The Subterranean Species of the Vjetrenica Cave System in Bosnia and Herzegovina
Abstract
1. Introduction
History of Biological Studies of Vjetrenica Cave
2. Geographical Setting and Description of the Vjetrenica Cave System
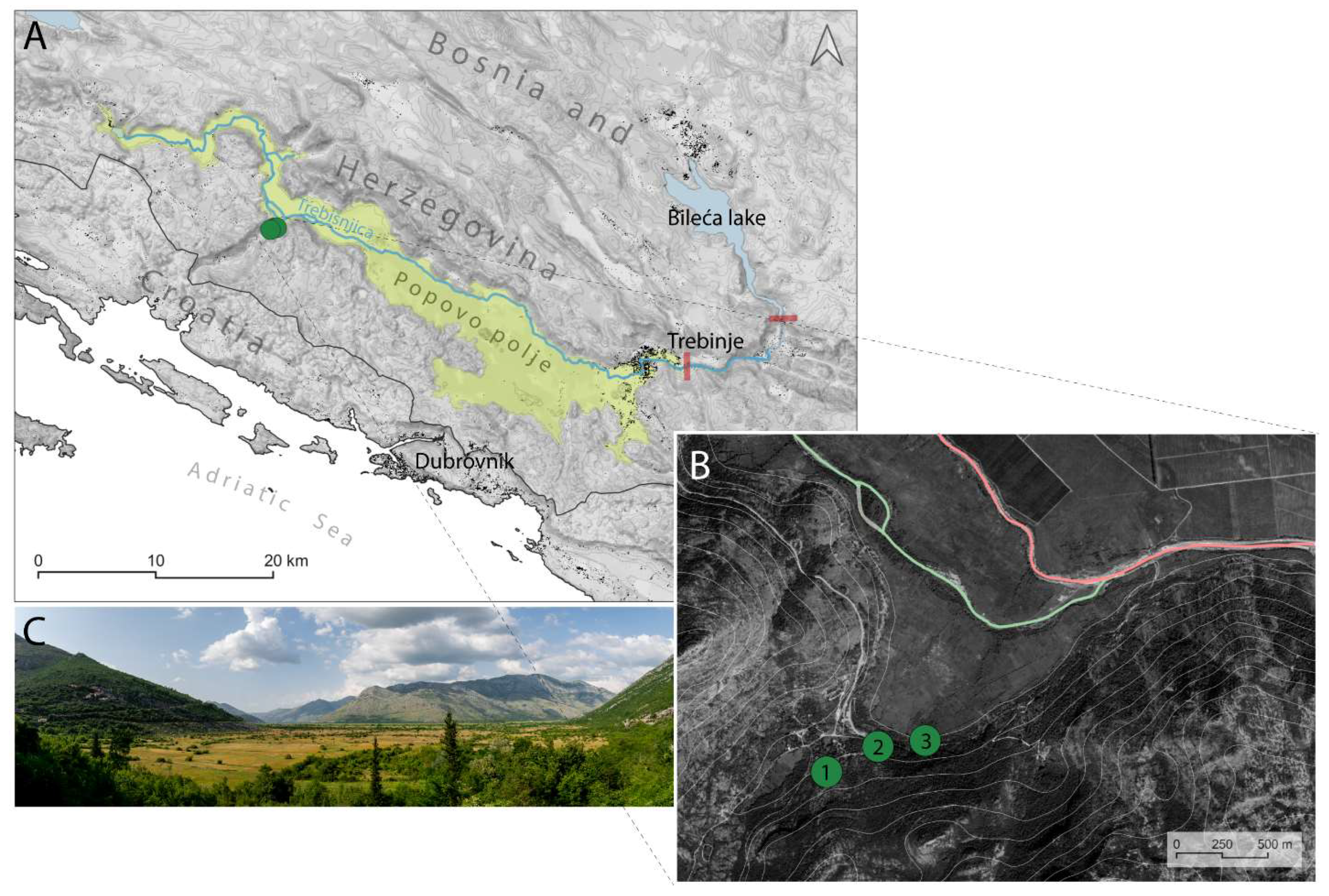
2.1. Geographic Setting
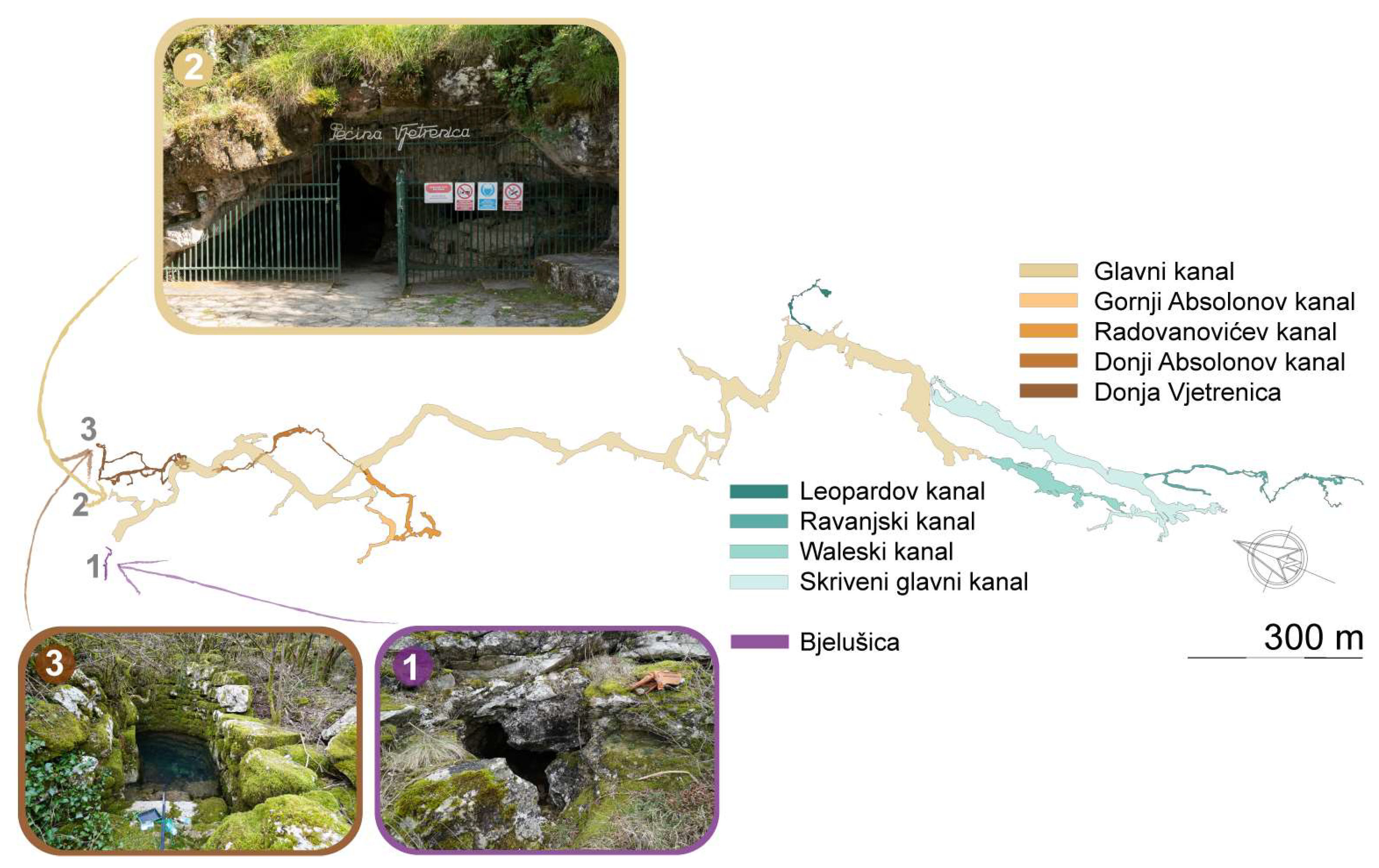
2.2. The Vjetrenica Cave System
3. Compiling the List of Taxa
4. The Overview of Troglobiotic Species in the Vjetrenica Cave System
4.1. General Overview
4.2. Comments to Selected Aquatic Taxonomic Groups

4.3. Comments on Selected Terrestrial Taxonomic Groups
5. Comments on Some of the Non-Troglobiotic Species Adding to the Conservation Importance of the Vjetrenica Cave System
6. Discussion
6.1. General Overview and Significance of the New Species List
6.2. Monitoring of the Subterranean Communities
6.3. Past, Present, and Future Threats and Conservation
6.4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culver, D.C.; Deharveng, L.; Bedos, A.; Lewis, J.J.; Madden, M.; Reddell, J.R.; Sket, B.; Trontelj, P.; White, D. The Mid-Latitude Biodiversity Ridge in Terrestrial Cave Fauna. Ecography 2006, 29, 120–128. [Google Scholar] [CrossRef]
- Zagmajster, M.; Malard, F.; Eme, D.; Culver, D.C. Subterranean biodiversity patterns from global to regional scales. In Cave Ecology, 1st ed.; Moldovan, O.T., Kovač, L., Halse, S., Eds.; Ecological Studies; Springer International Publishing: Cham, Switzerland, 2018; pp. 195–227. [Google Scholar]
- Schmidt, F. Beitrag zu Krain’s Fauna. Leptodirus Hochenwartii, n. g., n. sp. Illyrisches Blatt. 1832, 3, 9–10. [Google Scholar]
- Zagmajster, M.; Culver, D.C.; Sket, B. Species richness patterns of obligate subterranean beetles (Insecta: Coleoptera) in a global biodiversity hotspot–effect of scale and sampling intensity. Divers. Distrib. 2008, 14, 95–105. [Google Scholar] [CrossRef]
- Sket, B. Diversity Patterns in Dinaric Karst. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 228–238. [Google Scholar]
- Bregović, P.; Zagmajster, M. Understanding Hotspots within a Global Hotspot—Identifying the Drivers of Regional Species Richness Patterns in Terrestrial Subterranean Habitats. Insect Conserv. Divers. 2016, 9, 268–281. [Google Scholar] [CrossRef]
- Borko, Š.; Altermatt, F.; Zagmajster, M.; Fišer, C. A Hotspot of Groundwater Amphipod Diversity on a Crossroad of Evolutionary Radiations. Divers. Distrib. 2022, 28, 2765–2777. [Google Scholar] [CrossRef]
- Zagmajster, M.; Polak, S.; Fišer, C. Postojna-Planina Cave System in Slovenia, a Hotspot of Subterranean Biodiversity and a Cradle of Speleobiology. Diversity 2021, 13, 271. [Google Scholar] [CrossRef]
- Ozimec, R.; Lučić, I. The Vjetrenica cave (Bosnia & Herzegovina)–one of the world’s most prominent biodiversity hotspots for cave-dwelling fauna. Subterr. Biol. 2009, 7, 17–24. [Google Scholar]
- Lučić, I. Vjetrenica: Pogled u Dušu Zemelje, 1st ed.; Lučić, I.: Zagreb, Croatia, 2003; p. 322. [Google Scholar]
- Sket, B. Životinjski svijet Vjetrenice. In Vjetrenica: Pogled u Dušu Zemlje, 1st ed.; Lučić, I., Ed.; Lučić, I.: Zagreb, Croatia, 2003; pp. 147–248. [Google Scholar]
- Ozimec, R.; Baković, N.; Bakšić, D.; Basara, D.; Bevanda, L.; Brajković, H.; Brancelj, A.; Erhard, C.; Gašić, Z.; Grego, J.; et al. Vjetrenica—Cave Biodiversity Hotspot of the Dinarides; Ozimec, R., Ed.; Public Enterprise Vjetrenica: Ravno, Bosnia and Herzegovina; ADIPA: Zagreb, Croatia, 2021; p. 356. [Google Scholar]
- Lučić, I. Presvlačenje krša. Povijest Poznavanja Dinarskog Krša na Primjeru Popovog Polja, 1st ed.; Synopsis: Zagreb, Croatia, 2018; p. 680. [Google Scholar]
- Malez, M.; Pepeonik, Z. Entdeckung des ganzen Skelettes eines fossilen Leoparden in der Vjetrenica—Höhle auf dem Popovo Polje (Herzegowina). Bull. Sci. Que Sect. A 1969, 14, 5–6. [Google Scholar]
- Miculinić, K. Fossil Remains of Leopard (Panthera pardus) from Vjetrenica Cave, Popovo Polje, BiH. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2012. [Google Scholar]
- Diedrich, C.G. Late Pleistocene leopards across Europe–northernmost European German population, highest elevated records in the Swiss Alps, complete skeletons in the Bosnia Herzegowina Dinarids and comparison to the Ice Age cave art. Quat. Sci. Rev. 2013, 76, 167–193. [Google Scholar] [CrossRef]
- Korać, J.V. Trebinje. Istorijski Pregled. Knjiga I; Zavičajni Muzej: Trebinje, Bosnia and Herzegovina, 1966; p. 240. [Google Scholar]
- Pamučina, J. Rizvanbegovića. In Ljetopisi; Čokorilo, P., Pamučina, J., Skenderova, S., Eds.; Veselin Masleša: Sarajevo, Bosnia and Herzegovina, 1976; pp. 83–88. [Google Scholar]
- Riedel, J. Eine Ventarole in der Herzegovina. Mitteilungen Der Sect. Für Höhlenkunde 1888, 2, 13–16. [Google Scholar]
- Cvijić, J. Karst, Geografska Monografija, 1st ed.; Državna štamparija: Beograd, Srbija, 1895; p. 176. [Google Scholar]
- Katzer, F. Geologischer Fuerer durch Bosnien und die Herzegovina. In Herausgegeben Anlässlich des IX. Internationalen Geologencongresses von der Landesregierung in Sarajevo, 1st ed.; Landesdruckerei: Sarajevo, Bosnia and Herzegovina, 1903; p. 280. [Google Scholar]
- Richter, E. Prilozi zemljopisu Bosne i Hercegovine. Glas. Zemalj. Muzeja 1905, 7, 257–414. [Google Scholar]
- Polak, S. Importance of discovery of the first cave beetle Leptodirus hochenwartii Schmidt, 1832. Endins 2005, 28, 71–80. [Google Scholar]
- Absolon, K. Výsledky Výskumných cest po Bálkaně. Časopis Morav. Mus. Zemsk. 1916, 2, 245–249. [Google Scholar]
- Nosek, A. Die Arachniden der herzegowinischen Höhlen. Verh. Zool.-Bot. Ges. Wien. 1905, 55, 212–221. [Google Scholar] [CrossRef][Green Version]
- Schäferna, K. O novem slepem blesivci Typhlogammarus n. sbg. Vestn. Kral. Ceske Spol. Nauk. Praha 1906, 26, 1–25. [Google Scholar]
- Müller, J. Diagnosen neuer Höhlensilphiden. Zool. Anz. 1910, 36, 184–186. [Google Scholar]
- Müller, J. Zwei neue Höhlensilphiden aus den österreichischen Karstländern. Wien. Entomol. Ztg. 1911, 30, 175–176. [Google Scholar]
- Absolon, K. Über Scotoplanetes arenstorffianus nov. subg., nov. spec., eine neue Anophthalmentype (Coleoptera Carabidae) aus dem Ponor-Gebiete der Trebišnjica in Südosthercegovina. Koleopterol. Rundsch. 1913, 2, 93–100. [Google Scholar]
- Absolon, K. Les grandes amphipodes aveugles dans les grottes Balkaniques. Compte Rendu de la 51e Session, Association Francaise Pour l’Avancement des Sciences 1927, 51, 291–295. [Google Scholar]
- Hadži, J. Contribution to the knowledge of fauna of cave Vjetrenica. (Pseudoscorpionidea: Neobisium (Blothus) vjetrenicae sp. n., Opilionidea: Travunia vjetrenicae sp. n., Nelima troglodytes Roewer). Glas. Srp. Kralj. Akad. 1932, 75, 103–157. [Google Scholar]
- Karaman, S.L. Beitrag zur Kenntnis der Süsswasser-Amphipoden. Prirodosl. Razpr. 1932, 2, 179–232. [Google Scholar]
- Karaman, S. Über zwei neue Isopoden der Gruppe Asellus aus Jugoslavien. Extr. Recl. Trav. Offer. A J. Georg. 1933, 1, 103–113. [Google Scholar]
- Stammer, H.J. Einige seltene oder neue Höhlentiere. Zool. Anz. 1933, 6, 263–266. [Google Scholar]
- Wolf, B. Animalium Cavernarum Catalogus. II.—Cavernarum Catalogus; Junk Verl: Wien, Austria, 1937; p. 616. [Google Scholar]
- Karaman, S. Dve nove vrste podzemnih amfipoda Popova Polja u Hercegovini. O nekim amfipodima—Izopodima Balkana i. njihovoj sistematici [CLXIII]. In Posebna Izdanja. Odjeljenje Prirodno-Matematickih Nauka; Srpska Akademija Nauka: Belgrade, Yugoslavia, 1950; pp. 101–118. [Google Scholar]
- Karaman, S. Prilozi poznavanju nifarga Hercegovine i južne Dalmacije. Prirodosl. Istraživanja 1952, 25, 45–55. [Google Scholar]
- Culver, D.C.; Sket, B. Hotspots of subterranean biodiversity in caves and wells. J. Cave Karst Stud. 2000, 62, 11–17. [Google Scholar]
- Pretner, E. Kako zaštititi pećinsku faunu Vjetrenice kod Zavale? In Proceedings of the Treći Jugoslavenski Speleološki Kongres, Sarajevo, Jugoslavija, 21–27 June 1962; pp. 169–174. [Google Scholar]
- Cvijić, J. Das Karstphänomen. Versuch einer morphologischen Monographie. Geogr. Abh. 1898, 5, 217–329. [Google Scholar]
- Bonacci, O. Karst Hydrology with Special References to the Dinaric Karst, 1st ed.; Springer: Berlin, Germany, 1987; p. 194. [Google Scholar]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Wiley & Sons Ltd.: West Sussex, UK, 2007; p. 562. [Google Scholar]
- Milanović, P. Karst of Eastern Herzegovina, the Dubrovnik Littoral and Western Montenegro. Env. Earth Sci. 2015, 74, 15–35. [Google Scholar] [CrossRef]
- Milanović, P. Karst of East Herzegovina and Dubrovnik Littoral, 1st ed.; Springer: Cham, Switzerland, 2023; p. 316. [Google Scholar]
- Bonacci, O. Hazards caused by natural and anthropogenic changes of catchment area in karst. Nat. Hazards Earth Syst. Sci. 2004, 4, 655–661. [Google Scholar] [CrossRef]
- Roje-Bonacci, T.; Bonacci, O. The possible negative consequences of underground dam and reservoir construction and operation in coastal karst areas: An example of the hydro-electric power plant (HEPP) Ombla near Dubrovnik (Croatia). Nat. Hazards Earth Syst. Sci. 2013, 13, 2041–2052. [Google Scholar] [CrossRef]
- Milanović, P. Hidrologija Karsta i Metode Istraživanja, HE ‘Trebišnjica’, 1st ed.; Institut za korišćenje i zaštitu voda na kršu: Trebinje, Jugoslavija, 1979; p. 302. [Google Scholar]
- Milanović, P. Uticaj hidrosistema Trebišnjica na režim površinskih i podzemnih voda u Popovom polju. Naš krš 1983, IX/14-15, 41–52. [Google Scholar]
- Milanović, P. Water Resources Engineering in Karst, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 328. [Google Scholar]
- Stevanović, Z.; Eftimi, R. Karstic sources of water supply for large consumers in Southeastern Europe–sustainability, disputes and advantages. Geol. Croat. 2010, 63, 179–185. [Google Scholar] [CrossRef]
- Čučković, S. The influence of the change in the water-course regime of the Trebišnjica water-system on the fauna of the karst underground regions. Naš Krš 1983, 9, 129–142. [Google Scholar]
- Bilandžija, H.; Puljas, S.; Gerdol, M. Hidden from our sight, but not from our impact: The conservation issues of Cave Bivalves. Preprints 2021, e2021050023. [Google Scholar] [CrossRef]
- Reier, S.; Bogutskaya, N.; Palandačić, A. Comparative Phylogeography of Phoxinus, Delminichthys, Phoxinellus and Telestes in Dinaric Karst: Which factors have influenced their current distributions? Diversity 2022, 14, 526. [Google Scholar] [CrossRef]
- Bakšić, D.; Paar, D.; Buzjak, N.; Lučić, I. Microclimatic monitoring in Vjetrenica Cave, B&H. In Proceedings of the International Scientific Symposium “Man and Karst”, Bijakovići, Međugorje, Bosnia and Herzegovina, 13–16 October 2011; Lučić, I., Mulaomerović, J., Eds.; Centre for Karst and Speleology: Sarajevo, Bosnia and Herzegovina, 2011; pp. 13–14. [Google Scholar]
- Čičić, S. Geološka građa terena šire okoline Popova polja i pećine Vjetrenica. Naš krš 2002, 35, 3–16. [Google Scholar]
- Resulović, H.; Vlahinić, M. Characteristics of soil and specificities of drainage of karstic field Popovo Polje (Yugoslavia). Poljopr. I Šumarstvo 1983, 29, 65–79. [Google Scholar]
- Spahić, M. Pećina Vjetrenica u Popovu polju–novo shvatanje speleogeneze. Acta Geogr. Bosniae Et Herzegovinae 2015, 4, 55–67. [Google Scholar]
- Sket, B. The nature of biodiversity in hypogean waters and how it is endangered. Biodivers. Conserv. 1999, 8, 1319–1338. [Google Scholar] [CrossRef]
- Bilandžija, H.; Morton, B.; Podnar, M.; Četković, H. Evolutionary history of relict Congeria (Bivalvia: Dreissenidae): Unearthing the subterranean biodiversity of the Dinaric Karst. Front. Zool. 2013, 10, 5. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Ten Tove, H.A.; Sket, B.; Zakšek, V.; Trontelj, P.; Rouse, G.W. Evolution of the unique freshwater cave dwelling tube worm Marifugia cavatica (Annelida: Serpulidae). Syst. Biodivers. 2009, 7, 389–401. [Google Scholar] [CrossRef]
- Zagmajster, M.; Delić, T.; Prevorčnik, S.; Zakšek, V. New records and unusual morphology of the cave hydrozoan Velkovrhia enigmatica Matjašič & Sket, 1971 (Cnidaria: Hydrozoa: Bougainvilliidae). Nat. Slov. 2013, 15, 13–22. [Google Scholar]
- Trontelj, P.; Blejec, A.; Fišer, C. Ecomorphological convergence of cave communities. Evolution 2012, 66, 3852–3865. [Google Scholar] [CrossRef] [PubMed]
- Fišer, C.; Blejec, A.; Trontelj, P. Niche-based mechanisms operating within extreme habitats: A case study of subterranean amphipod communities. Biol. Lett. 2012, 8, 578–581. [Google Scholar] [CrossRef]
- Karaman, G. XXXVIII. Contribution to the Knowledge of the Amphipoda. On the genus Typhlogammarus (Schäferna) (fam. Gammaridae) from Yugoslavia. Fragm. Balc. Musei Maced. Sci. Nat. 1972, 9, 21–34. [Google Scholar]
- Zakšek, V.; Sket, B.; Trontelj, P. Phylogeny of the cave shrimp Troglocaris: Evidence of a young connection between Balkans and Caucasus. Mol. Phylogenet. Evol. 2007, 42, 223–235. [Google Scholar] [CrossRef]
- Christodoulou, M.; Anastasiadou, C.; Jugovic, J.; Tzomos, T. Freshwater shrimps (Atyidae, Palaemonidae, Typhlocarididae) in the broader mediterranean region: Distribution, life strategies, threats, conservation challenges and taxonomic issues. In A Global Overview of the Conservation of Freshwater Decapod Crustaceans; Springer: Cham, Switzerland, 2016; pp. 199–236. [Google Scholar]
- Wittmann, K.J.; Ariani, A.P.; Daneliya, M. The Mysidae (Crustacea: Peracarida: Mysida) in fresh and oligohaline waters of the Mediterranean. Taxonomy, biogeography, and bioinvasion. Zootaxa 2016, 4142, 1–70. [Google Scholar] [CrossRef]
- Recknagel, H.; Trontelj, P. From cave dragons to genomics: Advancements in the study of subterranean tetrapods. BioScience 2022, 72, 54–266. [Google Scholar] [CrossRef] [PubMed]
- Recknagel, H.; Zakšek, V.; Delić, T.; Gorički, Š.; Trontelj, P. Multiple transitions between realms shape relict lineages of Proteus cave salamanders. Mol. Ecol. 2023, 1–16. [Google Scholar] [CrossRef]
- Sket, B. The cave hygropetric-a little known habitat and its inhabitants. Arch. Für Hydrobiol. 2004, 160, 413–425. [Google Scholar] [CrossRef]
- Giachino, P.M.; Vailati, D. Kircheria beroni, a new genus and new species of subterranean hygropetricolous Leptodirinae from Albania (Coleoptera, Cholevidae). Subterr. Biol. 2006, 4, 103–116. [Google Scholar]
- Engel, A.S.; Paoletti, M.G.; Beggio, M.; Dorigo, L.; Pamio, A.; Gomiero, T.; Furlan, C.; Brilli, M.; Dreon, A.L.; Bertoni, R.; et al. Comparative microbial community composition from secondary carbonate (moonmilk) deposits: Implications for the Cansiliella servadeii cave hygropetric food web. Int. J. Speleol. 2013, 42, 181–192. [Google Scholar] [CrossRef]
- Delić, T. First record of a specialized hygropetricolous cave beetle, genus Croatodirus (Coleoptera: Leiodidae), in Slovenia. Nat. Slo. 2017, 19, 55–61. [Google Scholar]
- Lukić, M.; Fišer, C.; Delić, T.; Bilandžija, H.; Pavlek, M.; Komerički, A.; Dražina, T.; Jalžić, B.; Ozimec, R.; Slapnik, R.; et al. Subterranean Fauna of the Lukina Jama–Trojama Cave System in Croatia: The Deepest Cave in the Dinaric Karst. Diversity 2023, 15, 726. [Google Scholar] [CrossRef]
- Vargovitsh, R.S. Cave Water Walker: An Extremely Troglomorphic Troglaphorura gladiator gen. et sp. nov. (Collembola, Onychiuridae) from Snezhnaya Cave in the Caucasus. Zootaxa 2019, 4619, 267–284. [Google Scholar] [CrossRef]
- Vargovitsh, R.S. Deep troglomorphy: New arrhopalitidae (Collembola: Symphypleona) of different life forms from the Snezhnaya cave system in the Caucasus. Diversity 2022, 14, 678. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Jalžić, B.; Erichsen, E. Adaptation of the mouthparts in some subterranean Cholevinae (Coleoptera, Leiodidae). Nat. Croat. 2004, 13, 1–18. [Google Scholar]
- Perreau, M.; Pavićević, D. The genus Hadesia Müller, 1911 and the phylogeny of Anthroherponina (Coleoptera, Leiodidae, Cholevinae, Leptodirini). In Advances in the Studies of the Fauna of the Balkan Peninsula, 1st ed.; Pavićević, D., Perreau, M., Eds.; Institute for Nature Conservation of Serbia: Beograd, Serbia, 2008; pp. 215–239. [Google Scholar]
- Polak, S.; Delić, T.; Kostanjšek, R.; Trontelj, P. Molecular phylogeny of the cave beetle genus Hadesia (Coleoptera: Leiodidae: Cholevinae: Leptodirini), with a description of a new species from Montenegro. Arthropod Syst. Phylogeny 2016, 74, 241–254. [Google Scholar] [CrossRef]
- Moravec, J.M.; Mlejnek, R.M. Nauticiella stygivaga gen. n. et sp. n., a new amphibiontic cavernicolous beetle from the Vjetrenica Cave, Herzegovina (Coleoptera: Leiodidae: Cholevinae: Leptodirini). Acta Soc. Zool. Bohem. 2002, 66, 293–302. [Google Scholar]
- Delić, T.; Lohaj, R.; Brestovansky, J.; Čaha, D.; Jalžić, B. Questioning the monophyly of Anthroherponina (Coleoptera: Leiodidae: Cholevinae: Leptodirini) and description of three new, ecologically ultraspecialized subterranean species. Zool. J. Linn. Soc. 2023, in press. [Google Scholar]
- Karaman, G.S. On two new or interesting Amphipoda from Italy and Montenegro (contribution to the knowledge of the Amphipoda 290). Ecol. Montenegrina 2016, 8, 1–16. [Google Scholar] [CrossRef]
- Sket, B.; Dovc, P.; Jalzic, B.; Kerovec, M.; Kucinic, M.; Trontelj, P. A cave leech (Hirudinea, Erpobdellidae) from Croatia with unique morphological features. Zool. Scr. 2001, 30, 223–229. [Google Scholar] [CrossRef]
- Antić, D.; Dražina, T.; Rađa, T.; Lučić, L.; Makarov, S. Review of the genus Typhloiulus Latzel, 1884 in the Dinaric region, with a description of four new species and the first description of the male of Typhloiulus insularis Strasser, 1938 (Diplopoda: Julida: Julidae). Zootaxa 2018, 4455, 258–294. [Google Scholar] [CrossRef] [PubMed]
- Lakota, J.; Lohaj, R.; Dunay, G. Taxonomical and ecological notes on the genus Scotoplanetes Absolon, with the description of a new species from Montenegro (Coleoptera: Carabidae: Trechini). Nat. Cro. 2010, 19, 99–110. [Google Scholar]
- Pavićević, D.; Popović, M. A new species of Scotoplanetes Absolon, 1913 (Coleoptera; Carabidae; Trechini) from Durmitor Mt., Montenegro. Biol. Serb. 2023, 45, 1–5. [Google Scholar]
- Absolon, K.; Kratochvíl, J. Zur Kenntnis der höhlenbewohnenden Araneae der illyrischen Karstgebiete. Mitteilungen Über Höhlen Und Karstforschung 1932, 3, 73–81. [Google Scholar]
- Deeleman-Reinhold, C.L. The genus Rhode and the harpacteine genera Stalagtia, Folkia, Minotauria and Kaemis (Araneae, Dysteridae) of Yugoslavia and Crete, with remarks on the genus Harpactea. Rev. Arachnol. 1993, 10, 105–135. [Google Scholar]
- Kury, A.B.; Mendes, A.C. Taxonomic status of the European genera of Travuniidae (Arachnida, Opiliones, Laniatores). Mun. Ent. Zool. 2007, 2, 1–14. [Google Scholar]
- Condé, B. Eukoenenia remyi n. sp., palpigrade cavernicole d’Herzégovine. Ann. Spéléol. 1974, 29, 53–56. [Google Scholar]
- Dányi, L.; Balázs, G.; Tuf, I.H. Taxonomic status and behavioural documentation of the troglobiont Lithobius matulici (Myriapoda, Chilopoda) from the Dinaric Alps: Are there semiaquatic centipedes in caves? ZooKeys 2019, 848, 1–20. [Google Scholar] [CrossRef]
- Zapparoli, M. The present knowledge on the European fauna of Lithobiomorpha (Chilopoda). Bull. Brit. Myr. Grp. 2003, 19, 20–41. [Google Scholar]
- Njunjić, I.; Perreau, M.; Hendriks, K.; Schilthuizen, M.; Deharveng, L. The cave beetle genus Anthroherpon is polyphyletic; molecular phylogenetics and description of Graciliella n. gen.(Leiodidae, Leptodirini). Contrib. Zool. 2016, 85, 337–359. [Google Scholar] [CrossRef]
- Lohaj, R.; Lakota, J.; Quéinnec, E.; Pavićević, D.; Čeplík, D. Studies on Adriaphaenops Noesske with the description of five new species from the Dinarides (Coleoptera: Carabidae: Trechini). Zootaxa 2016, 4205, 501–531. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. 2023. Available online: https://www.iucnredlist.org (accessed on 8 June 2023).
- Palandačić, A.; Matschiner, M.; Zupančič, P.; Snoj, A. Fish migrate underground: The example of Delminichthys adspersus (Cyprinidae). Mol. Ecol. 2012, 21, 1658–1671. [Google Scholar] [CrossRef]
- Mazija, M.; Rnjak, D. Rezultati istraživanja šišmiša u odabranim skloništima na dijeu Popovog polja u općini Ravno, Bosna i Hercegovina. Hypsugo 2016, 1, 20–29. [Google Scholar]
- Zagmajster, M. Zanimivosti Vjetrenice na Popovem polju. Glas podzemlja 2006, 1, 66–67. [Google Scholar]
- Pipan, T.; Culver, D.C. Epikarst communities: Biodiversity hotspots and potential water tracers. Environ. Geol. 2007, 53, 265–269. [Google Scholar] [CrossRef]
- Culver, D.C.; Brancelj, A.; Pipan, T. Epikarst communities. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: London, UK, 2019; pp. 399–406. [Google Scholar]
- Trontelj, P.; Douady, C.J.; Fišer, C.; Gibert, J.; Gorički, Š.; Lefébure, T.; Zakšek, V. A molecular test for cryptic diversity in ground water: How large are the ranges of macro-stygobionts? Freshw. Biol. 2009, 54, 727–744. [Google Scholar] [CrossRef]
- Lukić, M.; Delić, T.; Pavlek, M.; Deharveng, L.; Zagmajster, M. Distribution pattern and radiation of the European subterranean genus Verhoefiella (Collembola, Entomobryidae). Zool. Scr. 2020, 49, 86–100. [Google Scholar] [CrossRef]
- Hlebec, D.; Podnar, M.; Kučinić, M.; Harms, D. Molecular analyses of pseudoscorpions in a subterranean biodiversity hotspot reveal cryptic diversity and microendemism. Sci. Rep. 2023, 13, 430. [Google Scholar]
- Sket, B. Prilog za zaštitu nekih speleobioloških značajnih objekata. Naš Krš 1983, 9, 123–127. [Google Scholar]
- Piano, E.; Nicolosi, G.; Mammola, S.; Balestra, V.; Baroni, B.; Bellopede, R.; Cumino, E.; Muzzulini, N.; Piquet, A.; Isaia, M. A literature-based database of the natural heritage, the ecological status and tourism-related impacts in show caves worldwide. Nat. Conserv. 2020, 50, 159–174. [Google Scholar] [CrossRef]
- Culver, D.C.; Sket, B. Biological monitoring in caves. Acta Carsologica 2002, 31, 55–64. [Google Scholar] [CrossRef]
- Mulec, J. Lampenflora. In Encyclopedia of Caves, 3rd ed.; White, W.B., Culver, D.C., Pipan, T., Eds.; Academic Press: London, UK, 2019; pp. 635–641. [Google Scholar]
- Davor Baković, V.D. Direktora Vjetrenice: Rekordna Prošla Godina, Optimistično Čekamo Novu Ljetnu Sezonu. Available online: https://visitbih.ba/davor-bakovic-v-d-direktora-vjetrenice-rekordna-prosla-godina-optimisticno-cekamo-novu-ljetnu-sezonu/ (accessed on 28 July 2023).
- Paunović, A.S.; Bokić, Z.; Braeković, B. Investigations of the susceptibility of peach cvs, clingstone peaches and nectarines to powdery mildew (Sphaerotheca pannosa (Wall) Lev var persicae) in ecological conditions of Popovo Polje. Jugosl. Vocar. 1985, 19, 131–137. [Google Scholar]
- Ćustovic, H.; Cero, M. Condition of Ameliorative Issues and Land Consolidation Measures in Area of Popovo Polje Area, Bosnia and Herzegovina; Unpublished Work. Available online: https://www.fao.org/fileadmin/user_upload/reu/europe/documents/LANDNET/2007/Bosnia.pdf. (accessed on 28 July 2023).
- Darwall, W.; Carrizo, S.; Numa, C.; Barrios, V.; Freyhof, J.; Smith, K. Freshwater Key Biodiversity Areas in the Mediterranean Basin Hotspot: Informing Species Conservation and Development Planning in Freshwater Ecosystems (Vol. 52), 1st ed.; IUCN: Gland, Switzerland, 2014; pp. 1–86. [Google Scholar]
- Freyhof, J. Threatened Freshwater Fishes and Molluscs of the Balkan, Potential Impact of Hydropower Projects. ECA Watch Austria & EuroNatur; Unpublished Report. Available online: https://www.balkanrivers.net/uploads/legacy/Threatened-freshwater-fishes-and-molluscs_final.pdf (accessed on 23 June 2023).
- Oikonomou, A.; Leprieur, F.; Leonardos, I.D. Biogeography of freshwater fishes of the Balkan Peninsula. Hydrobiologia 2014, 738, 205–220. [Google Scholar] [CrossRef]
- Sket, B.; Paragamian, K.; Trontelj, P. A census of the obligate subterranean fauna of the Balkan Peninsula. In Balkan Biodiversity, 1st ed.; Griffiths, H.I., Kryštufek, B., Reed, J.M., Eds.; Springer: Amsterdam, The Netherlands, 2004; pp. 309–322. [Google Scholar]
- Gerhardt, A. Sensitivity towards Nitrate: Comparison of groundwater versus surface water crustaceans. J. Soil. Water. Sci. 2020, 4, 112–121. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Di Marzio, W.D.; Fiasca, B.; Galassi, D.M.P.; Korbel, K.; Iepure, S.; Pereira, J.L.; Reboleira, A.S.P.; Schmidt, S.I.; Hose, G.C. Recommendations for ecotoxicity testing with stygobiotic species in the framework of groundwater environmental risk assessment. Sci. Total Environ. 2019, 681, 292–304. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Fišer, Ž.; Dolar, A.; Novak, S.; Drobne, D.; Bračko, G.; Fišer, C. Screening of NaCl salinity sensitivity across eight species of subterranean amphipod genus Niphargus. Ecotoxicol. Environ. Saf. 2022, 236, 113456. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.; Sircar, I. Environmental politics in the Western Balkans: River basin management and non-governmental organisation (NGO) activity in Herzegovina. Env. Polit. 2010, 19, 808–830. [Google Scholar] [CrossRef]
- Naimark, N.M.; Case, H. Yugoslavia and Its Historians: Understanding the Balkan Wars of the 1990s, 1st ed.; Stanford University Press: Stanford, CA, USA, 2003; p. 296. [Google Scholar]
- Ozimec, R. Otvoren prvi biospeleološki muzej u svijetu: Biospeleološki muzej Vjetrenica. Subterranea Croat. 2016, 14, 49–50. [Google Scholar]
- World Heritage Convention Tentative List (Vjetrenica Cave). Available online: https://whc.unesco.org/en/tentativelists/1975/ (accessed on 15 June 2023).
- Milanović, P.; Glišić, R.; Ðorđević, B.; Dašić, T.; Sudar, N. Uticaj delimičnog prevođenja voda iz slivova Bune i Bregave u sliv Trebišnjice. Vodoprivreda 2012, 44, 255–257. [Google Scholar]
- Mirković, U.B. Techno-economic analysis for the construction of the supply tunnel of hpp “Dabar” with the application of TBM technology. Tehnika 2019, 74, 359–366. [Google Scholar] [CrossRef]
- Posljedice Megaprojekta “Gornji Horizonti”: Ostaje li Hercegovina bez Vode i Kako Izbjeći Apokalipsu. Available online: https://www.klix.ba/vijesti/bih/posljedice-megaprojekta-gornji-horizonti-ostaje-li-hercegovina-bez-vode-i-kako-izbjeci-apokalipsu/200916105 (accessed on 28 July 2023).
- Projekat Gornji Horizonti: Početak Kraja Neretve i Hercegovine Kakvu Poznajemo? Available online: https://abrasmedia.info/projekat-gornji-horizonti-pocetak-kraja-neretve-i-hercegovine-kakvu-poznajemo/ (accessed on 28 July 2023).
- Fišer, C.; Borko, Š.; Delić, T.; Kos, A.; Premate, E.; Zagmajster, M.; Altermatt, F. The European green deal misses Europe’s subterranean biodiversity hotspots. Nat. Ecol. Evol. 2022, 6, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Brunke, M. Colmation and depth filtration within streambeds: Retention of particles in hyporheic interstices. Int. Rev. Hydrobiol. 1999, 84, 99–117. [Google Scholar] [CrossRef]
- Brenna, A.; Surian, N.; Mao, L. Alteration of gravel-bed river morphodynamics in response to multiple anthropogenic disturbances: Insights from the sediment-starved Parma River (northern Italy). Geomorphology 2021, 389, 107845. [Google Scholar] [CrossRef]
- Borko, Š.; Trontelj, P.; Seehausen, O.; Moškrič, A.; Fišer, C. A subterranean adaptive radiation of amphipods in Europe. Nat. Commun. 2021, 12, 3688. [Google Scholar] [CrossRef]



| Higher Group | Family | Species | A/T | Cave/Spring | Year | Red List | |||
|---|---|---|---|---|---|---|---|---|---|
| V | B | L | IUCN | BIH | |||||
| Rhabdocoella | Scutariellidae | Scutariella stammeri Matjašič, 1958 | A | ✔* | / | ||||
| Stygodyticola hadzii Matjašič, 1958 | A | ✔* | / | ||||||
| Subtelsonia perianalis Matjašič, 1958 | A | ✔ | / | ||||||
| Troglocaridicola spelaeocaridis Matjašič, 1958 | A | ✔ | / | ||||||
| Troglocaridicola capreolaria herzegovinensis Matjašič, 1970 | A | ✔ | / | ||||||
| Tricladida | Geoplanidae | Rhynchodemus sp. | T | ✔ | 2021 | ||||
| Trematoda | Stenakridae | Caudotestis protei (Prudhoe, 1945) Yamaguti, 1958 | A | ✔* | / | ||||
| Nemertea | Prostomatidae | Prostoma hercegovinense Tarman, 1961 | A | ✔* | / | ||||
| Polychaeta | Serpulidae | Marifugia cavatica Absolon & Hrabe, 1930 | A | ✔ | ✔ | 2021 | |||
| Hirudinea | Erpobdellidae | Dina absoloni Johansson, 1913 | A | ✔* | 2021 | ||||
| Gastropoda | Cyclophoridae | Pholeoteras euthrix Sturany, 1904 | T | ✔ | ✔ | 2016 | LC | ||
| Ellobiidae | Zospeum troglobalcanicum Absolon, 1916 | T | ✔ | 2016 | |||||
| Emmericiidae | Emmericia ventricosa Brusina, 1870 | A | ✔ | / | VU | ||||
| Hydrobiidae | Kerkia briani Rysiewska & Osikowski, 2020 | A | ✔ | ✔ | 2020 | ||||
| Narentiana vjetrenicae Radoman, 1973 | A | ✔* | ✔* | / | EN | ||||
| Pseudamnicola troglobia Bole, 1961 | A | ✔ | ✔ | ✔ | / | ||||
| Moitessieriidae | Lanzaia vjetrenicae Kuščer, 1933 | A | ✔* | ✔ | / | VU | |||
| Paladilhiopsis absoloni (Wagner, 1914) | A | ✔ | ✔ | / | LC | ||||
| Iglicopsis butoti Falniowski & Hofman, 2021 | A | ✔ | 2010 | ||||||
| Orientalinidae | Radomaniola montana (Radoman, 1973) | A | ✔ | ✔ | / | ||||
| Pristilomatidae | Vitrea spelaea (Wagner, 1914) | T | ✔ | 2016 | EN | ||||
| Gyralina candida (Wagner, 1909) | A | ✔ | ✔ | / | |||||
| Spelaeoconchidae | Spelaeoconcha paganettii polymorpha Wagner, 1914 | T | ✔ | ✔* | 2016 | LC | |||
| Ferussaciidae | Cecilioides spelaea Wagner, 1914 | T | ✔ | ||||||
| Agardhiellidae | Agardhiella biarmata (O.Boettger, 1880) | T | ✔ | ||||||
| Zonitidae | Aegopis spelaeus Wagner, 1914 | T | ✔ | ✔* | 2016 | NT | |||
| Bivalvia | Dreissenidae | Congeria kusceri Bole, 1962 | A | ✔ | ✔ | / | VU | ||
| Diplopoda | Glomeridellidae | Typhloglomeris coeca Verhoeff, 1898 | T | ✔ | ✔ | ✔ | 2014 | ||
| Julidae | Typhloiulus edentulus Attems, 1951 | T | ✔* | / | |||||
| Polydesmidae | Brachydesmus stygivagus Verhoeff, 1899 | T | ✔ | ✔ | 2021 | ||||
| Trichopolydesmidae | Verhoeffodesmus sp. | T | ✔ | 2021 | |||||
| Chilopoda | Lithobiidae | Lithobius matulici Verhoeff, 1899 | T | ✔ | ✔ | 2021 | |||
| Lithobius sketi Matic & Darabantu, 1968 | T | ✔* | 2021 | ||||||
| Eupolybothrus leostygis (Verhoeff, 1899) | T | ✔ | / | ||||||
| Palpigradi | Eukoeneniidae | Eukoenenia remyi Conde, 1974 | T | ✔* | 2014 | ||||
| Acari | Labidostommatidae | Labidostomma longipes Willmann, 1940 | T | ✔ | ✔ | 2020 | |||
| Opiliones | Sironidae | Cyphophthalmus sp. | T | ✔ | ✔ | 2021 | |||
| Travuniidae | Travunia vjetrenicae Hadži, 1933 | T | ✔* | 2021 | |||||
| Pseudoscorpiones | Chtonidae | Chthonius occultus Beier, 1939 | T | ✔ | 2013 | EN | |||
| Neobisium vjetrenicae Hadži, 1932 | T | ✔* | / | EN | |||||
| Roncus anophthalmus (Ellingsen, 2013) | T | ✔ | / | ||||||
| Araneae | Dysderidae | Stalagtia hercegovinensis (Nosek, 1905) | T | ✔* | ✔ | 2020 | |||
| Stalitella noseki Absolon & Kratochvil, 1933 | T | ✔* | / | ||||||
| Linyphiidae | Troglohyphantes salax (Kulczynski, 1914) | T | ✔ | ✔ | / | ||||
| Nesticidae | Kryptonesticus fagei (Kratochvil, 1933) | T | ✔ | / | |||||
| Copepoda | Cyclopidae | Acanthocyclops troglophilus (Kiefer, 1932) | A | ✔* | / | ||||
| Diacyclops charon (Kiefer, 1931) | A | ✔ | / | ||||||
| Diacyclops karamani (Kiefer, 1932) | A | ✔* | / | ||||||
| Diacyclops tantalus (Kiefer, 1937) | A | ✔* | / | ||||||
| Eucyclops inarmatus Kiefer, 1932 | A | ✔* | ✔ | / | |||||
| Diaptomidae | Troglodiaptomus sketi Petkovski, 1978 | A | ✔ | / | |||||
| Ostracoda | Cyprididae | Pseudocypridopsis hartmanni Petkovski et al., 2009 | A | ✔* | / | ||||
| Pseudocypridopsis sywulai Petkovski et al., 2009 | A | ✔ | / | ||||||
| Entocytheridae | Sphaeromicola stammeri Klie, 1930 | A | ✔ | / | |||||
| Amphipoda | Hadziidae | Hadzia fragilis Karaman S., 1932 | A | ✔* | 2021 | DD | |||
| Niphargidae | Niphargus hercegovinensis Karaman S., 1950 | A | ✔ | 2000 | DD | ||||
| Niphargus kolombatovici Karaman S., 1950 | A | ✔ | 2003 | DD | |||||
| Niphargus factor Karaman G. & Sket, 1990 | A | ✔* | 2005 | ||||||
| Niphargus boskovici Karaman S., 1952 | A | ✔* | ✔ | 2021 | DD | ||||
| Niphargus trullipes Sket, 1958 | A | ✔* | 2021 | DD | |||||
| Niphargus vjetrenicensis Karaman S., 1932 | A | ✔* | ✔ | 2021 | DD | ||||
| Niphargus balcanicus (Absolon, 1927) | A | ✔* | ✔ | 2021 | DD | ||||
| Niphargus cvijici Karaman S., 1950 | A | ✔ | / | DD | |||||
| Niphargus zavalanus Karaman S., 1950 | A | ✔* | / | DD | |||||
| Typhlogammaridae | Typhlogammarus mrazeki (Schäferna, 1907) | A | ✔ | 2021 | |||||
| Isopoda | Asellidae | Proasellus hercegovinensis (Karaman S., 1933) | A | ✔* | ✔ | ✔ | 2021 | ||
| Proasellus anophtalmus (Karaman S., 1934) | A | ✔ | ✔ | / | |||||
| Microparasellidae | Microcharon sp. | A | ✔ | / | |||||
| Sphaeromatidae | Monolistra hercegoviniensis Absolon, 1916 | A | ✔* | 2021 | |||||
| Trichoniscidae | Alpioniscus heroldii (Verhoeff, 1931) | T | ✔ | ✔ | 2021 | ||||
| Cyphonethes herzegowinensis (Verhoeff, 1900) | T | ✔ | ✔ | 2021 | |||||
| Decapoda | Atyidae | Spelaeocaris pretneri Matjašič, 1956 | A | ✔ | 2003 | ||||
| Spelaeocaris hercegovinensis (Babić, 1922) | A | ✔* | ✔ | 2021 | |||||
| Troglocaris anophthalma periadriatica Jugovic et al., 2012 | A | ✔* | ✔ | 2021 | |||||
| Mysida | Mysidae | Troglomysis vjetrenicensis Stammer, 1933 | A | ✔* | ✔ | 2000 | |||
| Collembola | Entomobryidae | Verhoeffiella verdemontana Lukić & Deharveng, 2018 | T | ✔ | ✔ | 2014 | |||
| Verhoeffiella longicornis (Absolon, 1900) | T | ✔ | 2021 | ||||||
| Diplura | Campodeidae | Plusiocampa remyi Condé, 1947 | T | ✔* | 2021 | ||||
| Thysanura | Nicoletiidae | Coletinia sp. | T | ✔ | / | ||||
| Coleoptera | Carabidae | Neotrechus dalmatinus dalmatinus (Miller L., 1861) | T | ✔ | ✔ | ✔ | 2021 | ||
| Scotoplanetes arenstorffianus Absolon, 1913 | T | ✔* | 2021 | EN | |||||
| Adriaphaenops pretneri Scheibel, 1935 | T | ✔* | / | EN | |||||
| Neotrechus suturalis otiosus (Obenberger, 1917) | T | ✔ | ✔ | / | |||||
| Speluncarius anophthalmus (Reitter, 1886) | T | ✔ | / | ||||||
| Leiodidae | Speonesiotes schweitzeri Jeannel, 1941 | T | ✔ | ✔* | 2014 | ||||
| Speonesiotes narentinus latitarsis (Apfelbeck, 1919) | T | ✔ | ✔ | / | |||||
| Graciliella apfelbecki apfelbecki (Müller J., 1910) | T | ✔* | 2021 | ||||||
| Hadesia vasiceki Müller J., 1911 | T | ✔* | 2021 | ||||||
| Nauticiella stygivaga Moravec & Mlejnek, 2002 | T | ✔* | 2021 | ||||||
| Anthroherpon primitivum (Absolon, 1913) | T | ✔ | / | ||||||
| Staphylinidae | Troglamaurops ganglbaueri (Winkler, 1925) | T | ✔ | 2021 | |||||
| Nonveilleria sp. | T | ✔ | / | ||||||
| Urodela | Proteidae | Proteus anguinus Laurenti, 1768 | A | ✔ | ✔ | 2021 | VU | EN | |
| 85 | 26 | 22 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delić, T.; Pipan, T.; Ozimec, R.; Culver, D.C.; Zagmajster, M. The Subterranean Species of the Vjetrenica Cave System in Bosnia and Herzegovina. Diversity 2023, 15, 912. https://doi.org/10.3390/d15080912
Delić T, Pipan T, Ozimec R, Culver DC, Zagmajster M. The Subterranean Species of the Vjetrenica Cave System in Bosnia and Herzegovina. Diversity. 2023; 15(8):912. https://doi.org/10.3390/d15080912
Chicago/Turabian StyleDelić, Teo, Tanja Pipan, Roman Ozimec, David C. Culver, and Maja Zagmajster. 2023. "The Subterranean Species of the Vjetrenica Cave System in Bosnia and Herzegovina" Diversity 15, no. 8: 912. https://doi.org/10.3390/d15080912
APA StyleDelić, T., Pipan, T., Ozimec, R., Culver, D. C., & Zagmajster, M. (2023). The Subterranean Species of the Vjetrenica Cave System in Bosnia and Herzegovina. Diversity, 15(8), 912. https://doi.org/10.3390/d15080912









