Abstract
In highly modified coastal environments, such as commercial harbours, the installation of artificial habitats has garnered support as a means of enhancing local biological recruitment and connectivity. The success of these measures depends largely on the patterns of species colonisation. Using post-installation monitoring data, we compared the composition of assemblages of invertebrates colonising artificial habitats that were immersed for different periods (~6 vs. ~18 months) in three commercial harbours along the French Mediterranean coast. The artificial habitats were colonised by taxonomically diverse invertebrate assemblages of ecological and economic importance, including molluscs, crustaceans, and echinoids. Composition differed significantly with the immersion time of the artificial habitats, with total abundance, species richness, and evenness being significantly higher after ~18 than after ~6 months of immersion, indicating that long periods are necessary to enrich these new habitats with economically and ecologically important species. These results can inform restoration protocols and emphasise the value of post-installation monitoring programs.
1. Introduction
Habitat degradation and loss threaten population persistence, biodiversity, and the functioning of ecosystems [1,2,3]. Ecosystem managers face the challenge of implementing conservation and restoration initiatives in altered environments [4] where the effects of climate change exacerbate the impacts of coastal development [5,6,7,8]. In systems where it is determined that ecosystem thresholds have been crossed as a result of human impacts and where changes are irreversible, such as on heavily modified coastlines within large grey infrastructures (e.g., ports, harbours, commercial marinas), options for their management as ‘novel ecosystems’ may be considered to manipulate them and fulfil desired ecological conditions or functions [4,9,10].
The installation of artificial habitats with ecologically-engineered elements has been widely advocated and implemented for replacement of lost or degraded natural habitat, ecological conservation, biodiversity enhancement, and improvement of ecosystem services [11,12,13,14]. Specific goals of artificial habitats may include supporting local biodiversity and communities of fish or invertebrates of commercial or ecological interest [12,15,16,17,18,19], building ecosystem resilience, and enhancing ecological connectivity [4,20,21,22].
Evidence shows the efficacy of these artificial habitats in attracting marine organisms at different development stages, from larvae to adults, although the patterns of colonisation are context-dependent [17,20,22,23,24]. These patterns can depend on processes of community assembly and succession that are determined, among others, by the timing of species colonisation and interactions among species [25,26,27]. It is therefore anticipated that implementing artificial habitats in degraded ecosystems can facilitate or accelerate successional processes that foster the establishment and maintenance of diverse communities [24,26,28].
Evaluating the colonisation process of artificial habitats is key for assessing their use in ecologically degraded coastal ecosystems. In this study, we examine the composition (structure and diversity) of invertebrate assemblages colonising artificial habitats after two distinct immersion periods: 5.5–7 months (Year 1), and 17.5–19.5 months (Year 2).
The artificial habitats (Dock Biohut®; Ecocean SAS, Montpellier, Paris) were designed to provide ecological nursery habitat within commercial harbours and marinas [17,22]. We used a subset of existing monitoring data from these artificial habitats in three spatially distinct commercial harbours along the French Mediterranean coast where post-installation sampling replication allowed for comparison of colonisation across years. We compared invertebrate assemblages found in artificial habitats in Years 1 and 2 and hypothesised that the species composition of invertebrates would differ across time periods and that abundance and taxonomic diversity would increase with immersion time.
2. Materials and Methods
2.1. Study Sites
This study uses ecological monitoring data from three large commercial harbours in the Gulf of Lion along the French Mediterranean coast, separated by distances of 29 to 204 km, namely Le Barcarès (42.7980° N, 3.0375° E), Port-Vendres (42.5190° N, 3.1089° E), and Grand Port Maritime de Marseille (43.3448° N, 5.3377° E). Each of these three harbours has >200 vessel moorings and has been operating commercially for >40 years, although the physical and environmental characteristics of each harbour vary across a range of parameters (Table 1).

Table 1.
Characteristics of the three study sites (harbours) and Dock Biohut sampling.
We extracted this subset of data from a large monitoring database comprising data from Biohuts installed in 21 harbours and marinas across 19 French cities and in Monaco between 2013 and 2017 (Table S1). The subset was selected to allow for sufficient replication of artificial habitats within harbours across the years.
2.2. Sampling Unit and Protocol
Biohuts were composed of two adjoined carbon-steel alloy cages (50 × 80 × 12.5 cm; combined cages depth 25 cm) and attached to the dockside (Figure 1). One cage was filled with empty oyster shells to provide complex substrate and was positioned against the dock (2.5 cm mesh-size); the outward-facing adjoining cage was left empty (5 cm mesh size) to keep out large mobile predatory fish.
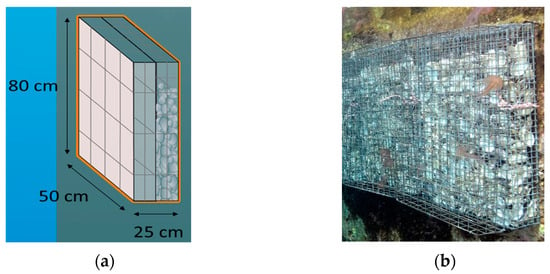
Figure 1.
Dimensions (a) and image (b) of Dock Biohut structures, composed of two carbon-steel alloy cages: inner-cage filled with oyster shells (2.5 cm mesh) and empty outer-cage (5 cm mesh).
In March and June 2013, all the sampled Biohuts were installed in each harbour, submerged just below the surface of the water. Assemblages were sampled on randomly selected Biohuts at least 20 m apart, either 5.5–7 (Year 1) or 17.5–19.5 months after installation (Year 2; Table 1). Because of the number of remaining Biohuts available in Year 2, the number of sampled Biohuts were different among years. During Year 1, 30 Biohuts were sampled (9 in Le Bacarès, 12 in Marseille and 9 in Port-Vendres), whereas 16 were sampled in Year 2 (4 in Le Bacarès, 7 in Marseille, and 5 in Port-Vendres). During monitoring, the Biohuts were encased with a PVC net (2 mm mesh) by divers to prevent loss of organisms during removal and lifted from the water onto the adjoining dock. Biohuts were then disassembled, the organisms identified to the lowest taxonomic level possible, and they were counted. The sampling protocol did not allow us to sample for macroalgal cover or biomass and we focused the study on consumers.
2.3. Data Analysis
We fitted generalised linear mixed models (GLMM) with time period as fixed factor (two levels: Year 1, Year 2) and harboured a random factor [29] on univariate data. We used this structure to model the biodiversity of invertebrate assemblages (species richness, Shannon diversity, Pielou’s evenness), the abundance of specific taxa (Bivalvia, Gastropoda, Malacostraca, Ophiuroidea), and the abundance of commercially exploited taxa that contributed >5% to the total invertebrate abundance (e.g., the palaemonid shrimp Palaemon spp.; the variegated scallop Mimachlamys varia [30]). Mixed-effects models that estimate parameters based on residual maximum likelihood were used due to their capacity to more appropriately handle unbalanced designs (particularly with random effects) than alternative approaches using observed and expected mean squares or error strata [31]. The count data of classes and total abundance were fitted using a negative binomial distribution to accommodate alternative exponential distributions of residuals due to evidence of overdispersion (with glmer.nb in lme4). Temporal variation in species richness was modelled with a Poisson distribution due to exponential variance, but within the assumed bounds of dispersion (glmer in lme4). Temporal variation in Shannon diversity and Pielou’s evenness was assessed with Gaussian models and a constant variance structure due to heteroscedasticity between time periods. Model assumptions were assessed visually using diagnostic plots of Pearson residuals. Variation in the multivariate taxonomic composition of invertebrate assemblages through time was tested using a two-way nested PERMANOVA (maximum permutations = 9999) and then visualised with non-metric multidimensional scaling (nMDS) based on a Bray-Curtis dissimilarity matrix of log (x + 1) transformed data. We used Monte Carlo sampling to estimate differences due to limited available unique permutations (360) and unconverged permutation versus Monte Carlo P-values [32].
Before running PERMANOVA, the homogeneity of residuals was tested using PERMDISP with time period (fixed) and harbour (random) as factors. Similarity percentage analysis (SIMPER) was also performed using Primer v6 with PERMANOVA+ [32,33]. The data were log (x + 1) transformed to quantify, (1) overall similarity across harbours across the time periods, and (2) mean similarity within or dissimilarity between harbours across time periods. SIMPER was also used to identify those species contributing consistently to similarity or dissimilarity (similarity or dissimilarity/standard deviation ≥ 2).
3. Results
A total of 48 invertebrate taxa from 39 families, 8 classes, and 5 phyla were recorded in Biohut structures across both survey periods (Tables S1 and S2). All animals were classified as native to the Mediterranean [34]. There were significant differences between Year 1 and Year 2 in total abundance (z (1,42) = 2.36, p = 0.02), species richness (s (1,42) = 2.28, p = 0.02), and Pielou’s evenness (s (1,42) = 2.07, p = 0.04), but not Shannon diversity (Tables S3 and S4). Abundance and species richness were higher in Year 2 than Year 1 (Figure 2).
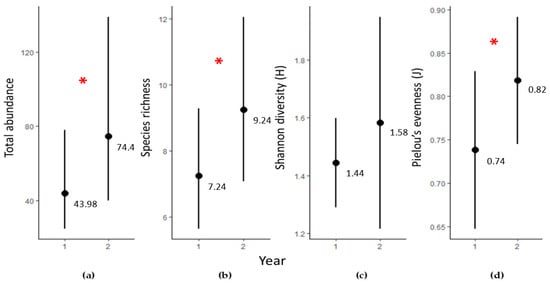
Figure 2.
Temporal variation (fitted values ±95% confidence intervals) in: (a) the total abundance (number of individuals/0.1 m3 of artificial structure); (b) species richness (number of taxa/0.1m3 of artificial structure); (c) Shannon diversity; and (d) Pielou’s evenness of invertebrate assemblages in artificial Dock Biohut structures within Year 1 and Year 2 since installation. Significant differences between time periods of each metric are indicated with asterisks (red * indicates p ≤ 0.05).
The taxonomic composition of invertebrate assemblages varied between Year 1 and Year 2. Non-metric MDS ordination showed distinct clusters between Year 1 and Year 2 (Figure 3). For both Le Bacarès and Port-Vendres, the Year 2 data were closer to each other than for the Year 1. The stress value (0.07, 0.1, and 0.08, respectively) provides a good representation of our data in reduced dimensions. PERMANOVA (F (1,40) = 3.569; p < 0.05) analysis revealed variation in taxonomic composition of invertebrate assemblages from Year 1 to Year 2 (Figure 3). However, PERMDISP analysis showed significant differences in the mean distance from centroids among the groups (F (5,40) = 11.099; p < 0.001), indicating that results from PERMANOVA should be interpreted with caution.
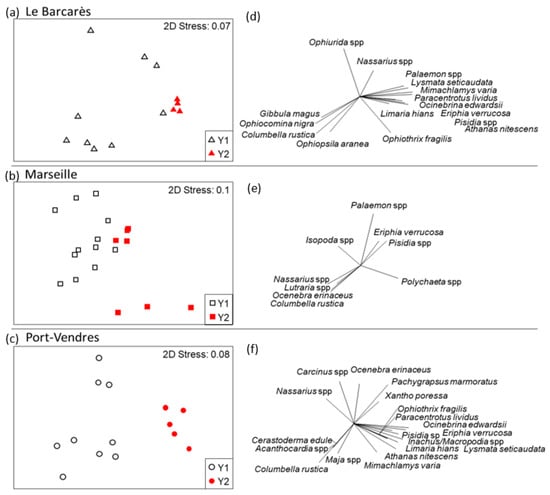
Figure 3.
Non-metric multidimensional scaling analysis showing: Variation in taxonomic composition of invertebrate assemblages among surveyed Dock Biohut structures in each harbour between years (Y1 and Y2) since installation ((a–c); log x + 1 transformed data); and the relative contribution of species to variation at each harbour ((d–f); >0.5 Pearson correlation).
Changes in assemblage composition between Year 1 and Year 2 caused an overall increase in taxonomic similarity of assemblages across all harbours (average assemblage similarity: Year 1, 28%; Year 2, 37%), with an average 78% dissimilarity in species composition between years. This overall increase was likely driven largely by increased similarity in taxonomic composition of assemblages at La Bacarès (Year 1, 28%; Year 2, 72%) and Port-Vendres (Year 1, 47%; Year 2, 70%), and not Marseille where similarity decreased (Year 1, 39%; Year 2, 31%; Table 2). In Year 1, only the variegated scallop Mimachlamys varia contributed consistently to assemblage similarity among Biohuts in Port-Vendres. However, in Year 2, six species consistently characterised species assemblages in Le Barcarès and eight species in Port-Vendres. In Marseille, no species consistently contributed to assemblage similarity in either year.

Table 2.
Similarity Percentage analysis of invertebrate assemblages in surveyed Dock Biohuts through time. Species consistently contributing to the average similarity within (sim/SD > 2), and dissimilarity between (diss/SD > 2) harbours from Year 1 (Y1) to Year 2 (Y2) identified in one-way SIMPER analysis are shown.
There was an overall increase in the abundance of Malacostraca (z (1,42) = 4.50, p < 0.0001), but not in Bivalvia, Gastropoda, or Ophiuroidea (Figure 4; Tables S3 and S4). Of 11 surveyed taxa identified as potentially commercially exploited [30], only two contributed to >5% of the total invertebrate abundance—Palaemon spp.(palaemonid shrimp) and M. varia—but neither varied in abundance significantly across years (Table S5). The remaining nine species (Carcinus spp.; common cockle Cerastoderma edule; black squat lobster Galathea squamifera; small periwinkle Melarhaphe neritoides; European flat oyster Ostrea edulis; purple sea urchin Paracentrotus lividus; Periclimenes spp. shrimp; bristle worm Polychaeta spp.; common cuttlefish Sepia officinalis) each accounted for <2% of the total surveyed invertebrate abundance (Table S6).

Figure 4.
Temporal variation (fitted values ±95% confidence intervals) in the total abundance (number of individuals/0.1m3 of artificial structure) of (a) Malacostraca; (b) Gastropoda; (c) Bivalvia; and (d) Ophiuroidea in surveyed Dock Biohut structures within Year 1 and Year 2 since installation. Significant differences between time periods are indicated with asterisks (red *** p < 0.001).
4. Discussion
The examination of post-installation monitoring data found that artificial habitats (Dock Biohut) hosted taxonomically diverse assemblages of invertebrate species, including molluscs, crustaceans, and echinoids of ecological, commercial, and social interest. Our analysis aims to complement the already-existing studies focused on the fish species associated with artificial habitats [17,18,19,22]. Communities develop and are structured over time, whereby pioneering species initially colonise areas, with the abundance and composition of colonising assemblages depending on interacting factors including habitat size and connectivity, the proximity of source populations, local hydrodynamics, inter-annual temporal variation in larval supply, and competitive interactions with other species [20,27,35,36,37].
Community development in restoration or conservation ecology would likely be time-dependent in achieving desired endpoints of biodiversity, productivity, and species-specific configurations [26,38]. The immersion time of artificial habitats is a known, influential predictor of community composition due to processes of faunal succession [25,27,39,40]. In our study, the results indicated that community changes through time, likely due to spatially and temporally variable colonisation by different species [27,39]. The results showed differences in the colonisation and recruitment of organisms in the Biohuts between Year 1 and Year 2 of immersion, indicating the capacity of artificial habitats to support local biodiversity enhancement via the recruitment of organisms in highly modified harbours.
We found significantly greater total abundance, species richness, species evenness, and abundance of crustaceans in artificial habitats across the three spatially distinct harbours after a longer period of immersion. Multivariate analysis of our data also showed differences in artificial habitats assemblages between Year 1 and Year 2. Indeed, significant variation in composition between the invertebrate assemblages sampled in Year 1 and Year 2 after deployment of the Dock Biohuts indicates the processes of community development and highlights the role of habitat soak-time in determining the outcome of artificial habitat installation initiatives [24,26,27].
Our analyses revealed an increase in the similarity in composition both within and among assemblages in two of the three spatially distinct harbours between Year 1 and Year 2, and an overall increase in abundance of crustaceans—a group of ecologically important organisms due to their role in food-web dynamics [41]—and their influence on the behaviour of settlement-stage larval organisms [42,43,44,45,46]. Species composition was highly variable in the first year across all harbours, but in Year 2, assemblage structure became similar within and between Port-Vendres and Le Barcarès, with the dominance of molluscs, crustaceans, and echinoderms. These similarities and the differences with Marseille harbour could be explained by the environmental characteristics of Marseille harbour, which is the largest and the deepest harbour of this study, and it is not directly influenced by outflow from the Rhone River, which delivers organic matter and sediment into the other two study harbours. Furthermore, the Biohuts of the Marseille harbour were positioned at a greater distance from the harbour entrance than those of the other two harbours. Finally, the differences in species assemblages could also be due to the local availability of species, e.g., the ecological concept of species pool [47,48]. We observed an overall increase in abundance of Malacostraca, while the abundance of other predominant classes (gastropods, bivalves and brittlestars) remained consistent, carrying implications for efforts targeting ecological restoration [26,41,46]. Crustaceans are key components of the diets of a range of macroinvertebrates and finfish [41], and an increase in their abundance may have implications for local food-web dynamics [49]. Similarly, crustaceans can create a loud and acoustically complex biophony, producing acoustic cues used by settlement-stage larvae of fish and invertebrates that likely further enhances community development [42,43,44,45,46,50]. For example, the estimated detection distance of snaps of the shrimp, Athanas nitescens, characteristic of Biohut invertebrate assemblages in Le Barcarès and Port-Vendres by Year 2, can be up to 40 m [51]. As such, shifts towards greater abundance of crustaceans may have a disproportionate role in the maintenance, development, and function of locally diverse ecological communities [26,41], and may point towards opportunities for passive acoustic monitoring of community development where intrusive survey techniques are less desirable [51,52,53].
Our results indicate that provided that the artificial habitats do not simply concentrate organisms, they may enhance local productivity and biodiversity in highly modified areas within relatively short periods of time [54]. Similarly, the observed differences in assemblage composition through time suggests that where specific species configurations are desired endpoints for habitat restoration, understanding how local communities are structured over time will likely enable pragmatic management goal setting [26,27]. Many species of crustaceans are also highly valued commercial and recreational fisheries resources [30]. Where artificial habitats can enhance rather than relocate local productivity, they may provide opportunities for harvesting species in support of fisheries enhancement initiatives [12,55,56], for the live-trade of ornamental organisms [18], or for aquaculture [57].
Artificial habitats can enhance the ecological capacity of highly modified areas of coastline such as large commercial ports and marinas by providing habitats for marine life at different stages of life-history and migration [4,10,19,22]. The nursery capacity of artificial habitats in large commercial ports has been shown previously for diverse assemblages of juvenile finfishes, with typically higher abundance and species richness on artificial habitat structures than on adjacent bare surfaces [17,19,22]. The availability of fine-scale structural complexity, such as is created by caged oyster shells in the focal Biohut structures, can provide refugia and enhance the survival of small-bodied and/or juvenile stage organisms when their risk of mortality is highest [22,58]. Furthermore, the colonisation, abundance and species diversity of macroinvertebrate fauna can be directly associated with availability and structural characteristics of habitats [28,59,60,61,62,63,64]. Investigating existing ecological monitoring data, our results provide insights into the relatively short-term capacity of artificial habitats to attract and maintain diverse assemblages of invertebrates. Moreover, our results highlight the role of habitat duration in community development and changes, and the establishment of biodiversity in highly modified commercial harbours. Our results also suggest that the environmental and physical characteristics of the harbours equipped with artificial habitat structures can also facilitate the colonisation by specific invertebrate assemblages. Furthermore, longer temporal studies comparing the colonisation of artificial habitats against background levels of diversity and productivity would enable greater understanding of their capacity to augment the ecological function of modified systems [54]. This includes improving our understanding of their role as ecological steppingstones for enhanced connectivity, and the ecological processes determining positive feedbacks and alternative states across degraded systems [15,38].
Biodiversity conservation and restoration are widely supported management goals [26,65,66], with species diversity being considered important for promoting ecosystem resilience via the maintenance of critical ecosystem functioning during disturbance (due to functional redundancy and response diversity [67,68]). Increasingly, efforts to restore or replace nursery habitats are viewed as a key component of the conservation of biodiversity and management of productive systems [5,69]. Our results indicate that periods longer than 7 months are necessary to enrich these artificial habitats of economically and ecologically important species. Finally, given the ecological importance of invertebrates in trophic dynamics and community development [41,45], experimental research considering the influence of variation in invertebrate assemblage composition through time on the recruitment of teleost fishes may aid in the understanding of the capacity for complementary acoustic ecological enhancement programs [46,70,71].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15040505/s1, Table S1: Surveyed species recorded in Biohut structures in Le Barcarès (BA), Port-Vendres (PV), and Grand Port maritime de Marseille (MA) in 2013 and 2014; Table S2: Invertebrate species surveyed between 2013 and 2017 in artificial structures (Dock Biohut: D; Pontoon Biohut, P) installed within 21 harbours, in 19 cities in France and Monaco during monitoring (total = 115 spp.); Table S3: Temporal comparisons (with 95% confidence intervals: CI) of invertebrate assemblages in Dock Biohut across harbours (random factor) in Year 1 to Year 2 (linear mixed effects models). Significant metrics shown in bold; Table S4: Mean ± SE total abundance, biodiversity, and abundance of classes of invertebrates surveyed within Biohut structures in year 1 and year 2 since installation; Table S5: Temporal comparisons (with 95% confidence intervals: CI) of potentially exploited species surveyed contributing > 5% of the total abundance of invertebrate assemblages in surveyed Biohuts in Year 1 and Year 2 (linear mixed effects models) [30]; Table S6: Mean ± SE total abundance of commercially exploitable species in surveyed Dock Biohut structures in Year 1 and Year 2 [30].
Author Contributions
Conceptualisation, L.E.R., S.D.S., A.N.R., G.L., A.G., P.B. and P.L.; methodology, L.E.R., A.V., S.D.S., A.N.R., G.L., A.G., P.B. and P.L.; software, L.E.R., A.V. and F.R.; validation, A.V., L.E.R., S.D.S. and F.R.; formal analysis, A.V., L.E.R., S.D.S. and F.R.; investigation, A.G., L.B. and E.A.; resources, A.G., L.B. and E.A.; data curation, G.L., A.G. and E.A.; writing—original draft preparation, L.E.R. and A.V.; writing—review and editing, all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge the Agence de l’Eau Rhône Méditerranée Corse for their support and their implication on restauration project in the Mediterranean sea. We thank harbour managers of Le Barcarès, Port-Vendres, and the Grand Port Maritime de Marseille for permissions to carry out the study, and Remy Dubas and Sebastien Fonbonne for field and logistical support.
Conflicts of Interest
Authors GL, AG and EA are employed by the company Ecocean SAS. Ecocean funded a joint meeting in the UK and supported AV and LR’s time to write this article. The remaining authors declare that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of Foundation Species: Consequences for the Structure and Dynamics of Forested Ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.C.; Kaplan, J.O.; Fuller, D.Q.; Vavrus, S.; Klein Goldewijk, K.; Verburg, P.H. Used Planet: A Global History. Proc. Natl. Acad. Sci. USA 2013, 110, 7978–7985. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Higgs, E.; Hall, C.M.; Bridgewater, P.; Chapin III, F.S.; Ellis, E.C.; Ewel, J.J.; Hallett, L.M.; Harris, J.; Hulvey, K.B.; et al. Managing the Whole Landscape: Historical, Hybrid, and Novel Ecosystems. Front. Ecol. Environ. 2014, 12, 557–564. [Google Scholar] [CrossRef]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The Identification, Conservation, and Management of Estuarine and Marine Nurseries for Fish and Invertebrates: A Better Understanding of the Habitats That Serve as Nurseries for Marine Species and the Factors That Create Site-Specific Variability in Nursery Quality Will Improve Conservation and Management of These Areas. BioScience 2001, 51, 633–641. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J.; et al. Climate Change, Human Impacts, and the Resilience of Coral Reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-Term Decline of the Populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères Coast (France, North-Western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Townhill, B.; Pinnegar, J.; Tinker, J.; Jones, M.; Simpson, S.; Stebbing, P.; Dye, S. Non-Native Marine Species in North-West Europe: Developing an Approach to Assess Future Spread Using Regional Downscaled Climate Projections. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1035–1050. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel Ecosystems: Theoretical and Management Aspects of the New Ecological World Order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Ido, S.; Shimrit, P.-F. Blue Is the New Green—Ecological Enhancement of Concrete Based Coastal and Marine Infrastructure. Ecol. Eng. 2015, 84, 260–272. [Google Scholar] [CrossRef]
- Grove, R.S.; Sonu, C.J.; Nakamura, M. Design and Engineering of Manufactured Habitats for Fisheries Enhancement. In Artificial Habitats for Marine and Freshwater Fisheries; Elsevier: Amsterdam, The Netherlands, 1991; pp. 109–152. ISBN 978-0-08-057117-1. [Google Scholar]
- Bell, J.D.; Bartley, D.M.; Lorenzen, K.; Loneragan, N.R. Restocking and Stock Enhancement of Coastal Fisheries: Potential, Problems and Progress. Fish. Res. 2006, 80, 1–8. [Google Scholar] [CrossRef]
- Morris, R.L.; Konlechner, T.M.; Ghisalberti, M.; Swearer, S.E. From Grey to Green: Efficacy of Eco-Engineering Solutions for Nature-Based Coastal Defence. Glob. Chang. Biol. 2018, 24, 1827–1842. [Google Scholar] [CrossRef] [PubMed]
- Firth, L.B.; Airoldi, L.; Bulleri, F.; Challinor, S.; Chee, S.-Y.; Evans, A.J.; Hanley, M.E.; Knights, A.M.; O’Shaughnessy, K.; Thompson, R.C.; et al. Greening of Grey Infrastructure Should Not Be Used as a Trojan Horse to Facilitate Coastal Development. J. Appl. Ecol. 2020, 57, 1762–1768. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime Shifts, Resilience, and Biodiversity in Ecosystem Management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Baine, M. Artificial Reefs: A Review of Their Design, Application, Management and Performance. Ocean Coast. Manag. 2001, 44, 241–259. [Google Scholar] [CrossRef]
- Mercader, M.; Mercière, A.; Saragoni, G.; Cheminée, A.; Crec’hriou, R.; Pastor, J.; Rider, M.; Dubas, R.; Lecaillon, G.; Boissery, P.; et al. Small Artificial Habitats to Enhance the Nursery Function for Juvenile Fish in a Large Commercial Port of the Mediterranean. Ecol. Eng. 2017, 105, 78–86. [Google Scholar] [CrossRef]
- Bell, J.D.; Clua, E.; Hair, C.A.; Galzin, R.; Doherty, P.J. The Capture and Culture of Post-Larval Fish and Invertebrates for the Marine Ornamental Trade. Rev. Fish. Sci. 2009, 17, 223–240. [Google Scholar] [CrossRef]
- Mercader, M.; Fontcuberta, A.; Mercière, A.; Saragoni, G.; Boissery, P.; Bérenger, L.; Dubas, R.; Lecaillon, G.; Pastor, J.; Lenfant, P. Observation of Juvenile Dusky Groupers (Epinephelus marginatus) in Artificial Habitats of North-Western Mediterranean Harbors. Mar. Biodivers. 2017, 47, 371–372. [Google Scholar] [CrossRef]
- Higgins, E.; Scheibling, R.E.; Desilets, K.M.; Metaxas, A. Benthic Community Succession on Artificial and Natural Coral Reefs in the Northern Gulf of Aqaba, Red Sea. PLoS ONE 2019, 14, e0212842. [Google Scholar] [CrossRef]
- Sutton, S.G.; Bushnell, S.L. Socio-Economic Aspects of Artificial Reefs: Considerations for the Great Barrier Reef Marine Park. Ocean Coast. Manag. 2007, 50, 829–846. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Darnaude, A.; Gudefin, A.; Neveu, R.; Verdoit-Jarraya, M.; Boissery, P.; Lenfant, P. Potential Use of Marinas as Nursery Grounds by Rocky Fishes: Insights from Four Diplodus Species in the Mediterranean. Mar. Ecol. Prog. Ser. 2016, 547, 193–209. [Google Scholar] [CrossRef]
- Mercader, M.; Rider, M.; Cheminée, A.; Pastor, J.; Zawadzki, A.; Mercière, A.; Crec’hriou, R.; Verdoit-Jarraya, M.; Lenfant, P. Spatial Distribution of Juvenile Fish along an Artificialized Seascape, Insights from Common Coastal Species in the Northwestern Mediterranean Sea. Mar. Environ. Res. 2018, 137, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Komyakova, V.; Chamberlain, D.; Jones, G.P.; Swearer, S.E. Assessing the Performance of Artificial Reefs as Substitute Habitat for Temperate Reef Fishes: Implications for Reef Design and Placement. Sci. Total Environ. 2019, 668, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, G.; Mackay, R.J.; Smith, I.M. Evolutionary and ecological strategies of animals in annual temporary pools. Evol. Ecol. Strateg. Anim. Annu. Tempor. POOLS 1980, 58, 97–206. [Google Scholar]
- Palmer, M.A.; Ambrose, R.F.; Poff, N.L. Ecological Theory and Community Restoration Ecology. Restor. Ecol. 1997, 5, 291–300. [Google Scholar] [CrossRef]
- Young, T.P.; Chase, J.M.; Huddleston, R.T. Community Succession and Assembly: Comparing, Contrasting and Combining Paradigms in the Context of Ecological Restoration. Ecol. Restor. 2001, 19, 5–18. [Google Scholar] [CrossRef]
- Hauser, A.; Attrill, M.; Cotton, P. Effects of Habitat Complexity on the Diversity and Abundance of Macrofauna Colonising Artificial Kelp Holdfasts. Mar. Ecol. Prog. Ser. 2006, 325, 93–100. [Google Scholar] [CrossRef]
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 13 May 2018).
- FAO. ASFIS List of Species for Fishery Statistics Purposes. Fisheries and Aquaculture Division [Online]. Rome. Available online: https://www.fao.org/fishery/en/collection/asfis/en (accessed on 1 August 2021).
- Logan, M. Biostatistical Design and Analysis Using R: A Practical Guide, 1st ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-1-4051-9008-4. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; Primer-E Ltd.: Plymouth, UK, 2014. [Google Scholar]
- Palomares, M.L.D.; Pauly, D. Search SeaLifeBase. Available online: https://www.sealifebase.ca/ (accessed on 25 January 2023).
- Sale, P.F.; Doherty, P.J.; Eckert, G.J.; Douglas, W.A.; Ferrell, D.J. Large Scale Spatial and Temporal Variation in Recruitment to Fish Populations on Coral Reefs. Oecologia 1984, 64, 191–198. [Google Scholar] [CrossRef]
- Caffey, H.M. Spatial and Temporal Variation in Settlement and Recruitment of Intertidal Barnacles. Ecol. Monogr. 1985, 55, 313–332. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P. (Eds.) Ecological Assembly Rules: Perspectives, Advances, Retreats; Cambridge University Press: Cambridge, UK, 1999; ISBN 978-0-521-65235-3. [Google Scholar]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative States and Positive Feedbacks in Restoration Ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef]
- Wiens, J.A. On Understanding a Non-Equilibrium World: Myth and Reality in Community Patterns and Processes. In 25. On Understanding a Non-Equilibrium World: Myth and Reality in Community Patterns and Processes; Princeton University Press: Princeton, NJ, USA, 2014; pp. 439–457. ISBN 978-1-4008-5708-1. [Google Scholar]
- Schneider, D.W.; Frost, T.M. Habitat Duration and Community Structure in Temporary Ponds. J. N. Am. Benthol. Soc. 1996, 15, 64–86. [Google Scholar] [CrossRef]
- Szaniawska, A. Function and Importance of Crustaceans. In Baltic Crustaceans; Szaniawska, A., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 185–188. ISBN 978-3-319-56354-1. [Google Scholar]
- Simpson, S.D.; Meekan, M.; Montgomery, J.; McCauley, R.; Jeffs, A. Homeward Sound. Science 2005, 308, 221. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.C.; Jeffs, A.; Simpson, S.D.; Meekan, M.; Tindle, C. Sound as an Orientation Cue for the Pelagic Larvae of Reef Fishes and Decapod Crustaceans. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 2006; Volume 51, pp. 143–196. ISBN 978-0-12-026152-9. [Google Scholar]
- Stanley, J.A.; Radford, C.A.; Jeffs, A.G. Induction of Settlement in Crab Megalopae by Ambient Underwater Reef Sound. Behav. Ecol. 2010, 21, 113–120. [Google Scholar] [CrossRef]
- Lillis, A.; Eggleston, D.B.; Bohnenstiehl, D.R. Oyster Larvae Settle in Response to Habitat-Associated Underwater Sounds. PLoS ONE 2013, 8, e79337. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, E.; Berten, L.; Rigo, P.; Aubrun, F.; Nedelec, S.L.; Simpson, S.D.; Lecchini, D. The Influence of Various Reef Sounds on Coral-Fish Larvae Behaviour. J. Fish Biol. 2015, 86, 1507–1518. [Google Scholar] [CrossRef]
- Cornell, H.V.; Harrison, S.P. What Are Species Pools and When Are They Important? Annu. Rev. Ecol. Evol. Syst. 2014, 45, 45–67. [Google Scholar] [CrossRef]
- Shen, T.-J.; Chen, Y.; Chen, Y.-F. Estimating Species Pools for a Single Ecological Assemblage. BMC Ecol. 2017, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Leitão, F.; Santos, M.N.; Monteiro, C.C. Contribution of Artificial Reefs to the Diet of the White Sea Bream (Diplodus sargus). ICES J. Mar. Sci. 2007, 64, 473–478. [Google Scholar] [CrossRef]
- Simpson, S.D.; Meekan, M.G.; McCauley, R.D.; Jeffs, A. Attraction of Settlement-Stage Coral Reef Fishes to Reef Noise. Mar. Ecol. Prog. Ser. 2004, 276, 263–268. [Google Scholar] [CrossRef]
- Coquereau, L.; Grall, J.; Chauvaud, L.; Gervaise, C.; Clavier, J.; Jolivet, A.; Di Iorio, L. Sound Production and Associated Behaviours of Benthic Invertebrates from a Coastal Habitat in the North-East Atlantic. Mar. Biol. 2016, 163, 127. [Google Scholar] [CrossRef]
- Nedelec, S.L.; Simpson, S.D.; Holderied, M.; Radford, A.N.; Lecellier, G.; Radford, C.; Lecchini, D. Soundscapes and Living Communities in Coral Reefs: Temporal and Spatial Variation. Mar. Ecol. Prog. Ser. 2015, 524, 125–135. [Google Scholar] [CrossRef]
- Gervaise, C.; Lossent, J.; Valentini-Poirier, C.A.; Boissery, P.; Noel, C.; Di Iorio, L. Three-Dimensional Mapping of the Benthic Invertebrates Biophony with a Compact Four-Hydrophones Array. Appl. Acoust. 2019, 148, 175–193. [Google Scholar] [CrossRef]
- Pickering, H.; Whitmarsh, D. Artificial Reefs and Fisheries Exploitation: A Review of the ‘Attraction versus Production’ Debate, the Influence of Design and Its Significance for Policy. Fish. Res. 1997, 31, 39–59. [Google Scholar] [CrossRef]
- Bell, J.D.; Munro, J.L.; Nash, W.J.; Rothlisberg, P.C.; Loneragan, N.R.; Ward, R.D.; Andrew, N.R. Restocking and Stock Enhancement of Marine Invertebrate Fisheries. Adv. Mar. Biol. 2005, 49, xi–xii. [Google Scholar] [CrossRef]
- Richardson, L.E.; Lenfant, P.; Clarke, L.J.; Fontcuberta, A.; Gudefin, A.; Lecaillon, G.; Le Vay, L.; Radford, A.N.; Simpson, S.D. Examining Current Best-Practices for the Use of Wild Post-Larvae Capture, Culture, and Release for Fisheries Enhancement. Front. Mar. Sci. 2023, 9, 1058497. [Google Scholar] [CrossRef]
- Hair, C.; Bell, J.; Doherty, P. The Use of Wild-Caught Juveniles in Coastal Aquaculture and Its Application to Coral Reef Fishes. Responsible Mar. Aquac. 2002, 327–353. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. Body Size and Mortality Rates in Coral Reef Fishes: A Three-Phase Relationship. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161858. [Google Scholar] [CrossRef]
- Heck, K.L.; Orth, R.J. Seagrass habitats: The role of habitat complexity, competition and predation in structuring associated fish and motile macroinvertebrate assemblages. In Estuarine Perspectives; Elsevier: Amsterdam, The Netherlands, 1980; pp. 449–464. ISBN 978-0-12-404060-1. [Google Scholar]
- O’Connor, N.A. The Effects of Habitat Complexity on the Macroinvertebrates Colonising Wood Substrates in a Lowland Stream. Oecologia 1991, 85, 504–512. [Google Scholar] [CrossRef]
- Attrill, M.J.; Strong, J.A.; Rowden, A.A. Are Macroinvertebrate Communities Influenced by Seagrass Structural Complexity? Ecography 2000, 23, 114–121. [Google Scholar] [CrossRef]
- Warfe, D.M.; Barmuta, L.A. Habitat Structural Complexity Mediates Food Web Dynamics in a Freshwater Macrophyte Community. Oecologia 2006, 150, 141–154. [Google Scholar] [CrossRef]
- García-Sanz, S.; Tuya, F.; Navarro, P.G.; Angulo-Preckler, C.; Haroun, R.J. Post Larval, Short-Term, Colonization Patterns: The Effect of Substratum Complexity across Subtidal, Adjacent, Habitats. Estuar. Coast. Shelf Sci. 2012, 112, 183–191. [Google Scholar] [CrossRef]
- Fabricius, K.E.; De’ath, G.; Noonan, S.; Uthicke, S. Ecological Effects of Ocean Acidification and Habitat Complexity on Reef-Associated Macroinvertebrate Communities. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132479. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global Biodiversity Conservation Priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; de Bello, F.; Bennett, J.A.; Fibich, P.; Finerty, G.E.; Götzenberger, L.; Hiiesalu, I.; Kasari, L.; Lepš, J.; Májeková, M.; et al. Applying the Dark Diversity Concept to Nature Conservation: Dark Diversity and Nature Conservation. Conserv. Biol. 2017, 31, 40–47. [Google Scholar] [CrossRef]
- Walker, B.H. Biodiversity and Ecological Redundancy. Conserv. Biol. 1992, 6, 18–23. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response Diversity, Ecosystem Change, and Resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Norton, D.A. Towards a Conceptual Framework for Restoration Ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Mukhin, A.; Chernetsov, N.; Kishkinev, D. Acoustic Information as a Distant Cue for Habitat Recognition by Nocturnally Migrating Passerines during Landfall. Behav. Ecol. 2008, 19, 716–723. [Google Scholar] [CrossRef]
- Moeslund, J.E.; Brunbjerg, A.K.; Clausen, K.K.; Dalby, L.; Fløjgaard, C.; Juel, A.; Lenoir, J. Using Dark Diversity and Plant Characteristics to Guide Conservation and Restoration. J. Appl. Ecol. 2017, 54, 1730–1741. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).