Annual Dynamics of a Layered Phytoplankton Structure in a Meromictic Lagoon Partially Isolated from the White Sea
Abstract
:1. Introduction
2. Materials and Methods
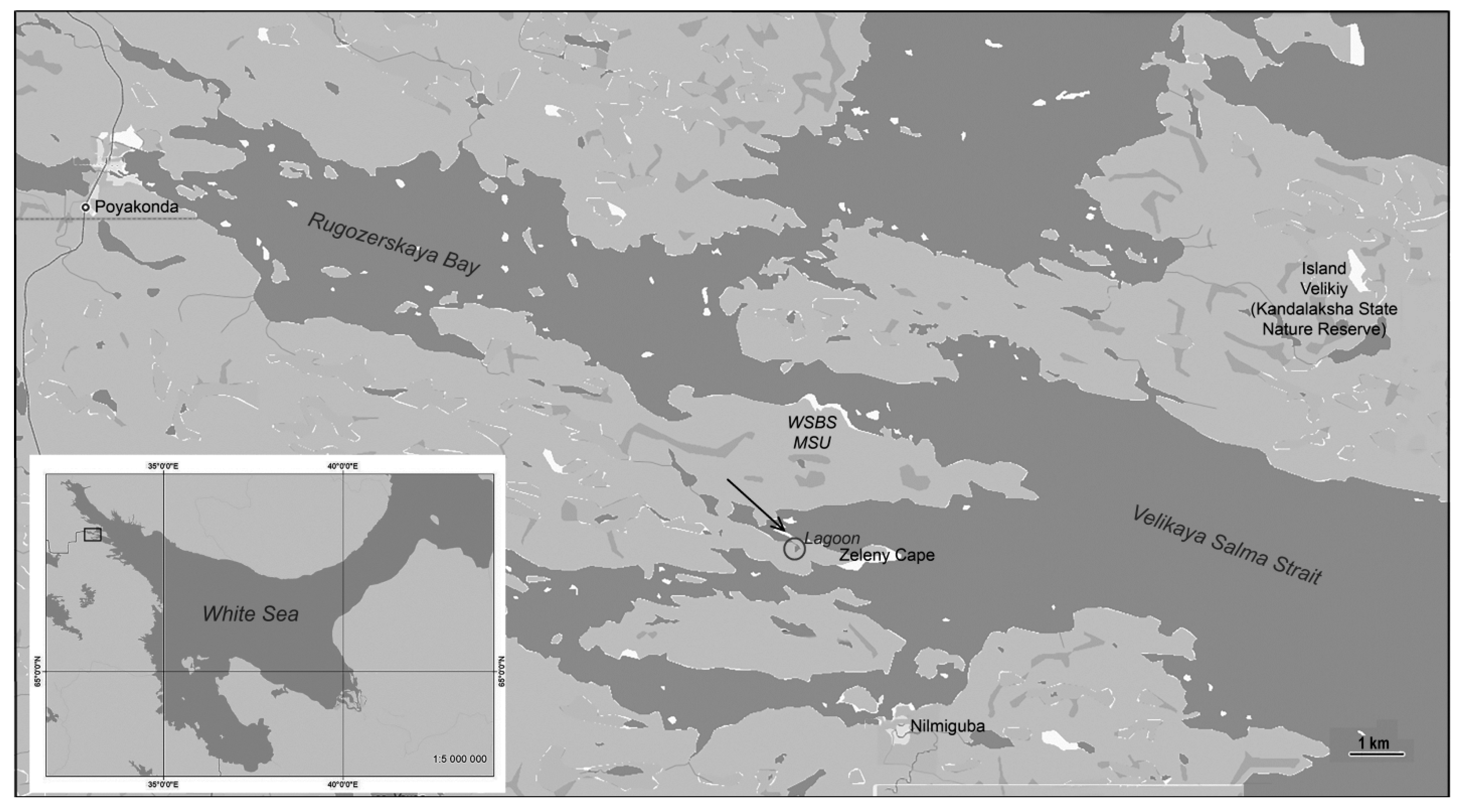
2.1. Characteristics of the Study Area
2.2. Sampling
2.3. Sample Processing
2.4. Statistical Analysis
3. Results
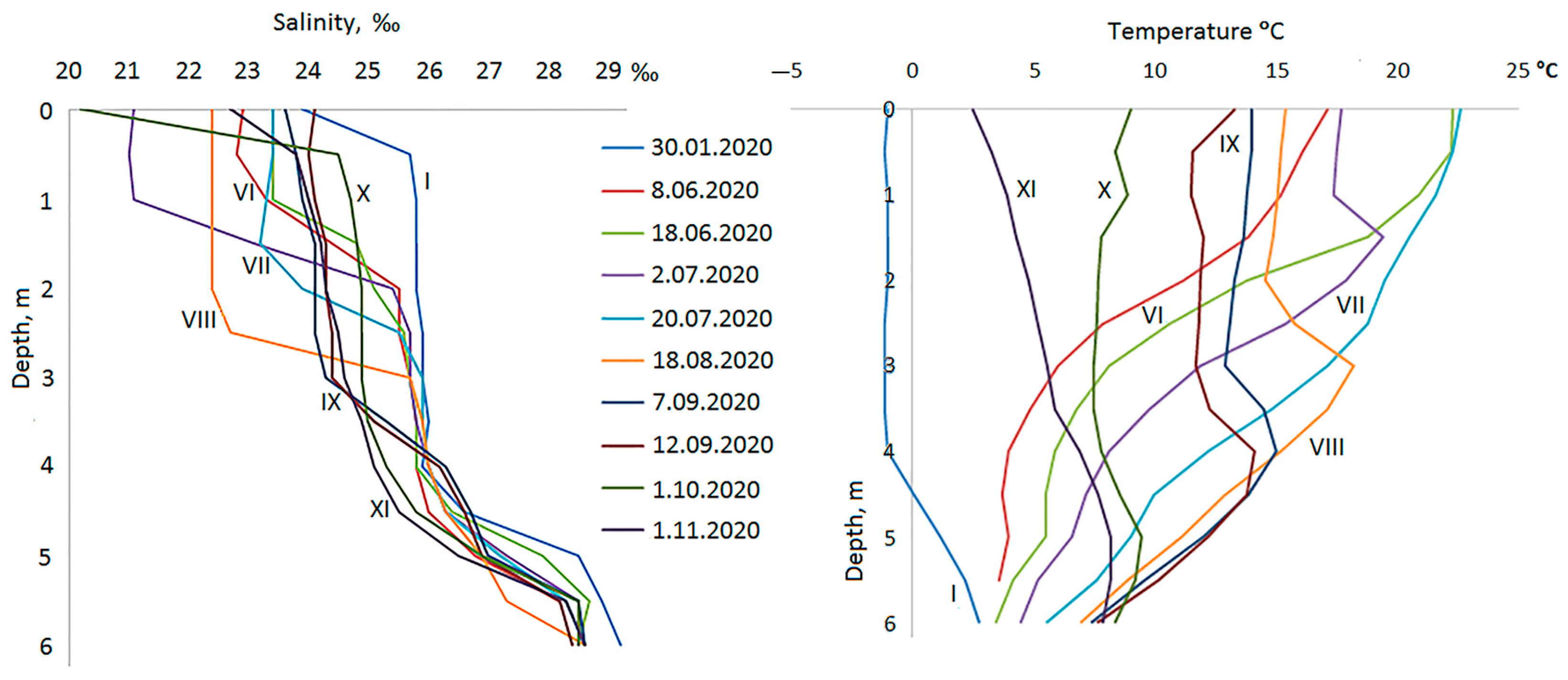
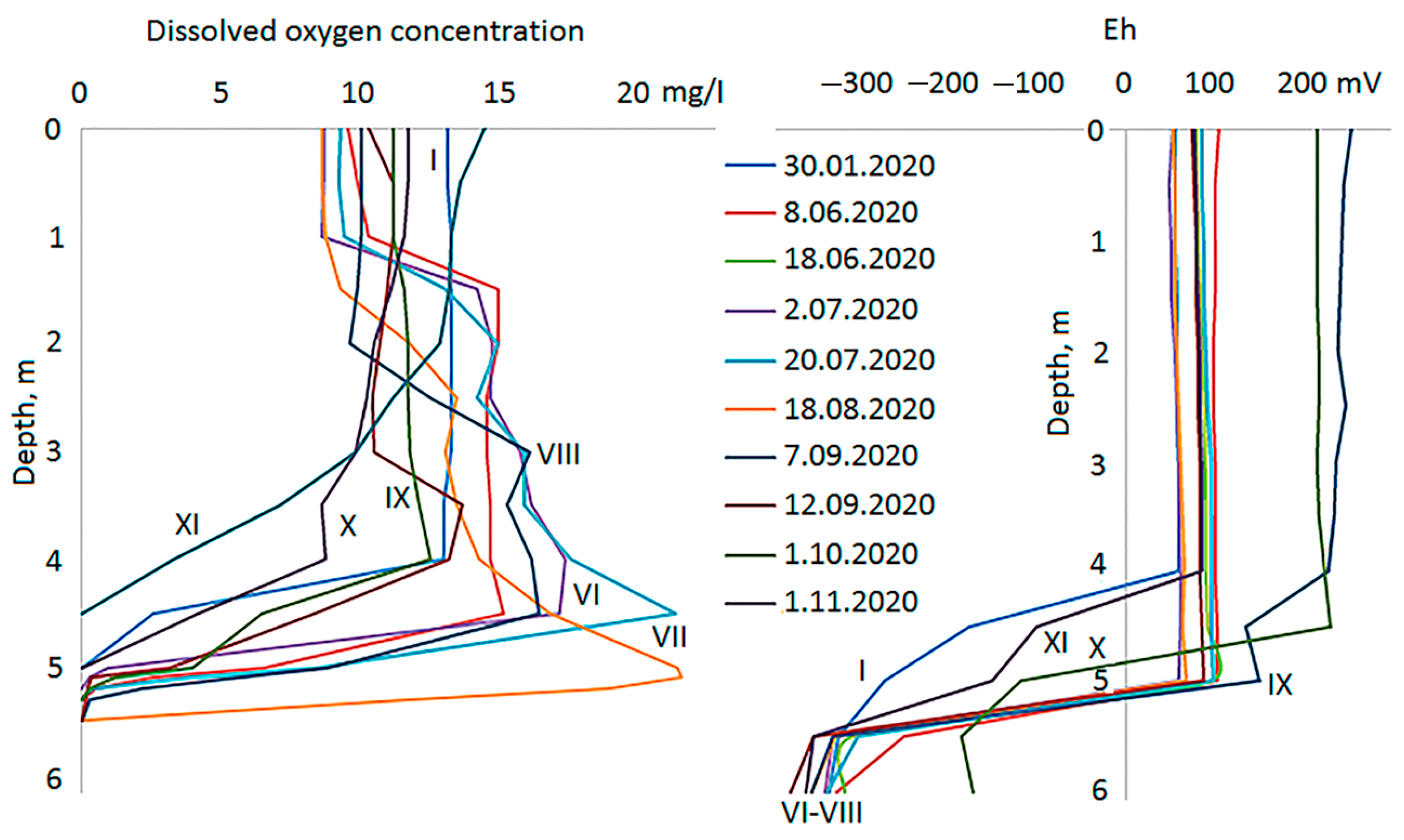
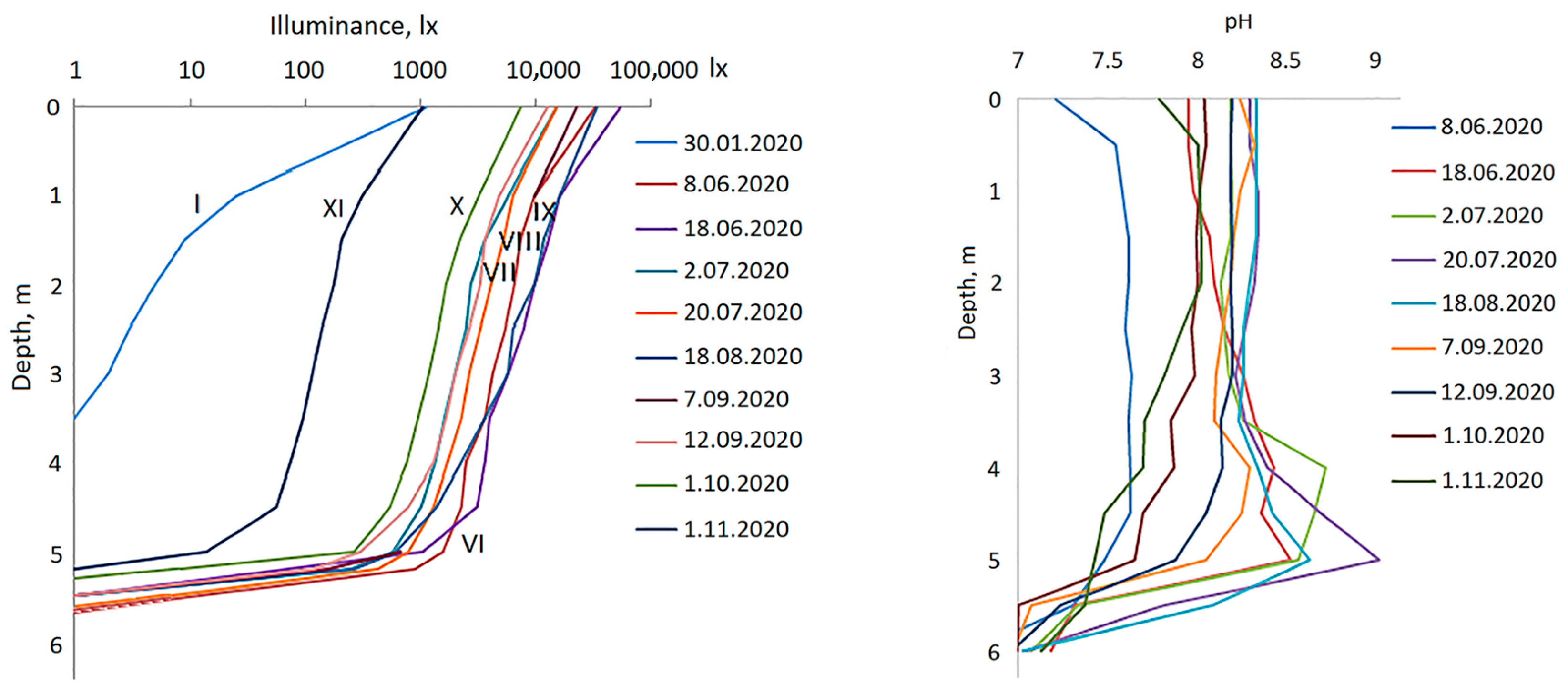
3.1. Hydrological Conditions
3.2. Taxonomic Composition of Algal Flora
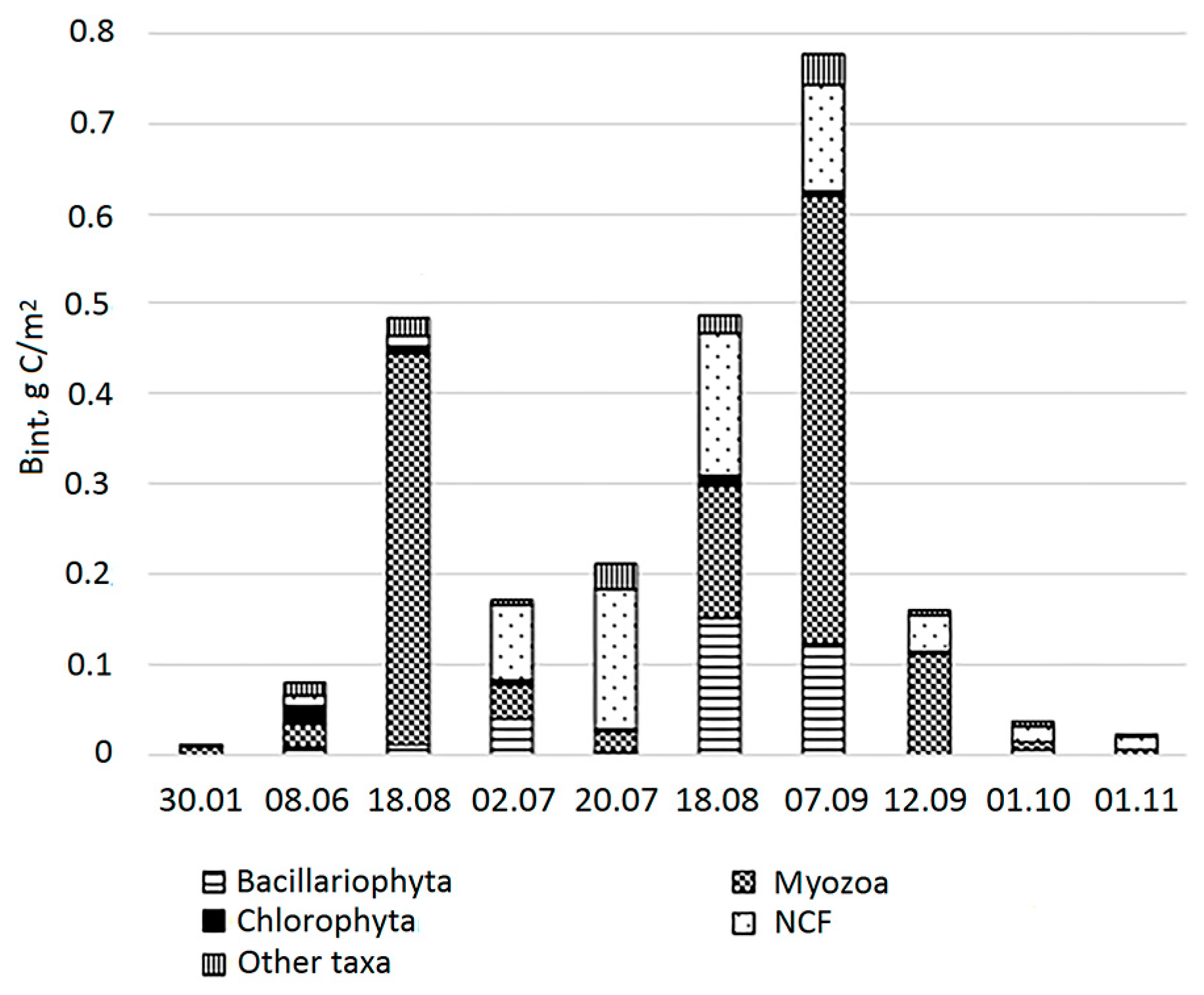
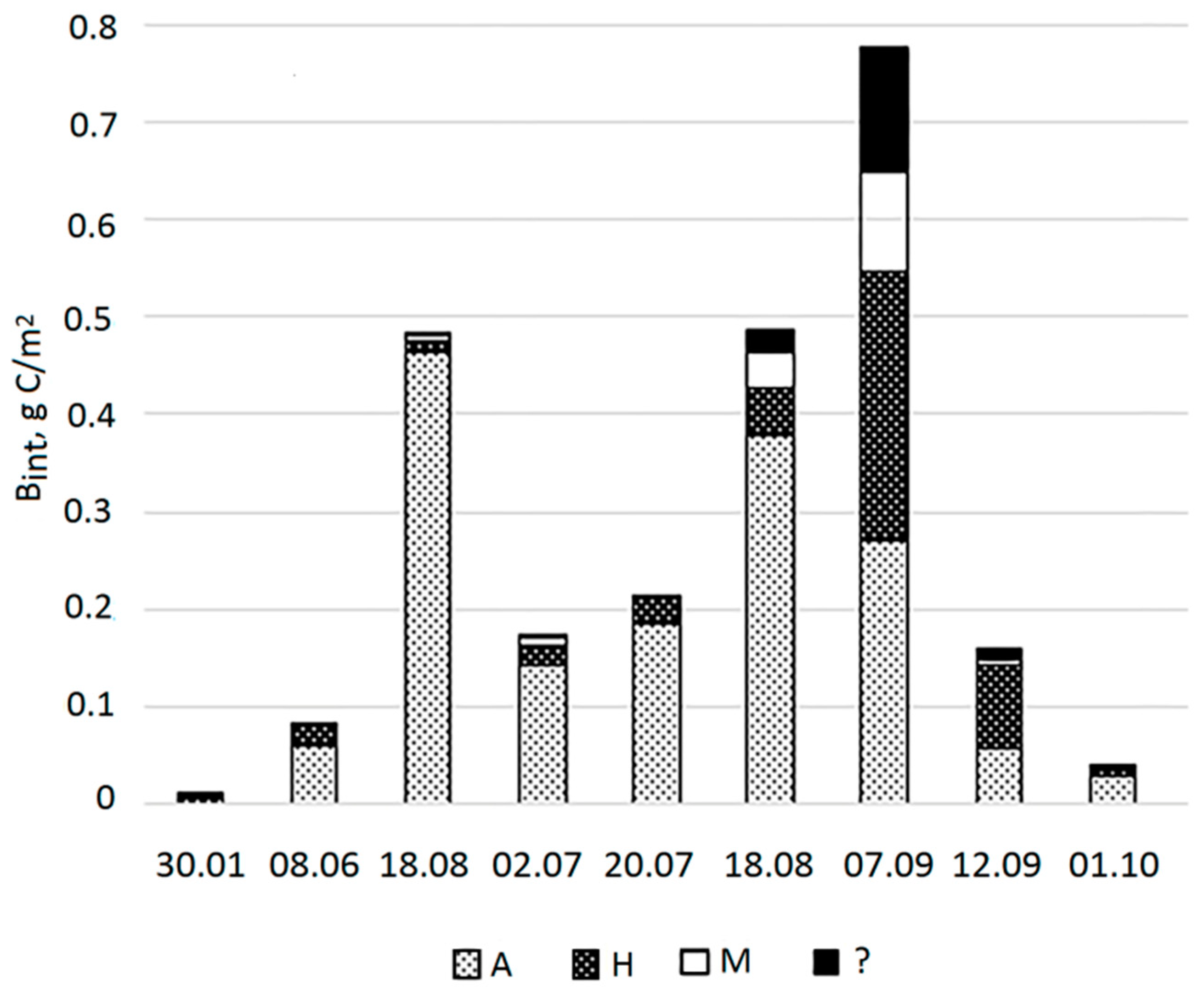
3.3. Biomass and Dominant Taxa in the Lagoon
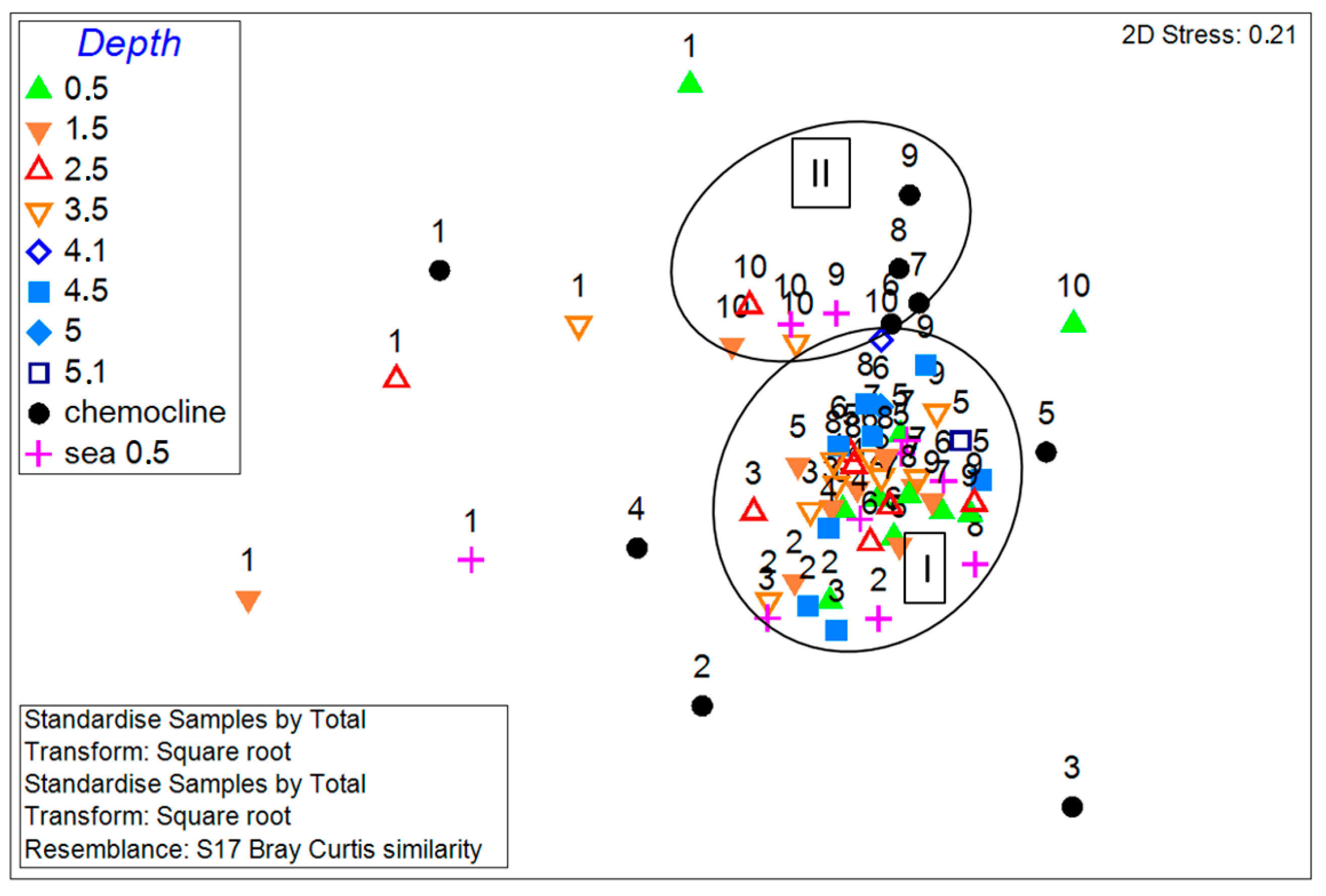
3.4. Phytoplankton Communities in the Lagoon and Sea
3.5. The Structure of the PhP in the Surface Layer of the Lagoon and the Sea
3.6. Environmental Factors That Determine the Dynamics and Structure of Phytoplankton
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krasnova, E.; Voronov, D.; Frolova, N.; Pantyulin, A.; Samsonov, T. Salt lakes separated from the White Sea. EARSeL eProc. 2015, 14, 8–22. [Google Scholar] [CrossRef]
- Krasnova, E.D. Ecology of meromictic lakes of Russia. 1. Coastal marine waterbodies. Water Resour. 2021, 48, 427–438. [Google Scholar] [CrossRef]
- Hakala, A. Meromixis as a part of lake evolution—Observations and a revised classification of true meromictic lakes in Finland. Boreal Environ. Res. 2004, 9, 37–53. [Google Scholar]
- Judd, K.E.; Adams, H.E.; Bosch, N.S.; Kling, G.W. A case history: Effects of mixing regime on nutrient dynamics and community structure in Third Sister Lake, Michigan during late winter and early spring 2003. Lake Reserv. Manag. 2005, 21, 316–329. [Google Scholar] [CrossRef]
- Ciglenečki, I.; Ljubešić, Z.; Janeković, I.; Batistić, M. Rogoznica Lake, a Euxinic Marine Lake on the Adriatic Coast (Croatia) that Fluctuates between Anoxic Holomictic and Meromictic Conditions. In Ecology of Meromictic Lakes. Ecological Studies; Gulati, R., Zadereev, E., Degermendzhi, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 228. [Google Scholar] [CrossRef]
- Melack, J.M.; Jellison, R.; MacIntyre, S.; Hollibaugh, J.T. Mono Lake: Plankton Dynamics over Three Decades of Meromixis or Monomixis. In Ecology of Meromictic Lakes. Ecological Studies; Gulati, R., Zadereev, E., Degermendzhi, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 228, pp. 325–351. [Google Scholar] [CrossRef]
- Zadereev, E.S.; Boehrer, B.; Gulati, R.D. Biological and ecological features, trophic structure and energy flow in meromictic lakes. In Ecology of Meromictic Lakes. Ecological Studies; Gulati, R., Zadereev, E., Degermendzhi, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 228, pp. 61–86. [Google Scholar]
- Zotina, T.A.; Tolomeyev, A.P.; Degermendzhy, N.N. Lake Shira, a Siberian salt lake: Ecosystem, structure and function. 1. Major physico-chemical and biological features. Intern. J. Salt Lake Res. 1999, 8, 211–232. [Google Scholar] [CrossRef]
- Degermendzhy, A.G.; Belolipetsky, V.M.; Zotina, T.A.; Gulati, R.D. Formation of the vertical heterogeneity in the Lake Shira ecosystem: The biological mechanisms and mathematical model. Aquat. Ecol. 2002, 36, 271–297. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Kosolapov, D.B.; Degermendzhy, N.N.; Zotina, T.A.; Romanenko, A.V. Phytoplankton, bacterial production and protozoan bacterivory in stratified, brackish-water Lake Shira (Khakasia, Siberia). Aquat. Ecol. 2002, 36, 205–218. [Google Scholar] [CrossRef]
- Wilk-Woźniak, E.; Żurek, R. Phytoplankton and its relationships with chemical parameters and zooplankton in meromictic Piaseczno reservoir, Southern Poland. Aquat. Ecol. 2006, 40, 165–176. [Google Scholar] [CrossRef]
- Khromechek, E.B.; Barkhatov, Y.V.; Rogozin, D.Y. Distribution of ciliates and Cryptomonas in the chemocline region of saline meromictic Lake Shunet (Siberia, Russia). Aquat. Ecol. 2010, 44, 497–511. [Google Scholar] [CrossRef]
- Makeeva, E.G.A. Algae of Lake Shira. In Natural Complex and Biodiversity of the “Lake Shira” Section of the "Khakasskiy” Reserve; Nepomnyaschiy, V.V., Ed.; Khakassky Publish House: Abakan, Russia, 2011; pp. 150–173. (In Russian) [Google Scholar]
- Rogozin, D.; Zadereev, E.; Prokopkin, I.; Tolomeev, A.; Barkhatov, Y.; Khromechek, E.; Degermendzhi, N.; Drobotov, A.; Degermendzhi, A. Comparative Study of the Stability of Stratification and the Food Web Structure in the Meromictic Lakes Shira and Shunet (South Siberia, Russia). In Ecology of Meromictic Lakes. Ecological Studies; Gulati, R., Zadereev, E., Degermendzhi, A., Eds.; Springer: Cham, Switzerland, 2017; Volume 228, pp. 89–124. [Google Scholar]
- Gorbunov, M.Y.; Umanskaya, M.V. The vertical distribution of prokaryotic phototrophic plankton in the Nizhnii Pond in Samara Botanic Garden. Samar. Luka Byul. 2007, 16, 144–155. [Google Scholar]
- Gorbunov, M.Y.; Umanskaya, M.V. Planktonic microbial communities of the meromictic and holomictic basins of lake Kichier. In Proceedings of the IV International Conference “Ozernye Ecosistemy: Biologicheskie Process, Antropogennaya Transformatsiya, Kachestvo Vody”, Minsk-Naroch, Belarus, 12–17 September 2011; p. 57. [Google Scholar]
- Gorlenko, V.M.; Buryukhaev, S.P.; Matyugina, E.B.; Borzenko, S.V.; Namsaraev, Z.B.; Bryantseva, I.A.; Boldareva, E.N.; Sorokin, D.Y.; Namsaraev, B.B. Microbial communities of the stratified soda Lake Doroninskoe (Transbaikal region). Microbiology 2010, 79, 390–401. [Google Scholar] [CrossRef]
- Malešević, N.; Ciglenečki, I.; Bura-Nakić, E.; Caric, M.; Radic, I.D.; Hrustić, E.; Viličić, D.; Ljubesic, Z. Diatoms in the extreme euxinic environment (Rogoznica Lake, Eastern Adriatic coast). Acta Bot. Croat. 2015, 74, 333–343. [Google Scholar] [CrossRef]
- Schanz, F.; Stalder, S. Phytoplankton summer dynamics: Sedimentation in the themally stratified Lake Cadagno. In Lake Cadagno: A Meromictic Alpine Lake; Peduzzi, R., Bachofen, R., Tonolla, M., Eds.; DocumentaDell’istituto di Idrobiologia: Pallanza, Italy, 1998; Volume 63, pp. 71–76. [Google Scholar]
- Kiss, K.T.; Acs, E.; Szabό, K.E.; Miracle, M.; Vicente, E. Morphological observations on Cyclotella distinguenda Hustedt and C. delicatula Hustedt from the core sample of a meromictic karstic lake of Spain (Lake La Cruz) with aspects of their ecology. Diatom Res. 2007, 22, 287–308. [Google Scholar] [CrossRef]
- Lange, E.K. Phytoplankton community of the meromictic Lake Mogilnoye (Kildin Island, Barents Sea). In Proceedings of the Thesis of International Scientific Conference “Nature of Marine Arctic: Modern Challenges and the Role of Science”, Murmansk, Russia, 10–12 March 2010; pp. 126–128. (In Russian). [Google Scholar]
- Ivanova, D.A.; Krasnova, E.D.; Voronov, D.A.; Radchenko, I.G. Seasonal dynamics of algal flora in the stratified Kislo-Sladkoe Lake partially separated from the White Sea. Oceanology 2022, 62, 207–220. [Google Scholar] [CrossRef]
- Camacho, A. On the occurrence and ecological features of deep chlorophyll maxima (DCM) in Spanish stratified lakes. Limnetica 2006, 25, 453–478. [Google Scholar] [CrossRef]
- Dokulil, M. Seasonal and spatial distribution of cryptophycean species in the deep, stratifying, alpine lake Mondsee and their role in the food web. Hydrobiologia 1988, 161, 185–201. [Google Scholar] [CrossRef]
- Gervais, F. Ecology of cryptophytes coexisting near a freshwater chemocline. Freshw. Biol. 1998, 39, 61–78. [Google Scholar] [CrossRef]
- Klaveness, D. Biology and ecology of the Cryptophyceae: Status and challenges. Biol. Oceanogr. 1989, 6, 257–270. [Google Scholar]
- Krasnova, E.D.; Pantyulin, A.N.; Matorin, D.N.; Todorenko, D.A.; Belevich, T.A.; Milyutina, I.A.; Voronov, D.A. Blooming of the cryptomonad alga Rhodomonas sp. (Cryptophyta, Pyrenomonadaceae) in the redox zone of the basins separating from the White Sea. Microbiology 2014, 83, 270–277. [Google Scholar] [CrossRef]
- Barkhatov, Y.V.; Khromechek, E.B.; Zykov, V.V.; Rogozin, D.Y. Cryptophytes of Lake Shira (Khakassia, Russia): Explosive growth during breakdown of meromixis. Hydrobiologia 2022, 849, 3373–3387. [Google Scholar] [CrossRef]
- Chupakov, A.V.; Chupakova, A.A.; Moreva, O.Y.; Shirokova, L.S.; Zabelina, S.A.; Vorobieva, T.Y.; Klimov, S.I.; Brovko, O.S.; Pokrovsky, O.S. Allochthonous and autochthonous carbon in deep, organic-rich and organic-poor lakes of the Europian Russian subarctic. Boreal Environ. Res. 2017, 22, 213–230. [Google Scholar]
- Savvichev, A.; Kokryatskaya, N.; Zabelina, S.; Rusanov, I.; Zakharova, E.; Veslopolova, E.; Lunina, O.; Patutina, E.; Bumazhkin, B.; Gruzdev, D.; et al. Microbial processes of the carbon and sulfur cycles in an ice-covered, iron-rich meromictic lake Svetloe (Arkhangelsk region, Russia). Environ. Microbiol. 2017, 19, 659–672. [Google Scholar] [CrossRef]
- Motylkova, I.V.; Konovalova, N.V. Spring Phytoplankton of the Tunaicha Lake (South Sakhalin). In Chteniya Pamyati V.Ya. Levanidova (Readings in Memory of V.Ya. Levanidov); Dal’nauka: Vladivostok, Russia, 2003; pp. 287–294. [Google Scholar]
- Motylkova, I.V.; Konovalova, N.V. Seasonal dynamics of phytoplankton in a lagoon-type lake Izmenchivoye (Southeast Sakhalin). Russ. J. Mar. Biol. 2010, 36, 86–92. [Google Scholar] [CrossRef]
- Motylkova, I.V.; Konovalova, N.V. Structure and seasonal dynamics of phytoplankton in Ptichye lake of the lagoon-type (south Sakhalin). In The Researches of the Aquatic Biological Resources of Kamchatka and the North-West Part of the Pacific Ocean; Kamchatka Research Institute of Fisheries and Oceanography: Petropavlovsk-Kamchatsky, Russia, 2018; pp. 3–76, (In Russian). [Google Scholar] [CrossRef]
- Konovalova, N.V.; Motylkova, I.V. The Phytoplankton of Tunaicha Lake (Southern Sakhalin). In Proceedings of the 21st International Symposium on Okhotsk Sea and Sea Ice, Monbetsu, Japan, 19–24 February 2006; Okhotsk Sea and Cold Ocean Research Association: Mombetsu, Japan, 2006; pp. 200–204. [Google Scholar]
- Ezhova, E.E.; Lange, E.K.; Polunina, Y.Y.; Kravtsov, V.A.; Emelyanov, E.M. Plankton and benthos of the meromictic Lake Mogilnoye (Kildin Island, Barents Sea). In Proceedings of the International Scientific Conference “Nature of Marine Arctic: Modern Challenges and Role of Science”, Murmansk, Russia, 10–12 March 2010; pp. 71–73. (In Russian). [Google Scholar]
- Motylkova, I.V.; Konovalova, N.V. The Composition and Structure of Phytoplankton in the Busse Lagoon, Southeastern Sakhalin Island. Russ. J. Mar. Biol. 2021, 47, 337–345. [Google Scholar] [CrossRef]
- Burch, M.D. Annual cycle of phytoplankton in Ace Lake, an ice covered, saline meromictic lake. Hydrobiologia 1988, 165, 59–75. [Google Scholar] [CrossRef]
- Krasnova, E.D.; Voronov, D.A.; Mardashova, M.V.; Pantulin, A.N.; Frolova, N.L. Long-term variability of physical and chemical parameters in a partially isolated lagoon on the Cape Zeleny (Karelian Coast of the White Sea). In Proceedings of the IV International Scientific and Practical Conference “Marine Education and Research (MARESEDU-2015)”, Moscow, Russia, 19–24 October 2015; pp. 451–454. (In Russian). [Google Scholar]
- Mardashova, M.V.; Voronov, D.A.; Krasnova, E.D. Benthic Communities of Coastal Water Bodies at Different Stages of Isolation from the White Sea in the Vicinity of the White Sea Biological Station, Moscow State University, Kandalaksha Bay, White Sea. Biol. Bull. 2020, 47, 1133–1152. [Google Scholar] [CrossRef]
- Luybeznova, N.V.; Mardashova, M.V. Features of growth of Ophioglossum vulgatum L. at the White Sea coasts. Phytodiversity East. Eur. 2017, XI, 74–80. (In Russian) [Google Scholar]
- Grouzdev, D.; Gaisin, V.; Lunina, O.; Krutkina, M.; Krasnova, E.; Voronov, D.; Baslerov, R.; Sigalevich, P.; Savvichev, A.; Gorlenko, V. Microbial communities of stratified aquatic ecosystems of Kandalaksha Bay (White Sea) shed light on the evolutionary history of green and brown morphotypes of Chlorobiota. FEMS Micriobiol. Ecol. 2022, 98, fiac103. [Google Scholar] [CrossRef] [PubMed]
- Krasnova, E.D.; Voronov, D.A. Influence of chemocline on vertical unevenness of zooplankton in coastal stratified water bodies separated from the White Sea. In Proceedings of the X International Conference «Marine Research and Education, MARESEDU-2021», Moscow, Russia, 24–28 October 2021; PolyPRESS: Tver, Russia, 2022; pp. 82–86. (In Russian). [Google Scholar]
- Shaporenko, S.I.; Korneeva, G.A.; Pantyulin, A.N.; Pertsova, N.M. Specific features of the ecosystems of isolated waterbodies of the White Sea Kandalaksha Bay. Vodn. Resur. 2005, 32, 517–532. [Google Scholar]
- Krasnova, E.; Matorin, D.; Belevich, T.; Efimova, L.; Kharcheva, A.; Kokryatskaya, N.; Losyuk, G.; Todorenko, D.; Voronov, D.; Patsaeva, S. The characteristic pattern of multiple colored layers in coastal stratified lakes in the process of separation from the White Sea. Chin. J. Ocean. Limn. 2018, 36, 1962–1977. [Google Scholar] [CrossRef]
- Vasilenko, A.N. Study of the level regime of water bodies of the north-western coast of the White Sea. In Proceedings of the IV International Scientific and Practical Conference “Marine Education and Research (MARESEDU-2015)”, Moscow, Russia, 19–24 October 2015; pp. 468–470. (In Russian). [Google Scholar]
- Available online: World-weather.ru (accessed on 10 July 2023). (In Russian).
- Sournia, A. (Ed.) Phytoplankton Manual. Monographs on Oceanographic Methodology 1978; UNESCO: Paris, France, 1978; Volume 6, 337p. [Google Scholar]
- Vinogradov, M.E. (Ed.) Modern Methods for Quantifying the Distribution of Marine Plankton; Nauka: Moscow, Russia, 1983; 280p. (In Russian) [Google Scholar]
- Guiry, M.D.; Guiry, G.M. (Eds.) AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2016; Available online: https://www.algaebase.org (accessed on 31 August 2023).
- Nordic Microalgae and Aquatic Protozoa. Electronic Database. Swedish Meteorological and Hydrological Institute (SMHI). Available online: http://nordicmicroalgae.org (accessed on 8 August 2023).
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protest plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Okolodkov, Y.N. Differentication of phototrophic and heterotrophic dinoflagellates (Dinophyceae) by epifluorescence microscopy in the northern Greenland Sea. Bot. J. Russ. Acad. Sci. 1999, 4, 53–61. [Google Scholar]
- Clarke, R.K.; Gorley, R.N. PRIMER V6: User Manual—Tutorial; Plymouth Marine Laboratory: Plymouth, UK, 2006; 190p. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Krasnova, E.D.; Kharcheva, A.V.; Milyutina, I.A.; Voronov, D.A.; Patsaeva, S.V. Study of microbial communities in redox zone of meromictic lakes isolated from the White Sea using spectral and molecular methods. J. Mar. Biol. Assoc. 2015, 95, 1579–1590. [Google Scholar] [CrossRef]
- Ilyash, L.V.; Belevich, T.A.; Zhitina, L.S.; Radchenko, I.G.; Ratkova, T.N. Phytoplankton of the White Sea. In Biogeochemistry of the Atmosphere, Ice and Water of the White Sea. The Handbook of Environmental Chemistry; Lisitsyn, A., Gordeev, V., Eds.; Springer: Cham, Switzerland, 2018; Volume 81. [Google Scholar] [CrossRef]
- Sazhin, A.F.; Sapozhnikov, F.V.; Rat’kova, T.N.; Romanova, N.D.; Shevchenko, V.P.; Filippov, A.S. The inhabitants of the spring ice, under-icewater, and sediments of the White Sea in the estuarine zone of the Severnaya Dvina River. Oceanology 2011, 51, 295–305. [Google Scholar] [CrossRef]
- Nikishova, E.R.; Radchenko, I.G.; Belevich, T.A. Small Photosynthetic Flagellates of the White Sea: Seasonal Dynamics and Their Role in Plankton and Ice Communities. Moscow Univ. Biol. Sci. Bull. 2020, 75, 147–152. [Google Scholar] [CrossRef]
- Radchenko, I.; Smirnov, V.; Ilyash, L.; Sukhotin, A. Phytoplankton dynamics in a subarctic fjord during the under-ice—Open water transition. Mar. Environ. Res. 2021, 164, 105242. [Google Scholar] [CrossRef]
- Ilyash, L.V.; Radchenko, I.G.; Shevchenko, V.P.; Zdorovennov, R.E.; Pantyulin, A.N. Contrasting summer phytoplankton communities in stratified and mixed waters of the White Sea. Oceanology 2014, 54, 730–738. [Google Scholar] [CrossRef]
- Ilyash, L.V.; Zhitina, L.S.; Belevich, T.A.; Shevchenko, V.P.; Kravchishina, M.D.; Pantyulin, A.N.; Tolstikov, A.V.; Tchultsova, A.L. Spatial distribution of phytoplankton in the White Sea during atypical dinoflagellates domination (July 2009). Oceanology 2016, 56, 372–381. [Google Scholar] [CrossRef]
- Radchenko, I.G.; Smirnov, V.V.; Usov, N.V.; Sukhotin, A.A. Seasonal dynamics of phytoplankton in the Chupa Inlet (Kandalaksha bay, White Sea). Mosc. Univ. Biol. Sci. Bull. 2022, 77, 32–39. [Google Scholar] [CrossRef]
- Stoecker, D.K.; Lavrentyev, P.J. Mixotrophic Plankton in the Polar Seas: A Pan-Arctic Review. Rev. Front. Mar. Sci. 2018, 5, 292. [Google Scholar] [CrossRef]
- Plotnikov, A.O.; Selivanova, E.A.; Khlopko, Y.A.; Voronov, D.A.; Matorin, D.N.; Todorenko, D.A.; Krasnova, E.D. Structure and Functioning of Plankton Communities of Phototrophic and Mixotrophic Protists in the Coastal Lagoon “Lake Kislo-Sladkoe” (White Sea, Karelian Coast). Izv. Ross. Akad. Nauk. Seriya Geogr. 2022, 86, 985–1001. (In Russian) [Google Scholar]
- Rogozin, D.Y.; Tarnovsky, M.O.; Belolipetskii, V.M.; Zykov, V.V.; Zadereev, E.S.; Tolomeev, A.P.; Drobotov, A.V.; Barkhatov, Y.V.; Gaevsky, N.A.; Gorbaneva, T.B.; et al. Disturbance of meromixis in saline Lake Shira (Siberia, Russia): Possible reasons and ecosystem response. Limnologica 2017, 66, 12–23. [Google Scholar] [CrossRef]
- Lange, E.K. Spatial and temporal variability of phytoplankton indicators of the relict lake Mogilnoye (Kildin Island, the Barents Sea). In Proceedings of the All-Russian Conference with International Participation “XXIX Coastal Conference: Field-Based and Theoretical Research in Shore Use Practice”, Kaliningrad, Russia, 18–24 April 2022; pp. 428–430. (In Russian). [Google Scholar]
- Saini, J.S.; Hassler, C.; Cable, R.; Fourquez, M.; Danza, F.; Roman, S.; Tonolla, M.; Storelli, N.; Jacquet, S.; Zdobnov, E.M.; et al. Bacterial, phytoplankton, and viral dynamics of meromictic Lake Cadagno offer insights into the Proterozoic ocean microbial loop. MBio 2022, 13, e0005222. [Google Scholar] [CrossRef]


| Phylum | Class | Number of Species |
|---|---|---|
| Myozoa | Dinophyceae | 68 |
| Oxyrrhidophyceae | 1 | |
| Bacillariophyta | Bacillariophyceae | 61 |
| Mediophyceae | 34 | |
| Coscinodiscophyceae | 9 | |
| Cyanobacteria | Cyanophyceae | 25 |
| Chlorophyta | Pyramimonadophyceae | 7 |
| Chlorophyceae | 3 | |
| Chlorodendrophyceae | 2 | |
| Trebouxiophyceae | 1 | |
| Ochrophyta | Chrysophyceae | 4 |
| Dictyochophyceae | 4 | |
| Euglenozoa | Euglenophyceae | 3 |
| Cercozoa | Imbricatea | 1 |
| Thecofilosea | 1 | |
| Charophyta | Zygnematophyceae | 1 |
| Haptophyta | Coccolithophyceae | 1 |
| Cryptista | Katablepharidophyceae | 1 |
| Cryptophyceae | not identified |
| Biotope (Depth, m) | BC, mg C/m3 | Taxon | Mode of Nutrition |
|---|---|---|---|
| 30 January 2020 | |||
| 0.5 | 0.06 | Protoperidinium brevipes (0.58) | P |
| 1.5 (Zeu) | 0.23 | cf. Planktolyngbya limnetica (0.78) | P |
| 2.5 | 0.16 | Oscillatoria sp.3 (0.19) | P |
| Gymnodinium wulffii (0.15) | H | ||
| NCF 10 μm (0.15) | P | ||
| 3.5 | 0.92 | Gymnodinium wulffii (0.33) | H |
| NCF 9–14 μm (0.21) | P | ||
| Dinophyta spp. (0.18) | ? | ||
| 4.5 (Zchem) | 16.60 | Gymnodinium wulffii (0.65) | H |
| Gyrodinium spirale (0.35) | H | ||
| Sea | 0.47 | Dinophysis acuminata (0.57) | M |
| Phalacroma rotundatum (0.21) | H | ||
| 8 June 2020 | |||
| 0.5 | 10.63 | NCF 3–5 μm (0.21) | P |
| Pyramimonas cf. diskoicola (0.20) | P | ||
| Heterocapsa rotundata (0.16) | P | ||
| 1.5 | 15.82 | Ebria tripartita (0.26) | H |
| Heterocapsa rotundata (0.12) | P | ||
| Diplopsalis lenticula (0.11) | H | ||
| 3.5 | 10.30 | Pyramimonas cf. diskoicola (0.38) | P |
| Heterocapsa rotundata (0.11) | P | ||
| 4.5 | 8.87 | Gyrodinium spirale (0.21) | H |
| NCF 6–8 μm (0.17) | P | ||
| Heterocapsa rotundata (0.12) | P | ||
| 5.2 (Zchem; Zeu) | 80.37 | Tetraselmis cordiformis (0.35) | P |
| Gymnodinium arcticum (0.18) | P | ||
| Skeletonema costatum (0.11) | P | ||
| Sea | 10.22 | Heterocapsa rotundata (0.16) | P |
| Pyramimonas cf. diskoicola (0.11) | P | ||
| Skeletonema costatum (0.11) | P | ||
| Unidentified cryptomonad cells 10–20 μm (0.11) | P | ||
| 18 June 2020 | |||
| 0.5 | 25.46 | Cyclotella choctawhatcheeana (0.55) | P |
| 1.5 | 23.55 | Ebria tripartita (0.30) | H |
| Cyclotella choctawhatcheeana (0.21) | P | ||
| 2.5 | 3.17 | Protoperidinium pellucidum (0.23) | H |
| 3.5 | 7.56 | Dinophysis norvegica (0.22) | M |
| Ebria tripartita (0.17) | H | ||
| Dinophysis acuminata (0.12) | M | ||
| 4.5 | 7.26 | Tripos arcticus (0.31) | P |
| Bunch of unidentified species with green cells 3 μm (0.29) | P | ||
| 5 (Zchem; Zeu) | 1730.91 | Gymnodinium arcticum (0.97) | P |
| Sea | 98.09 | Skeletonema costatum (0.75) | P |
| 2 July 2020 | |||
| 0.5 | 12.44 | Flagellate non det. 10–14 μm (0.30) | P |
| Heterocapsa rotundata (0.14) | P | ||
| Pyramimonas cf. diskoicola (0.14) | P | ||
| Cyclotella choctawhatcheeana (0.12) | P | ||
| 1.5 | 21.10 | Diplopsalis lenticula (0.37) | H |
| 3.5 | 13.90 | Cyclotella choctawhatcheeana (0.63) | P |
| 4.5 | 88.42 | Cyclotella choctawhatcheeana (0.33) | P |
| Bunch of unidentified species with green cells 3 μm (0.29) | P | ||
| NCF 3–5 μm (0.11) | P | ||
| 5 (Zchem) | 197.19 | Flagellate non det. 5 μm (0.54) | P |
| NCF 3–5 μm (0.29) | P | ||
| Sea | 35.25 | Heterocapsa rotundata (0.47) | P |
| Skeletonema costatum (0.14) | P | ||
| 20 July 2020 | |||
| 0.5 | 10.82 | Heterocapsa rotundata (0.24) | P |
| Pyramimonas cf. diskoicola (0.14) | P | ||
| 1.5 | 12.93 | Diplopsalis lenticula (0.19) | H |
| NCF 13 μm (0.11) | P | ||
| 2.5 | 25.75 | Gonyaulax spinifera (0.30) | P |
| Ebria tripartita (0.11) | H | ||
| 3.5 | 5.43 | NCF 3–5 μm (0.23) | P |
| Ebria tripartita (0.20) | H | ||
| Akashiwo sanguinea (0.15) | P | ||
| NCF 6–8 μm (0.13) | P | ||
| 4.5 | 24.90 | Ebria tripartita (0.36) | H |
| NCF 3–5 μm (0.23) | P | ||
| NCF 6–8 μm (0.18) | P | ||
| 5.1 | 261.85 | Unidentified species with green oval cells 5–6 μm (0.73) | P |
| NCF 3–5 μm (0.12) | P | ||
| 5.4 (Zchem; Zeu) | 182.94 | Unidentified species with green oval cells 5–6 μm (0.82) | P |
| Sea | 14.08 | Kryptoperidinium triquetrum vegetative cells and spores (0.18) | P |
| Unidentified cryptomonad cells 10–20 μm (0.14) | ? | ||
| NCF 6–8 μm (0.14) | P | ||
| NCF 3–5 μm (0.11) | P | ||
| 18 August 2020 | |||
| 0.5 | 124.77 | Heterocapsa rotundata (0.42) | P |
| Tripos arcticus (0.16) | P | ||
| 1.5 | 15.18 | Heterocapsa rotundata (0.24) | P |
| Kryptoperidinium triquetrum vegetative cells and spores (0.22) | P | ||
| NCF 6–8 μm (0.12) | P | ||
| NCF 3–5 μm (0.12) | P | ||
| 2.5 | 46.49 | Kryptoperidinium triquetrum (0.18) | P |
| Kryptoperidinium triquetrum spores (0.17) | P | ||
| Heterocapsa rotundata (0.11) | P | ||
| 3.5 | 151.06 | Cyclotella choctawhatcheeana (0.89) | P |
| 4.5 | 36.15 | Unidentified species with green oval cells 5–6 μm (0.22) | P |
| NCF 3–5 μm (0.20) | P | ||
| Ebria tripartita (0.11) | H | ||
| 5 | 280.03 | Unidentified species with green oval cells 5–6 μm (0.39) | P |
| NCF 3–5 μm (0.33) | P | ||
| 5.4 (Zchem; Zeu) | 303.40 | Unidentified species with green oval cells 5–6 μm (0.53) | P |
| Oxyrrhis marina (0.32) | H | ||
| Sea | 20.22 | Heterocapsa rotundata (0.36) | P |
| Pyramimonas cf. diskoicola (0.12) | P | ||
| 7 September 2020 | |||
| 0.5 | 21.70 | Kryptoperidinium triquetrum spores (0.20) | P |
| Lepidodinium chlorophorum (0.19) | P | ||
| Cyclotella choctawhatcheeana (0.14) | P | ||
| Heterocapsa rotundata (0.11) | P | ||
| 1.5 | 120.56 | Cyclotella choctawhatcheeana (0.31) | P |
| Kryptoperidinium triquetrum spores (0.28) | P | ||
| Kryptoperidinium triquetrum (0.16) | P | ||
| 2.5 | 31.38 | Cyclotella choctawhatcheeana (0.40) | P |
| Kryptoperidinium triquetrum vegetative cells and spores (0.29) | P | ||
| 3.5 | 130.55 | Cyclotella choctawhatcheeana (0.41) | P |
| Kryptoperidinium triquetrum vegetative cells and spores (0.25) | P | ||
| Ebria tripartita (0.12) | H | ||
| 4.5 | 45.75 | Ebria tripartita (0.28) | H |
| Micracanthodinium claytonii (0.25) | H | ||
| 5.1 (Zchem; Zeu) | 1492.99 | Oxyrrhis marina/Lebouridinium glaucum (0.44) | H |
| Gymnodinium spp. 8–15 μm (0.25) | ? | ||
| Unidentified species with green oval cells 5–6 μm (0.21) | P | ||
| Sea | 17.23 | Pyramimonas cf. diskoicola (0.18) | P |
| Unidentified cryptomonad cells 10–20 μm (0.11) | ? | ||
| 12 September 2020 | |||
| 0.5 | 15.54 | Kryptoperidinium triquetrum vegetative cells and spores (0.44) | P |
| Heterocapsa rotundata (0.14) | P | ||
| Unidentified cryptomonad cells 6–10 μm (0.12) | ? | ||
| 1.5 | 5.79 | Protoperidinium bipes (0.14) | H |
| Tripos fusus (0.11) | P | ||
| Gonyaulaxspinifera (0.11) | P | ||
| 2.5 | 8.58 | Gonyaulaxspinifera (0.13) | P |
| 3.5 | 19.89 | Micracanthodiniumclaytonii (0.33) | H |
| NCF 6–8 μm (0.13) | P | ||
| Gonyaulax spinifera (0.11) | P | ||
| 4.5 | 32.36 | NCF 6–8 μm (0.57) | P |
| NCF 3–5 μm (0.25) | P | ||
| 5.1 (Zchem; Zeu) | 310.55 | Oxyrrhis marina (0.75) | H |
| NCF 3–5 μm (0.11) | P | ||
| Sea | 18.77 | Unidentified cryptomonad cells 10–20 μm (0.36) | ? |
| Unidentified cryptomonad cells 6–10 μm (0.25) | ? | ||
| 1 October 2020 | |||
| 0.5 | 12.16 | Unidentified cryptomonad cells 10–20 μm (0.34) | ? |
| Unidentified cryptomonad cells 6–10 μm (0.18) | ? | ||
| Cyclotella choctawhatcheeana (0.18) | P | ||
| Gyrodinium spp. 21–32 μm (0.11) | ? | ||
| 1.5 | 13.78 | NCF 6–8 μm (0.60) | P |
| Cyclotella choctawhatcheeana (0.16) | P | ||
| 2.5 | 5.97 | NCF 6–8 μm (0.31) | P |
| Cyclotella choctawhatcheeana (0.20) | P | ||
| NCF 10 μm (0.13) | P | ||
| Heterocapsarotundata (0.11) | P | ||
| 3.5 | 2.91 | Cyclotella choctawhatcheeana (0.31) | P |
| NCF 6–8 μm (0.21) | P | ||
| NCF 3–5μm (0.17) | P | ||
| 4.5 | 3.84 | NCF 6–8 μm (0.41) | P |
| NCF 3–5 μm (0.22) | P | ||
| Unidentified cryptomonad cells 10–20 μm (0.12) | ? | ||
| 4.9 (Zchem) | 35.98 | Oxyrrhis marina (0.94) | H |
| Sea | 1.45 | Unidentified cryptomonad cells 10–20 μm (0.19) | ? |
| NCF 6–8 μm (0.16) | P | ||
| NCF11 μm (0.11) | P | ||
| 1 November 2020 | |||
| 0.5 | 3.84 | Ebria tripartia (0.31) | H |
| Melosira sp. (0.15) | P | ||
| 1.5 | 19.76 | NCF 10 μm (0.59) | P |
| 2.5 | 0.92 | Melosira arctica (0.26) | P |
| Unidentified cryptomonad cells 6–10 μm (0.19) | ? | ||
| Protoperidinium brevipes (0.13) | H | ||
| Odontella aurita (0.11) | P | ||
| 3.5 | 1.04 | Gymnodinium spp. 11–15 μm (0.22) | ? |
| Unidentified cryptomonad cells 10–20 μm (0.19) | ? | ||
| Unidentified cryptomonad cells 6–10 μm (0.15) | ? | ||
| 4.1 | 6.48 | Gymnodinium spp. 11–15 μm (0.27) | ? |
| Unidentified cryptomonad cells 10–20 μm (0.19) | ? | ||
| Micracanthodinium claytonii (0.18) | H | ||
| Unidentified cryptomonad cells 6–10 μm (0.13) | ? | ||
| Sea | 0.69 | NCF 10 μm (0.13) | P |
| Date | Average Similarity (%) | Number of Taxa Cumulatively Contributing 90–92% | Taxa Contributing at Least 9% to Similarity, and Their Percentage Contribution |
|---|---|---|---|
| 30 January 2020 | 7 | 7 | Gymnodinium wulffii (46), Paralia sulcata (17) |
| 8 June 2020 | 38 | 19 | Heterocapsa rotundata (14), Pyramimonas cf. diskoicola (10) |
| 18 June 2020 | 37 | 16 | Cyclotella choctawhatcheeana (14), Ebria tripartita (12), Synechocystis salina (11), Gymnodinium arcticum (11) |
| 2 July 2020 | 40 | 10 | Cyclotella choctawhatcheeana (30), Ollicola vangoorii (11) |
| 20 July 2020 | 42 | 19 | Unidentified cryptomonad cells 6–10 μm (11), Ebria tripartita (11), Cyclotella choctawhatcheeana (10) |
| 18 August 2020 | 40 | 19 | Cyclotella choctawhatcheeana (13) |
| 7 September 2020 | 45 | 25 | Cyclotella choctawhatcheeana (13), Kryptoperidinium triquetrum spore (11) |
| 12 September 2020 | 46 | 21 | Kryptoperidinium triquetrum (9) |
| 1 October 2020 | 41 | 9 | Cyclotella choctawhatcheeana (27) Unidentified cryptomonad cells 10–20 μm (17) Unidentified cryptomonad cells 6–10 μm (17) Kryptoperidinium triquetrum (11) |
| 1 November 2020 | 32 | 13 | Gymnodinium spp. 11–15 μm (18), cf. Peridiniella danica (12), Monoraphidium contortum (11) |
| Average Similarity (%) | Number of Taxa Cumulatively Contributing 90–92% | Taxa Contributing at Least 9% to Similarity, and Their Percentage Contribution |
|---|---|---|
| 30 January 2020 | ||
| 10 | 1 | Protoperidinium brevipes (100) |
| 8 June 2020 | ||
| 42 | 10 | Heterocapsarotundata (21), Pyramimonas cf. diskoicola (13), Unidentified cryptomonad cells 10–20 μm (13), Ollicola vangoorii (11) |
| 18 June 2020 | ||
| 23 | 9 | Ebria tripartita (18), Euglenozoa cells 21–40 μm (14), Unidentified cryptomonad cells 10–20 μm (11), Kryptoperidinium triquetrum (11) |
| 2 July 2020 | ||
| 42 | 9 | Heterocapsa rotundata (21), Pyramimonas cf. diskoicola (13) |
| 20 July 2020 | ||
| 47 | 14 | Heterocapsa rotundata (9), Gymnodinium spp. 16–20 μm (9) |
| 18 August 2020 | ||
| 47 | 9 | Heterocapsa rotundata (18), Pyramimonas cf. diskoicola (11) |
| 7 September 2020 | ||
| 40 | 9 | Heterocapsarotundata (16), Cyclotella choctawhatcheeana (14), Unidentified cryptomonad cells 6–10 μm (13), Pyramimonas cf. diskoicola (13) |
| 12 September 2020 | ||
| 43 | 6 | Heterocapsa rotundata (23), Unidentified cryptomonad cells 6–10 μm (22), Unidentified cryptomonad cells 10–20 μm (17), Pyramimonas cf. diskoicola (14), Cyclotella choctawhatcheeana (11) |
| 1 October 2020 | ||
| 32 | 4 | Unidentified cryptomonad cells 10–20 μm (34), Unidentified cryptomonad cells 6–10 μm (26), Cyclotella choctawhatcheeana (18), Kryptoperidinium triquetrum (17) |
| 1 November 2020 | ||
| 25 | 7 | Kryptoperidinium triquetrum (19), Cocconeis costata (18), Monoraphidium contortum (16), Thalassionema nitzschioides(15), Paulinella ovalis (11), Pterosperma sp.1 (11), Cyclotella choctawhatcheeana (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radchenko, I.G.; Aksenova, V.A.; Voronov, D.A.; Rostanets, D.V.; Krasnova, E.D. Annual Dynamics of a Layered Phytoplankton Structure in a Meromictic Lagoon Partially Isolated from the White Sea. Diversity 2023, 15, 1009. https://doi.org/10.3390/d15091009
Radchenko IG, Aksenova VA, Voronov DA, Rostanets DV, Krasnova ED. Annual Dynamics of a Layered Phytoplankton Structure in a Meromictic Lagoon Partially Isolated from the White Sea. Diversity. 2023; 15(9):1009. https://doi.org/10.3390/d15091009
Chicago/Turabian StyleRadchenko, Irina G., Vasilisa A. Aksenova, Dmitry A. Voronov, Dmitry Viktorovich Rostanets, and Elena Dmitrievna Krasnova. 2023. "Annual Dynamics of a Layered Phytoplankton Structure in a Meromictic Lagoon Partially Isolated from the White Sea" Diversity 15, no. 9: 1009. https://doi.org/10.3390/d15091009
APA StyleRadchenko, I. G., Aksenova, V. A., Voronov, D. A., Rostanets, D. V., & Krasnova, E. D. (2023). Annual Dynamics of a Layered Phytoplankton Structure in a Meromictic Lagoon Partially Isolated from the White Sea. Diversity, 15(9), 1009. https://doi.org/10.3390/d15091009






