Revealing the Stygobiotic and Crenobiotic Molluscan Diversity in the Caucasus: Part IV—Crenobiotic Belgrandiellinae Radoman, 1983 (Mollusca, Hydrobiidae) from Georgia †
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Study
2.3. Statistical Analysis
2.4. Molecular Phylogenetic Methods
2.5. Species Conservation Status
2.6. Abbreviations Used in the Text
| ANSP | Academy of Natural Sciences of Drexel University, Philadelphia, USA |
| AOO | Area of occupancy |
| EOO | Extent of occurrence |
| IEE | A.N. Severtzov Institute of Ecology and Evolution |
| ISU | Ilia State University, Tbilisi, Georgia |
| JGS | collection Jozef Grego, Banská Bystrica, Slovakia |
| FLMNH-UF | The Florida Museum of Natural History, Gainesville, Florida |
| LDA | Linear Discriminant Analysis |
| MNHN | Muséum national d’Histoire naturelle, Paris, France |
| MSU | Lomonosov Moscow State University |
| NHMUK | Natural History Museum London, UK |
| NHMW | Naturhistorisches Museum Wien, Austria |
| NMBE | Naturhistorisches Museum Bern, Switzerland |
| PIN | Yu. A. Orlov Paleontological Museum of the Paleontological Institute |
| RAS | Russian Academy of Sciences |
| WSBS | White Sea Biological Station |
| ZMH | Zoological Museum University Hamburg |
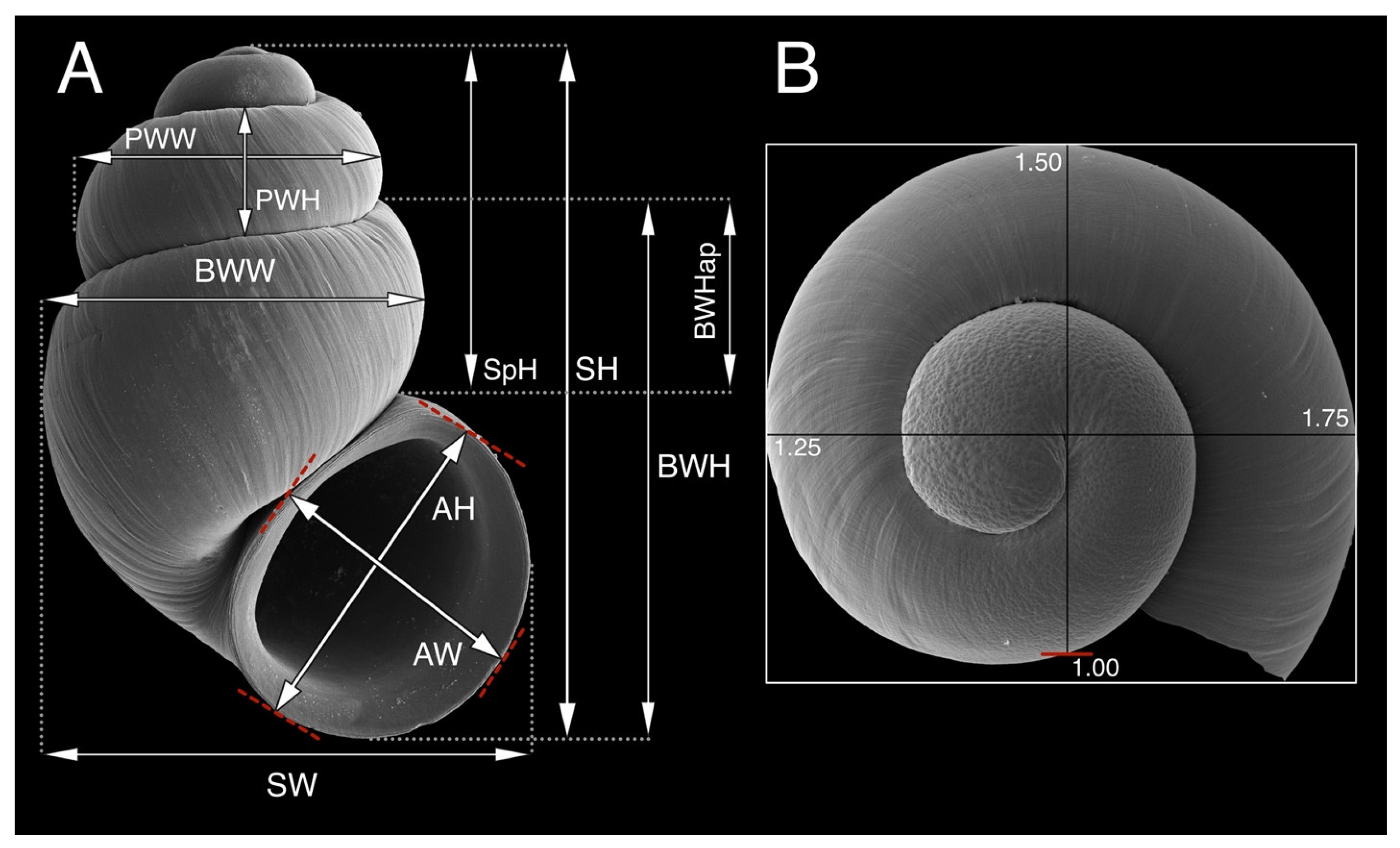
| Abbreviations related to the shell morphology | |
| AH | Aperture height |
| AW | Aperture width |
| BWH | Body whorl heigh |
| BWHap | Body whorl height above aperture |
| BWW | Body whorl width |
| PWH | Penultimate whorl height |
| PWW | Penultimate whorl width |
| SH | Shell height |
| SW | Shell width |
| SpH | Spire height |
3. Results
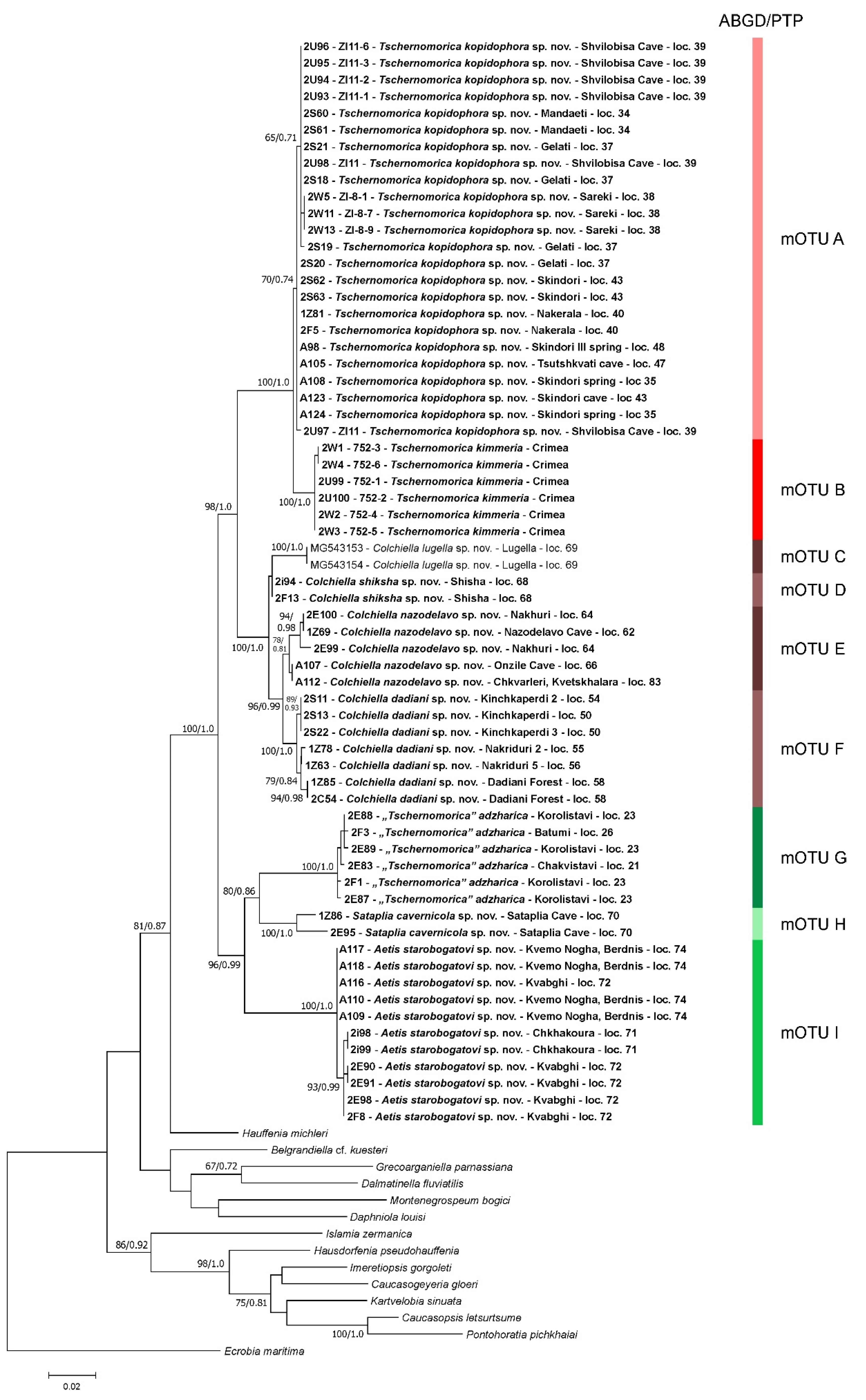
3.1. Molecular Phylogeny
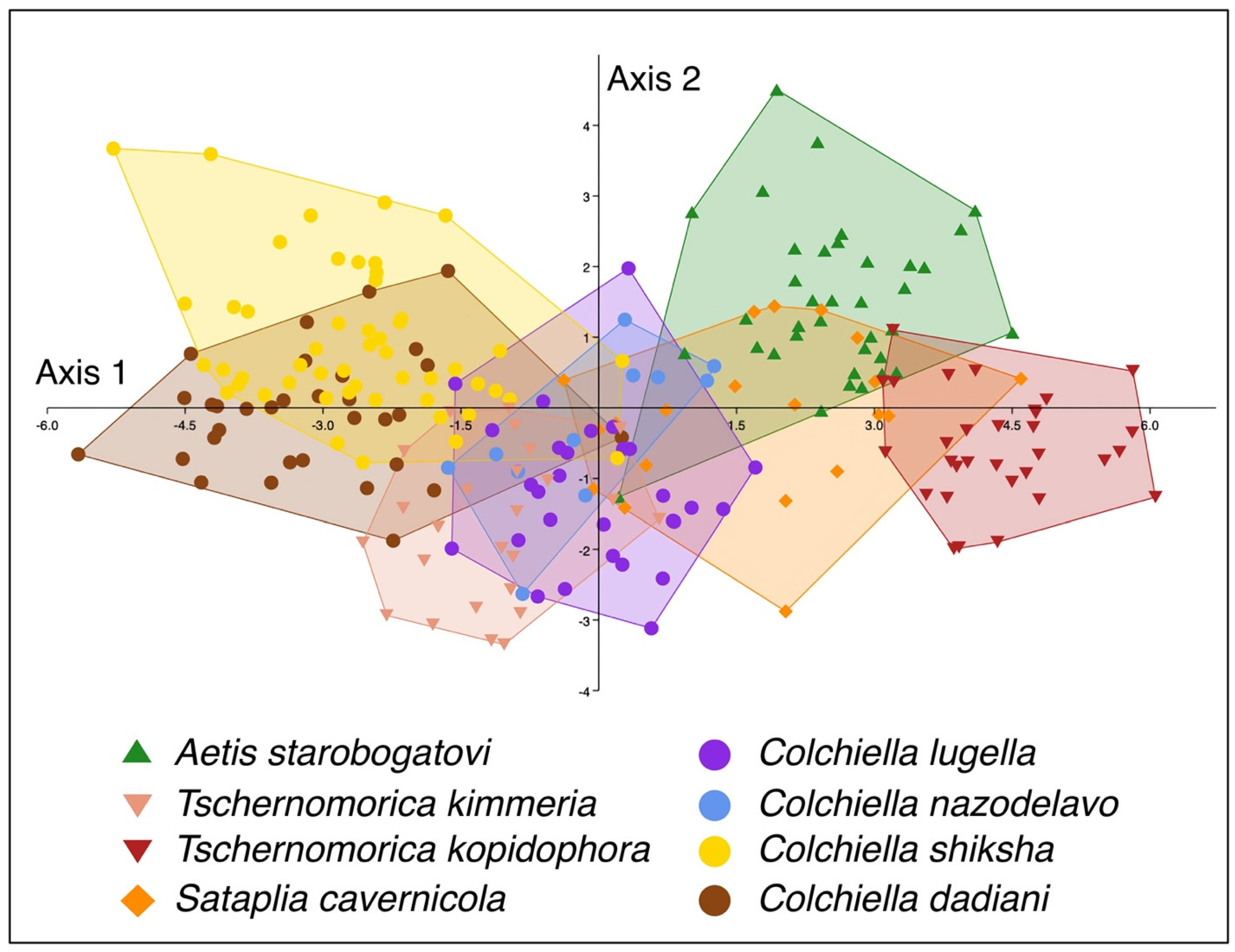
3.2. Morphologcal Analysis
3.3. Taxonomic Accounts
- Class: GASTROPODA Cuvier, 1795
- Subclass: Caenogastropoda Cox, 1960
- Clade: Hypsogastropoda Ponder & Lindberg, 1997
- Order: Littorinimorpha Golikov and Starobogatov, 1975
- Superfamily: Truncatelloidea Gray, 1840
- Family: Hydrobiidae Stimpson, 1865
- Subfamily: Belgrandiellinae Radoman, 1983
3.4. Genus Tschernomorica Vinarski & Palatov, 2019
3.4.1. Diagnosis
3.4.2. Distribution
3.4.3. Habitat
3.4.4. Remarks
3.5. Tschernomorica kimmeria Vinarski & Palatov, 2019.
3.5.1. Molecular Diagnosis
3.5.2. Distribution
3.5.3. Conservation Status
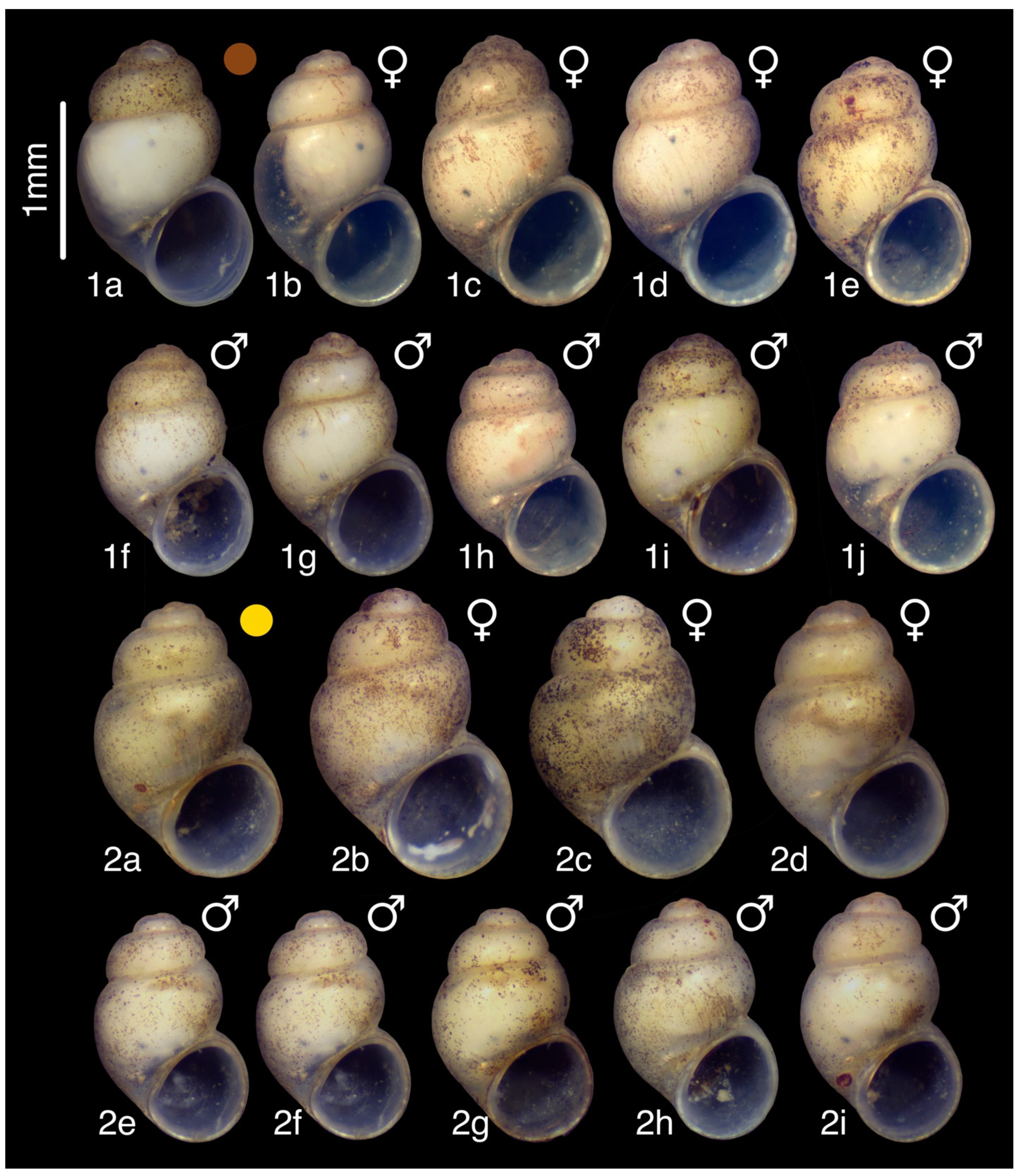
3.6. “Tschernomorica” adsharica (Lindholm, 1913)
- Tschernomorica adsharica Vinarski & Palatov, 2019 [9]
- Agrafia adsharica Grego et al., 2017 [25]
- Belgrandiella adsharica Vinarski & Kantor, 2016 [24]
- Belgrandiella adsharica Barjadze et al., 2015 [23]
- Belgrandiella adsharica Kantor et al., 2010 [22]
- Belgrandiella adsharica Bole & Velkovrh, 1983 [21]
- Belgrandiella adsharica Schütt & Şeşen, 1993 [6]
- Bythinella adsharica Lindholm, 1913 [1]
3.6.1. Material Examined
3.6.2. Molecular Diagnosis
3.6.3. Distribution
3.6.4. Conservation Status
3.6.5. Remarks
3.7. Tschernomorica kopidophora Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:706E6847-5231-4D3E-9AFA-DE4A7AAF4F7D
3.7.1. Material Examined
3.7.2. Diagnosis
3.7.3. Description
3.7.4. Variation
3.7.5. Etymology
3.7.6. Distribution
3.7.7. Habitat
3.7.8. Conservation Status
3.7.9. Remarks

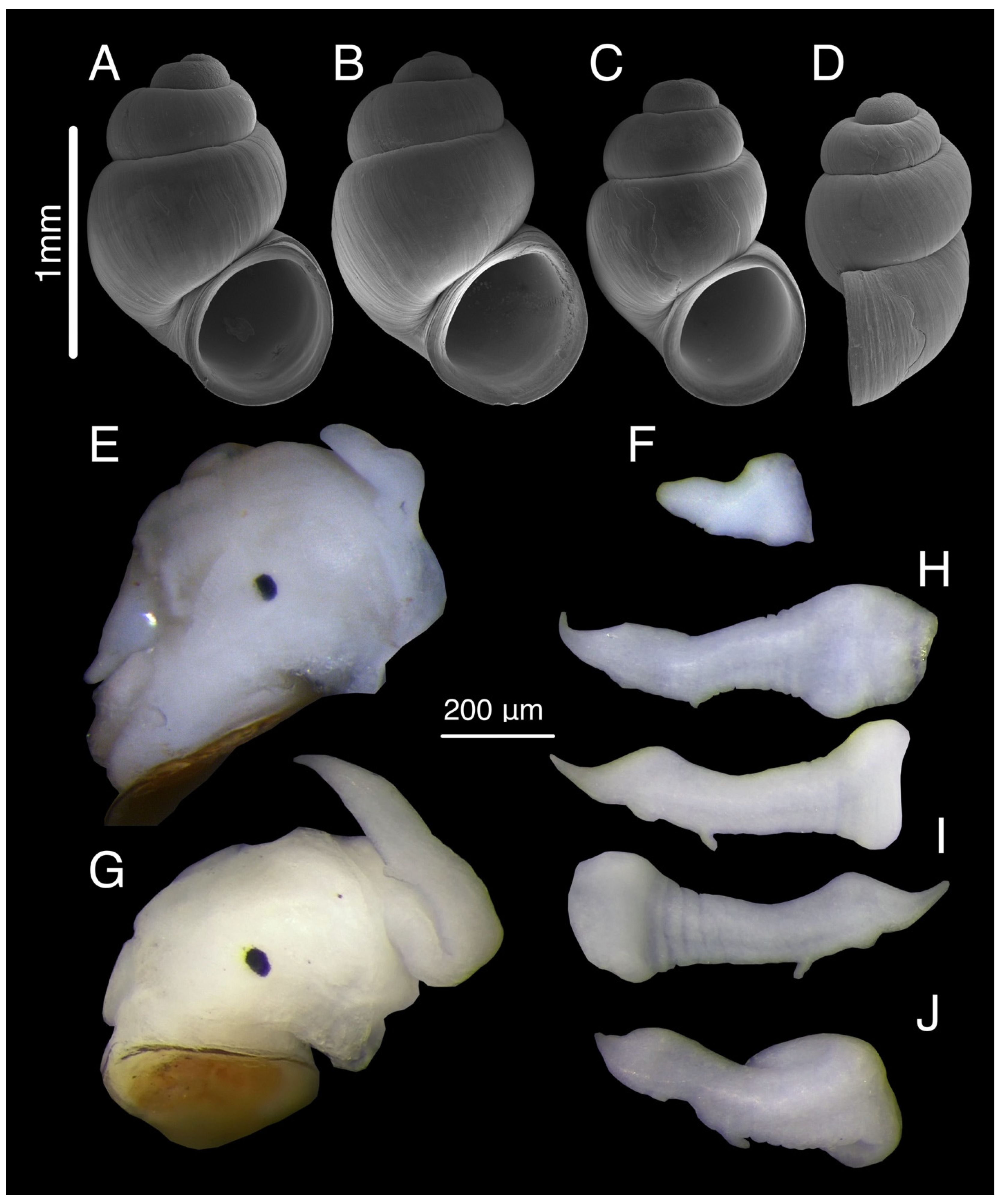
3.8. Genus Colchiella Chertoprud, Grego & Mumladze, gen. nov.
- LSIDurn:lsid:zoobank.org:act:44AE0561-C4EE-41AC-87CD-B9896AA827F3
3.8.1. Distribution
3.8.2. Remarks
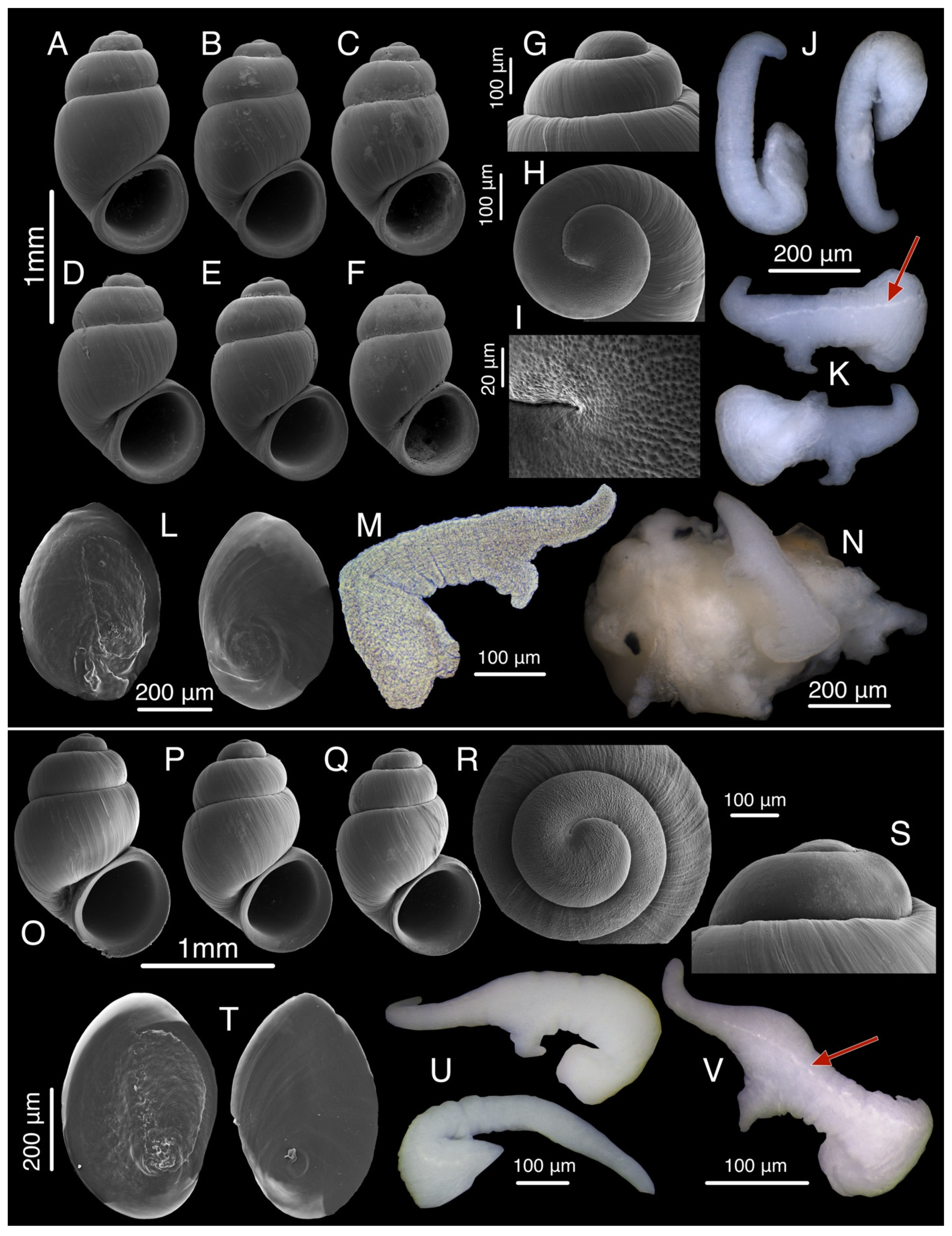
3.9. Colchiella dadiani Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:5DD19D22-B2B8-401E-B282-B78C8D4CE6EE
3.9.1. Material Examined
3.9.2. Other Material
3.9.3. Diagnosis
3.9.4. Description
3.9.5. Etymology
3.9.6. Habitat
3.9.7. Distribution
3.9.8. Conservation Status
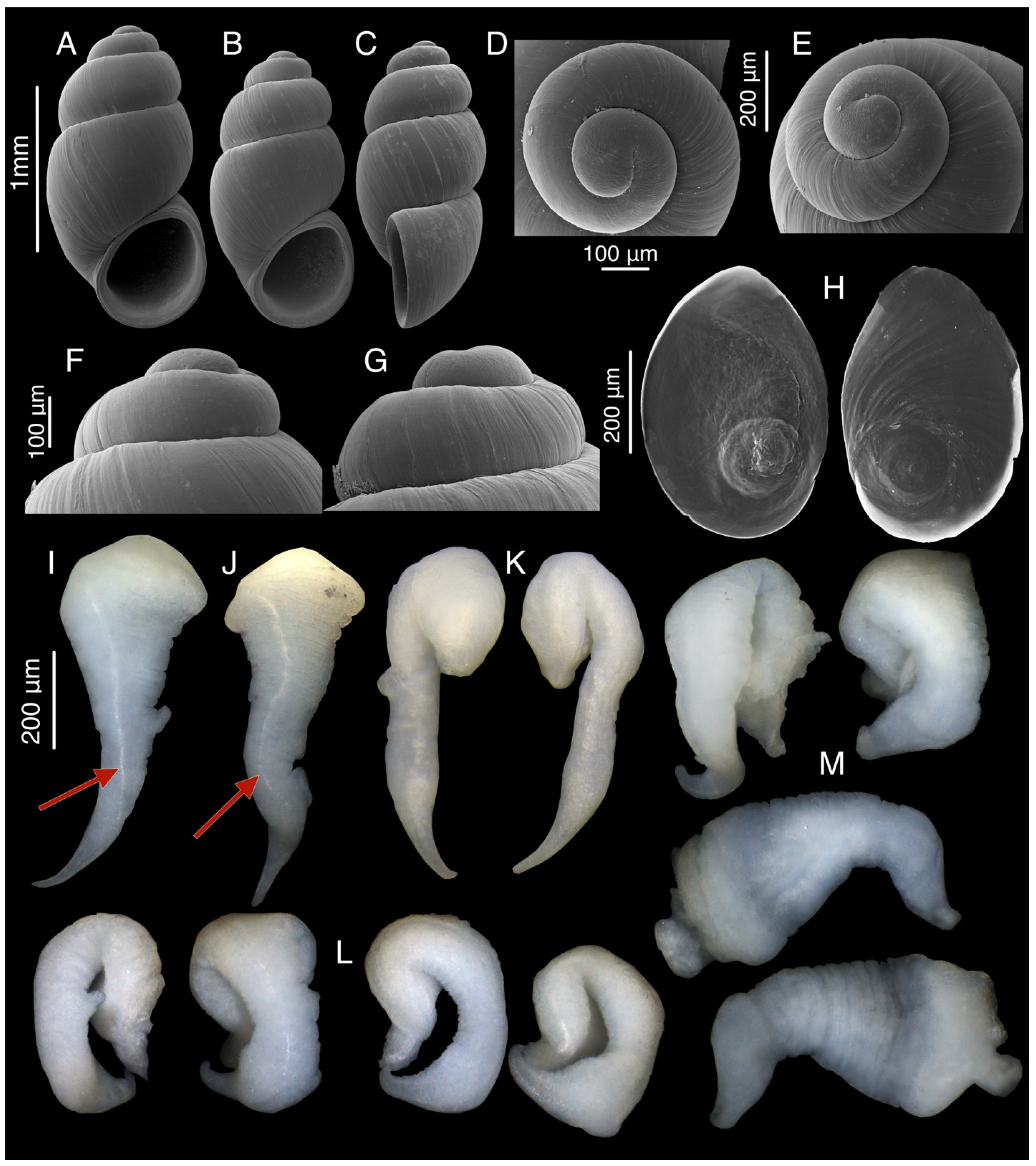
3.10. Colchiella nazodelavo Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:1729E817-AEE4-4922-9A0E-B79755897819
3.10.1. Material Examined
3.10.2. Other Material
3.10.3. Diagnosis
3.10.4. Description
3.10.5. Etymology
3.10.6. Habitat
3.10.7. Distribution
3.10.8. Conservation Status
3.11. Colchiella shiksha Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:BE784866-0790-43FE-9D28-725096B2CB51

3.11.1. Diagnosis
3.11.2. Description
3.11.3. Etymology
3.11.4. Habitat
3.11.5. Distribution
3.11.6. Conservation Status
3.11.7. Remarks
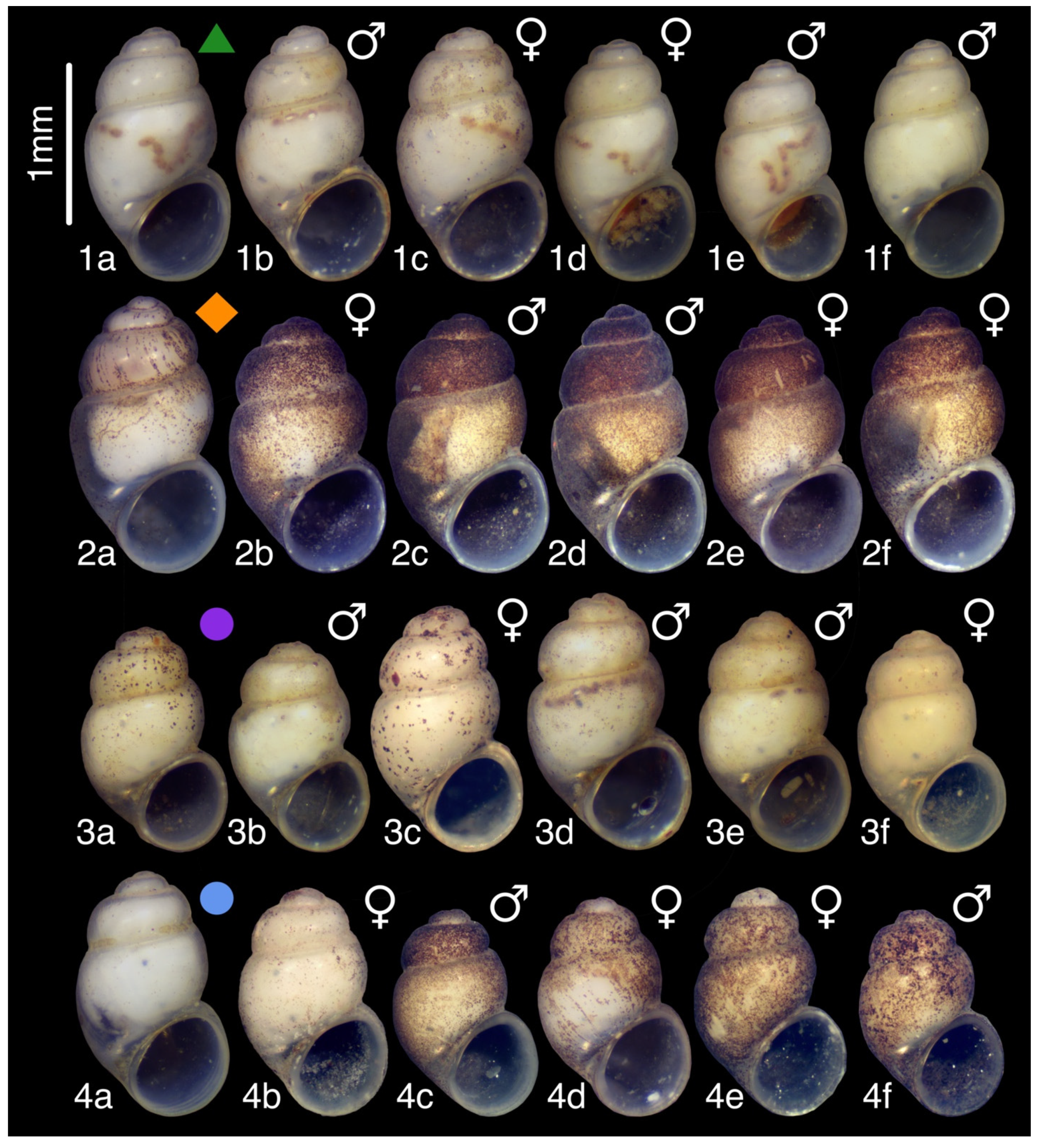
3.12. Colchiella lugella Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:31A039DA-36BC-470B-ACBF-1CFD1CF1BD34
3.12.1. Diagnosis
3.12.2. Description
3.12.3. Etymology
3.12.4. Habitat
3.12.5. Distribution
3.12.6. Conservation Status
3.13. Genus Sataplia Chertoprud, Grego & Mumladze, gen. nov.
- LSIDurn:lsid:zoobank.org:act:263F3855-8DBB-4028-8632-F653DBA7F3F7
3.13.1. Diagnosis
3.13.2. Etymology
3.14. Sataplia cavernicola Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:B3CD32D0-41C2-4E24-8F96-5459B50BFFA4
3.14.1. Description

3.14.2. Etymology
3.14.3. Habitat
3.14.4. Distribution
3.14.5. Conservation Status
3.15. Genus Aetis Chertoprud, Grego & Mumladze, gen. nov.
- LSIDurn:lsid:zoobank.org:act:EE474F38-7C58-447B-B978-AA15E2D7FB61
3.15.1. Diagnosis
3.15.2. Etymology
3.15.3. Distribution
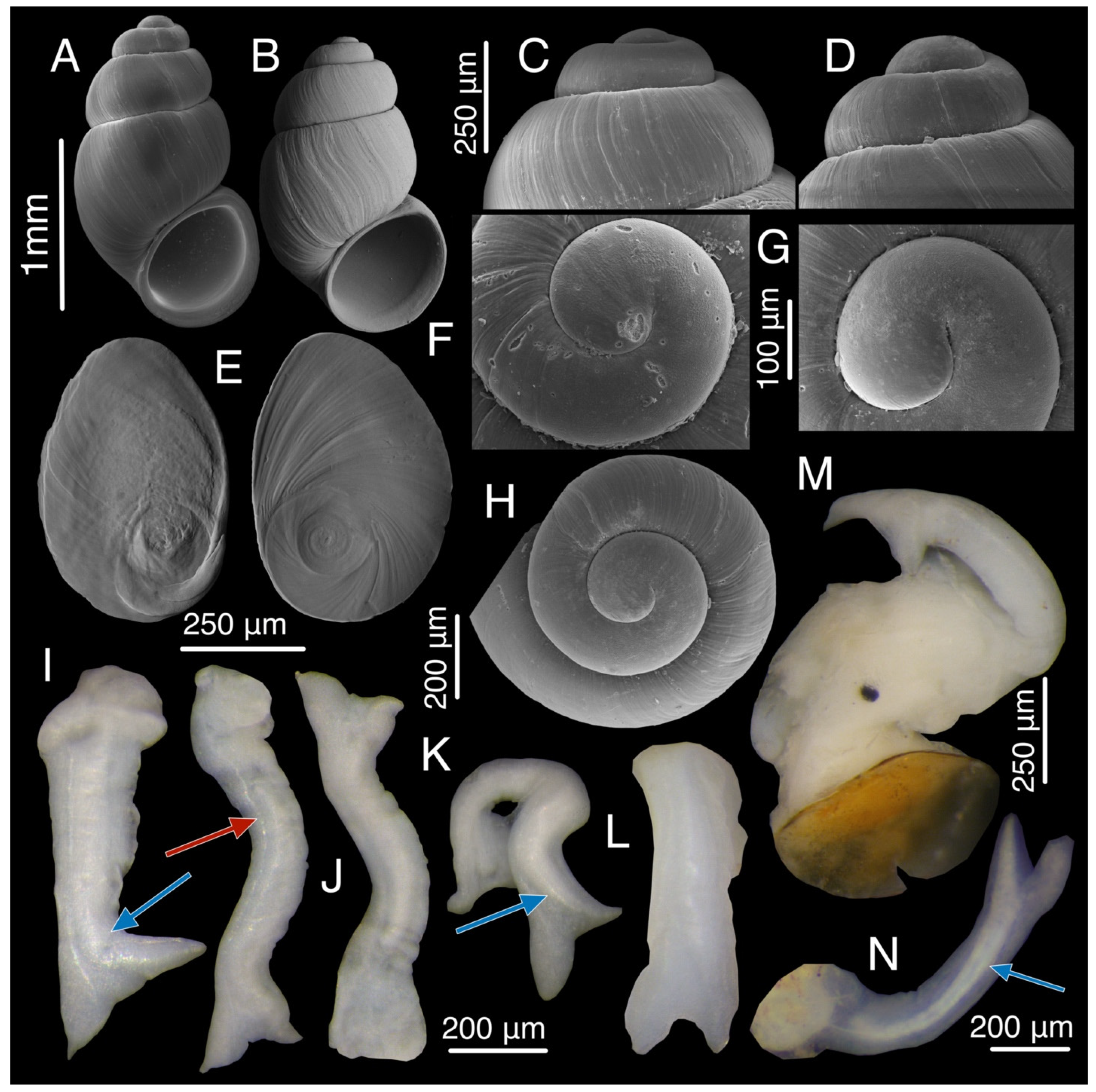
3.16. Aetis starobogatovi Chertoprud, Grego & Mumladze, sp. nov.
- LSIDurn:lsid:zoobank.org:act:DB4ED2C2-3149-4C69-9DC6-9470F408C38
3.16.1. Description
3.16.2. Variation
3.16.3. Etymology
3.16.4. Habitat
3.16.5. Distribution
3.16.6. Conservation Status

4. Discussion
4.1. Habitats of Caucasian Belgandiellinae
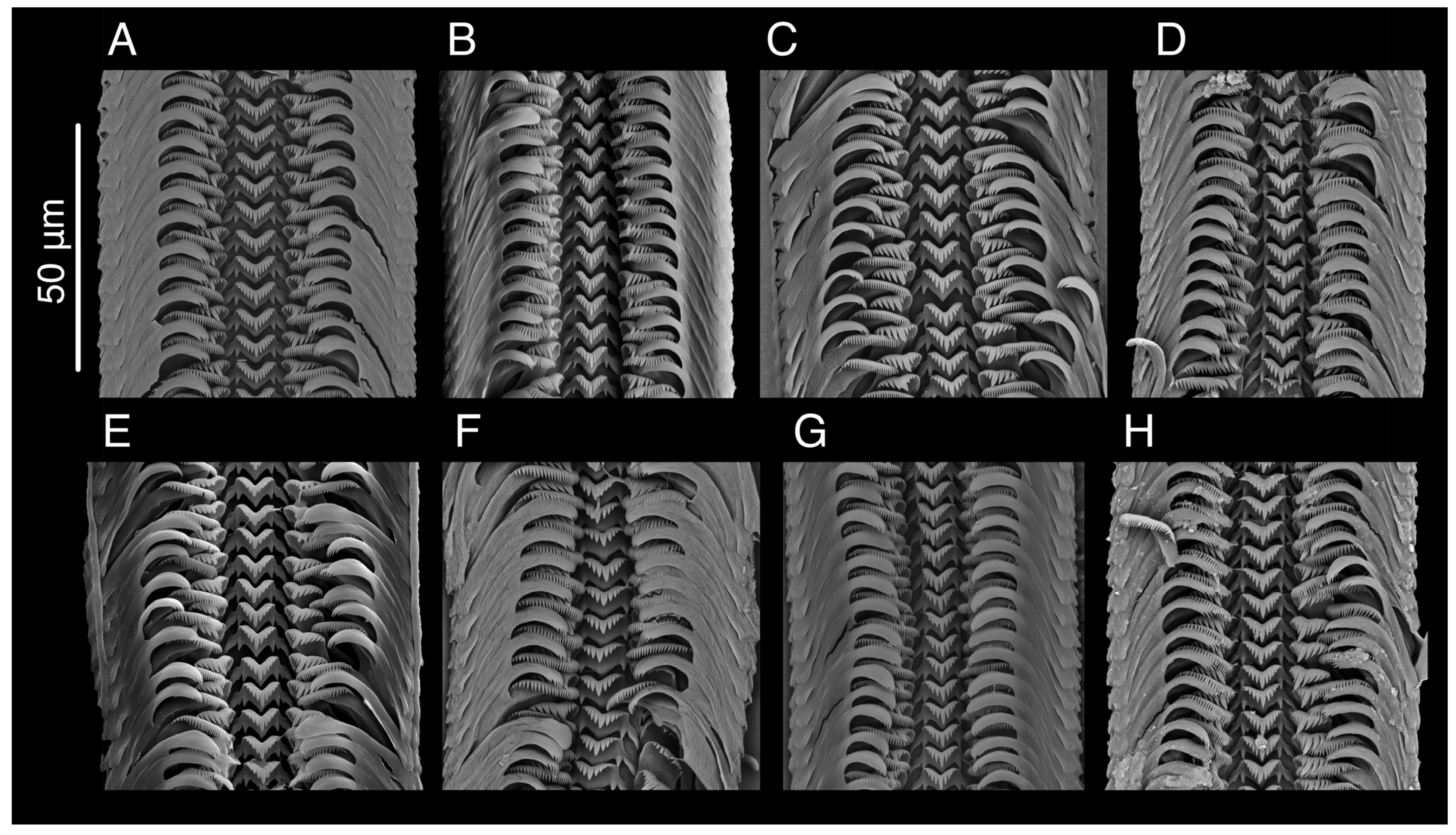
4.2. Shell Morphology
4.3. Historical Biogeography

4.4. Extinction Threats and Conservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindholm, W.A. Beschreibung neuer Arten und Formen aus dem Kaukasus-Gebiete. Nachricht. Der Deuts. Malakozool. Gesells. 1913, 45, 17–23, 62–69. Available online: https://www.biodiversitylibrary.org/page/15107980 (accessed on 22 December 2022).
- Shadin, V.I. Die Süßwassermollusken aud der Rion-Höhle bei Kutais (Transkaukasien, Georgien). Arc. Für Mollusk. 1932, 64, 12–14. [Google Scholar]
- Tzvetkov, B.N. A note on cave molluscs of South Caucasus. Byull. Moskovsk. Obshch. Isp. Prir. Otd. Biol. 1940, 49, 57–59. [Google Scholar]
- Shadin, V.I. Mollusks of Fresh and Brackish Waters of the USSR; Academy of Sciences of the U.S.S.R.: Moscow, Russia, 1952. [Google Scholar]
- Starobogatov, Y.I. Contribution to molluscs from subterranean waters of the Caucasus. Byulleten Mosk. Obs. Ispyt. Prir. Otd. Biol. 1962, 67, 42–54. [Google Scholar]
- Schütt, H.; Şeşen, R. Pseudamnicola species and other freshwater gastropods (Mollusca, Gastropoda) from East Anatolia (Turkey), the Ukraine and the Lebanon. Basteria 1993, 57, 161–171. [Google Scholar]
- Vinarski, M.V.; Palatov, D.M.; Glöer, P. Revision of ‘Horatia’ snails (Mollusca: Gastropoda: Hydrobiidaesensulato) from South Caucasus with description of two new genera. J. Nat. Hist. 2014, 48, 2237–2253. [Google Scholar] [CrossRef]
- Chertoprud, E.S.; Palatov, D.M.; Borisov, R.R.; Marinskiy, V.V.; Bizin, M.S.; Dbar, R.S. Distribution and a comparative analysis of the aquatic invertebrate fauna in caves of the western Caucasus. Subterr. Biol. 2016, 18, 49–70. [Google Scholar] [CrossRef]
- Vinarski, M.V.; Palatov, D.M. A survey of the Belgrandiella-like gastropods of the Northern Black Sea Region (Mollusca, Gastropoda, Hydrobiidae s.l.): Morphological variability and morphospecies. Zool. Zh. 2019, 98, 988–1002. [Google Scholar]
- Grego, J.; Mumladze, L.; Falniowski, A.; Osikowski, A.; Rysiewska, A.; Palatov, D.M.; Hofman, S. Revealing the stygobiotic and crenobiotic molluscan biodiversity hotspot in Caucasus: Part I. The phylogeny of stygobiotic Sadlerianinae Szarowska, 2006 (Mollusca, Gastropoda, Hydrobiidae) from Georgia with descriptions of five new genera and twenty-one new species. ZooKeys 2020, 955, 1–77. [Google Scholar]
- Chertoprud, E.M.; Palatov, D.M.; Vinarski, M.V. Revealing the stygobiont and crenobiont Mollusca biodiversity hotspot in Caucasus: Part II. Sitnikovia gen. nov., a new genus of stygobiont microsnails (Gastropoda: Hydrobiidae) from Georgia. Zoosyst. Rossica 2020, 29, 258–266. [Google Scholar] [CrossRef]
- Chertoprud, E.M.; Palatov, D.M.; Vinarski, M.V. Revealing the stygobiont and crenobiont Mollusca biodiversity hotspot in the Caucasus: Part III. Revision of stygobiont microsnails (Mollusca: Gastropoda: Hydrobiidae) from the Russian part of Western Transcaucasia, with the description of new taxa. Zootaxa 2021, 5005, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Arconada, B.; Ramos, M.A. The Iberico-Balearic region: One of the areas of highest Hydrobiidae (Gastropoda, Prosobrancha, Rissooidea) diversity in Europe. Graellsia 2003, 59, 91–104. [Google Scholar] [CrossRef][Green Version]
- Arconada, B.; Delicado, B.; Ramos, Á.M. A new genus andtwo new species of Hydrobiidae (Mollusca, Caenogastropoda) from the Iberian Peninsula. J. Nat. Hist. 2007, 41, 29–32. [Google Scholar] [CrossRef]
- Haase, M. Differentiation of selected species of Belgrandiella and the redefined genus Graziana (Gastropoda: Hydrobiidae). Zool. J. Linn. Soc. 1994, 111, 219–246. [Google Scholar] [CrossRef]
- Haase, M. The radiation of spring snails of the genus Belgrandiella in Austria (Mollusca: Caenogastropoda: Hydrobiidae). Hydrobiologia 1996, 319, 119–129. [Google Scholar] [CrossRef]
- Radoman, P. Nochmals über die Gattung Pseudamnicola und schliesslisch die Gattung Orientalia n. gen. Arch. Moll. 1972, 102, 195–200. [Google Scholar]
- Radoman, P. Hydrobioidea a Superfamily of Prosobranchia (Gastropoda). I. Systematics; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 1983. [Google Scholar]
- Falniowski, A.; Beran, L.; Belgrandiella, A.J. Wagner, 1928 (Caenogastropoda: Truncatelloidea: Hydrobiidae): How many endemics? Folia Malacol. 2015, 23, 187–191. [Google Scholar] [CrossRef]
- Radoman, P. New classification of fresh and brackish water Prosobranchia from the Balkans and Asia Minor. Posebna Izdanja Prirodn. Mus. Beograd. 1973, 32, 1–30. [Google Scholar]
- Bole, J.; Velkovrh, F. Mollusca from continental subterranean aquatic habitats. In Stygofauna mundi; Botosaneanu, P., Ed.; E.J. Brill: Leiden, The Netherlands, 1986; pp. 177–208. [Google Scholar]
- Kantor, Y.I.; Vinarski, M.V.; Schileyko, A.A.; Sysoyev, A.V. Continental Molluscs of Russia and Adjacent Territories [Internet]. Version 2.3.1. 2010. Available online: http://www.ruthenica.com/categorie-8 (accessed on 20 December 2022).
- Barjadze, S.; Murvanidze, M.; Arabuli, T.; Mumladze, L.; Pkhakadze, V.; Djanashvili, R.; Salakaia, M. Annotated List of Invertebrates of the Georgia Karst Caves; Georgian Academic Book: Tbilisi, Georgia, 2015. [Google Scholar]
- Vinarski, M.V.; Kantor, Y.I. Analytical Catalogue of Fresh and Brackish Water Molluscs of Russia and Adjacent Countries; Tovarishchestvo Nauchnyckh Izdanii KMK: Moscow, Russia, 2016. [Google Scholar]
- Grego, J.; Hofman, S.; Mumladze, L.; Falniowski, A. Agrafia Szarowska et Falniowski, 2011 (Caenogastropoda: Hydrobiidae) in the Caucasus. Folia Malacol. 2017, 237–25247. [Google Scholar] [CrossRef]
- Grego, J.; Glöer, P.; Erőss, Z.P.; Fehér, Z. Six new subterranean freshwater gastropod species from northern Albania and some new records from Albania and Kosovo (Mollusca, Gastropoda, Moitessieriidae and Hydrobiidae). Subterr. Biol. 2017, 23, 85–107. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.M.; Chen, C.-E.; Wu, C.; Kuang, T.-F.; Xing, X.-G.; Li, L.; Liu, W.-J.; Yan, Y.-L. The Pomatiopsidae of Hunan, China (Gastropoda: Rissoacea). Malacologia 1992, 34, 143–342. [Google Scholar]
- Hershler, R.; Ponder, W.F. A review of morphological characters of Hydrobioid snails. Contrib. Zool. 1998, 600, 1–55. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST-palaeontological statistics, ver. 1.89. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Elliott, J.M.; Hurley, M.A. The functional relationship between body size and growth rate in fish. Funct. Ecol. 1995, 9, 625–627. [Google Scholar] [CrossRef]
- Szarowska, M.; Osikowski, A.; Hofman, S.; Falniowski, A. Pseudamnicola Paulucci, 1878 (Caenogastropoda: Truncatelloidea) from the Aegean Islands: A long or short story? Org. Divers. Evol. 2016, 16, 121–139. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic. Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Xia, X. Data Analysis in Molecular Biology and Evolution; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2000. [Google Scholar]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v.2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. System. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1.3.1. 2010. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 26 November 2022).
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatic 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2011, 21, 1864–1877. [Google Scholar] [CrossRef]
- Merckelbach, L.; Borges, L. Make every species count: FastaChar software for rapid determination of molecular diagnostic characters to describe species. Mol. Ecol. Res. 2020, 20, 1761–1768. [Google Scholar] [CrossRef]
- Wilke, T. Salenthydrobia gen. nov. (Rissooidea: Hydrobiidae): A potential relict of the Messinian Salinity Crisis. Zool. J. Linn. Soc. 2003, 137, 319–336. [Google Scholar] [CrossRef]
- Wilke, T.; Davis, G.M. Infraspecific mitochondrial sequence diversity in Hydrobia ulvae and Hydrobia ventrosa (Hydrobiidae: Rissoacea: Gastropoda): Do their different life histories affect biogeographic patterns and gene flow? Biol. J. Linn. Soc. 2000, 70, 89–105. [Google Scholar] [CrossRef]
- Falniowski, A.; Szarowska, M.; Sirbu, I.; Hillebrand, A.; Baciu, M. Heleobia dobrogica (Grossu & Negrea, 1989) (Gastropoda: Rissooidea: Cochliopidae) and the estimated time of its isolation in a continental analogue of hydrothermal vents. Molluscan Res. 2008, 28, 165–170. [Google Scholar]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comp. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Szarowska, M.; Falniowski, A. An unusual, flagellum-bearing hydrobiid snail (Gastropoda: Rissooidea: Hydrobiidae) from Greece, with descriptions of a new genus and a new species. J. Nat. Hist. 2011, 45, 2231–2246. [Google Scholar] [CrossRef]
- Wilke, T.; Davis, G.M.; Falniowski, A.; Giusti, F.; Bodon, M.; Szarowska, M. Molecular systematics of Hydrobiidae (Mollusca: Gastropoda: Rissooidea): Testing monophyly and phylogenetic relationships. Proc. Acad. Nat. Sci. Phila. 2001, 151, 1–21. [Google Scholar] [CrossRef]
- Osikowski, A.; Hofman, S.; Rysiewska, A.; Sket, B.; Prevorčnik, S.; Falniowski, A. A case of biodiversity overestimation in the Balkan Belgrandiella, A.J. Wagner, 1927 (Caenogastropoda: Hydrobiidae): Molecular divergence not paralleled by high morphological variation. J. Nat. Hist. 2018, 52, 323–344. [Google Scholar] [CrossRef]
- Falniowski, A.; Szarowska, M. Phylogenetic relationships of Dalmatinella fluviatilis Radoman, 1973 (Caenogastropoda: Rissooidea). Folia Malacol. 2013, 21, 1–7. [Google Scholar] [CrossRef][Green Version]
- Szarowska, M.; Hofman, S.; Osikowski, A.; Falniowski, A. Daphniola Radoman, 1973 (Caenogastropoda: Truncatelloidea) at east Aegean islands. Folia Malacol. 2014, 22, 269–275. [Google Scholar] [CrossRef]
- Hofman, S.; Grego, J.; Fehér, Z.; Erőss, Z.P.; Rysiewska, A.; Osikowski, A.; Falniowski, A. New data on the valvatiform-shelled Hydrobiidae (Caenogastropoda, Truncatelloidea) from southern Greece. ZooKeys 2021, 1062, 31–47. [Google Scholar] [CrossRef]
- Osikowski, A.; Hofman, S.; Georgiev, D.; Kalcheva, S.; Falniowski, A. Aquatic snails Ecrobia maritima (Milaschewitsch, 1916) and E. ventrosa (Montagu, 1803) (Caenogastropoda: Hydrobiidae) in the east Mediterranean and Black Sea. Annal. Zool. 2016, 66, 477–486. [Google Scholar] [CrossRef]
- Falniowski, A.; Szarowska, M. A new genus and new species of valvatiform hydrobiid (Rissooidea; Caenogastropoda) from Greece. Moll. Res. 2011, 31, 189–199. [Google Scholar]
- Falniowski, A.; Georgiev, D.; Osikowski, A.; Hofman, S. Radiation of Grossuana Radoman, 1973 (Caenogastropoda: Truncatelloidea) in the Balkans. J. Moll. Stud. 2016, 82, 305–313. [Google Scholar] [CrossRef]
- Rysiewska, A.; Prevorčnik, S.; Osikowski, A.; Hofman, S.; Beran, L.; Falniowski, A. Phylogenetic relationships in Kerkia and introgression between Hauffenia and Kerkia(Caenogastropoda: Hydrobiidae). J. Zool. Syst. Evol. Res. 2017, 55, 106–117. [Google Scholar] [CrossRef]
- Szarowska, M.; Falniowski, A. Horatia Bourguignat, 1887: Is this genus really phylogenetically very close to Radomaniola Szarowska, 2006 (Caenogastropoda: Truncatelloidea)? Folia Malacol. 2014, 22, 31–39. [Google Scholar] [CrossRef]
- Hofman, S.; Rysiewska, A.; Osikowski, A.; Grego, J.; Sket, B.; Prevorčnik, S.; Zagmajster, M.; Falniowski, A. Phylogenetic relationships of the Balkan Moitessieriidae (Caenogastropoda: Truncatelloidea). Zootaxa 2018, 4486, 311–339. [Google Scholar] [CrossRef]
- Beran, L.; Osikowski, A.; Hofman, S.; Falniowski, A. Islamia zermanica (Radoman, 1973) (Caenogastropoda: Hydrobidae): Morphological and molecular distinctness. Folia Malacol. 2016, 24, 25–30. [Google Scholar] [CrossRef]
- Falniowski, A.; Pešić, V.; Glöer, P. Montenegrospeum Pešić et Glöer, 2013: A representative of Moitessieriidae? Folia Malacol. 2014, 22, 263–268. [Google Scholar] [CrossRef]
- Grego, J.; Glöer, P.; Rysiewska, A.; Hofman, S.; Falniowski, A. A new Montenegrospeum species from south Croatia (Mollusca: Gastropoda: Hydrobiidae). Folia Malacol. 2018, 26, 25–34. [Google Scholar] [CrossRef][Green Version]
- Rysiewska, A.; Georgiev, D.; Osikowski, A.; Hofman, S.; Falniowski, A. Pontobelgrandiella Radoman, 1973 (Caenogastropoda: Hydrobiidae): A recent invader of subterranean waters? J. Conch. 2016, 42, 193–203. [Google Scholar]
- Szarowska, M.; Falniowski, A. Species distinctness of Sadleriana robici (Clessin, 1890) (Gastropoda: Rissooidea). Folia Malacol. 2013, 21, 127–133. [Google Scholar] [CrossRef]
- Hofman, S.; Osikowski, A.; Rysiewska, A.; Grego, J.; Glöer, P.; Dmitrović, D.; Falniowski, A. Sarajana Radoman, 1975 (Caenogastropoda: Truncatelloidea): Premature invalidation of a genus. J. Conch. 2019, 43, 407–418. [Google Scholar]
- Jaszczyńska, A.; Hofman, S.; Erőss, Z.P.; Fehér, Z.; Grego, J. Phylogenetic relatioships of Terranigra Radoman, 1978 (Truncatelloidea: Hydrobiidae). Folia Malacol. 2023; in press. [Google Scholar]
- Cardoso, P.; Borges, P.A.; Triantis, K.A.; Ferrández, M.A.; Martín, J.L. Adapting the IUCN Red List criteria for invertebrates. Biol. Conserv. 2011, 144, 2432–2440. [Google Scholar] [CrossRef]
- Bache, F.; Popescu, S.M.; Rabineau, M.; Gorini, C.; Suc, J.P.; Clauzon, G.; Olivet, J.L.; Rubino, J.L.; Melinte-Dobrinescu, M.C.; Estrada, F.; et al. A two-step process for the reflooding of the Mediterranean after the Messinian Salinity Crisis. Basin Res. 2011, 24, 125–153. [Google Scholar] [CrossRef]
- Krijgsman, W.; Tesakov, A.; Yanina, T.; Lazarev, S.; Danukalova, G.; Van Baak, C.G.C.; Agustí, J.; Alçiçek, M.C.; Aliyeva, E.; Bista, D.; et al. Quaternary time scales for the Pontocaspian domain: Interbasinal connectivity and faunal evolution. Earth-Sci. Rev. 2019, 188, 1–40. [Google Scholar] [CrossRef]
- Glöer, P.; Slavevska-Stamenković, V. Bythinella melovskii n. sp., a news pecies from R. Macedonia (Gastropoda: Hydrobiidae). Ecol. Montenegr. 2015, 2, 150–154. [Google Scholar] [CrossRef]
- Georgiev, D.; Glöer, P. A new species of Bythinella from Strandzha mountain, SE Bulgaria (Gastropoda: Risooidea). Ecol. Montenegr. 2014, 1, 78–81. [Google Scholar] [CrossRef]
- Glöer, P.; Hirschfelder, H.J. New Freshwater molluscs from Crete, Greece (Gastropoda: Hydrobiidae, Bythinellidae, Valvatidae). Ecol. Montenegr. 2019, 20, 1023. [Google Scholar] [CrossRef][Green Version]
- Falniowski, A.; Lewarne, B.; Rysiewska, A.; Osikowski, A.; Hofman, S. Crenobiont, stygophile and stygobiont molluscs in the hydrographic area of the Trebišnjica River Basin. ZooKeys 2021, 1047, 61–89. [Google Scholar] [CrossRef]
- Kabat, A.R.; Hershler, R. The prosobranch snail family Hydrobiidae (Gastropoda: Rissooidea): Review of classification and supraspecific taxa. Smiths. Contrib. Zool. 1993, 547, 1–94. [Google Scholar] [CrossRef]
- Szarowska, M. Molecular phylogeny, systematics and morphological character evolution in the Balkan Rissooidea (Caenogastropoda). Folia Malacol. 2006, 14, 99–168. [Google Scholar] [CrossRef]
- Szarowska, M.; Falniowski, A. There is no philosopher’s stone: Coup de grace for the morphology-based systematics in the rissooidean gastropods? In Proceedings of the 5th Congress of the European Malacological Societies, Ponta Delgada, Portugal, 2–6 September 2008; p. 28. [Google Scholar]
- Falniowski, A. Species Distinction and Speciation in Hydrobioid Gastropods (Mollusca: Caenogastropoda: Truncatelloidea). Archiv Zool. Stud. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Garcia-Castellanos, D.; Micallef, A.; Estrada, F.; Camerlenghie, A.; Ercilla, G.; Periáñez, R.; Abrilf, J.M. The Zanclean megaflood of the Mediterranean—Searching for independent evidence. Earth-Sci. Rev. 2020, 201, 103061. [Google Scholar] [CrossRef]
- Krezsek, C.; Schleder, Z.; Bega, Z.; Ionescu, G.; Tari, G. The Messinian sea-level fall in the western Black Sea: Small or large? Insights from offshore Romania. Petroleum Geosci. 2016, 22, 392–399. [Google Scholar] [CrossRef]
- Grothe, A.; Andreetto, F.; Reichart, G.J.; Wolthers, M.; Van Baak, C.G.C.; Vasiliev, I.; Stoica, M.; Sangiorgi, F.; Middelburg, J.J.; Davies, G.R.; et al. Paratethys pacing of the Messinian Salinity Crisis: Low salinity waters contributing to gypsum precipitation? Earth Planet. Sci. Lett. 2020, 532, 116029. [Google Scholar] [CrossRef]
- Marin, I.N. The Quaternary speciation in the Caucasus: A new cryptic species of stygobiotic amphipod of the genus Niphargus (Crustacea: Amphipoda: Niphargidae) from the Kumistavi (Prometheus) Cave, Western Georgia. Arthropoda Sel. 2020, 29, 419–432. [Google Scholar] [CrossRef]
- Marin, I.N.; Krylenko, S.; Palatov, D. The Caucasian relicts: A new species of the genus Niphargus (Crustacea: Amphipoda: Niphargidae) from the Gelendzhik-Tuapse area of the Russian southwestern Caucasus. Zootaxa 2021, 4963, zootaxa.4963.3.5. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.N.; Palatov, D. Cryptic refugee on the northern slope of the Greater Caucasian Ridge: Discovery of Niphargus (Crustacea: Amphipoda: Niphargidae) in the North Ossetia–Alania, North Caucasus, separated from its relatives in the late Miocene. Zool. Anz. 2021, 292, 163–183. [Google Scholar] [CrossRef]
- Marin, I.N.; Turbanov, I.S.; Prokopov, G.A.; Palatov, D.M. A New Species of the Genus Niphargus Schiødte, 1849 (Crustacea: Amphipoda: Niphargidae) from Groundwater Habitats of the Tarkhankut Upland, Crimean Peninsula. Diversity 2022, 14, 1010. [Google Scholar] [CrossRef]
- Palatov, D.M.; Sokolova, A.M. Two new stygobiotic species of the genus Proasellus(Crustacea: Isopoda: Asellidae) from the North Caucasus. Invert. Zool. 2021, 18, 481–501. [Google Scholar] [CrossRef]
- Marin, I.N.; Barjadze, S. A new species of stygobiotic atyid shrimps of the genus Xiphocaridinella (Crustacea: Decapoda: Atyidae) from the Racha-Lechkhumi and Kvemo Svaneti, with a new record of X. kumistavi from the Imereti, Western Georgia. Invert. Zool. 2022, 19, 24–34. [Google Scholar] [CrossRef]
- Zakšek, V.; Sket, B.; Trontelj, P. Phylogeny of the cave shrimp Troglocaris: Evidence of a young connection between Balkans and Caucasus. Mol. Phylogenet. Evol. 2007, 42, 223–235. [Google Scholar] [CrossRef]
- Marin, I.N.; Krylenko, S.V.; Palatov, D.M. Euxinian relict amphipods of the Eastern Paratethys in the subterranean fauna of coastal habitats of the Northern Black Sea region. Invertebr. Zool. 2021, 18, 247–320. [Google Scholar] [CrossRef]
- Rouchy, J.M.; Caruso, A. The Messinian salinity crisis in the Mediterranean basin: A reassessment of the data and an integrated scenario. Sedimentary Geol. 2006, 188–189, 35–67. [Google Scholar] [CrossRef]
- Couto, D.D.; Popescu, S.; Suc, J.; Melinte-Dobrinescu, M.C.; Barhoun, N.; Gorini, C.; Jolivet, L.; Poort, J.; Jouannic, G.; Auxietre, J.L. Lago Mare and the Messinian Salinity Crisis: Evidence from the Alboran Sea (S. Spain). Mar. Pet. Geol. 2014, 52, 57–76. [Google Scholar] [CrossRef]
- Bartol, J.; Govers, R. Flexure due to the Messinian-Pontian sea level drop in the Black Sea. Geochem. Geophys. Geosyst. 2009, 10, 1–14. [Google Scholar] [CrossRef]
- Garcıa-Alix, A.; Minwer-Barakat, R.; Suarez, E.M.; Freudenthal, M.; Aguirre, J.; Kaya, F. Updating the Europe–Africa small mammal exchange during the late Messinian. J. Biogeogr. 2016, 43, 1336–1348. [Google Scholar] [CrossRef]
- Krijgsman, W.; Capella, W.; Simon, D.; Hilgen, F.J.; Kouwenhoven, T.J.; Meijer, P.T.; Sierro, F.J.; Tulbure, M.A.; van den Berg, B.C.J.; van der Schee, M.; et al. The Gibraltar Corridor: Watergate of the Messinian Salinity Crisis. Marine Geol. 2018, 403, 238–246. [Google Scholar] [CrossRef]
- Kikvidze, Z.; Ohsawa, M. Richness of Colchic vegetation: Comparison between refugia of south-western and East Asia. BMC Ecology 2001, 1, 1–10. Available online: http://www.biomedcentral.com/1472-6785/1/6 (accessed on 20 December 2022). [CrossRef] [PubMed]
- Connor, S.E.; Kvavadze, E.V. Modelling late quaternary changes in plant distribution, vegetation and climate using pollen data from Georgia, Caucasus. J. Biogeogr. 2009, 36, 529–545. [Google Scholar] [CrossRef]
- Tarkhnishvili, D.; Gavashelishvili, A.; Mumladze, L. Palaeoclimatic models help to understand current distribution of Caucasian forest species. Biol. J. Linn. Soc. 2012, 105, 231–248. [Google Scholar] [CrossRef]
- Nieber, M.T.; Hausdorf, B. Phylogeography of the land snail genus Circassina(Gastropoda: Hygromiidae) implies multiple Pleistocene refugia in the western Caucasus region. Mol. Phylogenet. Evol. 2015, 93, 129–142. [Google Scholar] [CrossRef]
- Sękiewicz, K.; Danelia, I.; Farzaliyev, V.; Gholizadeh, H.; Iszkuło, G.; Naqinezhad, A.; Ramezani, E.; Thomas, P.A.; Tomaszewski, D.; Walas, Ł.; et al. Past climatic refugia and landscape resistance explain spatial genetic structure in Oriental beech in the South Caucasus. Ecol. Evol. 2022, 12, e9320. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, W.; Hilgen, F.; Raffi, I.; Sierro, F.J.; Wilson, D.S. Chronology, causes and progression of the Messinian salinity crisis. Nature 1999, 400, 652–655. [Google Scholar] [CrossRef]
- Laermanns, H.; Kelterbaum, D.; May, S.M.; Elashvili, M.; Opitz, S.; Hülle, D.; Rölkens, J.; Verheul, J.; Riedesel, S.; Brückner, H. Mid-to Late Holocene landscape changes in the Rioni Delta area (Kolkheti lowlands, W Georgia). Quat. Int. 2018, 465 Part A, 85–98. [Google Scholar] [CrossRef]
- Gobejishvili, R.; Lomidze, N.; Tielidze, L. Chapter 12—Late Pleistocene (Würmian) Glaciations of the Caucasus. Dev. Quarter. Sci. 2011, 15, 141–147. [Google Scholar]
- Birkun, A.; Atudorei, A.; Gamgebeli, T.; Dedeoglu, S.G.; Movchan, N.; Nikolova, A.; Okus, E.; Yurenko, Y. Marine Litter in the Black Sea Region: A Review of the Problem; Black Sea Commission Publications 2007-1, BSC Report 2007; Black Sea Commission Publications: Istanbul, Turkey, 2007; p. 160. ISBN 978-9944-245-32-6. [Google Scholar]
- Ryan, W.B.F.; Major, C.O.; Lericolais, G.; Goldstein, S.L. Catastrophic Flooding of the Black Sea. Ann. Rev. Earth Planet Sci. 2003, 31, 525–554. [Google Scholar] [CrossRef]
- Rendoš, M.; Delić, T.; Copilaș-Ciocianuc, D.; Fišer, C. First insight into cryptic diversity of a Caucasian subterranean amphipod of the genus Niphargus (Crustacea: Amphipoda: Niphargidae). Zool. Anz. 2021, 290, 1–11. [Google Scholar] [CrossRef]
- Richling, I.; Malkowsky, Y.; Kuhn, Y.; Niederhöfer, H.-J.; Boeters, H.D. A vanishing hotspot—impact of molecular insights on the diversity of Central European Bythiospeum Bourguignat, 1882 (Mollusca: Gastropoda: Truncatelloidea). Org. Divers. Evol. 2016, 17, 67–85. [Google Scholar] [CrossRef]
- Haase, M.; Grego, J.; Erőss, Z.P.; Farkas, R.; Fehér, Z. On the origin and diversification of the stygobiotic freshwater snail genus Hauffenia (Caenogastropoda: Hydrobiidae) with special focus on the northern species and the description of two new species. Europ. J. Taxon. 2021, 775, 143–184. [Google Scholar] [CrossRef]
- Anistratenko, V.V.; Palatov, D.M.; Chertoprud, E.M.; Sitnikova, T.Y.; Anistratenko, O.Y.; Clewing, C.; Vinarski, M.V. Keyhole into a Lost World: The First Purely Freshwater Species of the Ponto-Caspian Genus Clathrocaspia (Caenogastropoda: Hydrobiidae). Diversity 2022, 14, 232. [Google Scholar] [CrossRef]
- Páll-Gergely, B.; Grego, J. A Georgian and an Iranian new species of Renea, G. Nevill, 1880 enormously extend the genus’s distribution (Gastropoda: Caenogastropoda: Aciculidae). Zootaxa 2022, 5188, 596–600. [Google Scholar] [CrossRef]
- Zallot, E.; Fehér, Z.; Bamberger, S.; Gittenberger, E. Cochlostoma revised: The subgenus Lovcenia Zallot et al., 2015 (Caenogastropoda, Cochlostomatidae). Europ. J. Tax. 2018, 464, 1–25. [Google Scholar] [CrossRef]
- Czaja, A.; Cardoza-Martínez, G.F.; Meza-Sánchez, I.; Estrada-Rodríguez, J.L.; Saenz-Mata, J.; Becerra-López, J.; Romero-Méndez, U.; Estrada-Arellano, J.R.; Garza-Martínez, M.Á.; Dávila Paulín, J.A. New genus, two new species and new records of subterranean freshwater snails (Caenogastropoda; Cochliopidae and Lithoglyphidae) from Coahuila and Durango, Northern Mexico. Subterr. Biol. 2019, 29, 89–102. [Google Scholar] [CrossRef]
- Mammola, S.; Cardoso, P.; Culver, D.C.; Deharveng, L.; Ferreira, R.L.; Fišer, C.; Galassi, D.M.P.; Griebler, C.; Halse, S.; Humphreys, W.F.; et al. Scientists’ Warning on the Conservation of Subterranean Ecosystems. BioScience 2019, 69, 641–650. [Google Scholar] [CrossRef]
- Souza Silva, M.; Martins, R.P.; Ferreira, R.L. Cave Conservation Priority Index to Adopt a rapid protection strategy: A case study in Brazilian Atlantic rain forest. Environ. Manag. 2015, 55, 279–295. [Google Scholar] [CrossRef]
- Polak, S.; Pipan, T. The Subterranean Fauna of Križna Jama, Slovenia. Diversity 2021, 13, 210. [Google Scholar] [CrossRef]
- Gillieson, D.S.; Gunn, J.; Auler, A.; Bolger, T. (Eds.) Guidelines for Cave and Karst Protection, 2nd ed.; International Union of Speleology and Gland, Switzerland, IUCN: Postojna, Slovenia, 2022; 112p, ISBN 978-0-646-84911-9. [Google Scholar]
- Eusébio, R.P.; Taiti, S. Species conservation profiles of cave-adapted terrestrial isopods from Portugal. Biodiver. Data J. 2022, 10, e78796. [Google Scholar]
- Mumladze, L.; Japoshvili, B.; Anderson, E.P. Faunal biodiversity research in the Republic of Georgia: A short review of trends, gaps, and needs in the Caucasus biodiversity hotspot. Biologia 2020, 75, 1385–1397. [Google Scholar] [CrossRef]
- Mumladze, L.; Cameron, R.A.; Pokryszko, B.M. Endemic land molluscs in Georgia (Caucasus): How well are they protected by existing reserves and national parks? J. Molluscan Stud. 2014, 80, 67–73. [Google Scholar] [CrossRef]
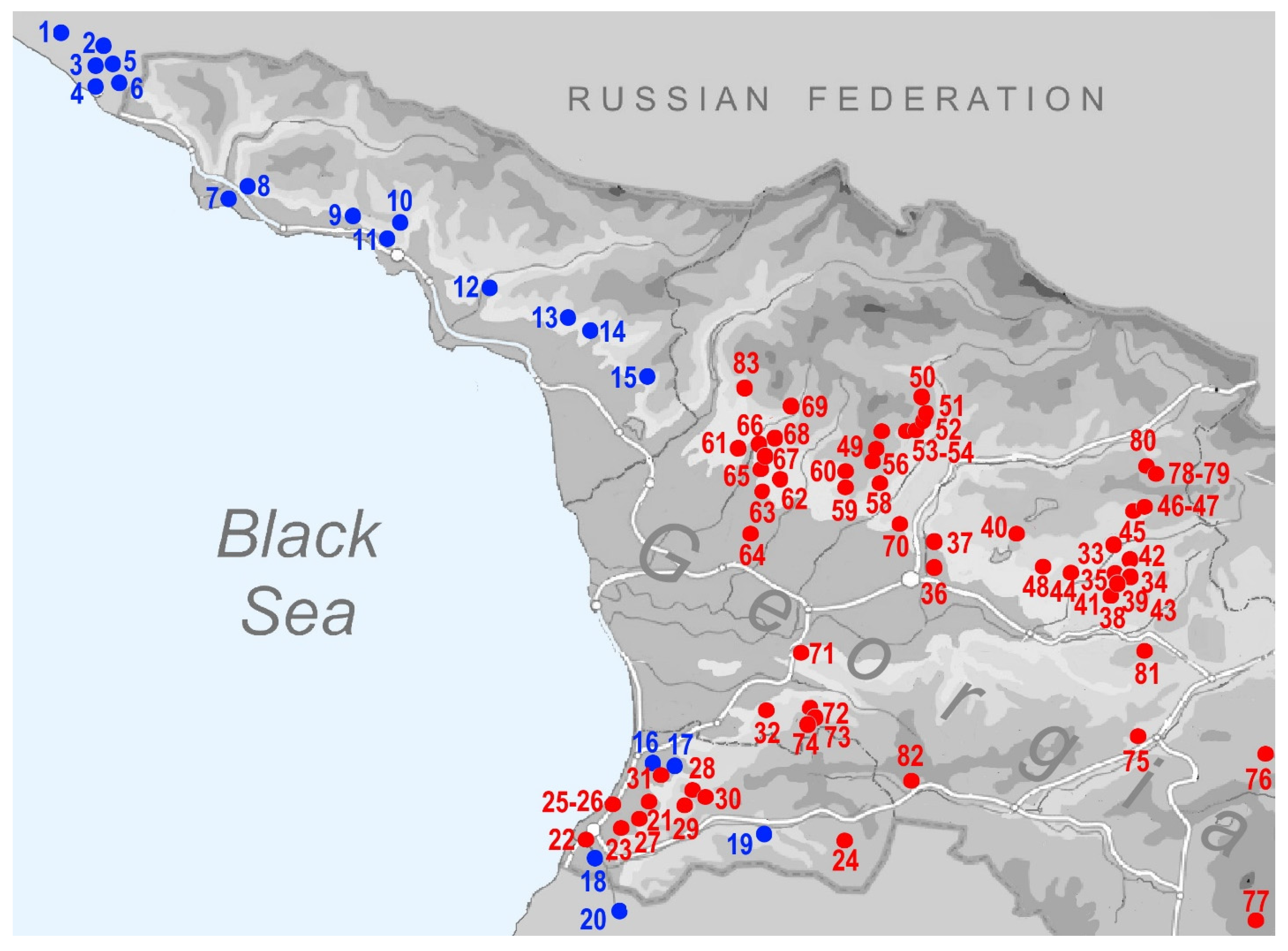





| No Map | Genus | Species | Region | Georgia: Locality | Latitude | Longitude | Altitude (m) | Leg. | Date |
|---|---|---|---|---|---|---|---|---|---|
| 21 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Chakvistavi | 41.676890° | 41.859046° | 348 | M | 19.09.2017. |
| 22 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Charnali | 41.555460° | 41.608890° | 92 | M | 12.07.2014. |
| 23a | “Tschernomorica” | adsharica Lindholm, 1913 | A | Korolistavi, small stream 1 | 41.638380° | 41.745750° | 152 | M | 03.08.2019. |
| 23b | “Tschernomorica” | adsharica Lindholm, 1913 | A | Korolistavi, small stream 2 | 41.641870° | 41.758510° | 394 | M | 03.08.2019. |
| 23c | “Tschernomorica” | adsharica Lindholm, 1913 | A | Korolistavi, rivulet | 41.652680° | 41.762480° | 542 | M | 17.09.2017. |
| 24 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Kveda Chkhutuneti, near Karimana Waterfall | 41.499270° | 41.839810° | 411 | M | 22.09.2017. |
| 25 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Makhinjauri, Batumi, left side of the Shua Street | 41.675487° | 41.706926° | 34 | G | 17.08.2017. |
| 26 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Makinjauri, Batumi Botanical Garden | 41.698350° | 41.717801° | 94 | M | 18.09.2017. |
| 27 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Mtirala Nat. Park, small stream | 41.680917° | 41.860360° | 276 | M | 19.09.2017. |
| 28 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Mtirala National Park, (mt11) | 41.679162° | 41.886043° | 440 | M | 02.03.2014. |
| 29 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Mtirala National Park, Georgia (mt3) | 41.669210° | 41.853620° | 746 | M | 02.03.2014. |
| 30 | “Tschernomorica” | adsharica Lindholm, 1913 | A | Mtirala National Park, Georgia (mt9) | 41.679703° | 41.888040° | 436 | M | 02.03.2014. |
| 31 | “Tschernomorica” | cf.adsharica Lindholm, 1913 | I | Vakijvari, right tributary rivulet of Natanebi River, | 41.906740° | 42.162170° | 470 | M | 22.08.2014. |
| 32 | “Tschernomorica” | cf.adsharica Lindholm, 1913 | A | Sameba, Georgia | 41.799500° | 41.863570° | 47 | M | 19.09.2016. |
| 33 | Tschernomorica | kopidophora sp. nov. | I | Chiatura region, Ghrodo Cave and spring | 42.307671° | 43.327316° | 433 | M | 21.10.2021. |
| 34 | Tschernomorica | kopidophora sp. nov. | I | Chiatura region, Mandaeti, Unnamed Spring, | 42.164197° | 43.317441° | 794 | M | 22.10.2021. |
| 35a | Tschernomorica | kopidophora sp. nov. | I | Chiatura region, Skindori, karst spring, | 42.241431° | 43.258988° | 545 | M | 21.10.2021. |
| 35b | Tschernomorica | kopidophora sp. nov. | I | Chiatura region, Skindori, karst spring, | 42.241431° | 43.258988° | 545 | C, G, O | 13.09.2022. |
| 36 | Tschernomorica | kopidophora sp. nov. | I | Kuatisi, Iazoni Cave, right bank of river Tskalsitela | 42.271811° | 42.734153° | 132 | G, M, O | 01.05.2018. |
| 36 | Tschernomorica | kopidophora sp. nov. | I | Kuatisi, Iazoni Cave, right bank of river Tskalsitela | 42.271811° | 42.734153° | 132 | G, M | 13.10.2019. |
| 37 | Tschernomorica | kopidophora sp. nov. | I | Mostsameta, Gelati spring above road to Monastery | 42.297183° | 42.770364° | 387 | G, S | 21.10.2021. |
| 38 | Tschernomorica | kopidophora sp. nov. | I | Sareki W, cave spring on the left bank of Jruchula river | 42.325356° | 43.267953° | 443 | C | 27.08.2021. |
| 39 | Tschernomorica | kopidophora sp. nov. | I | Shvilobisa Cave between Bunikauri and Tabagrebi | 42.325356° | 43.267953° | 628 | C | 29.08.2021. |
| 40a | Tschernomorica | kopidophora sp. nov. | I | spring under Nakerala pass above road, travaertine fall | 42.382927° | 43.012168° | 953 | M | 14.08.2016. |
| 40b | Tschernomorica | kopidophora sp. nov. | I | spring under Nakerala pass above road, travaertine fall | 42.382927° | 43.012168° | 953 | M | 28.07.2017. |
| 40c | Tschernomorica | kopidophora sp. nov. | I | spring under Nakerala pass above road, travaertine fall | 42.382927° | 43.012168° | 953 | G, M, O | 04.05.2018 |
| 40d | Tschernomorica | kopidophora sp. nov. | I | spring under Nakerala pass above road, travaertine fall | 42.382927° | 43.012168° | 953 | C, G, O | 10.09.2022. |
| 41 | Tschernomorica | kopidophora sp. nov. | I | Sveri village, spring near the road | 42.23574° | 43311485° | 551 | C, G, O | 13.09.2022. |
| 42a | Tschernomorica | cf. kopidophora sp. nov. | I | Chiatura region, Itkhvisi village. karst spring. | 42.289192° | 43.350163° | 671 | M | 21.10.2021. |
| 42b | Tschernomorica | cf. kopidophora sp. nov. | I | Chiatura region, Itkhvisi village. karst spring. | 42.289192° | 43.350163° | 671 | C, G, O | 13.09.2022. |
| 43 | Tschernomorica | cf. kopidophora sp. nov. | I | Chiatura region, Skindori, in cave | 42.241454° | 43.258937° | 548 | C, G, O | 13.09.2022. |
| 44 | Tschernomorica | cf. kopidophora sp. nov. | I | Mukhura, 6km E of Tkibuli, ssmall stream in Mukhura | 42.325160° | 43.065930° | 687 | M | 28.07.2017. |
| 45 | Tschernomorica | cf. kopidophora sp. nov. | I | right triburaty spring o Jurchula river, near Kvemo Khevi | 42.390500° | 43.359820° | 553 | M | 26.07.2017. |
| 46 | Tschernomorica | cf. kopidophora sp. nov. | I | stream Jurchula N of Kvemo Khevi at Jurchi Monastery | 42.394964° | 43.364591° | 544 | M | 26.07.2017. |
| 47 | Tschernomorica | cf. kopidophora sp. nov. | I | Tsushkvati, Tsutskhvati cave (Maghara) | 42.272718° | 42.852900° | 392 | C, G, O | 10.09.2022. |
| 48 | Tschernomorica | cf. kopidophora sp. nov. | I | Chiatura region, Skindori III, spring fom cave with pipes | 42.250153° | 43.273786° | 557 | C, G, O | 13.09.2022. |
| 78 | Tschernomorica | cf. kopidophora sp. nov. | R | Skhmeri Plateau, Kheuri seepage and spring | 42.484475° | 43.429302° | 1732 | C, G, O | 11.09.2022. |
| 79 | Tschernomorica | cf. kopidophora sp. nov. | R | Skhmeri Plateau, Kheuri seepage and spring | 42.486177° | 43.426250° | 1704 | C, G, O | 11.09.2022. |
| 80 | Tschernomorica | cf. kopidophora sp. nov. | R | Skhmeri, Shkhrimeri Cave at left tributary of Kheuri | 42.489233° | 43.401644° | 1662 | G, M | 05.05.2018. |
| 49 | Colchiella | dadiani sp. nov. | I | Imereti, Turchu Gamosadivari Basin, Nakriduri 3 spring | 42.478030° | 42.512797° | 875 | G, M, O | 03.05.2018. |
| 50 | Colchiella | dadiani sp. nov. | I | Kinchkaperd iabout 1,5 km NE of the Kinchkha Waterfall | 42.513206° | 42.558801° | 923 | G, S | 15.10.2021. |
| 50a | Colchiella | dadiani sp. nov. | I | Kinchkaperdi about 1,8 km NE of the Kinchkha Waterfall | 42.517252° | 42.559959° | 978 | G, S | 15.10.2021. |
| 50b | Colchiella | dadiani sp. nov. | I | Kinchkaperdi about 2 km NE of the Kinchkha Waterfall | 42.518485° | 42.557299° | 960 | G, S | 15.10.2021. |
| 51 | Colchiella | dadiani sp. nov. | I | Kinchkaperdi, Spring at right tributary of Saskitsvilo River | 42.519975° | 42.556215° | 976 | G, S | 15.10.2021. |
| 52a | Colchiella | dadiani sp. nov. | I | Kinchkhaperdi, about 1 km NE of the Kinchkha Waterfall | 42.502183° | 42.559525° | 874 | G, M, O | 02.05.2018. |
| 52b | Colchiella | dadiani sp. nov. | I | Kinchkhaperdi, about 1 km NE of the Kinchkha Waterfall | 42.502183° | 42.559525° | 874 | G, S | 15.10.2021. |
| 53 | Colchiella | dadiani sp. nov. | I | Kinchkhaperdi, about 1,1 km NE of the Kinchkha Waterfall | 42.502265° | 42.559821° | 874 | G | 26.07.2017 |
| 54 | Colchiella | dadiani sp. nov. | I | Saskitsvilo, Thurchismtha, spring of Okatse and Cave | 42.497017° | 42.547122° | 1044 | G, M, O | 02.05.2018. |
| 55 | Colchiella | dadiani sp. nov. | I | Turchu Gamosadivar Basin, Nakriduri 2 spring ford | 42.477581° | 42.512194° | 866 | G, M, O | 03.05.2018. |
| 56 | Colchiella | dadiani sp. nov. | I | Turchu Gamosadivar Basin, Upskhero spring lake | 42.463150° | 42.500967° | 886 | G, M, O | 03.05.2018. |
| 57 | Colchiella | dadiani sp. nov. | I | Turchu Gamosadivari Basin, Nakriduri 4 travertine spring | 42.478030° | 42.512797° | 875 | G, M, O | 03.05.2018. |
| 58 | Colchiella | dadiani sp. nov. | I | Zeda Gordi, spring in Dadiani Forest Park in Okatse OkatseCanyon | 42.456286° | 42.529152° | 642 | G | 11.08.2017. |
| 58 | Colchiella | dadiani sp. nov. | I | Zeda Gordi, spring in Dadiani Forest Park in Okatse OkatseCanyon | 42.456286° | 42.529152° | 642 | G, M, O | 01.05.2018. |
| 58 | Colchiella | dadiani sp. nov. | I | Zeda Gordi, spring in Dadiani Forest Park in Okatse OkatseCanyon | 42.456286° | 42.529152° | 642 | G, S | 15.10.2021. |
| 59 | Colchiella | cf. dadiani sp. nov. | S | Pirveli Balda, spring at bank of Toba River near waterfall | 42.476505° | 42.458445° | 677 | G | 14.10.2019. |
| 60 | Colchiella | cf. dadiani sp. nov. | S | Pirveli Balda, spring in village above road | 42.484039° | 42.398128° | 296 | G, M, O | 09.05.2018. |
| 62 | Colchiella | nazodelavo sp. nov. | S | Chkhorotsku, Nazodelavo Cave | 42.505189° | 42.220847° | 277 | G, M, O | 11.05.2018. |
| 64 | Colchiella | nazodelavo sp. nov. | S | Kotianteti, Nakhuri stream | 42.322830° | 42.154000° | 73 | M | 05.08.2019. |
| 83a | Colchiella | nazodelavo sp. nov. | S | Chkvaleri, Kvatskalara spring | 42.721250° | 42.091639° | 383 | G, S | 19.10.2021. |
| 83b | Colchiella | nazodelavo. nov. | S | Chkvaleri, Kvatskalara spring | 42.721250° | 42.091639° | 383 | G, S | 19.10.2021. |
| 61 | Colchiella | cf. nazodelavo o sp. nov. | S | Chkhorotsku, Letsurtsume, Letsurtsume Cave, conglomerates | 42.539228° | 42.113400° | 176 | G, M, O | 10.05.2018. |
| 63 | Colchiella | cf. nazodelavo sp. nov. | S | Garakha, Garakha Cave, comnglomerates | 42.177567° | 42.529869° | 56 | G, S | 17.09.2021. |
| 65 | Colchiella | cf. nazodelavo nov. | S | Nakiani, spring near road to Abano Cave, conglomerates | 42.518472° | 42.202833° | 216 | G, S | 18.10.2021. |
| 67 | Colchiella | cf. nazodelavo p. nov. | S | Small cave Hupina with the spring and reservoir | 42.554095° | 42.036681° | 232 | C, G, O | 17.09.2022. |
| 66 | Colchiella | kopidophora sp. nov. | S | Cave Onzile and spring at left bank of Chanitskali River | 42.529654° | 42.046006° | 180 | C, G, O | 17.09.2022. |
| 68a | Colchiella | shiksha sp. nov. | S | Mukhuri, Shiksha spring | 42.629811° | 42.190467° | 233 | G, M, O | 10.05.2018. |
| 68b | Colchiella | shiksha sp. nov. | S | Mukhuri, Shiksha spring | 42.629811° | 42.190467° | 233 | G, M B | 12.10.2019. |
| 68c | Colchiella | shiksha sp. nov. | S | Mukhuri, Shiksha spring | 42.629811° | 42.190467° | 233 | C, G, O | 16.09.2022. |
| 69 | Colchiella | lugella sp. nov. | S | Mukhuri, spring Lugella at bank of Khobsitskali River | 42.654430° | 42.224117° | 321 | G | 13.08.2017. |
| 69 | Colchiella | lugella sp. nov. | S | Mukhuri, spring Lugella at bank of Khobsitskali River | 42.654430° | 42.224117° | 321 | C, G, O | 10.05.2018. |
| 69 | Colchiella | lugella sp. nov. | S | Mukhuri, spring Lugella at bank of Khobsitskali River | 42.654430° | 42.224117° | 321 | C, G, O | 16.09.2022. |
| 70a | Sataplia | cavernicola sp. nov. | I | Banoja, Sataplia Cave, well in cave with water inlet | 42.311641° | 42.675151° | 467 | G, M, O | 01.05.2018. |
| 70b | Sataplia | cavernicola sp. nov. | I | Banoja, Sataplia Cave, rivulet in the end of cave | 42.311641° | 42.675151° | 467 | M | 23.03.2014. |
| 70c | Sataplia | cavernicola sp. nov. | I | Banoja, Sataplia Cave, rivulet in the end of cave | 42.311641° | 42.675151° | 467 | M | 23.03.2014. |
| 70d | Sataplia | cavernicola sp. nov. | I | Banoja, Sataplia Cave, water inlet at the end of cave | 42.311641° | 42.675151° | 467 | G, M, O | 01.05.2018. |
| 70e | Sataplia | cf. cavernicola sp. nov. | I | Banoja, rivulet above the spring in the village | 42.280479° | 42.665173° | 168 | G, M, O | 01.05.2018. |
| 70d | Sataplia | cf. cavernicola sp. nov. | I | Kumistavi, Prometheus Cave | 42.600860° | 42.376636° | 174 | G, M, O | 01.05.2018. |
| 71 | Aetis | starobogatovi sp. nov. | I | Khevistskali river, south to the village Kvemo Nogha | 42.063440° | 42.269450° | 53 | M | 01.09.2017. |
| 72a | Aetis | starobogatovi sp. nov. | G | Kvabghi spring, | 41.925174° | 42.387773° | 477 | C, G, O | 14.09.2022. |
| 72b | Aetis | starobogatovi sp. nov. | G | Kvabghi, at Chokhatauri-Bakhmaro road, above Gubazuli | 41.924991° | 42.387607° | 458 | M | 01.09.2017. |
| 32a | Aetis | starobogatovi sp. nov. | G | Small stream near village Chkhakoura, | 41.909827° | 42.387033° | 945 | M | 30.08.2017. |
| 32b | Aetis | starobogatovi sp. nov. | G | Small stream near village Chkhakoura, | 41.947869° | 41.947869° | 46 | M | 24.05.2017. |
| 74a | Aetis | starobogatovi sp. nov. | G | Spring Berdnis Tskharo near Zemo Nogha, pipe+ stream | 42.063471° | 42.269417° | 50 | C, G, O | 14.09.2022. |
| 74b | Aetis | cf. starobogatovi sp. nov. | G | spring by side of the Chokhatauri-Bakhmaro road | 41.891203° | 42.371960° | 1368 | C, G, O | 14.09.2022. |
| 74c | Aetis | cf. starobogatovi sp. nov. | G | spring by side of the Chokhatauri-Bakhmaro road | 41.909662° | 42.386778° | 945 | C, G, O | 14.09.2022. |
| 75 | Tschernomorica | sp.1 indet. | J | Kvabiskhevi, junct.of Khitakhevi and Gura Riv. to Mtsvane | 41.802557° | 43.318725° | 46 | M | 24.09.2017. |
| 76 | Tschernomorica | sp.2 indet. | K | River Khrami near Nakhiduri village, | 41.478612° | 44.696655° | 412 | M | 25.06.2017. |
| 77 | Tschernomorica | sp.3 indet. | K | Rivulet 5 km south from the village Saparlo | 41.275130° | 44.312940° | 1049 | M | 21.06.2017. |
| 81 | Tschernomorica | sp.5 indet. | I | Small stream in the village Vardzia 10km S of Zestafoni | 42.014699° | 43.091673° | 555 | M | 27.07.2017. |
| 82 | Tschernomorica | sp.6 indet. | A | stream near the Zvare; Right tributary of Ajarischkali River | 41.62721° | 41.976750° | 232 | M | 22.09.2017. |
| Character/Index | Species | |||||||
|---|---|---|---|---|---|---|---|---|
| Tschernomorica | Tschernomorica | Colchiella | Colchiella | Colchiella | Colchiella | Aetis | Sataplia | |
| kimmeria (n = 25) | kopidophora (n = 31) | dadiani (n = 32) | shiksha (n = 51) | nazodelavo (n = 11) | lugella (n = 29) | starobogatovi (n = 37) | cavernicola (n = 20) | |
| Whorl number (WhN) | 3.10–3.75 | 3.9–4.25 | 3.30–4.00 | 3.75–4.20 | 3.45–3.80 | 3.60–4.10 | 3.75–4.25 | 3.80–4.25 |
| 3.58 ± 0.19 | 4.04 ± 0.10 | 3.74 ± 0.20 | 3.73 ± 0.21 | 3.62 ± 0.14 | 3.90 ± 0.13 | 3.90 ± 0.15 | 4.05 ± 0.13 | |
| Shell height, mm (SH) | 1.34–1.93 | 1.61–1.91 | 1.48–1.84 | 1.46–2.00 | 1.34–1.60 | 1.37–1.74 | 1.43–1.76 | 1.60–1.79 |
| 1.52 ± 0.15 | 1.76 ± 0.08 | 1.65 ± 0.10 | 1.71 ± 0.14 | 1.50 ± 0.08 | 1.59 ± 0.09 | 1.60 ± 0.07 | 1.69 ± 0.06 | |
| Shell width, mm (SW) | 0.86–1.21 | 0.86–1.03 | 1.00–1.26 | 0.98–1.36 | 0.88–1.00 | 0.90–1.14 | 0.84–1.02 | 0.93–1.05 |
| 1.01 ± 0.08 | 0.95 ± 0.04 | 1.13 ± 0.07 | 1.16 ± 0.09 | 0.96 ± 0.04 | 1.01 ± 0.05 | 0.94 ± 0.04 | 1.00 ± 0.04 | |
| Body whorl height, mm (BWH) | 1.04–1.50 | 1.22–1.40 | 1.20–1.45 | 1.19–1.56 | 1.07–1.26 | 1.07–1.31 | 1.15–1.40 | 1.10–1.39 |
| 1.18 ± 0.10 | 1.29 ± 0.05 | 1.31 ± 0.06 | 1.35 ± 0.09 | 1.19 ± 0.07 | 1.20 ± 0.06 | 1.26 ± 0.06 | 1.27 ± 0.06 | |
| Body whorl width, mm (BWW) | 0.73–0.94 | 0.80–0.92 | 0.82–1.04 | 0.82–1.09 | 0.75–0.88 | 0.78–0.94 | 0.75–0.93 | 0.82–0.96 |
| 0.82 ± 0.05 | 0.87 ± 0.03 | 0.94 ± 0.06 | 0.98 ± 0.07 | 0.83 ± 0.04 | 0.87 ± 0.05 | 0.83 ± 0.04 | 0.90 ± 0.03 | |
| Body whorl height above aperture, mm (BWHap) | 0.35–0.59 | 0.50–0.60 | 0.45–0.58 | 0.44–0.60 | 0.38–0.53 | 0.37–0.57 | 0.45–0.55 | 0.43–0.60 |
| 0.44 ± 0.05 | 0.55 ± 0.02 | 0.50 ± 0.04 | 0.53 ± 0.05 | 0.47 ± 0.05 | 0.47 ± 0.04 | 0.51 ± 0.03 | 0.52 ± 0.04 | |
| Spire height, mm (SpH) | 0.66–1.01 | 0.92–1.14 | 0.72–1.00 | 0.74–1.07 | 0.67–0.90 | 0.69–1.00 | 0.74–0.92 | 0.85–1.10 |
| 0.78 ± 0.09 | 1.02 ± 0.06 | 0.84 ± 0.08 | 0.89 ± 0.09 | 0.77 ± 0.07 | 0.86 ± 0.07 | 0.84 ± 0.04 | 0.94 ± 0.06 | |
| Penultimate whorl height, mm (PWH) | 0.22–0.42 | 0.23–0.38 | 0.23–0.36 | 0.22–0.40 | 0.23–0.30 | 0.25–0.37 | 0.23–0.35 | 0.25–0.35 |
| 0.29 ± 0.05 | 0.34 ± 0.03 | 0.29 ± 0.03 | 0.30 ± 0.04 | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.29 ± 0.03 | 0.32 ± 0.03 | |
| Penultimate whorl width, mm (PWW) | 0.58–0.79 | 0.66–0.78 | 0.64–0.83 | 0.64–0.89 | 0.58–0.70 | 0.61–0.80 | 0.59–0.72 | 0.69–0.79 |
| 0.66 ± 0.05 | 0.72 ± 0.03 | 0.72 ± 0.05 | 0.74 ± 0.06 | 0.64 ± 0.04 | 0.70 ± 0.04 | 0.64 ± 0.03 | 0.73 ± 0.03 | |
| Aperture height, mm (AH) | 0.63–0.82 | 0.66–0.80 | 0.74–0.90 | 0.70–0.92 | 0.65–0.76 | 0.65–0.80 | 0.62–0.82 | 0.70–0.82 |
| 0.71 ± 0.04 | 0.73 ± 0.04 | 0.81 ± 0.04 | 0.81 ± 0.06 | 0.72 ± 0.03 | 0.74 ± 0.05 | 0.72 ± 0.05 | 0.76 ± 0.03 | |
| Aperture width, mm (AW) | 0.53–0.69 | 0.51–0.62 | 0.60–0.72 | 0.57–0.74 | 0.53–0.60 | 0.5–0.64 | 0.48–0.67 | 0.50–0.63 |
| 0.59 ± 0.04 | 0.56 ± 0.03 | 0.67 ± 0.03 | 0.65 ± 0.04 | 0.57 ± 0.02 | 0.57 ± 0.04 | 0.55 ± 0.04 | 0.58 ± 0.03 | |
| SW/SH | 0.62–0.73 | 0.51–0.58 | 0.62–0.74 | 0.59–0.73 | 0.61–0.67 | 0.60–0.68 | 0.56–0.63 | 0.52–0.66 |
| 0.67 ± 0.03 | 0.54 ± 0.02 | 0.68 ± 0.03 | 0.68 ± 0.03 | 0.64 ± 0.02 | 0.64 ± 0.02 | 0.59 ± 0.01 | 0.59 ± 0.03 | |
| BWH/SH | 0.73–0.84 | 0.70–0.78 | 0.75–0.85 | 0.76–0.85 | 0.77–0.83 | 0.70–0.79 | 0.75–0.85 | 0.69–0.80 |
| 0.78 ± 0.02 | 0.74 ± 0.02 | 0.80 ± 0.02 | 0.79 ± 0.02 | 0.80 ± 0.02 | 0.75 ± 0.02 | 0.79 ± 0.02 | 0.76 ± 0.03 | |
| SpH/SH | 0.44–0.56 | 0.55–0.62 | 0.46–0.56 | 0.48–0.56 | 0.48–0.56 | 0.50–0.59 | 0.50–0.56 | 0.53–0.61 |
| 0.51 ± 0.03 | 0.58 ± 0.02 | 0.51 ± 0.02 | 0.52 ± 0.02 | 0.51 ± 0.02 | 0.54 ± 0.02 | 0.53 ± 0.01 | 0.56 ± 0.02 | |
| AH/SH | 0.42–0.51 | 0.38–0.45 | 0.46–0.53 | 0.43–0.52 | 0.45–0.52 | 0.40–0.50 | 0.42–0.50 | 0.42–0.47 |
| 0.47 ± 0.02 | 0.41 ± 0.02 | 0.49 ± 0.02 | 0.48 ± 0.02 | 0.48 ± 0.02 | 0.46 ± 0.02 | 0.45 ± 0.02 | 0.45 ± 0.01 | |
| AH/AW | 1.14–1.28 | 1.21–1.41 | 1.14–1.33 | 1.14–1.32 | 1.19–1.34 | 1.05–1.38 | 1.12–1.48 | 1.17–1.42 |
| 1.22 ± 0.04 | 1.29 ± 0.05 | 1.22 ± 0.04 | 1.25 ± 0.04 | 1.26 ± 0.04 | 1.29 ± 0.07 | 1.32 ± 0.06 | 1.30 ± 0.06 | |
| PWH/SH | 0.15–0.23 | 0.14–0.22 | 0.15–0.20 | 0.15–0.21 | 0.16–0.19 | 0.16–0.22 | 0.15–0.21 | 0.16–0.20 |
| 0.19 ± 0.02 | 0.19 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.18 ± 0.01 | 0.19 ± 0.01 | |
| PWH/BWH | 0.19–0.30 | 0.18–0.30 | 0.18–0.26 | 0.18–0.26 | 0.19–0.25 | 0.21–0.30 | 0.17–0.28 | 0.21–0.29 |
| 0.25 ± 0.03 | 0.26 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.22 ± 0.02 | 0.26 ± 0.02 | 0.23 ± 0.02 | 0.25 ± 0.02 | |
| PWW/BWW | 0.68–0.90 | 0.79–0.87 | 0.72–0.90 | 0.70–1.00 | 0.74–0.80 | 0.71–0.87 | 0.71–0.97 | 0.78–0.87 |
| 0.80 ± 0.05 | 0.83 ± 0.02 | 0.77 ± 0.03 | 0.76 ± 0.04 | 0.77 ± 0.02 | 0.81 ± 0.04 | 0.81 ± 0.06 | 0.81 ± 0.02 | |
| Species | COI/H3 GB Numbers | References |
|---|---|---|
| Aetis starobogatovi Chertoprud, Grego & Mumladze, sp. nov. | OQ396620-30/OQ401670-80 | present study |
| Agrafia wiktori Szarowska et Falniowski, 2011 | JF906762 | Szarowska & Falniowski, 2011 [54] |
| Alzoniella finalina Giusti & Bodon, 1984 | AF367650 | Wilke et al., 2001 [55] |
| Avenionia brevis berenguieri (Draparnaud, 1805) | AF367638 | Wilke et al., 2001 [55] |
| Belgrandiella cf. kuesteri (Boeters, 1970) | MG551325/MG551366 | Osikowski et al., 2018 [56] |
| Belgrandiella cf. robusta Radoman, 1975 | MG551331 | Osikowski et al., 2018 [56] |
| Caucasogeyeria gloeri Grego & Mumladze, 2020 | MT406097/MT410523 | Grego et al., 2020 [10] |
| Caucasopsis letsurtsume Grego & Mumladze, 2020 | MT406082/MT410510 | Grego et al., 2020 [10] |
| Colchiella dadiani Chertoprud, Grego & Mumladze, sp. nov. | OQ396613-19/OQ401663-69 | present study |
| Colchiella lugella Chertoprud, Grego & Mumladze, sp. nov. | MG543150-51/MG543153-54 | Grego et al., 2017 [25] |
| Colchiella nazodelavo Chertoprud, Grego & Mumladze, sp. nov. | OQ396608-12/OQ401658-62 | present study |
| Colchiella shiksha Chertoprud, Grego & Mumladze, sp. nov. | OQ396606-07/OQ401656-57 | present study |
| Dalmatinella fluviatilis Radoman, 1973 | KC344541 | Falniowski & Szarowska, 2013 [57] |
| Daphniola louisi Falniowski & Szarowska, 2000 | KM887915/MZ265364 | Szarowska et al., 2014 [58]/Hofman et al., 2021 [59] |
| Ecrobia maritima (Milaschewitsch, 1916) | KX355830/MG551322 | Osikowski et al., 2016 [60]/Grego et al., 2017 [25] |
| Fissuria boui Boeters, 1981 | AF367654 | Wilke et al., 2001 [55] |
| Graecoarganiella parnassiana Falniowski & Szarowska, 2011 | JN202352/MZ265362 | Falniowski & Szarowska, 2011 [61]/Hofman et al., 2021 [59] |
| Graziana alpestris (Frauenfeld, 1863) | AF367641 | Wilke et al., 2001 [55] |
| Grossuana angeltsekovi Glöer & Georgiev, 2009 | KU201090 | Falniowski et al., 2016 [62] |
| Hauffenia michleri Kuščer, 1932 | KY087865/KY087878 | Rysiewska et al., 2017 [63] |
| Hausdorfenia pseudohauffenia Grego & Mumladze, 2020 | MT406102/MT410528 | Grego et al., 2020 [10] |
| Horatia klecakiana Bourguignat 1887 | KJ159128 | Szarowska & Falniowski, 2014 [64] |
| Iglica cf. gracilis (Clessin, 1882) | MH720988 | Hofman et al., 2018 [65] |
| Imeretiopsis gorgoleti Grego & Mumladze, 2020 | MT406092/MT410520 | Grego et al., 2020 [10] |
| Islamia zermanica (Radoman, 1973) | KU662362/MG551320 | Beran et al., 2016 [66]/Grego et al., 2017 [25] |
| Kartvelobia sinuata Grego & Mumladze, 2020 | MT406091/MT410517 | Grego et al., 2020 [10] |
| Montenegrospeum bogici (Pešić & Glöer, 2012) | KM875510/MG880218 | Falniowski et al., 2014 [67]/Grego et al., 2018 [68] |
| Paladilhiopsis grobbeni Kuscer, 1928 | MH720991 | Hofman et al., 2018 [65] |
| Peringia ulvae (Pennant, 1777) | AF118302 | Wilke and Davis, 2000 [51] |
| Pontobelgrandiella sp. Radoman, 1978 | KU497012 | Rysiewska et al., 2016 [69] |
| Pontohoratia pichkhaiai Grego & Mumladze, 2020 | MT406087/MT410513 | Grego et al., 2020 [10] |
| Sadleriana fluminensis (Küster, 1853) | KF193067 | Szarowska & Falniowski, 2013 [70] |
| Salenthydrobia ferrerii Wilke, 2003 | AF449213 | Wilke, 2003 [50] |
| Sarajana apfelbecki (Brancsik, 1888) | MN031432 | Hofman et al., 2019 [71] |
| Sataplia cavernicola Chertoprud, Grego & Mumladze, sp. nov. | OQ396631-32/OQ401681-82 | present study |
| Terranigra kosovica Radoman, 1978 | xxx | Jaszczyńska et al. in press [72] |
| “Tschernomorica” adsharica (Lindholm, 1913) | OQ396633-38/OQ401683-88 | present study |
| Tschernomorica kimmeria Vinarski & Palatov, 2019 | OQ396600-05/OQ401650-55 | present study |
| Tschernomorica kopidophora Chertoprud, Grego & Mumladze, sp. nov. | OQ396576-99/OQ401626-49 | present study |
| A | B | C | D | E | F | G | H | I | |
|---|---|---|---|---|---|---|---|---|---|
| A | 0.002 | 0.007 | 0.010 | 0.003 | 0.003 | 0.003 | 0.020 | 0.020 | 0.031 |
| B | 0.015 | 0.001 | 0.017 | 0.010 | 0.010 | 0.010 | 0.027 | 0.027 | 0.038 |
| C | 0.078 | 0.085 | 0.000 | 0.007 | 0.007 | 0.007 | 0.031 | 0.031 | 0.041 |
| D | 0.069 | 0.075 | 0.021 | 0.000 | 0.000 | 0.000 | 0.024 | 0.024 | 0.034 |
| E | 0.084 | 0.092 | 0.047 | 0.029 | 0.016 | 0.000 | 0.024 | 0.024 | 0.034 |
| F | 0.092 | 0.096 | 0.048 | 0.027 | 0.028 | 0.006 | 0.024 | 0.024 | 0.034 |
| G | 0.106 | 0.113 | 0.100 | 0.092 | 0.109 | 0.114 | 0.003 | 0.020 | 0.031 |
| H | 0.104 | 0.106 | 0.097 | 0.092 | 0.104 | 0.108 | 0.106 | 0.043 | 0.017 |
| I | 0.104 | 0.109 | 0.099 | 0.090 | 0.103 | 0.108 | 0.102 | 0.096 | 0.010 |
| Source of Variation | df | SS | MS | Pseudo-F | p | Unique | Variance Component (%) |
|---|---|---|---|---|---|---|---|
| Perms | |||||||
| SPECIES | 7 | 2121.8 | 303.11 | 29.473 | 0.001 | 998 | 49.6 |
| Residual variation | 228 | 2344.9 | 10.284 | 50.4 |
| Character | Axis 1 | Axis 2 | Axis 3 |
|---|---|---|---|
| SH | 0.004 | 0.025 | 0.076 |
| SW | −0.029 | 0.016 | 0.035 |
| BWH | −0.006 | 0.038 | 0.040 |
| BWHap | 0.004 | 0.018 | 0.024 |
| SpH | 0.015 | 0.005 | 0.062 |
| PWW | −0.005 | 0.000 | 0.036 |
| PWH | 0.004 | −0.006 | 0.016 |
| BWW | −0.014 | 0.020 | 0.040 |
| AH | −0.012 | 0.016 | 0.021 |
| AW | −0.015 | 0.007 | 0.017 |
| WhN | 0.050 | 0.020 | 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chertoprud, E.; Grego, J.; Mumladze, L.; Hofman, S.; Palatov, D.; Osikowski, A.; Jaszczyńska, A.; Falniowski, A. Revealing the Stygobiotic and Crenobiotic Molluscan Diversity in the Caucasus: Part IV—Crenobiotic Belgrandiellinae Radoman, 1983 (Mollusca, Hydrobiidae) from Georgia. Diversity 2023, 15, 450. https://doi.org/10.3390/d15030450
Chertoprud E, Grego J, Mumladze L, Hofman S, Palatov D, Osikowski A, Jaszczyńska A, Falniowski A. Revealing the Stygobiotic and Crenobiotic Molluscan Diversity in the Caucasus: Part IV—Crenobiotic Belgrandiellinae Radoman, 1983 (Mollusca, Hydrobiidae) from Georgia. Diversity. 2023; 15(3):450. https://doi.org/10.3390/d15030450
Chicago/Turabian StyleChertoprud, Elizaveta, Jozef Grego, Levan Mumladze, Sebastian Hofman, Dmitry Palatov, Artur Osikowski, Aleksandra Jaszczyńska, and Andrzej Falniowski. 2023. "Revealing the Stygobiotic and Crenobiotic Molluscan Diversity in the Caucasus: Part IV—Crenobiotic Belgrandiellinae Radoman, 1983 (Mollusca, Hydrobiidae) from Georgia" Diversity 15, no. 3: 450. https://doi.org/10.3390/d15030450
APA StyleChertoprud, E., Grego, J., Mumladze, L., Hofman, S., Palatov, D., Osikowski, A., Jaszczyńska, A., & Falniowski, A. (2023). Revealing the Stygobiotic and Crenobiotic Molluscan Diversity in the Caucasus: Part IV—Crenobiotic Belgrandiellinae Radoman, 1983 (Mollusca, Hydrobiidae) from Georgia. Diversity, 15(3), 450. https://doi.org/10.3390/d15030450









