Morphological and Molecular Study of the Fish Parasitic Crustaceans Cymothoa indica and Mothocya collettei (Isopoda: Cymothoidae), with New Distribution Records
Abstract
1. Introduction
2. Materials and Methods
2.1. Material for Morphological Examination
2.2. Molecular Analysis
3. Results
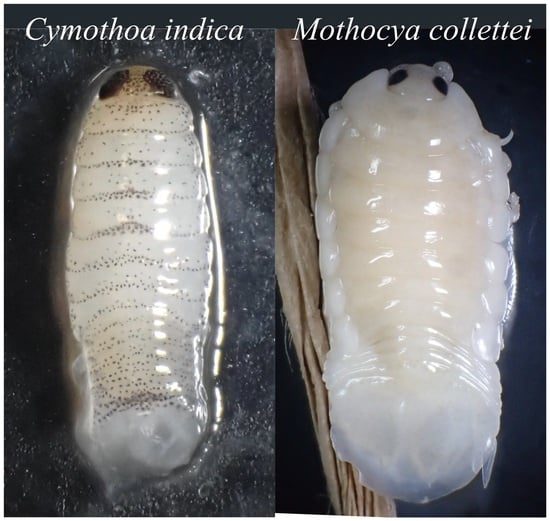
4. Description
- Order: Isopoda Latreille, 1816
- Superfamily: Cymothooidea Leach, 1814
- Family: Cymothoidae Leach, 1814
- Genus: Cymothoa Fabricius, 1793
- Cymothoa indica Schioedte and Meinert, 1884
- [New standard Japanese name: Minami-uonoe]
- Genus: Mothocya Costa in Hope, 1851.
- Mothocya collettei Bruce, 1986.
- [New standard Japanese name: Okizayori-eranushi].
5. Discussion
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyko, C.; Bruce, N.; Hadfield, K.; Merrin, K.; Ota, Y.; Poore, G.; Taiti, S. World Marine, Freshwater and Terrestrial Isopod Crustaceans Database. Cymothoidae Leach, 1818. World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=118274 (accessed on 22 January 2023).
- Zhang, Z.Q.; Hooper, J.N.A.; Van Soest, R.W.M.; Pisera, A.; Crowther, A.L.; Tyler, S.; Schilling, S.; Eschmeyer, W.N.; Fong, J.D.; Blackburn, D.C.; et al. Animal biodiversity: An outline of higher-level classification and taxonomic richness. Zootaxa 2011, 3148, 7–12. [Google Scholar] [CrossRef]
- Yamauchi, T. Cymothoid isopods (Isopoda: Cymothoidae) from fishes in Japanese waters. Cancer 2016, 25, 113–119. (In Japanese) [Google Scholar]
- Smit, N.J.; Bruce, N.L.; Hadfield, K.A. Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae. Int. J. Parasitol. Parasites Wildl. 2014, 3, 188–197. [Google Scholar] [CrossRef]
- Brusca, R.C. Studies on the cymothoid fish symbionts of the eastern Pacific (Isopoda, Cymothoidae) I. Biology of Nerocila californica. Crustaceana 1978, 34, 141–154. [Google Scholar] [CrossRef]
- Brusca, R.C. Studies on the cymothoid fish symbionts of the eastern Pacific (Crustacea: Isopoda: Cymothoidae). II Systematics and biology of Lironeca vulgaris Stimpson. Allan Hancock Found. Publ. Occas. Pap. New Ser. 1978, 2, 1–19. [Google Scholar]
- Brusca, R.C. A monograph on the Isopoda Cymothoidae (Crustacea) of the eastern Pacific. Zool. J. Linn. Soc. 1981, 73, 117–199. [Google Scholar] [CrossRef]
- Fujita, H.; Umino, T.; Saito, N. Molecular identification of the aegathoid stage of Anilocra clupei (Isopoda: Cymothoidae) parasitizing sweeper Pempheris sp. (Perciformes: Pempheridae). Crustac. Res. 2021, 50, 29–31. [Google Scholar] [CrossRef]
- Fujita, H.; Kawai, K.; Deville, D.; Umino, T. Quatrefoil light traps for free-swimming stages of cymothoid parasitic isopods and seasonal variation in their species compositions in the Seto Inland Sea, Japan. Int. J. Parasitol. Parasites Wildl. 2023, 20, 12–19. [Google Scholar] [CrossRef]
- Fujita, H.; Kawai, K.; Taniguchi, R.; Tomano, S.; Sanchez, G.; Kuramochi, T.; Umino, T. Infestation of the parasitic isopod Mothocya parvostis on juveniles of the black sea bream Acanthopagrus schlegelii as an optional intermediate host in Hiroshima Bay. Zool. Sci. 2020, 37, 544–553. [Google Scholar] [CrossRef]
- Fujita, H.; Saito, N.; Okuno, J.; Moritaki, T.; Yamauchi, T. New distribution record of the Fish parasitic crustacean Cinusa nippon (Isopoda: Cymothoidae), with a description of the manca. Jpn. J. Appl. Entomol. Zool. 2023, 67, 37–45. [Google Scholar] [CrossRef]
- Bruce, N.L. Revision of the isopod crustacean genus Mothocya Costa, in Hope, 1851 (Cymothoidae, Flabellifera), parasitic on marine fishes. J. Nat. Hist. 1986, 20, 1089–1192. [Google Scholar] [CrossRef]
- Welicky, R.L.; Smit, N.J. Redescription and molecular characterisation of the fish ectoparasite Anilocra capensis Leach, 1818 (Isopoda: Cymothoidae), with description of six new species of Anilocra Leach, 1818 from Africa. Parasites Vectors 2019, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Aneesh, P.T.; Kawai, K.; Kitamura, S.I.; Shimomura, M.; Umino, T.; Ohtsuka, S. Redescription and molecular characterization of Mothocya parvostis Bruce, 1986 (Crustacea: Isopoda: Cymothoidae) parasitic on Japanese halfbeak, Hyporhamphus sajori (Temminck & Schlegel, 1846) (Hemiramphidae) with Mothocya sajori Bruce, 1986 placed into synonymy. Zootaxa 2023, 5277, 259–286. [Google Scholar] [PubMed]
- Seno, H. Atherinidae. In Fishes of Japan with Pictorial Keys to the Species, 3rd ed.; Nakabo, T., Ed.; Tokai University Press: Hadano, Japan, 2013; pp. 642–644. (In Japanese) [Google Scholar]
- Takahashi, H. New method of specimen drawing. Nanyo Seibutu 2011, 16, 71–73. (In Japanese) [Google Scholar]
- Aneesh, P.T.; Helna, A.K.; Trilles, J.P.; Chandra, K. Occurrence and redescription of Anilocra leptosoma Bleeker, 1857 (Crustacea: Isopoda: Cymothoidae) parasitizing the clupeid fish Tenualosa toli (Valenciennes) from the Arabian Sea, India. Mar. Biodivers. 2019, 49, 443–450. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene-sequences and a compilation of conserved polymerase chain-reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Löytynoja, A.; Goldman, N. webPRANK: A phylogeny-aware multiple sequence aligner with interactive alignment browser. BMC Bioinform. 2010, 11, e579. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Martin, M.B.; Bruce, N.L.; Nowak, B.F. Review of the fish-parasitic genus Cymothoa Fabricius, 1793 (Crustacea: Isopoda: Cymothoidae) from Australia. Zootaxa 2016, 4119, 1–72. [Google Scholar] [CrossRef]
- Al-Zubaidy, A.B.; Mhaisen, F.T. The blue spot mullet Moolgarda seheli (Forsskål, 1775) a new host for the crustacean parasite Cymothoa indica. Am. J. Biol. Life Sci. 2014, 2, 58–62. [Google Scholar]
- Martin, M.B. Taxonomy and Phylogeny of the Buccal-Attaching Cymothoidae (Crustacea: Isopoda) of Australia. Ph.D. Thesis, University of Tasmania, Launceston, Australia, 2015. [Google Scholar]
- Aneesh, P.T.; Kappalli, S.; Kottarathil, H.A.; Gopinathan, A. Mothocya renardi (Bleeker, 1857) (Crustacea: Isopoda: Cymothoidae) parasitising Strongylura leiura (Bleeker) (Belonidae) off the Malabar coast of India: Redescription, occurrence and life-cycle. Syst. Parasitol. 2016, 93, 583–599. [Google Scholar] [CrossRef]
- Jones, C.M.; Miller, T.L.; Grutter, A.S.; Cribb, T.H. Natatory-stage cymothoid isopods: Description, molecular identification and evolution of attachment. Int. J. Parasitol. 2008, 38, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.K.; Mohanty, S.R.; Behera, R.K.; Seth, J.K.; Mohapatra, A. First record of Mothocya renardi and Mothocya collettei (Isopoda: Cymothoidae) from northern part of East Coast of India and new host record of Mothocya collettei. J. Parasit. Dis. 2021, 45, 651–654. [Google Scholar] [CrossRef]
- Aneesh, P.T.; Kottarathil, H.A.; Kappaili, S. Branchial cymothoids infesting the marine food fishes of Malabar coast. J. Parasit. Dis. 2016, 40, 1270–1277. [Google Scholar] [CrossRef]
- Bruce, N.L.; Wong, H.P.S. An overview of the marine Isopoda (Crustacea) of Singapore. Raffles Bull. Zool. 2015, 31, 152–168. [Google Scholar]
- Hata, H.; Sogabe, A.; Tada, S.; Nishimoto, R.; Nakano, R.; Kohya, N.; Takeshima, H.; Kawanishi, R. Molecular phylogeny of obligate fish parasites of the family Cymothoidae (Isopoda, Crustacea): Evolution of the attachment mode to host fish and the habitat shift from saline water to freshwater. Mar. Biol. 2017, 164, 105. [Google Scholar] [CrossRef]
- Nagasawa, K. Mothocya collettei Bruce, 1986 (Isopoda, Cymothoidae), a marine fish parasite new to Japan. Crustaceana 2017, 90, 613–616. [Google Scholar] [CrossRef]
- Hadfield, K.A.; Bruce, N.L.; Smit, N.J. Review of Mothocya Costa, in Hope, 1851 (Crustacea: Isopoda: Cymothoidae) from southern Africa, with the description of a new species. Afr. Zool. 2015, 50, 147–163. [Google Scholar] [CrossRef]
- Saito, N.; Yamauchi, T. A new species and new host records of the genus Elthusa (Crustacea: Isopoda: Cymothoidae) from Japan. Crustac. Res. 2016, 45, 59–67. [Google Scholar] [CrossRef]
- Nagasawa, K. Cinusa nippon n. sp. (Isopoda: Cymothoidae) parasitic in the buccal cavity of coastal puffers (Tetraodontiformes: Tetraodontidae) from Japan, with the first specimen-based record of the isopod genus from the Pacific region. Zool. Sci. 2021, 38, 359–369. [Google Scholar] [CrossRef]
- Nagasawa, K. Norileca aff. triangulata (Isopoda: Cymothoidae), a branchial cavity parasite of the African sailfin flyingfish, Parexocoetus mento (Beloniformes: Exocoetidae), from southern Japan, with a new Japanese record of the isopod genus. Crustac. Res. 2021, 50, 55–64. [Google Scholar]
- Kawanishi, R.; Miyazaki, Y.; Satoh, P. Mothocya kaorui n. sp. (Crustacea: Isopoda: Cymothoidae), a fish-parasitic isopod with unique antennules from the Izu Islands, Japan. Syst. Parasitol. 2023, 100, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.H.; Bunkley-Williams, L.; Dyer, W.G. Metazoan parasites of some Okinawan coral reef fishes with a general comparison to the parasites of Caribbean coral reef fishes. Galaxea 1996, 13, 1–13. [Google Scholar]
- Nagasawa, K. Anilocra prionuri (Isopoda: Cymothoidae), a marine fish ectoparasite, from the northern Ryukyu Islands, southern Japan, with a note on a skin wound of infected fish. Crustac. Res. 2018, 47, 29–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fujita, H.; Kawai, K.; Deville, D.; Umino, T. Molecular and morphological characterizations of the fish parasitic isopod Mothocya parvostis (Crustacea: Cymothoidae) parasitizing optional intermediate hosts: Juveniles of the cobaltcap silverside Hypoatherina tsurugae and yellowfin seabream Acanthopagrus latus. Zool. Stud. 2023, 62, e21. [Google Scholar]
- Shen, Y.Y.; Chen, X.; Murphy, R.W. Assessing DNA Barcoding as a Tool for Species Identification and Data Quality Control. PLoS ONE 2013, 8, e57125. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, H. Morphological and Molecular Study of the Fish Parasitic Crustaceans Cymothoa indica and Mothocya collettei (Isopoda: Cymothoidae), with New Distribution Records. Diversity 2023, 15, 969. https://doi.org/10.3390/d15090969
Fujita H. Morphological and Molecular Study of the Fish Parasitic Crustaceans Cymothoa indica and Mothocya collettei (Isopoda: Cymothoidae), with New Distribution Records. Diversity. 2023; 15(9):969. https://doi.org/10.3390/d15090969
Chicago/Turabian StyleFujita, Hiroki. 2023. "Morphological and Molecular Study of the Fish Parasitic Crustaceans Cymothoa indica and Mothocya collettei (Isopoda: Cymothoidae), with New Distribution Records" Diversity 15, no. 9: 969. https://doi.org/10.3390/d15090969
APA StyleFujita, H. (2023). Morphological and Molecular Study of the Fish Parasitic Crustaceans Cymothoa indica and Mothocya collettei (Isopoda: Cymothoidae), with New Distribution Records. Diversity, 15(9), 969. https://doi.org/10.3390/d15090969