Abstract
The taxonomic placements of Chaenomeles Lindl. (Rosaceae) and their intrageneric species have long been controversial. This research aims to explore the palynomorphological characters of all Chaenomeles extant species in detail and to compare the results with phylogenetic relationships and the taxonomic classification scheme. The pollen morphology of 30 individuals of six taxa of Chaenomeles was investigated using both light microscopy and scanning electron microscopy. The pollens were measured, observed and statistically analyzed for 12 quantitative features and 2 qualitative ones. The study revealed that the Chaenomeles pollens are monad, tri-colporate, medium in size (P = 32.78–42.74 μm, E = 30.42–36.31 μm) and prolatespheroidal to subprolate in shape (P/E = 0.98–1.35). Based on exine ornamentation observed under SEM, two sexine sculpture types (type I—striate with microperforations, type II—striate with macroperforations) and two subtypes (twisted-striate, reticulate-striate) were recognized in the genus. Statistical analysis identified some pollen characteristics with diagnostic importance, including pollen shape, colpus dimension, length or direction of striae and diameter of perforation. These characteristics may have diagnostic and taxonomic value for the genus Chaenomeles and the family Rosaceae. An artificial key to studied species, based on pollen micromorphological attributes, is also provided. The obtained result was basically consistent with that of molecular studies published earlier. This study, for the first time, provides palynological evidence for the hybrid origin hypothesis of C. sinensis and supports the placement of this species in the monotypic genus Pseudocydonia.
1. Introduction
The genus Chaenomeles Lindley belongs to the tribe Maleae of the family Rosaceae and is a small group of trees and shrubs distributed in temperate areas of East Asia [,,]. Chaenomeles species have long been widely appreciated as ornamentals in Asia and Europe, and their fruits, commonly known as Mugua in China, have demonstrated considerable potential in the food and pharmaceutical industries [,,]. According to the classifications of Flora of China [], it comprises five wild diploid (2n = 34) species, which are C. sinensis (Dum.Cours.) Koehne, C. cathayensis (Hemsl.) Schneider, C. japonica (Thunb.) Lindl., C. speciosa (Sweet) Nakai and C. thibetica Yü [,,]. Interspecific hybrids are of common occurrence in all possible combinations between three of the species (C. cathayensis, C. japonica and C. speciosa), and hybrid taxa produced from artificial breeding have consequently been described [,]. Molecular and morphological evidence of spontaneous interspecific hybridization has also been reported between C. cathayensis and C. speciosa, which are sympatrically distributed in Yunnan province [,].
The taxonomic placements of Chaenomeles or the intrageneric species remain controversial. This genus was initially established in 1822 by Lindley based on the type species C. japonica. The last member, C. thibetica Yü, was described in 1963 [], and was not in the checklist of Maloideae until 1990 []. Morphologically, Chaenomeles was considered closely related to Cydonia Mill. and Docynia Decne since they share multi-ovulate carpels, a character distinct from the remainders in Maleae []. Based on molecular evidence, Chaenomeles, nevertheless, has an uncertain position, either grouped with Cydonia, then sistered together to a combined clade of Sorbus L., Photinia Lindl. and Stranvaesia Lindl. [] (Zhang et al. unpublished), or sistered to the clade of Docynia and Malus Mill. [,]. Weber (1964) reported that Cydonia, Malus and Pyrus L. could hybridize among themselves; thus, the various positions of Chaenomeles in different datasets suggests that the evolutionary origin of this genus may be complex, and likely involved hybridization [].
In Chaenomeles, the taxonomic status of C. sinensis has been changed over time and still remains debatable. This species was firstly assigned to Cydonia by Roemer (1847) []. Koehne (1890) [], followed by Rehder (1940) and Yü (1974) [], transferred it to Chaenomeles due to the glabrous fruits. Since C. sinensis is morphologically intermediate in some characteristics between Cydonia and its congeners, Schneider (1906) [] established a new section in Chaenomele for C. sinensis, and later proposed to erect a new monotypic genus, viz. Pseudocydonia (C. K. Schneid.) C. K. Schneid., including the one species Pseudocydonia sinensis (Thouin) C. K. Schneid. This arrangement was widely accepted by Hillier (1981) [], Robertson et al. (1991) [], Kalkman (2004) [] and Potter et al. (2007) []. Loutfy et al. (1999) [], based on seed coat anatomy, support the merging of C. sinensis in the genus Cydonia as presented in Mabberley (1997) []. Nevertheless, current classification in Flora of China [] and The Plant List (www.plantlist.org, (accessed on 22 March 2022)) reclassified this species to Chaenomeles and listed Pseudocydonia sinensis as a synonym. Therefore, the major disagreements about its status focus on whether C. sinensis should be treated as a monotypic genus or a species of Chaenomeles. In molecular phylogenetic studies, recent efforts to resolve the relationships in Chaenomeles obtained incongruent results. Liu et al. (2019) [] and Sun et al. (2020) [], based on the whole plastome, resolved Chaenomeles as a monophyletic group with C. sinensis occupying a basal position. In contrast, Xiang et al. (2017) [] and Zhang et al. (unpublished), based on hundreds of low-copy nuclear genes, rejected the monophyly of Chaenomeles; they inferred C. sinensis and Cydonia to be closely related, while the rest of the taxa in Chaenomeles were understood to form a separate clade. Considering the topological conflict, Lo and Donoghue (2012) [] speculated that C. sinensis likely originated from hybridization. As for the three species traditionally assigned to Chaenomeles, several molecular studies found that the distance between C. cathayensis and C. japonica was the most distant, with C. speciosa occupying an intermediate position [,], whereas others instead supported a sister relationship of C. japonica and C. cathayensis [,]. In addition to the uncertain relationships mentioned above, the position of C. thibetica and hybrid taxa (e.g., C. ×superba) also remains to be clarified. As a consequence, the infrageneric relationships and taxonomy of Chaenomeles have not been confidently resolved, owing to their similar overlapping morphological characters and the molecular evidence.
Pollen morphology has great significance in plant taxonomy. It is well acknowledged that pollen morphological characters, as extra diagnostic traits, are often utilized in taxonomy or phylogenetic relationship reconstruction of Rosaceae at the genus [,,,,] and species [,,,,] levels. However, the only palynological study carried out so far on Chaenomeles handled pollens by a conventional method [], which failed to illustrate the excellent pollen features by SEM (with obvious shrinkage and deformation). Therefore, the morphologically and functionally important details (pollen size, sexine ornamentation, aperture state, etc.) about the pollen morphology of Chaenomeles are still scarce, and the taxonomic value of qualitative and quantitative data has not been evaluated.
Therefore, the present study was designed with the aims (1) to document and illustrate the morphology of optimally preserved pollen materials of the taxonomically problematic genus Chaenomeles using both light and scanning electron microscopy; (2) to identify informative (qualitative and quantitative) pollen features of taxonomical significance; (3) to categorize Chaenomeles taxa based on statistical analysis (PCA and cluster analysis), and evaluate whether the results support molecular-based phylogeny, viz. testing the hybrid origin hypothesis of C. sinensis from the perspective of palynology.
2. Materials and Methods
2.1. Plant Sampling and Identification
We collected 30 Chaenomeles species samples covering all currently accepted taxa of Chaenomeles, including five species and one interspecific hybrid cultivar. The collected living materials were photographed (Figure 1) and identified on the basis of characters documented in Flora of China []. The botanical names were conformed from the International Plant Name Index (http://www.ipni.org (accessed on 22 March 2022)) and The Plant List (http://www.theplantlist.org/ (accessed on 22 March 2022)). Properly dried and mounted specimens were submitted to the Herbarium of Henan Agricultural University (HEAC) and the voucher numbers, along with the collection area and collector, are given in Table S1. Pollen grains from fresh mature but not dehiscent anthers were taken with sterilized forceps.
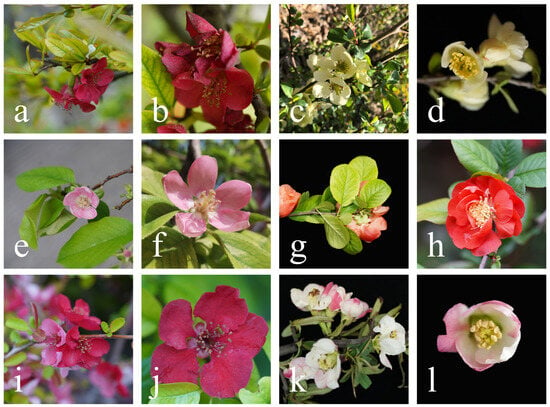
Figure 1.
Inflorescence morphology of Chaenomeles cathayensis (a,b), C. japonica (c,d), C. sinensis (e,f), C. speciosa (g,h), C. ×superba (i,j) and C. thibetica (k,l).
2.2. Observations Using LM
For light microscopy studies, pollens were treated by standard acetolysis according to Erdtman’s method []. The pollens collected from each sample were washed and then immersed in glacial acetic acid. Glycerin jelly slides were prepared by a common procedure proposed by Bryant, Jones and Mildenhall (1990) [], using glycerin-based gel (stained with 1% safranin) from the sediment that contained pollen. We observed the slides using a light microscope (LM; BX41 Laboratory Microscope, Olympus, Melville, USA) at magnification ×1000. Six quantitative pollen characteristics (polar axis (P), equatorial diameter (E), colpus width (CW), colpus length (CL), endoaperture diameter (ED) and exine thickness (ET)) were measured using a digital camera for microscopes (MDX-30, Shinwoo Optics, Anyang, Korea) based on 25 pollen grains (n = 25) per sample.
2.3. Observations Using SEM
For SEM observations, we employed the critical point drying method (CPD) [] to prepare pollen grains. The collected fresh anthers were fixed in 2.5% glutaraldehyde at room temperature, then post-fixed with 1% osmium tetroxide (0.1 M) at 4 °C for 2 h. All samples were subsequently dehydrated through an ethanol series, and finally rinsed with isoamyl acetate for 15 min. After dehydration, the anthers were critical-point dried and mounted on aluminum stubs using double-adhesive carbon tape. The pollen grains within the open locules of anthers were carefully expelled with dissecting needles. Using an ion-sputtering device (MC1000, Hitachi, Tokyo, Japan), the stubs were sputter-coated with gold for 180 s. The digital images of pollen grains at different magnifications were taken using SEM (S-4700, Hitachi, Tokyo, Japan), and the accelerating voltage ranged from 3–10 kV. The size measurements of quantitative pollen characteristics, including the equatorial diameter in polar view (EDPV), the widths of ridges (WRs) and valleys (WVs) of sexine ornamentations, the distance between the apices of two colpi (d), the endoaperture diameter (ED), the number of ridges per 25 µm2 at the equatorial region of the exine (NR) and perforation diameter (PD), on SEM images of twenty-five pollen grains per sample, were made using Smile View software (ver. 2.1, JEOL Ltd., Tokyo, Japan). Polar area indices (PAI) were calculated as the ratio of d to EDPV. The descriptive terminology of ornamentation was mainly used by following previous studies [,,,].
2.4. Data Analysis
Palynological characters of the 30 individuals belonging to six taxa of Chaenomeles, comprising 13 quantitative characters (P, E, EDPV, P/E, PAI, CL, CW, ED, ET, WV, WR, NR, PD) and 2 qualitative characters (exine sculpture type and amb) were chosen for statistical analysis. Cluster analysis, based on a standardized pollen character data matrix and utilizing an unweighted pair-group method with arithmetic mean (UPGMA) [], was performed to group Chaenomeles taxa and individuals into clusters on the basis of overall pollen character similarity. Palynological data of the outgroup species (Cydonia oblonga Mill.) were obtained from Radović et al. (2017) []. Principal component analysis (PCA) was employed to evaluate if pollen characters allowed taxa to be grouped, and to identify the characters that contribute most to the pollen morphological variability. We used untransformed and centered data to produce a covariance matrix and extract three eigenvectors from the matrix. We plotted the eigenvalues in a two-dimensional plot along the first two PCs. All computations were performed using the hclust function implemented in R (ver. 4.0.2, R Development Core Team 2020) for cluster analysis and the R vegan package for PCA. The relationships of quantitative pollen characteristics were determined by Pearson’s correlation coefficients (Sokal and Rohlf, 1995) [] and their variations among taxa were summarized by boxplot analysis. For individual pollen grain character, we utilized one-factor analysis of variance (ANOVA) to assess differences among all species studied. When critical differences were observed, multiple comparisons were applied with Tukey’s test. The pollen terminology mostly followed that of Hebda and Chinnappa (1994) [], Punt et al. (2007) [], and we used Song et al. (2017) [] to define the polar area index and the range of pollen size.
3. Results
The pollen morphology of all extant Chaenomeles species is described in terms of pollen size, shape, amb, colpus width, colpus length, polar area index, exine thickness, width of valleys, width of ridges, number of ridges per 25 µm2 at the equatorial region of the exine, diameter of perforation and sexine ornamentation as follows. Pollen grains of Chaenomeles samples observed under LM and SEM are illustrated in Figure 2 and Figure 3, respectively, and the pollen characteristics are summarized in Table 1 and Table 2.
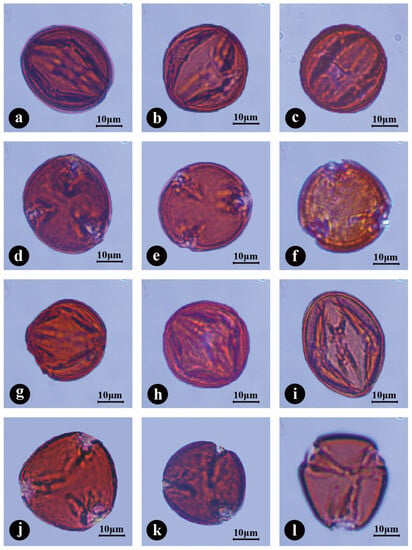
Figure 2.
LM micrographs of pollen grains of Chaenomeles. (a–c, g–i) Variations of pollen shape in the equatorial view. (d–f, j–l) The outline variations in polar view of pollen grains. (a,d) Chaenomeles cathayensis. (b,e) C. japonica. (c,f) C. sinensis. (g,j) C. speciosa. (h,k) C. ×superba. (i,l) C. thibetica. Scale bars—10 μm.
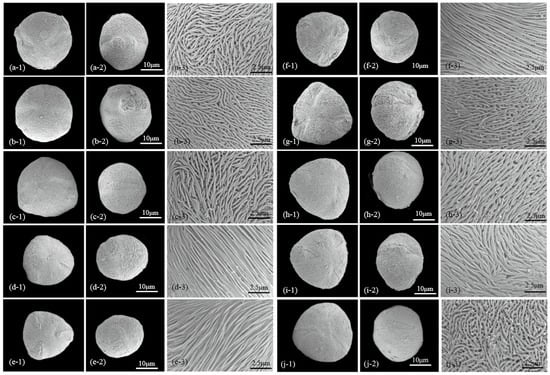
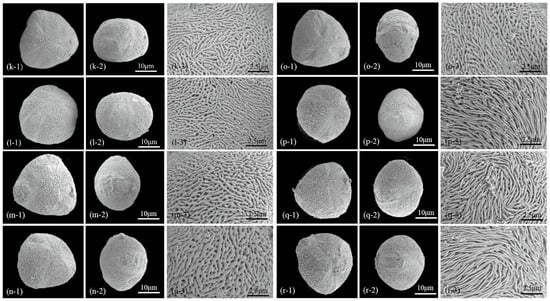
Figure 3.
Scanning electron micrographs of Chaenomeles. Pollen shape (polar view (a1–j1) and equatorial view (a2–j2), scale bars—10 μm) and exine pattern (a3–j3) (scale bars—2.5 μm) in representative individuals of Chaenomeles cathayensis (a–c), C. japonica (d–f), C. sinensis (g–i) and C. speciosa (j). Pollen shape (polar view (k1–r1) and equatorial view (k2–r2), scale bars—10 μm) and exine pattern (k3–r3) (scale bars—2.5 μm) in representative individuals of C. speciosa (k–l), C. ×superba (m–o) and C. thibetica (p–r).

Table 1.
Overview of major pollen characters of all samples studied in Chaenomeles.

Table 2.
Measurements of additional pollen quantitative characteristics, including equatorial diameter in polar view (EDPV), polar area index (PAI) of the pollen grains, number of ridges per 25 µm2 at the equatorial region of exine (NR), width of ridge (WR), width of valley (WV), diameter of perforation (PR) of sexine ornamentations (n = 20) in taxa of Chaenomeles.
3.1. Shape and Size
Chaenomeles has isopolar, radially symmetrical and medium-sized pollen grains (Table 1). With respect to polar diameter, the largest value was observed in C. thibetica (P = 40.61 ± 1.48 μm), and the smallest in C. sinensis (P = 33.98 ± 0.83 μm). For equatorial diameter, the variation was less evident, with the maximum value observed in C. japonica (E = 34.92 ± 1.04 μm) and the minimum value examined in C. speciosa (E = 32.11 ± 0.93 μm). The shape of the pollen grains, obtained through the ratio of polar diameter and equatorial diameter (P/E), varies from prolatespheroidal to subprolate (P/E = 0.98–1.35; Figure 2). The outline in the polar view (amb) is mostly subcircular and subangular in C. japonica and C. speciosa, subcircular grains were mainly observed in C. cathayensis, circular and subangular grains were mainly observed in C. sinensis, and subangular grains were mainly observed in C. ×superba and C. thibetica (Table 1). The variation in shape and size is wider in ACE pollen grains (P = 32.78–42.74 μm, P/E = 0.98–1.35) than in CPD pollen grains (P = 16.36–25.93 μm, P/E = 0.66–0.87). According to the results of the ANOVA, significant differences were observed in pollen diameter and P/E ratio at the species level (Figure 2). The frequency of deformed pollens varied among taxa and ranged from 1.65% (C. cathayensis) to 17.50% (C. ×superba) in non-hybrids. In hybrid C. ×superba, the observed percentage of deformed pollen (14 grains in total) was higher than in the parental species.
3.2. Polar Area and Apertures
The polar area is generally small (PAI = 0.23–0.35) in Chaenomeles species, with that of C. sinensis being the largest and significantly larger than that of the other species, and that of C. thibetica being the smallest (Figure 3p–r; Table 2). All taxa produce tri-colporate pollen grains in monads (Figure 2 and Figure 3). Simple colpi were arranged meridionally and symmetrically, with acute and pointed ends. The colpi are slightly invaginate (shallow), but distinct in the LM equatorial view. Their lengths (CL) are long and fairly varied, from 28.44 μm (C. sinensis; Figure 2c) to 34.30 μm (C. thibetica; Figure 2i), and constitute, on average, 78.02–88.75% of the polar diameter. The colpi length exhibit strong correlation with the polar axis length (r = 0.84, p < 0.001). Colpi are fusiform in outline and their width is diverse and mostly widest in the equatorial region. The significantly wider colpi (equatorial extent) in Chaenomeles occurred in C. sinensis (8.53 μm, Figure 2f) and C. ×superba (7.69 μm, Figure 2k), while the narrowest ones were found in C. speciosa (7.06 μm, Figure 2j). All species have fusiform colpus opercula. The opercula are wide and flattened to faintly convex at the equator, with corrugated surfaces (Figure 2). Endoapertures were observed in all studied taxa. They are circular or elliptic in outline, and usually located in the middle of colpi, with diameters ranging from 5.71 to 8.80 μm (Figure 2 and Figure 3; Table 1).
3.3. Exine Ornamentation
All Chaenomeles taxa have a sexine ornamentation pattern consisting of supratectal ridges and valleys, with perforations of varying sizes occurring in the valleys (Figure 3). A distinct zone of densely packed ridges, regularly aligned and parallel to the colpus, occurs at the margins of the colpus. The number of ridges in 25 μm2 ranges between 22 and 54. Two types (I, II) and two subtypes (II-A, II-B) of sexine ornamentation can be recognized based on the elongation patterns of the striae and the diameter of perforations (PR) (Table 1 and Table 2, Figure 3). The exine thickness varies from 0.58 to 1.29 μm (Table 1; Figure 2).
- Type I striate with microperforations:
- Ridges are long, continuously elongated with few anastomoses, and nearly all run parallel to colpi and continue over the poles. The extension of striae before ending or changing direction are usually more than 10 μm. This type contains tectal microperforations similar to pinpricks (0.03–0.06 μm in diameter), which were often obscured by ridges but clearly visible at poles. Type I ornamentation was exclusively observed in C. sinensis (Table 1; Figure 3g–i).
- Type II striate with macroperforations: this type is subdivided according to the length of ridges.
Subtype II-A (twisted striate): ridges are medium to long in length, 0.14–0.22 μm wide, and mostly oriented parallel to the colpus and pole axis, but regularly, they also form some curving and looping (fingerprint-like twists) at the inter-colpium region. This type includes pollen grains with wider valleys as well as larger perforations (0.12–0.30 μm in diameter, Figure 2). Taxa with subtype II-A ornamentation are: C. cathayensis (Figure 3a–c), C. japonica (Figure 3d–f), C. thibetica (Figure 3p–r).
Subtype II-B (reticulate striate): ridges are short, 0.13–0.23 μm wide, forming a complex interwoven pattern. The extensions of ridges before an end or a change in direction are usually less than 4 μm. The perforations at the bottoms of valleys are 0.12–0.20 μm in diameter. Taxa with subtype II-B ornamentation are: C. speciosa (Figure 3j–l), C. ×superba (Figure 3m–o).
| Pollen artificial key | |
| 1 Exine sculpture striate, perforations are minute pinholes of small diameters | C. sinensis |
| 1* Exine sculpture striate to striate-reticulate, perforations of larger diameters | 2 |
| 2 Striae medium to long, largely parallel to colpi, with some looping and crossing | 3 |
| 2* Striae short, weaving and crossing | 5 |
| 3 Pollen shape spherical to prolatespheroidal (average P/E ratio 1.03) | C. japonica |
| 3* Pollen shape subprolate to prolate (average P/E ratio ≧ 1.17) | 4 |
| 4 Amb in polar view subangular, PAI less than 0.26 | C. thibetica |
| 4* Amb in polar view subcircular, PAI more than 0.27 | C. cathayensis |
| 5 Pollen wall relatively thin; average exine thickness 0.75 (0.58–0.88) μm | C. ×superba |
| 5* Pollen wall relatively thick; average exine thickness 0.89 (0.75–1.00) μm | C. speciosa |
3.4. Multivariate Analysis
Statistically significant differences were observed among the Chaenomeles taxon based on the quantitative pollen grain features analyzed here (p < 0.05). The statistical analyses are illustrated and summarized in Figure 4. The PCA graph (Figure 5) presented the projections of the pollen characteristics in a multi-dimensional space and the first and second components accounted for 68.8% of the total variance. PC1 explains 49.9% of the overall variance and is related to the polar area index, number of ridges per 25 µm2, diameter of perforation, amb and colpus length, whereas PC2, which accounted for 18.9% of the variance, is associated with exine sculpture type, equatorial diameter in polar view, polar axis and colpus width (Table 3). Overall, the number of ridges per 25 µm2 at the equatorial region of the exine and equatorial diameter were the most distinctive and valuable characters, since they accounted for the largest amount of relative variation. C. sinensis, which has the unique sexine ornamentation type I, is positioned on the negative side of the PC 1 axis and is more separated from the other species in the PCA plot. The taxa of sexine ornamentation type II-B, including C. ×superba and C. speciose, are grouped on the negative side of the PC 2 axis. C. thibetica and C. cathayensis are in close similarity with regard to the data from P, P/E, CL, WV, PR and are distributed on the positive side of the PC 2 axis (Figure 5).
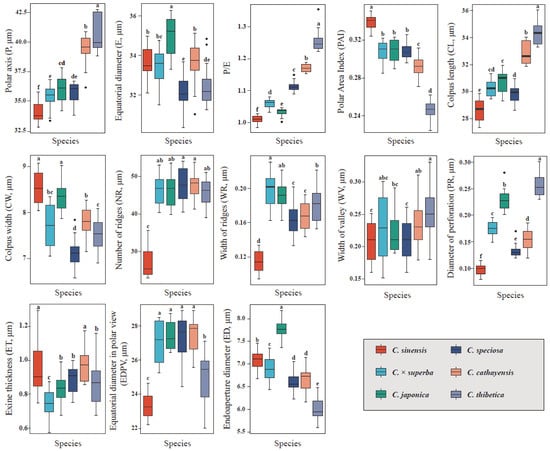
Figure 4.
Boxplot and dot–plot graphs for the quantitative pollen characters in the studied Chaenomeles taxa. Different superscript letters demonstrate statistically significant differences between species (p < 0.05). The black dots located outside the whiskers of the box plot are considered outliers.
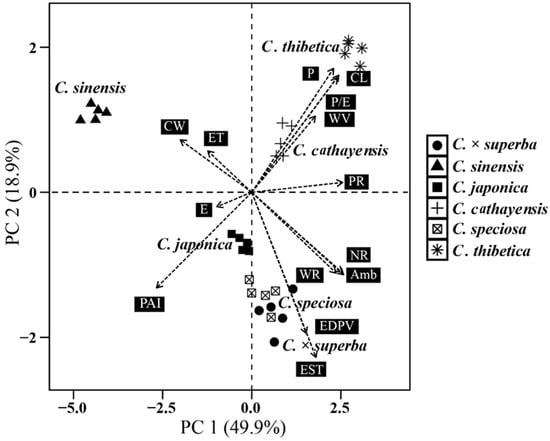
Figure 5.
Principal component analysis (PCA) based on the pollen features of Chaenomeles. P polar axis; E equatorial diameter; CW colpus width; CL colpus length; PAI polar area index; ET exine thickness; WV width of valley; WR width of ridge; NR number of ridges per 25 µm2 at the equatorial region of exine; PR diameter of perforation; EDPV equatorial diameter in polar view; Amb c circular, sa subangular, sc subcircular, sm semi–angular; EST exine sculpture type, type I striate with microperforations, type II striate with microperforations, subtype II-A type II with twisted striate, subtype II-B type II with reticulate striate.

Table 3.
The results of the principal component (PC) analysis based on the pollen qualitative and quantitative characters of Chaenomeles species.
The cluster analysis revealed that the palynological data obviously divides all of the taxa and individuals studied into three groups, concurrent with the PCA. The group comprising C. sinensis was firstly set apart from the other taxa, due to its significantly lower values of P/E ratio, colpus length, width of ridges and perforation diameter, and its larger polar area (Figure 3 and Figure 6). The taxa with exine sculpture subtype II-A, comprising samples of C. thibetica, C. cathayensis, C. japonica, formed a second group. Within this group, C. thibetica and C. cathayensis are in close similarity as to pollen and colpus dimensions and are distributed on the positive side of PC 1 (Figure 5 and Figure 6). The rest of the taxa (C. ×superba, C. speciosa) sharing exine sculpture subtype II-B and similar amb are clustered in the third group (Figure 5 and Figure 6). The samples from the same species tend to be clustered together, except for C. superba-AD5, which grouped with two samples of C. speciosa.
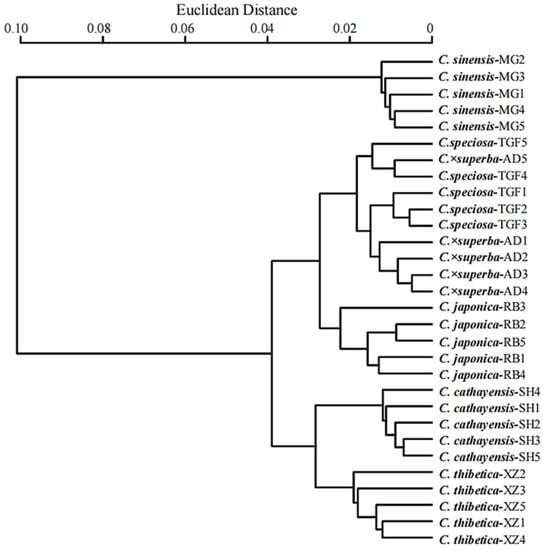
Figure 6.
UPGMA dendrogram of Chaenomeles samples constructed based on pollen morphological characteristics.
4. Discussion
In this study, LM and SEM were applied to observe the pollen morphological features of extant species of Chaenomeles in China. Statistical analyses of the morphological variability were undertaken for the first time to identify qualitative and quantitative characteristics that were valuable and informative to distinguish Chaenomeles species.
Pollen grains of Chaenomeles are medium in size with a prolatespheroidal to subprolate shape and are tri-colporate, in accord with the overall pollen features of Rosaceae [,,,,]. However, we discovered a statistically significant variance in pollen size and shape between species (Figure 2 and Figure 3). Chaenomeles species also differ with regard to polar surface area and colpus size (Figure 3). These results indicated that pollen and colpus dimensions, which are controlled to a large extent by genes, are suitable characters for species characterization. The only previous palynological study of Chaenomeles simply followed an air-drying method and reported a range of pollen sizes (P: 33.24–48.27μm; E: 18.93–25.58μm), differing to a large extent from those we obtained here []. In fact, preparation treatments can affect pollen size and shape [,,,,,]. In comparison with the previous SEM images of Zang and Ma (2004) [], we believe that the pollen grains of Chaenomeles were highly fragile and showed pressure distortion (pollen wall folding back along the apertures) after direct air drying, which possibly led to biases in measurement. Therefore, suitable artificial drying methods should be used to fix and further observe the natural pollen state of this genus. In this study, ACE and CPD methods were both employed for pollen preparation, and the latter is superior in maintaining the natural pollen size and shape, leaving the apertural membranes undestroyed []. It is suggested here that CPD pollen is always smaller than ACE pollen (mean by 49.12%; range: 28.48–68.44%; Table 2) like other palynological studies have found [,,]. In general, the pollen size of Chaenomeles was larger than its most closely related genera, as reported earlier, including Cydonia [], Photinia [], Sorbus [] and Dichotomanthes Kurz [].
Previous palynological studies demonstrated that sexine ornamentation features are highly variable and informative for Rosaceae pollen at the genus as well at the species level [,,,,,,,,]. The most important features include the number, interval, width, length and direction of striae, as well as the diameter and density of perforations [,,,,]. Our results do not support the diagnostic significance of the number, interval and width of striae, since except for C. sinensis, other taxa from Chaenomeles were in close similarity based on measurements of NR, WV and WR, whereas features such as the length or direction of striae and diameter of perforation, as a result of their considerable variations, were identified to be of taxonomic value and informative in species delimitation (Table 2, Figure 3). Similar results were obtained from the pollen study on different genera (Spiraea L., Sorbaria (Ser.) A. Braun, Rosa L., etc.) within the family Rosaceae [,,]. Hebda and Chinnappa (1994) [] classified the pollen sculpturing type of Rosaceae into six major categories: striate macroperforate, striate microperforate, tuberculate, microverrucate, verrucate and perforate without supratectal features. They included Chaenomeles in type I (the third subtype), which were described as having short ridges (waving and crossing) separated by valleys with larger perforations in the 0.1–0.2 μm diameter range. Nevertheless, there is mounting evidence that even in this small genus, the inclusion of taxa into one type is too general. According to Zang and Ma (2014) [], the above-mentioned type I was only quoted for C. japonica, and they newly distinguished two types of exine sculpture, one characterized by fine stripes nearly parallel to each other (e.g., C. sinensis), and the other with irregular striae and rare or no perforations (e.g., C. thibetica). Such division is not adopted here, firstly because it was based on air-dried pollens, which in comparison with the CPD pollens we obtained here (Figure 3) were subjected to obvious distortion and shrinkage (e.g., E decreased by 14.51–52.09%), leaving apertures unrecognized and perforations obscured, and secondly because it is descriptive, relying neither on measurements nor statistical analysis of exine sculpture characters, and is thus difficult to compare across studies. Notwithstanding, our study agreed with Zang and Ma (2014) [] in that species from Chaenomeles also belong to sculpture types other than type I as well as to types that are not described by Hebda and Chinnappa (1994) [].
In the current study, we distinguished two types and two subtypes of sculpture in Chaenomeles based on ridge patterns and perforation size (macroperforation/microperforation). While a majority of the members in Chaenomeles possess striate type II, C. sinensis is clearly distinct in having microperforations and conspicuous long ridges parallel to the colpus (type I; Figure 3g–i). In addition, C. sinensis can be separated as a basal group on the basis of significant differences in the qualitative pollen features we analyzed here (Figure 5 and Figure 6). In fact, C. sinensis was previously treated as Pseudocydonia, a monotypic genus closely related to Cydonia. The taxonomical position of Pseudocydonia has been the subject of much debate [,]. Previous phylogenetic studies revealed strong conflicts between chloroplast and nuclear data in the placement of C. sinensis [,,,,], indicating that this species might originate via hybridization between members of the ancestral lineages of Cydonia and Chaenomeles. Pollen evidence from our study supports the hybrid origin hypothesis, as the pollen type of C. sinensis is intermediate between that of typical Chaenomeles (sharing type II) and Cydonia in that it has conspicuous striate resembling Chaenomeles, and longitudinal fine ridges and tectal microperforations resembling Cydonia [,]. Furthermore, C. sinensis also shows some morphological resemblance to both of the putative parental taxa, with the basally fused styles and 25 or fewer stamens of Cydonia and the serrate leaves, glabrous fruits, and deciduous sepals of Chaenomeles [,,]. Since C. sinensis is morphologically anomalous in either Cydonia or Chaenomeles, this study supports the placement of this species in the monotypic genus Pseudocydonia.
As for the rest of the taxa, C. cathayensis, C. japonica and C. thibetica belonged to the same exine ornamentation subtype (type II-A; Figure 3a–f,p–r); yet they were discriminated as separate species based on the significant differences in perforation diameter (PR) and ridge width (WR) (Figure 4). The statistical analysis supported the closer relationship between C. cathayensis and C. thibetica, since they were more similar in pollen shape (P/E ratio), colpus length (CL) and colpus width (CW) (Figure 2). This corroborates the results of Lo and Donoghue (2012) [] and Sun et al. (2020) [], which, based on molecular phylogenetic analysis, inferred C. thibetica and C. cathayensis as sister species with high bootstrap support. In this study, pollen grains of the hybrid taxa C. ×superba and parents (C. japonica × C. speciosa) were examined in detail for the first time using both LM and SEM. The majority of diagnostic pollen features studied (P/E, CL, CW, PR) for C. ×superba are intermediate between that of C. japonica and C. speciosa (Table 1 and Table 2; Figure 2). However, the exine pattern of C. ×superba was classified as striate-reticulate with macroperforations, identical to that of C. speciosa (subtype II-B; Figure 3m–o, j–l). In addition, C. ×superba have comparably thinner pollen walls than that of other Chaenomeles species, including the parents. When observing SEM images, we noticed a higher proportion of deformed pollen grains for C. ×superba (17.50% abnormal pollen grains) than for the remaining taxa. The pollen deformation included mainly the loss of turgor pressure and changes in pollen shape. This feature was frequently found in ornamental plants and hybrids, and has been reported in other hybrid members of related genera (Malus, Crataegus L., etc.) in Rosaceae [,,].
5. Conclusions
Chaenomeles pollen grains are medium sized, prolatespheroidal to subprolate and tri-colporate, with striate sexine ornamentation. The pollen features of size, polar area, colpus dimension and sexine sculpture were verified by statistical analysis to be useful criteria for delimiting species. Two sexine sculpture types (type I—striate with microperforations, type II—striate with macroperforations) and two subtypes (twisted-striate, reticulate-striate) were recognized in the species studied depending on direction and length of the ridge patterns, which might be of systematic importance. C. sinensis is clearly distinct from the other Chaenomeles species on the basis of the unique sexine ornamentation type I, as well as the significant differences in qualitative pollen features we analyzed, which supports the placement of this species in the monotypic genus Pseudocydonia. This study is the first comprehensive investigation of the pollen morphology of Chaenomeles species based on LM and SEM and demonstrated the diagnostic significance of pollen features in the taxonomy of closely related species. Furthermore, the present data is beneficial to a comprehensive understanding of pollen morphology in Chaenomeles as part of ongoing palynological studies of Rosaceae that strive to provide an overview of the family’s pollen micromorphology and character evolution.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15090960/s1, Table S1: Sampling details of the Chaenomeles species.
Author Contributions
Conceptualization, Z.H. and Y.W.; methodology, Y.W.; software, Z.H.; validation, Z.H. and Y.W.; formal analysis, Z.H.; investigation, W.M.; resources, L.T.; data curation, J.L.; writing—original draft preparation, Z.H.; writing—review and editing, Y.W.; visualization, J.L.; supervision, Y.Z.; project administration, P.G.; funding acquisition, F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Henan Province Major Research Fund of Public Welfare (No. 201300110900), Postdoctoral Research Grant in Henan Province (No. 201901020), 2022 Provincial Science and Technology Research and Development Plan Joint Fund (application research) Project (No. 222103810010), Postgraduate Education Reform and Quality Improvement Project of Henan Province (No. HNYJS2020JD08) and Academic Degrees & Gradate Education Reform Project of Henan Province (No. 2021SJGLX154Y).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Mingwan Li, Shuai Wang and Huinan Li for collecting plant materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weber, C. Cultivars in the genus Chaenomeles. Arnoldia 1964, 23, 17–75. [Google Scholar]
- Gu, C.; Li, C.; Lu, L.; Jiang, S.; Alexander, C.; Bartholomew, B.; Brach, A.R.; Boufford, D.E.; Ikeda, H.; Ohba, H.; et al. Rosaceae. In Flora of China; Wu, Z., Raven, P., Hong, D., Eds.; Science Press: Beijing, China, 2003; pp. 46–434. [Google Scholar]
- Sun, J.; Wang, Y.; Liu, Y.; Xu, C.; Yuan, Q.; Guo, L.; Huang, L. Evolutionary and phylogenetic aspects of the chloroplast genome of Chaenomeles species. Sci. Rep. 2020, 10, 11466. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Li, H.; Zhong, P.X.; Xu, Y.J.; Li, C.H.; Ji, K.X.; Wang, L.S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; He, J.; Nisar, M.F.; Li, H.; Wan, C. Phytochemical and Pharmacological Properties of Chaenomeles speciosa: An Edible Medicinal Chinese Mugua. Evid.-Based Complement. Altern. Med. 2018, 2018, 9591845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, S.Y.; Zhu, Z.Z.; He, J.G. Recent advances in valorization of Chaenomeles fruit: A review of botanical profile, phytochemistry, advanced extraction technologies and bioactivities. Trends Food Sci. Technol. 2019, 91, 467–482. [Google Scholar] [CrossRef]
- Phipps, J.B.; Robertson, K.R.; Smith, P.G.; Rohrer, J.R. A checklist of the subfamily Maloideae (Rosaceae). Can. J. Bot. 1990, 68, 2209–2269. [Google Scholar] [CrossRef]
- Rumpunen, K. Chaenomeles: A potential new fruit crop for Northern Europe. In Proceedings of the New Crops & New Uses Fifth National Symposium, Atlanta, GA, USA, 10–13 November 2001. [Google Scholar]
- Bartish, I.V.; Garkava, L.P.; Rumpunen, K.; Nybom, H. Phylogenetic relationships and differentiation among and within populations of Chaenomeles Lindl. (Rosaceae) estimated with RAPDs and isozymes. Theor. Appl. Genet. 2002, 101, 554–563. [Google Scholar] [CrossRef]
- Yü, T.T.; Kuan, K.C. Taxa nova Rosacearum sinicarum (I). Acta Phytotaxa. Sin. 1963, 8, 202–234. [Google Scholar]
- Kalkman, C. The phylogeny of the Rosaceae. Bot. J. Linn. Soc. 1988, 98, 37–59. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, C.H.; Hu, Y.; Wen, J.; Li, S.; Yi, T.; Chen, H.; Xiang, J.; Ma, H. Evolution of Rosaceae Fruit Types Based on Nuclear Phylogeny in the Context of Geological Times and Genome Duplication. Mol. Biol. Evol. 2017, 34, 262–281. [Google Scholar] [CrossRef]
- Lo, E.Y.Y.; Donoghue, M.J. Expanded phylogenetic and dating analyses of the apples and their relatives. Mol. Phylogenet. Evol. 2012, 63, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shao, W.; Zheng, L.; Huang, J.B. First Report of Brown Rot Caused by Monilia mumecola on Chaenomeles lagenaria in China. Plant Dis. 2019, 103, 2137. [Google Scholar] [CrossRef]
- Loutfy, M.; El–Mashad, A.; Kamel, E. Studies in the Maloideae (Rosaceae) Chaenomeles Lindley and Cydonia Miller. Taeckholmia 1999, 19, 97–114. [Google Scholar] [CrossRef]
- Roemer, M.J. Familiarumn Aturaliumr Egni Vegetabilis Synopses Monographicae; Landes–Industrie–comptoir: Weimar, Germany, 1847; Volume 4. [Google Scholar]
- Koehne, E. Die Gattungen der Pomaceen; Wissenschaftliche Beilage zum Programm des Falk–Realgymnasi ums zu Berlin: Berlin, Germany, 1890. [Google Scholar]
- Yü, T.T. (Ed.) Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1974; pp. 1–66, Tomus 36. (In Chinese with Latin Name). [Google Scholar]
- Schneider, C.K. LVII. Species varietatesque Pomacearum novae. Repert. Nov. Specierum Regni Veg. Feddes Reper. 1906, 3, 177–183. [Google Scholar] [CrossRef]
- Hillier, H.G. Hillier’s Manual of Trees and Shrubs, 5th ed.; Hillier Nurseries (Winchester) Limited: Ampfield, UK, 1981. [Google Scholar]
- Robertson, K.R.; Phipps, J.B.; Rohrer, J.R.; Smith, P.G. A synopsis of genera in Maloideae (Rosaceae). Syst. Bot. 1991, 16, 376–394. [Google Scholar] [CrossRef]
- Kalkman, C. Rosaceae. In The Families and Genera of Vascular Plants VI Flowering Plants Dicotyledons Celastrales, Oxalidales, Rosales, Cornales, Ericales; Kubitzki, K., Ed.; Springer: New York, NY, USA, 2004; pp. 343–386. [Google Scholar]
- Potter, D.; Eriksson, T.; Evans, R.C.; Oh, S.; Smedmark, J.E.E.; Morgan, D.R.; Campbell, C.S. Phylogeny and classification of Rosaceae. Plant Syst. Evol. 2007, 266, 5–43. [Google Scholar] [CrossRef]
- Loutfy, N.F.; Awad, O.M.; El–Masry, A.G.; Kandil, G.M. Study on rodents infestation in Alexandria and prevalence of Trichinella spiralis infection among them. J. Egypt Soc. Parasitol. 1999, 29, 897–909. [Google Scholar]
- Mabberley, D.J. The Plant–Book, A Portable Dictionary of the Vascular Plants; Cambridge Univ. Press: Cambridge, UK, 1997. [Google Scholar]
- Garkava, L.P.; Rumpunen, K.; Bartish, I.V. Genetic relationships in Chaenomeles (Rosaceae) revealed by isozyme analysis. Sci. Hortic. 2000, 85, 21–35. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of Rosaceae in Canada. Rev. Palaeobot. Palyno. 1990, 64, 103–108. [Google Scholar] [CrossRef]
- Hebda, R.J.; Chinnappa, C.C. Studies on pollen morphology of Rosaceae. Acta Bot. Gall. 1994, 141, 183–193. [Google Scholar] [CrossRef]
- Lee, S.T.; Jung, Y.J.; Lee, J.H. Palynological relationship between Pentactina rupicola Nakai and its relative taxa. Korean J. Plant Taxon. 1993, 23, 149–159. [Google Scholar] [CrossRef]
- Song, J.H.; Moon, H.K.; Hong, S.P. Pollen morphology of the tribe Sorbarieae (Rosaceae). Plant Syst. Evol. 2016, 302, 853–869. [Google Scholar] [CrossRef]
- Song, J.H.; Oak, M.K.; Roh, H.S.; Hong, S.P. Morphology of pollen and orbicules in the tribe Spiraeeae (Rosaceae) and its systematic implications. Grana 2017, 56, 351–367. [Google Scholar] [CrossRef]
- Eide, F.Y. Key for northwest European Rosaceae pollen. Grana 1981, 20, 101–118. [Google Scholar] [CrossRef]
- Ueda, Y. Pollen surface morphology in the genus Rosa related genera. Jpn. J. Palynol. 1992, 38, 94–105. [Google Scholar]
- Wrońska-Pilarek, D.; Jagodziński, A.M. Systematic importance of pollen morphological features of selected species from the genus Rosa (Rosaceae). Plant Syst. Evol. 2011, 295, 55–72. [Google Scholar] [CrossRef]
- Shi, W.; Wen, J.; Lutz, S. Pollen morphology of the Maddenia clade of Prunus and its taxonomic and phylogenetic implications. J. Syst. Evol. 2013, 51, 164–183. [Google Scholar] [CrossRef]
- Wrońska-Pilarek, D.; Bocianowski, J.; Jagodziński, A.M. Comparison of pollen grain morphological features of selected species of the genus Crataegus (Rosaceae) and their spontaneous hybrids. Bot. J. Linn. Soc. 2013, 172, 555–571. [Google Scholar] [CrossRef]
- Zang, D.K.; Ma, Y. Pollen morphology and phylogenetic significance of Pseudocydonia and related genera. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2014, 38, 13–16. [Google Scholar]
- Erdtman, G. The acetolysis method. A revised description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Bryant VM, J.; Jones, J.G.; Mildenhall, D.C. Forensic palynology in the United States of America. Palynology 1990, 14, 193–208. [Google Scholar] [CrossRef]
- Moon, H.K.; Vinckier, S.; Smets, E.; Huysmans, S. Comparative pollen morphology and ultrastructure of Mentheae subtribe Nepetinae (Lamiaceae). Rev. Palaeobot. Palyno. 2008, 149, 174–186. [Google Scholar] [CrossRef]
- Erdtman, G. On pollen and spore terminology. J. Palaeosci. 1952, 1, 169–176. [Google Scholar] [CrossRef]
- Erdtman, G. Handbook of Palynology; Hafner Publishing C: New York, NY, USA, 1969. [Google Scholar]
- Ferguson, I.K.; Banks, H. Tetrad pollen in the subfamily Caesalpinioideae (Leguminosae) and its significance. Rev. Palaeobot. Palyno. 1994, 83, 31–42. [Google Scholar] [CrossRef]
- Halbritter, H.; Ulrich, S.; Grímsson, F.; Weber, M.; Zetter, R.; Hesse, M.; Frosch-Radivo, A. Illustrated Pollen Terminology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mete, D.; Şahin, A.A.; Hamzaoğlu, E.; Pinar, N.M. Pollen morphology applied to species delimitation of Turkish Dianthus L. (Caryophyllaceae). Palynology 2021, 45, 599–625. [Google Scholar] [CrossRef]
- Radović, A.; Nikolić, D.; Cerović, R.; Milatović, D.; Đorđević, B.; Zec, G. Unusual growth of pollen tubes in the ovary of quince (Cydonia oblonga Mill.). Acta. Sci. Pol-Hortoru. 2017, 16, 133–138. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman: San Francisco, CA, USA, 1995. [Google Scholar]
- Punt, W.; Hoen, P.P.; Blackmore, S.; Nilsson, S.; Le Thomas, A. Glossary of pollen and spore terminology. Rev. Palaeobot. Palyno. 2007, 143, 1–846. [Google Scholar] [CrossRef]
- Chissoe, W.F.; Vezey, E.L.; Skvarla, J.J. Hexamethyldisilazane as a drying agent for pollen scanning electron microscopy. Biotech. Histochem. 1994, 69, 192–198. [Google Scholar] [CrossRef]
- Schols, P.; Es, K.; D’hondt, C.; Merckx, V.; Smets, E.; Huysmans, S. A new enzymebased method for the treatment of fragile pollen grains collected from herbarium material. Taxon 2004, 53, 777–782. [Google Scholar] [CrossRef]
- Lens, F.; Dressler, S.; Vinckier, S.; Janssens, S.; Dessein, S.; Van Evelghem, L.; Smets, E. Palynological variation in balsaminoid Ericales. I. Marcgraviaceae. Ann. Bot. 2005, 96, 1047–1060. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Saha, S.; Kostina, O.; Muravnik, L.; Mitra, A. Replacing critical point drying with a low–cost chemical drying provides comparable surface image quality of glandular trichomes from leaves of Millingtonia hortensis L. f. in scanning electron micrograph. Appl. Microsc. 2020, 50, 15. [Google Scholar] [CrossRef] [PubMed]
- Demissew, S.; Harley, M.M. Trichome, seed surface and pollen characters in Stachys (Labiatae) in Tropical Africa. In Advances in Labiatae Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 1992; pp. 149–166. [Google Scholar]
- Reitsma, T.J. Size modification of recent pollen grains under different treatments. Rev. Palaeobot. Palyno. 1969, 9, 175–202. [Google Scholar] [CrossRef]
- Pathak, M.L.; Idrees, M.; Gao, Y.; Gao, X. A taxonomic revision of Photinia integrifolia (Rosaceae). Phytotaxa 2019, 401, 179–189. [Google Scholar] [CrossRef]
- Hayrapetyan, A.M.; Sonyan, H.H.; Muradyan, A.H. Pollen of Trees and Shrubs of Armenia (Angiospermae. XIII. Rosaceae. Genus Sorbus). Natl. Acad. Sci. Armen. Rep. 2022, 122, 57–64. [Google Scholar]
- Lihua, Z.; Xun, G.; Zhengyi, W. The karyomorphology and systematic position of the Chinese endemic genus Dichotomanthes. Acta Bot. Yunnanica 2000, 22, 282–285. [Google Scholar]
- Chung, K.S.; Elisens, W.J.; Skvarla, J.J. Pollen morphology and its phylogenetic significance in tribe Sanguisorbeae (Rosaceae). Plant Syst. Evol 2010, 285, 139–148. [Google Scholar] [CrossRef]
- Lee, S.; Heo, K.I.; Cho, J.; Lee, C.; Chen, W.; Kim, S.C. New insights into pollen morphology and its implications in the phylogeny of Sanguisorba L. (Rosaceae; Sanguisorbeae). Plant Syst. Evol. 2011, 291, 227–242. [Google Scholar] [CrossRef]
- Aldasoro, J.J.; Aedo, C.; Navarro, C. Phylogenetic and phytogeographical relationships in Maloideae (Rosaceae) based on morphological and anatomical characters. Blumea 2005, 50, 3–32. [Google Scholar] [CrossRef]
- Campbell, C.S.; Evans, R.C.; Morgan, D.R.; Dickinson, T.A.; Arsenault, M.P. Phylogeny of subtribe Pyrinae (formerly the Maloideae, Rosaceae): Limited resolution of a complex evolutionary history. Plant Syst. Evol. 2007, 266, 119–145. [Google Scholar] [CrossRef]
- Fedoronchuk, M.M.; Savitsky, V.D. Comparativeand morphological analysis of pollen for genera of the family Rosaceae Juss. of the Ukrainian flora. Ukr. Bot. Zh. 1987, 44, 32–38. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).