Spatiotemporal Variability in Fish Assemblages in a Coastal and Estuarine System in the Tropical Eastern Pacific during the Anthropause
Abstract
:1. Introduction
2. Materials and Methods
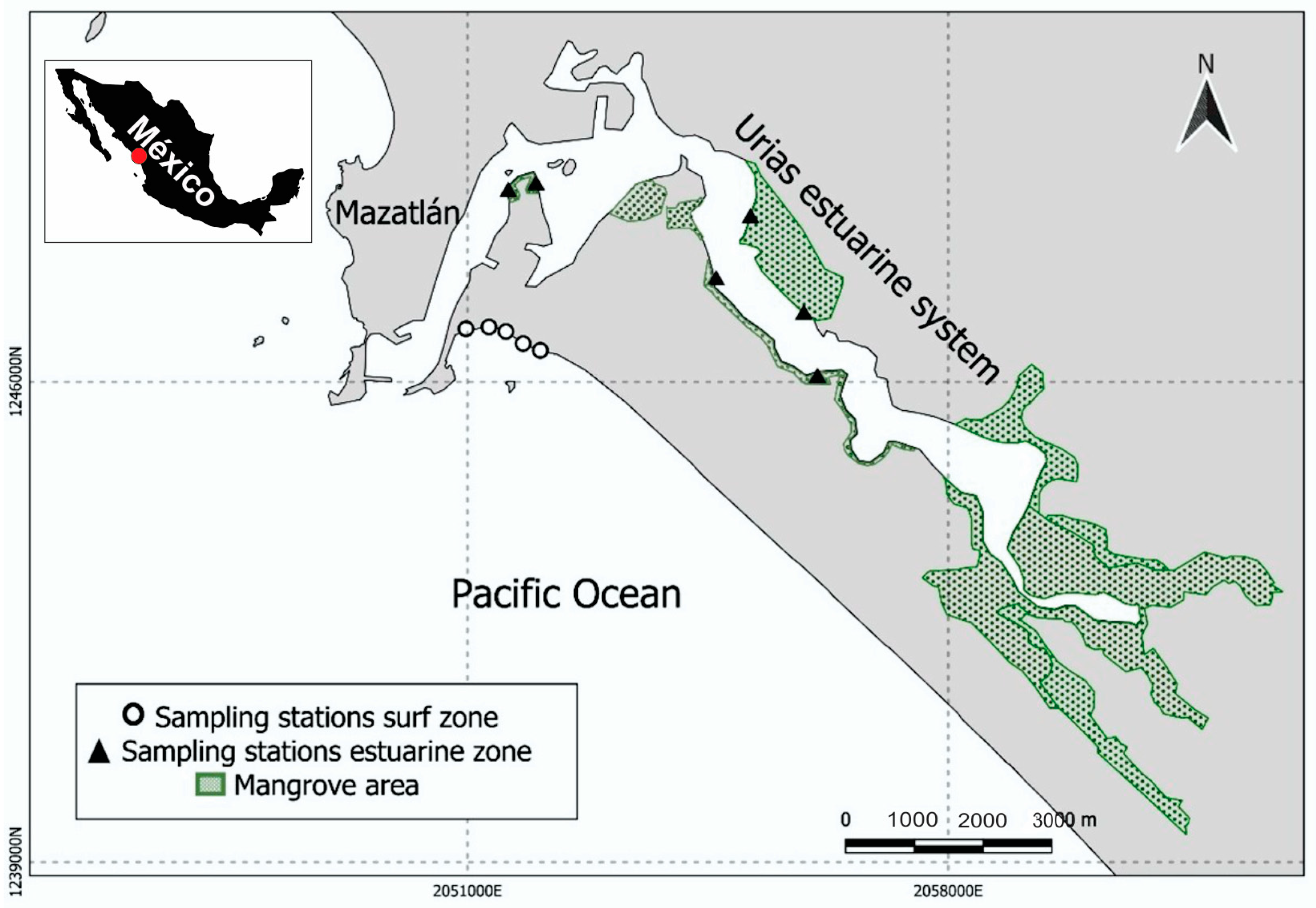
2.1. Study Area
2.2. Sampling Design
2.3. Analysis of Environmental Data
2.4. Analysis of Fish Assemblages
3. Results
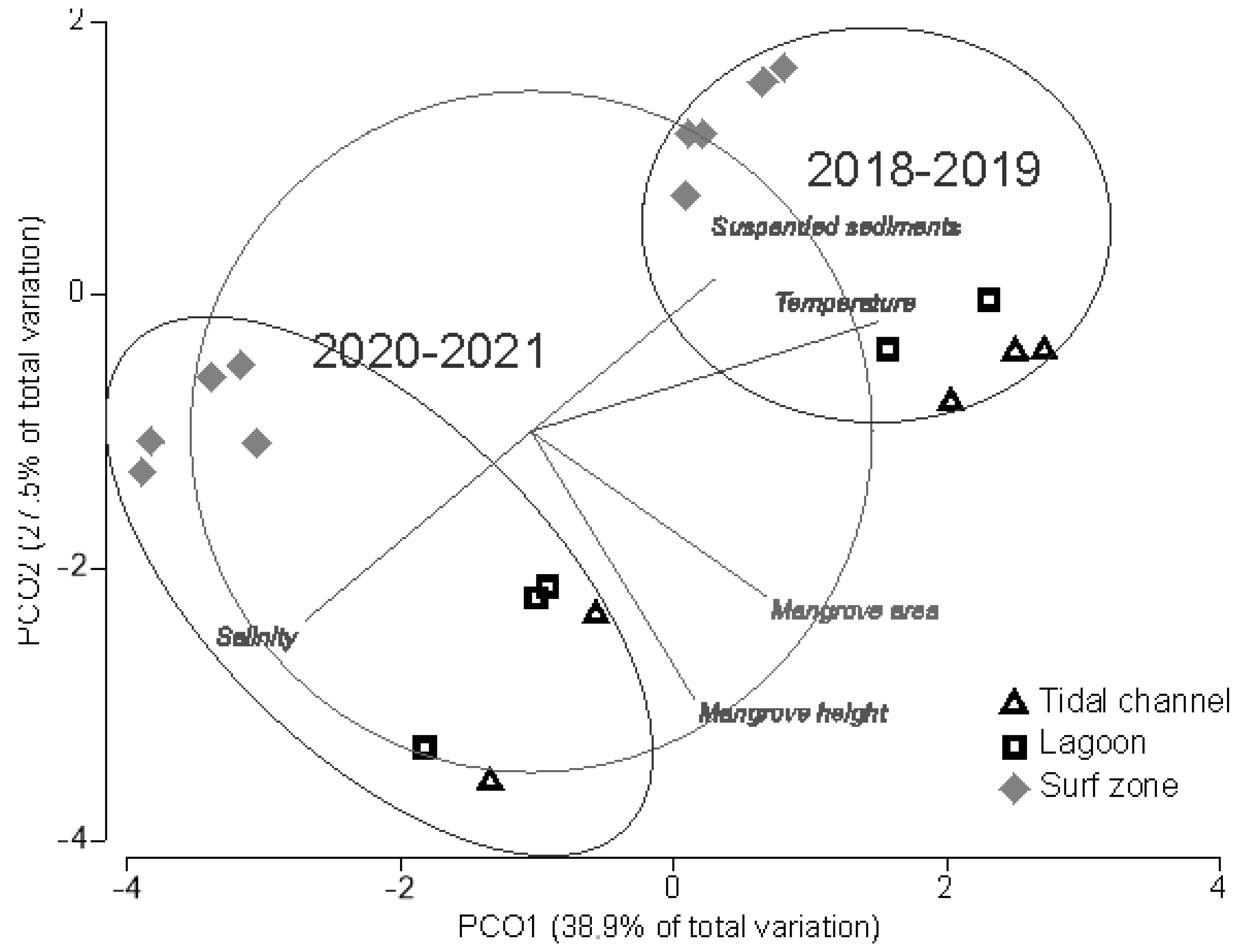
3.1. Analysis of Environmental Data
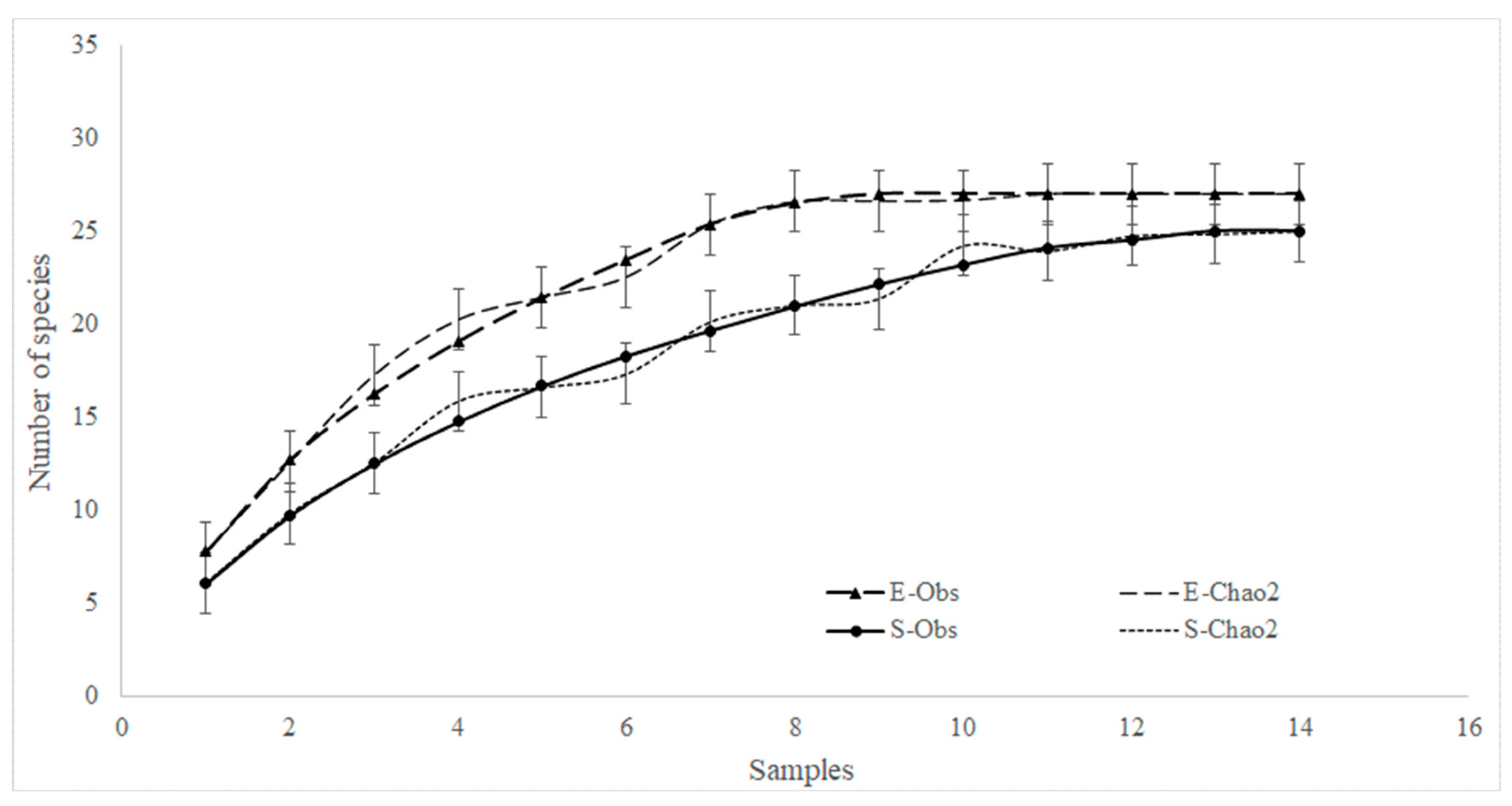
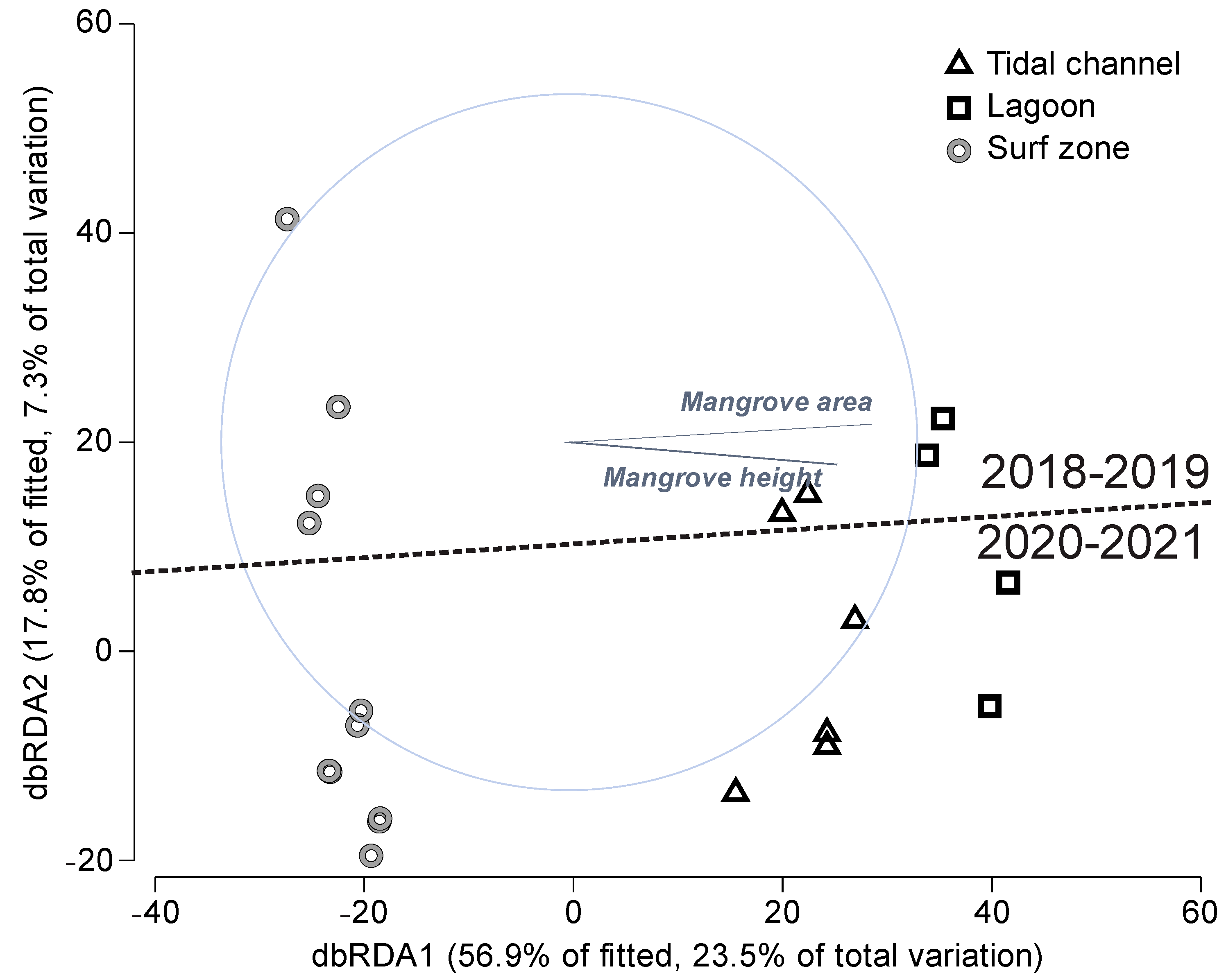
3.2. Analysis of Fish Assemblages
4. Discussion
4.1. Environmental Data and Habitat Characteristics
4.2. Fish Assemblages
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junk, W.J.; Brown, M.; Campbell, I.C.; Finlayson, M.; Gopal, B.; Ramberg, L.; Warner, B.G. The Comparative Biodiversity of Seven Globally Important Wetlands: A Synthesis. Aquat. Sci. 2006, 68, 400–414. [Google Scholar] [CrossRef]
- Tockner, K.; Stanford, J.A. Riverine Flood Plains: Present State and Future Trends. Environ. Conserv. 2002, 29, 308–330. [Google Scholar] [CrossRef]
- Junk, W.J.; Wantzen, K.M. The Flood Pulse Concept: New Aspects, Approaches and Applications-an Update. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries, Food and Agriculture Organization and Mekong River Commission, FAO Regional. Phnom Penh, Kingdom of Cambodia, 11–14 February 2004; pp. 117–149. [Google Scholar]
- Welcomme, R.L. Inland Fisheries: Ecology and Management. Food and Agriculture Organisation of the United Nations; Blackwell Science: Rome, Italy, 2001. [Google Scholar]
- Chu, C.; Minns, C.K.; Lester, N.P.; Mandrak, N.E. An Updated Assessment of Human Activities, the Environment, and Freshwater Fish Biodiversity in Canada. Can. J. Fish. Aquat. Sci. 2014, 72, 135–148. [Google Scholar] [CrossRef]
- Lara-Lara, R.; Arreola-Lizarraga, J.; Calderon-Aguilera, L.; Camacho-Ibar, V.; Lanza-Espino, D.L.G.; Escofet-Giansone, A.; Espejel, I.; Guzmán-Arroyo, M.; Ladah, L.; López-Hernández, M.; et al. Los Ecosistemas Costeros, Insulares y Epicontinentales. J. Comp. Capital. Nat. México 2008, 1, 109–134. [Google Scholar]
- Chaudhuri, A.; Mukherjee, S.; Homechaudhuri, S. Dinámica Estacional de Comunidades de Peces En Una Marisma Intermareal de Sundarbans Indio. Sci. Mar. 2013, 77, 301–311. [Google Scholar] [CrossRef]
- Newton, A.; Brito, A.C.; Icely, J.D.; Derolez, V.; Clara, I.; Angus, S.; Schernewski, G.; Inácio, M.; Lillebø, A.I.; Sousa, A.I.; et al. Assessing, Quantifying and Valuing the Ecosystem Services of Coastal Lagoons. J. Nat. Conserv. 2018, 44, 50–65. [Google Scholar] [CrossRef]
- Sreekanth, G.B.; Jaiswar, A.K.; Zacharia, P.U.; Pazhayamadom, D.G.; Chakraborty, S.K. Effect of Environment on Spatio-Temporal Structuring of Fish Assemblages in a Monsoon-Influenced Tropical Estuary. Environ. Monit. Assess. 2019, 191, 1–27. [Google Scholar] [CrossRef]
- Anthony, A.; Atwood, J.; August, P.; Byron, C.; Cobb, S.; Foster, C.; Fry, C.; Gold, A.; Hagos, K.; Heffner, L. Coastal Lagoons and Climate Change: Ecological and Social Ramifications in US Atlantic and Gulf Coast Ecosystems. Ecol. Soc. 2009, 14, 8. [Google Scholar] [CrossRef]
- Bayley, P.B. Understanding Large River: Floodplain Ecosystems. Bioscience 1995, 45, 153–158. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Bhattacharyya, S. Impact of COVID-19 Lockdown on the Socioenvironmental Scenario of Indian Sundarban. In Environmental Resilience and Transformation in Times of COVID-19: Climate Change Effects on Environmental Functionality; Elsevier: Amsterdam, The Netherlands, 2021; pp. 25–36. [Google Scholar] [CrossRef]
- Zambrano-Monserrate, M.A.; Ruano, M.A.; Sanchez-Alcalde, L. Indirect Effects of COVID-19 on the Environment. Sci. Total Environ. 2020, 728, 138813. [Google Scholar] [CrossRef]
- World Health Organization Archived: WHO Timeline—COVID-19. Available online: https://www.who.int/news/item/27-04-2020-who-timeline—COVID-19 (accessed on 27 August 2022).
- Arora, S.; Bhaukhandi, K.D.; Mishra, P.K. Coronavirus Lockdown Helped the Environment to Bounce Back. Sci. Total Environ. 2020, 742, 140573. [Google Scholar] [CrossRef]
- Mallik, A.; Chakraborty, P.; Bhushan, S.; Nayak, B.B. Impact of COVID-19 Lockdown on Aquatic Environment and Fishing Community: Boon or Bane? Mar. Policy 2022, 141, 105088. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Rawtani, D.; Hussain, C.M. Environmental Perspective of COVID-19. Sci. Total Environ. 2020, 728, 138870. [Google Scholar] [CrossRef] [PubMed]
- Amezcua, F.; Ramirez, M.; Flores-Verdugo, F. Classification and Comparison of Five Estuaries in the Southeast Gulf of California Based on Environmental Variables and Fish Assemblages. Bull. Mar. Sci. 2019, 95, 139–159. [Google Scholar] [CrossRef]
- Bennett, N.J.; Finkbeiner, E.M.; Ban, N.C.; Belhabib, D.; Jupiter, S.D.; Kittinger, J.N.; Mangubhai, S.; Scholtens, J.; Gill, D.; Christie, P. The COVID-19 Pandemic, Small-Scale Fisheries and Coastal Fishing Communities. Coast. Manag. 2020, 48, 336–347. [Google Scholar] [CrossRef]
- Peces y Mariscos Comerciales | Biodiversidad Mexicana. Available online: https://www.biodiversidad.gob.mx/diversidad/alimentos/peces (accessed on 3 May 2023).
- Sbarbati, P. Impacto de la Pandemia de Enfermedad Por Coronavirus (COVID-19) en la Industria del Turismo de Cruceros en el Gran Caribe; Documentos de Proyectos(LC/TS.2022/66); Comisión Económica para América Latina y el Caribe (CEPAL): Santiago, Chile, 2022. [Google Scholar]
- Eliana, P.; Barleta; Ricardo, J. Sánchez Informe Portuario 2020: El Impacto de La Pandemia Del COVID-19 en El Comercio Marítimo, Transbordo y Throughput de Los Puertos de Contenedores de América Latina y El Caribe. Available online: https://perfil.cepal.org/l/es/pub/2020_ES.pdf (accessed on 22 May 2023).
- Amezcua-Linares, F. Peces Demersales Del Pacífico de México; Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2009; ISBN 978-970-764-558-5. [Google Scholar]
- Roy, P.S.; Williams, R.J.; Jones, A.R.; Yassini, I.; Gibbs, P.J.; Coates, B.; West, R.J.; Scanes, P.R.; Hudson, J.P.; Nichol, S. Structure and Function of South-East Australian Estuaries. Estuar. Coast. Shelf Sci. 2001, 53, 351–384. [Google Scholar] [CrossRef]
- Valderrama-Landeros, L.; Flores-Verdugo, F.; Rodríguez-Sobreyra, R.; Kovacs, J.M.; Flores-de-Santiago, F. Extrapolating Canopy Phenology Information Using Sentinel-2 Data and the Google Earth Engine Platform to Identify the Optimal Dates for Remotely Sensed Image Acquisition of Semiarid Mangroves. J. Environ. Manag. 2021, 279, 111617. [Google Scholar] [CrossRef]
- Amezcua, F. Introduction on Managing Fisheries in Estuarine Systems of Mexico and Central America. In Fisheries Management of Mexican and Central American Estuaries; Amezcua, F., Bellgraph, B., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–28. ISBN 978-94-017-8916-5. [Google Scholar]
- Vasavilbazo Saucedo, A.; Covantes Rodríguez, C. Construcción Social de Inostenibilidad En El Estero de Urías, Mazatlán, Sinaloa, 1st ed.; Universidad Autónoma de Sinaloa: Mazatlán, Mexico, 2012; ISBN 9780415475976. [Google Scholar]
- Fischer, W.; Krupp, F.; Schneider, W.; Sommer, C.; Carpenter, K.; Niem, V. Guía FAO Para La Identificación de Especies Para Los Fines de La Pesca, Pacífico Centro-Oriental; FAO: Rome, Italy, 1995; ISBN 92-5-303408-4. [Google Scholar]
- Mishra, D.R.; Kumar, A.; Muduli, P.R.; Acharyya, T.; Acharya, P.; Singh, S.; Rastogi, G. Landfall Season Is Critical to the Impact of a Cyclone on a Monsoon-Regulated Tropical Coastal Lagoon. Sci. Total Environ. 2021, 770, 145235. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Primer + for Permanova: Guide to Software and Statistical Methods. 214; Primer-e: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). Wiley StatsRef: Stat. Ref. Online 2017, 1, 1–15. [Google Scholar] [CrossRef]
- Tolimieri, N.; Holmes, E.E.; Williams, G.D.; Pacunski, R.; Lowry, D. Population Assessment Using Multivariate Time-Series Analysis: A Case Study of Rockfishes in Puget Sound. Ecol. Evol. 2017, 7, 2846–2860. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity, 1st ed.; Blakwell Publishing: Oxford, UK, 2004; ISBN 0-632-05633-9. [Google Scholar]
- Collins, N.R.; Williams, R. Zooplankton Communities in the Bristol Channel and Severn Estuary. Mar. Ecol. Prog. Ser. 1982, 9, 1–11. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Similarity-Based Testing for Community Pattern: The Two-Way Layout with No Replication. Mar. Biol. 1994, 118, 167–176. [Google Scholar] [CrossRef]
- Jost, L. Partitioning Diversity into Independent Alpha and Beta Components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Hsieh, T.C. Proposing a Resolution to Debates on Diversity Partitioning. Ecology 2012, 93, 2037–2051. [Google Scholar] [CrossRef]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Jaccard, P. The Distribution of The Flora in The Alpine Zone.1. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed.; Plumouth Marine Laboratory, Ed.; Plymouth Marine Laboratory: Plymouth, UK, 2001; ISBN 1855311402, 9781855311404. [Google Scholar]
- Dray, S.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; Wagner, H.H. Package “Adespatial”. R Package Version 2018, 82, 3–8. [Google Scholar]
- Baselga, A. Partitioning the Turnover and Nestedness Components of Beta Diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Website. Available online: https://www.r-project.org/ (accessed on 12 May 2022).
- Bates, A.E.; Primack, R.B.; Biggar, B.S.; Bird, T.J.; Clinton, M.E.; Command, R.J.; Richards, C.; Shellard, M.; Geraldi, N.R.; Vergara, V.; et al. Global COVID-19 Lockdown Highlights Humans as Both Threats and Custodians of the Environment. Biol. Conserv. 2021, 263, 109175. [Google Scholar] [CrossRef]
- Gilby, B.L.; Henderson, C.J.; Olds, A.D.; Ballantyne, J.A.; Bingham, E.L.; Elliott, B.B.; Jones, T.R.; Kimber, O.; Mosman, J.D.; Schlacher, T.A. Potentially Negative Ecological Consequences of Animal Redistribution on Beaches during COVID-19 Lockdown. Biol. Conserv. 2021, 253, 108926. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Calvario-Martínez, O. Evaluación Espacial y Estacional Del Estado Trófico En El Sistema Estuarino Urías, Mazatlán, México. Ideas Cienc. Ing. 2019, 1, 9–26. [Google Scholar]
- Zúñiga-Zataráin, C.R. Capacidad de Carga del Estero de Urías; Sinaloa POR: Mazatlán, Mexico, 2011; p. 167. [Google Scholar]
- Serrano, D.; Valle-Levinson, A. Effects of River Discharge and the California Current on Pycnocline Depth at the Eastern Entrance to the Gulf of California. Cont. Shelf Res. 2021, 215, 104356. [Google Scholar] [CrossRef]
- Paez-Ozuna, F.; Montaño-Ley, Y.; Bojorquez-Leyva, H. Intercambio de Agua, Fosforo y Material Suspendido Entre El Sistema Lagunar Del Puerto de Mazatlán y Las Aguas Costeras Adyacentes. Rev. Int. Contam. Ambient. 2010, 6, 19–32. [Google Scholar]
- Jayaram, C.; Roy, R.; Chacko, N.; Swain, D.; Punnana, R.; Bandyopadhyay, S.; Choudhury, S.B.; Dutta, D. Anomalous Reduction of the Total Suspended Matter During the COVID-19 Lockdown in the Hooghly Estuarine System. Front. Mar. Sci. 2021, 8, 454. [Google Scholar] [CrossRef]
- Azidane, H.; Primadona, N.P.; Boko, M.; Elbouhaddioui, M.; Magrane, B. Impacts of COVID-19 Lockdowns on Water Quality along the Coast of Morocco. Water Pract. Technol. 2023, 18, 901–910. [Google Scholar] [CrossRef]
- Patterson Edward, J.K.; Jayanthi, M.; Malleshappa, H.; Immaculate Jeyasanta, K.; Laju, R.L.; Patterson, J.; Diraviya Raj, K.; Mathews, G.; Marimuthu, A.S.; Grimsditch, G. COVID-19 Lockdown Improved the Health of Coastal Environment and Enhanced the Population of Reef-Fish. Mar. Pollut. Bull. 2021, 165, 112124. [Google Scholar] [CrossRef]
- Al Shehhi, M.R.; Abdul Samad, Y. Effects of the COVID-19 Pandemic on the Oceans. Remote Sens. Lett. 2021, 12, 325–334. [Google Scholar] [CrossRef]
- Sala, M.M.; Peters, F.; Sebastián, M.; Cardelús, C.; Calvo, E.; Marrasé, C.; Massana, R.; Pelejero, C.; Sala-Coromina, J.; Vaqué, D.; et al. COVID-19 Lockdown Moderately Increased Oligotrophy at a Marine Coastal Site. Sci. Total Environ. 2022, 812, 151443. [Google Scholar] [CrossRef]
- Center, N.C.P. NOAA’s Climate Prediction Center. Available online: https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php (accessed on 21 October 2022).
- Ayvazian, S.G.; Hyndes, G.A. Surf-Zone Fish Assemblages in South-Western Australia: Do Adjacent Nearshore Habitats and the Warm Leeuwin Current Influence the Characteristics of the Fish Fauna? Mar. Biol. 1995, 122, 527–536. [Google Scholar] [CrossRef]
- Olán-González, M.; Reyes-Bonilla, H.; Arreola-Alarcon, I.M.; Uribe, R.V.; Olivier, D. COVID-19 Lockdown Reveals Fish Density May Be Much Higher in Marine Reserves. bioRxiv 2022, 492376. [Google Scholar] [CrossRef]
- Funes-Rodríguez, R.; Zárate-Villafranco, A.; Hinojosa-Medina, A.; González-Armas, R.; Hernández-Trujillo, S. Mesopelagic Fish Larval Assemblages during El Niño-Southern Oscillation (1997–2001) in the Southern Part of the California Current. Fish. Oceanogr. 2011, 20, 329–346. [Google Scholar] [CrossRef]
- Andreotti, G.F.; Alves, J.C.; Alves, D.C.; Agostinho, A.A.; Gomes, L.C. The Response of Fish Functional Diversity to the El Niño Southern Oscillation (ENSO) in a Neotropical Floodplain. Hydrobiologia 2021, 848, 1207–1218. [Google Scholar] [CrossRef]
- Pittman, S.; Mcalpine, C.; Pittman, K. Linking Fish and Prawns to Their Environment: A Hierarchical Landscape Approach. Mar. Ecol. Prog. Ser. 2004, 283, 233–254. [Google Scholar] [CrossRef]
- Pittman, S. Linking Fish and Prawns to Their Environment in Shallow Water-Marine Landscapes; University of Oxford: Oxford, UK, 2003. [Google Scholar]
- Whitfield, A.K.; Pattrick, P. Habitat Type and Nursery Function for Coastal Marine Fish Species, with Emphasis on the Eastern Cape Region, South Africa. Estuar. Coast. Shelf Sci. 2015, 160, 49–59. [Google Scholar] [CrossRef]
- Barletta, M.; Barletta-Bergan, A.; Saint-Paul, U.; Hubold, G. The Role of Salinity in Structuring the Fish Assemblages in a Tropical Estuary. J. Fish. Biol. 2005, 66, 45–72. [Google Scholar] [CrossRef]
- Vega-Cendejas, M.E.; Hernández De Santillana, M. Fish Community Structure and Dynamics in a Coastal Hypersaline Lagoon: Rio Lagartos, Yucatan, Mexico. Estuar. Coast. Shelf Sci. 2004, 60, 285–299. [Google Scholar] [CrossRef]
- Faunce, C.H.; Serafy, J.E.; Lorenz, J.J. Density-Habitat Relationships of Mangrove Creek Fishes within the Southeastern Saline Everglades (USA), with Reference to Managed Freshwater Releases. Wetl. Ecol. Manag. 2004, 12, 377–394. [Google Scholar] [CrossRef]
- Moraes, L.E.; Paes, E.; Garcia, A.; Möller, O.; Vieira, J. Delayed Response of Fish Abundance to Environmental Changes: A Novel Multivariate Time-Lag Approach. Mar. Ecol. Prog. Ser. 2012, 456, 159–168. [Google Scholar] [CrossRef]
- Hofmann, E.E.; Powell, T.M. Environmental Effects-Marine Fisheries S23 Environmental Variability Effects ON Marine Fisheries: Four Case Histories. Ecol. Appl. 1998, 8, 23–32. [Google Scholar] [CrossRef]
| Family | Species | aEZ | aSZ | aT | bEZ | bSZ | bT |
|---|---|---|---|---|---|---|---|
| Elopidae | Elops affinis | 10 | 1 | 11 | 4.31 | 1.01 | 5.33 |
| Pristigasteridae | Pliosteostoma lutipinnis | 0 | 101 | 101 | 0 | 17.53 | 17.53 |
| Opisthopterus dovii | 0 | 215 | 215 | 0 | 2.19 | 2.19 | |
| Engraulidae | Anchoa naus | 5 | 0 | 5 | 0.1 | 0 | 0.1 |
| Anchoa walkeri | 0 | 65 | 65 | 0 | 1.54 | 1.54 | |
| Clupeidae | Harengula thrissina | 0 | 8 | 8 | 0 | 0.97 | 0.97 |
| Lile stolifera | 2930 | 0 | 2930 | 115.61 | 0 | 115.61 | |
| Opisthonema libertate | 0 | 3 | 3 | 0 | 0.06 | 0.06 | |
| Poeciliidae | Poeciliopsis latidens | 5 | 0 | 5 | 0.001 | 0 | 0.001 |
| Gobiidae | Ctenogobius sagittula | 13 | 1 | 14 | 0.09 | 0 | 0.09 |
| Gobiosoma paradoxum | 9 | 0 | 9 | 0.02 | 0 | 0.02 | |
| Rhinogobiops nicholsii | 5 | 0 | 5 | 0.006 | 0 | 0.006 | |
| Mugilidae | Mugil curema | 93 | 10 | 103 | 33.92 | 4.36 | 38.28 |
| Mugil cephalus | 40 | 0 | 40 | 2.18 | 0 | 2.18 | |
| Cichlidae | Oreochromis sp. | 1 | 1 | 2 | 0.015 | 0.015 | 0.03 |
| Atherinopsidae | Leuresthes sp. | 77 | 92 | 169 | 0.13 | 0.30 | 0.43 |
| Atherinella sp. | 1 | 0 | 1 | 0.002 | 0 | 0.002 | |
| Carangidae | Caranx caninus | 9 | 799 | 808 | 1.31 | 27.69 | 29.00 |
| Oligoplites saurus | 1 | 0 | 1 | 0.13 | 0 | 0.13 | |
| Oligoplites refulgens | 2 | 15 | 17 | 0.04 | 0.15 | 0.19 | |
| Selene brevoortii | 0 | 1 | 1 | 0 | 0.1 | 0.1 | |
| Trachinotus kennedyi | 0 | 9 | 9 | 0 | 0.22 | 0.22 | |
| Trachinotus paitensis | 6 | 60 | 66 | 0.36 | 2.04 | 2.40 | |
| Hemiramphidae | Hemiramphus saltator | 0 | 1 | 1 | 0 | 0.09 | 0.09 |
| Hyporhamphus rosae | 5 | 494 | 499 | 0.59 | 50.34 | 50.93 | |
| Belonidae | Tylosurus pacificus | 0 | 1 | 1 | 0 | 5.08 | 5.08 |
| Cyclopsettidae | Citharichthys gilberti | 1 | 0 | 1 | 0.02 | 0 | 0.02 |
| Gerreidae | Diapterus brevirostris | 0 | 5 | 5 | 0 | 0.1 | 0.1 |
| Eucinostomus dowii | 74 | 0 | 74 | 3.95 | 0 | 3.95 | |
| Eucinostomus currani | 59 | 7 | 66 | 2.76 | 1.13 | 3.88 | |
| Eucinostomus entomelas | 286 | 0 | 286 | 9.39 | 0 | 9.39 | |
| Eucinostomus gracilis | 195 | 0 | 195 | 7.11 | 0 | 7.11 | |
| Gerres simillimus | 12 | 0 | 12 | 7.37 | 0 | 7.37 | |
| Haemulidae | Rhencus macracanthus | 6 | 0 | 6 | 0.18 | 0 | 0.18 |
| Lutjanidae | Lutjanus argentiventris | 3 | 0 | 3 | 0.52 | 0 | 0.52 |
| Lutjanus colorado | 6 | 6 | 12 | 2.25 | 8.48 | 10.74 | |
| Lutjanus novemfasciatus | 1 | 0 | 1 | 2.02 | 0 | 2.02 | |
| Scianidae | Menticirrhus elongatus | 0 | 32 | 32 | 0 | 1.57 | 1.57 |
| Umbrina xanti | 0 | 47 | 47 | 0 | 2.97 | 2.97 | |
| Polynemidae | Polydactylus approximans | 0 | 115 | 115 | 0 | 3.88 | 3.88 |
| Polydactylus opercularis | 0 | 2 | 2 | 0 | 0.23 | 0.23 |
| Response Variable | Select Variables | % DV | AIC | R2 |
|---|---|---|---|---|
| Biomass | s(Temperature) * + Mangrove area * + Period | 58.3 | 149.05 | 0.465 |
| 0D | s(Salinity) * + Season + Zone | 61.1 | 86.07 | 0.447 |
| 1D | s(Total matter suspended) + Season | 52.4 | 76.89 | 0.424 |
| 2D | s(Total matter suspended) + Season | 49.5 | 71.59 | 0.386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Álvarez, Y.; Ramírez-Ortiz, G.; Flores-de-Santiago, F.; Amezcua-Linares, F.; Muro-Torres, V.; Arceo-Carranza, D.; Amezcua, F. Spatiotemporal Variability in Fish Assemblages in a Coastal and Estuarine System in the Tropical Eastern Pacific during the Anthropause. Diversity 2023, 15, 934. https://doi.org/10.3390/d15080934
Hernández-Álvarez Y, Ramírez-Ortiz G, Flores-de-Santiago F, Amezcua-Linares F, Muro-Torres V, Arceo-Carranza D, Amezcua F. Spatiotemporal Variability in Fish Assemblages in a Coastal and Estuarine System in the Tropical Eastern Pacific during the Anthropause. Diversity. 2023; 15(8):934. https://doi.org/10.3390/d15080934
Chicago/Turabian StyleHernández-Álvarez, Yareli, Georgina Ramírez-Ortiz, Francisco Flores-de-Santiago, Felipe Amezcua-Linares, Victor Muro-Torres, Daniel Arceo-Carranza, and Felipe Amezcua. 2023. "Spatiotemporal Variability in Fish Assemblages in a Coastal and Estuarine System in the Tropical Eastern Pacific during the Anthropause" Diversity 15, no. 8: 934. https://doi.org/10.3390/d15080934
APA StyleHernández-Álvarez, Y., Ramírez-Ortiz, G., Flores-de-Santiago, F., Amezcua-Linares, F., Muro-Torres, V., Arceo-Carranza, D., & Amezcua, F. (2023). Spatiotemporal Variability in Fish Assemblages in a Coastal and Estuarine System in the Tropical Eastern Pacific during the Anthropause. Diversity, 15(8), 934. https://doi.org/10.3390/d15080934









