Variation of Gene Expression in the Endemic Dinaric Karst Cave-Dwelling Bivalve Mollusk Congeria kusceri during the Summer Season
Abstract
1. Introduction
2. Materials and Methods
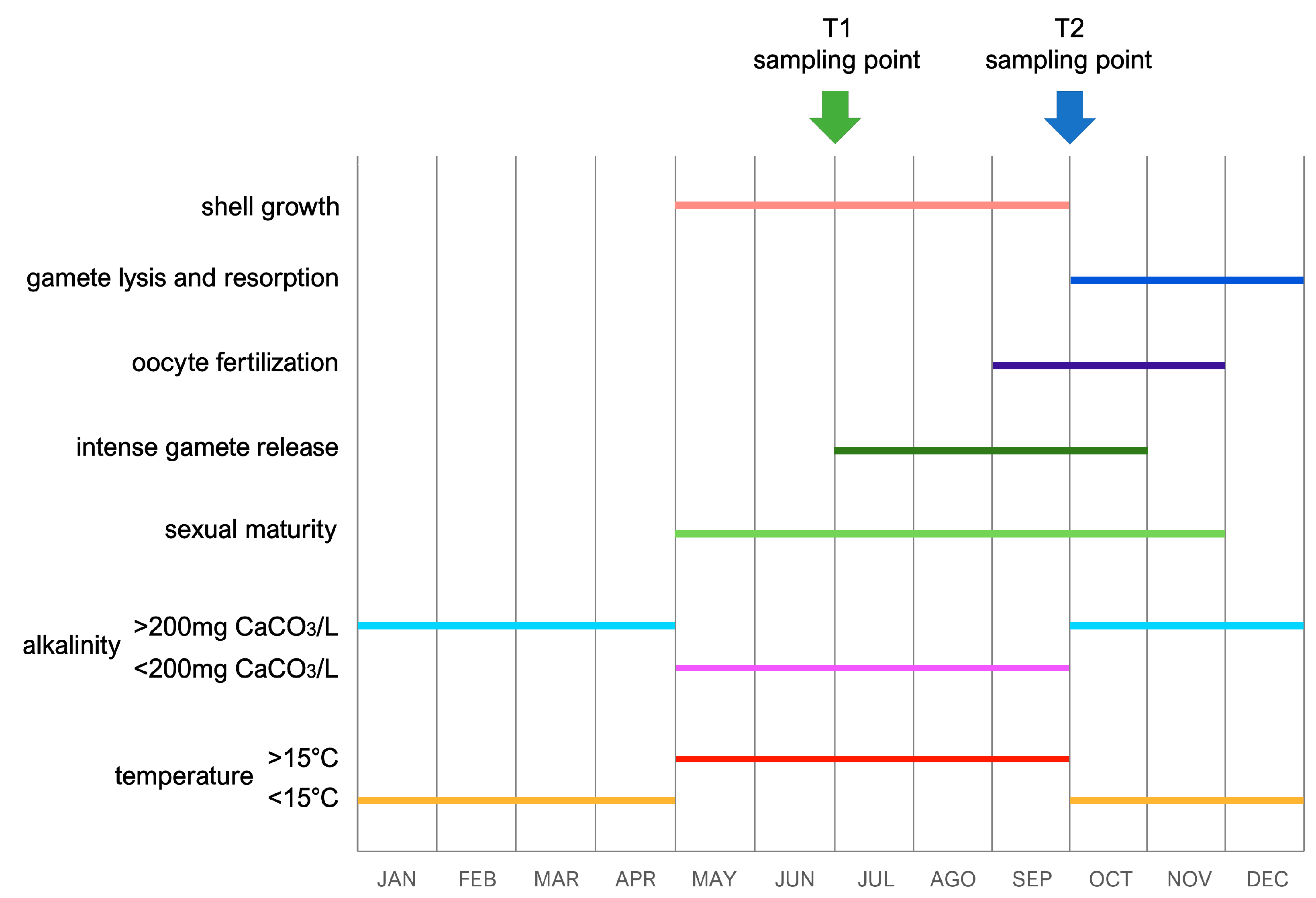
2.1. Sample Collection and RNA Extraction
2.2. Sequencing and Read Processing
2.3. De Novo Assembly, Quality Assessment, and Annotation of the Transcriptome
2.4. Differential Gene Expression Analysis
3. Results and Discussion
3.1. The Congeria kusceri Reference Transcriptome Assembly
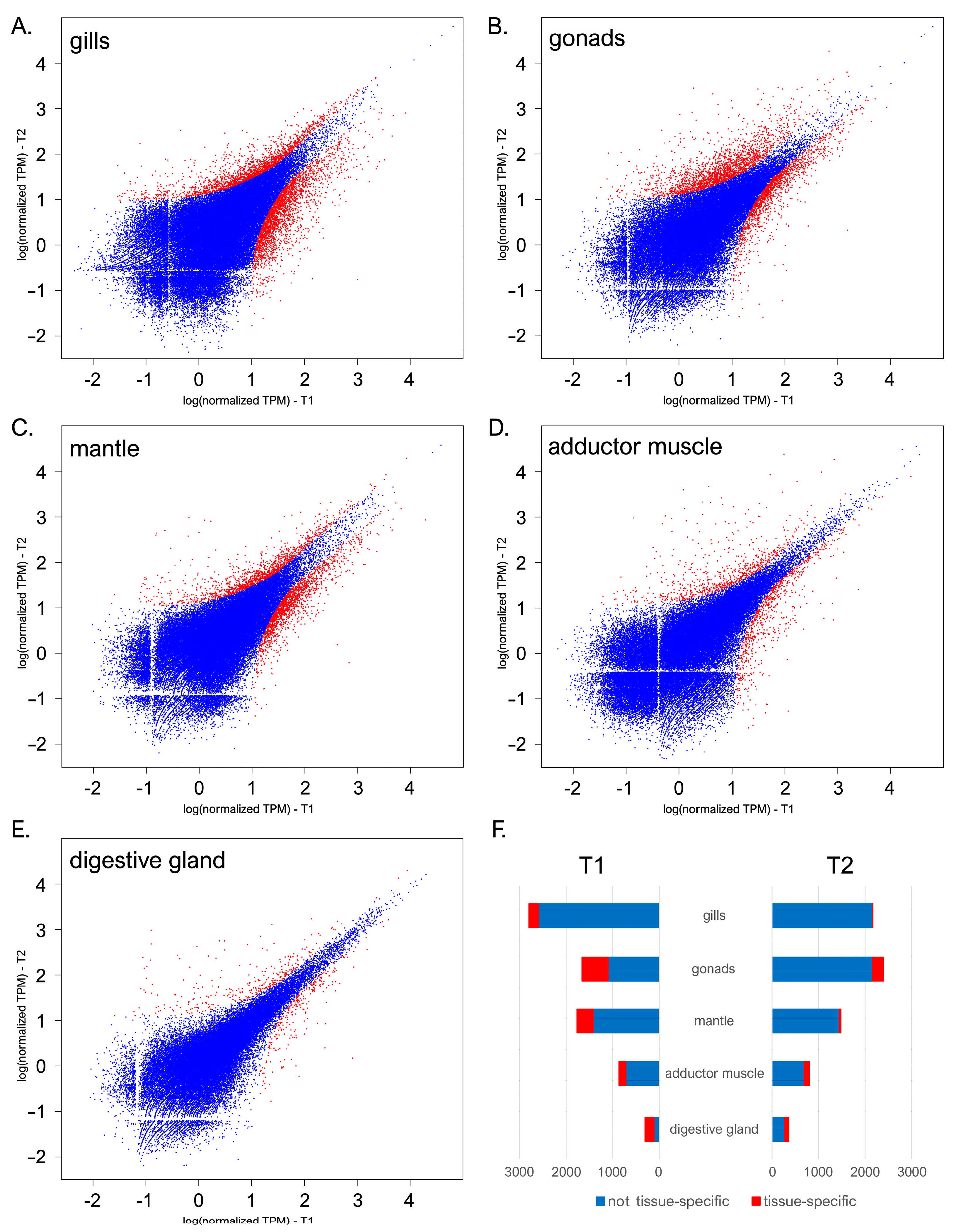
3.2. A General Overview of Gene Expression Changes Occurring from Early July to Late September
3.3. The Gonad Transcriptome Follows Opposing Trends of Expression Compared with Mantle and Gills
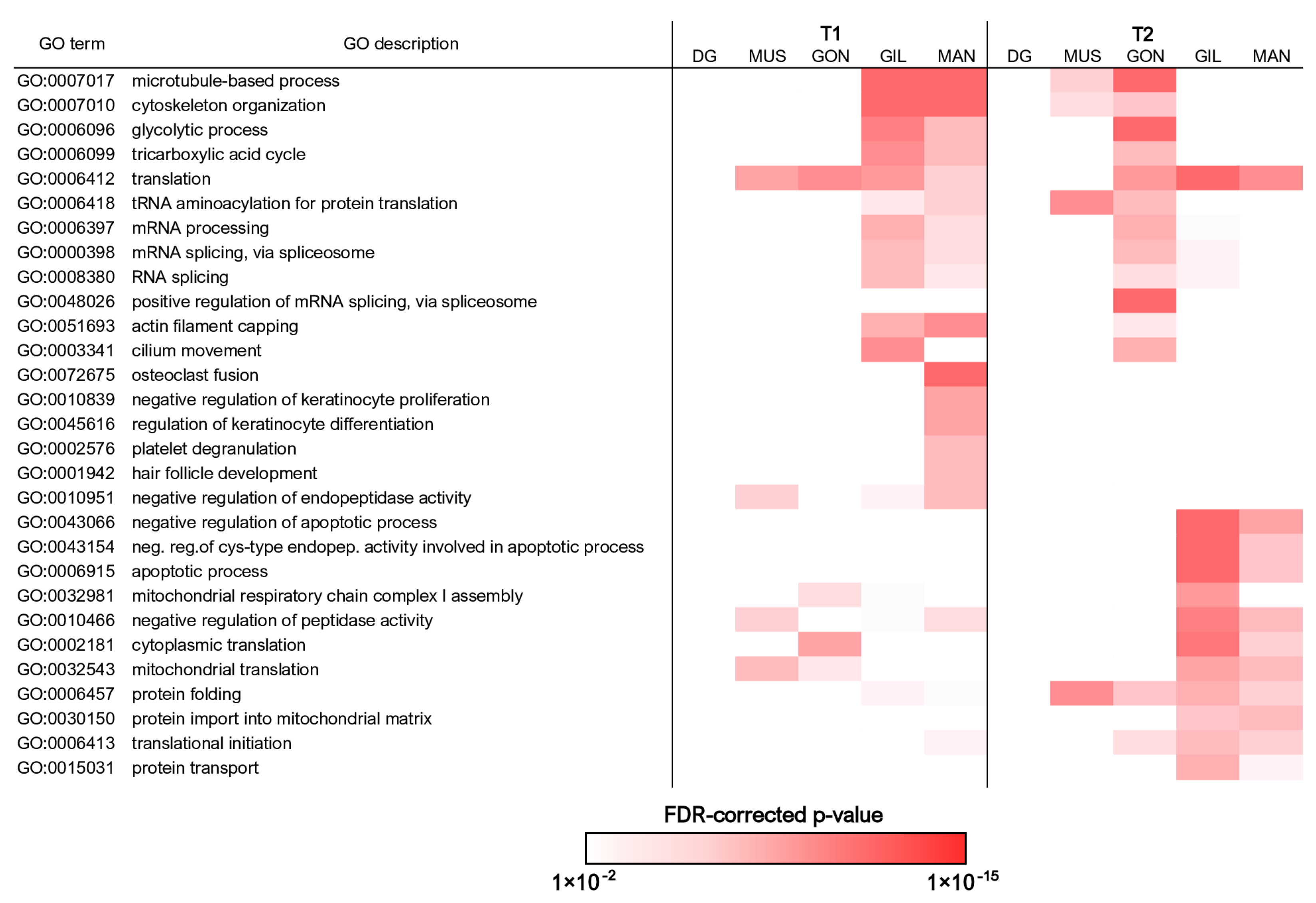
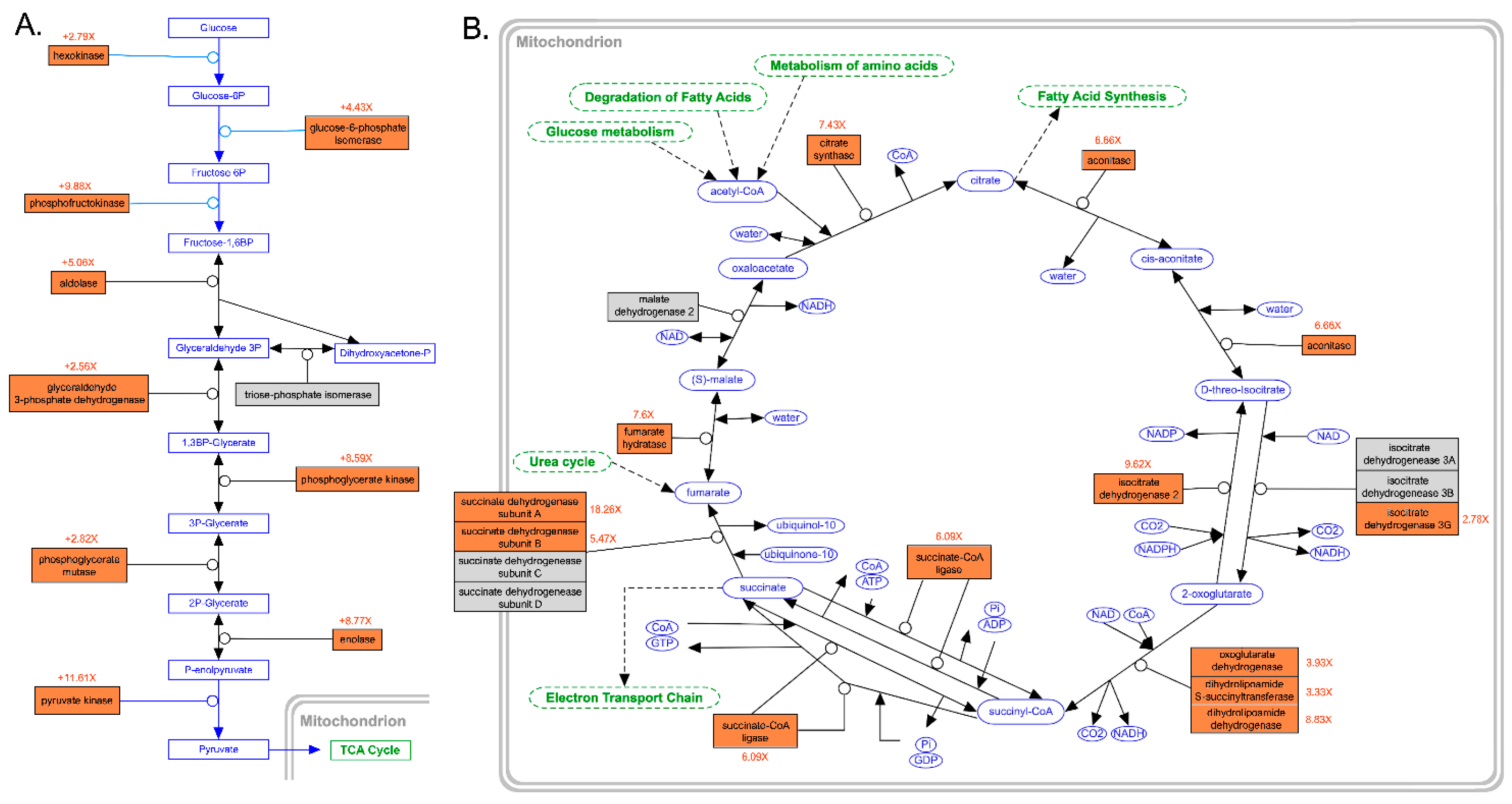
3.4. In-Depth Analysis of Differential Gene Expression in the Gills
3.5. In-Depth Analysis of Differential Gene Expression in the Gonads
3.6. In-Depth Analysis of Differential Gene Expression in the Mantle
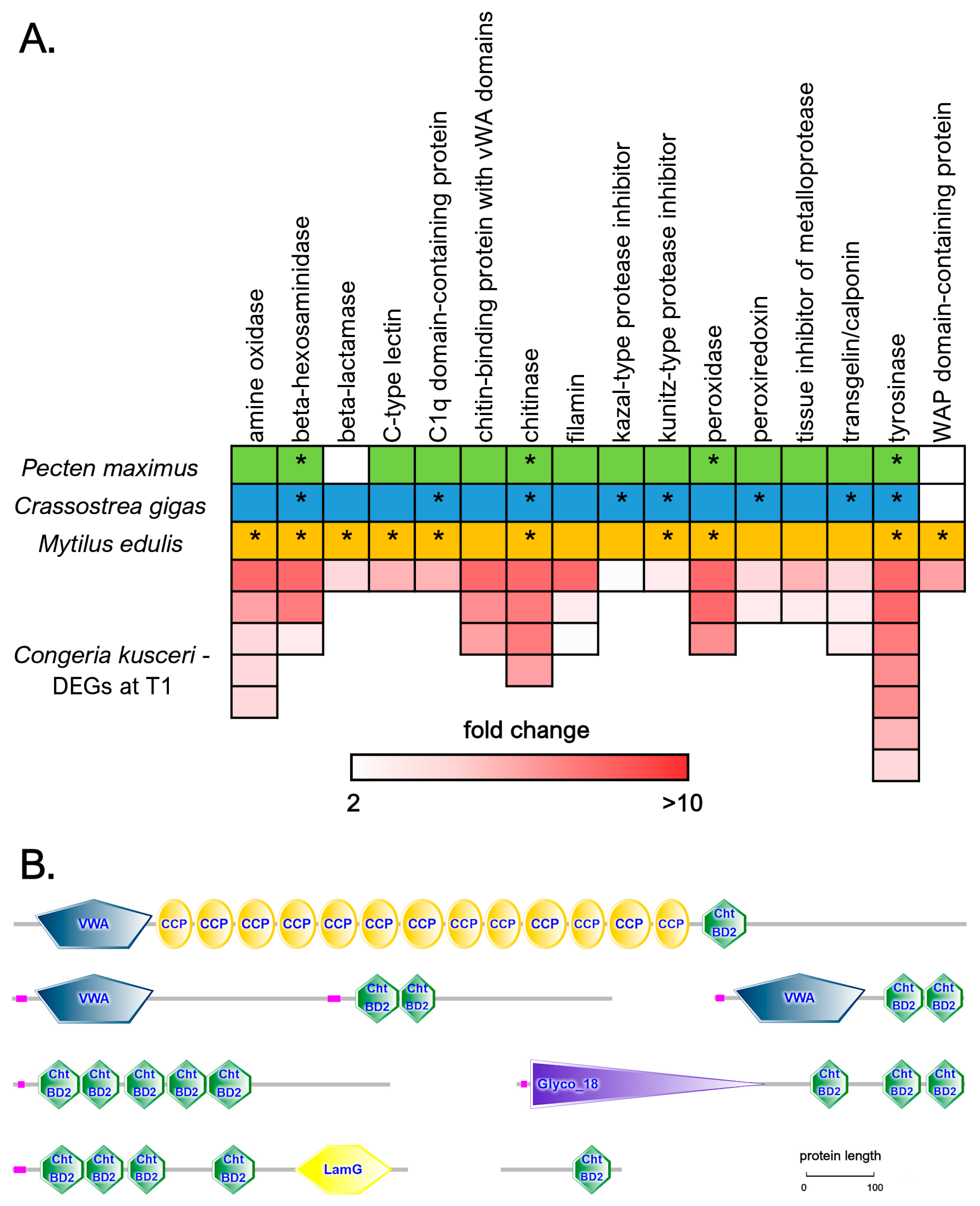
3.7. In-Depth Analysis of Differential Gene Expression in the Adductor Muscle
3.8. In-Depth Analysis of Differential Gene Expression in the Digestive Gland
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peck, L.S. Antarctic Marine Biodiversity: Adaptations, Environments and Responses to Change. In Oceanography and Marine Biology: An Annual Review; Hawkins, S.J., Evans, A.J., Dale, A.C., Firth, L.B., Smith, I.P., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Volume 56, pp. 105–236. ISBN 978-0-429-45445-5. [Google Scholar]
- Hourdez, S.; Jollivet, D. Metazoan Adaptation to Deep-Sea Hydrothermal Vents. In Life in Extreme Environments: Insights in Biological Capability; Huiskes, A.H.L., di Prisco, G., Edwards, H.G.M., Elster, J., Eds.; Ecological Reviews; Cambridge University Press: Cambridge, UK, 2020; pp. 42–67. ISBN 978-1-108-49856-2. [Google Scholar]
- Hüppop, K. Adaptation to Low Food. In Encyclopedia of Caves, 2nd ed.; White, W.B., Culver, D.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2012; pp. 1–9. [Google Scholar]
- Culver, D. Mollusks. In Encyclopedia of Caves; White, W.B., Culver, D.C., Eds.; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Rassam, H.; Moutaouakil, S.; Benaissa, H.; Albrecht, C.; Ghamizi, M. First Record of Pisidium Subtruncatum Malm, 1855 (Bivalvia, Sphaeriidae) in an African Cave. Subterr. Biol. 2020, 34, 99–108. [Google Scholar] [CrossRef]
- Turbanov, I.S.; Palatov, D.M.; Golovatch, S.I. The State of the Art of Biospeleology in Russia and Other Countries of the Former Soviet Union: A Review of the Cave (Endogean) Invertebrate Fauna. 1. Introduction—Crustacea. Entmol. Rev. 2016, 96, 926–963. [Google Scholar] [CrossRef]
- Simone, L.R.L.; Ferreira, R.L. Eupera Troglobia Sp. Nov.: The First Troglobitic Bivalve from the Americas (Mollusca, Bivalvia, Sphaeriidae). Subterr. Biol. 2022, 42, 165–184. [Google Scholar] [CrossRef]
- Bilandžija, H.; Morton, B.; Podnar, M.; Ćetković, H. Evolutionary History of Relict Congeria (Bivalvia: Dreissenidae): Unearthing the Subterranean Biodiversity of the Dinaric Karst. Front. Zool. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Stepien, C.A.; Morton, B.; Dabrowska, K.A.; Guarnera, R.A.; Radja, T.; Radja, B. Genetic Diversity and Evolutionary Relationships of the Troglodytic ‘Living Fossil’ Congeria Kusceri (Bivalvia: Dreissenidae). Mol. Ecol. 2001, 10, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Morton, B.; Velkovrh, F.; Sket, B. Biology and Anatomy of the ‘Living Fossil’ Congeria Kusceri (Bivalvia: Dreissenidae) from Subterranean Rivers and Caves in the Dinaric Karst of the Former Yugoslavia. J. Zool. 1998, 245, 147–174. [Google Scholar] [CrossRef]
- Puljas, S.; Peharda, M.; Morton, B.; Giljanović, N.Š.; Jurić, I. Growth and Longevity of the „Living Fossil” Congeria Kusceri (Bivalvia: Dreissenidae) from the Subterranean Dinaric Karst of Croatia. Mala 2014, 57, 353–364. [Google Scholar] [CrossRef]
- Morton, B.; Puljas, S. Life-History Strategy, with Ctenidial and Pallial Larval Brooding, of the Troglodytic ‘Living Fossil’ Congeria Kusceri (Bivalvia: Dreissenidae) from the Subterranean Dinaric Alpine Karst of Croatia. Biol. J. Linn. Soc. 2013, 108, 294–314. [Google Scholar] [CrossRef]
- Rađa, B.; Rađa, T. New Data about the Reproductive Cycle of Congeria Kusceri Bole, 1962 (Bivalves: Dreissenidae) from the Pit ‘Jama u Predolcu’ (Croatia). Ital. J. Zool. 2012, 79, 105–110. [Google Scholar] [CrossRef]
- Chichorro, F.; Urbano, F.; Teixeira, D.; Väre, H.; Pinto, T.; Brummitt, N.; He, X.; Hochkirch, A.; Hyvönen, J.; Kaila, L.; et al. Trait-Based Prediction of Extinction Risk across Terrestrial Taxa. Biol. Conserv. 2022, 274, 109738. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fišer, C.; Galassi, D.M.P.; Griebler, C.; Halse, S.; Humphreys, W.F.; et al. Scientists’ Warning on the Conservation of Subterranean Ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Vranješ, M.; Prskalo, M.; Džeba, T. Hidrologija i Hidrogeologija Sliva Neretve i Trebišnjice, Osvrt Na Izgradnju Dijela He Sustava-Gornji Horizonti. e-Zbonik Electron. Collect. Pap. Fac. Civ. Eng. 2013, 5, 1–23. [Google Scholar]
- Bilandžija, H.; Puljas, S.; Gerdol, M. Hidden from Our Sight, but Not from Our Impact: The Conservation Issues of Cave Bivalves. 2021. Available online: https://www.preprints.org/manuscript/202105.0023/v1 (accessed on 1 November 2022).
- Scapolatiello, A.; Rosani, U.; Manfrin, C.; Puljas, S.; Pallavicini, A.; Gerdol, M. Identification of Five Picorna-like Viruses Associated with the Endangered Cave-Dwelling Bivalve Congeria Kusceri (Bole, 1962). Invertebr. Surviv. J. 2022, 19, 28–36. [Google Scholar] [CrossRef]
- Bilandžija, H.; Jalžić, B. Dinaric Cave Clam, Congeria Kusceri Bole. In Red Book of Croatian Cave Dwelling Fauna; Ozimec, R., Zagreb, K.L., Eds.; Ministry of Culture, State Institute for Nature Protection: Zagreb, Croatia, 2009; pp. 67–68. [Google Scholar]
- Jovanović Glavaš, O.; Jalžić, B.; Bilandžija, H. Population Density, Habitat Dynamic and Aerial Survival of Relict Cave Bivalves from Genus Congeria in the Dinaric Karst. Int. J. Speleol. 2016, 46, 13–22. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotech. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Srivastava, A.; Malik, L.; Smith, T.; Sudbery, I.; Patro, R. Alevin Efficiently Estimates Accurate Gene Abundances from DscRNA-Seq Data. Genome Biol. 2019, 20, 65. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of MRNA Abundance Using RNA-Seq Data: RPKM Measure Is Inconsistent among Samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Gerdol, M.; Fujii, Y.; Hasan, I.; Koike, T.; Shimojo, S.; Spazzali, F.; Yamamoto, K.; Ozeki, Y.; Pallavicini, A.; Fujita, H. The Purplish Bifurcate Mussel Mytilisepta Virgata Gene Expression Atlas Reveals a Remarkable Tissue Functional Specialization. BMC Genom. 2017, 18, 590. [Google Scholar] [CrossRef]
- Greco, S.; D’agostino, E.; Manfrin, C.; Gaetano, A.; Furlanis, G.; Capanni, F.; Santovito, G.; Edomi, P.; Giulianini, P.; Gerdol, M. RNA-Sequencing Indicates High Hemocyanin Expression as a Key Strategy for Cold Adaptation in the Antarctic Amphipod Eusirus Cf. Giganteus Clade G3. Biocell 2021, 45, 1611–1619. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and Sensitive Protein Alignment Using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- UniProt: A Worldwide Hub of Protein Knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [CrossRef] [PubMed]
- Finn, R.D.; Mistry, J.; Tate, J.; Coggill, P.; Heger, A.; Pollington, J.E.; Gavin, O.L.; Gunasekaran, P.; Ceric, G.; Forslund, K.; et al. The Pfam Protein Families Database. Nucleic Acids Res. 2009, 38, D211–D222. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER Web Server: Interactive Sequence Similarity Searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing Genome Assembly and Annotation Completeness with Single-Copy Orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Kriventseva, E.V.; Kuznetsov, D.; Tegenfeldt, F.; Manni, M.; Dias, R.; Simão, F.A.; Zdobnov, E.M. OrthoDB V10: Sampling the Diversity of Animal, Plant, Fungal, Protist, Bacterial and Viral Genomes for Evolutionary and Functional Annotations of Orthologs. Nucleic Acids Res. 2019, 47, D807–D811. [Google Scholar] [CrossRef]
- Kal, A.J.; van Zonneveld, A.J.; Benes, V.; van den Berg, M.; Koerkamp, M.G.; Albermann, K.; Strack, N.; Ruijter, J.M.; Richter, A.; Dujon, B.; et al. Dynamics of Gene Expression Revealed by Comparison of Serial Analysis of Gene Expression Transcript Profiles from Yeast Grown on Two Different Carbon Sources. Mol. Biol. Cell 1999, 10, 1859–1872. [Google Scholar] [CrossRef]
- Falcon, S.; Gentleman, R. Hypergeometric Testing Used for Gene Set Enrichment Analysis. In Bioconductor Case Studies; Hahne, F., Huber, W., Gentleman, R., Falcon, S., Eds.; Use R! Springer: New York, NY, USA, 2008; pp. 207–220. ISBN 978-0-387-77240-0. [Google Scholar]
- Timmons, J.A.; Szkop, K.J.; Gallagher, I.J. Multiple Sources of Bias Confound Functional Enrichment Analysis of Global-Omics Data. Genome Biol. 2015, 16, 186. [Google Scholar] [CrossRef]
- Takeuchi, T.; Koyanagi, R.; Gyoja, F.; Kanda, M.; Hisata, K.; Fujie, M.; Goto, H.; Yamasaki, S.; Nagai, K.; Morino, Y.; et al. Bivalve-Specific Gene Expansion in the Pearl Oyster Genome: Implications of Adaptation to a Sessile Lifestyle. Zool. Lett. 2016, 2, 3. [Google Scholar] [CrossRef]
- Gerdol, M.; Moreira, R.; Cruz, F.; Gómez-Garrido, J.; Vlasova, A.; Rosani, U.; Venier, P.; Naranjo-Ortiz, M.A.; Murgarella, M.; Greco, S.; et al. Massive Gene Presence-Absence Variation Shapes an Open Pan-Genome in the Mediterranean Mussel. Genome Biol. 2020, 21, 275. [Google Scholar] [CrossRef]
- Schnoes, A.M.; Ream, D.C.; Thorman, A.W.; Babbitt, P.C.; Friedberg, I. Biases in the Experimental Annotations of Protein Function and Their Effect on Our Understanding of Protein Function Space. PLoS Comput. Biol. 2013, 9, e1003063. [Google Scholar] [CrossRef] [PubMed]
- Calcino, A.D.; de Oliveira, A.L.; Simakov, O.; Schwaha, T.; Zieger, E.; Wollesen, T.; Wanninger, A. The Quagga Mussel Genome and the Evolution of Freshwater Tolerance. DNA Res. 2019, 26, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, P.; Moreira, R.; Novoa, B.; Figueras, A. Differential Expression of Long Non-Coding RNA (LncRNA) in Mediterranean Mussel (Mytilus Galloprovincialis) Hemocytes under Immune Stimuli. Genes 2021, 12, 1393. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Li, Q.; Yu, H.; Kong, L.; Du, S. Transcriptional Profiling of Long Non-Coding RNAs in Mantle of Crassostrea Gigas and Their Association with Shell Pigmentation. Sci. Rep. 2018, 8, 1436. [Google Scholar] [CrossRef]
- Moreira, R.; Pereiro, P.; Canchaya, C.; Posada, D.; Figueras, A.; Novoa, B. RNA-Seq in Mytilus Galloprovincialis: Comparative Transcriptomics and Expression Profiles among Different Tissues. BMC Genom. 2015, 16, 728. [Google Scholar] [CrossRef]
- Verween, A.; Vincx, M.; Degraer, S. Growth Patterns of Mytilopsis Leucophaeata, an Invasive Biofouling Bivalve in Europe. Biofouling 2006, 22, 221–231. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Burlakova, L.E.; Padilla, D.K. Growth Rate and Longevity of Dreissena Polymorpha (PALLAS): A Review and Recommendations for Future Study. Shre 2006, 25, 23–32. [Google Scholar] [CrossRef]
- Zagoory, O.; Braiman, A.; Priel, Z. The Mechanism of Ciliary Stimulation by Acetylcholine. J. Gen. Physiol. 2002, 119, 329–339. [Google Scholar] [CrossRef]
- Pesin, J.A.; Orr-Weaver, T.L. Regulation of APC/C Activators in Mitosis and Meiosis. Annu. Rev. Cell Dev. Biol. 2008, 24, 475–499. [Google Scholar] [CrossRef]
- Jan, S.Z.; Jongejan, A.; Korver, C.M.; van Daalen, S.K.M.; van Pelt, A.M.M.; Repping, S.; Hamer, G. Distinct Prophase Arrest Mechanisms in Human Male Meiosis. Development 2018, 145, dev160614. [Google Scholar] [CrossRef]
- Kubiak, J.Z.; Weber, M.; de Pennart, H.; Winston, N.J.; Maro, B. The Metaphase II Arrest in Mouse Oocytes Is Controlled through Microtubule-Dependent Destruction of Cyclin B in the Presence of CSF. EMBO J. 1993, 12, 3773–3778. [Google Scholar] [CrossRef]
- Yang, X.; Song, M.; Wang, Y.; Tan, T.; Tian, Z.; Zhai, B.; Yang, X.; Tan, Y.; Cao, Y.; Dai, S.; et al. The Ubiquitin-Proteasome System Regulates Meiotic Chromosome Organization. Proc. Natl. Acad. Sci. USA 2022, 119, e2106902119. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Loktev, A.V.; Ban, K.H.; Jackson, P.K. Plk1 Regulates Activation of the Anaphase Promoting Complex by Phosphorylating and Triggering SCFbetaTrCP-Dependent Destruction of the APC Inhibitor Emi1. Mol. Biol. Cell. 2004, 15, 5623–5634. [Google Scholar] [CrossRef] [PubMed]
- Radonova, L.; Pauerova, T.; Jansova, D.; Danadova, J.; Skultety, M.; Kubelka, M.; Anger, M. Cyclin A1 in Oocytes Prevents Chromosome Segregation And Anaphase Entry. Sci. Rep. 2020, 10, 7455. [Google Scholar] [CrossRef] [PubMed]
- Kemphues, K.J.; Kaufman, T.C.; Raff, R.A.; Raff, E.C. The Testis-Specific Beta-Tubulin Subunit in Drosophila Melanogaster Has Multiple Functions in Spermatogenesis. Cell 1982, 31, 655–670. [Google Scholar] [CrossRef]
- Funk, M.C.; Bera, A.N.; Menchen, T.; Kuales, G.; Thriene, K.; Lienkamp, S.S.; Dengjel, J.; Omran, H.; Frank, M.; Arnold, S.J. Cyclin O (Ccno) Functions during Deuterosome-Mediated Centriole Amplification of Multiciliated Cells. EMBO J. 2015, 34, 1078–1089. [Google Scholar] [CrossRef]
- Cornes, E.; Bourdon, L.; Singh, M.; Mueller, F.; Quarato, P.; Wernersson, E.; Bienko, M.; Li, B.; Cecere, G. PiRNAs Initiate Transcriptional Silencing of Spermatogenic Genes during C. Elegans Germline Development. Dev. Cell 2022, 57, 180–196.e7. [Google Scholar] [CrossRef]
- Fu, T.J.; Peng, J.; Lee, G.; Price, D.H.; Flores, O. Cyclin K Functions as a CDK9 Regulatory Subunit and Participates in RNA Polymerase II Transcription. J. Biol. Chem. 1999, 274, 34527–34530. [Google Scholar] [CrossRef]
- Camasses, A.; Bogdanova, A.; Shevchenko, A.; Zachariae, W. The CCT Chaperonin Promotes Activation of the Anaphase-Promoting Complex through the Generation of Functional Cdc20. Mol. Cell 2003, 12, 87–100. [Google Scholar] [CrossRef]
- Arivalagan, J.; Yarra, T.; Marie, B.; Sleight, V.A.; Duvernois-Berthet, E.; Clark, M.S.; Marie, A.; Berland, S. Insights from the Shell Proteome: Biomineralization to Adaptation. Mol. Biol. Evol. 2017, 34, 66–77. [Google Scholar] [CrossRef]
- Yarra, T.; Blaxter, M.; Clark, M.S. A Bivalve Biomineralization Toolbox. Mol. Biol. Evol. 2021, 38, 4043–4055. [Google Scholar] [CrossRef]
- Feng, D.; Li, Q.; Yu, H.; Kong, L.; Du, S. Identification of Conserved Proteins from Diverse Shell Matrix Proteome in Crassostrea Gigas: Characterization of Genetic Bases Regulating Shell Formation. Sci. Rep. 2017, 7, 45754. [Google Scholar] [CrossRef]
- Song, N.; Li, J.; Li, B.; Pan, E.; Ma, Y. Transcriptome Analysis of the Bivalve Placuna Placenta Mantle Reveals Potential Biomineralization-Related Genes. Sci. Rep. 2022, 12, 4743. [Google Scholar] [CrossRef]
- Marie, B.; Joubert, C.; Tayalé, A.; Zanella-Cléon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different Secretory Repertoires Control the Biomineralization Processes of Prism and Nacre Deposition of the Pearl Oyster Shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef]
- Liao, Z.; Jiang, Y.; Sun, Q.; Fan, M.; Wang, J.; Liang, H. Microstructure and In-Depth Proteomic Analysis of Perna Viridis Shell. PLoS ONE 2019, 14, e0219699. [Google Scholar] [CrossRef]
- Joubert, C.; Piquemal, D.; Marie, B.; Manchon, L.; Pierrat, F.; Zanella-Cléon, I.; Cochennec-Laureau, N.; Gueguen, Y.; Montagnani, C. Transcriptome and Proteome Analysis of Pinctada Margaritifera Calcifying Mantle and Shell: Focus on Biomineralization. BMC Genom. 2010, 11, 613. [Google Scholar] [CrossRef]
- Suzuki, M.; Sakuda, S.; Nagasawa, H. Identification of Chitin in the Prismatic Layer of the Shell and a Chitin Synthase Gene from the Japanese Pearl Oyster, Pinctada Fucata. Biosci. Biotechnol. Biochem. 2007, 71, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.M.; Schönitzer, V.; Eichner, N.; Sumper, M. The Chitin Synthase Involved in Marine Bivalve Mollusk Shell Formation Contains a Myosin Domain. FEBS Lett. 2006, 580, 1846–1852. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Deng, Z.; Huang, G.; Fan, S.; Zhou, D.; Liu, B.; Zhang, B.; Yu, D. Molecular Characterization and Expression Analysis of Chitinase from the Pearl Oyster Pinctada Fucata. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 203, 141–148. [Google Scholar] [CrossRef]
- Xiong, X.; Cao, Y.; Li, Z.; Jiao, Y.; Du, X.; Zheng, Z. Transcriptome Analysis Reveals the Transition and Crosslinking of Immune Response and Biomineralization in Shell Damage Repair in Pearl Oyster. Aquac. Rep. 2021, 21, 100851. [Google Scholar] [CrossRef]
- Schönitzer, V.; Weiss, I.M. The Structure of Mollusc Larval Shells Formed in the Presence of the Chitin Synthase Inhibitor Nikkomycin Z. BMC Struct. Biol. 2007, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Iwashima, A.; Tsutsui, N.; Ohira, T.; Kogure, T.; Nagasawa, H. Identification and Characterisation of a Calcium Carbonate-Binding Protein, Blue Mussel Shell Protein (BMSP), from the Nacreous Layer. Chembiochem 2011, 12, 2478–2487. [Google Scholar] [CrossRef]
- Aguilera, F.; McDougall, C.; Degnan, B.M. Evolution of the Tyrosinase Gene Family in Bivalve Molluscs: Independent Expansion of the Mantle Gene Repertoire. Acta Biomater. 2014, 10, 3855–3865. [Google Scholar] [CrossRef]
- Ren, G.; Chen, C.; Jin, Y.; Zhang, G.; Hu, Y.; Shen, W. A Novel Tyrosinase Gene Plays a Potential Role in Modification the Shell Organic Matrix of the Triangle Mussel Hyriopsis Cumingii. Front. Physiol. 2020, 11, 100. [Google Scholar] [CrossRef]
- Miglioli, A.; Dumollard, R.; Balbi, T.; Besnardeau, L.; Canesi, L. Characterization of the Main Steps in First Shell Formation in Mytilus Galloprovincialis: Possible Role of Tyrosinase. Proc. R. Soc. B Biol. Sci. 2019, 286, 20192043. [Google Scholar] [CrossRef]
- Xiong, X.; Cao, Y.; Li, Z.; Huang, R.; Jiao, Y.; Zhao, L.; Du, X.; Zheng, Z. Insights from Tyrosinase into the Impacts of Modified Morphology of Calcium Carbonate on the Nacre Formation of Pearl Oysters. Front. Mar. Sci. 2022, 9, 1381. [Google Scholar] [CrossRef]
- Sun, Y.; Shan, X.; Li, D.; Liu, X.; Han, Z.; Qin, J.; Qiu, P.; Guan, B.; Tan, L.; Wei, M.; et al. Cloning, Characterization, and Expression Prolife Analysis of Tyrosinase Genes: Insights into Black Shell Formation in Cyclina Sinensis. Aquac. Res. 2023, 2023, e7412887. [Google Scholar] [CrossRef]
- Liu, J.; Yang, D.; Liu, S.; Li, S.; Xu, G.; Zheng, G.; Xie, L.; Zhang, R. Microarray: A Global Analysis of Biomineralization-Related Gene Expression Profiles during Larval Development in the Pearl Oyster, Pinctada Fucata. BMC Genom. 2015, 16, 325. [Google Scholar] [CrossRef]
- Yarra, T.; Ramesh, K.; Blaxter, M.; Hüning, A.; Melzner, F.; Clark, M.S. Transcriptomic Analysis of Shell Repair and Biomineralization in the Blue Mussel, Mytilus Edulis. BMC Genom. 2021, 22, 437. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Huang, J.; Paine, M.L.; Reinhold, V.N.; Chasteen, N.D. Structural Characterization of the Major Extrapallial Fluid Protein of the Mollusc Mytilus Edulis: Implications for Function. Biochemistry 2005, 44, 10720–10731. [Google Scholar] [CrossRef] [PubMed]
- Weiss, I.M.; Kaufmann, S.; Mann, K.; Fritz, M. Purification and Characterization of Perlucin and Perlustrin, Two New Proteins from the Shell of the Mollusc Haliotis Laevigata. Biochem. Biophys. Res. Commun. 2000, 267, 17–21. [Google Scholar] [CrossRef]
- Takeuchi, T.; Fujie, M.; Koyanagi, R.; Plasseraud, L.; Ziegler-Devin, I.; Brosse, N.; Broussard, C.; Satoh, N.; Marin, F. The ‘Shellome’ of the Crocus Clam Tridacna Crocea Emphasizes Essential Components of Mollusk Shell Biomineralization. Front. Genet. 2021, 12, 674539. [Google Scholar] [CrossRef] [PubMed]
- Oudot, M.; Neige, P.; Shir, I.B.; Schmidt, A.; Strugnell, J.M.; Plasseraud, L.; Broussard, C.; Hoffmann, R.; Lukeneder, A.; Marin, F. The Shell Matrix and Microstructure of the Ram’s Horn Squid: Molecular and Structural Characterization. J. Struct. Biol. 2020, 211, 107507. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Hu, H.; Li, J.; Yan, L.; Zhong, J.; Bai, Z.; Li, J. Hc-Transgelin Is a Novel Matrix Protein Gene Involved in the Shell Biomineralization of Triangle Sail Mussel (Hyriopsis Cumingii). Aquac. Fish. 2022. [Google Scholar] [CrossRef]
- Patwary, M.U.; Reith, M.; Kenchington, E.L. Isolation and Characterization of a CDNA Encoding an Actin Gene from Sea Scallop (Placopecten Magellanicus). J. Shellfish Res. 1996, 15, 265–270. [Google Scholar]
- Lin, J.; Patel, S.R.; Cheng, X.; Cho, E.A.; Levitan, I.; Ullenbruch, M.; Phan, S.H.; Park, J.M.; Dressler, G.R. Kielin/Chordin-like Protein, a Novel Enhancer of BMP Signaling, Attenuates Renal Fibrotic Disease. Nat. Med. 2005, 11, 387–393. [Google Scholar] [CrossRef]
- Lee, J.; Bandyopadhyay, J.; Lee, J.I.; Cho, I.; Park, D.; Cho, J.H. A Role for Peroxidasin PXN-1 in Aspects of C. Elegans Development. Mol. Cells 2015, 38, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Shelud’ko, N.S.; Matusovskaya, G.G.; Permyakova, T.V.; Matusovsky, O.S. Twitchin, a Thick-Filament Protein from Molluscan Catch Muscle, Interacts with F-Actin in a Phosphorylation-Dependent Way. Arch. Biochem. Biophys. 2004, 432, 269–277. [Google Scholar] [CrossRef]
- Yokoyama, M.; Matsuzawa, T.; Yoshikawa, T.; Nunomiya, A.; Yamaguchi, Y.; Yanai, K. Heparan Sulfate Controls Skeletal Muscle Differentiation and Motor Functions. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129707. [Google Scholar] [CrossRef]
- Le Chevalier, P.; Sellos, D.; Van Wormhoudt, A. Purification and Partial Characterization of Chymotrypsin-like Proteases from the Digestive Gland of the Scallop Pecten Maximus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1995, 110, 777–784. [Google Scholar] [CrossRef]
- Reid, R.G.B.; Rauchert, K. Proteolytic Enzymes in the Bivalve Mollusc Chlamys Hericius Gould. Comp. Biochem. Physiol. 1970, 35, 689–695. [Google Scholar] [CrossRef]
- Liberti, A.; Zucchetti, I.; Melillo, D.; Skapura, D.; Shibata, Y.; De Santis, R.; Pinto, M.R.; Litman, G.W.; Dishaw, L.J. Chitin Protects the Gut Epithelial Barrier in a Protochordate Model of DSS-Induced Colitis. Biol. Open 2018, 7, bio029355. [Google Scholar] [CrossRef] [PubMed]
- Gerdol, M.; Venier, P. An Updated Molecular Basis for Mussel Immunity. Fish Shellfish Immunol. 2015, 46, 17–38. [Google Scholar] [CrossRef]
- Fang, M.; Siciliano, N.A.; Hersperger, A.R.; Roscoe, F.; Hu, A.; Ma, X.; Shamsedeen, A.R.; Eisenlohr, L.C.; Sigal, L.J. Perforin-Dependent CD4+ T-Cell Cytotoxicity Contributes to Control a Murine Poxvirus Infection. Proc. Natl. Acad. Sci. USA 2012, 109, 9983–9988. [Google Scholar] [CrossRef]
- Calcino, A.D.; Kenny, N.J.; Gerdol, M. Single Individual Structural Variant Detection Uncovers Widespread Hemizygosity in Molluscs. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200153. [Google Scholar] [CrossRef] [PubMed]
- Lydeard, C.; Cowie, R.H.; Ponder, W.F.; Bogan, A.E.; Bouchet, P.; Clark, S.A.; Cummings, K.S.; Frest, T.J.; Gargominy, O.; Herbert, D.G.; et al. The Global Decline of Nonmarine Mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Feind, S.; Geist, J.; Kuehn, R. Glacial Perturbations Shaped the Genetic Population Structure of the Endangered Thick-Shelled River Mussel (Unio Crassus, Philipsson 1788) in Central and Northern Europe. Hydrobiologia 2018, 810, 177–189. [Google Scholar] [CrossRef]
- Smith, C.H.; Johnson, N.A.; Robertson, C.R.; Doyle, R.D.; Randklev, C.R. Establishing Conservation Units to Promote Recovery of Two Threatened Freshwater Mussel Species (Bivalvia: Unionida: Potamilus). Ecol. Evol. 2021, 11, 11102–11122. [Google Scholar] [CrossRef]
- Klymus, K.E.; Richter, C.A.; Thompson, N.; Hinck, J.E.; Jones, J.W. Metabarcoding Assays for the Detection of Freshwater Mussels (Unionida) with Environmental DNA. Environ. DNA 2021, 3, 231–247. [Google Scholar] [CrossRef]
- Gorički, Š.; Presetnik, P.; Prosenc-Zmrzljak, U.; Blatnik, M.; Gredar, T.; Kogovšek, B.; Koit, O.; Strah, S.; Bilandžija, H.; Jalžić, B.; et al. Environmental DNA in Subterranean Biology Update: From “Where?” To “How Many?”. ARPHA Conf. Abstr. 2018, 1, e29968. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Burlakova, L.E.; Karatayev, A.Y.; Mehler, K.; Seddon, M.; Sousa, R. Conservation of Freshwater Bivalves at the Global Scale: Diversity, Threats and Research Needs. Hydrobiologia 2018, 810, 1–14. [Google Scholar] [CrossRef]

| The Congeria kusceri Transcriptome | |
|---|---|
| number of assembled contigs | 298,442 |
| total assembly size (nt) | 97,960,735 |
| average contig size (nt) | 736.2 |
| N50 (nt) | 1316 |
| Ex90N50 (nt) | 2036 |
| complete BUSCOs (Metazoa ODB v.10) | 93.5% |
| duplicated BUSCOs (Metazoa ODB v.10) | 0.8% |
| fragmented BUSCOs (Metazoa ODB v.10) | 2.2% |
| missing BUSCOs (Metazoa ODB v.10) | 3.5% |
| contigs > 5 Kb | 920 |
| contigs > 10 Kb | 45 |
| longest assembled contig (nt) | 37,948 |
| contigs with Gene Ontology annotations | 17,635 |
| contigs with Pfam annotations | 22,890 |
| contigs with OrthoDB annotations | 26,941 |
| contigs with BLAST hits vs. UniprotKB | 17,997 |
| contigs with BLAST hits vs. Dreissena rostriformis | 41,753 |
| Gene Symbol | Full Name | FC | FDR p-Value | Role in Cell Cycle Progression and Meiosis |
|---|---|---|---|---|
| ANAPC2 | Anaphase Promoting Complex Subunit 2 | 8.49 | 0.0000206 | Component of the APC/C complex; promotes metaphase-anaphase transition by ubiquitinating and promoting proteasomal degradation of several cell cycle regulators. |
| ANAPC5 | Anaphase Promoting Complex Subunit 5 | 11.88 | 0.05 | Component of the APC/C complex; promotes metaphase-anaphase transition by ubiquitinating and promoting proteasomal degradation of several cell cycle regulators. |
| ANAPC7 | Anaphase Promoting Complex Subunit 7 | 5.48 | 0.0005 | Component of the APC/C complex; promotes metaphase-anaphase transition by ubiquitinating and promoting proteasomal degradation of several cell cycle regulators. |
| ASPM | Assembly Factor For Spindle Microtubules | 5.43 | 0.00248 | Regulates spindle assembly and meiotic progression. |
| AURKA | Aurora Kinase A | 2.13 | 0.00879 | Key regulator of various mitotic events. |
| BUB1B | BUB1 Mitotic Checkpoint Serine/Threonine Kinase B | 14.1 | 0.02 | Inhibits the APC/C complex, delaying the onset of anaphase and ensuring proper chromosome segregation. |
| CCNA2 | Cyclin A2 | 2.38 | 0.0000239 | Controls both the G1/S and the G2/M transition phases of the cell cycle. |
| CDC20 | Cell Division Cycle 20 | 20.39 | 0 | Co-activator of the APC/C complex. |
| CDC23 | Cell Division Cycle 23 | 23.98 | 0.03 | Component of the APC/C complex; promotes metaphase-anaphase transition by ubiquitinating and promoting proteasomal degradation of several cell cycle regulators. |
| HSF2BP | Heat Shock Transcription Factor 2 Binding Protein | 4.96 | 0.00293 | Modulates the localization of recombinases to meiotic double-strand break sites. |
| MEIOB | Meiosis Specific With OB-Fold | 4.08 | 0.03 | Stabilizes recombinases, allowing homologous recombination in meiosis I. |
| MELK | Maternal Embryonic Leucine Zipper Kinase | 5.28 | 0.00702 | Mediates the phosphorylation of CDC25B, promoting its localization to the centrosome and spindle poles. |
| MND1 | Meiotic nuclear division protein 1 homolog | 5.49 | 1.64 × 10−9 | Stimulates homologous strand assimilation, which is required for the resolution of meiotic double-strand breaks. |
| MTBP | MDM2 Binding Protein | 13.1 | 0.000457 | Promotes the degradation of CDC25C and delays cell cycle progression through the G2/M phase. |
| PKMYT1 | Protein Kinase, Membrane Associated Tyrosine/Threonine | 4.26 | 0.0000116 | Negatively regulates the G2/M transition by phosphorylating and inactivating CDK1. |
| PLK1 | Polo Like Kinase 1 | 3.09 | 0.05 | Regulates centrosome maturation and spindle assembly, the removal of cohesins from chromosome arms, the inactivation of APC/C inhibitors, mitotic exit, and cytokinesis. |
| RACK1 | Receptor For Activated C Kinase 1 | 12.07 | 0 | Prolongs the G0/G1 phase of the cell cycle. |
| SYCP1 | Synaptonemal Complex Protein 1 | 3.62 | 3.89× 10−10 | Required for the formation of synaptonemal complexes during meiosis. |
| UBB | ubiquitin B | 15.64 | 0 | Controls the turnover of chromosomal proteins, regulating chromosome axis length during meiotic chromosome reorganization. |
| ZC3HC1 | Zinc Finger C3HC-Type Containing 1 | 3.35 | 0.00513 | Controls mitotic entry by mediating the ubiquitination and degradation of cyclin B1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scapolatiello, A.; Manfrin, C.; Greco, S.; Rončević, T.; Pallavicini, A.; Puljas, S.; Gerdol, M. Variation of Gene Expression in the Endemic Dinaric Karst Cave-Dwelling Bivalve Mollusk Congeria kusceri during the Summer Season. Diversity 2023, 15, 707. https://doi.org/10.3390/d15060707
Scapolatiello A, Manfrin C, Greco S, Rončević T, Pallavicini A, Puljas S, Gerdol M. Variation of Gene Expression in the Endemic Dinaric Karst Cave-Dwelling Bivalve Mollusk Congeria kusceri during the Summer Season. Diversity. 2023; 15(6):707. https://doi.org/10.3390/d15060707
Chicago/Turabian StyleScapolatiello, Annalisa, Chiara Manfrin, Samuele Greco, Tomislav Rončević, Alberto Pallavicini, Sanja Puljas, and Marco Gerdol. 2023. "Variation of Gene Expression in the Endemic Dinaric Karst Cave-Dwelling Bivalve Mollusk Congeria kusceri during the Summer Season" Diversity 15, no. 6: 707. https://doi.org/10.3390/d15060707
APA StyleScapolatiello, A., Manfrin, C., Greco, S., Rončević, T., Pallavicini, A., Puljas, S., & Gerdol, M. (2023). Variation of Gene Expression in the Endemic Dinaric Karst Cave-Dwelling Bivalve Mollusk Congeria kusceri during the Summer Season. Diversity, 15(6), 707. https://doi.org/10.3390/d15060707










