
Cordoharpalus,
Amblystus,
Actephilus,
Acardystus,
Psammoharpalus,
Ooistus,
Asioharpalus,
Anamblystus and
Harpalobius subgroups; the
affinis stock are the
Pheuginus,
Pharalus,
Hypsinephus,
Mauriharpalus,
Calloharpalus,
Idioharpalus,
Artabas and
Harpalus subgroups. The belonging of these subgroups to one or another stock is determined, first of all, by different initial types of sclerotized armament of the internal sac of the aedeagus, to which all the diversity of armaments observed within each of these two taxonomic complexes can be reduced. The members of the
latus stock are characterized by a more complex type of the armament. It is present in its most complete form in species of the
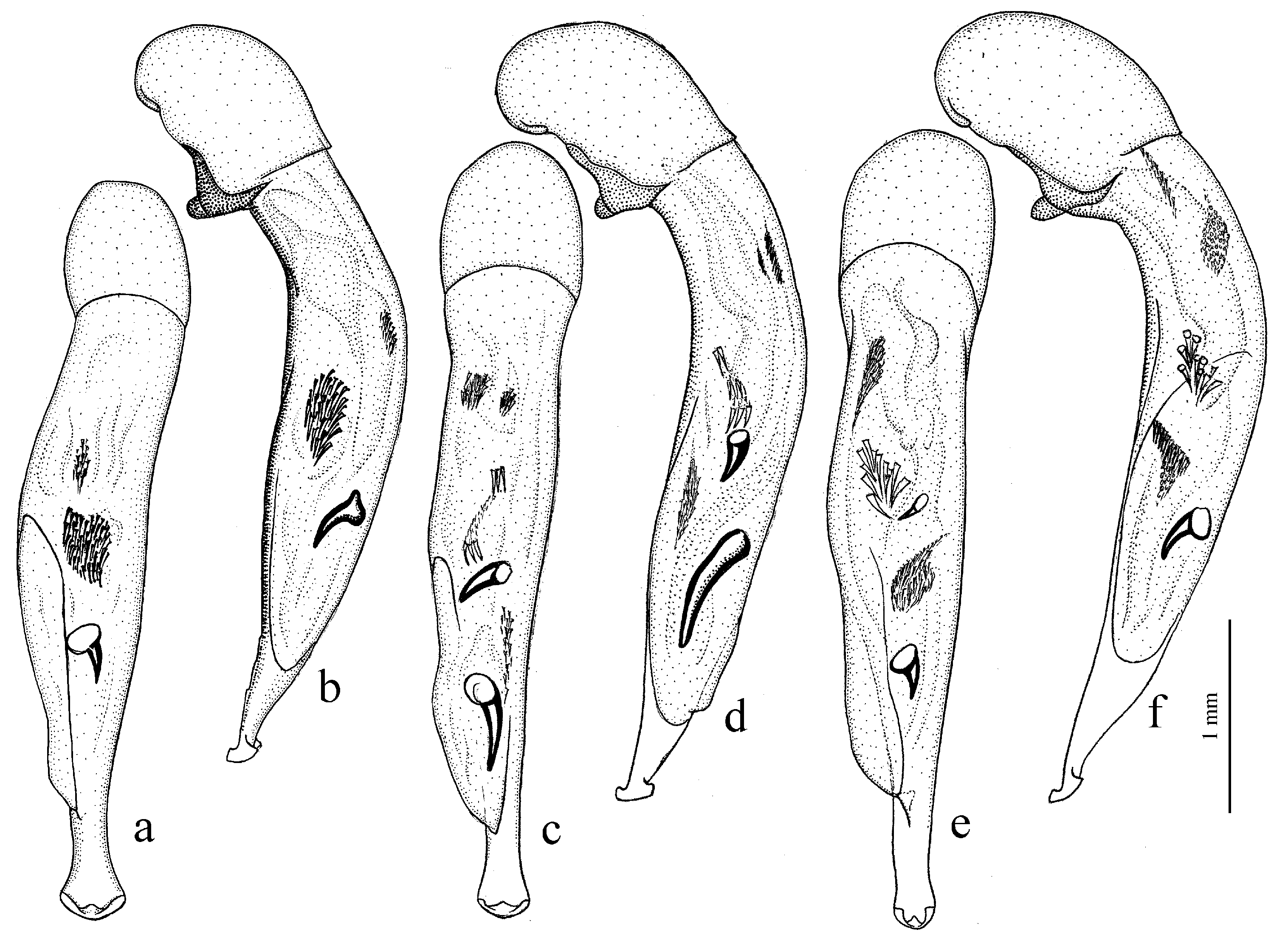
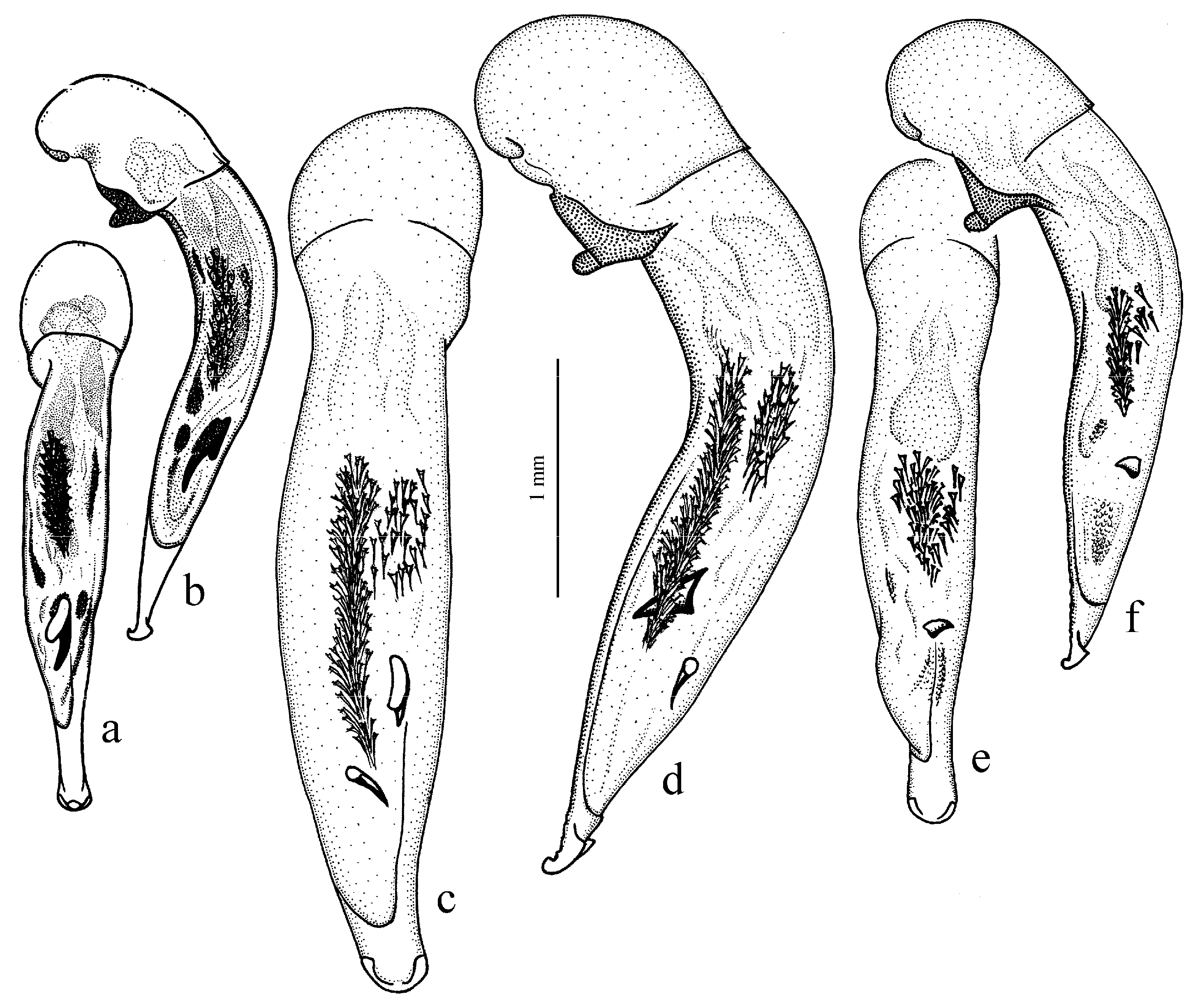
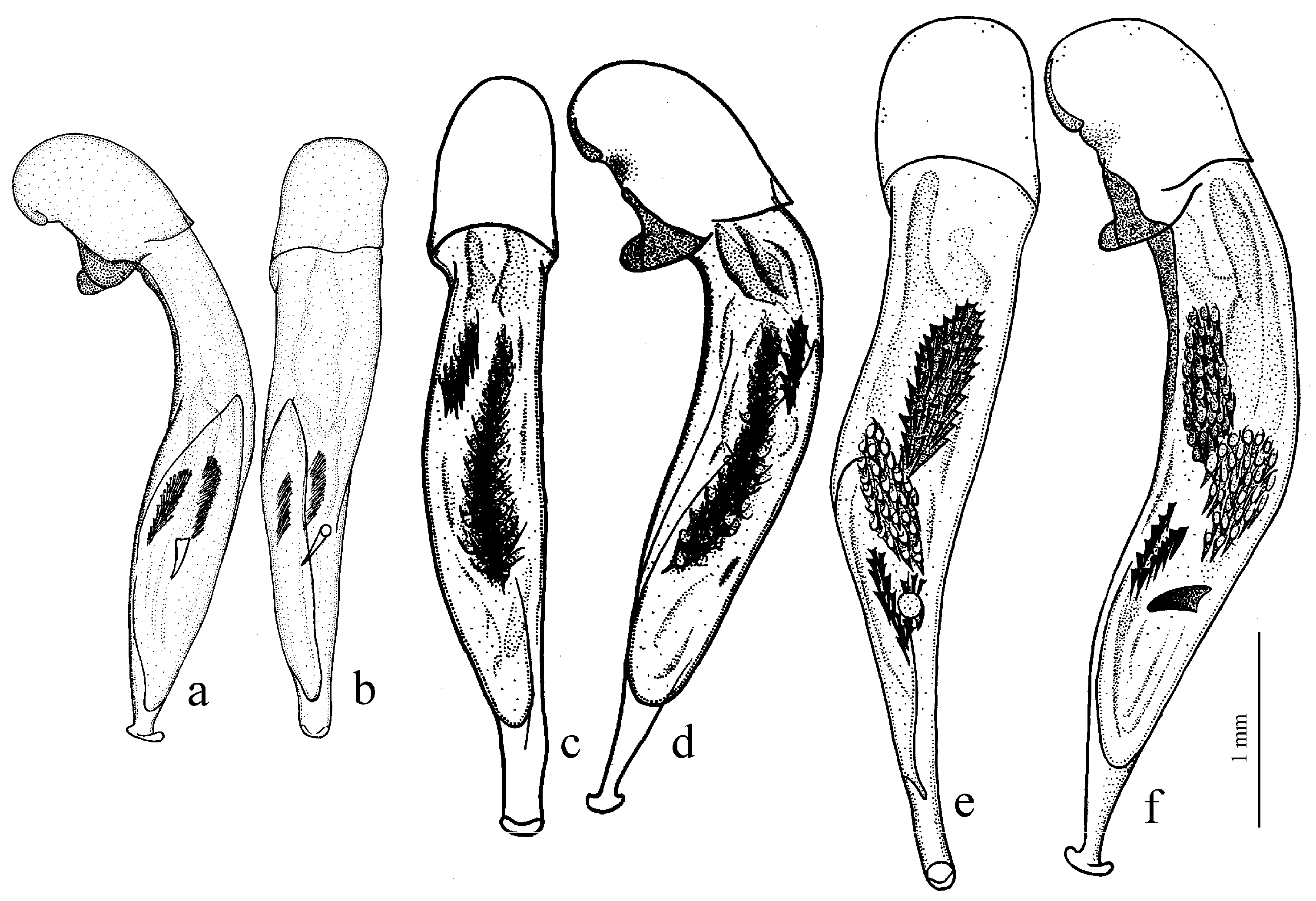
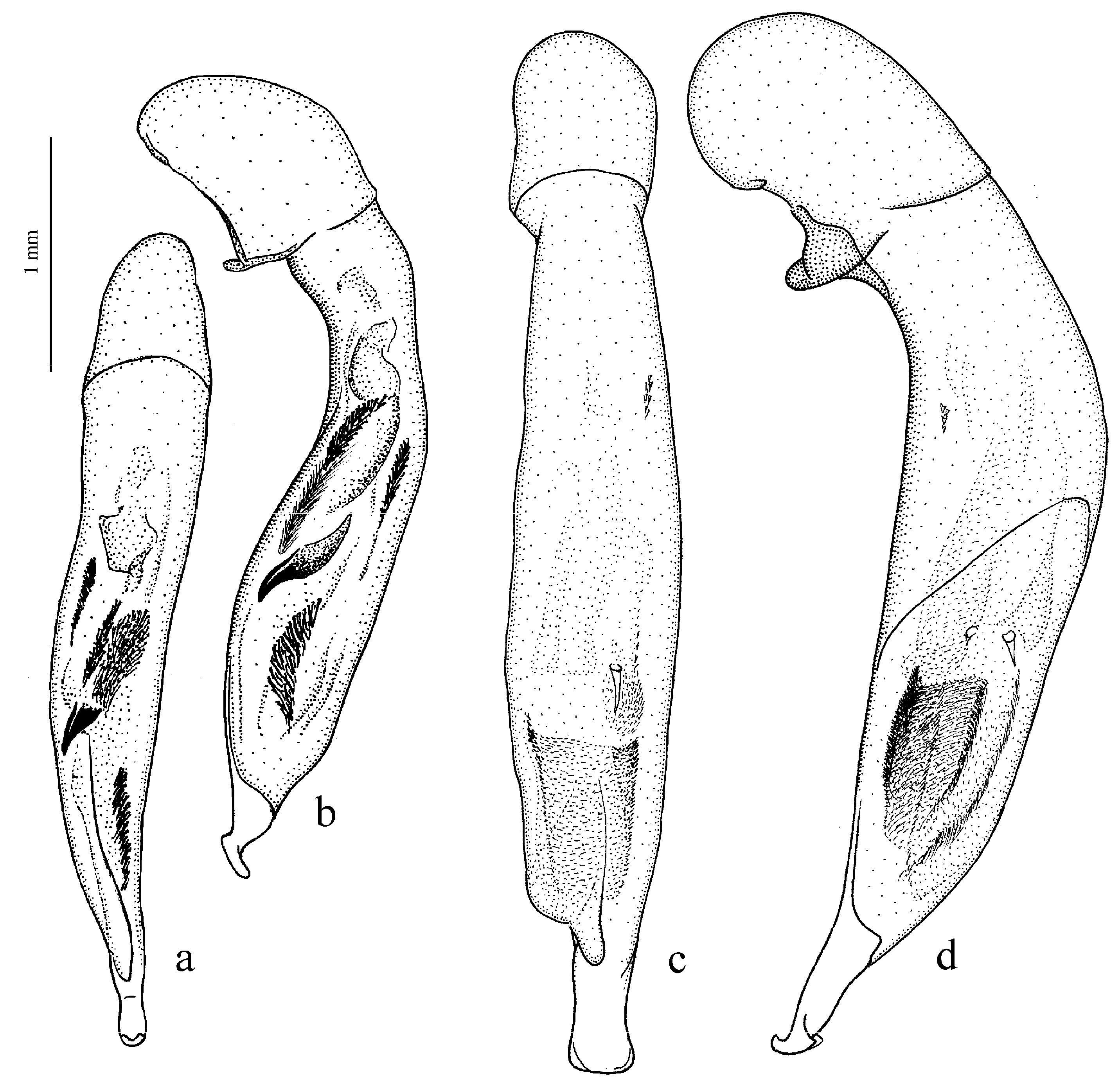
Amblystus subgroup (
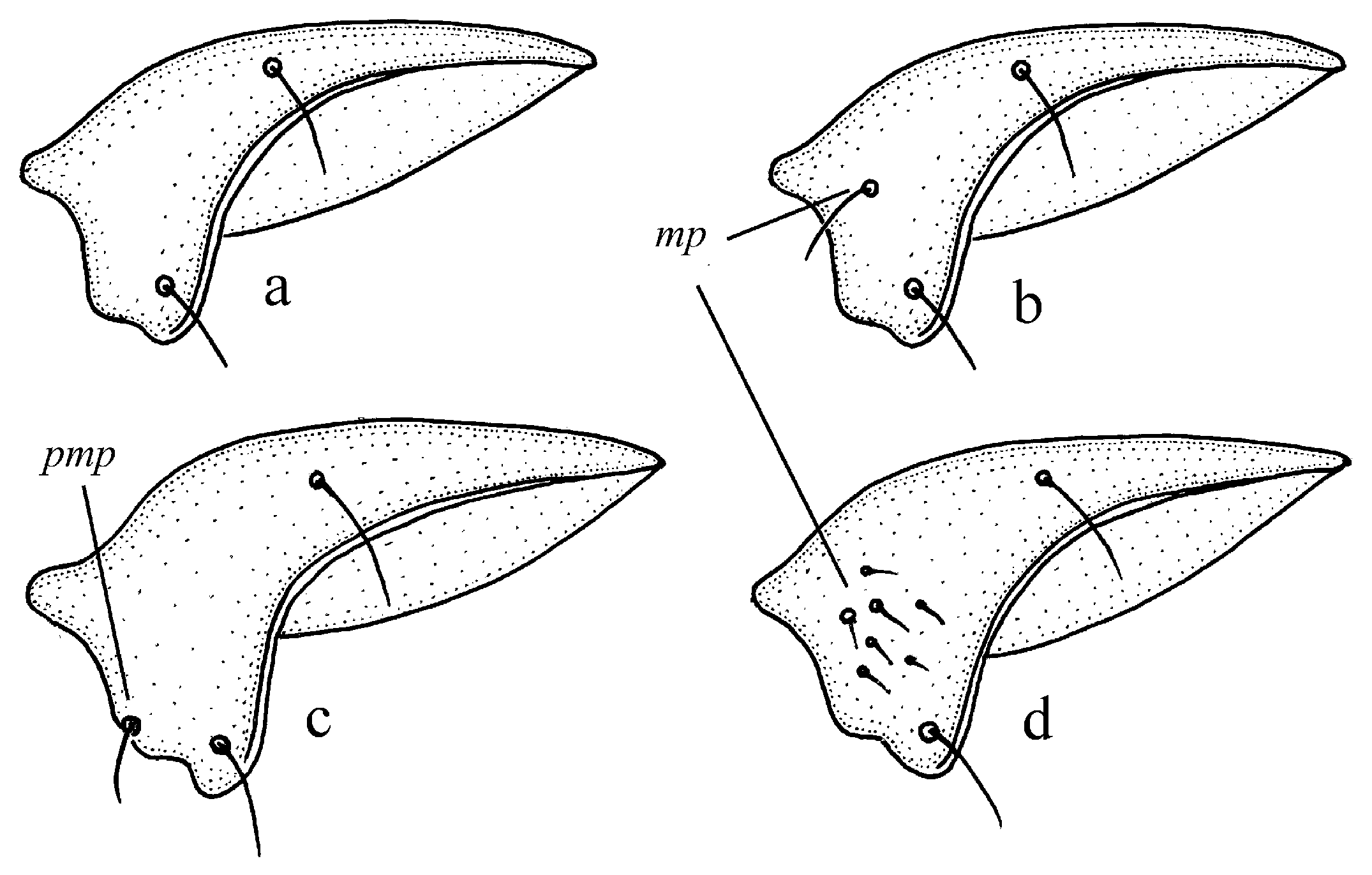
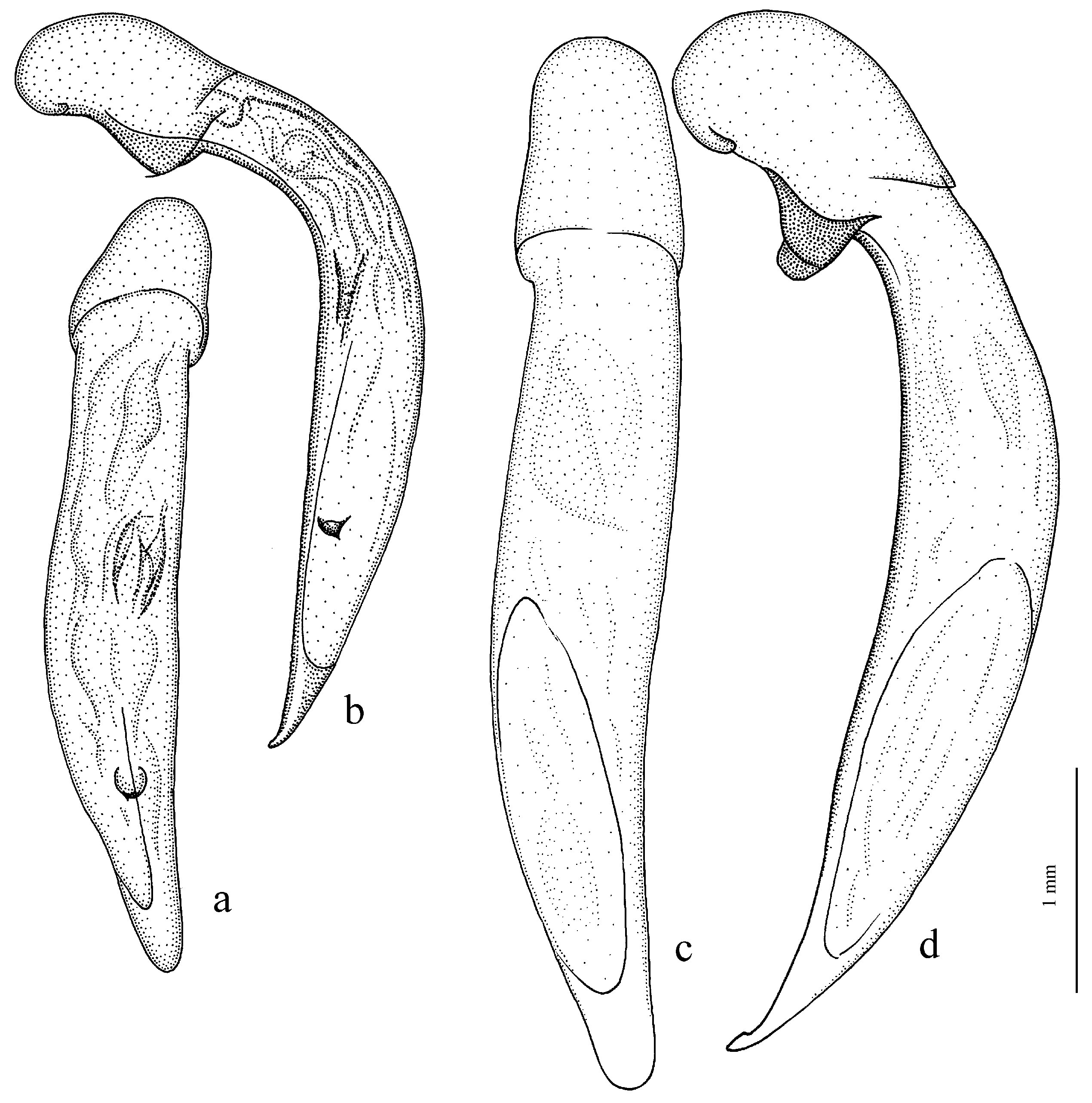
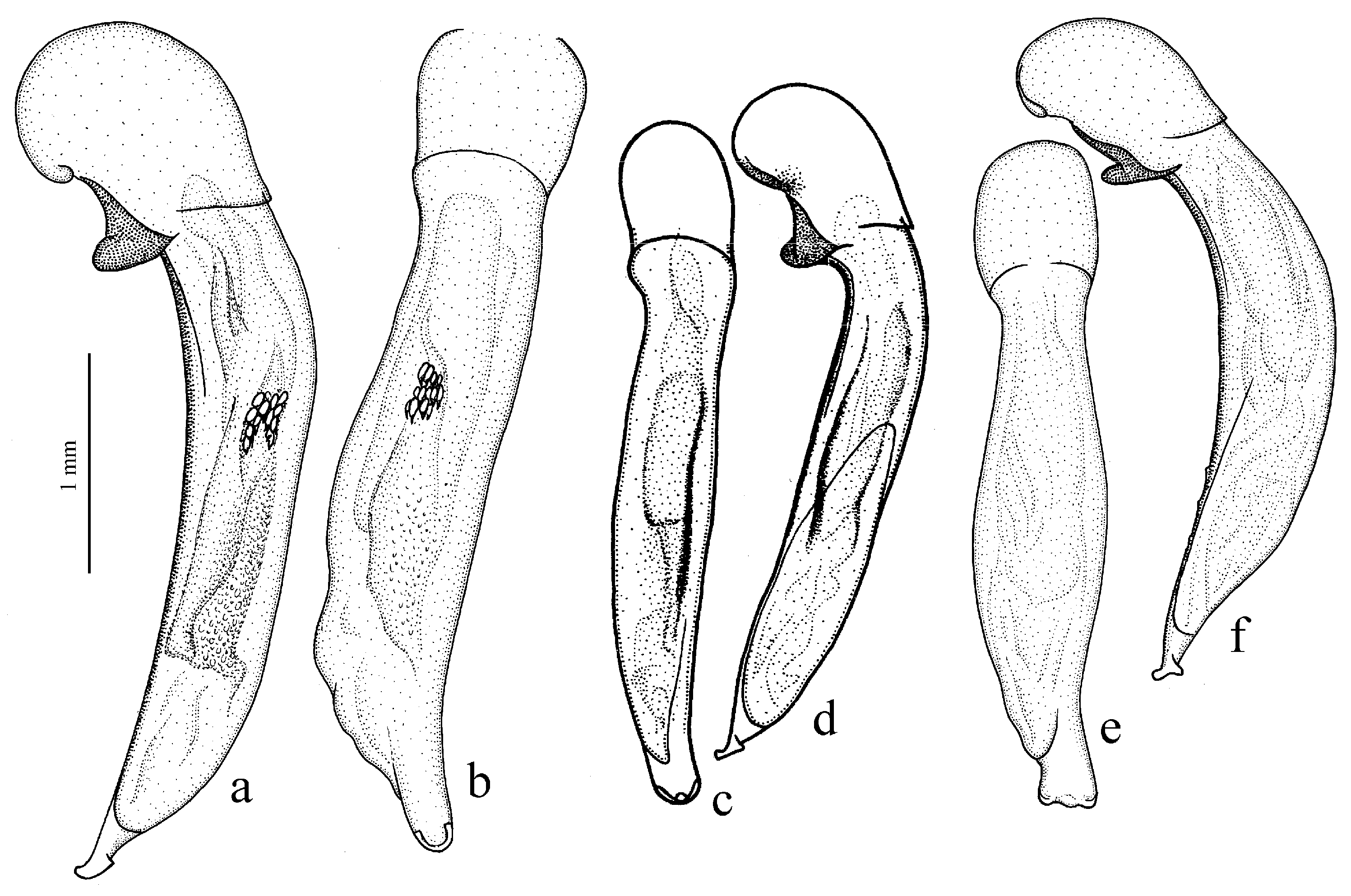
Figure 11c,d and Figure 35e–h), where the following sclerotic elements are clearly distinguished: (1) two separate spines (sometimes one spine), (2) one or several groups of small spines, more rarely spiny patches, medially, and (3) a separate apical spiny patch, usually on the right side of the median lobe of the aedeagus. This type of armament corresponds to the type that Lindroth [
15] described for the
fraternus species group. During the evolution of the taxa of this stock, the main elements of this type were generally preserved, although in some taxa, the armament was simplified, and even individual cases of its complete loss can be traced (for example, in some representatives of the
Acardystus,
Anamblystus and
Harpalobius subgroups). The
affinis stock is characterized by a simpler initial armament of the internal sac. In its most complete form, it is observed in some representatives of the
Pheuginus subgroup (Figure 50): (1) one or two separate spines, and (2) two spiny patches, usually located medially. In the course of the evolution of the taxa of this stock, in different phyletic lineages, a rather early loss of elements of this type armament, especially separate spines, is traced, although in some subgenera, for example, in the
Caloharpalus subgroup, new elements appeared, mainly spiny patches and groups of small or medium spines.
The differences between the two stocks are striking in ecology and distribution of the included taxa. The
latus stock, in addition to the taxa typical for open landscapes, also includes all known forest inhabiting species of the
Harpalus group. The species of open landscapes of this stock are mainly typical xerophilic species occurring in zonal steppe habitats. Among them, there is no species associated with saline soils and specialized desert forms. Only a few have adapted to living in almost pure sand along the banks of rivers [for example,
H. (
Acardystus)
flavescens and
H. (
Psammoharpalus)
kozlovi], less often seas [
H. (
Amblystus)
neglectus]. In zoogeographical terms, in addition to the Palaearctic taxa, this stock also includes the majority of the Holarctic and Nearctic species. On the contrary, only taxa inhabiting open landscapes, including the Mediterranean type, both steppe and desert, belong to the
affinis stock. Among them, species associated with azonal habitats clearly predominate, i.e., they are found in arid territories mainly along the banks of water bodies, in various wetter depressions, on saline soils and salt-marshes. The ranges of most of the species lie within the Palaearctic, concentrating in the Mediterranean region, western and middle Asia. The only exceptions are two species of the subgenus
Pharalus, two or three species of the predominantly Palaearctic subgenus
Brachyharpalus subg. n., and the nominotypical subspecies of the Holarctic
H. (
Harpalus)
amputatus, which all are distribured in North America. The latter species belongs to the nominotypical subgenus (as it is here treated), all other species of which are distributed in the Palaearctic. There is no doubt that the origin and subsequent evolution of these two stocks of the
Harpalus group took place in completely different geographical and ecological conditions [
8].
Afroharpalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. A monobasic subgroup, including only one Afrotropical subgenus.
Subgenus Afroharpalus subg. n.
Type species Harpalus fulvicornis Thunberg, 1806.
Diagnosis. Size small to large (length 4.9–12.5 mm). Body flattened or moderately convex, somewhat wide or elongate, brownish yellow to black, with or without metallic luster on dorsum. Pronotum with one lateral seta on each side before middle and with glabrous or (more rarely) setose basal edge; surface in most species almost impunctate, rarely (e.g., in H. massarti) densely punctate basally. Elytra impunctate and glabrous, with glabrous basal border; interval 3 generally with one discal setigerous pore, in some species this pore absent. Metepisternum elongate or slightly wider than long. Metacoxa generally without posteromedial setigerous pore, in some species this pore present. Metafemur usually with three, sometimes four, setigerous pores along posterior margin. Protibia with one ventroapical spine and with three (more rarely two) preapical spines on outer margin, isolated from spines on ventral surface of tibia; ventroapical tubercle in male generally absent, rarely (e.g., in H. fuscipennis) more or less prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally. Abdominal sternites glabrous; last visible abdominal sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with comparatively long terminal lamella (its length greater than width) and usually without apical capitulum (in some species apical capitulum more or less developed, horseshoe-shaped); internal sac generally with small spiny patches, in some species also with one or two separate spines or without any sclerotic elements.
Etymology. The subgeneric name is based on a combination of Africa and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises all Afrotropical
Harpalus (about 55 described species), with the exception of most Madagascan species, which are treated as members of the separate genus
Anisochirus Jeannel, 1946 [
55], and with the exception of
H. agnatus, which is treated as a member of the subgenus
Cryptophonus. Among them, the majority (52 species) are distributed in east and south Africa; two species,
H. sanctaehelenae Basilewsky, 1972, and
H. prosperus Basilewsky, 1972, were described from Saint Helena Island, and one species,
H. rivalsi Jeannel, 1948, seems to be endemic or introduced to Reunion Island [
55]. There are no common species for east and south Africa, though, according to preliminary data, some east African species are replaced in south Africa by very close vicariant taxa.
Fauna of east Africa includes 16 species:
H. impressus Roth, 1851,
H. asemus Basilewsky, 1946,
H. pseudoasemus Kataev, 2021,
H. merkli Kataev, 2021,
H. meteorus Basilewsky, 1946,
H. jeanneli Basilewsky, 1946,
H. somereni Basilewsky, 1946,
H. kibonoti Alluaud, 1926,
H. gilgil Basilewsky, 1946,
H. inconcinnus Chaudoir, 1876,
H. procognatus Lorenz, 1998,
H. frater Chaudoir, 1876,
H. gregoryi Alluaud, 1917 (
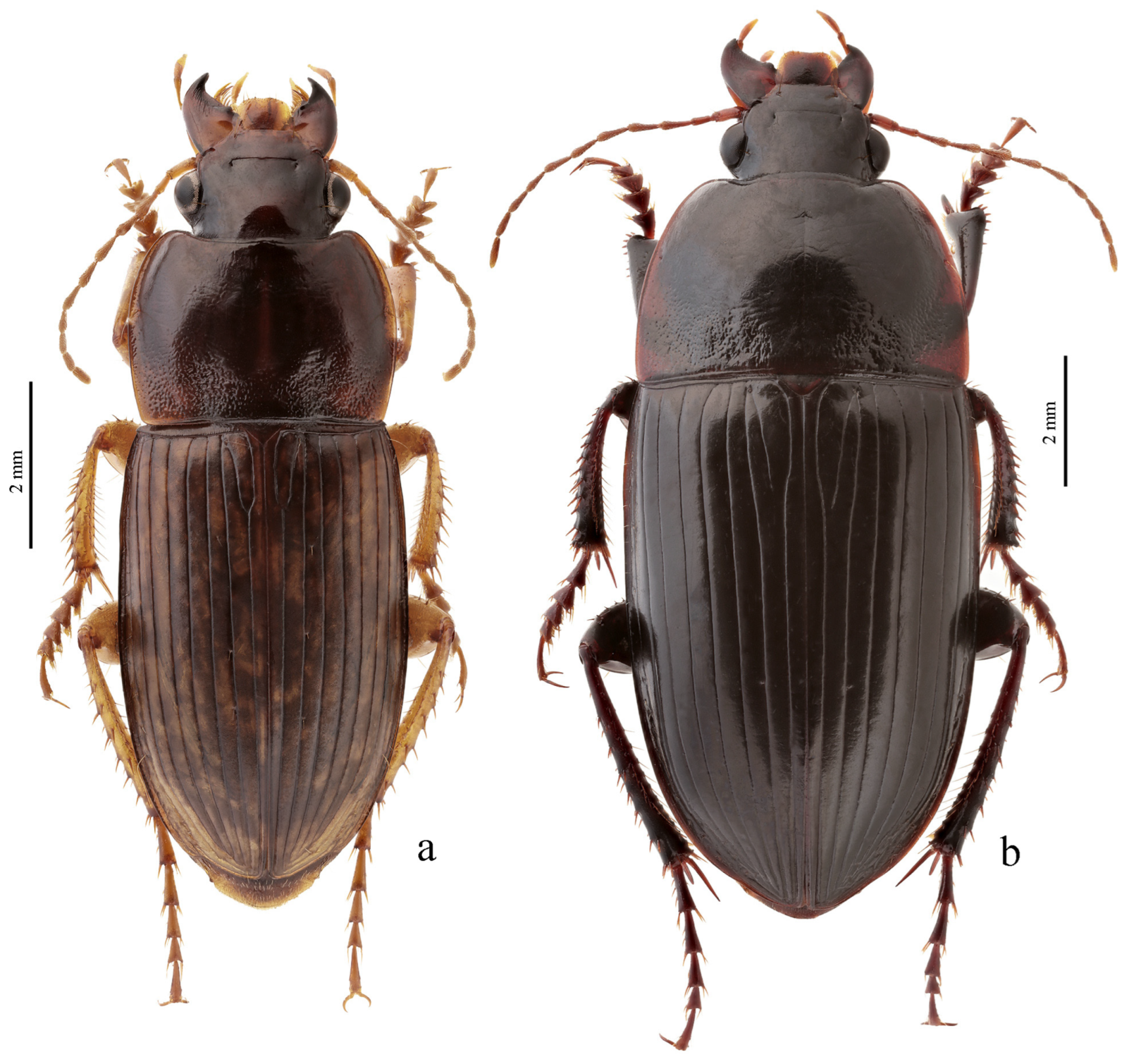
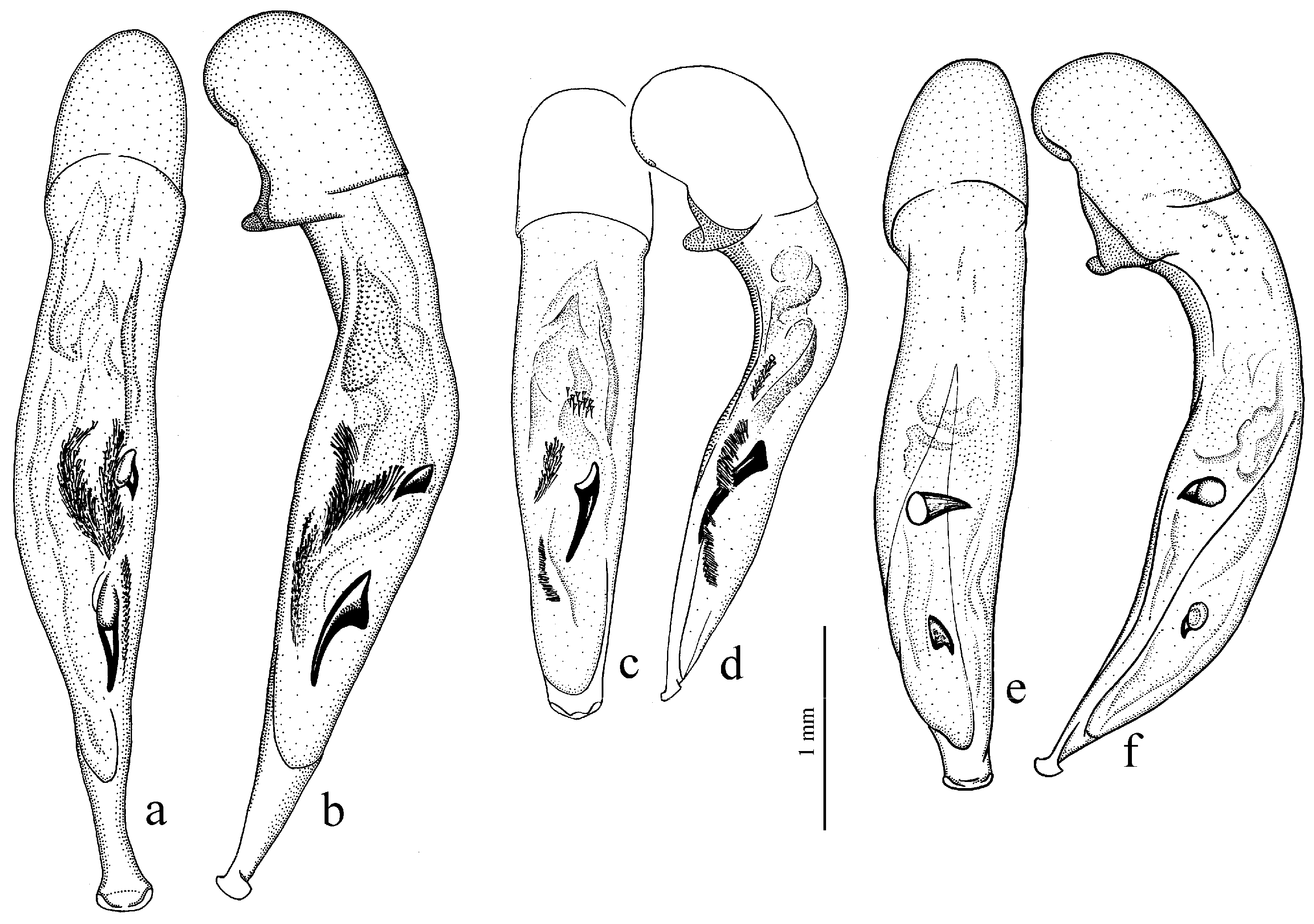
Figure 25e,f),
H. clarkei Kataev et Schmidt, 2020,
H. rougemonti Clarke, 1973 and
H. baleensis Clarke, 1973. Among these species,
H. asemus and
H. inconcinnus occur also in the southern part of the Arabian Peninsula (Yemen and Saudi Arabia).
Fauna of south Africa are more diverse and comprises 36 species:
H. fulvipennis Chaudoir, 1843,
H. exiguus Boheman, 1848,
H. minutissimus Facchini, 2015,
H. nanniscus Peringuey, 1896,
H. parvulus Dejean, 1829 (
Figure 25c,d),
H. fuscoaeneus Dejean, 1829,
H. massarti Burgeon, 1935,
H. natalicus Peringuey, 1896,
H. diversicollis Basilewsky, 1958,
H. nyassicus Basilewsky, 1946,
H. venator Boheman, 1848,
H. makhekensis Basilewsky, 1958,
H. subaeneus Boheman, 1848,
H. miles Peringuey, 1896,
H. defector Peringuey, 1896,
H. rufocinctus Chaudoir, 1843,
H. sinuaticollis Facchini, 2015,
H. fuscipennis Wiedeman, 1825,
H. fulvicornis Thunberg, 1806,
H. corrugatus Basilewsky, 1958,
H. basuto Basilewsky, 1958,
H. natalensis Boheman, 1848 (
Figure 26a),
H. parallelocollis Facchini, 2003,
H. basilewskyi Facchini, 2003,
H. rotundus Facchini, 2003,
H. fimetarius Dejean, 1829,
H. capicola Dejean, 1829,
H. kmecoi Facchini, 2003,
H. elliptipennis Facchini, 2003,
H. hybridus Boheman, 1848;
H. agilis Peringuey, 1896,
H. dubius Boheman, 1848,
H. lugubris Boheman, 1848,
H. angustipennis Boheman, 1848,
H. spurius Peringuey, 1896 and
H. spretus Peringuey, 1896. Two south African species,
H. parvulus and
H. fulvicornis, were introduced to Australia (the former species also to New Zealand).
Harpalus australasiae Dejean, 1829, originally described from Australia, appears to have also been introduced from Africa and appears to be conspecific with either
H. fuscoaeneus or
H. asemus [
55].
Ecology. As far as is known, all species of this group occur in various open habitats, mainly in mountainous areas.
Remarks.
Afroharpalus subg. n. corresponds to the
fulvicornis species group sensu Kataev [
8,
55]. This subgenus is in need of revision, so this review is preliminary. The only revision of the east and south African species was published by Basilewsky [
13], but after that, 18 new species were described [
55,
119,
152,
153,
154,
155,
156]. In addition,
Harpalus subphaedrus Basilewsky, 2005 is transferred to the subgenus
Anisotarsus Chaudoir, 1837, of the genus
Notiobia Perty, 1830 (subtribe Anisodactylina) [
103]. Although the Afrotropical species are somewhat variable in their morphology and appear to represent several different species groups, they are all included here in one subgenus since, in my opinion, they form a monophyletic unit, taxonomically isolated from all Holarctic congeners. The subgenus
Afroharpalus subg. n. probably occupies a basal position in the
Harpalus group and is characterized by an unmodified state of many morphological structures and generally by the absence of many apomorphies, which are observed in most of the Holarctic taxa; for example, the median lobe of many species lacks apical capitulum and has only two spines in internal sac; there are also no conspicuous sexual modifications of tibiae and last visible abdominal sternite. In structure of aedeagus, these species are very similar to members of
Pseudoophonus,
Semiophonus,
Zangoharpalus and
Cryptophonus [
46]. Further study may demonstrate that
Afroharpalus subg. n. warrants the status of a separate subgenus group.
Many Afrotropical species are very similar in their external morphology and can reliably be distinguished only by features of male genitalia.
Hyloharpalus Subgroup
Diagnosis. Body moderately convex, glabrous on dorsum; legs comparatively slender and long. Head smooth, at most very finely punctate dorsally. Pronotum with sides generally not sinuate basally (this character variable in
H. spadiceus), with one lateral seta on each side and with glabrous basal edge; surface either more or less punctate (mainly basally) or almost smooth, only with a few punctures in basal foveae. Elytra glabrous, generally impunctate, more rarely punctate along sides or throughout, with glabrous basal border; humeral denticle present in most members; interval 3 with one or several discal setigerous pores, rarely without pore; intervals 7 and 5 without preapical pores. Metacoxa generally without additional setae, more rarely with one or several additional setae medially. Metafemur with two to six setigerous pores along posterior margin. Protibia with one or two ventroapical spines and with three (more rarely four) preapical spines on outer margin, isolated from spines on ventral surface of tibia; ventroapical tubercle in male of most species not developed. Male mesotibia in most species with more or less distinct preapical callous thickening on inner margin (
Figure 15c). Tarsi glabrous, rarely (in
H. puetzi) setose dorsally; metatarsomere 1 average for genus or slightly more elongate. Abdominal sternites generally glabrous, in a few species with short additional setae; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with moderately long terminal lamella and distinct horseshoe-shaped apical capitulum (modified in
H. laevipes); internal sac generally with one separate spine, one to three groups of medium-sized spines and usually also with one or several spiny patches.
Composition and distribution. This subgroup comprises five subgenera with Holarctic, eastern Palaearctic and Nearctic distributions.
Ecology. Most species of this subgroup occur in forests or at forest edges.
Remarks. Many species of this subgroup are apterous, with short metepisterna, and have more or less distinct preapical callous thickening on inner margin of male mesotibia.
Subgenus Hyloharpalus subg. n.
Type species Harpalus laevipes Zetterstedt, 1828.
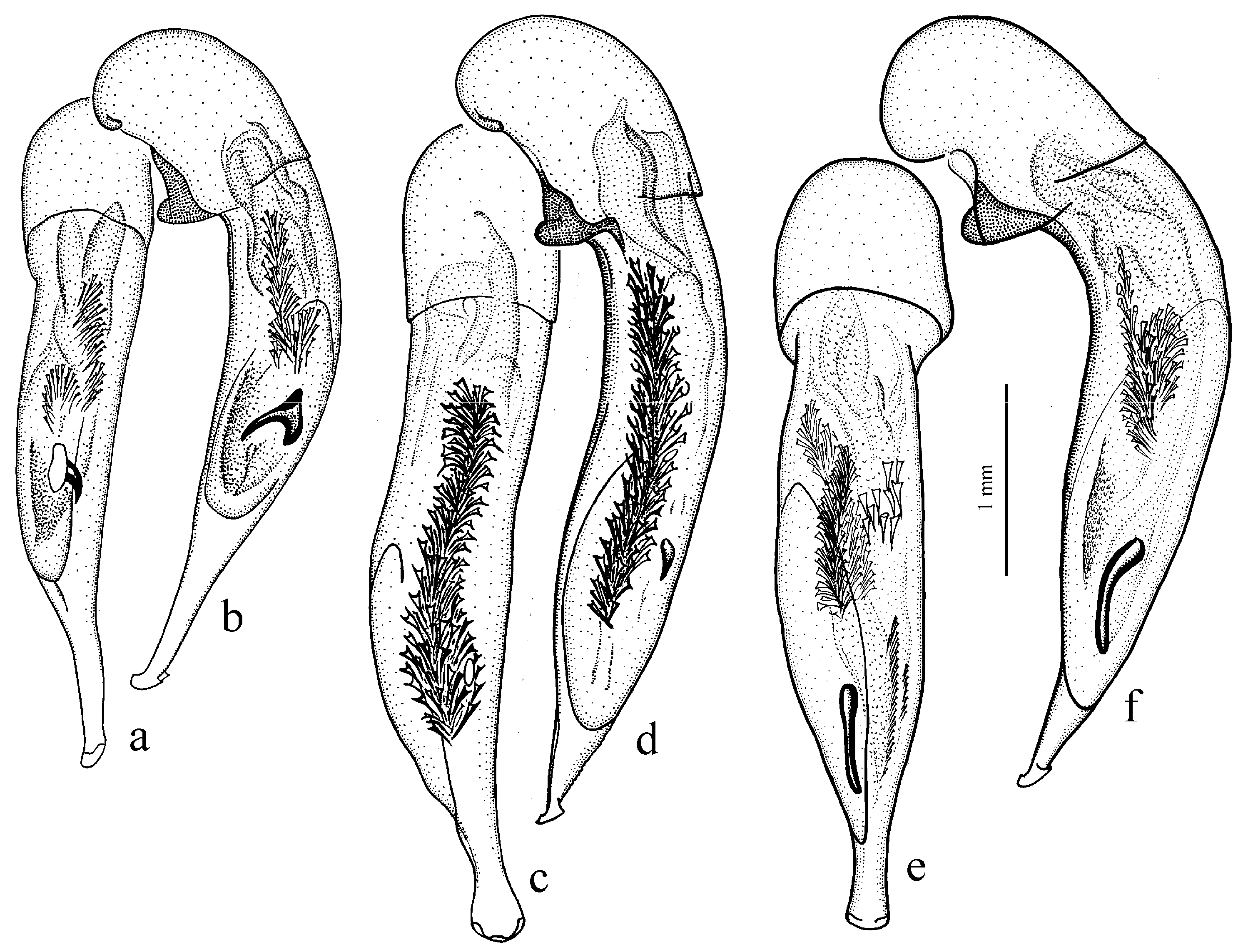
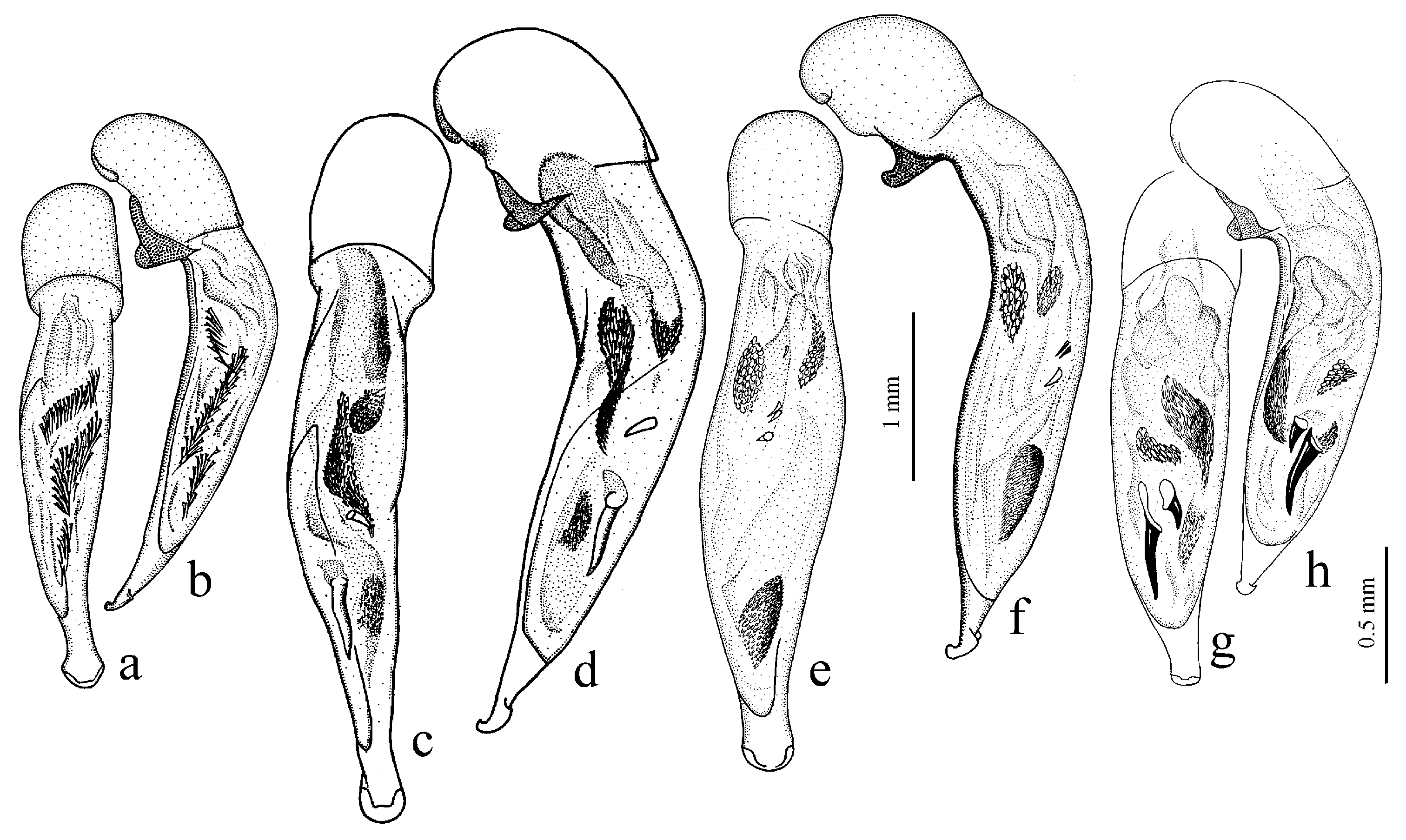
Diagnosis. Medium-sized to large (length 8.0–11.0 [12.5] mm). Body wide or elongate, dark brown to black, without metallic luster. Pronotal disc densely punctate along base. Elytra impunctate; interval 3 with several, up to six, large foveate discal setigerous pores, occasionally (usually on one interval) with one such pore or without pore; abbreviate (parascutellar) striole long, with or without basal pore; preapical sinuation shallow or somewhat deep with small denticle at its base; microsculpture consisting of isodiametric or weakly transverse meshes. Metepisternum either elongate, longer than wide, or short and wide, wider than long. Metacoxa with or without additional setae medially. Protibia with one ventroapical spine; ventroapical tubercle in male not or only slightly prominent. Tarsi glabrous dorsally. Internal sac of aedeagus with two compact groups of medium-sized spines and a medium-sized separate spine.
Etymology. The subgeneric name is a combination of the Greek húlē, meaning “wood, forest”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes the Holarctic
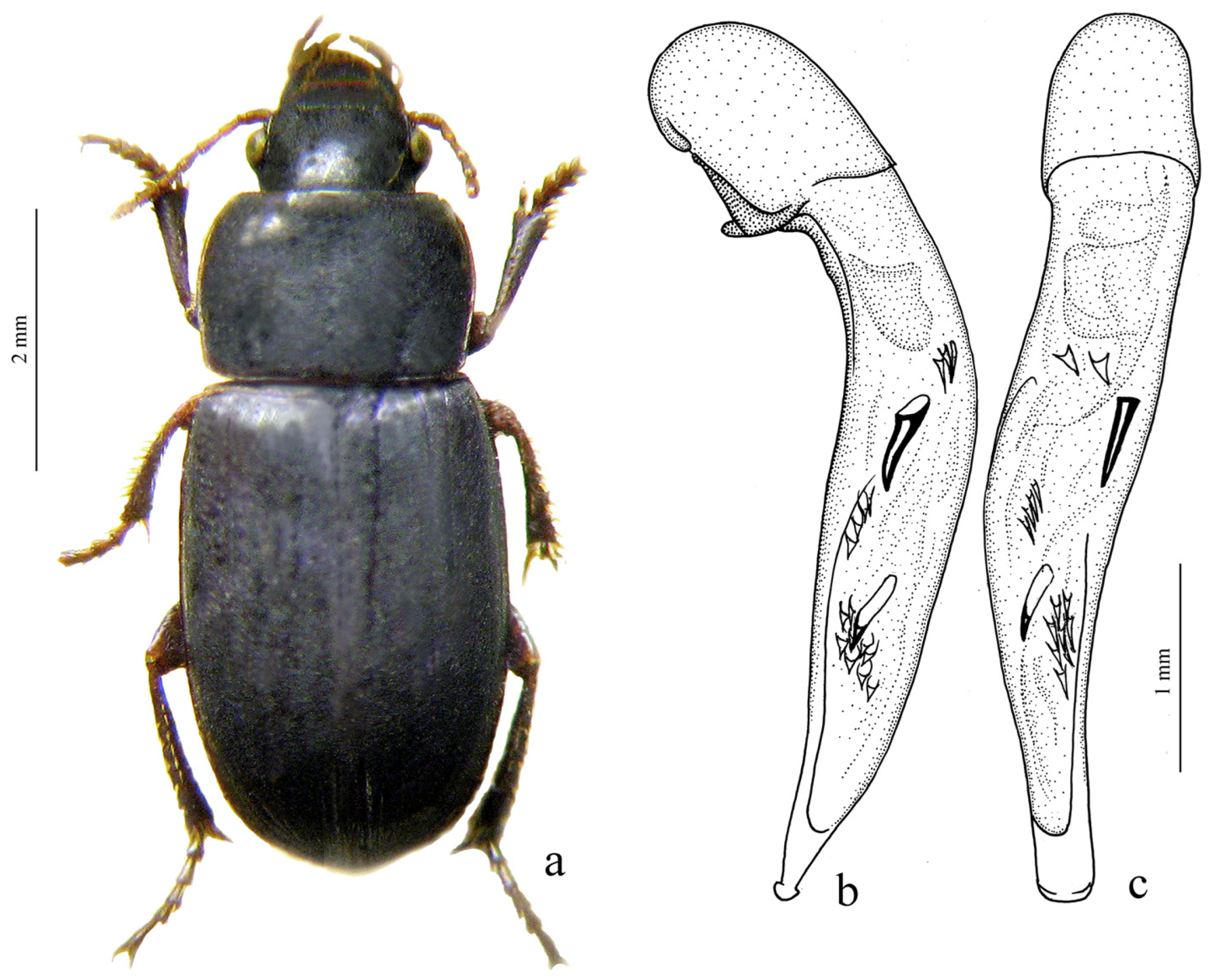
H. laevipes Zetterstedt, 1828 (
Figure 27a,b) and two east Asian species:
H. tibeticus Andrewes, 1930 (
Figure 26b), and
H. farkaci Kataev et Wrase, 1995.
Remarks. The species of this subgenus differ from the species of other subgenera of the
Hyloharpalus subgroup in the presence of usually several large foveate discal setigerous pores on elytral interval 3 and in the characteristic male genitalia with two compact groups of medium-sized spines and a medium-sized separate spine in the internal sac (as in
Figure 27a,b).
Hyloharpalus subg. n. corresponds to the
quadripunctatus (=
laevipes) species group sensu Kryzhanovskij et al. [
56], sensu Kataev [
8,
108] and sensu Kataev & Wrase [
116]. Noonan [
16] treated
H. quadripunctatus (=
H. laevipes), the only species occurring in North America, as a member of the monobasic
quadripunctatus group.
The genesis of this subgenus (as the
laevipes species group) was published by Kataev [
108].
Subgenus Sinoharpalus subg. n.
Type species Harpalus puetzi Kataev et Wrase, 1997.
Diagnosis. Medium-sized to large (length 9.2–12.4 mm). Body slightly elongate, dark brown to black, without metallic luster. Pronotum densely punctate along base. Elytra finely punctate or smooth; interval 3 generally with one (occasionally two) small discal setigerous pores; abbreviate (parascutellar) striole short (usually not longer than width of intervals 1 and 2 combined basally), with basal pore; preapical sinuation somewhat deep with small denticle at its base; microsculpture consisting of transverse or isodiametric meshes. Metepisternum short and wide, wider than long. Metacoxa with or without additional setae medially. Protibia with one ventroapical spine. Tarsi setose or glabrous dorsally. Internal sac of aedeagus with a large longitudinal compact group of medium-sized spines and a small or medium-sized separate spine.
Etymology. The subgeneric name is a combination of Sina (China) and the name of the carabid taxon Harpalus.
Composition and distribution. This group includes two described species from China:
H. puetzi Kataev et Wrase, 1997 from Shaanxi (
Figure 27c,d) and
H. hiekei Kataev et Wrase, 2010 (
Figure 28a) from Hubei and Sichuan. Several taxa of this subgenus also from China are still undescribed.
Remarks. This subgenus is very similar and apparently closely related to Hyloharpalus subg. n., differing mainly in elytra with a short abbreviate striole and with generally one discal setigerous pore on interval 3, and in the presence of only one large longitudinal group of medium-sized spines in internal sac; also in some members, tarsi are more or less setose dorsally and elytra finely punctate throughout or along sides.
Sinoharpalus subg. n. corresponds to the
puetzi species group sensu Kataev [
8].
Subgenus Macroharpalus subg. n.
Type species Erpeinus major Motschulsky, 1850.
Diagnosis. Comparatively large (length 10.0–13.7 [15.5] mm). Body stout, wide, dark brown to black, without metallic luster. Pronotal disc almost smooth, with very fine, occasionally indistinct micropunctation along base. Elytra impunctate; interval 3 with one discal setigerous pore; preapical sinuation shallow, without denticle at its base; microsculpture isodiametric. Metepisternum elongate, longer than wide. Metacoxa without additional setae medially. Metafemur with three (occasionally two and four) setigerous pores along posterior margin. Protibia with one or two ventroapical spines. Tarsi glabrous dorsally. Internal sac of aedeagus with one large separate spine, one to three groups of medium-sized spines, and a spiny patch apically.
Etymology. The subgenus name is a combination of the Greek makrós, meaning “big, large”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes the eastern Palaearctic
H. major (Motschulsky, 1850) (
Figure 27e,f) and three Nearctic species:
H. providens Casey, 1914,
H. animosus Casey, 1924 (
Figure 28b), and
H. laticeps LeConte, 1850.
Ecology. The most species of this subgenus occur mainly in forests;
H. animosus lives in mountains, subalpine and alpine zones, where it occurs in meadows, pastures, moraines and forest clearings [
140].
Remarks. This subgenus can be recognized among other subgenera of this subgroup in large stout body with almost smooth (without distinct punctation) pronotum.
Macroharpalus subg. n. corresponds to the Palaearctic
obesus species group sensu Kryzhanovskij et al. [
56], and to the
major species group sensu Kataev [
8], including both Palaearctic and Nearctic species. Lindroth [
15] included the Nearctic species of this subgenus in the
fraternus species group (=
Euharpalops), which he considered very widely. Noonan [
16] included
H. viduus (=
H. providens) and
H. animosus in the
viduus species group and treated
H. laticeps as a member of the closely related, monobasic
laticeps group based on some features of the male genitalia. I prefer to consider these three species as members of one subgenus. The Palaearctic
H. major is very similar to the Nearctic
H. providens both in external features and male genitalia.
Subgenus Meroharpalus subg. n.
Type species Harpalus fulvilabris Mannerheim, 1853.
Diagnosis. Medium-sized (length 7.4–9.5 [10.8] mm). Body elongate, dark brown to black, with metallic luster on dorsum. Pronotal disc densely punctate along base. Elytra impunctate; interval 3 either without discal setigerous pore or with one, occasionally two, pores; basal pore present; abbreviate (parascutellar) striole comparatively long; preapical sinuation shallow, without denticle at its base; microsculpture isodiametric, in male highly obliterate on disc. Metepisternum elongate or as long as wide. Metacoxa without additional setae medially. Protibia with one ventroapical spine; ventroapical tubercle in male not developed or slightly prominent. Tarsi glabrous dorsally. Internal sac of aedeagus with one or two medium-sized separate spines, one or two groups of medium-sized spines and a spiny patch apically.
Etymology. The subgeneric name is a combination of the Greek méros, meaning “part, portion”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes two Nearctic species:
H. fulvilabris Mannerheim, 1853 (
Figure 29a), and
H. megacephalus LeConte, 1848.
Remarks. The species of this subgenus are recognizable among other members of the Hyloharpalus subgroup by the metallic luster on dorsum and the characteristic male genitalia.
This subgenus corresponds to the
fulvilabris species group sensu Lindroth [
15]. Noonan [
16] included
H. fulvilabris together with
H. spadiceus in the
spadiceus group and treated
H. megacephalus together with
H. nigritarsis as members of the separate
nigritarsis group; according to my data, the latter species in his interpretation is actually a complex of several taxa. The position of
H. megacephalus, known to me only from one female, needs further study.
Subgenus Ameroharpalus subg. n.
Type species Harpalus spadiceus Dejean, 1829.
Diagnosis. Medium-sized (length 7.7–9.0 [10.5] mm). Body elongate, dark brown to black, without metallic luster. Pronotal disc with very fine, often more or less obliterate punctation along base. Elytra impunctate; interval 3 with one discal setigerous pore; abbreviate (parascutellar) striole short or moderately long, with basal pore; preapical sinuation shallow, without denticle at its base; microsculpture clearly transverse, in male highly obliterate on disc. Metepisternum short and wide, wider than long. Metacoxa without additional setae medially. Protibia with one ventroapical spine; ventroapical tubercle in male not prominent. Tarsi glabrous dorsally. Internal sac of aedeagus with a large group of medium-sized spines, one medium-sized separate spine and a spiny patch apically.
Etymology. The subgeneric name is based on a combination of America and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only the Nearctic
H. spadiceus Dejean, 1829 (
Figure 29b), distributed in the mountains of the east of the continent (Appalachians and Black Mountains). This wingless species is represented by several geographical forms, the status of which requires further study.
Remarks. This subgenus corresponds to the
spadiceus species group sensu Lindroth [
15]. The only included species of this subgenus differs from the species of
Meroharpalus subg. n. in clearly transverse elytral microsculpture, metepisternum wider than long and dorsum dark, without metallic luster; in addition, its pronotum is with finer, often more or less obliterate punctation along base.
Amblystus Subgroup
Diagnosis. Body moderately convex or flattened. Head impunctate or punctate, glabrous or sparsely setose on dorsum; legs comparatively slender and long. Pronotum with sides sinuate or not sinuate basally, with one lateral seta on each side (in H. chobautianus with an additional setigerous pore before basal angles) and with basal edge glabrous or setose; surface in most species more or less coarsely and densely punctate, rarely impunctate. Elytra either impunctate and glabrous or punctate and pubescent; basal border glabrous or setose, humeral denticle in most species present; interval 3 generally with one discal setigerous pore; intervals 7 and 5 with or without preapical pores. Metacoxa generally without additional setae medially, rarely (for example, in H. neglectus with additional setae). Metafemur with three to ten setigerous pores along posterior margin. Protibia with one ventroapical spine and with three or four preapical spines on outer margin, isolated from spines on ventral surface of protibia; ventroapical tubercle in male not prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally. Abdominal sternites usually glabrous, more rarely with additional long setae; last visible abdominal sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen, more rarely slightly blunted in male. Median lobe of aedeagus with a moderately long terminal lamella and a distinct horseshoe-shaped apical capitulum; internal sac usually with two (rarely one) more or less large separate spines, also a group of medium-sized or small spines and generally one or several spiny patches.
Composition and distribution. This subgroup comprises six Palaearctic subgenera, with most species distributed in the western part of the Palaearctic, mainly in mountainous regions; one monobasic subgenus is endemic to mountains in the Chinese province of Sichuan.
Remarks. This subgroup is very similar to Hyloharpalus subgroup in many distinctive characters listed in their diagnoses, but male mesotibia lacks preapical callous thickening on inner margin. Since this feature is somewhat variable within Hyloharpalus subgroup, the relationship and composition of these two subgroups need further study.
The Amblystus subgroup includes many morphologically distinct mountain wingless species, the ranges of which are sometimes significantly distant from each other.
Subgenus Drymoharpalus subg. n.
Type species Harpalus atratus Latreille, 1804.
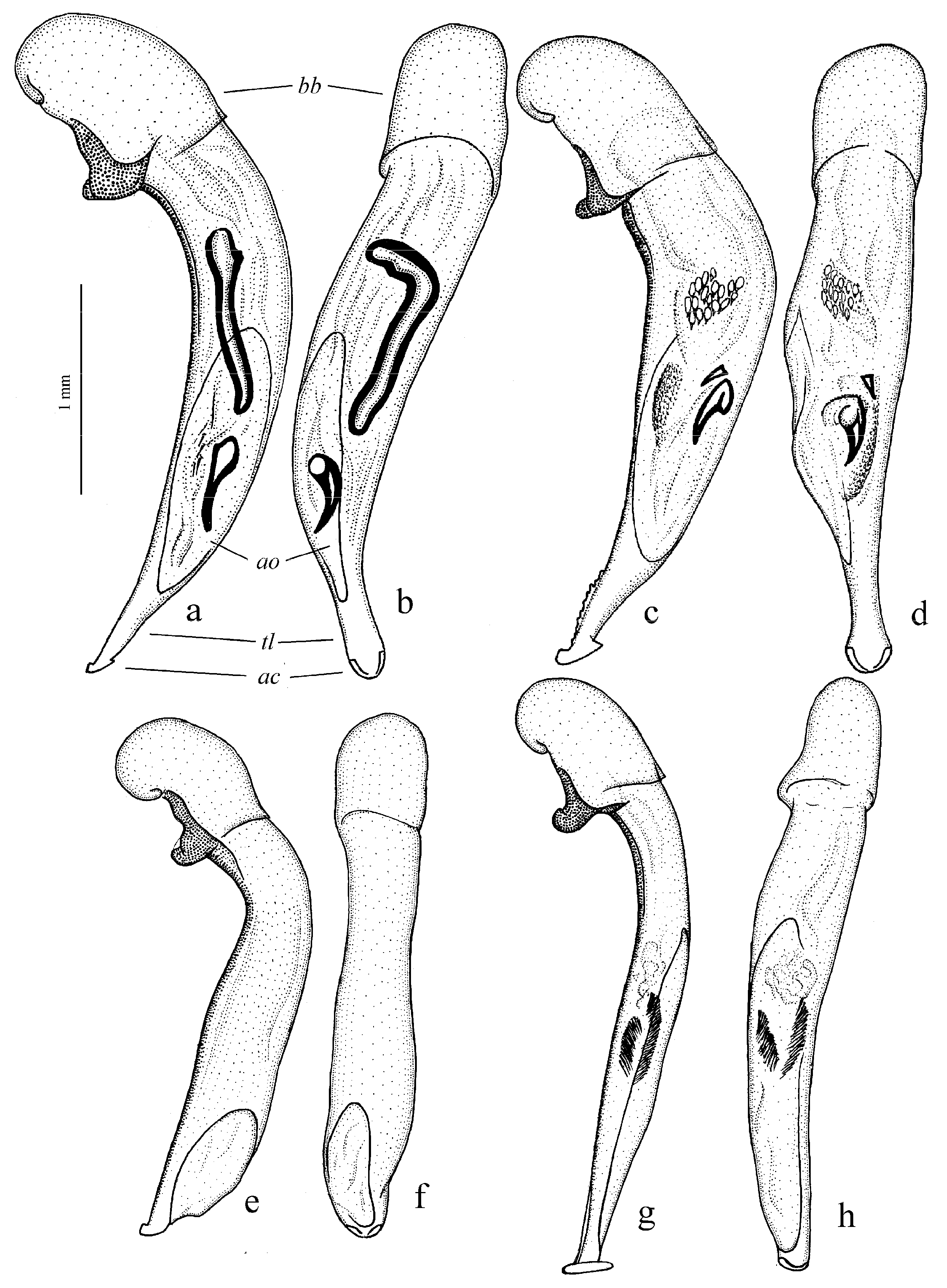
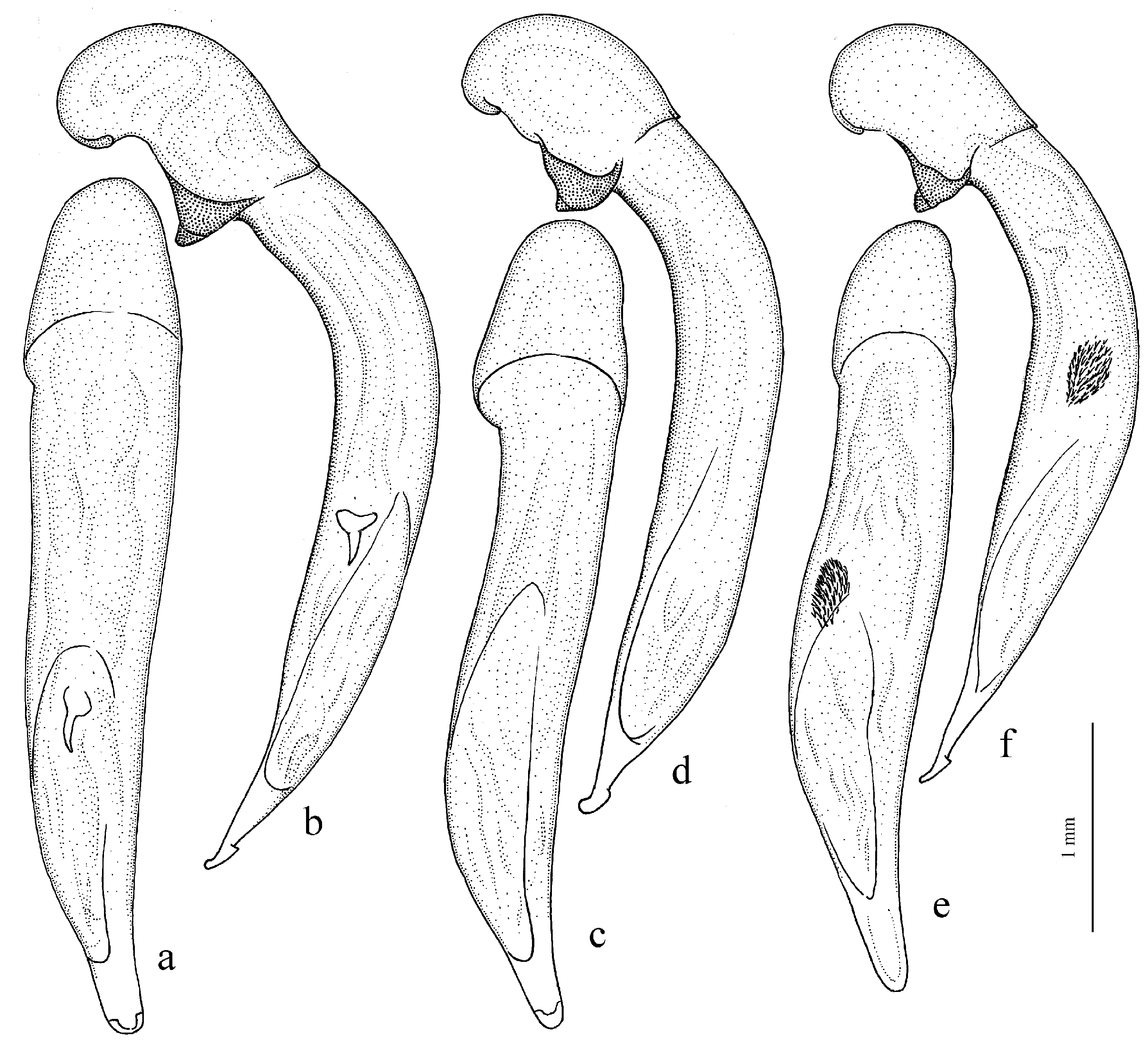
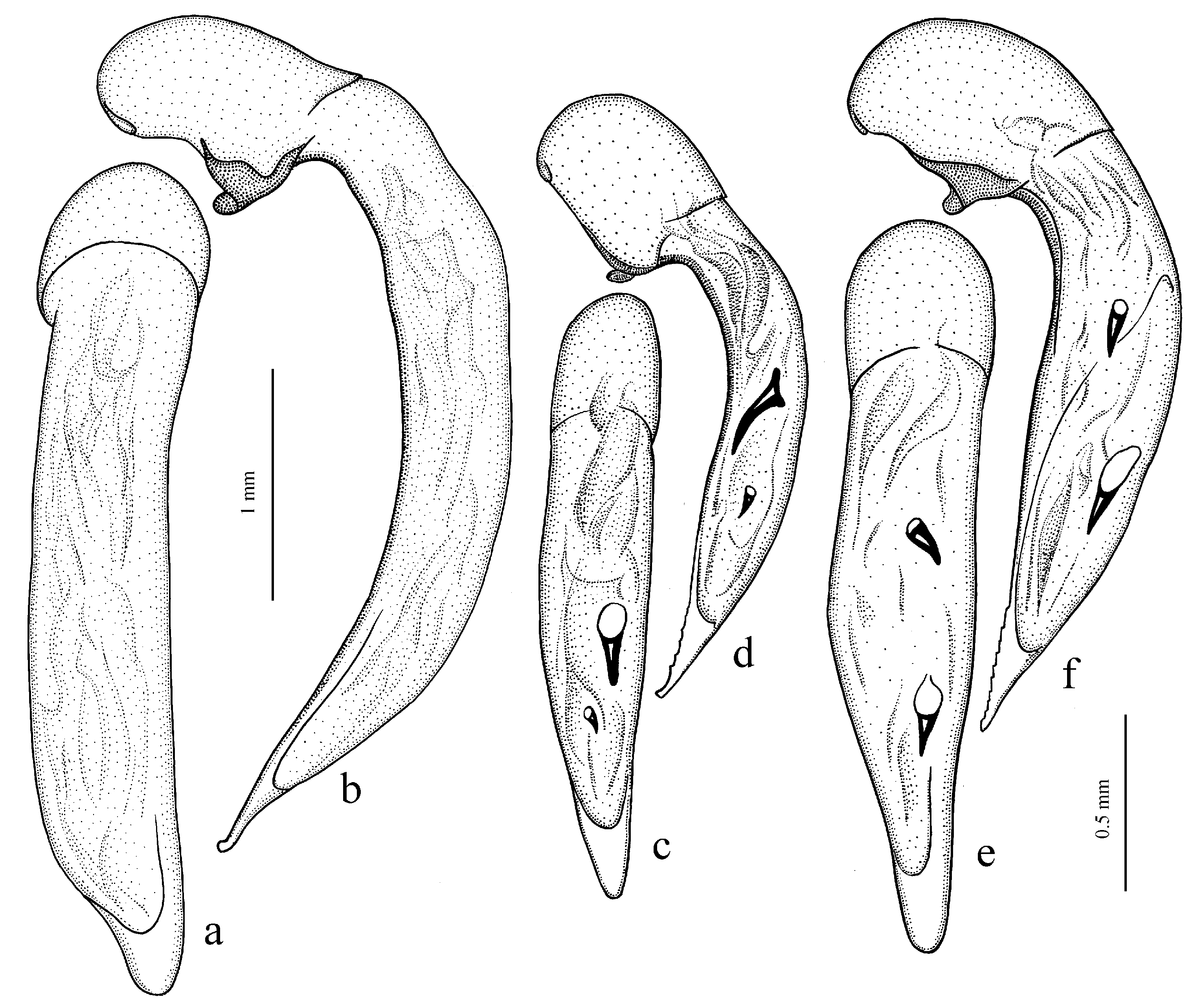
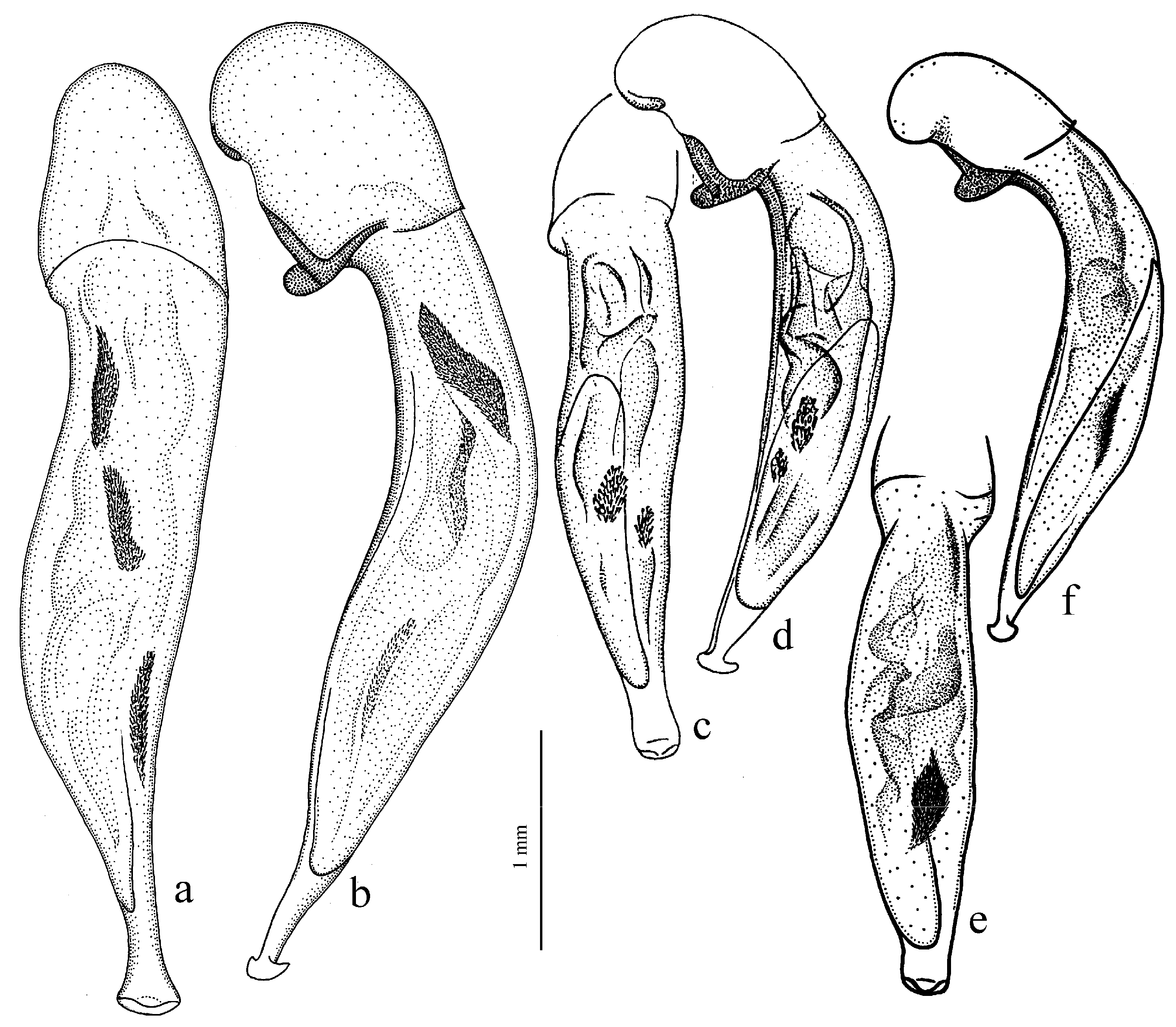
Diagnosis. Comparatively large size (length 10.0–13.0 mm). Head impunctate and glabrous. Body moderately wide, dark brown to black, without metallic luster. Pronotal basal edge glabrous. Elytra impunctate and glabrous, with glabrous basal border and distinct humeral denticle; preapical sinuation shallow; intervals 7 and 5 without preapical pores. Metepisternum elongate, longer than wide. Abdominal sternites glabrous. Internal sac of aedeagus with one large separate spine and two groups of small spines medially (Figure 32a,b).
Etymology. The subgeneric name is a combination of the Greek drymós, meaning “wood, forest”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only one winged species,
H. atratus Latreille, 1804 (
Figure 30a and Figure 32a,b), from the western Palaearctic.
Ecology. The only representative of this subgenus usually occurs in deciduous forests.
Remarks. The monobasic Drymoharpalus subg. n. is similar to members of Epiharpalus in general habitus and having glabrous basal edge of pronotum and abdominal sternites, but markedly differs from it in elongate metepisternum and only one separate spine in internal sac of aedeagus.
This subgenus corresponds to the
atratus species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8,
109].
Subgenus Epiharpalus Reitter, 1900
Epiharpalus Reitter, 1900 [
38] (pp. 75, 80) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus punctipennis Mulsant, 1852, designated by Antoine [
40].
Harpaloxys Reitter, 1900 [
38] (pp. 75, 94) (as a subgenus of
Harpalus Latreille, 1802). Type species:
Harpalus cardioderus Putzeys, 1872 (=
H. ebeninus Heyden, 1870), designated by Noonan [
6].
Epharpalus: Jakobson, 1907 [
63] (p. 378) (print error).
Diagnosis. Medium-sized to moderately large (length 9.4–11.8 mm). Head impunctate and glabrous. Body moderately wide, dark brown to black, without metallic luster. Pronotal basal edge glabrous. Elytra either coarsely punctate and pubescent (laterally and apically) or impunctate and glabrous; basal border glabrous; humeral denticle present; preapical sinuation shallow or moderately deep, at most with traces of obtuse denticle at its base; intervals 7 and 5 without preapical pores. Metepisternum short and wide, wider than long. Abdominal sternites glabrous. Internal sac of aedeagus with two large separate spines, one or two groups of small spines medially and generally three spiny patches.
Composition and distribution. This subgenus includes two wingless species from west Europe:
H. ebeninus Heyden, 1870 (
Figure 31b and
Figure 32c,d), endemic to the Cantabrian Mountains in Spain, and
H. punctipennis Mulsant, 1852 (
Figure 31a and
Figure 32e,f), endemic to the Alpes-Maritimes on the border of France and Italy.
Ecology. The species occurs in mountain meadows and grasslands.
Remarks. Although the two included species are distinguished by elytral punctation, they are very similar in other characters and apparently closely related. So, they are included in one subgenus (the
punctipennis species group sensu Kataev [
8]).
Subgenus Caucasoharpalus subg. n.
Type species Omaseus aeneipennis Faldermann, 1836.
Diagnosis. Medium-sized (length 7.3–10.6 mm). Body slightly elongate, moderately convex, dark brown to black, with or without metallic luster. Head impunctate and glabrous. Pronotal basal edge glabrous. Elytra impunctate and glabrous, with glabrous basal border and prominent humeral denticle; preapical sinuation deep, with distinct denticle at its base; intervals 7 and 5 with or without preapical pores. Metepisternum short and wide, wider than long. Abdominal sternites glabrous. Internal sac of aedeagus with two separate closely spaced spines apically, one to three groups of small or medium-sized spines medially and a spiny patch apically.
Etymology. The subgeneric name is based on a combination of Caucasus and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes two wingless species from the West and Lesser Caucasus:
H. aeneipennis (Faldermann, 1836) (
Figure 30b and
Figure 33a,b) and
H. chrysopus Reitter, 1887 (
Figure 33c,d) (with the subspecies
H. ch. abasinus Rost, 1891 and
H. ch. contumax Lutshnik, 1933). Several taxa of this subgenus have not yet been described.
Ecology. Both species live in the mountains, both in the forest belt and higher, in alpine and subalpine meadows.
Remarks. This subgenus is morphologically very similar to Epiharpalus, but differs from it in the impunctate elytra combined with a deep preapical sinuation having a distinct denticle at the base and the characteristic male genitalia.
Caucasoharpalus subg. n. corresponds to the
aeneipennis species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8,
109].
Subgenus Calathoderus subg. n.
Type species Harpalus potanini Tschitschérine, 1906.
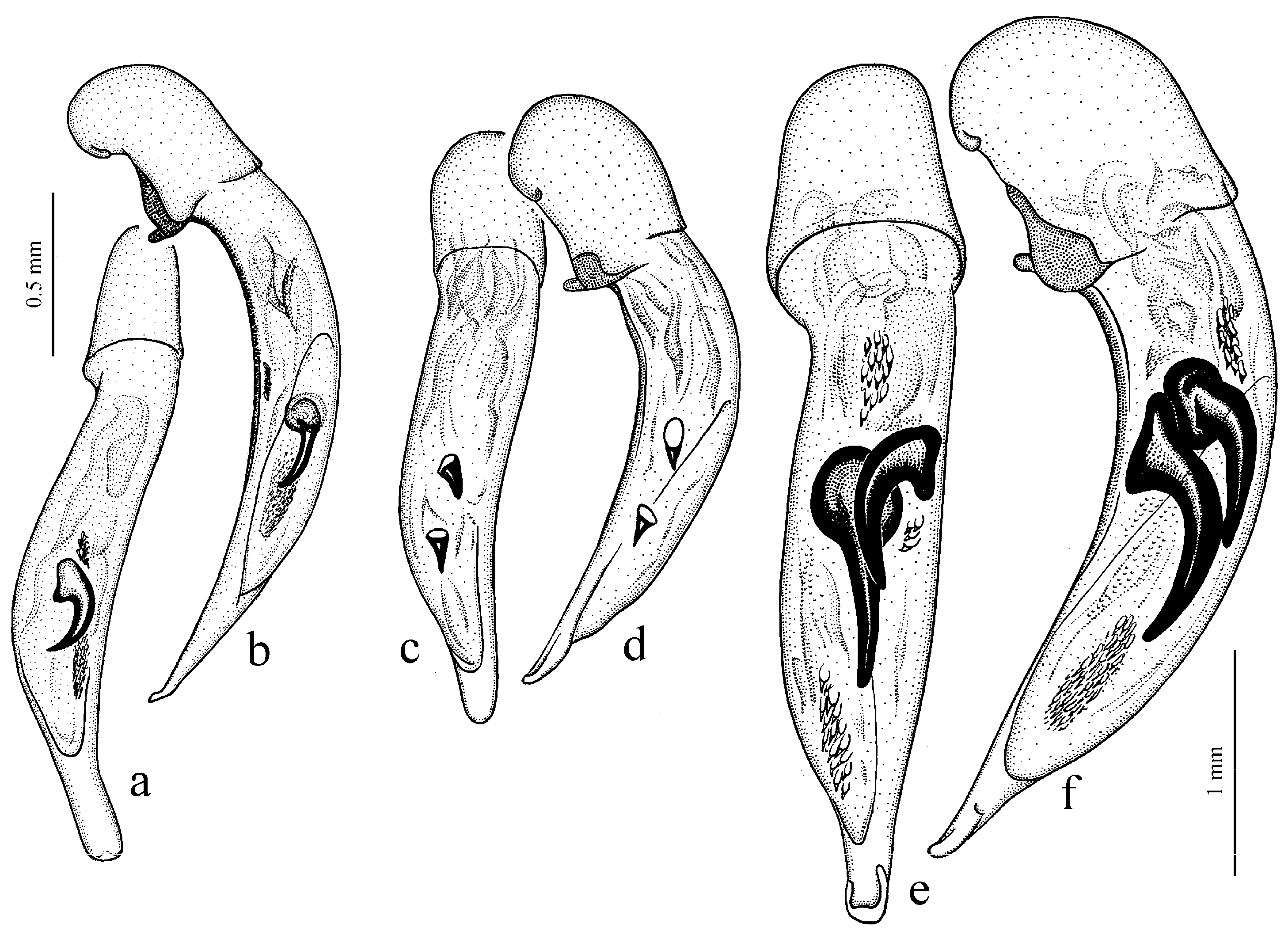
Diagnosis. Large-sized (length 10.8–14.0 mm). Body moderately wide and flat, black, without metallic luster; legs comparatively long and slender, with long and narrow, almost parallel-sided metatarsomeres. Head impunctate and glabrous. Pronotal basal edge glabrous. Elytra impunctate and glabrous, with glabrous basal border and without humeral denticle; preapical sinuation rather deep, with traces of obtuse denticle at its base; intervals 7 and 5 without preapical pores. Metepisternum short and wide, wider than long. Abdominal sternites glabrous. Internal sac of aedeagus with two large separate closely spaced spines, a small spiny patch medially and a larger spiny patch apically (
Figure 33e,f).
Etymology. The subgeneric name is based on a combination the name of the carabid taxon Calathus and the Greek dére, meaning “neck/notum”.
Composition and distribution. This subgenus includes one wingless species,
H. potanini Tschitschérine, 1906 (
Figure 33e,f and
Figure 34a), known from the mountains of the Chinese province of Sichuan.
Remarks. The position of this subgenus requires further study. It is remarkable in the appearance of the only included species, resembling some members of Calathus Bonelli, 1810, but similar in the morphology and probably related to Caucasoharpalus subg. n. Calathoderus subg. n. shares with the latter subgenus almost all its distinctive characters listed in the diagnosis, differing mainly in the larger and less convex body, the longer legs, the absence of prominent humeral denticle on elytra and in the characteristic male genitalia.
Calathoderus subg. n. corresponds to the
potanini species group sensu Kataev and Liang [
112] and sensu Kataev [
8,
109].
Subgenus Licinoderus Sainte-Claire Deville, 1905
Licinoderus Sainte-Claire Deville, 1905 [
157] (p. 114) (as a genus). Type species
Licinoderus chobauti Sainte-Claire Deville, 1905, by monotypy.
Neoharpalus Mateu, 1954 [
158] (p. 4) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus franzi Mateu, 1954, by monotypy.
Baeticoharpalus Serrano et Lecina, 2009 [
159] (p. 194) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus lopezi Serrano et Lecina, 2009, by original designation.
Diagnosis. Medium-sized (length 8.0–10.4 mm). Body slightly elongate, brown to black, with or without metallic luster on dorsum. Head impunctate and glabrous, or with fine punctures and very sparse setae dorsally; tempora setose or glabrous. Pronotal basal edge glabrous or setose. Elytra punctate and pubescent, with setose basal border and distinct humeral denticle; preapical sinuation shallow, without denticle at its base; interval 7 and 5 with or without preapical pores. Metepisternum short and wide, wider than long. Abdominal sternites with additional long setae. Internal sac of aedeagus with one or two large spines, one or two groups of small spines medially and generally a spiny patch apically.
Composition and distribution. This subgenus includes three wingless species from the Iberian Peninsula:
H. chobautianus Lutshnik, 1922 (
Figure 35a,b), endemic to the Pyrenees,
H. franzi Mateu, 1954 (
Figure 34b and
Figure 35c,d), endemic to the Cantabrian Mountains, and
H. lopezi Serrano et Lecina, 2009, endemic to the Baetic Mountains.
Ecology. All three species occur in open mountain habitats.
Remarks. The species of Licinoderus well differs from other members of this subgroup in having punctate and pubescent elytra combined with setose basal border and abdominal sternites with additional long setae.
Licinoderus is usually considered as a separate genus (e.g., [
43,
160]), although it has all the features of
Harpalus and should be included in this genus [
99]. Jeanne [
160] synonymized
Neoharpalus and
Licinoderus. The synonymy of
Baeticoharpalus and
Licinoderus was stated by Kataev [
109].
This subgenus corresponds to the
chobautianus species group sensu Kataev [
8,
109].
Subgenus Amblystus Motchulsky, 1864
Amblystus Motchulsky, 1864 [
61] (p. 209) (as a genus). Type species
Carabus rubripes Duftschmidt, 1812, by original designation.
Harpaloderus Reitter, 1900 [
38] (pp. 76, 100) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus sulphuripes Germar, 1824, designated by Habu [
29].
Diagnosis. Medium-sized (length 6.4–11.9 mm). Body moderately wide or elongate, dark brown to black, often with metallic luster on dorsum. Head impunctate and glabrous. Pronotal basal edge setose. Elytra impunctate and glabrous, with a more or less prominent humeral denticle; basal border generally glabrous, in some species setose; intervals 7 and 5 often with preapical pores. Metepisternum either elongate, longer than wide, or short and wide, wider than long. Abdominal sternites in most species with long additional setae, in some species glabrous. Internal sac of aedeagus with two more or less large separate spines, one or two groups of small spines medially and a spiny patch apically.
Composition and distribution. This subgenus comprises 17 winged and wingless species, distributed mainly in the western Palaearctic, with two centers of species diversity—one, larger, in the Mediterranean region, mainly in its western part, and the second, much smaller, in the western part of the Himalaya region; one species,
H. rubripes (Duftschmid, 1812) (
Figure 35g,h), has a trans-Palaearctic distribution.
The Mediterranean center of diversity includes 12 species, both winged and wingless:
H. rufipalpis Sturm, 1818 (
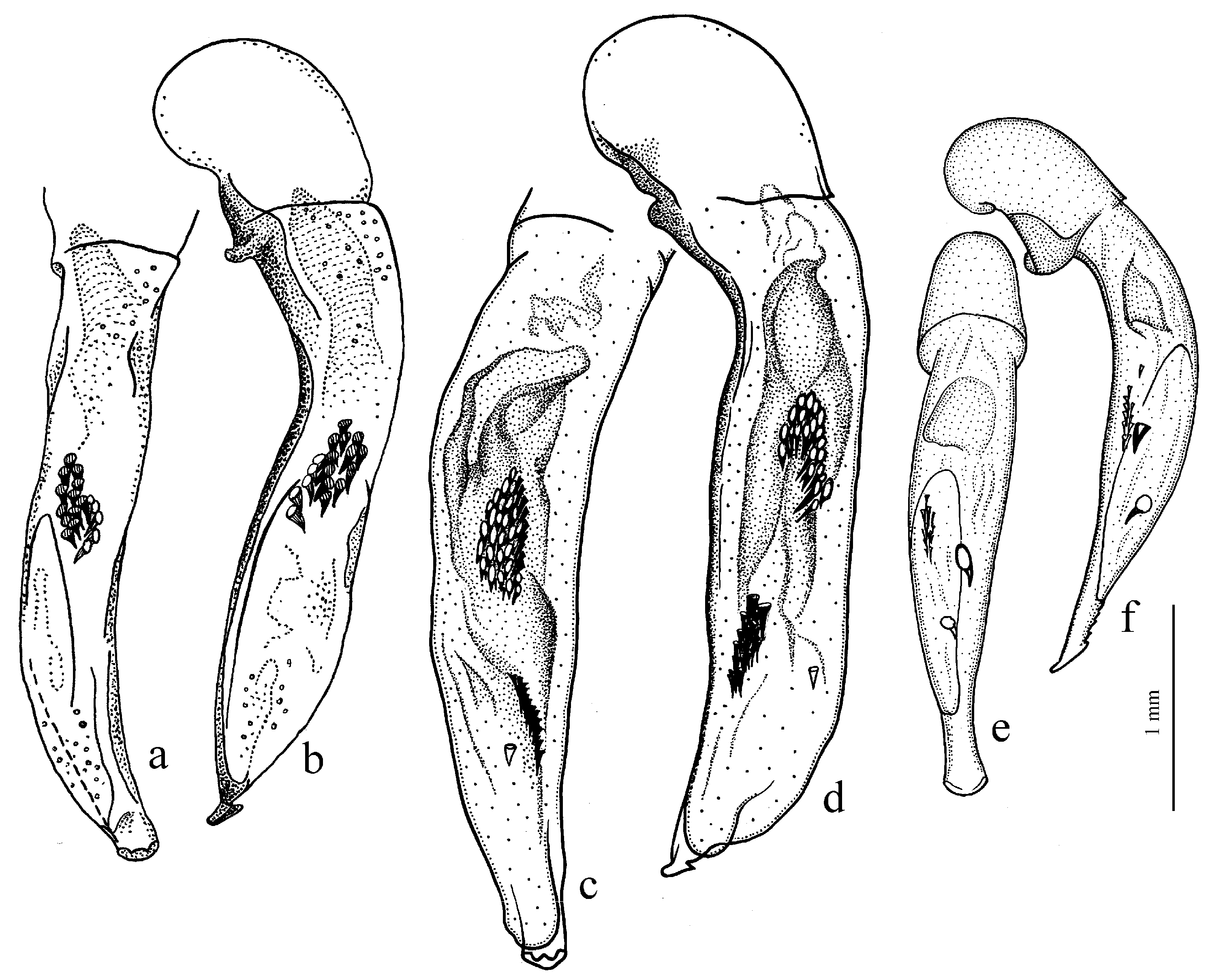
Figure 11c,d) (with the subspecies
H. r. montanellus Mateu, 1953,
H. r. machadoi Jeanne, 1970, and
H. r. lusitanicus Schatzmayr, 1943),
H. wagneri Schauberger, 1926,
H. nevadensis K. et J. Daniel, 1898,
H. rufitarsoides Schauberger, 1934,
H. dissitus Antoine, 1931,
H. wohlberedti Emden et Schauberger, 1932 (
Figure 36a),
H. bellieri Reiche, 1861,
H. decipiens Dejean, 1829 (with the subspecies
H. d. correiroi Schatzmayr, 1943 and
H. d. latianus Schauberger, 1923),
H. honestus (Duftschmid, 1812) (with the subspecies
H. h. creticus Maran, 1934),
H. sulphuripes Germar, 1824 (
Figure 35e,f) (with the subspecies
H. s. goudotii Dejean, 1829),
H. neglectus Audinet-Serville, 1821 (with the subspecies
H. n. mayeti Verdier, Quezel et Rioux, 1951 and
H. n. alluaudi Antoine, 1922) and
H. attenuatus Stephens, 1828.
The Himalayan center of diversity comprises four apterous species: H. indicola Bates, 1878 (with the subspecies H. i. uriensis Schauberger, 1933, H. i. kashmirensis Bates, 1889, H. i. kirschenhoferi Kataev, 2002 and H. i. shogranensis Kataev, 2002), H. calciatii Della Beffa, 1931, H. hartmanni Kataev, 2002 and H. morvani Kataev, 2002.
Ecology. The species of this subgenus inhabit predominantly the mountains, more rarely the lowlands, occurring in various open biotopes. Harpalus neglectus is a psammophilous species, occurring usually in dunes near the coast.
Remarks. The species of Amblystus are readily distinguished from other members of this subgroup in having setose pronotal basal edge combined with impunctate and glabrous elytra.
The species of this subgenus, especially the western Mediterranean taxa, require revision, so the exact status of some forms is not yet entirely clear.
This subgenus corresponds to the
Harpalus rufitarsis (=
H. rufipalpis) species group (=
Harpaloderus) sensu Schauberger [
64,
161,
162], who published the revision of the Mediterranean and European species of this subgenus known in that time. The Himalayan species (as the
honestus group) were revised by Kataev [
98].
Cordoharpalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. A monobasic subgroup, including only one Nearctic subgenus.
Subgenus Cordoharpalus Hatch, 1949
Cordoharpalus Hatch, 1949 [
74] (p. 87) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus cordifer Notman, 1919, by original designation.
Diagnosis. Medium-sized (length 7.5–9.0 [9.8] mm). Head micropunctate dorsally, glabrous. Body moderately convex, somewhat elongate, dark brown to black, without metallic luster. Pronotum with sides sinuate basally, with one lateral seta on each side and with glabrous, not setose, basal edge; surface coarsely punctate along base. Elytra impunctate and glabrous, without humeral denticle, with glabrous basal border; interval 3 without discal setigerous pore; intervals 7 and 5 without preapical pores. Metacoxa without additional setae medially. Metafemur with two or three setigerous pores along posterior margin. Protibia with three ventroapical spines and with three (more rarely four) preapical spines on outer margin, isolated from spines on ventral surface of tibia; ventroapical tubercle in male not prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally. Abdominal sternites glabrous; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with moderately long terminal lamella and distinct horseshoe-shaped apical capitulum; internal sac with two medium-sized separate spines and a group of narrow, medium-sized spines.
Composition and distribution. The only representative of this subgenus,
H. cordifer Notman, 1919 (
Figure 36b), is distributed along the northwestern coast of North America from Alaska to Oregon.
Ecology. This species inhabits mainly forests and their glades, sometimes thickets or shrubs in fields, occurring in shaded or open localities, with moderately dry soil covered with dense leaf litter and humus [
140].
Remarks. In combination of characters, the single subgenus of this subgroup is similar to the subgenera of the Hyloharpalus and Amblystus subgroups but differs from them in having three ventroapical spines on protibia. It is also distinguished from most of the members of the Hyloharpalus subgroup by having two separate spines in the internal sac of aedeagus.
The subgenus
Cordoharpalus corresponds to the monobasic
cordifer species group sensu Lindroth [
15] and sensu Kataev [
8]. Noonan [
16] also included in it
H. tadorcus Ball, 1972 (=
H. cordatus), the type species of the subgenus
Opadius, without a mentioning of the name of this subgenus in his revision. The relationships of these taxa were discussed by Kataev [
106].
Actephilus Subgroup
Diagnosis. Body more or less convex and wide; legs relatively short. Head impunctate and glabrous. Pronotum generally impunctate, with sides more or less rounded, not sinuate basally, with one lateral seta on each side and with setose or glabrous basal edge. Elytra impunctate and glabrous, with glabrous basal border and with or without humeral denticle; interval 3 with one to three discal setigerous pores or without pore; intervals 7 and 5 generally without preapical pores (such pores occasionally present in H. pseudoserripes). Metacoxa with or without additional setae medially. Metafemur with four to ten setigerous pores along posterior margin. Protibia with one ventroapical spine and with three to seven preapical spines on outer margin either isolated from spines on ventral surface of tibia or arranged with them in a single row; ventroapical tubercle in male not developed. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous ventrally. Abdominal sternites glabrous, more rarely with additional scattered long setae; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with somewhat short or moderately long terminal lamella and with a distinct horseshoe-shaped apical capitulum; internal sac usually with one or two large separate spines, also one to three groups of medium-sized spines and several spiny patches.
Composition and distribution. This subgroup comprises two Palaearctic subgenera.
Ecology. The members of this subgroup are confined to open habitats and most numerous in the steppe zone. They occur on dry, usually sandy or gravelly soil with sparse vegetation.
Remarks. The members of this subgroup are similar to those of the Hyloharpalus and Amblystus subgroups in the structure of the aedeagus but are generally characterized by a more or less convex and wide body with relatively short legs; pronotum in most species impunctate. The legs of some species are adapted for burrowing into the soil.
Subgenus Actephilus Stephens, 1833
Actephilus Stephens, 1833 [
163] (column 11) (as a genus). Type species
Carabus vernalis Paykull sensu Duftschmid, 1812 (=
Harpalus pumilus Sturm, 1818), designated by Westwood [
164].
Actophilus Agassiz, 1846 [
165] (pp. 6, 7) (unjustified emendation).
Euxenus Gistel, 1856 [
166] (p. 359) (as a genus). Type species
Carabus vernalis Paykull sensu Duftschmid, 1812 (=
Harpalus pumilus Sturm, 1818), by monotypy.
Diagnosis. Small-sized (length 3.9–6.6 mm). Body somewhat stout, brown to black, without metallic luster on dorsum. Pronotal basal edge setose. Elytra in most species without parascutellar pore, rarely (in H. masoreoides) such pore present; interval 3 either without discal setigerous pores or with one to three pores; humeral denticle present or absent. Metacoxa either without additional setigerous pores medially or with one or two such pores. Terminal lamella of aedeagus longer than wide.
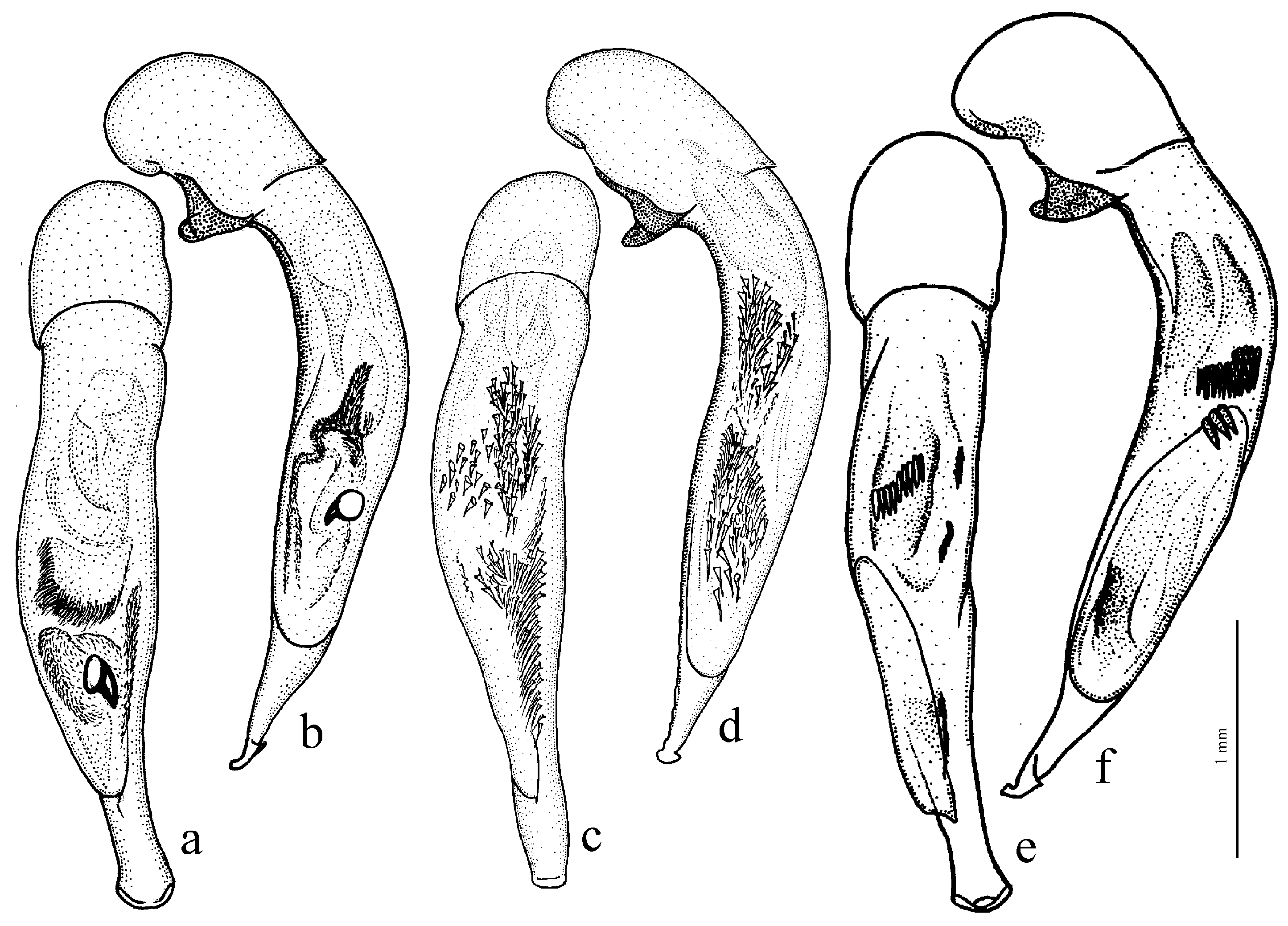
Composition and distribution. This group includes eleven species distributed mainly over the moderately arid areas of Eurasia from the Pyrenees in the west to the northern part of the Korean Peninsula in the east, with most species concentrated in southern Siberia, Mongolia and China:
H. picipennis (Duftschmid, 1812),
H. pumilus Sturm, 1818 (
Figure 37a and
Figure 38a,b),
H. lutshniki Schauberger, 1932,
H. masoreoides Bates, 1878,
H. pusillus (Motschulsky, 1850),
H. acupalpoides Reitter, 1900,
H. michaili Kataev, 1990,
H. longipalmatus Mordkovitsh, 1969,
H. minutulus Kataev et Liang, 2004,
H. alexandrae Kataev, 1990 and
H. sushenicus Kataev, 1990.
Ecology. Species of this group prefer dry open habitats, mostly with sandy soil.
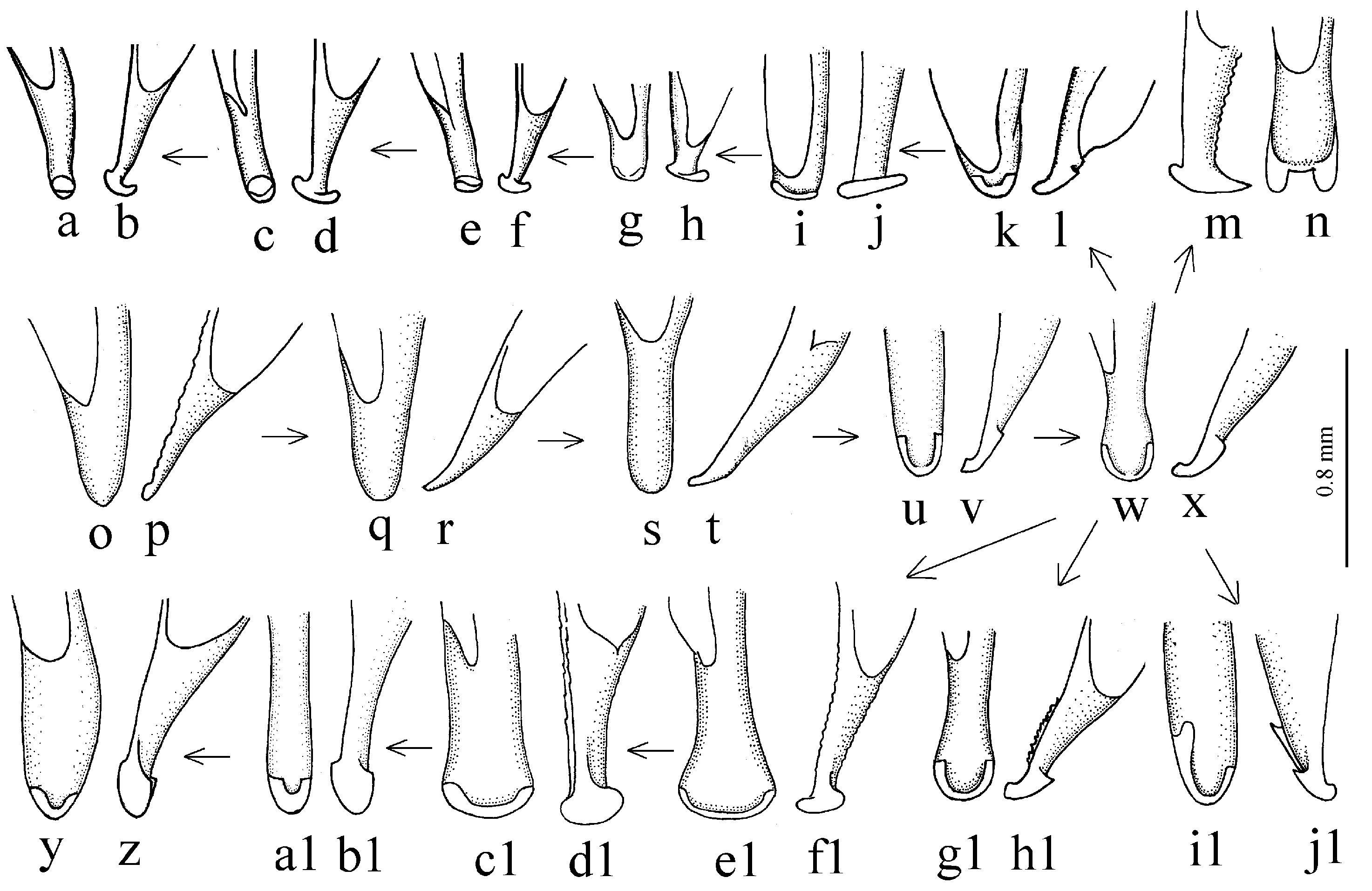
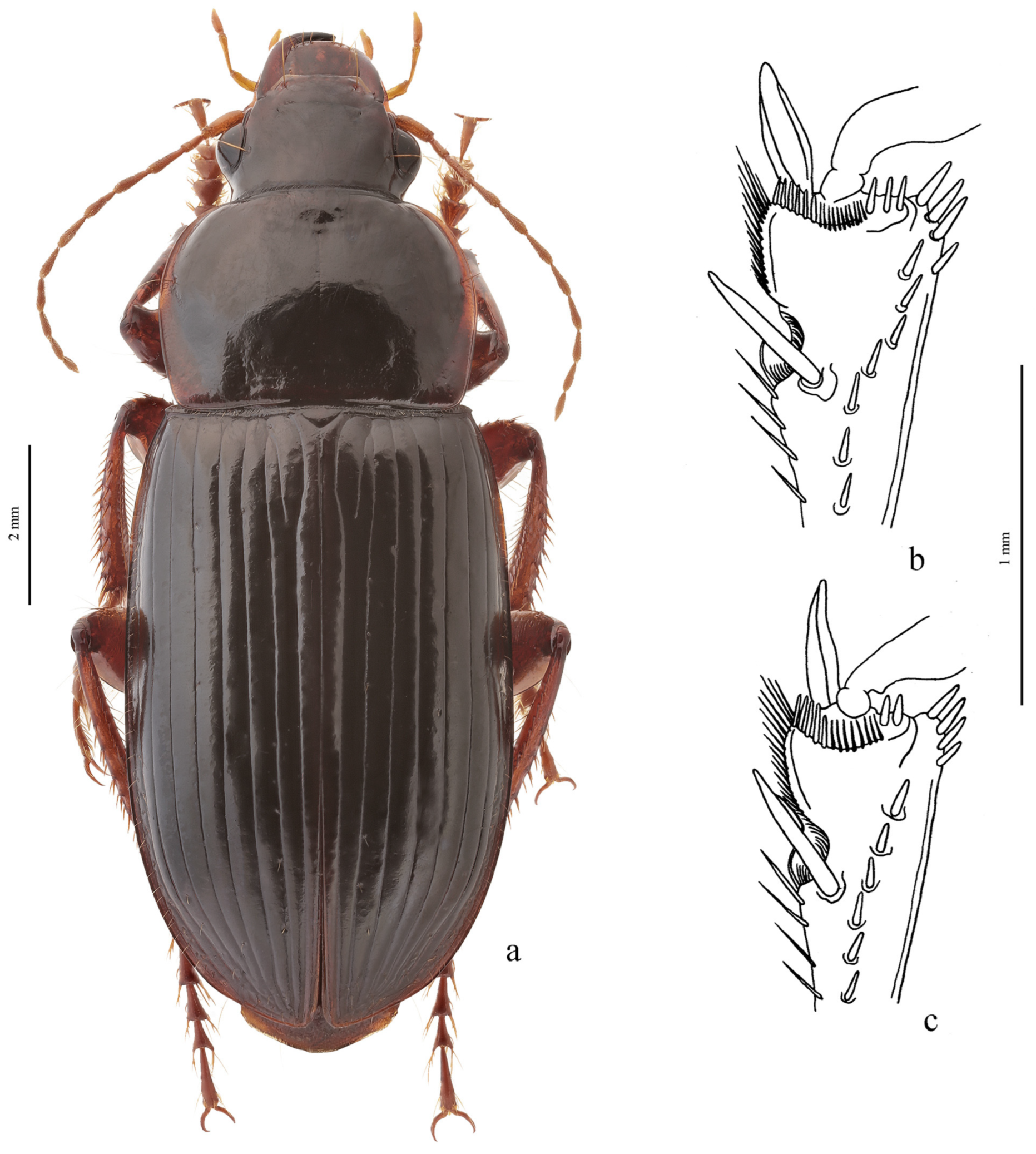
Remarks. In shaping the easily recognizable appearance of members of this subgenus, morphological adaptations played a significant role, facilitating the digging of beetles in light, mostly sandy soil (small size, compact body shape, widely rounded basal angles of the pronotum, shortened antennae and flattened protibiae—
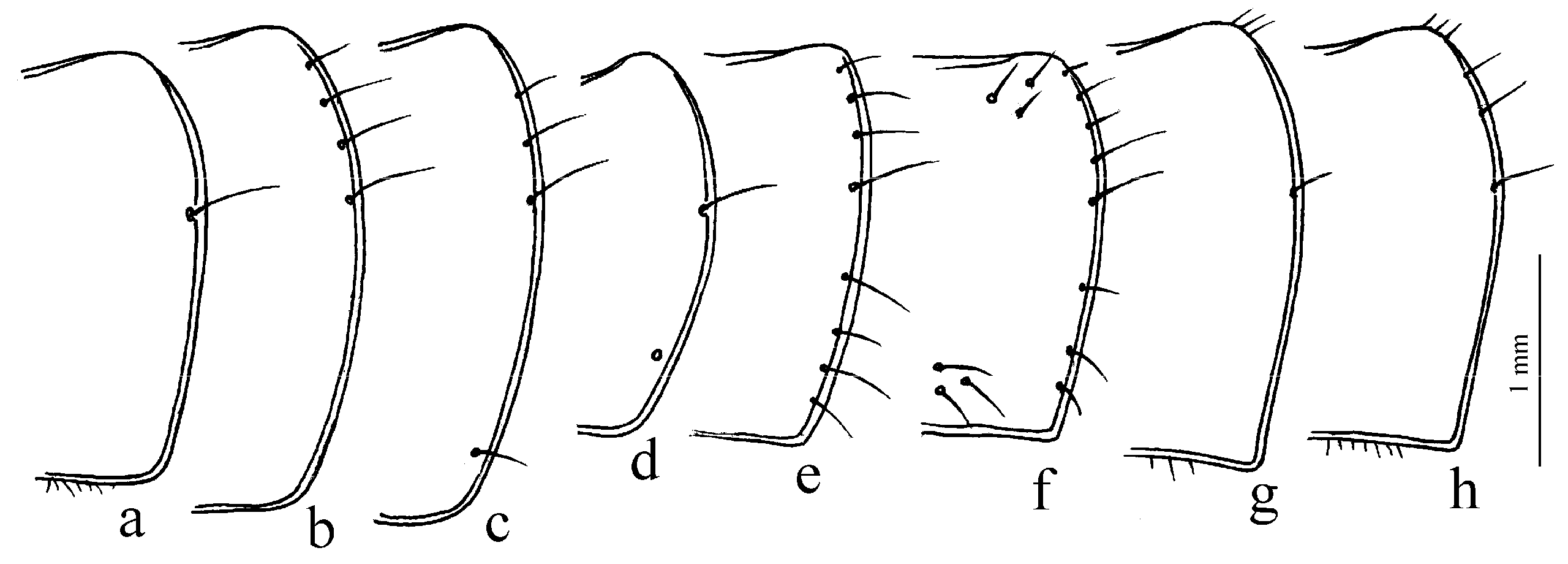
Figure 9g–l). The absence of discal and parascutelar pores on the elytra in many species may also be associated with a burrowing mode of life.
The species of this subgenus were revised by Schauberger [
68], and Kataev [
89] (as the
pumilus species group), with a subsequent addition [
111].
Subgenus Isoharpalus subg. n.
Type species Carabus serripes Quensel, 1806.
Diagnosis. Medium-sized to comparatively large (length 6.6–12.2 mm). Body stout, dark brown to black, without metallic luster or with weak bluish metallic tinge on dorsum. Pronotal basal edge setose or glabrous. Elytra with a basal (parascutellar) pore, one discal setigerous pore on interval 3 and generally with a humeral denticle. Terminal lamella of aedeagus somewhat short, at most slightly longer than wide.
Etymology. The subgeneric name is a combination of the Greek isos, meaning “equal”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes six Palaearctic species. Four of them are distributed mainly in the Thetyan region:
H. serripes Quensel, 1806 (
Figure 38c,d) (with the subspecies
H. s. ernsti Kataev, 1995),
H. pseudoserripes Reitter, 1900 (
Figure 37b),
H. politus Dejean, 1829 (with the subspecies
H. p. vasilinini Lutshnik, 1916) and
H. flavicornis Dejean, 1829 (
Figure 38e,f) (with the subspecies
H. f. tingens Reitter, 1900). The taxonomically more separated
H. vanemdeni Schauberger, 1932 and
H. beneshi Kataev et Wrase, 1997 are known from China. The true taxonomic position of these two species needs further study.
Ecology. Most members of this subgenus are moderately xerophilous species occurring mostly in dry meadow and steppe habitats.
Remarks.
Isoharpalus subg. n. corresponds to the
serripes species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8] together with the
beneshi species group sensu Kataev [
8]. In combination of characters, this subgenus is very similar to
Actephilus, differing from it mainly in larger body size, longer antennae, and a shorter terminal lamella of the aedeagus; elytra are with a parascutellar pore.
Acardystus Subgroup
Diagnosis. Body large- or medium-sized, more or less convex. Head impunctate and glabrous. Pronotum with one lateral seta on each side and with setose basal edge; surface usually impunctate, more rarely with fine punctures basally. Elytra impunctate and glabrous, with basal border glabrous or setose; humeral denticle generally prominent; interval 3 with one discal setigerous pore or without it; intervals 7 and 5 without preapical pores. Metacoxa with or without additional setae medially. Metafemur with four to twelve setigerous pores along posterior margin. Protibia notably widened and flattened apically, with one to three (rarely four) ventroapical spines and with five to six preapical spines on outer margin, forming a single row with spines on ventral surface of tibia or isolated from them; ventroapical tubercle in male absent or slightly prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally; length of metatarsomere 1 average for genus. Abdominal sternites generally with additional long setae; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with elongate terminal lamella and with a horseshoe-shaped apical capitulum; internal sac with one or two more or less large separate spines (absent in few species) and with two to three spiny patches.
Composition and distribution. This subgroup comprises one Nearctic and two Palaearctic subgenera.
Ecology. Most species of this subgroup occur in dry open habitats and are most common in the steppe and forest-steppe zones.
Remarks. Most members of this subgroup are characterized by a development of adaptations for burrowing, of which the most noticeable are characteristic changes in the structure of the protibia.
Subgenus Euharpalops Casey, 1924
Euharpalops Casey, 1924 [
72] (p. 116) (as a genus). Type species
Euharpalops wadei Casey 1924 (=
H. fraternus LeConte, 1852), by original designation.
Euharpalus: Hatch, 1953 [
167] (p. 170) (print error).
Diagnosis. Large-sized (length 10.0–14.5 [15.0] mm). Body stout, wide, dark brown to black, without metallic luster. Elytral basal border very finely setose (setae very short and sometimes barely visible); interval 3 with one discal setigerous pore (occasionally without pore). Protibia with generally three, rarely four ventroapical spines, and with five to six preapical spines on outer margin, isolated from spines on ventral surface of tibia. Internal sac of aedeagus with one large separate spine. Gonocoxite in female moderately wide and notably curved.
Composition and distribution. This group includes five Nearctic species:
H. fraternus LeConte, 1852 (
Figure 39a),
H. lecontei Casey, 1914,
H. reversus Casey, 1924,
H. lewisii LeConte, 1865 and
H. alienus Bates, 1878.
Ecology. The members of this group are most common in dry open habitats, usually on sandy soil covered with sparse vegetation.
Harpalus lewisii occurs also in open forests, subalpine and alpine meadows [
140].
Remarks. This subgenus is similar to Haploharpalus in many characters including stout body, setose basal elytral border and shape of gonocoxite but differs in less-specialized protibia with generally three ventroapical spines and with preapical spines on outer margin isolated from spines on ventral surface of tibia.
Lindroth [
15] included species of this subgenus in the
fraternus species group together with some Nearctic species of the subgenera
Hyloharpalus subg. n. and
Macroharpalus subg. n., based exclusively on the structures of the internal sac of the aedeagus. However, similar type of the armament of the internal sac is distributed among the Palaearctic species more widely and occurs with some modifications in many species of the
Hyloharpalus,
Amblystus,
Actephilus,
Acardystus and
Ooistus subgroups. Noonan [
16] considered
H. fraternus and
H. funerarius Csiki, 1932 (=
H. reversus) as members of the
fraternus subgroup (=
Euharpalops) of the
fraternus group and regarded
H. lewisi as a member of the related, monobasic
lewisi group.
The subgenus
Euharpalops, as it is treated here, corresponds to the
fraternus species group sensu Kataev [
8].
Subgenus Haploharpalus Schauberger, 1926
Haploharpalus Schauberger, 1926 [
64] (pp. 44, 45) (as a subgenus of
Acardystus Reitter, 1908). Type species
Harpalus froelichi Sturm 1818, designated by Habu [
29].
Diagnosis. Medium-sized to large (length 7.5–15.0 mm). Body stout, wide, dark brown to black, without metallic luster. Elytral basal border generally setose, rarely (in H. froelichi) glabrous; interval 3 with one discal setigerous pore. Protibia with one or two, very rarely three, ventroapical spines, and with at least five preapical spines on outer margin, forming in most species a single row with spines on ventral surface of tibia (in some specimens of H. melaneus spines of outer margin isolated from spines on lower surface). Internal sac of aedeagus with one or two separate spines, rarely without spines. Gonocoxite in female somewhat wide and notably curved.
Composition and distribution. This group comprises twelve Palaearctic species, most of which are widely distributed over steppe and forest-steppe zones of Eurasia, with the center of species diversity in south Siberia and Mongolia:
H. froelichii Sturm, 1818 (
Figure 40a,b),
H. raphaili Kataev, 1997,
H. hirtipes (Panzer, 1796),
H. zabroides Dejean, 1829,
H. tichonis Jacobson, 1907,
H. macronotus Tschitschérine, 1893 (
Figure 39b),
H. brevis Motschulsky, 1844,
H. brevicornis Germar, 1824 (
Figure 39c,d),
H. corporosus (Motschulsky, 1861),
H. alpivagus Tschitschérine, 1899,
H. alajensis Tschitschérine, 1898, and
H. melaneus Bates, 1878 (with the subspecies
H. m. sherpicus Kataev, 2002 and and
H. m. stoetzneri Schauberger, 1933).
Ecology. All species of this group occur in rather dry open habitats and are most common in steppe biotopes.
Remarks. The protibiae of the species of this subgenus demonstrate different degrees of development of adaptive changes for burrowing: from less-specialized, weakly widened apically in
H. melaneus to very specialized, strongly widened apically and with a lobed outer angle in
H. hirtipes (
Figure 9a–f).
The species of this subgenus were revised by Schauberger [
64,
143,
161] and Kataev [
54] (as the
hirtipes species group).
Subgenus Acardystus Reitter, 1908
Acardystus Reitter, 1908 [
39] (pp. 172, 173) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus rufus Brüggemann, 1873 (=
Carabus flavescens Piller et Mitterpacher, 1783), designated by Schauberger [
64].
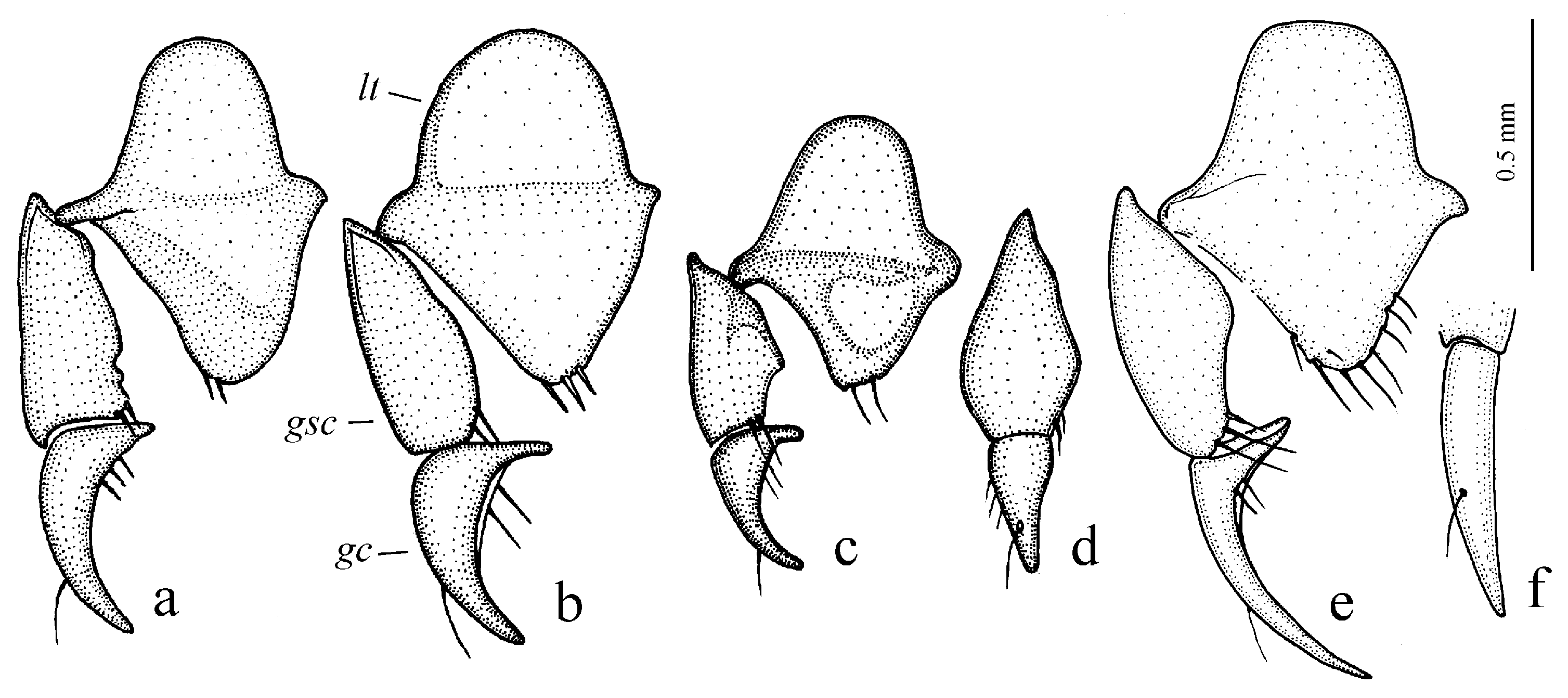
Diagnosis. Comparatively large-sized (length 9.5–12.7 mm). Body slightly elongate, yellow to light brown, without metallic luster on dorsum. Elytral basal border glabrous; interval 3 generally without discal setigerous pore (occasionally with one pore). Protibia with one ventroapical spine, and with several preapical spines on outer margin, forming a single row with spines on ventral surface of tibia (
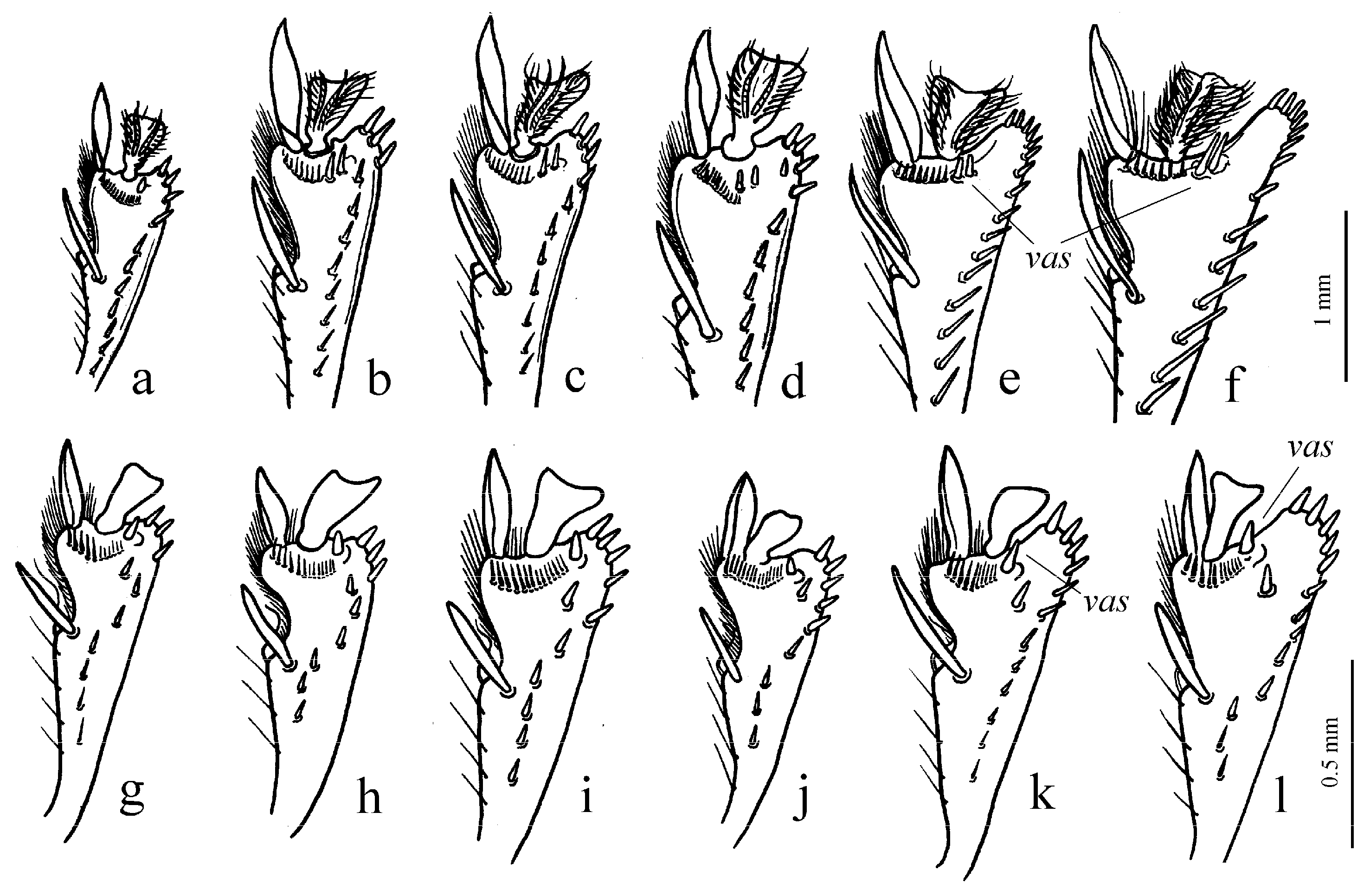
Figure 10b). Internal sac of the aedeagus without large separate spines, only with spiny patches (
Figure 40e,f). Gonocoxite in female long, narrow and weakly curved.
Composition and distribution. This subgenus includes only one species,
H. flavescens (Piller et Mitterpacher, 1783) (
Figure 40e,f and
Figure 41a), which is widely distributed over Europe, east to the Ural Mountain Range and west Kazakhstan.
Ecology. The only member of this subgenus is a specialized psammophilous species that occurs on sandy river banks and sea dunes. The species lives in pure sand, in which it burrows deeply.
Remarks. The single species of Acardystus differs from the members of two preceding subgenera in a combination of elongate light brown body, glabrous basal elytral border and shape of gonocoxite in female (long, narrow and weakly curved).
This subgenus corresponds to the
flavescens species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8].
Psammoharpalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. A monobasic subgroup, including only one eastern Palaearctic subgenus.
Subgenus Psammoharpalus subg. n.
Type species Harpalus kozlovi Kataev, 1993.
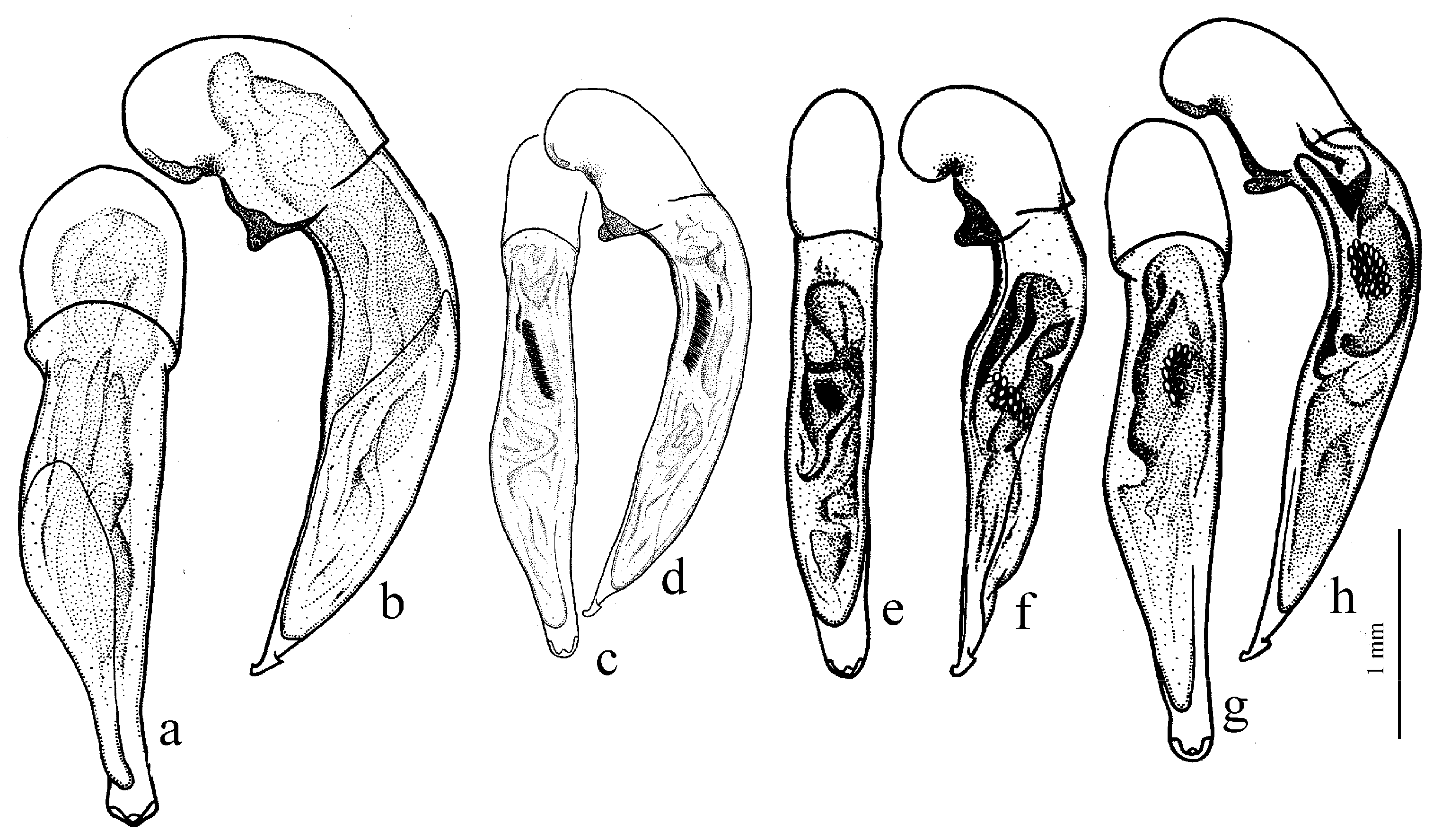
Diagnosis. Medium-sized (length 5.6–6.9 mm). Body convex, elongate, yellow to light brown, without metallic luster. Head impunctate and glabrous. Pronotum densely punctate basally, with one lateral seta on each side and with setose basal edge. Elytra impunctate and glabrous, with glabrous basal border and one discal setigerous pore on interval 3; intervals 7 and 5 without preapical pores; humeral denticle present, prominent. Metacoxa with one or several additional setae medially. Metafemur with seven to ten setigerous pores along posterior margin. Protibia with one ventroapical spine (longer than that of most other congeneres) and with three to five preapical spines on outer margin, generally isolated from spines on ventral surface of tibia (occasionally spines on outer margin passing on lower surface and forming a single row with spines of lower surface); ventroapical tubercle in male not developed. Male mesotibia without preapical callous thickening on inner margin. Abdominal sternites with additional long setae; last visible abdominal sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. In male aedeagus with elongate terminal lamella and with disc-shaped apical capitulum; internal sac with two separate spines and three spiny patches (
Figure 40g,h). Gonocoxite in female somewhat wide and notably curved.
Etymology. The subgeneric name is a combination of the Greek psámmos, meaning “sand”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only
H. kozlovi Kataev, 1993 from Qinghai, China (
Figure 40g,h and
Figure 41b).
Ecology. The single species is found on sandy river banks.
Remarks. Like H. (Acardystus) flavescens, the only species of this subgenus is also adapted for burrowing in river sands, but it is less specialized, with less modified protibiae and shorter and wider gonocoxites. Harpalus kozlovi is morphologically separated from other taxa, but its relationship with any of them has not yet been clarified; therefore, it is included in a separate subgenus and, accordingly, in a separate subgroup.
The subgenus
Psammoharpalus subg. n. corresponds to the
kozlovi species group sensu Kataev [
8].
Ooistus Subgroup
Diagnosis. Body medium-sized, flattened. Head impunctate and glabrous. Antennae either unicolorous, pale, or bicolorous, dark brown to black, with pale antennomeres 1–2. Pronotum generally impunctate, rarely vaguely punctate latero-basally, with sides rounded to slightly sinuate basally, with one lateral seta on each side and with basal edge glabrous, very rarely (occasionally in some species) with very short, poorly recognizable setae. Elytra impunctate and glabrous, with glabrous basal border; interval 3 generally with one discal pore, rarely without it; intervals 7 and 5 without or (more rarely) with preapical pores; humeral denticle present, more or less prominent. Metepisternum elongate or as wide as long, markedly narrowed basally. Metacoxae with or without additional setae medially. Metafemur with 3–12 setae along posterior margin. Protibia with one ventroapical spine and with three to four preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male not developed, rarely slightly prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally; length of metatarsomere 1 average for genus. Abdominal sternites usually without additional setae, very rarely with very short, poorly recognizable setae at base of sternites; last visible abdominal sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with elongate terminal lamella and pronounced horseshoe-shaped apical capitulum; internal sac with one or two separate spines (these spines occasionally absent), with several spiny patches and with or without a group of medium-sized spines.
Composition and distribution. This subgroup comprises one Palaearctic and one Nearctic subgenus.
Ecology. Members of this subgroup occur in open, moderately dry habitats with sparse vegetation, most commonly in steppes and prairies.
Subgenus Ooistus Motschulsky, 1864
Ooistus Motschulsky, 1864 [
61] (p. 209 (as a genus). Type species
Harpalus taciturnus Dejean, 1829, designated by Noonan [
6].
Diagnosis. Length of body 5.8–10.8 mm. Body slightly elongate or wide, dark brown to black, without or with weak metallic luster on dorsum. Pronotum generally impunctate even basally. Internal sac of aedeagus with one or two separate spines (occasionally absent in H. giacomazzoi) and with a few spiny patches, including usually two very peculiar, elongate patches in apical half of median lobe; groups of medium-sized spines absent.
Composition and distribution. This subgenus comprises 15 Palaearctic species, with the most species diversity in the steppe and forest-steppe zones:
H. anxius (Duftschmid, 1812),
H. angustitarsis Reitter, 1887,
H. anxioides Kataev, 1991,
H. convexus Faldermann, 1835,
H. kirgisicus Motschulsky, 1844,
H. amarellus Bates, 1891,
H. subcylindricus Dejean, 1829,
H. servus (Duftschmid, 1812) (
Figure 42a and
Figure 43c,d),
H. amplicollis Ménétriés, 1848 (
Figure 43e,f),
H. calathoides Motschulsky, 1844,
H. taciturnus Dejean, 1829 (
Figure 43a,b),
H. pulchrinulus Reitter, 1900,
H. amariformis Motschulsky, 1844,
H. egorovi Lafer, 1989 and
H. giacomazzoi Kataev et Wrase, 1996 (with the subspecies
H. g. gracilis Kataev et Liang, 2007).
Ecology. The members of this subgenus occur in open, moderately dry habitats, often on sandy soil with sparse vegetation, and are most common in steppes. Harpalus egorovi occurs in dry open biotopes within forests and along river banks.
Remarks. In addition to external morphology, this subgenus is well defined by the structure of the aedeagus with characteristic armament of the internal sac.
The species of this subgenus (as the
anxius species group) were revised by Kataev [
54], with subsequent addition [
112]. The taxonomy of some species was heretofore discussed by Mlynář [
53].
Subgenus Platyharpalus subg. n.
Type species Harpalus ventralis LeConte, 1848.
Diagnosis. Length of body [7.2] 7.5–8.2 [10.2] mm. Body moderately wide, dark brown to black, without metallic luster. Pronotum impunctate or vaguely punctate latero-basally. Internal sac of aedeagus with one or two separate spines and with a group of medium-sized spines in middle portion of median lobe in addition to spiny patches in apical portion of median lobe.
Etymology. The subgeneric name is a combination of the Greek platýs, meaning “flat”, and the name of the carabid taxon Harpalus.
Composition and distribution. The subgenus includes two Nearctic species:
H. ventralis LeConte, 1848 (
Figure 42b) and
H. indigens Casey, 1924.
Ecology. Both species occur in rather dry, open habitats with sandy or silty soil and sparse vegetation: grasslands, prairies, meadows, pastures, cultivated fields, along roadsides, sometimes in open forests [
140].
Remarks. This Nearctic subgenus is considered to be related to the Palaearctic subgenus Ooistus based on their external similarities, but its true relationship requires further study as the similarities may be convergent. These subgenera differ in the male genitalia, mainly in the armament of the internal sac of the aedeagus.
Platyharpalus subg. n. corresponds to the
ventralis species group sensu Lindroth [
15] and sensu Kataev [
8] and to the
ventralis subgroup of the
fraternus group sensu Noonan [
16].
Asioharpalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. A monobasic subgroup, including one eastern Palaearctic subgenus.
Subgenus Asioharpalus subg. n.
Type species Harpalus nigrans Morawitz, 1862.
Diagnosis. Medium-sized (length 6.2–8.7 mm). Body moderately convex, moderately wide or slightly elongate, brown to black, without metallic luster. Head impunctate and glabrous. Antennae more or less unicolorous, pale or infuscate. Pronotum punctate or impunctate basally, with one lateral seta on each side and with glabrous basal edge. Elytra impunctate and glabrous, with glabrous basal border and one discal setigerous pore on interval 3; intervals 7 and 5 without preapical pores; humeral denticle present, small. Metacoxa with additional setae medially. Metafemur with three to five setae along posterior margin. Protibia with one ventroapical spine and with three to four preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male not developed. Male mesotibia without preapical callous thickening on inner margin. Abdominal sternites glabrous; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with elongate terminal lamella and pronounced horseshoe-shaped apical capitulum; internal sac with one separate spine and one or two narrow spiny patches in apical half of median lobe.
Etymology. The subgeneric name is based on a combination of Asia and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises three eastern Palaearctic species:
H. nigrans Morawitz, 1862 (
Figure 44a and
Figure 45a,b),
H. sinuatus Tschitschérine, 1893 and
H. parasinuatus Kataev et Liang, 2007.
Ecology. The species of this group occur in dry, open habitats, mainly within or near forests.
Remarks.
Asioharpalus subg. n. corresponds to the
nigrans species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8] and to the
sinuatus species group sensu Kataev and Liang [
112]. The taxonomic position of this subgenus is still unclear. Although it is somewhat similar morphologically to the subgenus
Diaharpalus subg. n. of the
Harpalobius subgroup [
112],
Asioharpalus subg. n. is treated here as a member of a separate subgroup. The species of
Asioharpalus subg. n. distinctly differ from those of the
Harpalobius subgroup in having the antennae reddish brown, only weakly infuscate on antennomeres 2–7, the protibia of male without prominent ventroapical tubercle, and the aedeagus with different pattern of spiny patches in the internal sac. In structure of aedeagus,
Asioharpalus subg. n. is more similar to the subgenera of the
Actephilus,
Acardystus and
Ooistus subgroups.
Anamblystus Subgroup
Diagnosis. Body dark brown to black, without metallic luster. Head impunctate and glabrous. Antennae more or less unicolorous, pale or infuscate. Pronotum punctate or impunctate basally, with one lateral seta on each side; basal edge generally glabrous, rarely with very short, poorly recognizable setae. Elyra in most species impunctate and glabrous, rarely finely punctate and pubescent on lateral intervals, with glabrous basal border (occasionally setose in some species); interval 3 usually with one discal setigerous pore (occasionally without pore); intervals 7 and 5 in most species without preapical pores; basal (parascutellar) pore generally present (absent in H. albanicus). Metacoxa generally without additional setae medially, rarely with one or several such setae. Metafemur with three to eight setae along posterior margin. Protibia with one ventroapical spine and with three to six preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male not developed or very small. Male mesotibia without preapical callous thickening on inner margin. Abdominal sternites generally glabrous, in some species with very short, poorly recognizable setae along base of sternites, or with few longer setae; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with elongate terminal lamella and pronounced horseshoe-shaped, button-like or almost discoidal apical capitulum; internal sac with one or several spiny patches or groups of spines, without separate spines, rarely without any sclerotic elements.
Composition and distribution. This subgroup includes two subgenera: one Holarctic and one Palaearctic.
Ecology. This subgroup includes both species inhabiting forests and species occurring in dry, open habitats, including steppe ones.
Remarks. The members of this subgroup are characterized by the absence of pronounced sexual dimorphism in the pro- and mesotibia and abdominal sternite, the absence of separate spines in the internal sac of the aedeagus, and the absence of many distinctive features found in members of other subgroups (for example, punctation on head, setae on basal border of elytra and dorsal surface of tarsi, additional setae on pronotum, protibia specialized for burrowing, two and more ventroapical spines on protibia, etc.).
Subgenus Anamblystus subg. n.
Type species Carabus latus Linnaeus, 1758.
Diagnosis. Medium-sized (length 5.9–11.5 mm). Body more or less convex, slightly elongate. Pronotal base more or less densely punctate, rarely almost impunctate, in most species with glabrous basal edge (rarely, for example in H. rufiscapus and H. martini, basal edge setose). Elytra generally impunctate and glabrous (in H. torridoides finely punctate and pubescent laterally) and without preapical pores on intervals 7 and 5 (in H. marginellus with such pores); basal border generally glabrous (occasionally setose in some Nearctic species). Metacoxa without additional setae medially, rarely (for example in H. rufiscapus) with one or several such setae. Metafemur with three, sometimes four (in H. rufiscapus generally five, in H. martini up to twelve), setigerous pores along posterior margin. Protibia with three or four (in H. rufiscapus occasionally five, in H. martini up to six) preapical spines on outer margin. Abdominal sternites generally without additional moderately long setae (in H. martini with several such setae). Median lobe of aedeagus serrate on ventral side in many species.
Etymology. The subgeneric name is a combination of the Greek an-, meaning “not”, and the name of the carabid taxon Amblystus.
Composition and distribution. This subgenus includes about 22 species from Eurasia and North America. A more precise number of species included in this group cannot now be indicated, since the status of some American taxa requires a revision. Two species, H. solitaris Dejean, 1829 and H. nigritarsis C. Sahlberg, 1827 (with the subspecies H. n. proximus LeConte, 1848) have a Holarctic distribution.
According to my data, about 13 species are known only from North America: H. seclusus Casey, 1914, H. fanaticus Casey, 1924, H. atrichatus Hatch, 1949, H. herbivagus Say, 1823, H. pleuriticus Kirby, 1837, H. somnulentus Dejean, 1829, H. celox Casey, 1914, H. intactus Casey, 1914, H. fallax LeConte, 1859, H. carbonatus LeConte, 1860, H. uteanus Casey, 1914, H. martini Van Dyke, 1926 and H. aterrimus Casey, 1914.
The Palaearctic fauna includes the following species:
H. torridoides Reitter, 1900 (
Figure 43b),
H. latus (Linnaeus, 1758) (
Figure 45c,d),
H. ussuricus Mlynář, 1979,
H. marginellus Gyllenhal, 1827,
H. progrediens Schauberger, 1922,
H. luteicornis (Duftschmid, 1812),
H. xanthopus Gemminger et Harold, 1868 (with the subspecies
H. x. winkleri Schauberger, 1923) and the taxonomically more separated
H. rufiscapus Gebler, 1833.
Ecology. The species of this subgenus occur mainly in forests or near forests, less often in open landscapes, but, as a rule, in the forest zone; some are in the highlands; H. rufiscapus occurs in dry steppe habitats.
Remarks. Although the species of this subgenus show quite a wide variability in some distinctive characters, most of the included taxa form together a morphological continuum separated from species of other subgenera.
The taxonomic position of H. rufiscapus, distributed over the Eurasian steppe zone, needs further study since its morphological characteristics are somewhat intermediate between Anamblystus subg. n. and Homaloharpalus subg. n. It well differs from the most species of these subgenera also ecologically since it occurs in dry steppe habitats; the internal sac of its aedeagus is without any sclerotic elements.
The Nearctic H. martini, which possesses several characters unusual for the subgenus, as noted in the disanosis of the subgenus, is very similar to H. uteanus in other characters, including the structure of the aedeagus.
Anamblystus subg. n. corresponds to the Palaearctic
latus species group sensu Kryzhanovskij et al. [
56], the Nearctic
herbivagus species group sensu Lindroth [
15] and the
latus species group sensu Kataev [
8], including both Palaearctic and Nearctic species. Lindroth [
15] referred the Nearctic
herbivagus species group to the Palaearctic subgenus
Amblystus, but the type species of the latter subgenus,
H. rubripes, markedly differs both from the species of the
herbivagus group and from the Palaearctic species included originally together with
H. rubripes in the subgenus
Amblystus by Motschulsky [
61] and after him by Reitter [
38] and other researchers. In set of characters,
H. rubripes should be combined with the species related to
H. sulphuripes (the type species of of the subgenus
Harpaloderus). Therefore, the new subgenus
Anamblystus subg. n. is erected here for the
latus species group sensu Kataev [
8].
Noonan [
16] included most of the Nearctic species of
Anamblystus subg. n. in the
somnulentus species group, treating
H. somnulentus very widely, including
H. pleuriticus,
H. celox,
H. carbonarius and
H. uteanus as its synonyms. He considered
H. atrichatus as a member of the monobasic
atrichatus species group, based on the specific armament of the internal sac of the aedeagus, and
H. nigritarsis, which he also treated very widely, as a member of the
nigritarsis species group together with
H. megacephalus.
Subgenus Homaloharpalus subg. n.
Type species Carabus tardus Panzer, 1796.
Diagnosis. Medium-sized (length 5.4–10.5 mm). Body moderately convex, somewhat wide. Pronotal base generally impunctate, more rarely with obliterate punctures; basal edge not setose. Elytra impunctate and glabrous, without preapical pores on intervals 7 and 5; basal border glabrous. Metacoxa generally without additional setae, more rarely (in H. modestus) with one or two such setae medially. Metafemur with four (occasionally three) to seven setigerous pores along posterior margin. Protibia with five or six, very rarely three or four, preapical spines. Abdominal sternites without additional moderately long setae. Median lobe of aedeagus not serrate on ventral side.
Etymology. The subgeneric name is a combination of the Greek omalós, meaning “flat”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises eleven Palaearctic species, with most of them distributed in eastern Asia:
H. tardus (Panzer, 1796) (
Figure 45e,f),
H. tarsalis Mannerheim, 1825,
H. modestus Dejean, 1829,
H. bungii Chaudoir, 1844,
H. chasanensis Lafer, 1989,
H. tangutorum Kataev, 1993 (
Figure 46a),
H. praecurrens Schauberger, 1934,
H. stevensi Kataev, 2011,
H. vernicosus Kataev et Liang, 2007,
H. albanicus Reitter, 1900 and the taxonomically more separated
H. dudkoi Kataev, 2011.
Ecology. Species of this subgenus occur in various dry, open habitats, but usually near or within forests; they are most characteristic of the forest-steppe zone.
Remarks. This subgenus is very similar in morphology to the preceding one, differing mainly in the impunctate pronotal base in most species and usually in a more number of preapical spines on the outer margin of the protibia and in a more number of setae along the posterior margin of the metafemur, although the variability in each of these characters taken separately overlaps in the species of these two subgenera.
The subgenus
Homaloharpalus subg. n. corresponds to the
tardus species group sensu Kryzhanovskij et al. [
56], sensu Kataev [
8] and sensu Kataev and Liang [
112].
Harpalobius Subgroup
Diagnosis. Body brown to black, with or without green, blue or copper metallic luster on dorsum; elytra of some members bicolorous, with yellow pattern. Head impunctate and glabrous. Antennae generally bicolorous, with one or two basal antennomeres pale and other antennomeres distinctly infuscate, usually black. Pronotum with sides generally rounded, rarely slightly sinuate basally, with one lateral seta on each side and with basal edge generally glabrous, more rarely setose; surface generally impunctate, rarely with fine sparse or dense punctures basally. Elytra impunctate and glabrous, with glabrous basal border and with or without one discal setigerous pore on interval 3; intervals 7 and 5 without preapical pores; humeral denticle generally present, more or less prominent or very small, almost indistinct. Metacoxa with or without additional setae medially. Metafemur with four (rarely three) to fifteen setigerous pores along posterior margin. Protibia with one or two (very rarely three) ventroapical spines in transverse row and with three to five preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male more or less prominent. Male mesotibia without preapical callous thickening on inner margin or with small such thickening. Abdominal sternites glabrous or with additional long setae; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with elongate terminal lamella and promiment horseshoe-shaped apical capitulum; internal sac with one to three somewhat small spiny patches; separate spines absent.
Composition and distribution. This subgroup includes three Holarctic subgenera, each of which is distributed both in the Palaearctic and in the Nearctic.
Ecology. Most of the species of this subgroup live in open habitats with sandy or gravelly soil, both in the steppes and in the forest zone; many species occur in mountains.
Remarks. This subgroup is similar to the Anamblystus subgroup in many distinctive characters listed in their diagnoses but differs from it in having more or less prominent ventroapical tubercle in male protibia.
Subgenus Bactroharpalus subg. n.
Type species Harpalus cautus Dejean, 1829.
Diagnosis. Medium-sized (length 6.8–10.6 [11.0] mm). Body elongate or somewhat wide, without metallic luster and with unicolorous elytra. Pronotal basal edge glabrous, not setose. Humeral denticle prominent or almost indistinct. Elytral interval 3 with one discal pore. Metafemur with four (rarely three or five) setigerous pores along posterior margin. Protibia with generally two (very rarely one or three) ventroapical spines, and with four or five preapical spines on outer margin. Male mesotibia generally with a very small preapical callous thickening on inner margin. Abdominal sternites glabrous. Median lobe of aedeagus with rather short terminal lamella; internal sac in most species without sclerotic elements, in some species with one or two indistinct spiny patches (in H. ochropus with a distinct small spiny patch apically).
Etymology. The subgeneric name is a combination of the Greek baktron, meaning “stick”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes eight species. With the exception of
H. lederi Tschitschérine, 1899 (
Figure 47a,b), which is distributed in the northeast Palaearctic, all other species occur in North America:
H. cautus Dejean, 1829,
H. obnixus Casey, 1924 (
Figure 46b),
H. opacipennis (Haldeman, 1843),
H. plenalis Casey, 1914,
H. ellipsis LeConte, 1848,
H. balli Noonan, 1991 and
H. ochropus Kirby, 1837.
Ecology. Species of this subgenus occur in open habitats with sandy or gravelly soil.
Remarks. Within the Harpalobius subgroup, the members of Bactroharpalus subg. n. are recognizable by the combination of the characters listed in the diagnosis.
This subgenus corresponds to the Nearctic
opacipennis species group sensu Lindroth [
15], the Palaearctic
lederi species group sensu Kryzhanovskij et al. [
56] and the
cautus species group sensu Kataev [
8], including both Nearctic and Palaearctic species. Lindroth [
15] referred the Nearctic species of this subgenus (the
opacipennis species group) to the Palaearctic subgenus
Pheuginus, but the type species of the latter subgenus,
H. optabilis, differs markedly both from these Nearctic species and from the Palaearctic species originally included together with
H. optabilis in
Pheuginus by Motschulsky [
34] and after him by Reitter [
38] and other authors. Therefore, the new subgenus
Bactroharpalus subg. n. is erected here for species of the
cautus species group sensu Kataev [
8].
Noonan [
16] treated most species of
Bactroharpalus subg. n. (except for
H. obnixus,
H. plenalis and
H. opacipennis) together with the Nearctic species of the
Diaharpalus subg. n. as members of the
cautus species group. He included
H. obnixus and
H. plenalis in the
obnixus species group based on the absence of sclerotic armament in the internal sac of their aedeagi.
Harpalus opacipennis was considered by him as a member of the separate monobasic
opacipennis species group as showing no close cladistic relationships to other Nearctic species.
Subgenus Diaharpalus subg. n.
Type species Harpalus vittatus Gebler, 1833.
Diagnosis. Medium-sized (length 5.2–9.0 [9.7] mm). Body moderately convex, somewhat wide or slightly elongate, with or without metallic luster on dorsum; elytra of some members bicolorous, with yellow pattern. Pronotal basal edge generally glabrous, rarely setose. Humeral denticle generally more or less prominent. Elytral interval 3 with or without discal setigerous pore. Metafemur with four to six setigerous pores along posterior margin. Protibia with one ventroapical spine and with three to four (rarely five) preapical spines on outer margin. Male mesotibia without preapical callous thickening on inner margin. Abdominal sternites generally glabrous, rarely (in H. metarsius and occasionally in H. udege) with sparse additional somewhat long setae. Median lobe of aedeagus with short or moderately long terminal lamella and with one to three distinct small spiny patches (or groups of small spines) in internal sac medially.
Etymology. The subgeneric name is a combination of the Greek dia-, meaning “through, between”, and the name of the carabid taxon Harpalus.
Composition and distribution. The subgenus comprises 12 species from the eastern Palaearctic and the Nearctic regions. Among them,
H. vittatus Gebler, 1833 (
Figure 47c,d and
Figure 48a) (with the subspecies
H. v. kiselevi Kataev et Shilenkov, 1990 and
H. v. alaskensis Lindroth, 1968) are distributed both in Siberia and in North America (Alaska); most other (eight species) are known from Palaearctic Asia:
H. udege Lafer, 1989,
H. karakorum Jedlička, 1958,
H. metarsius Andrewes, 1930,
H. mlynari Kataev, 1990,
H. kaznakovi Kataev et Wrase, 1997 (with the subspecies
H. lilliputa Kataev et Liang, 2007),
H. alexeevi Kataev, 1990,
H. manas Kataev, 1990 and
H. plancyi Tschitschérine, 1897.
Three species are known from North America: H. innocuus LeConte, 1863, H. paululus Casey, 1914 and H. intactus Casey, 1924. The status of some American forms needs further study.
Ecology. Species of this subgenus occur in open, dry habitats with sandy or gravelly soil, both in the steppes and at the edge of the forests; many are distributed in mountainous areas.
Remarks. This subgenus is most similar in morphology, including the male genitalia, to the subgenus Harpalobius but distinctly distinguished from it by a lesser number of setae along the posterior margin of the protibia and the abdominal sternites without additional dense long setae.
In glabrous abdominal sternites of most species, Diaharpalus subg. n. is similar to Bactroharpalus subg. n., differing from it mainly in having only one ventroapical spine on the protibia, the male mesotibia without preapical callous thickening on the inner margin and the aedeagus with several distinct spiny patches (groups of small spines) in the internal sac.
The subgenus
Diaharpalus subg. n. corresponds to the
innocuus species group sensu Lindroth [
15] and to the
vittatus species group sensu Kataev [
8,
88] and sensu Kryzhanovskij et al. [
56].
The Palaearctic species of this subgenus were revised by Kataev [
88], with subsequent additions [
112,
116].
Subgenus Harpalobius Reitter, 1900
Harpalobius Reitter, 1900 [
38] (pp. 76, 103) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus fuscipalpis Sturm 1818, designated by Habu [
29].
Harpalellus Lindroth, 1968 [
15] (p. 815) (as a genus). Type species
Harpalus basilaris Kirby 1837 (=
H. fuscipalpis Sturm 1818), by original designation.
Diagnosis. Medium-sized to comparatively large (length 6.6–12.0 mm). Body moderately convex, somewhat wide or slightly elongate, with or without metallic luster on dorsum; elytra of some members bicolorous, with yellow pattern. Pronotal basal edge glabrous or setose. Humeral denticle more or less prominent. Elytral interval 3 with or without discal setigerous pore. Metafemur with seven to fifteen setigerous pores along posterior margin. Protibia with one or two ventroapical spines and with five (rarely four) preapical spines on outer margin. Male mesotibia without preapical callous thickening on inner margin. Abdominal sternites with numerous additional long setae. Median lobe of aedeagus with short terminal lamella and with one small spiny patch (group of small spines) in internal sac medially.
Composition and distribution. The subgenus includes four species. One of them, the Holarctic
H. fuscipalpis Sturm, 1818 (
Figure 47e,f), is widely distributed over Eurasia and North America; the other three species occur in the Palaearctic region:
H. fuscicornis Ménétriés, 1832,
H. inexspectatus Kataev, 1989 (
Figure 47g,h) and
H. viridanus Motschulsky, 1844 (
Figure 48b) (with the subspecies
H. v. angustibasis Kataev et Liang, 2007 and
H. v. staudingerianus Schauberger, 1932).
Ecology. All species of this subgenus occur in dry, open, mainly steppe habitats.
Remarks. This subgenus differs from two preceding subgenera in having numerous additional long setae on the abdominal sternites and the metafemur with a more number of setae along the posterior margin. The representatives of
Harpalobius and
Diaharpalus subg. n. demonstrate a certain parallelism in the variability of some characters, in particular, the appearance of a very similar light pattern on the elytra, the presence or absence of setae on the basal edge of the pronotum and a discal pore on the elytral interval 3 [
88].
The subgenus
Harpalobius, as here treated, corresponds to the
fuscipalpis species group sensu Kataev [
54] and sensu Kryzhanovskij et al. [
56].
Harpalobius and
Harpalellus are considered synonyms because the type species of these taxa are conspecific [
53,
54].
Harpalus fuscipalpis has aedeagus with apical orifice in dorsal position which is unusual for
Harpalus. Perhaps the origin of this species is the result of partial juvenilization of development [
118].
The species of this subgenus (as the
fuscipalpis species group) were revised by Kataev [
54], with subsequent addition [
112].
Pheuginus Subgroup
Diagnosis. Body brown to black, with or without green or blue metallic luster on dorsum. Head impunctate and glabrous, in Anophonus subg. n. more or less punctate and setose dorsally. Pronotum punctate or impunctate basally, with one lateral seta on each side and with basal edge generally setose, rarely glabrous. Elytra generally impunctate and glabrous, in some taxa more or less widely punctate and pubescent; basal border glabrous or setose; basal (parascutellar) pore present or absent in some taxa; interval 3 in most species with or without one discal setigerous pore, in some species with several discal pores; intervals 7 and 5 with or without preapical pores. Metacoxae with or without additional setae medially. Protibia generally with one, more rarely two, ventroapical spines and generally with three or four (more rarely five or six) preapical spines on outer margin, usually not forming a single row with spines on lover surface of tibia; ventroapical tubercle in male generally not prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous or setose dorsally; metatarsomere 1 often more elongate than average for genus. Abdominal sternites generally with more or less numerous additional setae, in some species glabrous; last visible sternites in most species without pronounced sexual dimorphism, in few species its apex truncate in male and slightly swollen in female. Median lobe of aedeagus with long or short terminal lamella and with pronounced more or less discoidal, more rarely horseshoe-shaped, apical capitulum; internal sac generally with one or two separate spines (more rarely without them), and usually also with one or two spiny patches; in some members without sclerotic elements.
Composition and distribution. This subgroup comprises twelve Palaearctic subgenera, with the largest subgenus and species diversity in Asia.
Ecology. Members of this subgroup occur in various open habitats, mainly in the steppes and deserts, as well as in the mountains. In deserts, the species are usually found near water.
Remarks. The subgenera included in this subgroup form a morphological continuum without distinct apomorphies common to all these subgenera. Many members are characterized by a median lobe of the aedeagus with a pronounced discoidal apical capitulum and by rather slender metatarsus with a comparatively long first tarsomere. The taxonomic position of some subgenera requires further study.
This subgroup corresponds to the
smaragdinus stock sensu Kataev [
104].
Subgenus Nephoharpalus Huang, Lei, Yan et Hu, 1996
Nephoharpalus Huang, Lei, Yan et Hu, 1996 [
168] (pp. 120, 123) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus jianyangensis Huang, Lei, Yan et Hu, 1996 (=
H. pallidipennis Morawitz, 1862), by original designation.
Diagnosis. Medium-sized (length 7.3–11.0 mm). Body moderately convex, slightly elongate, brown to black, with or without greenish or bluish metallic luster on elytra. Pronotum punctate basally, with sides sinuate or not sinuate basally; basal edge setose. Elytra impunctate and glabrous, with glabrous basal border; interval 3 generally with one discal setigerous pore (in H. pallidipennis with one or several discal pores); basal (parascutellar) pore present; intervals 7 and 5 without preapical pores. Prosternum with short setae. Metepisternum elongate, longer than wide. Protibia with one ventroapical spine. Tarsi glabrous dorsally or sparsely setose. Abdominal sternites with very short dense pubescence. Median lobe of aedeagus with discoidal apical capitulum and relatively short terminal lamella; internal sac with two parallel elongate groups of small spines and with or without a small sepatate spine medially.
Composition and distribution. This subgenus includes three Palaearctic species:
H. smaragdinus (Duftschmid, 1812) (Figure 50a,b),
H. pallidipennis Morawitz, 1862 (
Figure 11g,h and
Figure 49a) and
H. cyclogonus Chaudoir, 1844 (with the subspecies
H. c. olenini Poppius, 1906).
Ecology. Species of this subgenus occur on the plains, in dry, open habitats, both in meadows and steppes.
Remarks. The most distinctive characters of this subgenus are an elongate metepisternum, abdominal sternites with short dense pubescence and a characteristic aedeagus with a short terminal lamella and a large transverse apical capitulum.
The subgenus
Nephoharpalus, as here treated, corresponds to the
smaragdinus species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8]. Mlynář [
76] first drew attention to the close relationship between
H. smaragdinus and
H. pallidipennis.
Subgenus Pheuginus Motschulsky, 1844
Pheuginus Motschulsky, 1844 [
34] (tabl. between pp. 196 and 197, p. 197) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus optabilis Dejean, 1829, designated by Noonan [
6].
Conicus Motschulsky, 1844 [
34] (tabl. between pp. 196 and 197, p. 197) (as a taxon within
Harpalus Latreille, 1802). Type species
Harpalus acuminatus Motsch. 1844 (=
H. optabilis Dejean, 1829), designated by Noonan [
6].
Diagnosis. Medium-sized to large (length 8.4–13.5 mm). Body more or less convex, moderately wide or elongate, elliptical-shaped, without metallic luster. Pronotum punctate basally, with sides rounded; basal edge generally glabrous, more rarely setose (setae very short and barely noticeable). Elytra impunctate and glabrous, with glabrous basal border and with one discal setigerous pore on interval 3; basal (parascutellar) pore present; interval 7, or intervals 7 and 5, or intervals 7, 5 and 3 with row of preapical pores; these pores in
H. davidianus almost reaching basal elytral border (
Figure 6d). Prosternum with moderately long setae. Metepisternum elongate, longer than wide. Protibia with one ventroapical spine. Abdominal sternites with dense short pubescence. Tarsi glabrous or (in
H. davidianus) very finely setose dorsally (in the latter species also tibiae finely setose). Median lobe of aedeagus with discoidal apical capitulum and long terminal lamella; internal sac with two or three more or less large groups of medium-sized spines.
Composition and distribution. This subgenus includes three species from the eastern Palaearctic:
H. oodioides Dejean, 1829,
H. optabilis Dejean, 1829 (
Figure 49b and
Figure 50c,d) and
H. davidianus Tschitschérine, 1903 (with the subspecies
H. d. basharicus Schauberger, 1933).
Ecology. All three species occur mainly on the plains, in steppe habitats.
Remarks. The members of Pheuginus are similar to those of Nephoharpalus in dense short pubescence on abdominal sternites but differ in having preapical pores at least in interval 7 and a more convex body.
The species of this subgenus (as the
optabilis species group) were revised by Kataev [
54]. The taxonomy of some taxa was discussed by Mlynář [
53].
Subgenus Mesoharpalus subg. n.
Type species Harpalus gisellae Csiki, 1932.
Diagnosis. Medium-sized to large (length 7.6–12.3 mm). Body moderately convex, elongate, generally with green or violet metallic luster on dorsum. Pronotum impunctate or with more or less distinct punctures basally; sides sinuate basally; basal edge generally setose, more rarely glabrous. Elytra impunctate and glabrous, with glabrous basal border; interval 3 with one discal setigerous pore or without it; intervals 7 and 5 without preapical pores; basal (parascutellar) pore present. Prosternum with short setae. Metepisterna short and wide, at least as wide as long. Protibia with one ventroapical spine. Tarsi glabrous dorsally. Abdominal sternites almost glabrous, with a few short barely noticeable setae. Median lobe of aedeagus with discoidal apical capitulum and comparatively long terminal lamella; internal sac generally with one separate spine and with one to three groups of medium-sized spines (in H. zhdankoi without groups of spines, but with an additional separate spine).
Etymology. The subgeneric name is a combination of the Greek mésos, meaning “middle, medium”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes five allopatric Palaearctic species with more or less reduced wings:
H. mitridati Pliginsky, 1915 from the east European and Kazakh steppes, and
H. gisellae Csiki, 1932 (
Figure 50e,f),
H. kiritshenkoi Kataev, 1990 (
Figure 51a),
H. ovtshinnikovi Kataev, 1990 and
H. zhdankoi Kataev, 1990 from the Tien Shan.
Ecology. Species of this subgenus occur in steppe habitats, H. mitridati in lowlands, and other species in mountains.
Remarks. Mesoharpalus subg. n. differs from the two preceding subgenera in almost glabrous abdominal sternites and a short and wide metepisternum.
This subgenus corresponds to the
gisellae species group sensu Kataev [
8,
90] and sensu Kryzhanovskij et al. [
56]. A revision of the species of this group was published by Kataev [
90].
Subgenus Eremoharpalus subg. n.
Type species Harpalus remboides Solsky, 1874.
Diagnosis. Comparatively large-sized (length 9.3–12.3 mm). Body moderately convex, somewhat wide, without metallic luster; legs long and slender. Pronotum impunctate basally, with rounded sides and setose basal edge. Elytra impunctate and glabrous, with glabrous basal border and with one discal setigerous pore on interval 3; intervals 7 and 5 without preapical pores; basal (parascutellar) pore present. Metepisternum as long as or slightly longer than wide. Protibia with one ventroapical spine. Tarsi glabrous dorsally; metatarsomeres long, weakly widened posteriorly. Abdominal sternites almost glabrous, with a few short barely noticeable setae. Median lobe of aedeagus with discoidal apical capitulum and relatively long terminal lamella; internal sac with two or three spiny patches and without separate spines.
Etymology. The subgeneric name is a combination of the Greek erēmos, meaning “desert”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes three allopatric species from Eurasian deserts:
H. remboides Solsky, 1874 (
Figure 52a,b),
H. medvedevi Kataev, 2006 (
Figure 50b) and
H. araraticus Mlynář, 1979.
Ecology. Species of this subgenus inhabit dry steppes and deserts but occur not far from water, usually in riparian (tugai) forests or in the river coastal zone.
Remarks. This subgenus is most similar to Nephoharpalus and particularly to the subgenus Mesoharpalus subg. n. in most of the structural characters, including the glabrous basal border of elytra and the discoidal apical capitulun of aedeagus. The subgenera Eremoharpalus subg. n. and Mesoharpalus subg. n. also share the almost glabrous abdominal sternites and the short metepisterna. The members of Eremoharpalus subg. n. can be distinguished from those of Mesoharpalus subg. n. by the rounded sides of the pronotum and the absence of separate spines in the internal sac of the aedeagus.
Eremoharpalus subg. n. corresponds to the
remboides species group sensu Kryzhanovskij et al. [
56] and Kataev [
8,
104]. A revision of the species of this group was published by Kataev [
104].
Subgenus Oreoharpalus subg. n.
Type species Harpalus famelicus Tschitschérine, 1898.
Diagnosis. Medium-sized (length 7.7–11.3 mm). Body moderately convex, elongate, without metallic luster. Head impunctate and glabrous. Pronotum impunctate basally, with setose basal edge; sides sinuate or not sinuate basally. Elytra either more or less widely punctate and pubescent or impunctate and glabrous; basal border setose; interval 3 with one discal setigerous pore or without it; intervals 7 and 5 with or without preapical pores; abbreviate (parascutellar) striole often more or less reduced; basal (parascutellar) pore present or occasionally absent. Metepisternum as long as or slightly longer than wide. Protibia with one ventroapical spine; ventroapical tubercle in male slightly prominent. Tarsi glabrous dorsally. Abdominal sternites almost glabrous, with a few short barely noticeable setae. Median lobe of aedeagus with moderately long terminal lamella and discoidal or horseshoe-shaped apical capitulum; internal sac either without sclerotic armament or with one separate spine, or with two groups of small spines and also spiny patches.
Etymology. The subgeneric name is a combination of the Greek óros, meaning “mount”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes three wingless Palaearctic species endemic to the Hissar-Darvaz Mountains:
H. famelicus Tschitschérine, 1898 (
Figure 52c,d and
Figure 53a) (with the subspecies
H. f. fanensis Kataev et Wrase, 1993 and
H. f. loxophonoides Kataev et Wrase, 1993),
H. diligens Tschitschérine, 1898 and
H. strenuus Tschitschérine, 1898.
Ecology. Species of this subgenus occur in mountains, in open habitats at altitudes of 1000–4000 m.
Remarks. This subgenus is recognizable by the features listed in the diagnosis, among which the most important are elytra with setose basal border, pronotum with one ventroapical spine, short metepisterna and almost glabrous abdominal sternites.
The subgenus
Oreoharpalus subg. n. corresponds to the
famelicus species group sensu Kataev and Wrase [
114], sensu Kryzhanovskij et al. [
56], and sensu Kataev [
8]. A revision of the species of this group was published by Kataev and Wrase [
114].
Subgenus Hypsoharpalus subg. n.
Type species Harpalus arnoldii Kataev, 1988.
Diagnosis. Medium-sized (length 8.3–9.3 mm). Body moderately convex, somewhat wide or slightly elongate, without metallic luster. Head impunctate and glabrous. Pronotum impunctate basally, with setose basal edge; sides not sinuate basally. Elytra impunctate and glabrous, with glabrous basal border; interval 3 with or without one discal setigerous pore; intervals 7 and 5 without preapical pores; basal (parascutellar) pore present. Metepisternum short and wide, about as long as wide. Protibia with one ventroapical spine and with three or four preapical spines on outer margin, isolated from spines on ventral surface of tibia; ventroapical tubercle in male slightly prominent. Tarsi glabrous dorsally. Abdominal sternites almost glabrous, with a few short barely noticeable setae. Median lobe of aedeagus with a relatively short terminal lamella and with a horseshoe-shaped apical capitulum; internal sac without sclerotic elements or only with small spiny patches.
Etymology. The subgeneric name is a combination of the Greek húpsos, meaning “hight, altitude”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes two wingless Palaearctic species endemic to the Tien Shan:
H. kadyrbekovi Kataev, 1988 and
H. arnoldii Kataev, 1988 (
Figure 52e,f and
Figure 53b).
Ecology. Both species of this subgenus inhabit mountains, occurring in the forest and alpine belts at altitudes of 1800–3200 m.
Remarks. The subgenus
Hypsoharpalus subg. n. corresponds to the
kadyrbekovi species group sensu Kataev [
8]. The relationships of this subgenus are still unclear. In some characters (one ventroapical spine on protibia, short metepisternum and almost glabrous abdominal sternites),
Hypsoharpalus subg. n. is similar to
Oreoharpalus subg. n. but distinguished by the elytra with a glabrous basal border. The members of
Hypsoharpalus subg. n. markedly differ from those of
Oreoharpalus subg. n., which also have a glabrous basal border of the elytra, in the structure of the aedeagus with a short terminal lamella, a horseshoe-shaped apical capitulum and no spines in the internal sac.
Subgenus Anophonus subg. n.
Type species Ophonus cyanopterus Tschitschérine, 1897.
Diagnosis. Medium-sized (length 7.3–11.0 mm). Body moderately convex, elongate, with or without metallic luster. Head punctate and more or less setose dorsally. Pronotum punctate basally, with setose basal edge; sides sinuate or not sinuate basally. Elytra more or less widely punctate and pubescent, generally with setose basal border; interval 3 with one discal setigerous pore (can be difficult to distinguish against the background of punctation); basal (parascutellar) pore present. Metepisternum short and wide, wider than long. Protibia with one ventroapical spine. Tarsi densely setose dorsally. Abdominal sternites with additional somewhat long setae. Median lobe of aedeagus with discoidal apical capitulum; internal sac with one or two separate spines and one or several groups of small spines.
Etymology. The subgeneric name is a combination of the Greek an-, meaning “not”, and the name of the carabid taxon Ophonus.
Composition and distribution. This subgenus includes two wingless species endemic to the west Tien Shan:
H. cyanopterus (Tschitschérine, 1897) (
Figure 54a and Figure 56a,b) and
H. pterostichus (Reitter, 1900). Some new taxa of this subgenus are still undescribed.
Ecology. Both species occur in rather dry open habitats, mainly within the mountain belt of juniper forests.
Remarks.
Harpalus cyanopterus и
H. pterostichus have a punctate and pubescent body and densely setose tarsi, and based on these characters, they have long been considered representatives of the genus
Ophonus [
23,
38,
169]. However, paraglossae in these species are setose at margins, epilobes of mentum are narrow, basal labial palpomere is without an oblique carina, metacoxa is without a posteromedial pore, and, therefore, both species are included in the genus
Harpalus [
170]. Based on the features of their aedeagi and external morphology, they are treated as belonging to a separate subgenus within
Pheuginus subgroup. A closer relationship of this subgenus, which is distinguished by a punctate and pubescent body, including the head and tarsi, with other taxa of this subgroup requires further study.
This subgenus corresponds to the
cyanopterus species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8].
Subgenus Haloharpalus subg. n.
Type species Harpalus salinulus Reitter, 1900.
Diagnosis. Medium-sized (length 6.8–7.0 mm). Body moderately convex, slightly elongate, black, without metallic luster. Head impunctate and glabrous. Pronotum impunctate basally, with setose basal edge; sides not sinuate basally. Elytra impunctate and glabrous, with glabrous basal border and without discal setigerous pore on interval 3; basal (parascutellar) pore present; intervals 7 and 5 without preapical pores. Prosternum with moderately long setae. Metepisternum elongate, markedly longer than wide. Protibia with one ventroapical spine and four preapical spines on outer margin, isolated from spines on ventral surface of tibia. Tarsi glabrous dorsally. Abdominal sternites with additional long setae. Median lobe of aedeagus with a relatively short terminal lamella and with a button-like apical capitulum; internal sac with one or two separate spines and three or four groups of small spines (
Figure 55b,c).
Etymology. The subgeneric name is a combination of the Greek hals, meaning “salt”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only one very rare Palaearctic species,
H. salinulus Reitter 1900 (
Figure 55), endemic to central Anatolia.
Ecology. The only species of this subgenus is found on saline lands.
Remarks. This subgenus is well recognizable by unique combination of distinctive characters listed in the diagnosis, but its relationship with other taxa is still unclear.
The subgenus
Haloharpalus subg. n. corresponds to the
salinulus species group sensu Kataev [
8].
Subgenus Megaharpalus subg. n.
Type species Harpalus stoetznerianus Schauberger, 1932.
Diagnosis. Large-sized (length 14.6–15.7 mm). Body moderately convex, elongate, black, with bluish or greenish tinge on dorsum. Head impunctate and glabrous. Pronotum impunctate, with sides not sinuate basally, with one lateral seta on each side and with setose basal edge. Elytra impunctate and glabrous, with glabrous basal border and without discal setigerous pore on interval 3; basal (parascutellar) pore present; intervals 7 and 5 without preapical pores. Prosternum almost glabrous or with very short setae. Metepisternum short, wider than long. Metacoxa without additional setae medially. Protibia with two (occasionally three) ventroapical spines and with four preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male absent. Tarsi glabrous dorsally. Three last abdominal sternites (V–VII) glabrous; sternite IV densely setose; last visible sternite (VII) without pronounced sexual dimorphism, its apex in both sexes more or less rounded and only slightly swollen in female. Median lobe of aedeagus with an elongate terminal lamella and with a transverse, almost discoidal apical capitulum; internal sac with a wide spiny patch apically and occasionally also with one very small separate spine medially (
Figure 56c,d).
Etymology. The subgeneric name is a combination of the Greek mégas, meaning “big, large”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only the wingless eastern Palaearctic
H. stoetznerianus Schauberger, 1932 (
Figure 54b and
Figure 56c,d), known from the Chinese province of Sichuan.
Remarks. Like the preceding taxon, the only species of Megaharpalus subg. n. is also well recognizable by unique combination of distinctive characters listed in the diagnosis, including a large size and a setation of the abdominal sternites, but its relationship with other taxa is still unclear.
This subgenus corresponds to the
stoetznerianus species group sensu Kataev [
8].
Subgenus Aristoharpalus subg. n.
Type species Harpalus ingenuus Tschitschérine, 1898.
Diagnosis. Comparatively large-sized (length 9.1–13.0 mm). Body convex, stout and somewhat wide, with or without metallic luster on dorsum. Head impunctate and glabrous. Pronotum impunctate basally, with rounded sides and setose basal edge. Elytra punctate and pubescent basally and often laterally, with setose basal edge, without discal setigerous pore on interval 3 and without basal (parascutellar) pore; intervals 7 and 5 without preapical pores; preapical sinuation rather deep, with a denticle at its base. Metepisternum short and wide, wider than long (
Figure 7d,e). Protibia with two ventroapical spines; ventroapical tubercle in male not prominent. Tarsi glabrous dorsally. Abdominal sternites with additional long setae. Median lobe of aedeagus with a long terminal lamella and with a discoidal or horseshoe-shaped apical capitulum; internal sac with two more or less large separate spines, one or two groups of small or medium-sized spines and also spiny patches.
Etymology. The subgeneric name is a combination of the Greek áristos, meaning “best, noble”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes two wingless Palaearctic species endemic to the Pamir-Alai region:
H. ingenuus Tschitschérine, 1898 (
Figure 57a and
Figure 58a,b) (with the subspecies
H. i. lailakensis Kataev, 2006 and
H. i. alajanicus Jedlička, 1957) and
H. arcuatus Tschitschérine, 1898.
Ecology. Both species of this subgenus occur in mountains, in open habitats, at altitudes of 2000–3700 m.
Remarks. This subgenus is similar to the subgenera Oreoharpalus subg. n. and Cycloharpalus subg. n. in having the basal border of elytra densely setose, but it well differs from both in having a protibia with two ventroapical spines. Species of Aristoharpalus subg. n. are also recognizable by their elytra, which are finely punctate and setose basally and lack basal (parascutellar) and any discal pores.
The subgenus
Aristoharpalus subg. n. corresponds to the
ingenuus species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8,
104]. A revision of the species of this group was published by Kataev [
104].
Subgenus Cycloharpalus subg. n.
Type species Harpalus pulvinatus Ménétries, 1848.
Diagnosis. Medium-sized (length 6.7–10.5 mm). Body convex, somewhat wide, without metallic luster. Head impunctate and glabrous. Pronotum impunctate basally, with rounded sides and setose basal edge. Elytra impunctate and glabrous, with setose basal border; basal (parascutellar) pore present; interval 3 generally without discal setigerous pore; interval 7 and 5 with or without preapical pores. Metepisternum as long as or longer than wide. Protibia with one ventroapical spine; preapical spines on outer margin of tibia isolated from spines on ventral surface of tibia or forming a single row with them; ventroapical tubercle in male not developed. Tarsi glabrous dorsally. Abdominal sternites with additional long setae. Median lobe of aedeagus with a relatively short terminal lamella and an oblique horseshoe-shaped apical capitulum; internal sac with one or two separate spines and one or three groups of small spines.
Etymology. The subgeneric name is a combination of the Greek kýklos, meaning “circle”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes two Palaearctic species from deserts and semideserts of Transcaucasia, the lower Volga region and middle Asia:
H. pulvinatus Ménétriés, 1848 (
Figure 57b and
Figure 58c,d) (with the subspecies
H. p. lubricus Reitter, 1900) and
H. breviusculus Chaudoir, 1846.
Ecology. Both species of this subgenus inhabit lowland desert and semi-deserts but are usually found near water, in river floodplains or at lake shores.
Remarks. This subgenus is similar to Aristoharpalus subg. n. in the presence of setae on the basal border of the elytra and on the abdominal sternites but differs from it in the smaller body size, glabrous elytra without parascutellar pore, longer metepisterna, only one ventroapical spine on the protibia and aedeagus with a shorter terminal lamella.
The subgenus
Cycloharpalus subg. n. corresponds to the
pulvinatus species group sensu Kryzhanovskij et al. [
56] and the
breviusculus species group sensu Kataev [
8]. The taxonomy of
H. pulvinatus was discussed by Kataev [
93].
Subgenus Euryharpalus subg. n.
Type species Harpalus cisteloides Motschulsky, 1844.
Diagnosis. Medium-sized to large (length 8.7–13.1 mm). Body weakly convex, comparatively wide, without metallic luster. Head impunctate and glabrous. Pronotum impunctate or more or less distinctly punctate basally, with glabrous basal edge; sides not sinuate basally. Elytra impunctate and glabrous, with glabrous basal border and with one discal setigerous pore on interval 3; intervals 7 and 5 with or without preapical pores; basal (parascutellar) pore present. Metepisternum elongate, markedly longer than wide. Protibia with one ventroapical spine and four or five (rarely 6) preapical spines on outer margin, isolated from spines on ventral surface of tibia; ventroapical tubercle in male slightly prominent or indistinct. Tarsi glabrous dorsally. Abdominal sternites either almost glabrous, with a few short barely noticeable setae, or with dense, very short setation. Median lobe of aedeagus with a relatively short terminal lamella and with a horseshoe-shaped apical capitulum; internal sac either with one or two separate spines or with a group of small spines, or only small spiny patches.
Etymology. The subgeneric name is a combination of the Greek eurys, meaning “wide”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises four species from the eastern Palaearctic region:
H. cisteloides Motschulsky, 1844 (
Figure 58e,f) (with the subspecies
H. c. hurkai Divoky, Pulpan et Rebl, 1990 and
H. c. schouberti Tschitschérine, 1898),
H. aequicollis Motschulsky, 1844 (
Figure 59a),
H. heyrovskyi Jedlička, 1928 and the taxonomically more separated
H. compressus Motschulsky, 1844.
Ecology. Species of this subgenus inhabit the steppes and steppe meadows, both in the lowlands and in the mountains.
Remarks. Within the Pheuginus subgroup, the members of this subgenus are recognizable by having a glabrous basal border, an elongate metepisternum and an aedeagus with a horseshoe-shaped apical capitulum. In external characters, Euryharpalus subg. n. is most similar to the subgenus Pheuginus but sharply distinguished from it by the characteristics of the aedeagus. Its taxonopmic position requires further study.
The subgenus
Euryharpalus subg. n. corresponds to the
cisteloides species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8], but
H. kadyrbekovi, considered as a member of this group in the former publication, is included in the
Hypsoharpalus subg. n. The taxonomy of
H. aequicollis and
H. heyrovskyi was discussed by Kataev [
54].
Pharalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. A monobasic subgroup, including only one Nearctic subgenus.
Subgenus Pharalus Casey, 1914
Pharalus Casey, 1914 [
71] (p. 68) (as a genus). Type species
Pangus testaceus LeConte, 1853 (=
H. indianus Csiki, 1932), by original designation.
Diagnosis. Medium-sized (length 7.8–9.8 [10.7] mm). Body convex, slightly elongate or somewhat wide, unicolorous testaceous or reddish brown with metallic luster on dorsum. Head impunctate and glabrous. Pronotum finely punctate laterobasally or impunctate, with sides slightly sinuate basally, with one lateral seta on each side and with setose basal edge. Elytra impunctate and glabrous, with glabrous basal border; interval 3 with or without discal pore; intervals 7 and 5 without preapical pores. Prosternum almost glabrous, at most with a few very short setae apically. Metepisternum longer than wide. Metacoxa with one or several setae medially. Protibia with one or two (occasionally three) ventroapical spines and with four to six preapical spines on outer margin, forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male absent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally; metatarsomere 1 short. Abdominal sternites with additional long setae; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with elongate terminal lamella and with large discoidal apical capitulum; internal sac with spiny patches similar to that of members of Hypsinephus subgroup; large separate spines absent.
Composition and distribution. This subgenus includes two Nearctic species from the eastern part of North America:
H. indianus Csiki, 1932 (
Figure 59b) and
H. gravis LeConte, 1858.
Ecology. Both species of this subgenus occur in various open habitats, but
H. indianus prefers drier areas than
H. gravis, and usually areas with a significant proportion of sand in the soil, colonizing sand dunes as well [
140].
Remarks. This habitually well-recognized subgenus occupies a somewhat intermediate position in external morphology and male genitalia between the subgenera of Pheuginus and Hypsinephus subgroups.
Noonan [
16] treated
Pharalus as the
indianus subgroup of the
desertus group. This subgenus corresponds to the
indianus species group sensu Kataev [
8].
Hypsinephus Subgroup
Diagnosis. Head impunctate and glabrous (occasionally with short setae around basal foveae in Hemipangus subg. n.), tempora generally glabrous, in Hemipangus subg. n. and Loxophonus usually setose. Pronotum impunctate or punctate basally, with sides generally rounded, more rarely slightly sinuate basally, with one or several lateral setae on each side and with basal edge generally setose. Elytra in most species impunctate and glabrous, more rarely punctate and pubescent; basal border setose; basal (parascutellar) pore generally present (occasionally absent in some species); interval 3 with one or several discal setigerous pores (occasionally pore absent); in most species, interval 7, often also intervals 5 and 3, with short row of preapical setigerous pores (these pores not recognizable in most species with punctate elytra). Metacoxae with additional setae medially, rarely (in one species of subgenus Hypsinephus) also with a posteromedial pore. Protibia with one, more rarely two or three, ventroapical spines in a transverse row and with three or four preapical spines on outer margin, isolated from spines on ventral surface of tibia or forming with them a single row; ventroapical tubercle in male not prominent. Male mesotibia without preapical callous thickening on inner margin. Tarsi glabrous dorsally; metatarsomere 1 average for genus or slightly more elongate. Abdominal sternites with more or less numerous additional setae; last visible sternite generally without pronounced sexual dimorphism, in some species its apex slightly truncate in male and slightly swollen in female. Median lobe of aedeagus with more or less long terminal lamella and a horseshoe-shaped apical capitulum; internal sac generally with a characteristic more or less elongate spiny patch in apical half of median lobe or medially, usually in addition to spiny patches or groups of small spines; separate spines absent.
Composition and distribution. This subgroup comprises one Holarctic and four Palaearctic subgenera.
Ecology. Species of this subgroup inhabit open arid and semi-arid landscapes.
Remarks. In addition to the characteristic aedeagus, the main distinctive features of this subgroup are the setae on the pronotal basal edge, elytral basal border and abdominal sternites and a short row of preapical setigerous pores on the elytral interval 7.
Subgenus Hypsinephus Bates, 1878
Hypsinephus Bates, 1878 [
171] (p. 715) (as a genus). Type species
Hypsinephus ellipticus Bates, 1878 (=
H. salinus Dejean, 1829), by monotypy.
Rapahlus Lutshnik, 1922 [
21] (p. 61) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus salinus Dejean, 1829, by original designation.
Diagnosis. Medium-sized to large (length 8.2–14.0 mm). Body moderately convex, more or less elongate, brown to almost black, without metallic luster. Basal outer angles of mandibles not angularly prominent. Pronotum with one lateral seta on each side and with basal angles rounded or sharp at tip. Elytra impunctate and glabrous; intervals 3 and 5 with several (up to ten) discal setigerous pores along entire length; occasionally with additional discal pores also on intervals 2, 4 and 6. Metepisternum markedly longer than wide. Protibia with two or three ventroapical spines and with four preapical spines on outer margin, isolated from spines on ventral surface of tibia and not forming a single row with them (
Figure 60b,c).
Composition and distribution. This subgenus includes two species from the eastern Palaearctic region:
H. salinus Dejean, 1829 (
Figure 60a and
Figure 61a,b) (with the subspecies
H. s. agonus Tschitschérine, 1894 and
H. s. klementzae Kataev, 1984) and
H. lumbaris Mannerheim, 1825.
Ecology. Both species occur in dry steppe and semi-desert habitats, both on the plains and in the mountains.
Remarks. Within the subgroup, the members of this subgenus are recognizable by the elytra with numerous discal setigerous pores at least on the intervals 3 and 5. Interestingly, the metacoxa of H. salinus has a posteromedial pore, buth this pore is absent in H. lumbaris.
This subgenus corresponds to the
salinus species group sensu Kryzhanovskij et al. [
56] and to the
lumbaris species group sensu Kataev [
8]. The revision of
Hypsinephus was published by Kataev [
84].
Subgenus Brachyharpalus subg. n.
Type species Carabus autumnalis Duftschmid, 1812.
Diagnosis. Medium-sized (length 5.9–10.6 mm). Body moderately convex, often stout, brown to black, without metallic luster. Basal outer angles of mandibles not angularly prominent (Figure 63a). Pronotum with one lateral seta on each side and with basal angles rounded or blunted at tip. Elytra impunctate and glabrous; interval 3 with one to five discal setigerous pores (rarely without pores), interval 5 without discal pores (rarely with one or two pores basally). Metepisternum variable, elongate or wider than long (
Figure 7f). Protibia generally with one, rarely two, ventroapical spines and with three or four preapical spines on outer margin, usually isolated from spines on ventral surface of tibia and not forming a single row with them.
Etymology. The subgeneric name is a combination of the Greek brakhús, meaning “short”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises about 14 predominantly wingless species from the arid and semiarid regions of the Palaerctic and Nearctic, with four isolated faunal centers.
The largest of them, the European and Middle Eastern center, includes eight winged and wingless species:
H. autumnalis (Duftschmid, 1812) (
Figure 61c,d),
H. danieli Reitter, 1900,
H. brachypterus Tschitschérine, 1898,
H. anatolicus Tschitschérine, 1898 (with the subspecies
H. a. lydius Kataev et Wrase, 1997,
H. a. caricus Kataev et Wrase, 1997 and
H. a. lycius Kataev et Wrase, 1997),
H. triseriatus Fleischer, 1897 (with the subspecies
H. t. babunensis Mlynář, 1979),
H. kazanensis Jedlička, 1958 (
Figure 62a),
H. reflexus Putzeys, 1878 (with the subspecies
H. anadoluensis Kataev, 1993) and
H. foveiger Tschitschérine, 1895.
The second faunal center is located in the western part of the Himalayas and is represented there by only one wingless species,
H. kunarensis Kataev, 1993 (
Figure 61e,f).
The third, Tibetan, faunal center includes two sympatric wingless species endemic to Tibet: H. lama Kataev et Wrase, 1997 and H. ascetes Kataev et Wrase, 1997.
The fourth, Nearctic, faunal center includes three species (wingless or dimorphic) from western North America: H. desertus LeConte, 1859, H. furtivus LeConte, 1865 and the insufficiently studied H. durangoensis Bates, 1891.
Ecology. All species of this subgenus occur in relatively dry open habitats, most in dry steppes and semi-deserts, both in lowlands and in mountains.
Remarks. In combination of characters, this subgenus is very similar to Hypsinephus but differs from it in less number of discal setigerous pores (sometimes without pores) on the elytral intervals 3 and 5. Brachyharpalus subg. n. is distinguished from the monotypical Brachypangus by having the basal outer angles of the mandibles not prominent.
This subgenus corresponds to the
desertus group sensu Lindroth [
15], recognized for the Nearctic species, and to the
autumnalis species group sensu Mlynář [
76], sensu Kataev [
8,
93], sensu Kryzhanovskij et al. [
56] and sensu Kataev and Wrase [
116], recognized for the Palaearctic species. Noonan [
16] considered
H. desertus (with
H. furtivus as its synonym, the
furtivus morph) to be a single member of the
desertus subgroup of the
desertus species group, in which he included also the
indianus subgroup. According to my data,
H. furtivus distinctly differs from
H. desertus at least in having a short and wide (wider than long) metepisternum; in
H. desertus, the metepisternum is slightly longer than wide, markedly narrowed posteriorly. Noonan [
16] made no mention of this distinctive character in his revision.
The Palaearctic species of this subgenus were partly revised by Mlynář [
76], Kataev [
93] and Kataev and Wrase [
116]. Several Palaearctic species have not yet been described.
Subgenus Brachypangus Tschitschérine, 1898
Brachypangus Tschitschérine, 1898 [
172] (p. 174) (as a genus). Type species
Brachypangus antonowi Tschitschérine, 1898, by monotypy.
Diagnosis. Medium-sized (length 8.3–10.0 mm). Body convex and stout, brown to dark brown, without metallic luster. Basal outer angles of mandibles angularly prominent (
Figure 63b). Pronotum with one lateral seta on each side and with basal angles sharp, not blunted ar tip. Elytra impunctate and glabrous; interval 3 with one discal setigerous pore or without it; interval 5 without discal pores. Metepisternum short, wider than long. Protibia with one ventroapical spines and with three preapical spines on outer margin, isolated from spines on ventral surface of tibia and not forming a single row with them.
Composition and distribution. This subgenus includes only one very rare wingless Palaearctic species,
H. antonowi (Tschitschérine, 1898) (
Figure 62b), endemic to the Kopetdag.
Ecology. The single species of this subgenus occurs in dry, open habitats.
Remarks. In combination of characters, this subgenus coincides with Brachyharpalus subg. n., differing from it in the prominent basal outer angles of the mandibles, similar to those in Ophonus convexicollis (Ménétriés, 1832).
The subgenus
Brachypangus corresponds to the
antonowi species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8].
Subgenus Hemipangus subg. n.
Type species Harpalus klapperichi Jedlička, 1955.
Diagnosis. Medium-sized (length 8.3–10.6 mm). Body moderately convex, elongate, brown to black, without metallic luster. Tempora generally setose. Ligular sclerite in addition to two long ventroapical setae generally also with several short dorsal setae. Basal outer angles of mandibles not angularly prominent. Pronotum with one lateral seta on each side and with basal angles rounded or somewhat sharp. Elytra coarsely punctate and pubescent on lateral intervals; interval 3 with one to four discal setigerous pores, interval 5 without discal pores or with one or two pores basally. Metepisternum short, wider than long. Protibia with one ventroapical spine and with three or four preapical spines on outer margin, isolated from spines on ventral surface of tibia and not forming a single row with them.
Etymology. The subgeneric name is a combination of the Greek hēmi-, meaning “semi-, quasi-, half”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes two wingless Palaearctic species from Afghan and Tadjik Badakhshan:
H. klapperichi Jedlička, 1955 (Figure 65a,b) and
H. badakschanus Jedlička, 1955 (
Figure 64a).
Ecology. Both species occur in the highlands (2600–4200 m) in relatively humid habitats.
Remarks. This subgenus differs from Brachyharpalus subg. n. in the elytra coarsely punctate and setose on the lateral intervals and differs from Loxophonus in one lateral seta on each side of the pronotum.
The subgenus
Hemipangus subg. n. corresponds to the
klapperichi species group sensu Kataev [
8].
Subgenus Loxophonus Reitter, 1894
Loxophonus Reitter, 1894 [
173] (p. 124) (as a genus). Type species
Loxophonus setiporus Reitter, 1894, by monotypy.
Diagnosis. Medium-sized (length 8.5–11.0 mm). Body weakly convex, elongate, brown to almost black, without metallic luster. Tempora setose. Ligular sclerite in addition to two long ventroapical setae also with several short dorsal setae. Basal outer angles of mandibles not angularly prominent. Pronotum with several lateral setae on each side and with basal angles rounded. Elytra coarsely punctate and setose throughout or on odd intervals; interval 3 and 5 without discal setigerous pores (at least they not recognizable against background of punctation). Metepisternum short, as wide as or slightly wider than long. Protibia with one ventroapical spine and with three or four preapical spines on outer margin, isolated from spines on ventral surface of tibia and not forming a single row with them.
Composition and distribution. This subgenus includes two wingless allopatric Palaearctic species, endemic to the eastern Hissar-Darvaz Mountains:
H. setiporus (Reitter, 1894) (
Figure 65c,d) and
H. agakhaniantzi (Michailov, 1972) (
Figure 64b).
Ecology. Both species occur in relatively humid habitats, both in mountain forests and in the subalpine zone [
174].
Remarks. This subgenus is readily distinguished from other taxa of the Hypsynephus subgroup by the presence of several lateral setae on the pronotum.
The subgenus
Loxophonus corresponds to the
setiporus species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8], but
H. badakschanus, which was treated as a member of this group in the former publication, is included in the subgenus
Hemipangus subg. n. Mauriharpalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. This subgenus includes only one western Palaearctic subgenus.
Subgenus Mauriharpalus subg. n.
Type species Harpalus cardoni Antoine, 1922.
Diagnosis. Medium-sized (length 7.0–11.0 mm). Body moderately convex, elongate, brown to black, without metallic luster. Head impunctate, glabrous. Pronotum punctate basally, with one lateral seta on each side and with glabrous basal edge. Elytra impunctate and glabrous, with glabrous basal border; interval 3 generally with one, occasionally two, discal setigerous pores. Prosternum with very short setae. Protibia with one ventroapical spine and with three preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male prominent. Male mesotibia slightly curved, with distinct preapical callous thickening on inner margin. Abdominal sternites almost glabrous, only with very short setae basally; last visible sternite without pronounced sexual dimorphism, its apex in both sexes more or less rounded and not swollen. Median lobe of aedeagus with short terminal lamella and with almost discoidal apical capitulum; internal sac without distinct sclerotic elements (
Figure 65e,f).
Etymology. The subgeneric name is a combination of mauri (Moor), the name the ancient inhabitants of northwest Africa, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only
H. cardoni Antoine, 1922 (
Figure 65e,f) from the western Mediterranean region.
Ecology. The only species of this subgenus occur along the edges of small reservoirs on sandy soil, under stones or at the base of herbaceous plants [
40].
Remarks. The taxonomic position of H. cardoni requires further research since it demonstrates a unique combination of the distinctive characters dissimilar to any other known species. This species is characterized by an anal sternite without sexual differentiation; however, the male has a distinct callous thickening on the inner margin on the inner margin of mesotibia and a markedly prominent ventroapical tubercle on the protibia.
The subgenus
Mauriharpalus subg. n. corresponds to the
cardoni species group sensu Kataev [
8]. Antoine [
40] included
H. cardoni in one species group together with
H. numidicus, based mainly on coloration of the body, but the latter species differs in many characters, including several discal setigerous pores on the elytral interval 3 and the structure of the aedeagus, and it is apparently unrelated to
H. cardoni.
Caloharpalus Subgroup
Diagnosis. Head glabrous, impunctate or very finely punctate. Pronotum densely punctate basally, with one lateral seta on each side and with glabrous basal edge. Elytra either impunctate and glabrous or punctate and pubescent, with glabrous basal border; interval 3 with one to several discal setigerous pores. Prosternum with short or moderately long setae. Metacoxa with additional setae medially. Protibia with one ventroapical spine and with four to eight preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male indistinct or prominent. Male mesotibia generally with more or less distinct preapical callous thickening on inner margin (in some species this thickening almost not developed). Abdominal sternites generally with additional numerous setae; last visible sternite with pronounced sexual dimorphism: its apex rounded or subtruncate in male, and swollen, slightly expanded posteriorly in female. Median lobe of aedeagus generally with relatively long (at least longer than wide, but usually much longer) terminal lamella and apical capitulum of various shapes; armament of internal sac generally well developed, usually including a characteristic chain of short and wide spines, and with or without one separate spine.
Composition and distribution. This subgroup comprises three Palaearctic subgenera.
Ecology. Species of this subgroup inhabit mainly arid landscapes, but are usually found in fairly humid biotopes.
Remarks. This subgroup is similar to the following three subgroups (Idioharpalus, Artabas and Harpalus subgroups) in having additional setae on the abdominal sternites and the swollen apex of the last visible female sternite, among other common characters. The males of the Caloharpalus sugroup are also similar to those of the Harpalus subgroup in a more or less distinct preapical callous thickening on the inner margin of the mesotibia. The members of the Caloharpalus sugroup differ from members of these three subgroups in the characteristic male genitalia and also differ from the Idioharpalus subgroup and Harpalus subgroup in having only one ventroapical spine on the protibia, and from the Artabas subgroup in the unmodified mesotibia of the male. In addition, the Caloharpalus subgroup is distinct from the Harpalus subgroup in having a glabrous basal pronotal edge.
Subgenus Caloharpalus subg. n.
Type species Harpalus cupreus Dejean, 1829.
Diagnosis. Medium-sized to large (length 10.3–16.0 mm). Body moderately convex, somewhat elongate, with metallic luster on dorsum. Elytra impunctate and glabrous, with one discal setigerous pore on interval 3 and without preapical pores on intervals 5 and 7. Male profemur without a dense row of setae along inner anterior margin. Internal sac of aedeagus with a separate spine in addition to a longitudinal chain of spines and groups of small spines (spiny patches).
Etymology. The subgeneric name is a combination of the Greek kalós, meaning “beauty”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes three Palaearctic species:
H. cupreus Dejean, 1829 (Figure 67a,b) (with the subspecies
H. c. asaphus Antoine, 1940,
H. c. rhodopus Schauberger, 1930,
H. c. ragusae Müller, 1924,
H. c. fastuosus Faldermann, 1836) and
H. reitteri Wrase et Kataev, 2011 from the western Palaearctic, and
H. chalcentus Bates, 1873 (
Figure 66a) from eastern Asia.
Ecology. At least one of the species of this subgenus (H. cupreus) occurs in open, wet habitats, such as along the banks of ponds and swampy lowland areas with dense herbaceous vegetation.
Remarks. The least specialized subgenus within this subgroup, probably basal to the other two subgenera. It is characterized by the absence of the specialized elytral and protibial setations present in Baryharpalus subg. n. and Heteroharpalus subg. n.
The subgenus
Caloharpalus subg. n. corresponds to the
cupreus species group sensu Kataev [
8,
86] and Kryzhanovskij et al. [
56]. The taxonomy of the western Palaerctic species was studied by Schauberger [
161] and Puel [
175].
Subgenus Baryharpalus subg. n.
Type species Carabus dimidiatus Rossi, 1790.
Diagnosis. Medium-sized to large (length 9.7–13.6 mm). Body convex and stout, dark brown to black, with or without metallic luster on dorsum. Elytra either impunctate and glabrous or coarsely punctate and pubescent, with one discal setigerous pore on interval 3 and generally with several preapical pores on intervals 7 and 5, or only 7 (these pores absent in H. torosensis). Profemur of male without a dense row of setae along inner anterior margin (in some species with slightly more number of setae in male than in female). Internal sac of aedeagus with a longitudinal chain of spines and often also groups of medium-sized or small spines; a separate spine present or absent.
Etymology. The subgeneric name is a combination of the Greek barús, meaning “heavy”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises six western Palaearctic species, distributed mainly in the eastern Mediterranean:
H. dimidiatus (Rossi, 1790) (
Figure 67c,d),
H. caspius (Steven, 1806) (
Figure 66b),
H. karamani Apfelbeck, 1902,
H. murzini Kataev, 2008 and the taxonomically slightly more separated
H. trichophorus Tschitschérine, 1897 and
H. torosensis Jedlička, 1961.
Ecology. Species of this subgenus occur in moderately humid habitats, mainly near forests.
Remarks. This subgenus differs from Caloharpalus subg. n. mainly in a more convex and stout body, with elytra in most species (except for H. torosensis having punctate and pubescent elytra) with several preapical pores on the intervals 7 and 5. In punctate and pubescent elytra, H. trichophorus and H. torosensis are similar to the species of the subgenus Heteroharpalus subg. n. but distinguished from them by having protibia in male without a dense row of setae along inner anterior margin and elytral interval 3 with only one discal setigerous pore.
The subgenus
Baryharpalus subg. n. corresponds to the
dimidiatus species group sensu Kataev [
8,
86,
105] and sensu Kryzhanovskij et al. [
56].
The species with impunctate and glabrous elytra were revised by Schauberger [
65], Puel [
176] and Kataev [
105].
Subgenus Heteroharpalus subg. n.
Type species Harpalus metallinus Ménétries, 1839.
Diagnosis. Medium-sized to somewhat large (length 8.0–12.2 mm). Body moderately convex to rather flat, stout or elongate, brown to black, with or without metallic luster. Elytra generally more or less punctate and pubescent (punctures often arranged in rows along striae), more rarely (in
H. mauritanicus) impunctate and glabrous; interval 3 generally with several (up to five) discal setigerous pores (occasionally discal pores also present on interval 5 and 7, or discal pores indistinguishable among ordinary punctation); preapical pores on intervals 5 and 7 absent (present in
H. polyglyptus). Profemur of male with a more or less dense row of setae along inner anterior margin (
Figure 15a). Median lobe of aedeagus and its apical capitulum are very variable in shape in different species; internal sac in most species with groups of medium-sized or large spines, without separate spines.
Etymology. The subgeneric name is a combination of the Greek héteros, meaning “different, variable”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises eight western Palaearctic species. Seven of them are distributed mainly in the eastern Mediterranean:
H. metallinus Ménétriés, 1838 (
Figure 67e,f and
Figure 68a) (with the subspecies
H. pharisaeus Reiche et Saulcy, 1855),
H. caiphus Reiche et Saulcy, 1855),
H. jordanus Jedlička, 1964,
H. tithonus Reitter, 1900 (
Figure 67g),
H. tiridates Reitter, 1900 (
Figure 67h,i),
H. akbensis Jedlička, 1958,
H. polyglyptus Schaum, 1862, and one species, the taxonomically and geographically more separated
H. mauritanicus Gaubil, 1844, is known from Algeria.
Ecology. Species of this subgenus occur in deserts and semi-deserts, usually not far from water.
Remarks. This subgenus differs from the other two subgenera of the Caloharpalus subgroup in having a male profemur with a denser row of setae along the inner anterior margin; many species also have punctate elytra with several discal setigerous pores on the elytral interval 3.
This subgenus corresponds to the
metallinus species group sensu Jedlička [
177], sensu Kataev [
8,
86] and sensu Kryzhanovskij et al. [
56].
Idioharpalus Subgroup
Diagnosis. Same as for the subgenus.
Composition and distribution. A monobasic group, including only one western Palaearctic subgenus.
Subgenus Idioharpalus subg. n.
Type species Harpalus numidicus Bedel, 1893.
Diagnosis. Medium-sized (length 8.2–11.0 mm). Body moderately convex, somewhat elongate, brown to black, without metallic luster. Head impunctate and glabrous. Pronotum more or less widely punctate basally, with one lateral seta on each side and with glabrous basal edge. Elytra impunctate and glabrous, with glabrous basal border and with four to six discal setigerous pores on interval 3; intervals 7, occasionally also 5 and 3, with preapical pores. Prosternum with rather long setae. Profemur of male without a dense row of setae along inner anterior margin. Protibia with three, occasionally two or four, spines in a transverse row and with five or six (occasionally seven or eight) preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male not developed. Male mesotibia without a preapical callous thickening on inner margin. Abdominal sternites with more or less numerous additional setae; last visible sternite with sexual dimorphism: its apex subtruncate in male and slightly swollen (but not expanded posteriorly) in female. Median lobe of aedeagus with a short terminal lamella and a horseshoe-shaped apical capitulum; internal sac with a large group of long and narrow spines.
Etymology. The subgeneric name is a combination of the Greek idios, meaning “separate, special, one’s own”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus includes only the western Palaearctic
H. numidicus Bedel, 1893 (
Figure 68b), distributed in northwest Africa and in the south of the Iberian Peninsula.
Ecology. The only species of this subgenus occurs on fairly moist clayey soil near water bodies but at some distance from water [
40].
Remarks. This subgenus is somewhat intermediate in its morphology between the Caloharpalus subgroup and Artabas subgroup. Having generally three ventroapical spines on the protibia, it is similar to Smirnovia but differs in the presence of several discal setigerous pores on the elytral interval 3, as in many members of Heteroharpalus subg. n. and some Artabas. Unlike members of the Artabas subgroup, the only representative of Idioharpalus subg. n. has a large group of long and narrow spines in the internal sac of the aedeagus.
The subgenus
Idioharpalus subg. n. corresponds to the
numidicus species group sensu Kataev [
8].
Artabas Subgroup
Diagnosis. Head impunctate and glabrous or punctate and pubescent dorsally. Pronotum more or less punctate at least basally, with one or several lateral setae on each side and with setose or glabrous basal edge. Elytra either punctate and pubescent or impunctate and glabrous, generally with glabrous, very rarely setose, basal border; interval 3 generally with one discal setigerous pores, more rarely intervals 3, 5 and 7 also with several setigerous pores in middle of intervals. Prosternum generally with more or less long setae. Metatrochanteres with or without additional setae along posterior margin. Profemur of male without a dense row of setae along inner anterior margin. Protibia with one or three ventroapical spines in transverse row and with three to six preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male more or less prominent. Male mesotibia without a preapical callous thickening on inner margin. Abdominal sternites generally with more or less numerous additional setae; last visible sternite with pronounced sexual dimorphism: its apex rounded, truncate or concave in male and swollen and expanded posteriorly in female. Median lobe with somewhat short terminal lamella (at most slightly longer than wide) and a horseshoe-shaped apical capitulum; internal sac generally without sclerotic elements.
Composition and distribution. This subgroup comprises two western Palaearctic subgenera.
Ecology. The species of this subgroup occur on saline soils.
Remarks. In combination of distinctive characters, including male genitalia, this subgroup is most similar and apparently most closely related to the Harpalus subgroup, distinguishing from the latter by the presence of one or three ventroapical spines on the protibia, generally longer setae on the prosternum and the absence of a preapical callous thickening on the inner margin of the male mesotibia.
Subgenus Artabas Gozis, 1882
Artabas Gozis, 1882 [
138] (p. 287) (as a genus). Type species
Harpalus punctatostriatus Dejean, 1829, by monotypy.
Diagnosis. Medium-sized to somewhat large (length 5.2–12.5 mm). Body moderately convex, stout or moderately elongate, brown to black, with or without metallic luster on dorsum. Pronotum generally with several, more rarely one, lateral setae on each side (
Figure 5e,f). Elytra either punctate and pubescent or impunctate and glabrous; interval 3 generally with one discal setigerous pores adjacent to stria 2; intervals 3, 5 and 7 in some species also with several setigerous pores apically or along entire length in middle of intervals. Prosternum with comparatively long or moderately long setae. Protibia with one ventroapical spine.
Composition and distribution. This subgenus comprises 14 western Palaearctic species, distributed in the Thetyan region, mainly in the Mediterranean:
H. dispar Dejean, 1829 (
Figure 69a and
Figure 70a,b) [with the subspecies
H. d. splendens (Gebler, 1830) and
H. d. elegantulus Ménétriés, 1832],
H. kadleci Kataev et Wrase, 1995,
H. szalliesi Kataev et Wrase, 1995,
H. punctatostriatus Dejean, 1829 (
Figure 11e,f),
H. suturangulus Reitter, 1887,
H. basanicus J. Sahlberg, 1913,
H. wadiensis Jedlička, 1964,
H. rumelicus Apfelbeck, 1904,
H. petri Tschitschérine, 1902,
H. kabakianus Kataev, 1988,
H. vereschaginae Kataev, 1988, and the taxonomically more separated
H. pygmaeus Dejean, 1829 (
Figure 70c,d),
H. microthorax (Motschulsky, 1849) and
H. siculus Dejean, 1829.
Ecology. Almost all species of this subgenus are pronounced halophiles occurring along the shores of salt lakes or on salt marshes.
Remarks. Although
Artabas was originally erected for species with several lateral setae on pronotum, it should also include some species with only one such seta (
H. kabakianus,
H. vereschaginae,
H. pygmaeus,
H. microthorax and
H. siculus), since they all are very similar to each other in all other characters, including male genitalia. This is true at least for
H. kabakianus and
H. vereschaginae [
87]; the taxonomic position of
H. pygmaeus,
H. microthorax and
H. siculus needs further study, but now I also prefer to include them in
Artabas. All species of this subgenus are characterized by one ventroapical spine on the protibia.
The subgenus
Artabas corresponds to the
dispar species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8]. The taxonomy of some species of this subgenus was discussed by Kataev and Wrase [
115].
Subgenus Smirnovia Lutshnik, 1922
Smirnovia Lutshnik, 1922 [
21] (p. 62) (as a genus). Type species
Smirnovia tristis Lutshnik, 1922 (=
H. kandaharensis Jedlička, 1955), by original designation.
Diagnosis. Medium-sized (length 8.1–10.2 mm). Body moderately convex, somewhat elongate, black, without metallic luster. Pronotum with one lateral seta on each side. Elytra impunctate and glabrous, with one discal setigerous pore on interval 3 adjacent to stria 2. Prosternum with comparatively short setae. Protibia with three ventroapical spines in a transverse row. Median lobe of aedeagus strongly arcuate (
Figure 70e,f).
Composition and distribution. This subgenus includes only
H. kandaharensis Jedlička, 1955 (
Figure 69b and
Figure 70e,f), known from the deserts of southern Turan.
Ecology. The only species of this subgenus inhabits desert salt marshes.
Remarks. This subgenus is distinguished from
Artabas by having three ventroapical spines on the protibia and shorter setae on the prosternum [
85].
The subgenus
Smirnovia corresponds to the
lutshnikianus species group sensu Kryzhanovskij et al. [
56] and to the
kandaharensis species group sensu Kataev [
8].
Harpalus Subgroup
Diagnosis. Head impunctate and glabrous or punctate and pubescent dorsally. Pronotum punctate or impunctate basally, with one lateral seta on each side and with setose basal edge. Elytra either punctate and pubescent or impunctate and glabrous, generally with glabrous basal border; interval 3 with one discal setigerous pore (rarely pore absent). Metatrochanteres with or without additional setae along posterior margin. Protibia with two ventroapical spines and with four to seven preapical spines on outer margin, not forming a single row with spines on ventral surface of tibia; ventroapical tubercle in male prominent (
Figure 15b). Male mesotibia generally with a preapical callous thickening on inner margin (
Figure 15d). Tarsi glabrous or setose dorsally. Abdominal sternites generally with more or less numerous additional setae; last visible sternite with pronounced sexual dimorphism: its apex subtruncate or more or less deeply concave in male (
Figure 15e,g–i) and swollen and expanded posteriorly in female (
Figure 15f). Median lobe with somewhat short terminal lamella and a horseshoe-shaped apical capitulum; internal sac generally without large spines, in most species with small spiny patches and/or groups of small spines, often without any sclerotic elements.
Composition and distribution. This subgroup comprises one Holarctic and five Palaearctic subgenera.
Ecology. Representatives of this subgroup live in open landscapes, with many species within the steppe or desert zone, but they are usually found there in relatively humid places, less often in rather dry habitats. Some species are halophilous.
Remarks. This subgroup is well defined by the characters listed in the diagnosis and certainly represents a monophyletic unit. Its main synapomorphies are: the pronounced dimorphism of the last abdominal sternite, protibia and mesotibia, the setose basal edge of pronotum and two ventroapical spines on the protibia [
86].
Subgenus Pachyharpalus subg. n.
Type species Harpalus crates Bates, 1883.
Diagnosis. Large (length 11.5–15.8 mm). Body moderately convex, stout, brown to black, usually with metallic luster on dorsum, glabrous dorsally. Pronotum with rounded sides and widely rounded basal angles. Elytra impunctate, with glabrous basal border and with small humeral denticle; preapical sinuation indistinct; sutural angle of female slightly blunted. Metacoxa without additional setigerous pores medially. Metafemur with five to eight setigerous pores along posterior margin. Metatrochanteres without additional setae along posterior margin. Protibia with six or seven preapical spines on outer margin. Tarsi glabrous dorsally. Male mesotibia occasionally with very small, almost indistinct preapical callous thickening on inner margin. Apex of last visible abdominal sternite of male subtruncate. Median lobe of aedeagus with a group of small spines in internal sac medially (Figure 72a,b).
Etymology. The subgeneric name is a combination of the Greek pakhús, meaning “thick”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus only includes the eastern Palaearctic
H. crates Bates, 1883 (
Figure 71a and
Figure 72a,b), distributed in eastern Asia.
Ecology. The only species of this subgenus occurs in open, moderately dry habitats, mainly in meadows but also in various disturbed anthropogenic habitats.
Remarks. The single species of this subgenus is similar to species of the subgenus Harpalus s. str. with impunctate and glabrous elytra but differs from them mainly in having protibia with a larger number (six or seven) of preapical spines on the outer margin of the protibia, elytra with a humeral denticle, an indistinct preapical sinuation and a blunted (in female) sutural angle. In the latter character and in metacoxa without additional setigerous pores medially, Pachyharpalus subg. n. also differs from all other subgenera of the Harpalus subgroup.
The subgenus
Pachyharpalus subg. n. corresponds to the
crates species group sensu Kataev [
8,
86] and sensu Kryzhanovskij et al. [
56].
Subgenus Proteonus Fischer von Waldheim, 1829
Proteonus Fischer von Waldheim, 1829 [
134] (p. 21) (as a genus). Type species
Carabus distinguendus Duftschmid, 1812, designated by Bousquet [
135].
Lasioharpalus Reitter, 1900 [
38] (pp. 75, 86) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus subangulatus Reitter, 1900, designated by Antoine [
40].
Lasiarpalus: Jakobson, 1907 [
63] (p. 378) (print error).
Diagnosis. Medium-sized (length 7.5–10.8 mm). Body moderately convex, somewhat elongate, brown to black, often with metallic luster on dorsum, glabrous dorsally. Pronotum with distinct basal angles (sharp to narrowly rounded at apex) and sides usually straight or sinuate basally. Elytra impunctate, with glabrous basal margin; humeri more or less rounded, with a small denticle, or angulate, without denticle; preapical sinuation very shallow or indistinct; sutural angle of female generally acute and prominent posteriorly. Metacoxa with additional setigerous pores medially. Metafemur with five to seven setigerous pores along posterior margin. Metatrochanteres without additional setae along posterior margin. Protibia with four to five preapical spines on outer margin. Male mesotibia often with almost indistinct preapical callous thickening on inner margin. Tarsi glabrous dorsally. Apex of last visible abdominal sternite of male generally not markedly concave (at most very shallowly emarginate). Internal sac of aedeagus without sclerotic elements or with small spiny patches or a group of few small spines.
Composition and distribution. This subgenus comprises four western Palaearctic species, distributed mainly in the western part of the region:
H. distinguendus (Duftschmid, 1812) (
Figure 72c,d) (with the subspecies
H. kidanicus Kataev, 1989),
H. contemptus Dejean, 1829,
H. saxicola Dejean, 1829 and
H. angulatus Putzeys, 1878 (
Figure 71b and
Figure 72e,f) (with the subspecies
H. a. subangulatus Reitter, 1900 and
H. a. scytha Tschitschérine, 1899).
Ecology. Species of this subgenus occur in meadows and in steppe habitats, H. distinguendus is often also in destroyed antropogenic biotopes and agricultural fields.
Remarks. The species of this subgenus are very similar in their distinctive characters to those of Harpalus s. str. with impunctate and glabrous elytra and of Paraharpalus subg. n. but can be distinguished from them by the elytral humer either angulate, without denticle, or rounded, with a denticle at the tip; in addition, the pronotum is generally with more distinct basal angles and usually with straight or sinuate sides basally; Proteonus also differs from Harpalus s. str. in having the last abdominal sternite of male not markedly concave at the apex.
The subgenus
Proteonus corresponds to the
distinguendus species group sensu Kataev [
8,
86] and sensu Kryzhanovskij et al. [
56]. Some species of this subgenus were revised by Puel [
178] and Mlynář [
76]. The
distinguendus species group sensu Puel [
178] also includes the species of
Paraharpalus subg. n. Subgenus Paraharpalus subg. n.
Type species Harpalus oblitus Dejean, 1829.
Diagnosis. Medium-sized to large (length 7.1–12.4 mm). Body moderately convex, moderately robust to somewhat elongate, brown to black, often with metallic luster on dorsum, glabrous dorsally. Pronotum generally with rounded sides and widely rounded basal angles; more rarely basal angles more or less angulate, blunted at apex. Elytra impunctate, with glabrous basal border and generally with rounded humeral angle lacking denticle; preapical sinuation rather deep, often with a denticle at its base; sutural angle of female generally acute and prominent posteriorly. Metacoxa with additional setigerous pores medially. Metafemur with five to seven setigerous pores along posterior margin. Metatrochanteres without additional setae along posterior margin. Protibia with five to seven preapical spines on outer margin. Male mesotibia often with almost indistinct preapical callous thickening on inner margin. Tarsi glabrous dorsally. Apex of last visible abdominal sternite of male not concave (at most slightly truncate). Internal sac of aedeagus with characteristic armament consisting of one or two groups of small spines medially.
Etymology. The subgeneric name is a combination of the Greek pará, meaning “near”, and the name of the carabid taxon Harpalus.
Composition and distribution. This subgenus comprises six western Palaearctic species, distributed in the Tethyan region, mainly in the Mediterranean:
H. oblitus Dejean, 1829 (Figure 74a,b) (with the subspecies
H. o. patruelis Dejean, 1829),
H. akinini Tschitschérine, 1895,
H. lateralis Dejean, 1829,
H. smyrnensis Heyden, 1888 (
Figure 73a) (with the subspecies
H. s. raddei Tschitschérine, 1897 and
H. s. medicus Kataev, 1993),
H. quadratus Chaudoir, 1846 and
H. subtruncatus Chaudoir, 1846.
Ecology. The species of this subgenus inhabit arid landscapes, steppes and semi-deserts, but they usually occur in humid places; some species are found on saline soils.
Remarks. The species of this subgenus are very similar in their distinctive characters to those of Harpalus s. str. with impunctate and glabrous elytra differing from them in having the last abdominal sternite of male not concave and the aedeagus with a characteristic armament consisting of one or two groups of small spines.
The subgenus
Paraharpalus subg. n. corresponds to the
oblitus species group sensu Kataev [
8,
86,
92] and sensu Kryzhanovskij et al. [
56]. A revision of the species of this group was published by Kataev [
92]. Some species were previously revised by Puel [
178].
Subgenus Harpalus Latreille, 1802
Harpalus Latreille, 1802 [
10] (p. 92) (as a genus). Type species
Carabus proteus Paykull, 1790 (=
C. affinis Schrank, 1781), designated by Andrewes [
12], tentatively accepted as type species.
Harpaleus Billberg, 1820 [
121] (p. 23) (unjustified emendation).
Bioderus Motschulsky, 1848 [
59] (p. 487) (as a genus). Type species
Microderus petreus Motschulsky, 1844 (=
Anisodactylus obtusus, Gebler, 1833), by monotypy.
Harpalomerus Casey, 1914 [
71] (p. 76) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus amputatus Say, 1830, designated by Lindroth [
15].
Diagnosis. Medium-sized to large (length 7.0–13.0 mm). Body moderately convex, somewhat elongate, brown to black, often with metallic luster on dorsum, glabrous dorsally or pubescent on pronotum and elytra. Pronotum with more or less widely rounded sides and basal angles. Elytra either impunctate or punctate throughout or laterally; basal border glabrous (very rarely setose); humeral angle rounded, generally lacking denticle; preapical sinuation rather deep, often with a denticle at its base, rarely very shallow; sutural angle of female generally acute and prominent posteriorly. Metacoxa with additional setigerous pores medially, rarely without them. Metafemur with five to eight setigerous pores along posterior margin. Metatrochanteres without additional setae along posterior margin. Protibia with four to five preapical spines on outer margin. Tarsi generally glabrous or very sparsely setose dorsally. Apex of last visible abdominal sternite of male distinctly concave (
Figure 15g–i). Internal sac of aedeagus with groups of small spines and/or distinct spiny patches medially, in some species also with a separate spine.
Composition and distribution. This subgenus has a Holarctic distribution; it includes one Holarctic and 13 Palaearctic species, with two centers of diversity—in the western Mediterranean and in the eastern part of the Tethyan region; among them, one species,
H. affinis (Schrank, 1781) (
Figure 74c,d), has a trans-Palaearctic distribution.
The fauna of the west Mediterranean region includes two endemic species: H. semipunctatus Dejean, 1829 and H. lethierryi Reiche, 1859 (with the subspecies H. l. azrouanus Emden et Schauberger, 1932).
The fauna of the eastern part of the Tethyan region comprises eleven species, ten (except for
H. affinis) Palaearctic:
H. tjanschanicus Semenov, 1889,
H. glasunovi Kataev, 1987 (with the subspecies
H. g. spinulifer Kataev, 2006 and
H. g. opaculus Kataev, 2006),
H. anisodactyliformis Solsky, 1874,
H. bucharicus Tschitschérine, 1898,
H. michailovi Kataev, 1987,
H. kabakovi Kataev, 1987 (
Figure 73b),
H. caeruleatus Bates, 1878,
H. uniformis Motschulsky, 1844 (with the subspecies
H. u. staudingeri Jedlička, 1953),
H. ganssuensis Semenov, 1889 (
Figure 74e,f) and
H. erosus Mannerheim, 1825, and one Holarctic species:
H. amputatus Say, 1830 [with the Palaearctic subspecies
H. a. amputatoides Mlynář, 1979,
H. a. obtusus (Gebler, 1833), and
H. a. inschanicus Breit, 1914]; the nominotypical subspecies of the latter species is widely distributed in North America.
Ecology. The species of this subgenus, like the previous one, usually inhabit arid and semi-arid landscapes, steppes and semi-deserts, both on the plain and in the mountains, but they occur usually in humid places; some species occur on saline soils.
Remarks. The most important features of this subgenus, distinguishing it from other representatives of this subgroup, are as follows: the basal pronotal angles rounded, the elytra with a rounded humeral angle without denticle and with a deep preapical sinuation, the tarsi glabrous dorsally and the apex of the last abdominal sternite of male distinctly concave.
The nominotypical subgenus, as it is treated here, corresponds to the
affinis species group sensu Kataev [
8,
86] and sensu Kryzhanovskij et al. [
56]. A revision of this group was published by Kataev [
86], with a later addition [
104]. Lindroth [
15] and Noonan [
16] treated the single Nearctic species,
H. amputatus, as a member of the monobasic
amputatus species group corresponding to
Harpalomerus.
Subgenus Harpalophonus Ganglbauer, 1892
Harpalophonus Ganglbauer, 1892: 341, 346 [
36] (as a subgenus of
Ophonus Dejean, 1821). Type species
Harpalus hospes Sturm, 1818, by monotypy.
Diagnosis. Medium-sized to large (length 8.3–14.0 mm). Body moderately convex, somewhat elongate, brown to black, often with metallic luster on dorsum. Head impunctate and glabrous dorsally. Pronotum with rounded sides and widely rounded basal angles. Elytra punctate and pubescent throughout or laterally, with setose basal border and rounded humeral angle without denticle; preapical sinuation more or less deep, often with a denticle at its base; sutural angle of female generally acute and prominent posteriorly. Metacoxa with additional setigerous pores medially. Metafemur with five to seven setigerous pores along posterior margin. Metatrochanteres without additional setae along posterior margin. Protibia with four to five preapical spines on outer margin. Tarsi densely setose dorsally. Apex of last visible abdominal sternite of male distinctly concave. Internal sac of aedeagus with one or two groups of small spines medially, in some species also with a spiny patch.
Composition and distribution. This subgenus comprises seven western Palaearctic species, distributed in the Tethyan region:
H. hospes Sturm, 1818 (Figure 76a,b) [with the subspecies
H. h. ciscaucasicus Lutshnik, 1921 and
H. h. armenus (Daniel, 1904)],
H. kagyzmanicus Kataev, 1984,
H. mairei Peyerimhoff, 1928,
H. italus Schaum, 1860,
H. circumpunctatus Chaudoir, 1846,
H. terrestris (Motschulsky, 1844) and
H. stevenii Dejean, 1829 (
Figure 75a).
Ecology. Species of this subgenus inhabit arid and semi-arid territories, often found near water bodies, on more or less saline soils.
Remarks. This taxon was previously either included in the genus
Ophonus ([
14,
36,
37,
40], etc.) or considered as a subgenus or even a synonym of
Pseudoophonus [
6,
28,
29,
30,
38]. My research [
83,
86] fully confirmed the opinion of Mlynář [
53,
75] that
Harpalophonus is very similar and undoubtedly very closely related to the subgenus
Harpalus s. str. since the only character distinguishing it from the latter subgenus is densely punctate and pubescent tarsomeres [
86].
The subgenus
Harpalophonus corresponds to the
hospes species group sensu Kryzhanovskij et al. [
56] and sensu Kataev [
8]. The revision of the subgenus was published by Kataev [
83], with a subsequent addition [
110].
Hapalus altaicus Jedlička, 1968, which was regarded as a distinct species of
Harpalophonus in Kataev [
83], was later treated as a synonym of the nominotypical subspecies of
H. uniformis (Motschulsky, 1844) of the subgenus
Harpalus s. str. [86, 100).
Subgenus Cephalotypsis Tschitschérine, 1901
Cephalotypsis Tschitschérine, 1901 [
20] (p. 238) (as a subgenus of
Harpalus Latreille, 1802). Type species
Harpalus semenowi Tschitschérine, 1901, by monotypy.
Diagnosis. Large (length 11.2–14.5 mm). Body moderately convex, elongate, brown to dark brown, with metallic luster on dorsum. Head, pronotum and elytra punctate and pubescent dorsally. Pronotum with rounded sides and widely rounded basal angles. Elytra with setose basal border and rounded humeral angle lacking denticle; preapical sinuation absent; sutural angle of female generally acute and prominent posteriorly. Metacoxa with additional setigerous pores medially. Metafemur with numerous (more than ten) setigerous pores along posterior margin. Metatrochanteres with several additional setae along posterior margin. Protibia with five to six preapical spines on outer margin. Tarsi glabrous dorsally. Apex of last visible abdominal sternite of male distinctly concave. Internal sac with two groups of medium-sized spines, occasionally also with a small separate spine.
Composition and distribution. This subgenus includes two species from the Tethyan region of Asia:
H. semenowi Tschitschérine, 1901 and
H. kryzhanovskii Kataev, 1988 (
Figure 75b and
Figure 76c,d).
Ecology. Both species of this subgenus inhabit deserts.
Remarks. This subgenus is similar to the subgenera Harpalus s. str. and Harpalophonus in many characters including the general habitus, elytra punctate and pubescent, humeral angle rounded, without denticle and last abdominal sternite of male deeply concave. Cephalotypsis distinctly differs from them in having head punctate and pubescent, elytra without preapical sinuation, metafemur with numerous (more than ten) setigerous pores along posterior margin and metatrochanter with several additional setae along posterior margin. In addition, Cephalotypsis differs from Harpalophonus in tarsi glabrous dorsally.
The subgenus
Cephalotypsis corresponds to the
semenowi species group sensu Kataev [
8,
86] and sensu Kryzhanovskij et al. [
56].