Members of the Genus Beauveria Associated with Natural Populations of Locusts in Southern European Russia
Abstract
1. Introduction
2. Materials and Methods
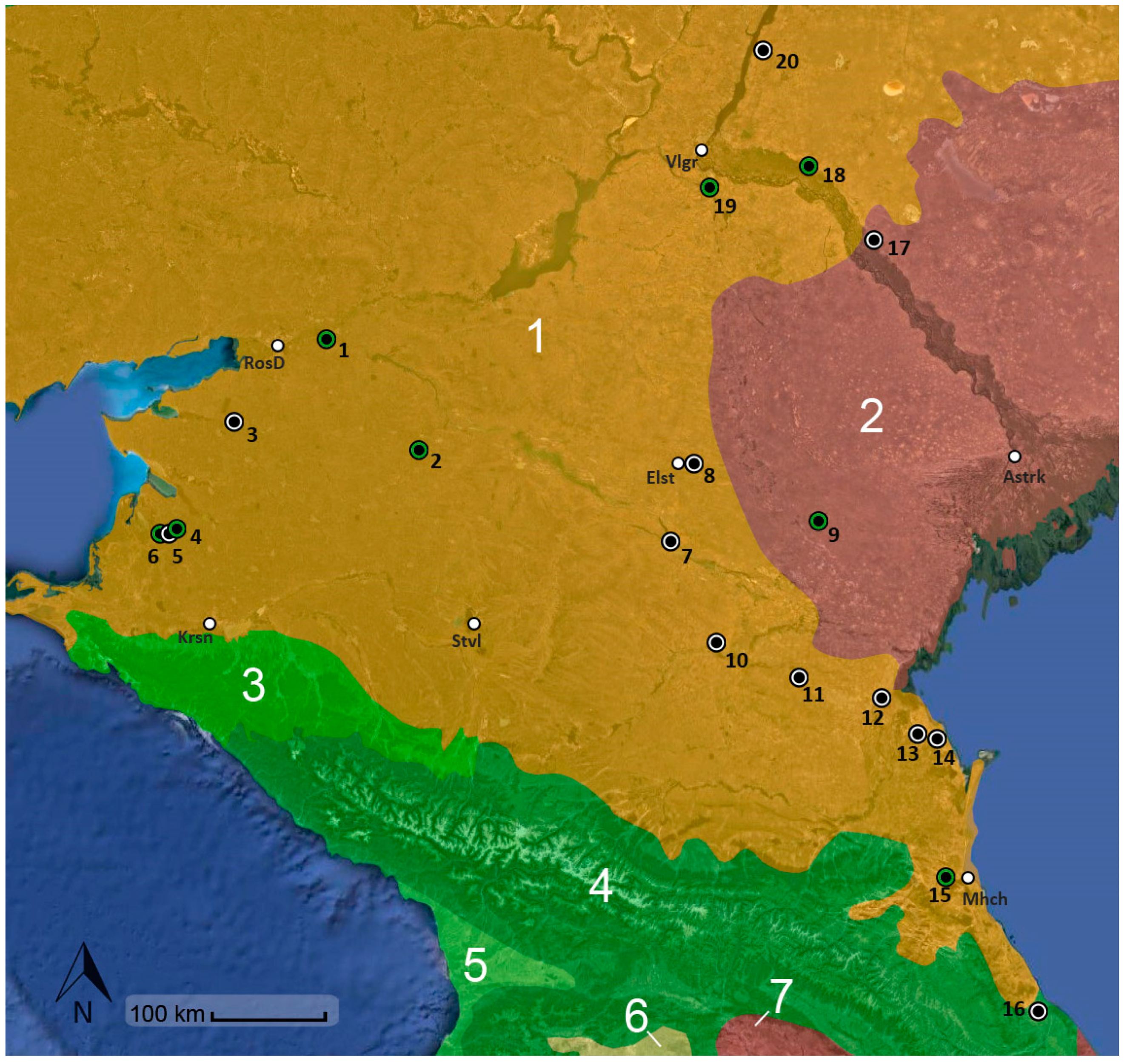
2.1. Insect Populations and Fungal Isolates
2.2. DNA Extraction, Amplification and Sequencing
2.3. Bioinformatic Analyses
2.4. Virulence Assay
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lachininsky, A.V.; Sergeev, M.G.; Childebaev, M.K.; Chernyakhovsky, M.E.; Lockwood, J.A.; Kambulin, V.E.; Gapparov, F.A. Locusts of Kazakhstan, Central Asia and Adjacent Territories; University of Wyoming: Laramie, WY, USA, 2002; p. 387. [Google Scholar]
- Govorov, D.N.; Zhivykh, A.V. Review of the Phytosanitary State of Agricultural Crops in the Russian Federation in 2019 and the Forecast of the Development of Pests in 2020; Rosselkhoztsentr: Moscow, Russia, 2020; p. 512. [Google Scholar]
- Hartig, R. Mittheilungen über Pilzkrankheiten der Insecten im Jahre 1868. Z. Für Forst-Und Jagdwes. 1869, 1, 476–500. [Google Scholar]
- Brongniart, C. Le criquet pèlerin, Acridium peregrinum Oliv. Ses métamorphoses. Son parasite cryptogame. Le Nat. 1891, 13, 232–233. [Google Scholar]
- Saccardo, P.A. Sylloge Fungorum, R; Friedländer & Sohn: Berlin, Germany, 1892; Volume 10, pp. 1–964. [Google Scholar]
- Bruner, L. Killing destructive locusts with fungus diseases. In Some Miscellaneous of the Work of the Division of Entomology; Government Printing Office: Washington, DC, USA, 1902; Volume VI, pp. 50–61. [Google Scholar]
- Reyes, G.M. An unreported fungus disease of the Phillippine Migratory Locust. Philippine J. Sci. 1932, 49, 407–418. [Google Scholar]
- Schaefer, E.E. The White fungus disease (Beauveria bassiana) among red locusts in South Africa, and some observations on the grey fungus disease (Empusa grylli). Sci. Bull. Dep. Agric. For. 1936, 160, 28. [Google Scholar]
- Balfour-Browne, F.L. The green muscardine disease of insects, with special reference to an epidemic in a swarm of locusts in Eritrea. Proc. R. Entomol. Soc. 1960, 35, 56–74. [Google Scholar]
- Roffey, J. The occurrence of the fungus Entomophthora grylli Fresenius on locusts and grasshoppers in Thailand. J. Invertebr. Pathol. 1968, 11, 237–241. [Google Scholar] [CrossRef]
- Samšiňáková, A.A.; Purrini, K. Studies on the natural infection of the grasshopper, Patanga succincta, by the fungus Metarhizium anisopliae in Thailand. J. Appl. Entomol. 1986, 102, 273–277. [Google Scholar] [CrossRef]
- Prior, C.; Carey, M.; Abraham, Y.J.; Moore, D.; Bateman, R.P. Development o f a bioassay method for the select ion of entomopathogenic fungi virulent to the desert locust, Shistocerca gregaria (Forskal). J. Appl. Entomol. 1995, 119, 567–573. [Google Scholar] [CrossRef]
- Bateman, R.; Carey, M.; Batt, D.; Prior, C.; Abraham, Y.; Moore, D.; Jenkins, N.; Fenlon, J. Screening for Virulent Isolates of Entomopathogenic Fungi Against the Desert Locust, Schistocerca gregaria Forskal. Biocontrol Sci. Technol. 1996, 6, 549–560. [Google Scholar] [CrossRef]
- Lomer, C.J.; Prior, C.; Kooyman, C. Development of Metarhizium spp. for the control of grasshoppers and locusts. Mem. Ent. Soc. Can. 1997, 129, 265–286. [Google Scholar] [CrossRef]
- Lomer, C.J.; Bateman, R.P.; Johnson, D.L.; Langewald, J.; Thomas, M.B. Biological control of locusts and grasshoppers. Annu. Rev. Entomol. 2001, 46, 667–702. [Google Scholar] [CrossRef]
- Shah, P.A.; Kooyman, C.; Paraïso, A. Surveys for fungal pathogens of locusts and grasshoppers in Africa and the Near East. Mem. Ent. Soc. Can. 1997, 129, 27–35. [Google Scholar] [CrossRef]
- Douthwaite, B.; Langewald, J.; Harris, J. Development and Commercialization of the Green Muscle Biopesticide; IITA: Ibadan, Nigeria, 2001; p. 23. [Google Scholar]
- van der Valk, H. Review of the Efficacy of Metarhizium anisopliae var. acridum against the Desert Locust; Desert Locust Technical Series; FAO: Rome, Italy, 2007; p. 77. [Google Scholar]
- Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Hernandez-Crespo, P.; Santiago-Alvarez, C. Entomopathogenic fungi associated with natural populations of the Moroccan locust Dociostaurus maroccanus (Thunberg) (Orthoptera: Gomphocerinae) and other Acridoidea in Spain. Biocontrol. Sci. Technol. 1997, 7, 357–364. [Google Scholar] [CrossRef]
- Nurzhanov, A.A.; Lachininsky, A.V. Entomopathogenic microorganisms of locusts in Uzbekistan. In Saranchovye—Ekologiya i Metody bor’by (Sbornik Nauchnyh Trudov); Shumakov, E.M., Ed.; All-Union Institute of Plant Protection (VIZR): Leningrad, USSR, 1987; pp. 62–69. [Google Scholar]
- Nurzhanov, A.A. Entomopathogenic Microorganisms of Locusts of Uzbekistan and Prospects for Their Use in Biological Plant Protection. Ph.D. Thesis, All-Union Institute of Plant Protection (VIZR), Leningrad, USSR, 1989. [Google Scholar]
- Lednev, G.R.; Levchenko, M.V. Muscardinoses of the Italian Locust in Novosibirsk Region. Plant Protection from Pests, Diseases and Weeds; St. Petersburg State Agrarian University: St. Petersburg, Russia, 2004; pp. 56–62. [Google Scholar]
- Rehner, S.A.; Minnis, A.M.; Sung, G.H.; Luangsaard, J.J.; Devotto, L.; Humber, R.A. Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 2011, 103, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Liu, M.; Huang, Z.X.; Yang, G.M.; Han, Y.F.; Liang, J.D.; Liang, Z.Q. Beauveria majiangensis, a new entomopathogenic fungus from Guizhou, China. Phytotaxa 2018, 333, 243–250. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Oliva, M.; Leiva, S.; Mendoza, J.E.; Bobadilla, L.; Angulo, G.; Calderon, M.S. Phylogeny and species delimitations in the entomopathogenic genus Beauveria (Hypocreales, Ascomycota), including the description of B. peruviensis sp. nov. MycoKeys 2019, 58, 47–68. [Google Scholar] [CrossRef]
- Kazartsev, I.A.; Lednev, G.R. Distribution and diversity of Beauveria in boreal forests of northern European Russia. Microorganisms 2021, 9, 1409. [Google Scholar] [CrossRef]
- Steinhaus, E.A. Principles of Insect Pathology; McGraw-Hill Book Company Inc.: New York, NY, USA, 1949; p. 757. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Rehner, S.A.; Buckley, E.A. Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar]
- Rehner, S.A.; Posada, F.; Buckley, E.P.; Infante, F.; Castillo, A.; Vega, F.E. Phylogenetic origins of African and Neotropical Beauveria bassiana s. l. pathogens of the coffee berry borer, Hypothenemus hampei. J. Invertebr. Pathol. 2006, 93, 11–21. [Google Scholar] [CrossRef]
- Malferrari, G.; Monferini, E.; DeBlasio, P.; Diaferia, G.; Saltini, G.; Del Vecchio, E.; Rossi-Bernardi, L.; Biunno, I. High-quality genomic DNA from human whole blood and mononuclear cells. Biotechniques 2002, 33, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; p. 512. [Google Scholar]
- Librado, P.; Rozas, J. DNASP V5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Weiß, C.H. StatSoft, Inc., Tulsa, OK.: STATISTICA, Version 8. AStA Adv. Stat. Anal. 2007, 91, 339–341. [Google Scholar] [CrossRef]
- Gapparov, F.A. Bioecological Features of Harmful Locusts’ Development in Uzbekistan and Measures to Control Them; Navruz: Tashkent, Uzbekistan, 2014; p. 336. [Google Scholar]
- Boomsma, J.J.; Jensen, A.B.; Meyling, N.V.; Eilenberg, J. Evolutionary interaction networks of insect pathogenic fungi. Annu. Rev. Entomol. 2014, 59, 467–485. [Google Scholar] [CrossRef]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol. Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Butt, T.M. Natural and Released Inoculum Levels of Entomopathogenic Fungal Biocontrol Agents in Soil in Relation to Risk Assessment and in Accordance with EU Regulations. Biocontrol Sci. Technol. 2010, 20, 503–552. [Google Scholar]
- Medo, J.; Michalko, J.; Medová, J.; Cagáň, Ľ. Phylogenetic structure and habitat associations of Beauveria species isolated from soils in Slovakia. J. Invertebr. Pathol. 2016, 140, 46–50. [Google Scholar] [CrossRef]
- Levchenko, M.V.; Kryukov, V.Y.; Lednev, G.R. Influence of host phase variability on the sensitivity of migratory locusts to entomopathogenic hyphomycetes. Inf. Bull. EPRS IOBC 2007, 38, 156–158. [Google Scholar]
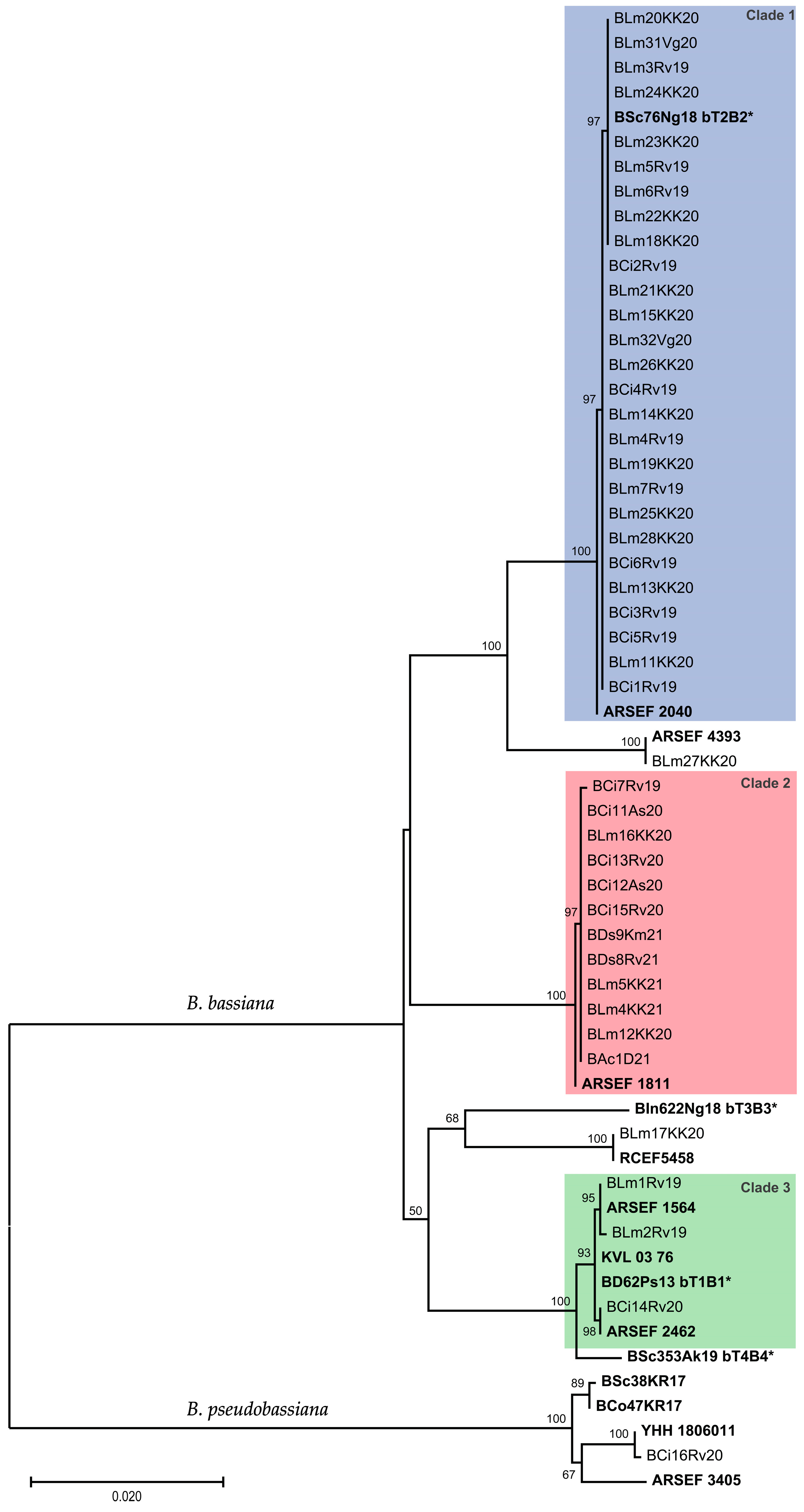
| Isolate | Host | Location | Clade *, Close Reference | LT50 | Mortality after 9 d, Mean ± SE |
|---|---|---|---|---|---|
| Beauveria bassiana | |||||
| BCi1Rv19 | C. italicus | Rostov region, forb–wormwood steppe | 1, ARSEF2040 | 5.0 ± 0.19 a | 100 a |
| BCi2Rv19 | 5.0 ± 0.41 a | 100 a | |||
| BCi3Rv19 | 7.0 ± 0.68 b | 100 a | |||
| BCi4Rv19 | 5.0 ± 0.91 ab | 100 a | |||
| BCi5Rv19 | 7.0 ± 0.44 b | 100 a | |||
| BCi6Rv19 | 7.0 ± 0.16 b | 100 a | |||
| BLm3Rv19 | L. migratoria | Rostov region, reedbeds | 5.0 ± 0.59 ab | 100 a | |
| BLm4Rv19 | 7.0 ± 0.18 b | 86.7 ± 6.7 a | |||
| BLm5Rv19 | 5.0 ± 0.23 a | 100 a | |||
| BLm6Rv19 | 5.0 ± 0.41 a | 100 a | |||
| BLm7Rv19 | 7.0 ± 0.14 b | 93.3 ± 6.7 a | |||
| BLm31Vg20 | Volgograd region, reedbeds | 5.0 ± 0.91 ab | 100 a | ||
| BLm32Vg20 | 5.0 ± 0.41 a | 86.7 ± 13.3 a | |||
| BLm11KK20 | Krasnodar krai, reedbeds | 7.0 ± 0.68 ab | 86.7 ± 13.3 a | ||
| BLm13KK20 | 5.0 ± 0.59 ab | 93.3 ± 6.7 a | |||
| BLm14KK20 | 5.0 ± 0.47 a | 93.3 ± 6.7 a | |||
| BLm15KK20 | 7.0 ± 0.19 b | 93.3 ± 6.7 a | |||
| BLm18KK20 | 5.0 ± 0.64 ab | 93.3 ± 6.7 a | |||
| BLm19KK20 | 5.0 ± 0.31 a | 93.3 ± 6.7 a | |||
| BLm20KK20 | 5.0 ± 0.0 a | 100 a | |||
| BLm21KK20 | 5.0 ± 0.41 a | 100 a | |||
| BLm22KK20 | 7.0 ± 0.30 b | 100 a | |||
| BLm23KK20 | 5.0 ± 1.00 ab | 100 a | |||
| BLm24KK20 | 7.0 ± 0.44 b | 100 a | |||
| BLm25KK20 | - | 46.7 ± 6.7 b | |||
| BLm26KK20 | 7.0 ± 0.33 b | 100 a | |||
| BLm28KK20 | 7.0 ± 0.61 ab | 93.3 ± 6.7 a | |||
| BCi7Rv19 | C. italicus | Rostov region, forb–wormwood steppe | 2, ARSEF1811 | 7.0 ± 0.61 ab | 86.7 ± 6.7 a |
| BCi13Rv20 | 5.0 ± 0.61 ab | 93.3 ± 6.7 a | |||
| BCi15Rv20 | 5.0 ± 0.51 ab | 100 a | |||
| BCi11As20 | Astrakhan region, forb–wormwood steppe | 5.0 ± 0.91 ab | 100 a | ||
| BCi12As20 | 5.0 ± 0.41 a | 100 a | |||
| BLm12KK20 | L. migratoria | Krasnodar krai, reedbeds | 7.0 ± 0.38 b | 93.3 ± 6.7 a | |
| BLm16KK20 | 5.0 ± 0.28 a | 100 a | |||
| BLm4KK21 | 7.0 ± 0.42 b | 100 a | |||
| BLm5KK21 | - | 23.8 ± 9.5 c | |||
| BDs8Rv21 | Dociostaurus sp. | Rostov region, forb–wormwood steppe | 7.0 ± 0.81 ab | 66.7 ± 12.6 b | |
| BDs9Km21 | D. marrocanus | Kalmykia, gramineous-forb steppe | 7.0 ± 0.38 b | 95.2 ± 4.8 a | |
| BAc1D21 | Acrididae sp. | Dagestan, forb steppe | 7.0 ± 0.40 b | 95.2 ± 4.8 a | |
| BLm1Rv19 | L. migratoria | Rostov region, reedbeds | 3, ARSEF1564 | 7.0 ± 0.30 b | 100 a |
| BLm2Rv19 | 5.0 ± 0.29 a | 100 a | |||
| BCi14Rv20 | C. italicus | 7.0 ± 0.44 b | 100 a | ||
| BLm17KK20 | L. migratoria | Krasnodar krai, reedbeds | – | 5.0 ± 1.05 ab | 86.7 ± 6.7 a |
| BLm27KK20 | L. migratoria | Krasnodar krai, reedbeds | – | 7.0 ± 0.24 b | 100 a |
| B. pseudobassiana | |||||
| BCi16Rv20 | C. italicus | Rostov region, forb–wormwood steppe | – | 5.0 ± 0.97 ab | 93.3 ± 6.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lednev, G.; Levchenko, M.; Kazartsev, I. Members of the Genus Beauveria Associated with Natural Populations of Locusts in Southern European Russia. Diversity 2023, 15, 930. https://doi.org/10.3390/d15080930
Lednev G, Levchenko M, Kazartsev I. Members of the Genus Beauveria Associated with Natural Populations of Locusts in Southern European Russia. Diversity. 2023; 15(8):930. https://doi.org/10.3390/d15080930
Chicago/Turabian StyleLednev, Georgy, Maxim Levchenko, and Igor Kazartsev. 2023. "Members of the Genus Beauveria Associated with Natural Populations of Locusts in Southern European Russia" Diversity 15, no. 8: 930. https://doi.org/10.3390/d15080930
APA StyleLednev, G., Levchenko, M., & Kazartsev, I. (2023). Members of the Genus Beauveria Associated with Natural Populations of Locusts in Southern European Russia. Diversity, 15(8), 930. https://doi.org/10.3390/d15080930





