Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis
Abstract
1. Introduction
1.1. Hypothesis 1: Breeding Failure Triggers Nest Change
1.2. Hypothesis 2: Nest Change Affects Breeding Outcomes
1.3. Hypothesis 3: Nest Change Affects Survival
2. Materials and Methods
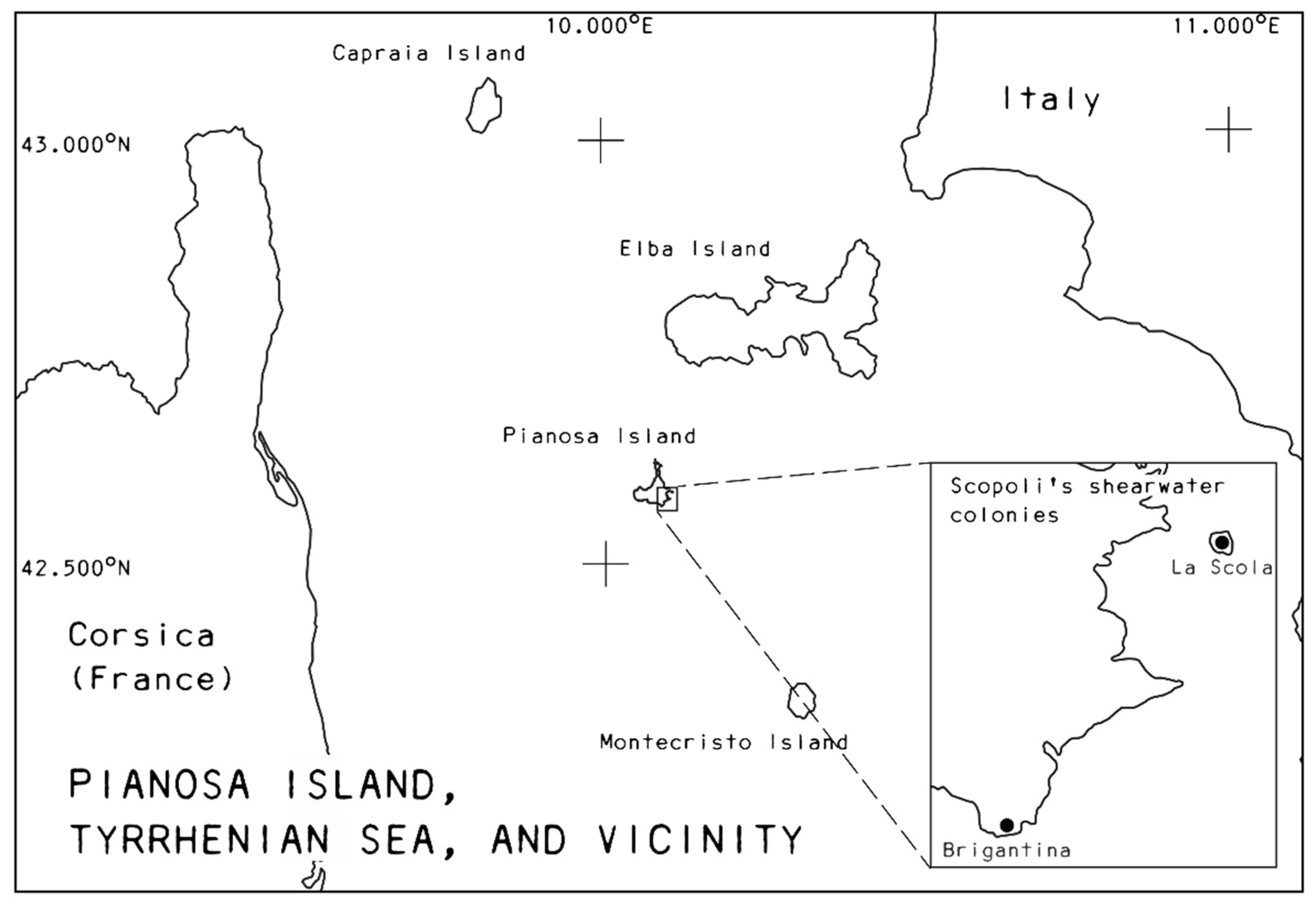
2.1. Study System and Data Collection
2.2. Statistical Analyses
- AS (apparent survival—Φ): the probability of surviving and not permanently emigrating from the colony between one breeding season and the next.
- NC (nest change probability—Ψ): the probability of changing the nest at the beginning of a new reproductive season.
- OLD (old nest): an individual breeding in its first nest.
- NEW (new nest): an individual breeding in a different nest from the first.
- BS (breeding success probability—Β): The species we studied lays only one egg per breeding season, so BS is the probability of reproducing successfully (i.e., with the chick fledging).
- Winner: An individual who has performed successful breeding in the previous season (its chick has fledged).
- Loser: an individual who has tried a breeding attempt in the previous season but failed.
- R (recapture probability—Ρ): the probability of being captured by the fieldworker during the reproductive season.
- RLE (reproductive life expectancy): The number of reproductive years after which a cohort’s death rate is 50%. It is the average reproductive lifespan of the sampled population.
3. Results
3.1. Factors Influencing Nest Change
3.2. Factors Influencing Breeding Success
3.3. Factors Influencing Survival
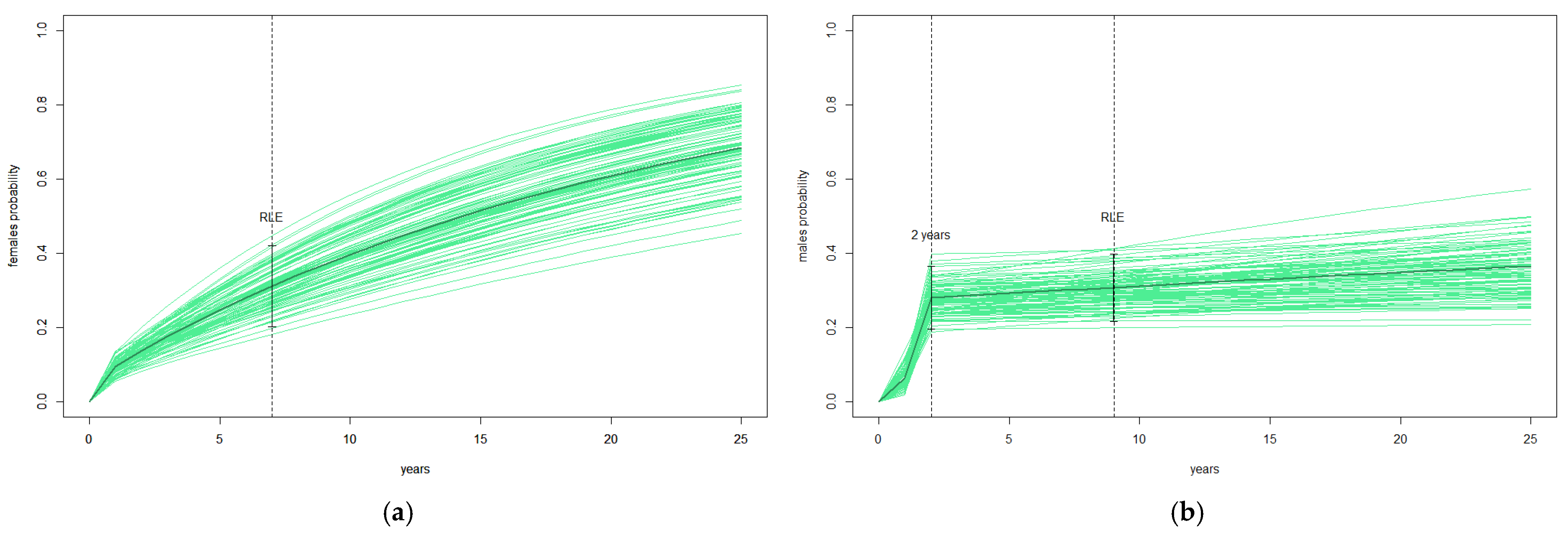
3.4. Nest Change and Reproductive Life Expectancy (RLE)
4. Discussion
4.1. Hypothesis 1: Breeding Failure Triggers Nest Change
4.2. Hypothesis 2: Nest Change Affects Breeding Outcome
4.3. Hypothesis 3: Nest Change Affects Survival
4.4. Effects within the Pairs
5. Conclusions and Future Developments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bried, J.; Jouventin, P. Site and Mate Choice in Seabirds: An evolutionary approach. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 263–306. [Google Scholar]
- Ramos, J.A.; Monteiro, L.R.; Sola, E.; Moniz, Z. Characteristics and competition for nest cavities in burrowing procellariiformes. Condor 1997, 99, 634–641. [Google Scholar] [CrossRef]
- Minias, P. Evolution of within-colony distribution patterns of birds in response to habitat structure. Behav. Ecol. Sociobiol. 2014, 68, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Lack, D.L. Ecological Adaptations for Breeding in Birds; Methuen and Company: London, UK, 1968. [Google Scholar]
- Wittenberger, J.F.; Tilson, R.L. The Evolution of Monogamy: Hypotheses and Evidence. Annu. Rev. Ecol. Syst. 1980, 11, 197–232. [Google Scholar] [CrossRef]
- Rowley, I. Re-mating in birds. In Mate Choice; Bateson, P., Ed.; Cambridge University Press: Cambridge, UK, 1983; pp. 331–360. [Google Scholar]
- Black, J.M. Partnerships in Birds: The Study of Monogamy; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Coulson, J.C. Colonial Breeding in Seabirds. In Biology of Marine Birds; Schreiber, E.A., Burger, J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 87–113. [Google Scholar]
- Hamilton, W.D. Geometry for the selfish herd. J. Theor. Biol. 1971, 31, 295–311. [Google Scholar] [CrossRef]
- Dubois, F.; Cézilly, F.; Pagel, M. Mate Fidelity and Coloniality in Waterbirds: A Comparative Analysis. Oecologia 1998, 116, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Warham, J. The Petrel: Their Ecology and Breeding Systems; Black, A., Ed.; Academic Press: London, UK, 1990. [Google Scholar]
- Parmelee, D.F.; Pietz, P.J. Philopatry, mate and nest-site fidelity in the brown skuas of Anvers Island, Antarctica. Condor 1987, 89, 916–919. [Google Scholar] [CrossRef]
- Kokko, H.; Harris, M.P.; Wanless, S. Competition for breeding sites and site-dependent population regulation in a highly colonial seabird, the common guillemot Uria aalge. J. Anim. Ecol. 2004, 73, 367–376. [Google Scholar] [CrossRef]
- Nelson, J.B. Contrasts in breeding strategies between some tropical and temperate marine pelecaniformes. Stud. Avian Biol. 1983, 8, 95–114. [Google Scholar]
- Schjørring, S.; Gregersen, J.; Bregnballe, T. Sex difference in criteria determining fidelity towards breeding sites in the great cormorant. J. Anim. Ecol. 2000, 69, 214–223. [Google Scholar] [CrossRef]
- Mougin, J.-L.; Despin, B.; Jouanin, C.; Roux, F. La fidelité au partenaire et au nid chez le Puffin cendre Calonectris diomedea borealis de l’ile Selvagem grande. Le Gerfault 1987, 77, 353–369. [Google Scholar]
- Mougin, J.-L. La fidelite au partenaire et au nid chez le Petrel de Bulwer Bulweria bulweri de l’Ile Selvagem Grande (30°09′ N, 15°52′ W). L’Oiseau Rev. Fr. d’Ornithologie 1990, 60, 224–232. [Google Scholar]
- Monteiro, L.R.; Ramos, J.A.; Furness, R.W.; Del Nevo, A.J. Movements, morphology, breeding, molt, diet and feeding of seabirdsin the Azores. Colonial Waterbirds 1996, 19, 82–97. [Google Scholar] [CrossRef]
- Patrick, S.C.; Weimerskirch, H. Reproductive success is driven by local site fidelity despite stronger specialisation by individuals for large-scale habitat preference. J. Anim. Ecol. 2017, 86, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ollason, J.C.; Dunnet, G.M. Age, Experience and Other Factors Affecting the Breeding Success of the Fulmar, Fulmarus glacialis, in Orkney. J. Anim. Ecol. 1978, 47, 961–976. [Google Scholar] [CrossRef]
- Greenwood, P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980, 28, 1140–1162. [Google Scholar] [CrossRef]
- Włodarczyk, R.; Wieloch, M.; Czyz, S.; Dolata, P.T.; Minias, P. Natal and breeding dispersal in Mute Swans Cygnus olor: Influence of sex, mate switching and reproductive success. Acta Ornithol. 2013, 48, 237–244. [Google Scholar] [CrossRef]
- Ens, B.J.; Safriel, U.N.; Harris, M.P. Divorce in the long-lived and monogamous oystercather, haematopus ostralegus incompatibility or choosing the better option? Anim. Behav. 1993, 45, 1199–1217. [Google Scholar] [CrossRef]
- Zack, S.; Stutchbury, B.J. Delayed Breeding in Avian Social Systems: The Role of Territory Quality and “Floater” Tactics. Behaviour 1992, 123, 194–219. [Google Scholar] [CrossRef]
- Switzer, P.V. Site fidelity in predictable and unpredictable habitats. Evol. Ecol. 1993, 7, 533–555. [Google Scholar] [CrossRef]
- Johannesen, E.; Perriman, L.; Steen, H. The effect of breeding success on nest and colony fidelity in the Little Penguin (Eudyptula minor) in Otago, New Zealand. Emu 2002, 102, 241–247. [Google Scholar] [CrossRef]
- Rogers, T.; Knight, C. Burrow and mate fidelity in the Little Penguin Eudyptula minor at Lion Island, New South Wales, Australia. IBIS 2006, 148, 801–806. [Google Scholar] [CrossRef]
- Naves, L.C.; Monnat, J.Y.; Cam, E. Breeding performance, mate fidelity, and nest site fidelity in a long-lived seabird: Behaving against the current? OIKOS 2006, 115, 263–276. [Google Scholar] [CrossRef]
- Mariné, M.; Cadiou, B. Breeding success, nest site fidelity and mate fidelity in the European Storm-petrel Hydrobates pelagicus. Seabird 2019, 32, 46–58. [Google Scholar]
- Robert, A.; Paiva, V.H.; Bolton, M.; Jiguet, F.; Bried, J. Nest fidelity is driven by multi-scale information in a long-lived seabird. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141692. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, K.; Dromzée, S.; Vidal, E. Relationships between nest-cavity and mate selection, reproductive performance and fidelity in the mediterranean endemic yelkouan shearwater puffinus yelkouan. Acta Ornithol. 2014, 49, 9–22. [Google Scholar] [CrossRef]
- Bradley, J.S.; Wooller, R.D.; Skira, I.J.; Serventy, D.L. The Influence of Mate Retention and Divorce Upon Reproductive Success in Short-Tailed Shearwaters Puffinus tenuirostris. J. Anim. Ecol. 1990, 59, 487–496. [Google Scholar] [CrossRef]
- Thibault, J.C. Nest-site tenacity and mate fidelity in relation to breeding success in cory’s shearwater Calonectris diomedea. Bird Study 1994, 41, 25–28. [Google Scholar] [CrossRef]
- Pyle, P.; Sydeman, W.J.; Hester, M. Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in a declining population of Cassin’s auklets. J. Anim. Ecol. 2001, 70, 1088–1097. [Google Scholar] [CrossRef]
- Greenwood, P.J.; Harvey, P.H. The natal and breeding dispersal of birds. Annu. Rev. Ecol. Syst. 1982, 13, 1–21. [Google Scholar] [CrossRef]
- Bried, J.; Pontier, D.; Jouventin, P. Mate fidelity in monogamous birds: A re-examination of the Procellariiformes. Anim. Behav. 2003, 65, 235–246. [Google Scholar] [CrossRef]
- Sacchi, M.; Santoro, S.; Culina, A.; Pollonara, E.; Cozzo, M.; Pezzo, F.; Baccetti, N. Sex-specific fitness consequences of mate change in Scopoli’s shearwaters (Calonectris diomedea). Anim. Behav. 2023, in press.
- Sangster, G.; Collinson, J.M.; Crochet, P.A.; Knox, A.G.; Parkin, D.T.; Votier, S.C. Taxonomic recommendations for British birds: Eighth report. IBIS 2012, 154, 874–883. [Google Scholar] [CrossRef]
- González-Solís, J.; Croxall, J.P.; Oro, D.; Ruiz, X. Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front. Ecol. Environ. 2007, 5, 297–301. [Google Scholar] [CrossRef]
- Navarro, J. Foraging segregation between two closely related shearwaters breeding in sympatry. Biol. Lett. 2009, 5, 545–548. [Google Scholar] [CrossRef]
- De Felipe, F.; Reyes-González, J.M.; Militão, T.; Neves, V.C.; Bried, J.; Oro, D.; Ramos, R.; González-Solís, J. Does sexual segregation occur during the nonbreeding period? A comparative analysis in spatial and feeding ecology of three Calonectris shearwaters. Ecol. Evol. 2019, 9, 10145–10162. [Google Scholar] [CrossRef]
- Morera-Pujol, V.; Catry, P.; Magalhães, M.; Péron, C.; Reyes-González, J.M.; Granadeiro, J.P.; Militão, T.; Dias, M.P.; Oro, D.; Dell’Omo, G.; et al. Methods to detect spatial biases in tracking studies caused by differential representativeness of individuals, populations and time. Divers. Distrib. 2023, 29, 19–38. [Google Scholar] [CrossRef]
- Mougin, J.-L. The Influence of Colony Characteristics on Some Breeding Parameters in the Cory’s Shearwater (Calonectris Diomedea Borealis). Ardeola 1999, 46, 45–51. [Google Scholar]
- Bried, J.; Dubois, M.-P.; Jarne, P.; Jouventin, P.; Santos, R.S. Does Competition for Nests Affect Genetic Monogamy in Cory’s Shearwater Calonectris Diomedea? J. Avian Biol. 2010, 41, 407–418. [Google Scholar] [CrossRef]
- Cachia Zammit, R.; Borg, J.J. Notes on the Breeding Biology of the Cory’s Shearwater in the Maltese Islands. Il-Merril 1987, 24, 1–9. [Google Scholar]
- Borg, J.J.; Sultana, J. Aspects of the Breeding Biology of Cory’s Shearwater (Calonectris Diomedea) in the Maltese Island. Die Vogelwarte 2000, 40, 258–264. [Google Scholar]
- Ristow, D.; Feldmann, F.; Scharlau, W.; Wink, M. Population Structure, Philopatry and Mortality of Cory’s Shearwater Calonectris d. Diomedea. Die Vogelwelt 1990, 111, 172–181. [Google Scholar]
- Swatschek, I.; Ristow, D.; Wink, M. Mate Fidelity and Parentage in Cory’s Shearwater Calonectris Diomedea-Field Studies and DNA Fingerprinting. Mol. Ecol. 1994, 3, 259–262. [Google Scholar] [CrossRef]
- Sanz-Aguilar, A.; Tavecchia, G.; Genovart, M.; Igual, J.M.; Oro, D.; Rouan, L.; Pradel, R. Studying the Reproductive Skipping Behavior in Long-Lived Birds by Adding Nest Inspection to Individual-Based Data. Ecol. Appl. 2011, 21, 555–564. [Google Scholar] [CrossRef]
- Gotti, C.; De Pascalis, F.; Zenatello, M.; Cecere, J.G.; Baccetti, N. Piano d’azione transfrontaliero per la conservazione della Berta maggiore e della Berta minore nel bacino ligure e alto-tirrenico. In Relazione Finale Convenzione ISPRA–PNAT; Progetto GIREPAM: Ozzano dell’Emilia (BO), Italy, 2020; 37p. [Google Scholar]
- Capizzi, D.; Baccetti, N.; Sposimo, P. Fifteen Years of Rat Eradication on Italian Islands. In Problematic Wildlife: A Cross-Disciplinary Approach; Springer: Cham, Switzerland, 2016; pp. 205–227. ISBN 9783319222462. [Google Scholar]
- Ristow, D.; Wink, M. Sexual dimorphism of Cory’s shearwater. Il-Merril 1980, 21, 9–12. [Google Scholar]
- Bretagnolle, V.; Lequette, B. Structural Variation in the Call of the Cory’s Shearwater (Calonectris diomedea, Aves, Procellariidae). Ethology 1990, 85, 313–323. [Google Scholar] [CrossRef]
- Curé, C.; Mathevon, N.; Aubin, T. Mate vocal recognition in the Scopoli’s shearwater Calonectris diomedea: Do females and males share the same acoustic code? Behav. Process. 2016, 128, 96–102. [Google Scholar] [CrossRef]
- Lebreton, J.D.; Pradel, R. Multistate recapture models: Modelling incomplete individual histories. J. Appl. Stat. 2002, 29, 353–369. [Google Scholar] [CrossRef]
- Pradel, R. Multievent: An extension of multistate capture-recapture models to uncertain states. Biometrics 2005, 61, 442–447. [Google Scholar] [CrossRef]
- Lebreton, J.D.; Nichols, J.D.; Barker, R.J.; Pradel, R.; Spendelow, J.A. Chapter 3 Modeling Individual Animal Histories with Multistate Capture-Recapture Models. In Advances in Ecological Research; Caswell, H., Ed.; Academic Press: Burlington, NJ, USA, 2009; Volume 41, pp. 87–173. ISBN 9780123749253. [Google Scholar]
- Choquet, R.; Lebreton, J.-D.; Gimenez, O.; Reboulet, A.-M.; Pradel, R. U-CARE: Utilities for performing goodness of fit tests and manipulating CApture-REcapture data. Ecography 2009, 32, 1071–1074. [Google Scholar] [CrossRef]
- Choquet, R.; Reboulet, A.; Lebreton, J.-D.; Gimenez, O.; Pradel, R. U-CARE 3.3 User’s Manual; CEFE: Montpellier, France, 2020. [Google Scholar]
- Choquet, R.; Nogue, E. E-SURGE 1.8 User’s Manual; UMR 5175; CEFE: Montpellier, France, 2011. [Google Scholar]
- Woller, R.; Bradley, S. Monogamy in a long-lived seabird: The Short-tailed Shearwater. In Partnerships in Birds: The Study of Monogamy; Black, J.M., Ed.; The Wildfowl and Wetlands Trust: Slimbridge, Glocestershire, UK, 1996; p. 223. [Google Scholar]
- Mougin, J.-L. Age-dependant adult survival in the Corys Shearwater (Calonectris diomedea). Avocetta 2002, 26, 7–9. [Google Scholar]
- Forslund, P.; Pärt, T. Age and reproduction in birds—Hypotheses and tests. Trends Ecol. Evol. 1995, 10, 374–378. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. A practical information-theoretic approach. In Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002; pp. 70–71. [Google Scholar]
- Dugger, K.M.; Ballard, G.; Ainley, D.G.; Barton, K.J. Effects of flipper bands on foraging behavior and survival of Adélie Penguins (Pygoscelis adeliae). Auk 2006, 123, 858–869. [Google Scholar] [CrossRef]
- Morin, D.J.; Yackulic, C.B.; Diffendorfer, J.E.; Lesmeister, D.B.; Nielsen, C.K.; Reid, J.; Schauber, E.M. Is your ad hoc model selection strategy affecting your multimodel inference? Ecosphere 2020, 11, e02997. [Google Scholar] [CrossRef]
- Seber, G.A.F. The Estimation of Animal Abundance and Related Parameters; Blackburn Press: Caldweel, NJ, USA, 1982; Volume 8. [Google Scholar]
- Gimenez, O.; Choquet, R.; Lamor, L.; Scofield, P.; Fletcher, D.; Lebreton, J.D.; Pradel, R. Efficient profile-likehood confidence intervals for capture-recapture models. J. Agric. Biol. Environ. Stat. 2005, 10, 184–196. [Google Scholar] [CrossRef]
- Tavecchia, G.; Viedma, C.; Martínez-Abraín, A.; Bartolomé, M.A.; Gómez, J.A.; Oro, D. Maximizing re-introduction success: Assessing the immediate cost of release in a threatened waterfowl. Biol. Conserv. 2009, 142, 3005–3012. [Google Scholar] [CrossRef]
- Santoro, S.; Green, A.J.; Figuerola, J. Immigration enhances fast growth of a newly established source Population. Ecology 2016, 97, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Kokko, H. Sex-biased dispersal: A review of the theory. Biol. Rev. 2019, 94, 721–736. [Google Scholar] [CrossRef]
- Vergara, P.; Aguirre, J.I.; Fargallo, J.A.; Dávila, J.A. Nest-site fidelity and breeding success in White Stork Ciconia ciconia. IBIS 2006, 148, 672–677. [Google Scholar] [CrossRef]
- Thibault, J.C.; Bretagnolle, V.; Rabouam, C. Calonectris diomedea Cory’s shearwater. Birds West. Palearct. Updat. 1997, 1, 75–98. [Google Scholar]
- Hipfner, J.M.; McFarlane-Tranquilla, L.A.; Addison, B. Experimental evidence that both timing and parental quality affect breeding success in a zooplanktivorous seabird. Auk 2010, 127, 195–203. [Google Scholar] [CrossRef]
- Chastel, O.; Weimerskirch, H.; Jouventin, P. Influence of body condition on reproductive decision and reproductive success in the Blue Petrel. Auk 1995, 112, 964–972. [Google Scholar] [CrossRef]
- Lescroël, A.; Dugger, K.M.; Ballard, G.; Ainley, D.G. Effects of individual quality, reproductive success and environmental variability on survival of a long-lived seabird. J. Anim. Ecol. 2009, 78, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.A.; Huntington, C.E.; Grubb, T.C. Age-specific reproductive success: Evidence for the selection hypothesis. Evolution 2004, 58, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, P.; Gimenez, O.; Doligez, B.; Pradel, R.; Garant, D.; Pelletier, F.; Bélisle, M. Assessment of individual and conspecific reproductive success as determinants of breeding dispersal of female tree swallows: A capture–recapture approach. Ecol. Evol. 2017, 7, 7334–7346. [Google Scholar] [CrossRef]
- Doligez, B.; Danchin, E.; Clobert, J.; Gustafsson, L. The use of conspecific reproductive success for breeding habitat selection in a non-colonial, hole-nesting species, the collared flycatcher. J. Anim. Ecol. 1999, 68, 1193–1206. [Google Scholar] [CrossRef]
- Oro, D.; Cam, E.; Pradel, R.; Martínez-Abraín, A. Influence of food availability on demography and local population dynamics in a long-lived seabird. Proc. R. Soc. B Biol. Sci. 2004, 271, 387–396. [Google Scholar] [CrossRef]
- Danchin, E.; Boulinier, T.; Massot, M. Conspecific Reproductive Success and Breeding Habitat Selection: Implications for the Study of Coloniality. Ecology 1998, 79, 2415–2428. [Google Scholar] [CrossRef]
- Brooke, M. The Manx Shearwater; Black, A., Ed.; A&C Black: London, UK, 2013. [Google Scholar]
- Barbraud, C.; Delord, K. Selection against immigrants in wild seabird populations. Ecol. Lett. 2021, 24, 84–93. [Google Scholar] [CrossRef]
- Jenouvrier, S.; Tavecchia, G.; Thibault, J.C.; Choquet, R.; Bretagnolle, V. Recruitment processes in long-lived species with delayed maturity: Estimating key demographic parameters. OIKOS 2008, 117, 620–628. [Google Scholar] [CrossRef]


| Species | Location | Period | Nest Fidelity | Nest Change | N | Sex | Reference |
|---|---|---|---|---|---|---|---|
| Cory’s shearwater | Selvagem grande (Madeira, Portugal) | 1981–1995 | 0.819 | 0.181 | 7861 | Males | [43] |
| 0.805 | 0.195 | 7861 | Females | ||||
| 0.813 | 0.187 | 15,722 | Combined | ||||
| Vila islet, (Azores archipelago, Portugal) | 2002–2008 | 0.890 | 625 | Combined | [44] | ||
| Scopoli’s shearwater | Lavezzi (Corsica, France) | 1978–1991 | 0.843 * | 74 | Males | [33] | |
| 0.757 * | 75 | Females | |||||
| 0.812 * | 149 | Combined | |||||
| Malta, Gozo, and Filfla (Maltese archipelago, Malta) | 1983–1985 | 0.963 | 0.037 | 22 | Males | [45] | |
| 0.981 | 0.019 | 22 | Females | ||||
| 0.971 | 0.029 | 22 | Combined | ||||
| 1983–1998 | 0.834 * | 194 | Males | [46] | |||
| 0.793 * | 154 | Females | |||||
| 0.818 * | 348 | Combined | |||||
| Southern Aegean Sea (Greece) | 1985–1989 | 0.960 | 0.040 | 33 | Males | [47] | |
| 0.924 | 0.076 | 37 | Females | ||||
| 0.946 | 0.054 | 70 | Combined | ||||
| Crete (Greece) | 1989–1993 | 0.990 | 0.010 | 1038 | Combined | [48] | |
| Pantaleu Island (Balearic Archipelago, Spain) | 2001–2008 | 0.040 ** (0.030–0.060) | ~200 | Combined | [49] |
| Initial State—Π | ||||
|---|---|---|---|---|
| OLD–loser | OLD–winner | NEW–loser | NEW–winner | |
| 1 − π | π | 0 | 0 | |
| Apparent Survival (AS)—Φ | ||||||
|---|---|---|---|---|---|---|
| t + 1 − q | OLD–loser | OLD–winner | NEW–loser | NEW–winner | Dead | |
| t | ||||||
| OLD–loser | φOL | 0 | 0 | 0 | 1 − φOL | |
| OLD–winner | 0 | φOW | 0 | 0 | 1 − φOW | |
| NEW–loser | 0 | 0 | φNL | 0 | 1 − φNL | |
| NEW–winner | 0 | 0 | 0 | φNW | 1 − φNW | |
| Dead | 0 | 0 | 0 | 0 | 1 | |
| Nest Change Probability (NC)—Ψ | ||||||
|---|---|---|---|---|---|---|
| t + 1 | OLD–loser | OLD–winner | NEW–loser | NEW–winner | Dead | |
| t + 1 − q | ||||||
| OLD–loser | 1 − ψ(OL→NL) | 0 | ψ(OL→NL) | 0 | 0 | |
| OLD–winner | 0 | 1 − ψ(OW→NW) | 0 | ψ(OW→NW) | 0 | |
| NEW–loser | 0 | 0 | 1 | 0 | 0 | |
| NEW–winner | 0 | 0 | 0 | 1 | 0 | |
| Dead | 0 | 0 | 0 | 0 | 1 | |
| Breeding Success Probability (BS)—Β | ||||||
|---|---|---|---|---|---|---|
| t + 1 | OLD–loser | OLD–winner | NEW–loser | NEW–winner | Dead | |
| t + 1 | ||||||
| OLD–loser | 1 − βOL | βOL | 0 | 0 | 0 | |
| OLD–winner | 1 − βOW | βOW | 0 | 0 | 0 | |
| NEW–loser | 0 | 0 | 1 − βNL | βNL | 0 | |
| NEW–winner | 0 | 0 | 1 − βNW | βNW | 0 | |
| Dead | 0 | 0 | 0 | 0 | 1 | |
| Recapture Probability (R)—Ρ | ||||||
|---|---|---|---|---|---|---|
| t + 1 | Not detected | Detected as OLD–loser | Detected as OLD–winner | Detected as NEW–loser | Detected as NEW–winner | |
| t + 1 | ||||||
| OLD–loser | 1 − ρOL | ρOL | 0 | 0 | 0 | |
| OLD–winner | 1 − ρOW | 0 | ρOW | 0 | 0 | |
| NEW–loser | 1 − ρNL | 0 | 0 | ρNL | 0 | |
| NEW–winner | 1 − ρNW | 0 | 0 | 0 | ρNW | |
| Dead | 1 | 0 | 0 | 0 | 0 | |
| Events | 0 | 1 | 2 | 3 | 4 | |
| Model Structure | Model Ranking | Parameter’s Ranking | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | ϕ | ψ | β | ρ | np | Dev | QAICc | ΔAICc | w_AICc | w_ϕ | w_ψ | w_β | w_ρ |
| F1 | f13 | f | f13 | t | 14 | 740.45 | 770.22 | 0.00 | 0.17 | 0.25 | 0.96 | 0.46 | 1.00 |
| F2 | a12 | f | f13 | t | 14 | 740.75 | 770.51 | 0.30 | 0.15 | 0.22 | |||
| F3 | a2 | f | f13 | t | 14 | 741.03 | 770.79 | 0.57 | 0.13 | 0.19 | |||
| F4 | i | f | f13 | t | 13 | 743.96 | 771.49 | 1.27 | 0.09 | 0.14 | |||
| F5 | f13 | f | i | t | 13 | 744.10 | 771.62 | 1.40 | 0.08 | 0.23 | |||
| F6 | f13 | f | a3 | t | 15 | 740.62 | 772.65 | 2.43 | 0.05 | 0.08 | |||
| F7 | f | f | f13 | t | 16 | 738.48 | 772.78 | 2.56 | 0.05 | 0.07 | |||
| F8 | f13 | f | f12 | t | 14 | 743.81 | 773.58 | 3.36 | 0.03 | 0.09 | |||
| F9 | f12 | f | f13 | t | 14 | 743.85 | 773.61 | 3.39 | 0.03 | 0.05 | |||
| F10 | f13 | f | a12 | t | 14 | 744.02 | 773.79 | 3.57 | 0.03 | 0.08 | |||
| Males | ϕ | ψ | β | ρ | np | Dev | QAICc | ΔAICc | w_AICc | w_ϕ | w_ψ | w_β | w_ρ |
| M1 | i | f + a3 | f | t | 16 | 829.00 | 863.23 | 0.00 | 0.20 | 0.35 | 0.67 | 0.37 | 1.00 |
| M2 | i | f + a3 | i | t | 14 | 834.64 | 864.35 | 1.12 | 0.11 | 0.21 | |||
| M3 | f13 | f + a3 | f | t | 17 | 828.13 | 864.65 | 1.43 | 0.10 | 0.17 | |||
| M4 | f12 | f + a3 | f | t | 17 | 828.36 | 864.88 | 1.65 | 0.09 | 0.15 | |||
| M5 | a12 | f + a3 | f | t | 17 | 828.77 | 865.29 | 2.06 | 0.07 | 0.12 | |||
| M6 | a2 | f + a3 | f | t | 17 | 828.98 | 865.50 | 2.28 | 0.06 | 0.11 | |||
| M7 | i | f + a3 | f + a12 | t | 17 | 828.99 | 865.51 | 2.29 | 0.06 | 0.12 | |||
| M8 | i | f + a3 | f | t | 15 | 834.02 | 865.98 | 2.75 | 0.05 | 0.17 | |||
| M9 | i | f + a3 | a2 | t | 15 | 834.08 | 866.04 | 2.82 | 0.05 | 0.09 | |||
| M10 | i | f + a3 | a12 | t | 15 | 834.52 | 866.48 | 3.25 | 0.04 | 0.07 | |||
| Sex | Age | Outcome of the Previous Reproduction | Nest Change Probability | |
|---|---|---|---|---|
| Females | Failed | 0.376 | 0.207–0.581 | |
| Successful | 0.000 | 0.000–0.037 | ||
| Males | After 1st repr. | Failed | 0.086 | 0.013–0.400 |
| After 1st repr. | Successful | 0.014 | 0.003–0.138 | |
| After 2nd repr. | Failed | 0.483 | 0.192–0.787 | |
| After 2nd repr. | Successful | 0.121 | 0.043–0.297 | |
| After 3rd+ repr. | Failed | 0.000 | 0.000–0.034 | |
| After 3rd+ repr. | Successful | 0.000 | 0.000–0.034 | |
| Sex | Nest | Outcome of the Previous Reproduction | Breeding Success Probability | |
|---|---|---|---|---|
| Females | Failed | 0.666 | 0.400–0.857 | |
| Successful | 0.889 | 0.825–0.932 | ||
| Males | First nest ever | Failed | 0.610 | 0.412–0.775 |
| First nest ever | Successful | 0.789 | 0.693–0.861 | |
| After nest change | Failed | 1.000 | 0.977–1.000 | |
| After nest change | Successful | 0.835 | 0.623–0.940 | |
| Sex | Model | Age | Outcome of the Previous Reproduction | Apparent Survival | |
|---|---|---|---|---|---|
| Females | f13 | Failed | 0.725 | 0.531–0.860 | |
| Successful | 0.897 | 0.834–0.939 | |||
| a12 | First or second repr. | 0.810 | 0.720–0.876 | ||
| Third+ repr. | 0.914 | 0.831–0.958 | |||
| Males | 0.895 | 0.851–0.927 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacchi, M.; Zenatello, M.; Pezzo, F.; Cozzo, M.; Pollonara, E.; Gotti, C.; De Faveri, A.; Baccetti, N. Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis. Diversity 2023, 15, 718. https://doi.org/10.3390/d15060718
Sacchi M, Zenatello M, Pezzo F, Cozzo M, Pollonara E, Gotti C, De Faveri A, Baccetti N. Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis. Diversity. 2023; 15(6):718. https://doi.org/10.3390/d15060718
Chicago/Turabian StyleSacchi, Massimo, Marco Zenatello, Francesco Pezzo, Mario Cozzo, Enrica Pollonara, Camilla Gotti, Adriano De Faveri, and Nicola Baccetti. 2023. "Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis" Diversity 15, no. 6: 718. https://doi.org/10.3390/d15060718
APA StyleSacchi, M., Zenatello, M., Pezzo, F., Cozzo, M., Pollonara, E., Gotti, C., De Faveri, A., & Baccetti, N. (2023). Nest Change and Individual Fitness in a Scopoli’s Shearwater Population: A Capture-Recapture Multistate Analysis. Diversity, 15(6), 718. https://doi.org/10.3390/d15060718







