First Worldwide Evidence of Bronchopulmonary Strongyle Nematodes and the First Report on Italy of Cryptosporidium sp. in Allochthonous Nutria (Myocastor coypus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methods
3. Results
| No. Fecal Samples | 308 a | 308 b | 304 c | 200 d | 438 e | 20 f | 252 g | 30 h | 16 i | 9 j | 108 k | 150 l | 153 m | 31 n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rectum (R)/Ground (G) | G | G | R | G | R | G | R | R | G | G | R | RG | R | G |
| Wild (W)/Captivity (C) | C | C | C | C | W | W | W | W | W | W | W | W | W | W |
| Taxon | ||||||||||||||
| Cryptosporidium sp. 1 | + | + | ||||||||||||
| C. myocastoris 2 | + | |||||||||||||
| C. ubiquitum 3 | + | |||||||||||||
| C. parvum 4 | + | |||||||||||||
| Eimeriidae indet. | + | + | ||||||||||||
| Eimeria sp. 5 | + | |||||||||||||
| E. nutriae 6 | + | + | + | |||||||||||
| E. myopotami 7 | + | + | ||||||||||||
| E. coypi 8 | + | + | + | + | ||||||||||
| E. seideli 9 | + | + | + | |||||||||||
| E. fluviatilis 10 | + | |||||||||||||
| Enterocytozoon bieneusi 11 | + | |||||||||||||
| Giardia sp. 12 | + | + | ||||||||||||
| G. duodenalis 13 | + | |||||||||||||
| Ascarididae indet. | + | |||||||||||||
| Muellerius vel. Angiostrongylus 14 | + | |||||||||||||
| Trichostrongylidae | + | |||||||||||||
| Strongyloidea indet. | + | |||||||||||||
| Trichostrongylus sp. 15 | + | + | ||||||||||||
| T. duretteae 16 | + | |||||||||||||
| Strongyloides sp. 17 | + | + | + | + | ||||||||||
| S. myopotami 18 | + | |||||||||||||
| Trichuris sp. 19 | + | + | ||||||||||||
| Fasciola hepatica 20 | + | + | + | + | ||||||||||
| Cestoda indet. | + |
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cocchi, R.; Riga, F. Linee Guida per il Controllo della Nutria (Myocastor coypus); No. 5; Ministero dell’Ambiente e della Tutela del Territorio, Servizio Conservazione Natura: Roma, Italy, 2001. (In Italian)
- Adhikari, P.; Kim, B.-J.; Hong, S.-H.; Lee, D.-H. Climate change induced habitat expansion of nutria (Myocastor coypus) in South Korea. Sci. Rep. 2022, 12, 3300. [Google Scholar] [CrossRef] [PubMed]
- Frainer, A.; McKie, B.G.; Amundsen, P.-A.; Knudsen, R.; Lafferty, K.D. Parasitism and the Biodiversity-Functioning Relationship. Trends Ecol. Evol. 2018, 33, 260–268. [Google Scholar] [CrossRef]
- Landaeta-Aqueveque, C.; Moreno Salas, L.; Henríquez, A.; Silva-de la Fuente, M.C.; González-Acuña, D. Parasites of Native and Invasive Rodents in Chile: Ecological and Human Health Needs. Front. Vet. Sci. 2021, 8, 643742. [Google Scholar] [CrossRef] [PubMed]
- Chalkowski, K.; Lepczyk, C.A.; Zohdy, S. Parasite Ecology of Invasive Species: Conceptual Framework and New Hypotheses. Trends Parasitol. 2018, 34, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Llaberia-Robledillo, M.; Balbuena, J.A.; Sarabeev, V.; Llopis-Belenguer, C. Changes in native and introduced host–parasite networks. Biol. Invasions 2022, 24, 543–555. [Google Scholar] [CrossRef]
- Capizzi, D.; Monaco, A.; Genovesi, P.; Scalera, R.; Carnevali, L. Impact of alien mammals on human health. In Invasive Species and Human Health; CAB International: Wallingford, UK, 2018; pp. 130–150. [Google Scholar] [CrossRef]
- Babero, B.B.; Lee, J.W. Studies on the helminths of nutria, Myocastor coypus (Molina), in Louisiana with checklist of other worm parasites from this host. J. Parasitol. 1961, 47, 378–390. [Google Scholar] [CrossRef]
- Newson, R.M.; Holmes, R.G. Some Ectoparasites of the Coypu (Myocastor coypus) in Eastern England. J. Anim. Ecol. 1968, 37, 471–481. [Google Scholar] [CrossRef]
- Howerth, E.W.; Reeves, A.J.; McElveen, M.R.; Austin, F.W. Survey for Selected Diseases in Nutria (Myocastor coypus) from Louisiana. J. Wildl. Dis. 1994, 30, 450–453. [Google Scholar] [CrossRef]
- Ménard, A.; Agoulon, A.; L’Hostis, M.; Rondelaud, D.; Collard, S.; Chauvin, A. Myocastor coypus as a reservoir host of Fasciola hepatica in France. Vet. Res. 2001, 32, 499–508. [Google Scholar] [CrossRef]
- Issia, L.; Pietrokovsky, S.; Sousa-Figueiredo, J.; Stothard, J.R.; Wisnivesky-Colli, C. Fasciola hepatica infections in livestock flock, guanacos and coypus in two wildlife reserves in Argentina. Vet. Parasitol. 2009, 165, 341–344. [Google Scholar] [CrossRef]
- Martino, P.; Radman, N.; Parrado, E.; Bautista, E.; Cisterna, C.; Silvestrini, M.; Corba, S. Note on the occurrence of parasites of the wild nutria (Myocastor coypus, Molina, 1782). Helminthologia 2012, 49, 164–168. [Google Scholar] [CrossRef]
- Martino, P.E.; Radman, N.E.; Gamboa, M.I.; Samartino, L.E.; Parrado, E.J. Ectoparasites from some Myocastor coypus (Molina, 1782) populations (Coypus or Nutria) in Argentina. Rev. Bras. Parasitol. Vet. 2018, 27, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Nechybová, S.; Langrová, I.; Tůmová, E. Parasites of Myocastor coypus—A Comparison in Farm Animals and Their Feral Counterparts. Sci. Agric. Bohem. 2018, 49, 21–25. [Google Scholar] [CrossRef]
- Lim, S.R.; Lee, D.H.; Park, S.Y.; Lee, S.; Kim, H.Y.; Lee, M.S.; Lee, J.R.; Han, J.E.; Kim, H.K.; Kim, J.H. Wild nutria (Myocastor coypus) is a potential reservoir of carbapenem-resistant and zoonotic Aeromonas spp. in Korea. Microorganisms 2019, 7, 224. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, D.; Wang, W.; Zhang, Y.; Jing, B.; Xu, C.; Chen, Y.; Qi, M.; Zhang, L. Occurrence and Multi-Locus Analysis of Giardia duodenalis in Coypus (Myocastor coypus) in China. Pathogens 2021, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Ježková, J.; Limpouchová, Z.; Prediger, J.; Holubová, N.; Sak, B.; Konečný, R.; Květoňová, D.; Hlàskovà, L.; Rost, M.; McEvoy, J.; et al. Cryptosporidium myocastoris n. sp. (Apicomplexa: Cryptosporidiidae), the Species Adapted to the Nutria (Myocastor coypus). Microorganisms 2021, 9, 813. [Google Scholar] [CrossRef]
- Nardoni, S.; Angelici, M.C.; Mugnaini, L.; Mancianti, F. Prevalence of Toxoplasma gondii infection in Myocastor coypus in a protected Italian wetland. Parasites Vectors 2011, 4, 240. [Google Scholar] [CrossRef]
- Serracca, L.; Battistini, R.; Rossini, I.; Mignone, W.; Peletto, S.; Boin, C.; Pistone, G.; Ercolini, R.; Ercolini, C. Molecular Investigation on the Presence of Hepatitis E Virus (HEV) in Wild Game in North-Western Italy. Food Environ. Virol. 2015, 7, 206–212. [Google Scholar] [CrossRef]
- Scaglione, F.E.; Pregel, P.; Masoero, L.; Caruso, C.; Starvaggi Cucuzza, L.; Dondo, A.; Zoppi, S.; Sereno, A.; Chiappino, L.; Zanet, S.; et al. Nutria (Myocastor coypus) health status in the natural park “La Mandria”. Anatomopathological and microbiological investigations. In Proceedings of the 72nd Convegno SISVet: Tutte le Novità in Ambito Veterinario, Torino, Italy, 20–22 June 2018; p. 239. [Google Scholar]
- Bertolino, S.; Cocchi, R. Piano di Gestione Nazionale della Nutria Myocastor coypus; Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2018. (In Italian) [Google Scholar]
- Bertelloni, F.; Cilia, G.; Turchi, B.; Pinzauti, P.; Cerri, D.; Fratini, F. Epidemiology of leptospirosis in North-Central Italy: Fifteen years of serological data (2002–2016). Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 14–22. [Google Scholar] [CrossRef]
- Arcangeli, G. La nutria selvatica quale potenziale “reservoir” di agenti trasmissibili all’uomo: Situazione in Italia e nel mondo. La gestione delle specie alloctone in Italia: Il caso della nutria e del gambero rosso della Louisiana. Quad. Padule Fucecchio 2002, 2, 31. (In Italian) [Google Scholar]
- Battisti, C.; Cento, M.; Fraticelli, F.; Hueting, S.; Muratore, S. Vertebrates in the “Palude di Torre Flavia” special protection area (Lazio, Central Italy): An updated checklist. Nat. Hist. Sci. 2021, 8, 3–28. [Google Scholar] [CrossRef]
- Amori, G.; Battisti, C. An invaded wet ecosystem in Central Italy: An arrangement and evidence for an alien food chain. Rend. Lincei 2008, 19, 161–171. [Google Scholar] [CrossRef]
- Ferri, V.; Battisti, C.; Soccini, C.; Santoro, R. A hotspot of xenodiversity: First evidence of an assemblage of non-native freshwater turtles in a suburban wetland in Central Italy. Lakes Reserv. Res. Manag. 2020, 25, 250–257. [Google Scholar] [CrossRef]
- Di Blasio, L.; Santoro, R.; Ferri, V.; Battisti, C.; Soccini, C.; Egidi, A.; Scalici, M. First successful reproduction of the Chinese striped-necked turtle Mauremys sinensis (Gray, 1834) in a European wetland. BioInvasions Rec. 2021, 10, 721–729. [Google Scholar] [CrossRef]
- Battisti, C. Biodiversità, Gestione, Conservazione di Un’area Umida del Litorale Tirrenico: La Palude di Torre Flavia; Provincia di Roma, Assessorato alle Politiche Agricole e dell’Ambiente, Gangemi Editore: Roma, Italy, 2006. (In Italian) [Google Scholar]
- Gundersen, G.; Johannesen, E.; Andreassen, H.; Ims, R. Source-sink dynamics: How sinks affect demography of sources. Ecol. Lett. 2001, 4, 14–21. [Google Scholar] [CrossRef]
- Battisti, C.; Marini, F.; Vignoli, L. A five-year cycle of coypu abundance in a remnant wetland: A case of sink population collapse? Hystrix 2015, 26, 37–40. [Google Scholar]
- Marini, F.; Gabrielli, E.; Montaudo, L.; Vecchi, M.; Santoro, R.; Battisti, C.; Carpaneto, G.M. Diet of coypu (Myocastor coypus) in a Mediterranean coastal wetland: A possible impact on threatened rushbeds? Vie Milieu 2013, 63, 97–103. [Google Scholar]
- De Michelis, S.; Ceschin, S.; Carosi, M.; Battisti, C. Coypu (Myocastor coypus Molina, 1782) feeding on algae: First evidence for Europe. Vie Milieu 2022, 72, 31–34. [Google Scholar]
- Marini, F.; Ceccobelli, S.; Battisti, C. Coypu (Myocastor coypus) in a Mediterranean remnant wetland: A pilot study of a yearly cycle with management implications. Wetl. Ecol. Manag. 2011, 19, 159–164. [Google Scholar] [CrossRef]
- Angelici, C.; Marini, F.; Battisti, C.; Bertolino, S.; Capizzi, D.; Monaco, A. Cumulative impact of rats and coypu on nesting waterbirds: First evidences from a small Mediterranean wetland (Central Italy). Vie Milieu 2012, 62, 137–141. [Google Scholar]
- Gallitelli, L.; Battisti, C.; Pietrelli, L.; Scalici, M. Anthropogenic particles in coypu (Myocastor coypus; Mammalia, Rodentia)’ faeces: First evidence and considerations about their use as track for detecting microplastic pollution. Environ. Sci. Pollut. Res. 2022, 29, 55293–55301. [Google Scholar] [CrossRef]
- Grillo, G.; Sartori, G.; Battisti, C.; Ferri, V.; Luiselli, L.; Amori, G.; Carpaneto, G.M. Attempted copulatory behaviour between two phylogenetically unrelated alien species (Coypu, Myocastor coypus, and Pond slider, Trachemys scripta): First evidence. Zool. Ecol. 2020, 30, 165–168. [Google Scholar] [CrossRef]
- Redolfi De Zan, L.; Battisti, C.; Carpaneto, G.M. Inter-annual and intra-seasonal patterns of abundance in a set of common waterbirds: Along term study in a Mediterranean wetland. Vie Milieu 2011, 61, 101–106. [Google Scholar]
- Guidi, A. Introduzione alla flora e alle comunità vegetali. In Biodiversità, Gestione E Conservazione di Un’area Umida del Litorale Tirrenico: La Palude di Torre Flavia; Battisti, C., Ed.; Provincia di Roma, Assessorato alle Politiche Agricole e dell’Ambiente, Gangemi Editore: Roma, Italy, 2006; pp. 169–187. (In Italian) [Google Scholar]
- Fanelli, G.; Bianco, P.M. Memoria Illustrativa della Carta della Vegetazione della Provincia di Roma; Provincia di Roma, Dip. VI-Governo del Territorio, Serv. 3., Sistema Informativo Geografico: Roma, Italy, 2007. (In Italian) [Google Scholar]
- Ioni, S.; Battisti, C.; Fanelli, G. Mapping vegetation dynamics on embryonic sand dunes: A fine-grained atlas for periodic plant monitoring in a Mediterranean protected area. Quad. Mus. Civ. St. Nat. Ferrara 2020, 8, 37–42. [Google Scholar]
- Blasi, C.; Michetti, L. Biodiversità e Clima. In Stato della Biodiversità in Italia; Contributo alla Strategia Nazionale per la Biodiversità; Blasi, C., Boitani, L., La Posta, S., Manes, F., Marchetti, M., Eds.; Ministero dell’Ambiente e della Tutela del territorio, F.lli Palombi Editori: Roma, Italy, 2005. (In Italian) [Google Scholar]
- Battisti, C.; Luiselli, L.; Teofili, C. Quantifying threats in a Mediterranean wetland: Are there any changes in their evaluation during a training course? Biodivers. Conserv. 2009, 18, 3053–3060. [Google Scholar] [CrossRef]
- Sabia, G.; Petta, L.; Moretti, F.; Ceccarelli, R. Combined statistical techniques for the water quality analysis of a natural wetland and evaluation of the potential implementation of a FWS for the area restoration: The Torre Flavia case study, Italy. Ecol. Indic. 2018, 84, 244–253. [Google Scholar] [CrossRef]
- Sloss, M.W.; Kemp, R.L. Parassiti in Medicina Veterinaria: Metodi di Identificazione ed Indagine Microscopica; Ed. Ermes: Milan, Italy, 1982. (In Italian) [Google Scholar]
- Ambrosi, M. Parassitologia Zootecnica; Edagricole: Bologna, Italy, 1995. (In Italian) [Google Scholar]
- De Carneri, I. Parassitologia Generale e Umana, 11th ed.; Casa Editrice Ambrosiana: Milan, Italy, 1992. (In Italian) [Google Scholar]
- Zajac, A.M.; Conboy, G. Veterinary Clinical Parasitology, 8th ed.; Wiley-Blackwell Publishing: Oxford, UK, 2012. [Google Scholar]
- Dytham, C. Choosing and Using Statistics: A Biologist’s Guide; John Wiley & Sons: Oxford, UK, 2011. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electr. 2001, 4, 9. [Google Scholar]
- Lewis, D.C.; Ball, S.J. Eimeria fluviatilis n.sp and other species of Eimeria in wild coypus in England. Syst. Parasitol. 1984, 6, 191–198. [Google Scholar] [CrossRef]
- Zanzani, S.A.; Di Cerbo, A.; Gazzonis, A.L.; Epis, S.; Invernizzi, A.; Tagliabue, S.; Manfredi, M.T. Parasitic and Bacterial Infections of Myocastor coypus in a Metropolitan Area of Northwestern Italy. J. Wildl. Dis. 2016, 52, 126–130. [Google Scholar] [CrossRef]
- Bollo, E.; Pregel, P.; Gennero, S.; Pizzoni, E.; Rosati, S.; Nebbia, P.; Biolatti, B. Health status of a population of nutria (Myocastor coypus) living in a protected area in Italy. Res. Vet. Sci. 2003, 75, 21–25. [Google Scholar] [CrossRef]
- Pedruzzi, L.; Schertler, A.; Giuntini, S.; Leggiero, I.; Mori, E. An update on the distribution of the coypu, Myocastor coypus, in Asia and Africa through published literature, citizen-science and online platforms. Mamm. Biol. 2022, 102, 109–118. [Google Scholar] [CrossRef]
- Murillo Peixoto-Couto, R.; Correa-Branco, A.; Cabrera-Miguel, M. First record of Myocastor coypus (Molina 1782) (Mammalia, Rodentia) for the state of Mato Grosso do Sul, Brazil, and its distribution in southern South America. Act. Biol. 2022, 44, 1–6. [Google Scholar] [CrossRef]
- Yu, F.; Cao, Y.; Wang, H.; Liu, Q.; Zhao, A.; Qi, M.; Zhang, L. Host-adaptation of the rare Enterocytozoon bieneusi genotype CHN4 in Myocastor coypus (Rodentia: Echimyidae) in China. Parasites Vectors 2020, 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, B.G.; Thies, M.L. Giardia in beaver (Castor canadensis) and nutria (Myocastor coypus) from east Texas. J. Parasitol. 2002, 88, 1254–1258. [Google Scholar] [CrossRef]
- El-Kouba, M.M.A.N.; Marques, S.M.T.; Pilati, C.; Hamann, W. Presence of Fasciola hepatica in Feral Nutria (Myocastor coypus) Living in a Public Park in Brazil. J. Zoo Wildl. Med. 2009, 40, 103–106. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Strongyloides—Biology. 2019. Available online: https://www.cdc.gov/parasites/strongyloides/biology.html (accessed on 1 December 2022).
- Cringoli, G.; Rinaldi, L.; Veneziano, V. Mappe con omogenea distribuzione dei punti—Point distribution maps. In Mappe Parassitologiche; University of Naples Federico II: Naples, Italy, 2001; Volume 4. (In Italian) [Google Scholar]
- Hoberg, E.P.; Kocan, A.A.; Rickard, L.G. Gastrointestinal Strongyles in Wild Ruminants. In Parasitic Diseases of Wild Mammals, 2nd ed.; Samuel, W.M., Pybus, M.J., Kocan, A.A., Eds.; Iowa State University Press: Ames, IA, USA, 2001. [Google Scholar]
- Buonfrate, D.; Angheben, A.; Gobbi, F.; Mistretta, M.; Degani, M.; Bisoffi, Z. Four clusters of Trichostrongylus infection diagnosed in a single center, in Italy. Infection 2017, 45, 233–236. [Google Scholar] [CrossRef]
- Zafari, S.; Mohtasebi, S.; Sazmand, A.; Bahari, A.; Sargison, N.D.; Verocai, G.G. The Prevalence and Control of Lungworms of Pastoral Ruminants in Iran. Pathogens 2022, 11, 1392. [Google Scholar] [CrossRef]
- Spratt, D.M. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: A review. Int. J. Parasitol. Parasites Wildl. 2015, 4, 178–189. [Google Scholar] [CrossRef]
- Federspiel, F.; Skovmand, S.; Skarphedinsson, S. Eosinophilic meningitis due to Angiostrongylus cantonensis in Europe. Int. J. Infect. Dis. 2020, 93, 28–39. [Google Scholar] [CrossRef]
- Morgan, E.R.; Modry, D.; Paredes-Esquivel, C.; Foronda, P.; Traversa, D. Angiostrongylosis in Animals and Humans in Europe. Pathogens 2021, 10, 1236. [Google Scholar] [CrossRef]
- Morgan, E.; Shaw, S. Angiostrongylus vasorum infection in dogs: Continuing spread and developments in diagnosis and treatment. J. Small Anim. Pract. 2010, 51, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Kiskaddon, R.M.; Renshaw, R.J.F. Human coccidiosis. J. Am. Med. Assoc. 1945, 128, 731–732. [Google Scholar] [CrossRef]
- Li, N.; Xiao, L.; Alderisio, K.; Elwin, K.; Cebelinski, E.; Chalmers, R.; Santin, M.; Fayer, R.; Kvac, M.; Ryan, U.; et al. Subtyping Cryptosporidium ubiquitum, a Zoonotic Pathogen Emerging in Humans. Emerg. Infect. Dis. 2014, 20, 217. [Google Scholar] [CrossRef] [PubMed]
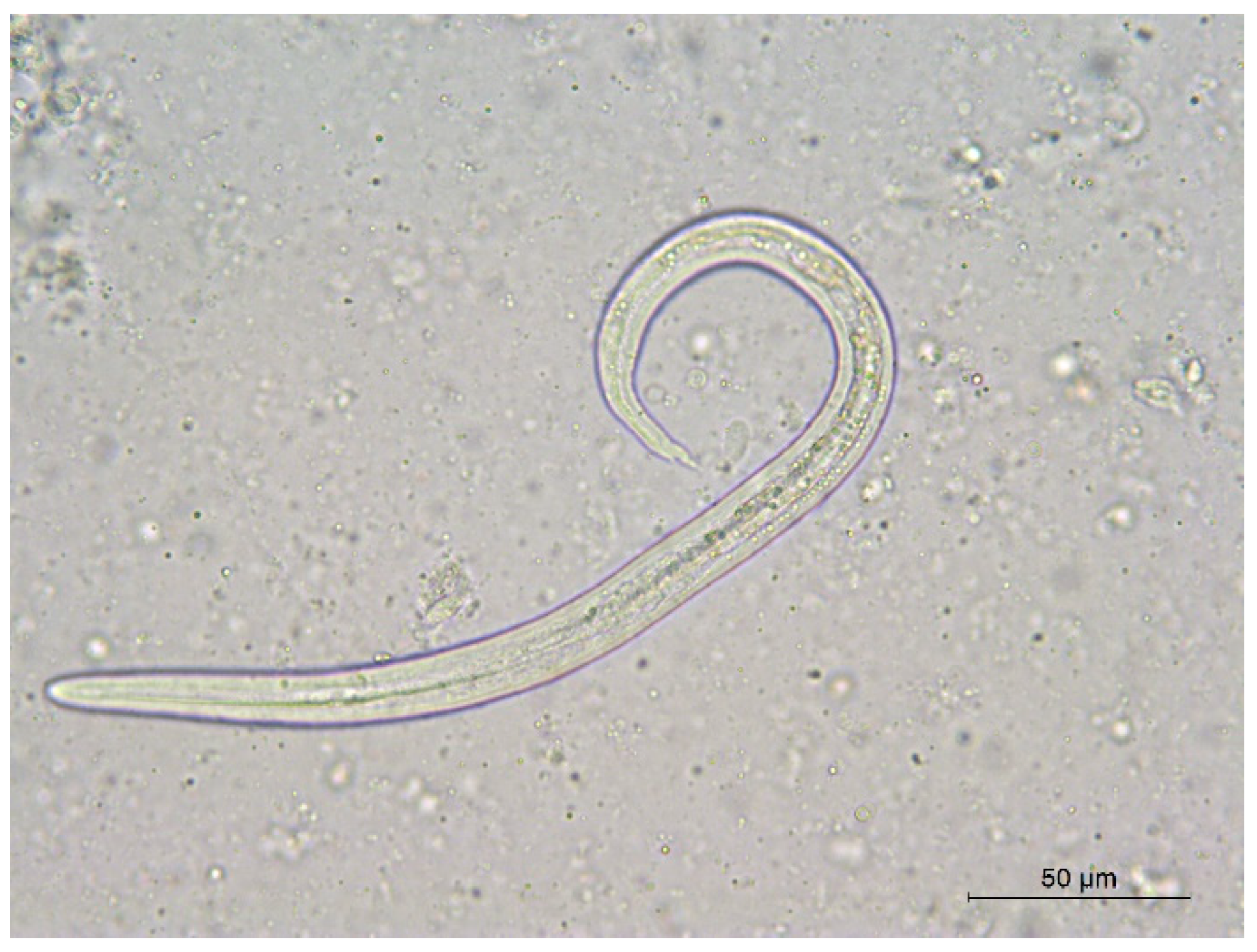
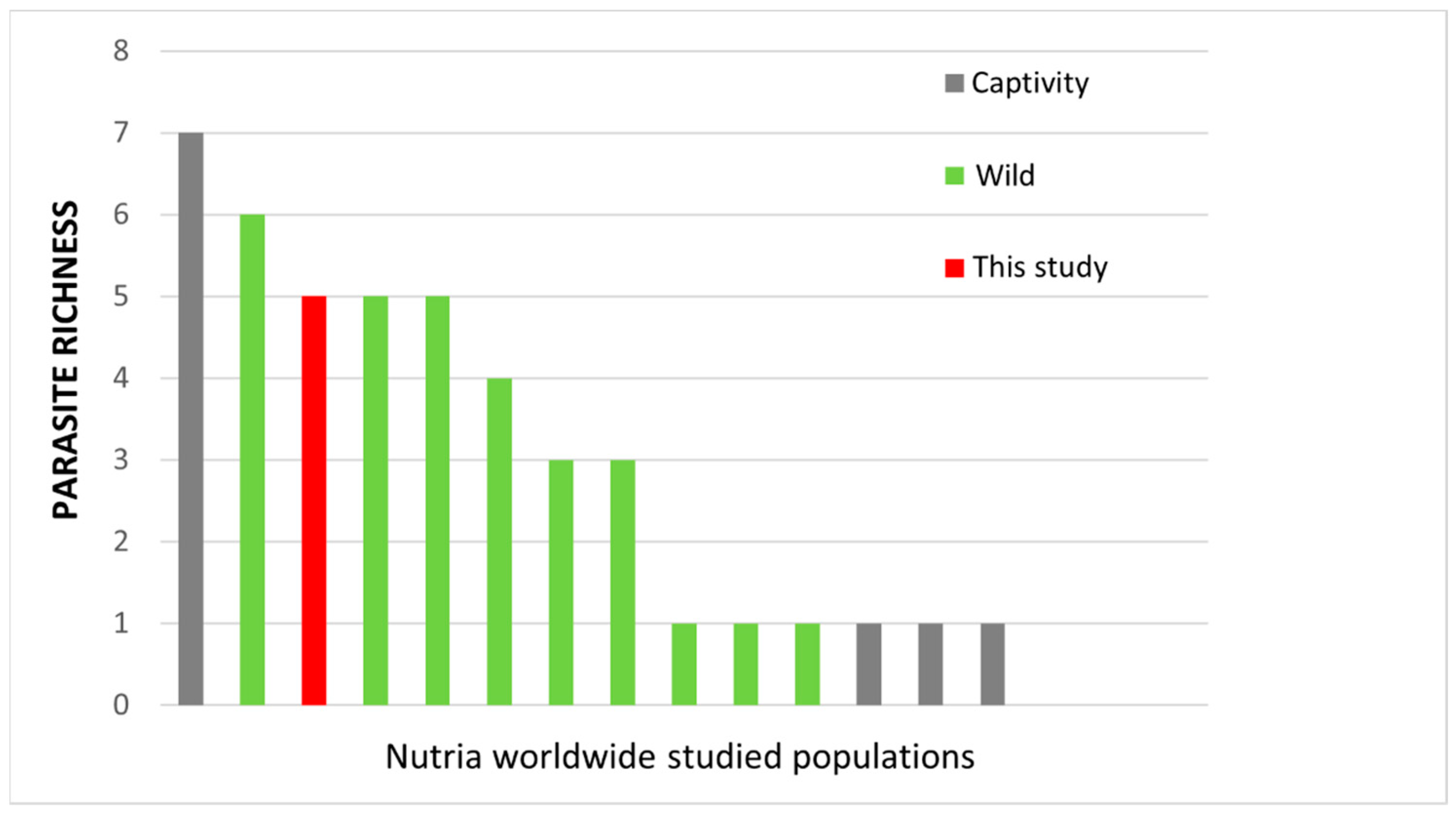
| Regions (Italy) | Piedmont a | Tuscany b | Lombardy c | Piedmont d | Lazio e |
|---|---|---|---|---|---|
| Biomatrices * | F/B/O/T | B/O/T | F/B/O/T | B/T | F |
| Taxon | |||||
| Coccidia indet. | + | ||||
| Cryptosporidium sp. 1 | + | ||||
| Eimeria sp. 2 | + | ||||
| Eimeria coypi 3 | + | ||||
| Eimeria seideli 4 | + | ||||
| Toxoplasma gondii 5 | + | + | + | + | |
| Muellerius vel. Angiostrongylus 6 | + | ||||
| Trichostrongylidae | + | ||||
| Strongyloides sp. 7 | + | + | |||
| Strongyloides myopotami 8 | + | ||||
| Trichostrongylus sp. 9 | + | ||||
| Trichostrongylus duretteae 10 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Michelis, S.; De Liberato, C.; Amoruso, C.; Battisti, C.; Carosi, M. First Worldwide Evidence of Bronchopulmonary Strongyle Nematodes and the First Report on Italy of Cryptosporidium sp. in Allochthonous Nutria (Myocastor coypus). Diversity 2023, 15, 611. https://doi.org/10.3390/d15050611
De Michelis S, De Liberato C, Amoruso C, Battisti C, Carosi M. First Worldwide Evidence of Bronchopulmonary Strongyle Nematodes and the First Report on Italy of Cryptosporidium sp. in Allochthonous Nutria (Myocastor coypus). Diversity. 2023; 15(5):611. https://doi.org/10.3390/d15050611
Chicago/Turabian StyleDe Michelis, Silvia, Claudio De Liberato, Cristina Amoruso, Corrado Battisti, and Monica Carosi. 2023. "First Worldwide Evidence of Bronchopulmonary Strongyle Nematodes and the First Report on Italy of Cryptosporidium sp. in Allochthonous Nutria (Myocastor coypus)" Diversity 15, no. 5: 611. https://doi.org/10.3390/d15050611
APA StyleDe Michelis, S., De Liberato, C., Amoruso, C., Battisti, C., & Carosi, M. (2023). First Worldwide Evidence of Bronchopulmonary Strongyle Nematodes and the First Report on Italy of Cryptosporidium sp. in Allochthonous Nutria (Myocastor coypus). Diversity, 15(5), 611. https://doi.org/10.3390/d15050611








