Abstract
This paper presents the current state of knowledge on the biological activity and possible medicinal applications of selected species of the genus Ganoderma: Ganoderma adspersum (Schulzer) Donk, Ganoderma applanatum (Pers.) Pat., Ganoderma carnosum Pat., Ganoderma lucidum (Curtis) P. Karst., Ganoderma pfeifferi Bres., Ganoderma resinaceum Boud. These inedible, wood-decaying fungi are pathogens that cause the enzymatic decomposition of wood. They are a valued natural medicinal resource and have been used in traditional Far Eastern medicine for centuries. Research conducted on these species using modern analytical methods has led to advances in knowledge of the potential therapeutic use of compounds isolated not only from basidiocarps but also from biomass obtained by in vitro methods. Recent pharmacological studies have confirmed the known traditional uses of these species, elucidated previously unknown mechanisms of biological action, and found evidence of new biological activities, such as anticancer, cytotoxic, antiallergic, and neuroprotective activities. Furthermore, the article updated the state of knowledge on the general mycological characteristics of these species.
1. Introduction
The family Ganodermataceae (Polyporales, Basidiomycota) stands out morphologically because of the structure of the basidiospores with double-walled and slightly to distinctly thick-walled with varied ornamentation [1,2]. Ganodermataceae is a large and complex family that has been studied for many decades, yet the species diversity, geographical distribution, species classification, and taxonomy of Ganodermataceae remain uncertain [2,3]. Ganoderma, the major genus in the Ganodermataceae, is characterized by a cosmopolitan distribution and is especially found in tropical and subtropical regions, including Africa, America, Asia, and Europe [3].
The name Ganoderma originates from Greek, meaning shiny, glowing skin (“gános”—shiny, glowing, brightness; and “dérma”—skin). However, it does not reflect the morphological features of all species of this genus as not all species have a bright and shiny surface [2,4].
The genus Ganoderma was first described in 1881 by the Finnish mycologist Petter Adolf Karsten and initially included only one species, Ganoderma lucidum (Curtis) P. Karst., whose former names were Boletus lucidus Curtis (1781) and Polyporus lucidus (Curtis) Fr. (1821) [5]. In 1889, Patouillard expanded this genus to 48 species, which were classified by the presence of pigmented spores, clinging tubes, and a shell-like cap [6].
Recently Sun et al. [2] mentioned that the genus Ganoderma includes 181 species that are both ecologically and economically important. In Europe, this genus is represented by seven species [4,7] and it was recorded on over 50 genera of trees (Table 1). Depending on the species, the basidiocarps appear singly or in groups, taking various shapes, such as console-shaped and hoof-shaped, and they are either attached to the side or growing out of the stem. Ganoderma spp. are saprotrophs or parasites [8]. Most members of Ganoderma are tree pathogens that cause root rot and basal stem rot, as well as wood-decay. These fungi cause the so-called uniform or mottled white rot of living and dead hardwoods and conifers, i.e., the enzymatic breakdown of wood components such as lignin, hemicelluloses, and cellulose [9]. Wood decay enzymes present in Ganoderma spp. are used in bioleaching and bioremediation [10,11,12]. Some Ganoderma species such as G. adspersum and other white-rot Badiomycota, associated yeasts, and bacteria, can cause very extensive selective delignification in the moist forest climate. This white wood-decay described was from Chile, and it is called „Palo podrido” because the decayed tree trunks (spongy-wet wood tissue consists of 97% cellulose) were often used as cattle and horses’ fodder (myco-fodder) [9].
The therapeutic effects of Ganoderma spp. have been known for thousands of years. They are most popular in traditional Chinese medicine, where they have been used since ancient times. This is evidenced by available references found in myths, ancient poems, and other literary works [13,14]. They are also traditionally used in some West African countries such as Nigeria in the treatment of skin diseases, hypertension, and digestive system diseases [15,16,17].
Numerous reports and studies have confirmed the multidirectional biological activity of extracts from various Ganoderma spp. and isolated compounds. The following properties have been proven anticancer, immunomodulatory, antioxidant [16], anti-inflammatory, antiallergic, neuroprotective, hepatoprotective, hypoglycemic, hypotensive, antimicrobial, antiviral, and antimalarial [17]. The most important groups of compounds found in Ganoderma spp. include triterpenes (Ganoderma triterpenes) and polysaccharides [18]. Until now, more than 300 triterpenes and 200 polysaccharides characterized by diverse chemical structures and biological activity have been isolated [19].
Compounds and extracts obtained from G. lucidum have presented by far the broadest spectrum of activities and most of the aforementioned properties, as confirmed by the presence of monographs of this species, e.g., in the European and American Pharmacopoeia. A monograph was also noted in the latest Polish Pharmacopoeia XII Supplement. However, other Ganoderma spp. also show characteristic lines of action, which is confirmed by the increasing number of scientific reports [20].

Table 1.
Nomenclature and comparison of occurrence of Ganoderma ssp.
Table 1.
Nomenclature and comparison of occurrence of Ganoderma ssp.
| Latin Names | Common English Names | Hosts in Europe (Genus) | Geographical Distribution | References |
|---|---|---|---|---|
| Ganoderma adspersum (Schulzer) Donk | Southern bracket | Acacia, Acer, Aesculus, Alianthus, Alnus, Arbutus, Armeniaca, Betula, Buxus, Buddleia, Calycanthus, Castanea, Casuarina, Carpinus, Cedrus, Celtis, Cercis, Cinamomum, Citrus, Eleagnus, Eucalyptus, Fagus, Ficus, Fraxinus, Genista, Gleditsia, Juglans, Lagerstroemia, Laurus, Larix, Morus, Olea, Picea, Pittosporum, Platanus, Populus, Prunus, Pyrus, Quercus, Salix, Schinus, Sophora, Sorbus, Spirea, Tamarix, Tecomaria, Tilia, Ulmus | Europe, most often in the southern and western part; North Africa; Asia | [3,7,21,22,23,24,25,26] |
| Ganoderma applanatum (Pers.) Pat. | Artist’s bracket | Abies, Acer, Aesculus, Alnus, Amygdalus, Arbutus, Armeniaca, Betula, Castanea, Carpinus, Cedrus, Celtis, Cerasus, Ceratonia, Cercis, Corylus, Crategus, Eleagnus, Eucalyptus, Fagus, Fraxinus, Hibiscus, Ilex, Juglans, Laburnum, Liliodendron, Lonicera, Malus, Morus, Picea, Pinus, Platanus, Populs, Prunus, Pterocarya, Pyracantha, Pyrus, Quercus, Robinia, Salix, Sambucus, Sophora, Sorbus, Syringa, Tamarix, Tilia, Ulmus | Europe, most often in the northern and central part; Asia; North Africa; North America | [3,7,21,22,23,24,26,27,28,29] |
| Ganoderma carnosum Pat. | – | Abies, Alnus, Betula, Carpinus, Cedrus, Corylus, Fagus, Larix, Malus, Picea, Pinus, Prunus, Pseudotsuga, Pyrus, Quercus, Taxus, Tilia, Tsuga | Europe | [3,7,21,22,23,24,25] |
| Ganoderma lucidum (Curtis) P. Karst. | Lacquered bracket | Abies, Acacia, Acer, Alnus, Aesculus, Arbutus, Armeniaca, Betula, Carpinus, Castanea, Cerasus, Corylus, Eriobotrya, Eucalyptus, Fagus, Ficus, Fraxinus, Juglans, Malus, Laurus, Larix, Olea, Padus, Picea, Pinus, Populus, Pyrus, Quercus, Robinia, Rosa, Salix, Tilia, Ulmus | Europe; Asia | [2,3,7,21,22,23,24,25] |
| Ganoderma pfeifferi Bres. | Beeswax bracket | Acer, Aesculus, Alnus, Carpinus, Fagus, Fraxinus, Prunus, Quercus, Tilia, Ulmus | Europe | [3,7,21,22,23,24,25,30] |
| Ganoderma resinaceum Boud. | – | Acacia, Acer, Aesculus, Alnus, Betula, Castanea, Catalpa, Celtis, Ceratonia, Cercis, Citrus, Cornus Eriobotrya, Eucalyptus, Fagus, Ficus, Fraxinus, Gleditsia, Juglans, Laurus, Lonicera, Liquidambar, Malus, Morus, Olea, Pistacia, Platanus, Populus, Prunus, Pyrus, Quercus, Robinia, Salix, Sophora, Sorbus, Tamarix, Tilia, Ulmus | Europe; Asia; North Africa | [3,7,21,22,23,24,25,26] |
2. Mycological Characteristics of Selected Ganoderma spp.
2.1. Ganoderma adspersum (Schulzer) Donk
This taxon was first described in 1878 by Schulzer, who gave it the name Polyporus adspersus. The present name, recognized by the Index Fungorum, was proposed in 1969 by Donk, who transferred this species to the genus Ganoderma.
Latin synonyms of the name southern bracket are Ganoderma europaeum Steyaert, P. adspersus, and Ganoderma australe (Fr.) Pat. sensu Ryvarden, Gilberdson, Telleria. The perennial basidiomata are semicircular and hoof-shaped, with a thickening at the base. They grow sideways to the tree, both individually and in groups. They are up to 50 cm wide and 20 cm thick and characterized by a pleated, lumpy, reddish-brown surface that turns brown-black over time. On the other hand, the round edge is white or creamy yellow. They have durable and hard skin, and their flesh is fibrous, corky, and red-brown in color.
The hymenophore is tubular and multi-layered, 6–8 cm thick, with no context layers between the tube layers. Tubes reach a length of 10–15 mm, and pores are round and fine and present in the amount of 3–4 per mm. The initial white or pale-yellow color of the pores becomes gray-brown with age. The basidiospores formed are ovoid-ellipsoid, truncate and pale brown, and their size is 8.5–12 × 5–7.5 µm [21], 8.8–12 × 5.5–8.1 µm [4], and 11–14.4 × 5.7–9.3 µm [7]. This species is inedible with a weak odor and imperceptible taste [8].
G. adspersum is found in forests as well as in parks, gardens, and roadside alleys and roadside. It grows on a wide range of living and dying angiosperms trees (it can form basidiomata even several years after the death of the host), and rarer on conifers (Table 1), (Figure 1). In Europe, it is found most often in the southern and western parts [22,23,24,25,26].

Figure 1.
(a) Ganoderma adspersum: one-year-old fruiting bodies in a wound at the base of the trunk of Aesculus hippocastanum, Poland, photo Andrzej Szczepkowski; (b) Ganoderma applanatum: fruiting bodies on a stump of Betula sp., Poland, photo Andrzej Szczepkowski; (c) Ganoderma carnosum: fruiting bodies at the base of the trunk of snag Abies alba, Poland, photo Jacek Nowicki; (d) Ganoderma lucidum: last year’s fruiting bodies on a log of Alnus glutinosa, Poland, photo Andrzej Szczepkowski; (e) Ganoderma pfeifferi: fruiting bodies on the trunk of Acer saccharinum, Poland, photo Andrzej Szczepkowski; (f) Ganoderma resinaceum: fruiting bodies on the trunk of Acer saccharinum, Poland, photo Andrzej Szczepkowski.
2.2. Ganoderma applanatum (Pers.) Pat.
This taxon was first described by Persoon in 1800/1799, who named it Boletus applanatus, whereas the present name, recognized by the index Fungorum, was given by Patouillard in 1887. This species is referred to as cartoonist’s or artist’s mushroom, hence the English names: artist’s bracket and artist’s conk. Such terms are not accidental—the snow-white underside of the fruiting bodies turns into a permanent dark brown color when touched, thus making it possible to write and draw on it is using a sharp tool. This way, unusual ornaments can be created using this species [27].
This species has about 50 Latin synonyms, including Boletus applanatus Pers., Ganoderma incrassatum (Berk.) Bres., Ungularia subganodermica Lázaro Ibiza. The common English names are Artist’s Bracket, Artist’s Conk, and Bear Bread. Perennial basidiomata, whose age can be assessed based on the number of tube layers visible in the cross-section, are semicircular, hoof-shaped, and most often flattened. They grow broadly and lateral to the tree. They grow in groups or singly. Usually, they are 10–20 cm (up to 40–50 cm) wide and 2–8 cm thick. Their surface is uneven and wavy, with various shades of brown. The edge is white and wavy, with clear zoning. The crustosed pileus surface is characterized by low durability and hardness, and its thickness is 1 mm. The basidiomata of this species have a homogeneous, elastic, and felt-cork context, which is reddish/purplish brown usually mottled with whitish streaks and patches. The hymenophore is initially white but turns cinnamon-brown with age, and it is tubular and multilayered, with context between the layers. Tubes are usually 5–13 mm long, and pores are round, 4–6 per mm, white at first, and pale yellow over time. The characteristic feature of the hymenophore is it turns brown when pressed. A reddish-brown exudation of spores is observed, which are ovoid-ellipsoid, apically truncate, papillary, pale brown, and up to 6–8.5 × 4.5–6 µm [21], 7–8.5 × 4.5–6 µm [4], 7–9.3 × 5.3–6.5 µm in size [7]. Characteristic galls may form on the hymenophore surface at the feeding site of Agathomyia wankowiczi Schnabl larvae. This species is inedible, with a mushroom odor and a bitter, slightly stinging taste [8].
G. applanatum is a saprotroph, a parasite of weakness, and a wound parasite. It causes severe white rot in wood. Unlike G. adspersum, it is a common species throughout the year. It is found in parks, forests, gardens, and cities. It most often grows on stumps and dead and living trunks and branches of a wide range of deciduous trees. Sometimes, it also grows on conifers (Table 1), (Figure 1). It is found throughout Europe, most often in the northern and central parts [3,7,21,22,23,24,25,26,28,29,31].
2.3. Ganoderma carnosum Pat.
This species was first described by Narcisse Théophile Patouillard in 1889. The name G. carnosum originated from the Latin word carnem, meaning “fleshy”. The Latin synonyms are as follows: Fomes carnosus (Pat.) Sacc., Ganoderma atkinsonii H. Jahn, Kotl., Pouzar, and Scindalma carnosum (Pat.) Kuntze.
The basidiomata are semicircular, up to 20 cm in diameter and 5 cm in thickness. They grow laterally to the tree and usually with a thick stipe of 1–25 cm long, 0.7–4.0 cm in diameter. Pileus sessile reaches up to 40 cm long, 25 cm wide and 5 cm thick. The surface of the basidioma is irregular and concentrically grooved, with visible thickening. It turns reddish-brown and then black-purple with time. The surface of the young basidiomata is shiny but becomes dull with time.
The tubular hymenophore, initially cream-colored, turns brown with time or contact. Small, round, or angular pores are present in the amount of 3–4 per mm. The tube layer is 2.5 cm thick and characterized by a chocolate-brown color. The context directly above this layer also turns dark brown, whereas the flesh under the skin and in the shaft is creamy and darkens over time. G. carnosum is characterized by a-pale brown discharge of spores, which are ellipsoidal, apically truncate and papillary,11–13.5 × 7.5–8.5 [32], 10–13 × 7–8.5 µm [21], 10–13 × 7–8 µm [4], 10.4–13.7 × 6.4–8.7 in size [7]. It is an inedible species and has no perceptible taste or smell. It exhibits parasitic and saprotrophic features. The main host/substratum for G. carnosum is Abies alba and Picea abies, more rarely other conifers and occasionally angiosperms (Table 1), (Figure 1). It grows at the base of living trees, with emerging roots, and lying trunks, but mainly on stumps. G. carnosum is distributed strictly in Europe [3,7,21,22,23,24,25,26].
2.4. Ganoderma lucidum (Curtis) P. Karst
This taxon was first described by William Curtis in 1781, who gave it the name Boletus lucidus. The current name, recognized by the Index Fungorum, was given by Petter Adolf Karsten in 1881. The name G. lucidum refers to the characteristic appearance of the basidiomata, as the Latin word lucidum means “bright, shiny.” Polish terms indicating a shiny surface and partially yellow color also refer to the appearance. Worldwide, G. lucidum is known by many local names. In China, the name Ling Zhi is used, in Japan it is Reishi, and in Korea, it is Youngzhi [33]. The name of this species has numerous Latin synonyms, including Agarico-igniarium trulla Paulet, Fomes lucidus (Curtis) Sacc, Ganoderma nitens Lázaro Ibiza, Polyporus lucidus (Curtis) Fr. The common English name is Lacquered Bracket. The basidiomata consist of a stem and a cap. They occur singly or in groups, and they are annuals. The hat, 5–20 cm wide and 2–5 cm thick, is flat, circular, and centrally or laterally stipitate. It has an uneven, glossy yellow-brown surface, which turns darker and reddish-brown with age. Irregular concentric zonings and grooves are also visible. The brim of the hat is light yellow and white, dull, and wavy. Usually, the stem is 5–12 × 1–3 cm. Its surface is uneven, lumpy, and reddish brown. The corky context can vary in color from whitish to cream, becoming brown to purple-brown. The hymenophore is tubular, ocher, or brownish in color. Tubes are 5–20 mm long and most often appear in one layer. Fine round pores (4–5 per mm) of cream color turn brown under pressure. A brownish spore rash is present. These spores are ellipsoid, apically truncate, papillary, -pale brown and their size is 7–11 × 6–8 µm [21], 8–11 × 6–8.5 µm [4], 11–14.1 × 6.8–8.3 [7]. This species is inedible, with a mushroom aroma and a bitter taste.
In addition to being a saprotroph, G. lucidum is a parasite that causes uniform white rot. The infested wood takes on a yellow shade. It is found in summer and fall in forests, parks, and gardens. Most often, it grows on live and dead deciduous trees, but occasionally on conifers (Table 1), (Figure 1). It is common and widespread in Europe [4,7,21].
2.5. Ganoderma pfeifferi Bres.
This species was first named by Giacomo Bresadola in 1889. The following are the Latin synonyms: Fomes laccatus Sacc., Ganoderma laccatum (Sacc.) Bourdot and Galzin. The English name (beeswax bracket) deserves attention, which directly refers to a characteristic feature: the secretion of a yellow and sweet-smelling waxy substance by its basidiomata.
The basidiomata of this species are perennial and reach up to 50 cm in diameter, and their thickness may be 5–20 cm. They grow broadly on the trunk of trees in the lower and middle part. The surface of the basidiomata is copper red or purple in color, with the cracked and wrinkled resinous layer. The edge is pale yellow and wavy. The hymenophore is tubular and covered with bright round pores in the amount of 4–6 per mm. With age, the pores darken, and irregular brown patches become visible. The tubes are up to 2 cm long, and their layer is characterized by a chocolate-brown color. The context above this layer is chestnut brown to amber in color. Similar to G. carnosum, the spore discharge is chocolate brown. Basidiospores can reach the size of 9–12 × 6–9 µm [21], 8.5–11.5 × 6–9 µm [4], 9.9–12.9 × 7.4–9.3 µm [7] and assume an ellipsoidal shape, apically truncate, pale brown. This species does not have a distinct flavor. A characteristic feature of G. pfeifferi that distinguishes them from other Ganoderma spp. is the release of a yellow waxy substance with a sweet smell in late winter and spring. In addition, the surface of the basidioma melts because of applying a burning match.
2.6. Ganoderma resinaceum Boud.
This taxon was first described in 1889 by the French mycologist Jean Louis Émile Boudier.
The name G. resinaceum species originates from the adjective resinaceum, which in Latin means “resinous” and refers to the characteristic sticky hardening liquid flowing from damaged basidiomata. This species has about 30 Latin synonyms, including Fomes areolatus (Murrill) Murrill and Ganoderma subperforatum G.F. Atk.
The basidiomata of this fungus are annual, rarely perennial, bracket-like with short or without stipe, 15–35 cm in diameter, and are usually 4–10 cm thick. They can grow in multitiered groups and connect with each other. The sterile surface of the basidioma is of brown to reddish brown color with a light yellowish edge.
The tubular hymenophore is covered with whitish or pale-yellow pores in young basidiomata, which turn light brown with age or damage. The number of pores are 2–4 per mm. The brown tubes are 8–20 mm long with round to angular pores at the ends. Context is pale brown with the darker layer close to the tube layer. A reddish-brown spore exudation is observed, which is ellipsoidal-ovate, smooth, finely rough, and apically truncated, pale brown. Their size is 9–11.5 × 4.5–7 µm [21], 9–11.5 × 5–7.5 µm [4], 10.3–14.4 × 6–8.2 µm [7]. This species is inedible. It has a bitter taste and a spicy, pungent smell.
A characteristic feature of G. resinaceum that distinguishes it from other species is the release of a thick yellow resin in response to damage or fracture. This resin hardens quickly to a hard glossy surface. It looks similar to G. pfeifferi, but the latter has a dark red-brown context, fissured-reticulate pileal surface, wider basidiospores, and a yellow, waxy layer with a sweet secretion by the pore surface in the winter or early in the spring. This species is a parasite that grows at the base of a wide number of living deciduous trees, especially on Quercus sp. (Table 1), (Figure 1). In Europe, it is found primarily in the southern and central parts of the continent [3,7,21,22,23,24,25,26].
3. Selected Biological Activities and Therapeutic Potential of Ganoderma spp.
3.1. Immunomodulatory Action
Many fungi species and their compounds that affect the immune system have been identified. Four main groups of compounds can act as immunomodulators: lectins, terpenoids, proteins, and polysaccharides. As a representative of the genus Ganoderma, G. lucidum is one of the most effective fungi with an immunomodulatory effect [34]. The primary immunomodulatory mechanisms of action of the substances present in it include mitogenicity and activation of immune effector cells such as T lymphocytes, macrophages, mast cells, and natural killer cells (NK cells), which leads to the production of cytokines, e.g., interleukins, interferons, tumor necrosis factor (TNF-α) [35].
Ganoderma spp. accumulate triterpenes such as ganoderic and ganodermic acids, ganoderol, ganodermanontriol, ganodermanondiol, and lucidon. These substances affect the immune system by stimulating the nuclear signaling pathway of NF-κB and mitogen-activated kinases [34]. Ganoderiol F (Figure 2), ganodermanondiol, and ganodermanontriol derived from G. lucidum spores are characterized by strong activity against the complement system, thus influencing the humoral immune system [36]. The chemical structures of selected terpenes whose biological activity is described in the following subsections are shown in Figure 2.
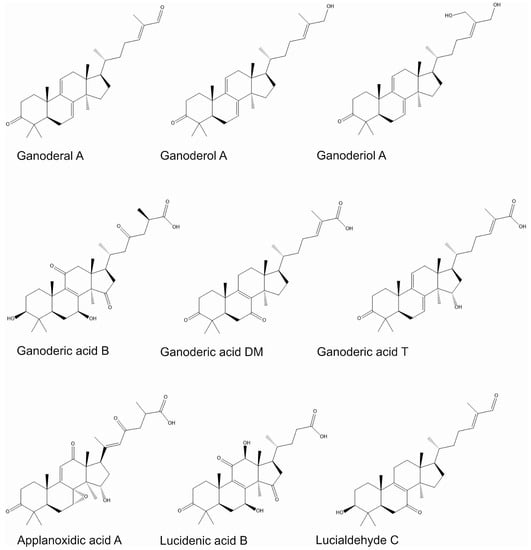
Figure 2.
The chemical structures of selected terpenes presented in Ganoderma spp.
One of the most widely recognized constituents found in Ganoderma lucidum is the Ling-Zhi-8 protein, also known as FIP-glu. This protein, derived from fungi, has been identified as the initial immunomodulatory protein isolated from this source. It consists of a sequence of 110 amino acids and exhibits immunosuppressive properties. Experimental evidence from a study conducted on mouse spleen cells reveals that Ling-Zhi-8 enhances the transcription of specific interleukins, namely IL-2, IL-3, and IL-4, as well as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and the receptor for IL-2. Moreover, Ling-Zhi-8 has demonstrated its effectiveness in mice by suppressing autoimmune reactions associated with diabetes and enhancing the longevity of allogeneic skin grafts. Notably, this protein exhibits a lower degree of nephrotoxicity when compared to other immunosuppressive substances such as cyclosporin A. These findings have been supported by various studies [34,37,38].
A significant class of compounds presented in Ganoderma lucidum is polysaccharides, which are represented by, e.g., ganoderan, heteroglycans, mannoglucans, and glycopeptides. These polysaccharides have been found to elicit immune responses by stimulating the production of interleukin-1 (IL-1), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). Additionally, they play a role in the activation of nuclear factor kappa B (NF-κB), a transcription factor involved in immune signaling [34]. In an animal study utilizing mice with immunosuppression induced by cyclophosphamide, the administration of low doses of polysaccharides derived from Ganoderma lucidum resulted in enhanced activity of immune effector cells. Furthermore, accelerated regeneration of bone marrow cells, red blood cells, and white blood cells was observed, indicating potential hematopoietic effects [39]. An experimental investigation conducted using the RAW 264.7 cell line has provided confirmation that the four polysaccharides derived from Ganoderma lucidum, namely GLP-I, GLP-II, GLP-III, and GLP-IV (Ganoderma Lucidum Polysaccharides), exhibit significant properties that enhance macrophage proliferation and stimulate the production of nitric oxide (NO). NO serves as one of the key mediators in nonspecific immune responses [40]. Among the polysaccharides identified in Ganoderma lucidum, LZ-D-1 fucogalactan stands out as a water-soluble compound. Its composition comprises L-fucose, D-glucose, and D-galactose in a molar ratio of 1:1:5, with a molecular weight of 28 kDa. In vitro studies assessing its activity have demonstrated that LZ-D-1 promotes the proliferation of murine lymphocytes, with increasing efficacy corresponding to higher doses administered. Remarkably, at a concentration of 500 µg/mL, LZ-D-1 exhibited an effect comparable to that of phytohemagglutinin, a reference compound used in the study [41]. Given the multifaceted mechanisms of action, Ganoderma lucidum polysaccharides (GLPs) may play a significant role in modulating the immune response against cancer, viruses, and bacteria [42].
Furthermore, G. applanatum is a promising source of immunomodulatory components. One of them is an exobiopolymer produced by G. applanatum mycelial cultures, which is 58.9% sugar and 17.1% protein. The most common sugars are mannose and glucose, and the major amino acids are serine, glycine, aspartic acid, and alanine. Tests carried out in an animal model confirmed the immunomodulatory and antitumor activities of the isolated exobiopolymer. After its application, an increase in the weight of the spleen and liver was observed in animals, which may indicate an increase in the number of macrophages in these organs. In addition, a significant increase in NK cell activity and the regulation of complement system functions were observed [43].
Two proteins derived from G. applanatum are also identified: FIP-gap1 and FIP-gap2. They have stronger immunomodulatory properties than the long-known Ling-Zhi-8 from G. lucidum. These proteins increased mouse splenocyte viability, interleukin IL-2 release, and expression of IFN-γ [44].
3.2. Anticancer and Cytotoxicity Effects
Extracts and compounds isolated from G. lucidum have proven anticancer activity. They function through numerous mechanisms and are effective against various types of cancer, including liver, breast, cervix, prostate, colon, lung, and soft tissue cancers. In some cases, they also enhance the effectiveness of synthetic drugs and increase the sensitivity of neoplastic cells to the treatment. The antitumor activity is mainly attributable to the triterpenes and polysaccharides of G. lucidum [45].
Among triterpenes, ganoderic acids T, V, W, X, Y (Figure 2), and Z obtained from mycelium G. lucidum have shown cytotoxic activity in vitro against liver cancer cells [45]. For lucialdehyde A, B, and C, in vitro cytotoxic activity against cell lines of various cancers has been proven LLC (Lewis’s lung cancer), T47D (breast cancer), Sarcoma 180 (soft tissue sarcoma in mice), Meth-A (murine fibrosarcoma). Lucialdehyde C (Figure 2) showed the strongest activity, reaching ED50 values of 10.7, 4.7, 7.1, and 3.8 µg/mL [46]. Ganoderic acid D inhibited the proliferation of cervical cancer cells (HeLa) by inducing apoptosis and inhibiting the cell cycle in the G2/M phase at IC50 = 17.3 µmol/L [47]. Ganoderic acids F and K demonstrated apoptotic activity against HeLa cells [48]. Ganoderic acid DM (Figure 2), demonstrated antitumor efficacy against MCF-7 breast cancer cells. It caused the arrest of the cell cycle in the G1 phase, induction of apoptosis, and a decrease in the protein level: CDK2, CDK6, cyclin D1, p-Rb, and c-Myc [49]. Previous studies have also shown its effectiveness in prostate cancer. Ganoderic acid DM (Figure 2) isolated from the ethanolic extract inhibited the activity of 5α-reductase and bound to the androgen receptor, preventing DHT from binding to this receptor. Thus, it effectively inhibited the growth of prostate cancer cells and blocked osteoclastogenesis, which is particularly beneficial because long-term antiandrogen treatment promotes increased bone loss, and bone metastases are often observed in prostate cancer, increasing the risk of fractures [50,51]. Ganoderiol F (Figure 2) prevented the proliferation of neoplastic cells in a study on liver cancer (HepG2, Huh 7) and leukemia (K562) cell lines [47].
The mechanisms of action of the triterpene fraction obtained from mycelium G. lucidum include the inhibition of protein kinase C activity and the activation of JNK and p38 MAP kinases. This results in the cell cycle arrest in the G2 phase, due to which the growth of Huh-7 cells of human liver cancer was inhibited [52]. Ethanol extracts inhibited cell proliferation by increasing the expression of the p21 protein involved in the regulation of the cell cycle in the G1 phase and inhibiting the activity of cyclin D1. Furthermore, induction of apoptosis was demonstrated in MCF-7 breast cancer cells by increasing the expression of the proapoptotic Bax protein of the Bcl-2 protein family [45]. Another study confirmed that the aqueous extract of G. lucidum inhibits the proliferation of both estrogen-dependent (MCF-7) and estrogen-dependent (MDA-MB-231) breast cancer cells [53].
The antitumor activity of GLPs results primarily from their immunomodulatory activity, e.g., stimulating the production of macrophages, NK cells, and T lymphocytes. These compounds also exhibit other properties, e.g., antiangiogenic activity [45,54].
In a study on a human lung cancer cell line, the complex of polysaccharides and peptides was shown to prevent angiogenesis through reduced secretion of proangiogenic factors such as vascular endothelial growth factor. However, a test conducted on human umbilical vein endothelial cells showed that the polysaccharide–peptide complex inhibited the proliferation of vascular endothelial cells [54]. In melanoma cells, GLPs were found to increase the expression of the major histocompatibility complex, leading to an increased presentation of antigens, and thus stimulating resistance to cancer development [19]. The antitumor activity of polysaccharides isolated from G. lucidum was observed in human colon cancer cells (HCT-116). Induction of tumor cell apoptosis was also observed, which is due to the activation of mitogen-activated protein kinase pathways. Polysaccharides increased the expression of the Bax/Bcl-2 protein, caspase 3, and poly (ADP-ribose) polymerase [55]. α-D-Glucan, a component of the G. lucidum cell wall, showed cytotoxic activity against HeLa cervical cancer cells [19,56]. In vivo studies showed that β-D-glucans, heteropolysaccharides, and glycoproteins isolated from G. lucidum show antitumor activity against sarcoma (Sarcoma 180) in mice [19].
The latest reports do not recommend the use of G. lucidum as a first-line treatment in cancer due to the lack of sufficient evidence but strongly emphasize the benefits of using it as a supportive treatment. Patients treated with chemotherapy or radiotherapy who received concomitant G. lucidum are more likely to respond to the treatment. Moreover, G. lucidum was well tolerated by most patients, and only a few cases of mild side effects, e.g., nausea, were observed [57]. G. lucidum has been shown to enhance the effect of some chemotherapy drugs; in particular, orally administered polysaccharides isolated from this species improved the effectiveness of cyclophosphamide treatment [45]. An in vitro study showed that G. lucidum extracts effectively increase the sensitivity of ovarian cancer cells to cisplatin [58].
Another argument in favor of the use of G. lucidum in combination therapy is the reduction in toxicity and side effects due to conventional treatment methods. Numerous studies have confirmed that G. lucidum has a radioprotective effect against healthy cells and supports the function of the immune system after radiotherapy. Furthermore, G. lucidum prevents radiation-induced DNA damage. Another study proved that the use of G. lucidum during cisplatin treatment reduces the nephrotoxicity of this drug [54]. In another study by Sułkowska-Ziaja et al., the cytotoxic effects of six Ganoderma species on 16F10 mouse melanoma cells were investigated and G. carnosum was noted as the most active extract with 43.29% at 0.15 mg/mL [59].
Some reports also indicate the anticancer properties of other Ganoderma species. In the analysis of the antiproliferative activity of G. applanatum, three compounds isolated from the ethanol extract were used C15-tetraol and ganoderic acid A. A dose-dependent inhibition of gastric cancer cell growth (SGC-7901) was observed. A mixture of these compounds at a concentration of 98.5% caused inhibition of cell growth. Each compound was also tested individually (concentration 300 µg/mL): C15-tetraol caused 55.4% inhibition, ganoderic acid A 32.7%, and ganoderic acid A 62.9% [60].
In a recent study conducted by Cao et al., one new triterpene was isolated from G. lucidum and it exhibited remarkable cytotoxic effects A549 (IC50: 15.38 μM) and HepG2 (IC50: 18.61 μM cell lines) [61]. In contrast to the results, ganodrenol derivatives, along with three known nor-triterpenoids were isolated from G. lucidum and displayed no cytotoxic effect on t LOVO, MCF-7, and RAW264.7 cells [62]. In a study performed by Zhong et al., the water-soluble polysaccharides from G. lucidum were positively affected by apoptosis pathways on Jurkat and HaCat cell lines and the expression levels of apoptotic genes (BAX and Bcl2) regulated (up or down) with the polysaccharide’s concentration (at 25 and 50 mg/L) [63]. However, in another study, the polysaccharides from G. lucidum showed no cytotoxic effect on CML and HeLa cells [64]. Peng et al. investigated the effect of one immunomodulatory peptide from G. microsporum on S. aureus methicyllin-resistant Staphylococcus aureus (MRSA) promoted expansion of myeloid-derived suppressor cells (MDSCs) to suppress T-cell proliferation and the peptide increased IL-6 and TNF-alpha levels and thus the author suggested that G. microsporum could be useful for periprosthetic joint infections induced by S. aureus [65]. Selected biological activities of terpenes from Ganoderma spp. and possible mechanisms of their activities are presented in Figure 3.
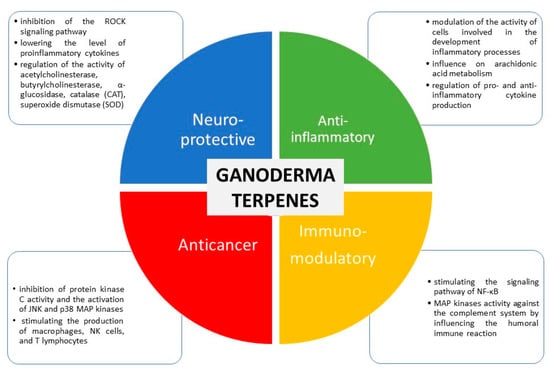
Figure 3.
Selected biological activities of terpenes from Ganoderma spp. and possible mechanisms of their activities (compiled based on scientific data).
3.3. Antioxidant Effects
Antioxidant activity has been demonstrated for many fungi species, including representatives of the genus Ganoderma. This activity is primarily attributable to phenolic compounds, polysaccharides, peptides, polysaccharide–peptide complexes, and triterpenes. Antioxidant compounds of fungal origin might minimize the production of reactive oxygen species and alleviate the oxidative stress associated with the pathogenesis of numerous diseases, such as cancer and atherosclerosis [66]. Therefore, Ganoderma spp. are an interesting example of a source of natural antioxidants, whose potential can be utilized in the prevention and treatment of various diseases, in the manufacture of food products, and the cosmetics industry [67].
Another study examined the antioxidant properties of four commonly known and used species, especially in medicine: G. applanatum, G. lucidum, Lentinus edodes, and Trametes versicolor. This study used extracts in which the content of polysaccharides, phenolic compounds, and proteins was determined using four tests: DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging, the ability to reduce iron ions, the inhibition of lipid peroxidation, and the ability to chelate iron ions. In each of the tests, G. applanatum showed the best results. This confirms that Ganoderma spp. have a high antioxidant potential. Furthermore, a strong correlation between antioxidant properties and the content of phenolic compounds and α-glucans in the extract was observed [68].
In another study, the antioxidant properties of the mycelial cultures of G. lucidum, G. applanatum, and G. carnosum were confirmed. In the DPPH radical scavenging test, G. lucidum extract turned out to be the most effective, neutralizing 57.12% of free radicals. In addition, G. applanatum and G. carnosum returned values of 20.35% and 17.04%, respectively. The phenolic compounds present in the mycelium of these species were chiefly responsible for the antioxidant activity [69].
Antioxidant activity has also been proven for G. adspersum, G. pfeifferi, and G. resinaceum. Among the various extracts (aqueous, ethanol, chloroform) from four species tested—G. applanatum, G. lucidum, G. pfeifferi, and G. resinaceum—the highest antioxidant potential was observed for aqueous and ethanol extracts of G. pfeifferi and G. applanatum, and the highest content of phenolic compounds was determined in the ethanolic extract of G. applanatum [67]. In an experiment comparing the antioxidant activity of the G. pfeifferi ethanol extract with that of ascorbic acid, a 500 µg/mL concentration of both samples resulted in more than 90% free radical capture [70]. In another study, the properties of extracts and isolated compounds of G. adspersum were evaluated. The highest antioxidant activity was demonstrated in all tests performed for the extract prepared using ethyl acetate. Among the four isolated compounds—applanoxidic acid G, applanoxidic acid E, applanoxidic acid A (Figure 2), and 22-stigmastenol—22-stigmastenol turned out to be the most effective antioxidant. In a test with β-carotene and linoleic acid, in which the ability to inhibit lipid peroxidation was examined, this compound achieved an IC50 of 160.39 ± 2.16 µg/mL [71]. For the extract prepared with ethyl acetate, the highest content of phenolic compounds was also observed, among which the highest content was observed for fumaric acid (14.3 µg/g) and caffeic acid (1.33 µg/g) with proven antioxidant activity [71,72].
In addition to phenolic compounds, polysaccharides are responsible for the antioxidant activity of Ganoderma spp. One of them is β-1,3-glucan, which is a component of the cell wall in G. lucidum. Low-molecular-weight β-1,3-glucan has been shown to prevent H2O2-induced damage to RAW264.7 cells and limit intracellular production of reactive oxygen species. Furthermore, it inhibits the activity of sphingomyelinase [73]. The key factors affecting the activity of polysaccharides are molecular weight, composition, type of glycosidic bond, and conformation. It is hypothesized that low-molecular-weight polysaccharides have a higher antioxidant activity, which is related to the number of hydroxyl groups [19]. Another study showed the antioxidant properties of G. applanatum polysaccharides, which was demonstrated in the DPPH test (77.5–81.9% at concentrations 0.1–1.0 mg/mL), the iron chelation test (15.7–89.0% at concentrations 0.1–20, 0 mg/mL), and the lipid peroxidation test (73.9–74.3% at concentrations of 5.0–20.0 mg/mL) [68].
However, peptides (GLP—G. lucidum peptide) are the primary antioxidant components of G. lucidum. In the hydroxyl radical scavenging test, the GLP peptide fraction was found to be more effective (IC50 = 0.025 mg/mL) than the polysaccharide and protein fractions with a higher molecular weight. Interestingly, GLP showed an antioxidant efficacy to synthetic antioxidant butylated hydroxytoluene comparable to that of soybean oil used at the same dose in the experiment. GLP may play an important role in inhibiting lipid peroxidation in biological systems due to its antioxidant, metal-chelating, and free radical scavenging effects [66].
Numerous reports also indicate the antioxidant properties of some triterpenes. Strong activity was demonstrated for many compounds isolated from G. lucidum: methyl ganoderan, lingzihina E, and lingzihina F [74]. In the aqueous extract of G. lucidum with antioxidant activity, the following compounds were identified: ganoderic acids A, B, C, and D (Figure 2), lucidene acid B, and ganodermanontriol [19]. The lanostane triterpenes present in G. adspersum—applanoxidic acids A and E—showed weak antioxidant properties [71].
The antioxidant properties have been studied also in unstable Fangina patients and those with a high risk of atherosclerosis. The assessed parameters were superoxide dismutase (SOD) and malondialdehyde (MDA) concentration, circulating endothelial cell (CEC) and endothelial progenitor cell (EPC) counts. After 90-days administration of 750 mg/day of polysaccharide peptide derived from G. lucidum, it was observed a significant reduction of MDA, CEC, and EPC counts in both stable angina patients and patients with a high risk of atherosclerosis. SOD was significantly increased instable angina group, although in the group of high-risk atherosclerosis, it registered only a slight increase and it was not statistically significant [75].
3.4. Anti-Inflammatory Effects
Inflammation in the body can be triggered by a wide range of factors: physical, chemical, or biological, to defend the body. Acute and chronic inflammation can accompany many diseases. An effective anti-inflammatory drug should inhibit the development of chronic inflammation without affecting the body’s homeostasis. To date, many herbal drugs targeting proinflammatory cytokines have been identified. One of the natural ingredients with proven anti-inflammatory properties is G. lucidum. Compounds obtained from this species may exert anti-inflammatory activity through various mechanisms: free radical scavenging and antioxidant activities, modulation of the activity of cells involved in the development of inflammatory processes, influence on arachidonic acid metabolism, and regulation of pro- and anti-inflammatory cytokine production [76].
A clear anti-inflammatory effect of the chloroform extract of G. lucidum was observed in a study conducted on mice. In this study, two models were used: carrageenan-induced acute inflammation and formalin-induced chronic inflammation. The extract used at the doses of 50 and 100 mg/kg in both models was characterized by an inflammatory inhibition capacity comparable to that of the reference drug diclofenac used at a dose of 10 mg/kg [35,77]. Another study tested the ability of the chloroform extract of G. resinaceum to inhibit the enzymes COX-1 and 12-LOX and showed that the extract has anti-inflammatory properties [67].
The anti-inflammatory compounds found in G. lucidum include, above all, triterpenes. The triterpene fraction inhibits the production of pro-inflammatory mediators such as TNF-α, interleukin 6, NO, and prostaglandin E2 (PGE2). Reduced NO and PGE2 secretion were due to the decreased expression of nitric oxide synthase and cyclooxygenase 2, respectively. The anti-inflammatory effect is primarily attributable to the inhibition of the NF-κB and AP-1 signaling pathways. The dominant group of triterpenes is ganoderic acids, among which the highest activity was demonstrated for ganoderic acid C [76,78].
Aromatic compounds isolated from G. lucidum have shown a significant anti-inflammatory effect, including meroterpenoids—lucidumin A, B, C, and D—and alkaloids –lucidomine E and ganocochlearin A. In a study on the murine macrophage RAW264.7 cell line, the ability of these compounds to inhibit the production of NO induced by lipopolysaccharide was studied, which demonstrated IC50 values ranging from 4.68 to 15.49 µM [79].
3.5. Antiallergic Effects
Antiallergic activity has been demonstrated by G. lucidum extracts, as well as some compounds isolated from this species, such as ganoderic acids, oleic acid, and cyclooctasulfur acid belonging to triterpenes [80]. A study showed that ganoderic acids C and D isolated from G. lucidum inhibit the release of histamine from mast cells [81]. The same effect was demonstrated for the chloroform extract of the G. lucidum culture medium. Cyclooctasulfur and oleic acid are responsible for the action of this extract. The mechanism of action of oleic acid, consisting of inhibition of histamine release and calcium uptake, results from the stabilization of the lipid bilayer of the cell membrane, whereas cyclooctasulfur affects membrane proteins [81,82,83]. The methanol extract of G. lucidum alleviates allergic pruritus through peripheral action. Furthermore, mast cells and H1 receptors have not been the primary sites of antipruritic action of this extract. It has been suggested to act through PAR2 and 5-HT2A receptors [81,84,85]. In patients with histamine-dependent allergic reactions, G. lucidum effectively regulates the balance between cytokines produced by Th1 and Th2 lymphocytes [35].
3.6. Neuroprotective Effects
Extensive research has demonstrated the wide-ranging neuroprotective effects of Ganoderma lucidum. Extracts of fruiting bodies exhibit significant potential in ameliorating the progression of neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Furthermore, G. lucidum has been found to have beneficial effects in the management of epilepsy and depression, and it plays a protective role in safeguarding nerve cells against damage during ischemic stroke. Moreover, it promotes the proliferation and differentiation of cells within the nervous system, highlighting its potential for neuroregenerative applications [86,87].
The protective effects of Ganoderma lucidum in Alzheimer’s disease involve multiple mechanisms. One key mechanism is its ability to prevent the excessive accumulation of harmful β-amyloid, achieved through inhibition of astrocyte activity, reduction of proinflammatory cytokine levels, improvement of mitochondrial function, antioxidant activity, and regulation of Ca2+ ion homeostasis. Notably, G. lucidum regulates the activity of crucial enzymes such as acetylcholinesterase, butyrylcholinesterase, α-glucosidase, catalase (CAT), and superoxide dismutase (SOD). Promising research results have been reported for both the aqueous extract and isolated polysaccharides (GLP) and triterpenes (GLT) of G. lucidum. Regarding triterpenes, one significant mechanism of action is the inhibition of the ROCK (Rho-associated protein kinase) signaling pathway, which is considered a primary factor in the development of Alzheimer’s disease. These findings highlight the potential therapeutic value of G. lucidum in the prevention and treatment of Alzheimer’s disease [86,88]. Parkinson’s disease is associated with the impaired function of dopaminergic neurons. Their destruction can be prevented using G. lucidum, preventing the damage caused by oxidative stress and limiting the apoptosis of dopaminergic neurons [63].
Ischemic stroke is currently one of the most common causes of death worldwide. As in the case of other nervous system dysfunctions, the beneficial effects of G. lucidum are attributable to its antioxidant, anti-inflammatory, enzyme, and mitochondrial-regulating properties. Studies have shown that G. lucidum sterols protect cortical neurons from hypoxia-induced damage after stroke [86,89].
The efficacy and safety of administration of G. lucidum’s spore powder was explored in a group of patients with Alzheimer’s disease. After 6 weeks of treatment, there have not been observed any symptoms or improvements compared to the placebo group. However, the negative outcome can be caused by short-term administration and small sample size [90].
3.7. Hepatoprotective Action
G. lucidum has both therapeutic and preventive properties in the case of liver damage. Preparations of G. lucidum have a beneficial effect on the liver in the case of poisoning with chemical substances, e.g., tetrachloromethane, α-amanitine, benzo(α)pyrene, and ethyl alcohol, but also in the case of nonalcoholic fatty liver disease and damage caused by radiation therapy. Two groups of compounds found in this species are responsible for the activities: triterpenes and polysaccharides [35,91]. Hepatoprotective properties have been demonstrated for triterpene compounds such as ganoderic acid R and S and ganosporic acid A, which have been confirmed in an in vitro study carried out on rat hepatocytes [35,92].
The extract of G. lucidum containing the complete triterpene fraction showed a strong protective effect against liver damage induced by tetrachloromethane and D-galactosamine. The use of this extract resulted in a decrease in the level of ALT (alanine aminotransferase) in serum and triglycerides (TG) in the liver. This hepatoprotective action may result from the increased activity of enzymes that remove free radicals from the liver [35].
A double-blind, randomized clinical trial conducted on patients with chronic hepatitis B confirmed the hepatoprotective properties of Ganopoly, which is comprised of GLPs [35]. In another study, GLPs were used in the case of liver damage induced by BCG infection, which resulted in a decrease in the ALT level. The hepatoprotective effect of GLPs is probably due to their anti-inflammatory properties and regulation of NO production [93]. The extracts of G. lucidum also showed a beneficial effect in the case of liver damage caused by ethyl alcohol. A study on rats confirmed that the use of this extract reduces the histological changes caused by acute liver damage. Furthermore, a decrease in the level of ALT and an increase in the activity of SOD and CAT were observed. Another study showed that the extract protects against liver damage by modulating the activity of ethanol-metabolizing enzymes and alleviating oxidative stress [94].
Hepatoprotective activity was also observed for G. applanatum, which was demonstrated by examining the liver of mice treated with benzo-α-pyrene. This compound is a polycyclic aromatic hydrocarbon that induces gene mutations, chromosome aberrations, and other genotoxic effects. Triterpenes with G. applanatum resulted in a reduction in the levels of alanine and asparagine transferase in blood, indicating liver damage, and a reduction in tissue damage. In addition, a reduction in the content of reactive oxygen species was observed, and the content of active glutathione increased significantly [95].
Triterpenes—ganoderiol B and lucidon A—isolated from the fruiting bodies of G. resinaceum inhibited the activity of liver enzymes aspartate aminotransferase and alanine in an in vitro study on hepatocellular carcinoma cells. This study reported that G. resinaceum triterpenoids have a protective effect on liver cells by influencing enzyme levels [96].
Another randomized, double-blind placebo-controlled study of hepatoprotective and antioxidant properties of G. lucidum was carried out on healthy volunteers. After 6 months of consumption, it has been noted a significant increase in total antioxidant capacity and a significant decrease in hepatic marker enzymes compared to the placebo group. Moreover, after abdominal ultrasonic examination, it was observed a redevelopment of the liver from a mild fatty condition to a normal state [97].
3.8. Hypoglycemic Effects
Hypoglycemic activity is demonstrated by numerous compounds present in the extracts of G. lucidum: polysaccharides, proteoglycans, proteins, and triterpenes. It is presumed that G. lucidum extracts may be an alternative adjuvant treatment for diabetes. The mechanism of action of polysaccharides is by increasing insulin levels and lowering blood glucose levels [98]. Perhaps these compounds accelerate the regeneration and renewal of damaged β cells in the pancreas and stimulate the secretion of pancreatic insulin. In addition, they regulate the expression of enzymes involved in glucogenogenesis and glycogenolysis. In a study on mice with streptozotocin-induced diabetes, polysaccharides have been found to have a beneficial effect on the wound-healing process, which is important in patients with diabetes because their healing processes are slow and there is a high risk of amputation. The effect of polysaccharides on the lipid profile was also observed: lowering the level of total cholesterol, TG, and LDL cholesterol, and increasing the level of HDL cholesterol. Therefore, GLPs may have a positive effect on diabetes coexisting with atherosclerosis or hyperlipidemia [98,99].
One of the polysaccharides identified is F31, a β-heteropolysaccharide that is comprised of glucose, mannose, xylose, arabinose, and galactose, with a molecular weight of 15.9 kDa [100]. Furthermore, ganoderan A and B isolated from an aqueous extract of G. lucidum showed antiglycemic properties [101]. The FYGL proteoglycan that is comprised of proteins and polysaccharides (78% glucose) in a 17:77 ratio in vitro inhibited the activity of the enzyme PTP1B (IC50 = 5.12 ± 0.05 µg/mL). PTP1B is involved in the regulation of the insulin-signaling pathway through the dephosphorylation of the insulin receptor and its substrates. The inhibition of PTP1B is a promising therapeutic target for the treatment of diabetes [102,103].
A study on mice with type 2 diabetes showed that FYGL is effective in regulating blood glucose levels and has a positive effect on the lipid profile; therefore, it is considered a good candidate in the treatment of type 2 diabetes with comorbid metabolic disorders [98,102,103]. Triterpenes inhibit the activity of aldose reductase and α-glucosidases, thus reducing postprandial hyperglycemia. Among the triterpenes that are aldose reductase inhibitors in the chloroform extract, 13 ganoderic acids and their 2 methyl esters have been identified. The strongest activity is shown by ganoderic acid Df (IC50 = 22.8 µM), ganoderic acid C2 (IC50 = 43.8 µM), and ganoderic acid A (IC50 = 119.2 µM). Ganoderol B, also isolated from the chloroform extract, is a strong inhibitor of α-glucosidase with an IC50 value of 119.8 µM. For comparison, the IC50 for acarbose is 521.5 µM [98,104,105]. The Ling-Zhi-8 protein is effective in type 1 diabetes due to its immunomodulatory properties [38,98]. The hypoglycemic effect was also observed in compounds obtained from other Ganoderma spp. Exopolymers of G. applanatum noticeably decrease blood glucose levels. In a study on rats with streptozotocin-induced diabetes, glucose levels were reduced by 22%. This effect may be due to an increase in insulin secretion by pancreatic cells [106]. Ergosterol peroxide isolated from G. applanatum can inhibit aldose reductase, which is proven in in vitro studies (at a level of 92.2% at a concentration of 15.4 µg/mL). This enzyme is involved in the development of diabetic complications (neuropathy and retinopathy); therefore, reductase inhibitors may be useful in their prevention [107].
The latest reports have indicated the hypoglycemic effect of extracts from two other species: G. pfeifferi and G. resinaceum. The aqueous extract of G. resinaceum led to a slight decrease in glycemia in alloxan-induced diabetic rats. The water and ethanol extract of G. resinaceum and the ethanol extract of G. pfeifferi prevented a significant increase in glycemia after the oral glucose loading test [108]. In the fruiting bodies of G. resinaceum, lanostane-type triterpenes were identified, which showed a strong inhibitory effect on α-glucosidases: resinacein C, ganoderic acid Y, lucialdehyde C (Figure 2), 7-oxo-ganoderic acid Z3, 7-oxo-ganoderic acid Z, and lucidadiol. The efficiencies of these compounds were compared with that of the positive control—acarbose, an antidiabetic drug that inhibits α-glucosidases. Furthermore, the presence of a double bond between the C-24 and C-25 atoms was found to be a necessary element of the structure of triterpenes to ensure the inhibition of α-glucosidases [109].
On the contrary, there was conducted the research on the effect of G. lucidum on blood parameters such as glucose or cholesterol level in women with fibromyalgia, which is a disorder related to i.e., high glucose level. Obtained results did not show statistically significant differences in any of the measures. Nevertheless, it can be caused by the administration of the low dose of active compounds compared to previous studies [110].
A double-blind, randomized, placebo-controlled trial was held also on patients with type 2 diabetes mellitus and metabolic syndrome. The participants received treatment of 3g of G. lucidum for 16 weeks. The assessed parameters were i.e., glycosylated hemoglobin [HbA1c] and fasting plasma glucose [FPG]. No significant improvements were noted after the whole course of administration [111].
3.9. Hypotensive Effects
Due to the numerous side effects of synthetic drugs used in the treatment of hypertension, natural and safe antihypertensive agents are increasingly being sought. Several fungi species have demonstrated such properties, including G. lucidum [112]. Reports have demonstrated that the methanolic extract of dried fruiting bodies of G. lucidum shows inhibitory activity against angiotensin-converting enzyme (ACE). The following triterpene compounds isolated from the extract were responsible for this effect: ganoderal A and B, and ganoderic acids B, D, F, H, K, S, and Y. (4.7 × 10−6 M) (Figure 2), [113]. Then, reports have indicated that the extract of G. lucidum prepared with hot water also shows ACE-inhibitory properties. This extract showed the strongest activity among the extracts of 14 species of fungi tested in this experiment. This activity has been suggested to be achieved through the presence of proteins that block the active site of ACE and prevent the conversion of angiotensin I to angiotensin II. In addition, the hypotensive effect of the extract may also result from other mechanisms such as regulation of NO production, enhancement of endothelial function, and free radical scavenging [114]. The drug losartan is an antagonist of the AT1 receptor for angiotensin II [114].
After 7 weeks of use, the extract reduced the pressure in the tested animals at a level comparable to that of the drug used. Additionally, the extract showed a beneficial effect on cerebral blood flow and the regulation of the balance between inhibitory and excitatory neurotransmitters. These properties indicate the nootropic potential of the extract studied [115]. Compared with the aqueous extract, the extract prepared from G. lucidum using proteases isolated from this species showed a stronger antihypertensive activity, which was used to hydrolyze proteins and obtain an ADR (autodigested Reishi extract) extract. Eleven peptides were identified in the extract analyzed, four of which were inhibitors of ACEs. There were three dipeptides: Ala-Tyr, Ser-Tyr, and Ile-Arg, and one tripeptide: Ile–Pro–Thr. Their IC50 values ranged from 73.1 µM to 162.7 µM. In vivo studies carried out on rats confirmed the hypotensive effect of the ADR extract and suggested the possibility of using it as a functional food or as part of hypertension therapy [116].
The high antihypertensive potential and the ability to inhibit ACE have been reported for mycelium G. lucidum and its proteins. The aqueous mycelium extract reached an IC50 value of 1.134 ± 0.036 mg/mL. This finding seems to be highly beneficial since obtaining biomass from mycelial cultures is several times faster than from growing fruiting bodies [117].
3.10. Antibacterial and Antifungal Effects
Triterpenes with a specific parent carbon skeleton are the primary compounds with antibacterial and antifungal activity found in Ganoderma spp. In addition, such properties are demonstrated by polyketides—including farnesyl quinone; peptides—ganodermin; polysaccharides—sacchachitin and chitosan; and steroid compounds [16].
Ganomycins A and B are isolated farnesyl hydroquinone derivatives from G. pfeifferi. Research has shown that they have antibacterial properties as they effectively inhibit the growth of some Gram-negative and Gram-positive bacterial strains, including Bacillus subtilis, Staphylococcus aureus, and Micrococcus flavus. The minimum inhibitory concentration (MIC) for ganomycin A and B against M. flavus was 2.5 µg/mL (ampicillin MIC: 0.25 µg/mL) [118].
In vitro tests showed that aqueous extracts of various Ganoderma spp., as well as triterpenes isolated from them, have a broad antimicrobial spectrum. Their effectiveness has been proven against numerous Gram-positive and Gram-negative bacteria, including Helicobacter pylori, which indicates the possible beneficial effect of these extracts in patients with chronic respiratory infections or peptic ulcer disease with the confirmed presence of H. pylori [35].
Colossolactone E and 23-hydroxycolossolactone E are triterpene compounds that have been isolated and found to possess significant activity against B. subtilis and Pseudomonas syringae [16]. Certain steroid compounds, such as ganodermadiol, ergosta-5,7,22-trien-3β-yl acetate, and ergosta-7,22-dien-3β-ol, have also demonstrated antimicrobial properties. These compounds exhibit effectiveness against B. subtilis and S. aureus, with a minimum inhibitory concentration (MIC) value ranging from 2.5 to 5 mg/mL [16,119]. Moreover, a study revealed that methanol and ethanol extracts of G. lucidum and G. applanatum exhibit stronger antimicrobial properties compared to aqueous extracts of these species. These extracts have shown effectiveness against Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, S. aureus, Bacillus cereus, and Actinomyces sp. Additionally, they have been confirmed to possess antifungal activity against various organisms, including Aspergillus niger, Candida albicans, Malassezia sympodialis, and Pache dermatitis [17].
Ganodermin is a protein with antifungal activity isolated from G. lucidum. It inhibits the growth of Botrytis cinerea, Fusarium oxysporum, and Physalospora piricola, with an IC50 value of 15.2, 12.4, and 18.1 µM, respectively [120].
Ethanolic extracts obtained from mycelium G. applanatum, G. carnosum, and G. lucidum also demonstrated antifungal activity. They were effective against Acremonium strictum, Aspergillus glaucus, Aspergillus flavus, Aspergillus fumigatus, Aspergillus nidulans, A. niger, Aspergillus terreus, and Trichoderma viride; however, the MIC and MFC values were several times higher than those of ketoconazole and fluconazole [16,69].
3.11. Antimalarial Activity
Triterpene compounds derived from Ganoderma spp., such as schisanlactone B, ganodermalactone F, and colossolactone E, have exhibited modest antimalarial activity. In tests against Plasmodium falciparum, their IC50 values ranged from 6.0 to 10.0 µM [16,121]. Similarly, terpenes isolated from ethyl acetate extract of G. lucidum demonstrated antimalarial properties. These terpenes include ganoderic acids DM, TR1 (Figure 2), and S, ganoderaldehyde TR, ganodermanondiol, and ganofuran B. Their IC50 values ranged from 6.0 to 20.0 µM [16,122].
3.12. Antiviral Effects
Antiviral properties have been demonstrated for numerous compounds derived from G. lucidum and G. pfeifferi, with their efficacy demonstrated against viruses such as HIV 1, HSV-1, HSV-2, HPV, hepatitis B virus (HBV), influenza A virus, and enterovirus 71 (EV71) [16]. Among triterpenes obtained from G. pfeifferi, ganodermadiol, lucidadiol, and G applanoxidic acid demonstrated the activity against influenza A virus in the Vero and MDCK cell lines, with an ED50 value of 0.22, 0.22, and 0.19 mmol/L. Ganoderon C, lucialdehyde B, and ergosta-7.22-dien-3β-ol were also effective, with an IC50 value of 2.6, 3.0, and 0.78 µg/mL, respectively. Activity against HSV-1 was demonstrated for ganodermadiol (ED50 = 0.068 mmol/L), ganoderon A (IC50 = 0.3 µg/mL), lucialdehyde B (IC50 = 0.075 µg/mL), ganoderol A (Figure 2), (IC50 = 0.75 µg/mL), ganoderal A (Figure 2), (IC50 = 0.03 µg/mL), and ergosta-7,22-dien-3β-ol (IC50 = 0.03 µg/mL) [16,123,124].
Ganoderiol F (Figure 2), and ganodermanontriol are isolated from methanolic extracts of G. lucidum fruiting bodies, which are effective against HIV-1 at a concentration of 7.8 µg/mL. Furthermore, ganoderic acids B, C1, α, and H; ganoderiol A and B; and 3β-5α-dihydroxy-6β-methoxy-ergosta-7.22-diene are HIV-1 protease inhibitors with moderate efficacy, with an IC50 of 0.17 ± 0.23 mM [125].
The efficacy against EV71 has been proven for two triterpenes derived from G. lucidum: lanosta-7,9 (11), 24-trien -3, one, 15; 26-dihydroxy (GLTA) and Y ganoderic acid (GLTB). The results of a study conducted on the RD cell line suggest that GLTA and GLTB prevent EV71 infection by interacting with the virus and blocking its adsorption into cells. Furthermore, these compounds significantly inhibit viral RNA replication [126].
A study carried out in the liver cell line HepG2215 confirmed the antiviral activity of ganoderic acid isolated from G. lucidum against HBV [127]. Polysaccharides of G. applanatum are also effective against HBV. In vitro studies showed that they inhibit viral DNA polymerase activity by 80% and anti-HBs antigen production by 60% [128].
The selected biological activities of the species included in this review are summarized in Table 2.

Table 2.
Selected biological activities of Ganoderma spp.
3.13. Other Activities
In a study conducted by Tang et al., the neurasthenia effect of G. lucidum polysaccharides was examined on 123 patients who were divided into two groups. The application of G. lucidum polysaccharides for 8 weeks resulted in a significant reduction in the sense of fatigue (28.3%) compared to the placebo group (20.1%). Moreover, the polysaccharides were well tolerated by the patients [129]. Conversely, in a study by Klupp et al. investigating the efficacy and safety of G. lucidum in treating cardiovascular risk among diabetic patients, the clinical results did not support the use of G. lucidum for reducing cardiovascular risk in this specific population [111].
4. Extraction of the Bioactive Components from Ganoderma spp.
Various types of extracts derived from Ganoderma have been employed in scientific research to investigate the diverse array of metabolites present within this fungus. These extracts possess distinct chemical profiles and utilize different extraction methods, enabling targeted exploration of specific metabolite classes and their associated biological activities [19]. The ethanolic extract is obtained by subjecting Ganoderma samples to extraction processes using ethanol as the solvent. This extract captures a broad spectrum of bioactive compounds, including terpenoids, polysaccharides, and proteins. Water extraction involves the immersion of Ganoderma samples in water to extract water-soluble compounds. This type of extract is particularly valuable for examining hydrophilic metabolites present in Ganoderma, such as polysaccharides, glycoproteins, and other water-soluble bioactive compounds [130]. Methanol extraction utilizes methanol as the solvent to extract a wide range of metabolites from Ganoderma. This extraction method is often employed for the analysis of secondary metabolites, including triterpenes, sterols, phenolic compounds, and other metabolites soluble in methanol. Hot water extraction involves subjecting Ganoderma samples to elevated temperatures to extract heat-stable metabolites. This method is commonly used for extracting thermostable bioactive compounds, particularly polysaccharides and other metabolites resistant to high temperatures. Supercritical fluid extraction employs supercritical carbon dioxide as the solvent to extract metabolites from Ganoderma. This advanced technique enables the extraction of lipophilic compounds, such as triterpenes and fatty acids, which are soluble in supercritical fluids [131].
5. Safety of Using Preparations Containing Ganoderma spp.
Several studies have been carried out on the effectiveness and safety of using G. lucidum preparations. In a study, 88 men with symptoms of lower urinary tract infection over 49 years of age were recruited, who received G. lucidum extract in a dose of 6 mg daily or placebo. At the end of the study, G. lucidum treatment was found to be well tolerated with no serious adverse effects [132]. The safety and tolerance study of G. lucidum also showed no effects. A total of 16 volunteers received 2.0 g of extract twice daily for 10 days. ECG tests, complete blood counts, and urine tests were performed. No undesirable side effects were observed in this group compared with the group receiving the placebo [133]. The water extract of G. lucidum and the polysaccharide fraction were tested for acute toxicity in mice, in which no serious or fatal effects were observed. Mice were orally administered an aqueous extract at a dose of 5000 mg/kg body weight for 30 days, and no changes in body weight were observed. In another study conducted by Gill and Rieder the toxic potentials of various doses of G. lucidum were assessed. The administration of high doses resulted in toxic effects on blood mononuclear cells, leading the authors to caution against the use of G. lucidum without careful consideration [134]. After taking Ganoderma powder for 1–2 months, Wanmuang et al. presented a case of fatal fulminant hepatitis [135]. Another patient taking Ganoderma reported chronic watery diarrhea and a diagnosis of non-Hodgkins lymphoma [136].
6. Cultivation of Ganoderma spp.
In recent years, the Ganoderma industry has experienced significant development and has introduced a wide range of products to the market [137]. However, certain issues have been reported regarding Ganoderma-based products, primarily due to variations in seasons, different substrate conditions, and stages of fruiting body development, all of which can impact product quality. The chemical composition of Ganoderma spp. can vary depending on factors such as the strain, cultivation conditions, and extraction methods [136]. Therefore, it is crucial to establish standardized and reproducible protocols for production processes to ensure consistent quality and safety of Ganoderma products [137]. To enhance productivity and reduce the reliance on chemical pest control, the breeding of new Ganoderma strains with higher yields and disease resistance is of great importance. Various breeding strategies are employed, including the identification, purification, and establishment of gene pools, followed by conventional breeding methods, radiation and mutant breeding techniques, cell and gene engineering breeding, and the evaluation of agronomic trials and biochemical characteristics [138].
7. Conclusions and Future Perspectives
Species belonging to the genus Ganoderma have a long history of use in Traditional Chinese Medicine (TCM), with Ganoderma lucidum being the most prominent species. According to TCM principles, the raw material derived from Ganoderma lucidum is believed to possess revitalizing and immune-enhancing effects, and it has been traditionally used for various conditions such as skin diseases, hypertension, and digestive disorders. Extensive research, including in vivo trials, has further substantiated the therapeutic potential of different species within this genus. Modern pharmacological investigations have provided evidence for numerous valuable activities associated with extracts and individual compounds obtained from Ganoderma species. These activities include antioxidant, anticancer, cytotoxic, immunomodulatory, anti-inflammatory, cardioprotective, anti-atherosclerotic, antidiabetic, hepatoprotective, and antimicrobial effects. The primary constituents responsible for these diverse actions are polysaccharides and terpene compounds. However, chemical insights suggest that isolating and purifying Ganoderma compounds and studying their individual, synergistic, or antagonistic effects on biological systems is necessary. Comprehensive clinical research, particularly focused on determining toxic doses and potential side effects, is crucial to establish the potential of Ganoderma compounds in the development of drugs for both common and rare diseases. Moreover, standardized testing and regulations should be implemented to ensure the safety and quality of pharmaceutical formulations containing Ganoderma products. Going forward, it is essential for medical professionals and scientists to take responsibility for educating others about the possible side effects and potential interactions of Ganoderma products with other drugs or chemicals.
Author Contributions
Conceptualization, K.S.-Z., M.B. and A.S.; methodology, K.S.-Z., M.B., A.S., M.T. and G.Z.; investigation, K.S.-Z., M.B., A.S. and G.Z.; writing—original draft preparation, K.S.-Z., M.B., A.S., M.T. and G.Z.; writing—review and editing, K.S.-Z., K.K. and B.M.; visualization, K.S.-Z., M.B., A.S. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The research was realized as a part of the research project supported by the Polish Ministry of Science and Higher Education (Grants PL: N42/DBS/000271).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Jacek Nowicki for permission to use photographs of Ganoderma carnosum.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjökvist, E.; Lindner, D.; Nakasone, K.; Niemelä, T.; Larsson, K.H.; Ryvarden, L.; et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef]
- Sun, Y.F.; Xing, J.H.; He, X.L.; Wu, D.M.; Song, C.G.; Liu, S.; Vlasák, J.; Gates, G.; Gibertoni, T.B.; Cui, B.K. Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud. Mycol. 2022, 101, 287–415. [Google Scholar] [CrossRef] [PubMed]
- Fryssouli, V.; Zervakis, G.I.; Polemis, E.; Typas, M.A. A global meta-analysis of ITS rDNA sequences from material belonging to the genus Ganoderma (Basidiomycota, Polyporales) including new data from selected taxa. MycoKeys 2020, 75, 71–143. [Google Scholar] [CrossRef]
- Bernicchia, A.; Gorjón, S.P. Polypores of the Mediterranean Region; Romar SRI: Rome, Italy, 2020. [Google Scholar]
- Karsten, P.A. Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Rev. Mycol. Toulouse 1881, 3, 16–19. [Google Scholar]
- Patouillard, N.T. Le genre Ganoderma. Bull. Soc. Mycol. France 1889, 5, 64–80. [Google Scholar]
- Rivoire, B.; Gannaz, M.; Pirlot, J.M. Polypores de France et d’Europe [Polypores of France and Europe]; Fédération Mycologique et Botanique Dauphiné-Savoie Annemasse Mycopolydev: Orlienas, France, 2020. [Google Scholar]
- Łakomy, P.; Kwaśna, H. Atlas Hub; Multico Oficyna Wydawnicza: Warszawa, Poland, 2008. [Google Scholar]
- Schwartze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategiesnof Wood Decay in Trees; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Loyd, A.L.; Held, B.W.; Linder, E.R.; Smith, J.A.; Blanchette, R.A. Elucidating wood decomposition by four species of Ganoderma from the United States. Fungal Biol. 2018, 122, 254–263. [Google Scholar] [CrossRef]
- Coelho-Moreira, J.D.S.; Brugnari, T.; Sá-Nakanishi, A.B.; Castoldi, R.; de Souza, C.G.M.; Bracht, A.; Peralta, R.M. Evaluation of diuron tolerance and biotransformation by the white-rot fungus Ganoderma lucidum. Fungal Biol. 2018, 122, 471–478. [Google Scholar] [CrossRef]
- Wang, H.; Deng, W.; Shen, M.H.; Yan, G.; Zhao, W.; Yang, Y. A laccase Gl-LAC-4 purified from white-rot fungus Ganoderma lucidum had a strong ability to degrade and detoxify the alkylphenol pollutants 4-n-octylphenol and 2-phenylphenol. J. Hazard. Mater. 2021, 408, 124775. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D. The perception of Ganoderma lucidum in Chinese and Western culture. Mycologist 2004, 18, 165–169. [Google Scholar] [CrossRef]
- Lin, Z. Ganoderma (Lingzhi) in Traditional Chinese Medicine and Chinese Culture. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1181, pp. 1–13. [Google Scholar]
- Wang, L.; Li, J.Q.; Zhang, J.; Li, Z.M.; Liu, H.G.; Wang, Y.Z. Traditional uses, chemical components and pharmacological activities of the genus Ganoderma P. Karst.: A review. RSC Adv. 2020, 10, 42084–42097. [Google Scholar] [CrossRef] [PubMed]
- Basnet, B.B.; Liu, L.; Bao, L.; Liu, H. Current and future perspective on antimicrobial and anti–parasitic activities of Ganoderma sp.: An update. Mycology 2017, 8, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, S.G.; Awotona, F.E. Studies on antimicrobial potentials of three Ganoderma species. Afr. J. Biomed. Res. 2010, 13, 133–139. [Google Scholar]
- Xia, Q.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef]
- Cör, D.; Knez, Ž.; Hrnčič, M.K. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum terpenoids and polysaccharides: A review. Molecules 2018, 23, 649. [Google Scholar] [CrossRef]
- Prezes Urzędu Rejestracji Produktów Leczniczych Wyrobów Medycznych i Produktów Biobójczych. Farmakopea Polska XII Tom II; Polskie Towarzystwo Farmaceutyczne: Warszawa, Poland, 2020. [Google Scholar]
- Ryvarden, L.; Melo, I. Poroid fungi of Europe; Fungiflora: Oslo, Norway, 2014. [Google Scholar]
- Sokół, S. The Ganodermataceae of Poland. In Taxonomy, Ecology and Distribution; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2000. [Google Scholar]
- Ryvarden, L.; Gilbertson, R.L. European Polypores; Part 1: Abortiporus-Lindtneria; Synopsis Fungorum: Oslo, Norway, 1993. [Google Scholar]
- Kotlaba, F. Zaměpisné Rozšiřeni a Ekologie Chorošů (Polyporales s.l.); Československa Academia: Praha, Czech Republic, 1984. [Google Scholar]
- Beck, T.; Gáper, J.; Šebesta, M.; Gáperová, S. Host preferences of wood-decaying fungi of the genus Ganoderma in the urban areas in Slovakia. Ann. Univ. Paedagog. Crac. Stud. Naturae 2018, 3, 22–37. [Google Scholar] [CrossRef]
- Luangharn, T.; Karunarathna, S.C.; Dutta, A.K.; Paloi, S.; Promputtha, I.; Hyde, K.D.; Xu, J.; Mortimer, P.E. Ganoderma (Ganodermataceae, Basidiomycota) Species from the Greater Mekong Subregion. J. Fungi 2021, 7, 819. [Google Scholar] [CrossRef]
- Szczepkowski, A. Arboreal fungi in a different light—The use of sporocarps. Stud. Mater. CEPL Rogowie 2012, 32, 171–189. [Google Scholar]
- Dai, Y.C. Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 2012, 53, 49–80. [Google Scholar] [CrossRef]
- Zhou, L.W.; Nakasone, K.K.; Burdsall, H.H.; Ginns, J.; Vlasak, J.; Miettinen, O.; Spirin, V.; Niemelä, T.; Yuan, H.S.; He, S.H.; et al. Polypore diversity in North America with an annotated checklist. Mycol. Progress 2016, 15, 771–790. [Google Scholar] [CrossRef]
- Szczepkowski, A.; Piętka, J. New localities and new host of Ganoderma pfeifferi in Poland. Acta Mycol. 2003, 38, 59–63. [Google Scholar] [CrossRef]
- Sułkowska–Ziaja, K.; Balik, M.; Muszyńska, B. Ganoderma applanatum—źródło cennych substancji prozdrowotnych. MIR 2019, 3, 147–155. [Google Scholar]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland; Heterobasidiomycetes, Aphyllophorales, Gasteromycetes; Verlag Mykologia: Lucerne, Switzerland, 1986; Volume 2. [Google Scholar]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F.F. Ganoderma lucidum (Lingzhi or Reishi): A Medicinal Mushroom. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- El Enshasy, H.A.; Hatti–Kaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef]
- Sanodiya, B.; Thakur, G.; Baghel, R.; Prasad, G.; Bisen, P. Ganoderma lucidum: A Potent Pharmacological Macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Min, B.S.; Gao, J.J.; Hattori, M.; Lee, H.K.; Kim, Y.H. Anticomplement activity of terpenoids from the spores of Ganoderma lucidum. Planta Med. 2001, 67, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Wang, X.F.; Bao, T.W.; Ran, L.; Lin, J.; Zhou, X.W. In vitro synthesis of a recombinant fungal immunomodulatory protein from Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (W. Curt.: Fr.) P. Karst. (Aphyllophoromycetideae) and analysis of its immunomodulatory activity. Int. J. Med. Mushrooms 2010, 12, 347–358. [Google Scholar] [CrossRef]
- Kino, K.; Mizumoto, K.; Sone, T.; Yamaji, T.; Watanabe, J.; Yamashita, A.; Yamaoka, K.; Shimizu, K.; Ko, K.; Tsunoo, H. An immunomodulating protein, Ling Zhi–8 (LZ–8) prevents insulitis in non–obese diabetic mice. Diabetologia 1990, 33, 713–718. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, X.L.; Chen, A.F.; Lin, Z. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, Z.; Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Zhou, K.; Yang, Y.; Zhou, S.; Jia, W.; Hao, R.; Pan, Y. Purification, NMR study and immunostimulating property of a fucogalactan from the fruiting bodies of Ganoderma lucidum. Planta Med. 2008, 74, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Yang, B.K.; Jeong, S.C.; Kim, S.M.; Song, C.H. Ganoderma applanatum: A promising mushroom for antitumor and immunomodulating activity. Phytother. Res. 2008, 22, 614–619. [Google Scholar] [CrossRef]
- Qu, Z.W.; Zhou, S.Y.; Guan, S.X.; Gao, R.; Duan, Z.W.; Zhang, X.; Sun, W.Y.; Fan, W.L.; Chen, S.S.; Chen, L.J.; et al. Recombinant expression and bioactivity comparison of four typical fungal immunomodulatory proteins from three main Ganoderma species. BMC Biotechnol. 2018, 18, 80. [Google Scholar] [CrossRef]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi–Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [PubMed]
- Gao, J.J.; Min, B.S.; Ahn, E.M.; Nakamura, N.; Lee, H.K.; Hattori, M. New triterpene aldehydes, lucialdehydes A–C, from Ganoderma lucidum and their cytotoxicity against murine and human tumor cells. Chem. Pharm. Bull. 2002, 50, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Men, X.; Lei, P. Review on anti–tumor effect of triterpene acid compounds. J. Cancer Res. Ther. 2014, 10, 14–19. [Google Scholar]
- Yue, Q.X.; Song, X.Y.; Ma, C.; Feng, L.X.; Guan, S.H.; Wu, W.Y.; Yang, M.; Jiang, B.H.; Liu, X.; Cui, Y.J.; et al. Effects of triterpenes from Ganoderma lucidum on protein expression profile of HeLa cells. Phytomedicine 2010, 17, 606–613. [Google Scholar] [CrossRef]
- Wu, G.S.; Lu, J.J.; Guo, J.J.; Li, Y.B.; Tan, W.; Dang, Y.Y.; Zhong, Z.F.; Xu, Z.T.; Chen, X.P.; Wang, Y.T. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 2012, 83, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shiono, J.; Shimizu, K.; Kukita, A.; Kukita, T.; Kondo, R. Ganoderic acid DM: Anti–androgenic osteoclastogenesis inhibitor. Bioorganic Med. Chem. Lett. 2009, 19, 2154–2157. [Google Scholar] [CrossRef]
- Korczak, J.; Litwiniuk, M.; Kusnierczak, M.; Kustra, K.; Kubisz, L.; Mańkowska-Wierzbicka, D.; Mardas, M.; Stelmach-Mardas, M. Complications of hormonal therapy (ADT—Androgen deprivation therapy) and the movement system in patients with prostate cancer. Lett. Oncol. Sci. 2020, 17, 1–6. [Google Scholar] [CrossRef][Green Version]
- Lin, S.; Li, C.H.; Lee, S.S.; Kan, L.S. Triterpene–enriched extracts from Ganoderma lucidum inhibit growth of hepatoma cells via suppressing protein kinase C, activating mitogen–activated protein kinases and G2–phase cell cycle arrest. Life Sci. 2003, 72, 2381–2390. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Fung, K.P.; Tse, G.M.K.; Leung, P.C.; Lau, C.B.S. Comparative studies of various Ganoderma species and their different parts with regard to their antitumor and immunomodulating activities in vitro. J. Altern. Complement. Med. 2006, 12, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.H.J.; Jesuthasan, A.C.; Bishop, K.S.; Glucina, M.P.; Ferguson, L.R. Anti–cancer activities of Ganoderma lucidum: Active ingredients and pathways. Funct. Foods Health Dis. 2013, 3, 48–65. [Google Scholar] [CrossRef]
- Liang, Z.; Yi, Y.; Guo, Y.; Wang, R.; Hu, Q.; Xiong, X. Chemical characterization and antitumor activities of polysaccharide extracted from Ganoderma lucidum. Int. J. Mol. Sci. 2014, 15, 9103–9116. [Google Scholar] [CrossRef] [PubMed]
- Wiater, A.; Paduch, R.; Choma, A.; Pleszczyńska, M.; Siwulski, M.; Dominik, J.; Janusz, G.; Tomczyk, M.; Szczodrak, J. Biological study on carboxymethylated (1→3)–α–d–glucans from fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2012, 51, 1014–1023. [Google Scholar] [CrossRef]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.; Chan, G. Ganoderma lucidum (Reishi mushroom) for cancer treatment (Review). Cochrane Database Syst. Rev. 2016, 4, CD007731. [Google Scholar]
- Zhao, S.; Ye, G.; Fu, G.; Cheng, J.X.; Yang, B.B.; Peng, C. Ganoderma lucidum exerts anti–tumor effects on ovarian cancer cells and enhances their sensitivity to cisplatin. Int. J. Oncol. 2011, 38, 1319–1327. [Google Scholar]
- Sułkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyżak, A.; Popiół, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszyńska, B. Bioactivity and mycochemical profile of extracts from mycelial cultures of Ganoderma spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Chen, Y.; Jiang, J.; Qin, Z. Cellular and molecular mechanisms of the Ganoderma applanatum extracts induces apoptosis on SGC–7901 gastric cancer cells. Cell Biochem. Funct. 2011, 29, 175–182. [Google Scholar] [CrossRef]
- Cao, L.; Jin, H.; Liang, Q.; Yang, H.; Li, S.; Liu, Z.; Yuan, Z. A new anti-tumor cytotoxic triterpene from Ganoderma lucidum. Nat. Prod. Res. 2022, 36, 4125–4131. [Google Scholar] [CrossRef]
- Li, D.; Leng, Y.; Liao, Z.; Hu, J.; Sun, Y.; Deng, S.; Wang, C.; Tian, X.; Zhou, J.; Wang, R. Nor-triterpenoids from the fruiting bodies of Ganoderma lucidum and their inhibitory activity against FAAH. Nat. Prod. Res. 2022, 24, 105161. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, J.; Mao, P.; He, C.; Yuan, D.; Chen, C.; Zhang, H.; Hu, J.; Zhang, J. Ganoderma lucidum polysaccharide inhibits the proliferation of leukemic cells through apoptosis. Acta Biochim. Pol. 2022, 69, 639–645. [Google Scholar] [CrossRef]
- Khalilova, G.A.; Turaev, A.S.; Mulkhitdinov, B.I.; Khaitmetova, S.B.; Normakhamatov, N.S. Cytotoxic effects and antitumor activity of polysaccharides isolated from the fruiting body of Ganoderma lucidum basidial mushroom. Pharm. Chem. J. 2022, 56, 1045–1048. [Google Scholar] [CrossRef]
- Peng, K.T.; Chen, J.L.; Kuo, L.T.; Yu, P.A.; Hsu, W.H.; Lee, C.W.; Chang, P.J.; Huang, T.Y. GMI, an immunomodulatory peptide from Ganoderma microsporum, restrains periprosthetic joint infections via modulating the functions of myeloid-derived suppressor cells and effector T cells. Int. J. Mol. Sci. 2021, 22, 6854. [Google Scholar] [CrossRef]
- Sun, J.; He, H.; Bi, J.X. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef] [PubMed]
- Rašeta, M.; Popović, M.; Beara, I.; Šibul, F.; Zengin, G.; Krstić, S.; Karaman, M. Anti–inflammatory, antioxidant and enzyme inhibition activities in correlation with mycochemical profile of selected indigenous Ganoderma spp. from Balkan region (Serbia). Chem. Biodivers. 2021, 18, e2000828. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J.L.D. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Cilerdzic, J.; Stajic, M.; Vukojevic, J. Potential of submergedly cultivated mycelia of Ganoderma spp. as antioxidant and antimicrobial agents. Curr. Pharm. Biotechnol. 2015, 17, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Jülich, W.; Lindequist, U.; Jansen, R.; Mothana, R. Biologically active compounds from Ganoderma pfeifferi DSM 13239. U.S. Patent 6,726,911, 27 April 2004. [Google Scholar]
- Tel–Çayan, G.; Öztürk, M.; Duru, M.E.; Rehman, M.U.; Adhikari, A.; Türkoğlu, A.; Choudhary, M.I. Phytochemical investigation, antioxidant and anticholinesterase activities of Ganoderma adspersum. Ind. Crops Prod. 2015, 76, 749–754. [Google Scholar] [CrossRef]
- Chen, J.H.; Ho, C.T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Kao, P.F.; Wang, S.H.; Hung, W.T.; Liao, Y.H.; Lin, C.M.; Yang, W.B. Structural characterization and antioxidative activity of low-molecular-weights beta-1,3-glucan from the residue of extracted Ganoderma lucidum fruiting bodies. J. Biomed. Biotechnol. 2012, 2012, 673764. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Sargowo, D.; Ovianti, N.; Susilowati, E.; Ubaidillah, N.; Nugraha, A.W.; Vitriyaturrida; Proboretno, K.S.; Failasufi, M.; Ramadhan, F.; Wulandari, H.; et al. The role of polysaccharide peptide of Ganoderma lucidum as a potent antioxidant against atherosclerosis in high risk and stable angina patients. Indian Heart J. 2018, 70, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Katyal, P.; Sharma, A.K. Suppression of inflammatory and allergic responses by pharmacologically potent fungus Ganoderma lucidum. Recent Pat. Inflamm. Allergy Drug Discov. 2015, 8, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Sabulal, B.; George, V.; Smina, T.P.; Janardhanan, K.K. Antioxidative and antiinflammatory activities of the chloroform extract of Ganoderma lucidum found in South India. Sci. Pharm. 2009, 77, 111–121. [Google Scholar] [CrossRef]
- Dudhgaonkar, S.; Thyagarajan, A.; Sliva, D. Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int. Immunopharmacol. 2009, 9, 1272–1280. [Google Scholar] [CrossRef]
- Lu, S.Y.; Peng, X.R.; Dong, J.R.; Yan, H.; Kong, Q.H.; Shi, Q.Q.; Li, D.S.; Zhou, L.; Li, Z.R.; Qiu, M.H. Aromatic constituents from Ganoderma lucidum and their neuroprotective and anti–inflammatory activities. Fitoterapia 2019, 134, 58–64. [Google Scholar] [CrossRef]
- Merdivan, S.; Lindequist, U. Medicinal mushrooms with antiallergic activities. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D., Tsay, H.S., Shyur, L.F., Wu, Y.C., Wang, S.Y., Eds.; Medicinal and Aromatic Plants of the World; Springer: Singapore, 2017. [Google Scholar]
- Kohda, H.; Tokumoto, W.; Sakamoto, K.; Fujii, M.; Hirai, Y.; Yamasaki, K.; Komoda, Y.; Nakamura, H.; Ishihara, S.; Uchida, M. The biologically active constituents of Ganoderma lucidum (Fr.) Karst. Histamine release—inhibitory triterpenes. Chem. Pharm. Bull. 1985, 33, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Tasaka, K.; Mio, M.; Izushi, K.; Akagi, M.; Makino, T. Anti–allergic constituents in the culture medium of Ganoderma lucidum. (II) The inhibitory effect of cyclooctasulfur on histamine release. Agents Actions 1988, 23, 157–160. [Google Scholar] [CrossRef]
- Tasaka, K.; Akagi, M.; Miyoshi, K.; Mio, M.; Makino, T. Anti–allergic constituents in the culture medium of Ganoderma lucidum. (I) Inhibitory effect of oleic acid on histamine release. Agents Actions 1988, 23, 153–156. [Google Scholar] [CrossRef]
- Andoh, T.; Zhang, Q.; Yamamoto, T.; Tayama, M.; Hattori, M.; Tanaka, K.; Kuraishi, Y. Inhibitory effects of the methanol extract of Ganoderma lucidum on mosquito allergy–induced itch–associated responses in mice. J. Pharmacol. Sci. 2010, 114, 292–297. [Google Scholar] [CrossRef]
- Zhang, Q.; Andoh, T.; Konno, M.; Lee, J.B.; Hattori, M.; Kuraishi, Y. Inhibitory effect of methanol extract of Ganoderma lucidum on acute itch–associated responses in mice. Biol. Pharm. Bull. 2010, 33, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Ma, A.; Yang, B. Preventive and therapeutic effect of Ganoderma (lingzhi) on brain injury. Adv. Exp. Med. Biol. 2019, 1182, 159–180. [Google Scholar]
- Zhao, C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Li, J.S.; Chang, M.W. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2019, 121, 1160–1178. [Google Scholar] [CrossRef]
- Yu, N.; Huang, Y.; Jiang, Y.; Zou, L.; Liu, X.; Liu, S.; Chen, F.; Luo, J.; Zhu, Y. Ganoderma lucidum triterpenoids (GLTs) reduce neuronal apoptosis via inhibition of ROCK signal pathway in APP/PS1 transgenic Alzheimer’s disease mice. Oxid. Med. Cell. Longev. 2020, 2020, 9894037. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Wang, S.Z.; He, Q.H.; Yuan, L.; Chen, A.F.; Lin, Z.B. Ganoderma total sterol (GS) and GS 1 protect rat cerebral cortical neurons from hypoxia/reoxygenation injury. Life Sci. 2005, 76, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Wang, L.H.; Wang, C.; Qin, L.H. Spore powder of Ganoderma lucidum for the treatment of Alzheimer disease: A pilot study. Medicine 2018, 97, e0636. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhong, D.; Yang, B. Preventive and therapeutic effect of Ganoderma (lingzhi) on liver injury. Adv. Exp. Med. Biol. 2019, 1182, 217–242. [Google Scholar] [PubMed]
- Hirotani, M.; Ino, C.; Furuya, T.; Shiro, M. Ganoderic acids T, S and R, new triterpenoids from the cultured mycelia of Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 2282–2285. [Google Scholar] [CrossRef]
- Zhang, G.L.; Wang, Y.H.; Ni, W.; Teng, H.L.; Lin, Z.B. Hepatoprotective role of Ganoderma lucidum polysaccharide against BCG-induced immune liver injury in mice. World J. Gastroenterol. 2002, 8, 728–733. [Google Scholar] [CrossRef]
- Jang, S.H.; Cho, S.; Yoon, H.M.; Jang, K.J.; Song, C.H.; Kim, C.H. Hepatoprotective evaluation of Ganoderma lucidum pharmacopuncture: In vivo studies of ethanol–induced acute liver injury. J. Pharmacopunct. 2014, 17, 16–24. [Google Scholar] [CrossRef]
- Ma, J.Q.; Liu, C.M.; Qin, Z.H.; Jiang, J.H.; Sun, Y.Z. Ganoderma applanatum terpenes protect mouse liver against benzo(α)pyren–induced oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2011, 31, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Liu, J.Q.; Han, Z.H.; Yuan, X.X.; Luo, H.R.; Qiu, M.H. Protective effects of triterpenoids from Ganoderma resinaceum on H2O2—Induced toxicity in HepG2 cells. Food Chem. 2013, 141, 920–926. [Google Scholar] [CrossRef]
- Chiu, H.F.; Fu, H.Y.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Triterpenoids and polysaccharide peptides-enriched Ganoderma lucidum: A randomized, double-blind placebo-controlled crossover study of its antioxidation and hepatoprotective efficacy in healthy volunteers. Pharm. Biol. 2017, 55, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Hsieh, J.F.; Chen, S.T. Anti–diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Zhong, Z. Antihyperglycemic effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2011, 12, 6135–6145. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, Q.; Zhang, J.; Xie, Y.; Cai, W.; Tan, J. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 down–regulated hepatic glucose regulatory enzymes in diabetic mice. J. Ethnopharmacol. 2017, 196, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Hikino, H.; Konno, C.; Mirin, Y.; Hayashi, T. Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruit bodies. Planta Med. 1985, 4, 339–340. [Google Scholar] [CrossRef]
- Teng, B.S.; Wang, C.D.; Zhang, D.; Wu, J.S.; Pan, D.; Pan, L.F.; Yang, H.J.; Zhou, P. Hypoglycemic effect and mechanism of a proteoglycan from Ganoderma lucidum on streptozotocin–induced type 2 diabetic rats. Eur. Rev. Med. Pharmacol. 2012, 16, 166–175. [Google Scholar]
- Wang, C.D.; Teng, B.S.; He, Y.M.; Wu, J.S.; Pan, D.; Pan, L.F.; Zhang, D.; Fan, Z.H.; Yang, H.J.; Zhou, P. Effect of a novel proteoglycan PTP1B inhibitor from Ganoderma lucidum on the amelioration of hyperglycaemia and dyslipidaemia in db/db mice. Br. J. Nutr. 2012, 108, 2014–2025. [Google Scholar] [CrossRef]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Structure–activity relationships of Ganoderma acids from Ganoderma lucidum as aldose reductase inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 7295–7297. [Google Scholar] [CrossRef]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderol B: A potent α–glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar] [CrossRef]
- Yang, B.K.; Jung, Y.S.; Song, C.H. Hypoglycemic effects of Ganoderma applanatum and Collybia confluens exo–polymers in streptozotocin–induced diabetic rats. Phytother. Res. 2007, 21, 1066–1069. [Google Scholar] [CrossRef]
- Lee, S.H.; Shim, S.H.; Kim, J.S.; Kang, S.S. Constituents from the fruiting bodies of Ganoderma applanatum and their aldose reductase inhibitory activity. Arch. Pharm. Res. 2006, 29, 479–483. [Google Scholar] [CrossRef]
- Rašeta, M.; Popović, M.; Čapo, I.; Stilinović, N.; Vukmirović, S.; Milošević, B.; Karaman, M. Antidiabetic effect of two different: Ganoderma species tested in alloxan diabetic rats. RSC Adv. 2020, 10, 10382–10393. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Zhao, J.; Chen, L.X.; Wang, S.F.; Wang, Y.; Li, S.P. Lanostane triterpenes from the mushroom Ganoderma resinaceum and their inhibitory activities against α–glucosidase. Phytochemistry 2018, 149, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Pazzi, F.; Adsuar, J.C.; Domínguez-Muñoz, F.J.; García-Gordillo, M.Á.; Gusi, N.; Collado-Mateo, D. Effects of Ganoderma lucidum and Ceratonia siliqua on blood glucose, lipid profile, and body composition in women with fibromyalgia. Nutr. Hosp. 2021, 38, 139–145. [Google Scholar] [PubMed]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci Rep. 2016, 11, 29540. [Google Scholar] [CrossRef]
- Mohamed Yahaya, N.F.; Rahman, M.A.; Abdullah, N. Therapeutic potential of mushrooms in preventing and ameliorating hypertension. Trends Food Sci. Technol. 2014, 39, 104–115. [Google Scholar] [CrossRef]
- Morigiwa, A.; Kitabatake, K.; Fujimoto, Y.; Ikekawa, N. Angiotensin converting enzyme–inhibitory triterpenes from Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 3025–3028. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of selected culinary–medicinal mushrooms for antioxidant and ACE inhibitory activities. Evid. Based Complement. Altern. Med. 2012, 2012, 464238. [Google Scholar] [CrossRef]
- Shevelev, O.B.; Seryapina, A.A.; Zavjalov, E.L.; Gerlinskaya, L.A.; Goryachkovskaya, T.N.; Slynko, N.M.; Kuibida, L.V.; Peltek, S.E.; Markel, A.L.; Moshkin, M.P. Hypotensive and neurometabolic effects of intragastric Reishi (Ganoderma lucidum) administration in hypertensive ISIAH rat strain. Phytomedicine 2018, 41, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.B.; Yamamoto, A.; Matsumoto, S.; Ito, H.; Igami, K.; Miyazaki, T.; Kondo, R.; Schimizu, K. Hypotensive effects and angiotensin–converting enzyme inhibitory peptides of reishi (Ganoderma lingzhi) auto–digested extract. Molecules 2014, 19, 13473–13485. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Ansor, N.; Abdullah, N.; Aminudin, N. Anti–angiotensin converting enzyme (ACE) proteins from mycelia of Ganoderma lucidum (Curtis) P. Karst. BMC Complement. Altern. Med. 2013, 13, 256. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Jansen, R.; Jülich, W.D.; Lindequist, U. Ganomycins A and B, new antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi. J. Nat. Prod. 2000, 63, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Vazirian, M.; Faramarzi, M.; Ebrahimi, S.; Esfahani, H.; Samadi, N.; Hosseini, S.A.; Asghari, A.; Manayi, A.; Mousazadeh, A.; Asef, M.R.; et al. Antimicrobial effect of the Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum (higher Basidiomycetes) and its main compounds. Int. J. Med. Mushrooms 2014, 16, 77–84. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum. Peptides 2006, 27, 27–30. [Google Scholar] [CrossRef]
- Lakornwong, W.; Kanokmedhakul, K.; Kanokmedhakul, S.; Kongsaeree, P.; Prabpai, S.; Sibounnavong, P.; Soytong, K. Triterpene lactones from cultures of Ganoderma sp. KM01. J. Nat. Prod. 2014, 77, 1545–1553. [Google Scholar] [CrossRef]
- Adams, M.; Christen, M.; Plitzko, I.; Zimmermann, S.; Brun, R.; Kaiser, M.; Hamburger, M. Antiplasmodial lanostanes from the Ganoderma lucidum mushroom. J. Nat. Prod. 2010, 73, 897–900. [Google Scholar] [CrossRef]
- Niedermeyer, T.H.J.; Lindequist, U.; Mentel, R.; Gördes, D.; Schmidt, E.; Thurow, K.; Lalk, M. Antiviral terpenoid constituents of Ganoderma pfeifferi. J. Nat. Prod. 2005, 68, 1728–1731. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Awadh Ali, N.A.; Jansen, R.; Wegner, U.; Mentel, R.; Lindequist, U. Antiviral lanostanoid triterpenes from the fungus Ganoderma pfeifferi. Fitoterapia 2003, 74, 177–180. [Google Scholar] [CrossRef]
- El–Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti–HIV–1 and anti–HIV–1–protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tao, J.; Yang, X.; Yang, Z.; Zhang, L.; Liu, H.; Wu, K.; Wu, J. Antiviral effects of two Ganoderma lucidum triterpenoids against enterovirus 71 infection. Biochem. Biophys. Res. Commun. 2014, 449, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wang, S.F. Anti–hepatitis B activities of ganoderic acid from Ganoderma lucidum. Biotechnol. Lett. 2006, 28, 837–841. [Google Scholar] [CrossRef]
- Gao, Y.; Chan, E.; Zhou, S. Immunomodulating activities of Ganoderma, a mushroom with medicinal properties. Food Rev. Int. 2004, 20, 123–161. [Google Scholar] [CrossRef]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A randomized, double-blind and placebo-controlled study of a Ganoderma lucidum polysaccharide extract in neurasthenia. J. Med. Food. 2005, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rašeta, M.; Mišković, J.; Čapelja, E.; Zapora, E.; Petrović Fabijan, A.; Knežević, P.; Karaman, M. Do Ganoderma species Represent novel sources of phenolic based antimicrobial agents? Molecules 2023, 6, 3264. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.J.; Lim, K.T. Mushroom-derived bioactive molecules as immunotherapeutic agents: A review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef]
- Noguchi, M.; Kakuma, T.; Tomiyasu, K.; Yamada, A.; Itoh, K.; Konishi, F.; Kumamoto, S.; Shimizu, K.; Kondo, R.; Matsuoka, K. Randomized clinical trial of an ethanol extract of Ganoderma lucidum in men with lower urinary tract symptoms. Asian J. Androl. 2008, 10, 777–785. [Google Scholar] [CrossRef]
- Wicks, S.M.; Tong, R.; Wang, C.Z.; O’Connor, M.; Karrison, T.; Li, S.; Moss, J.; Yuan, C.S. Safety and tolerability of Ganoderma lucidum in healthy subjects: A double-blind randomized placebo-controlled trial. Am. J. Chin. Med. 2007, 35, 407–414. [Google Scholar] [CrossRef]
- Gill, S.K.; Rieder, M.J. Toxicity of a traditional Chinese medicine, Ganoderma lucidum, in children with cancer. Can. J. Clin. Pharmacol. 2008, 15, e275–e285. [Google Scholar]
- Wanmuang, H.; Leopairut, J.; Kositchaiwat, C.; Wananukul, W.; Bunyaratvej, S. Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J. Med. Assoc. Thai. 2007, 90, 179–181. [Google Scholar] [PubMed]
- Suprasert, P.; Apichartpiyakul, C.; Sakonwasun, C.; Nitisuwanraksa, P.; Phuackchantuck, R. Clinical characteristics of gynecologic cancer patients who respond to salvage treatment with Lingzhi. Asian Pac. J. Cancer Prev. 2014, 15, 4193–4196. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, K.K.; Elkhateeb, W.A.; Karunarathna, S.C.; Cheng, C.R.; Bandara, A.R.; Kakumyan, P.; Hyde, K.D.; Daba, G.M.; Wen, T.C. Current status of global Ganoderma cultivation, products, industry and market. Mycosphere 2008, 9, 1025–1052. [Google Scholar] [CrossRef]
- Zhou, X.W.; Su, K.Q.; Zhang, Y.M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 2012, 93, 941–963. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).