Trophic Structure of the Soil-Dwelling Arthropod Communities at the Border of the Forest and the Steppe in the South of Western Siberia: Isotopic Data
Abstract
1. Introduction
- What is the trophic structure of the arthropod community in the “forest–edge–steppe” gradient, with special attention to ground beetles and spiders?
- What is the difference in the trophic niches of two main groups of predatory soil macroarthropods (ground beetles and spiders)? Are the food resources consumed by spiders different from those consumed by ground beetles? We assumed that the trophic niche of ground beetles should be wider than that of spiders because of the presence of omnivorous species among ground beetles (Hypothesis 1).
- Is the proportion of animals from the detrital food web (saprophages) higher in the diet of generalist predators in the forest ecosystem than in the steppe ecosystem? This should be reflected in higher δ13C and δ15N values of predators in the forest ecosystem (Hypothesis 2).
2. Materials and Methods
2.1. Brief Description of the Studied Communities of Predatory Soil-Dwelling Arthropods
2.2. Characteristics of the Study Area
2.3. Collection and Processing of Samples
3. Results
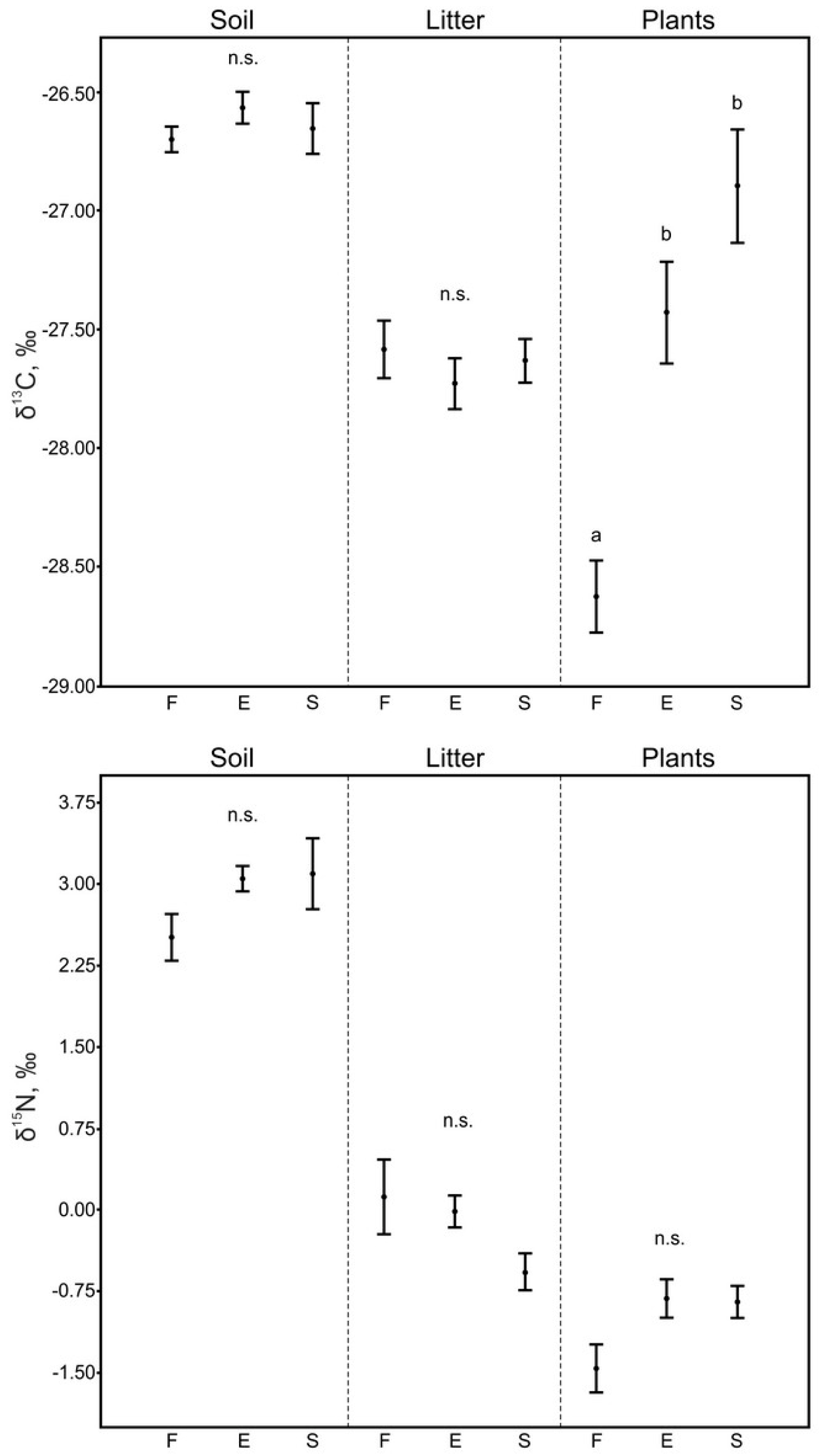
3.1. Soil and Litter
3.2. Plants
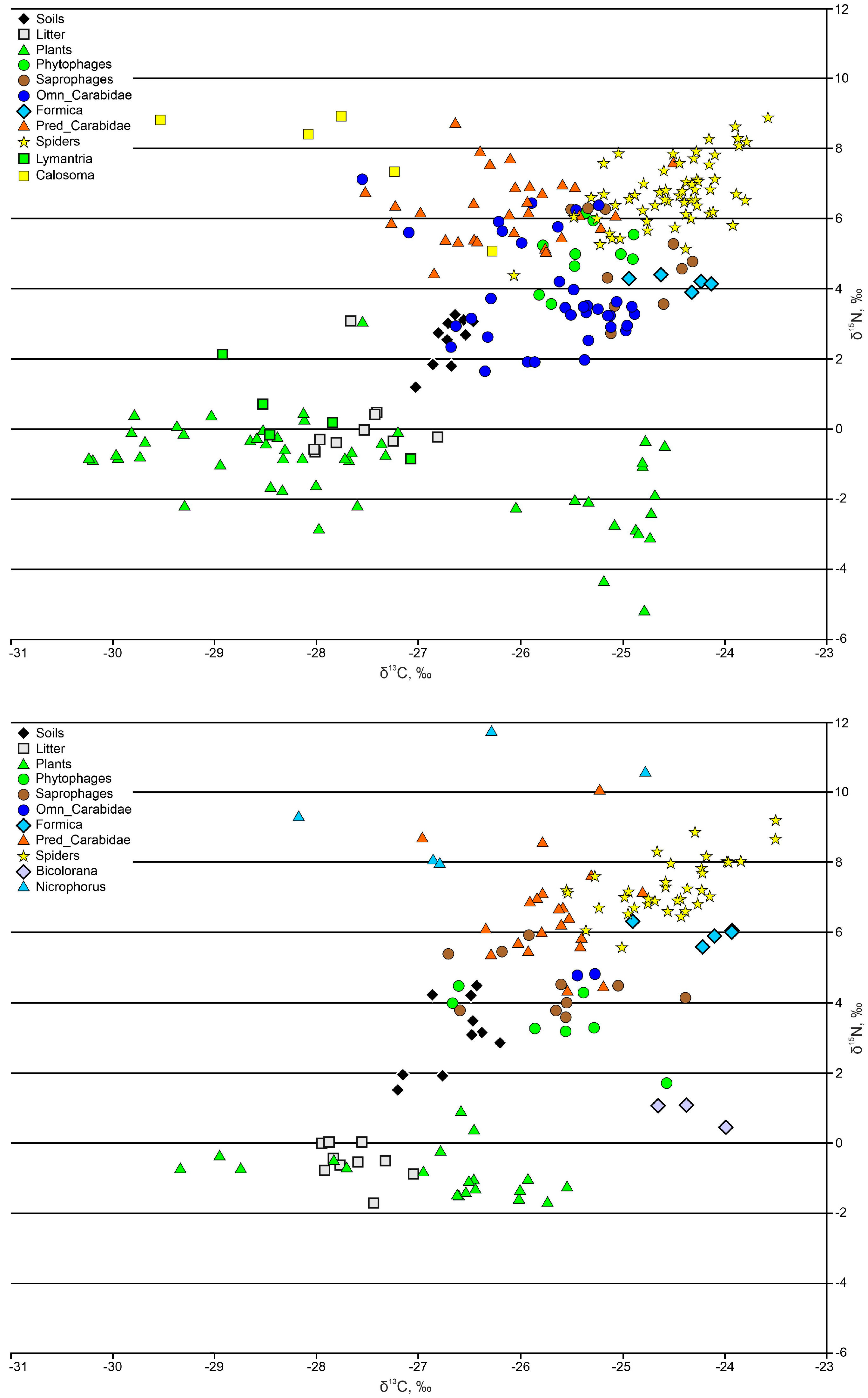
3.3. Arthropods
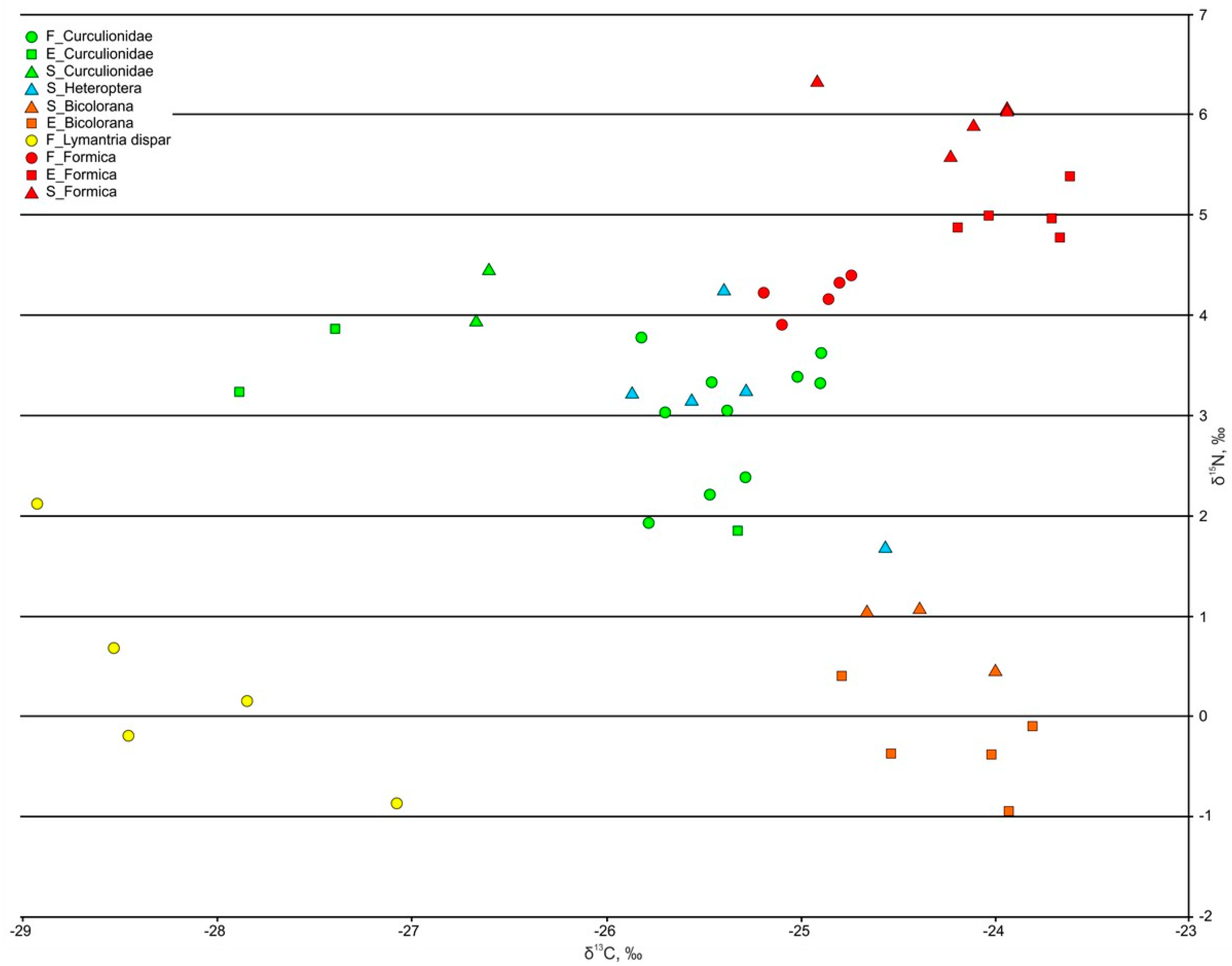
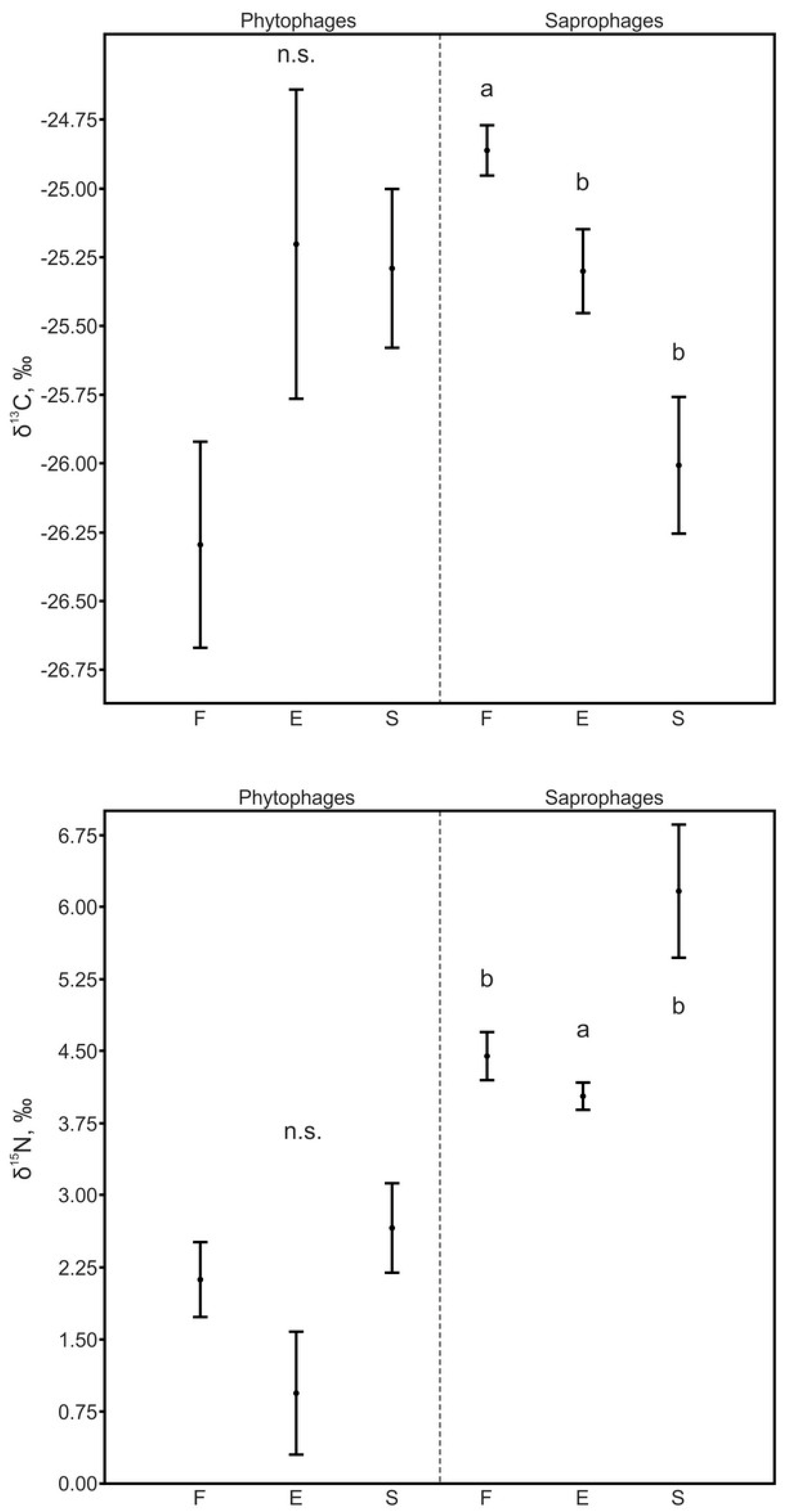
3.3.1. Phytophages and Saprophages
3.3.2. Ants
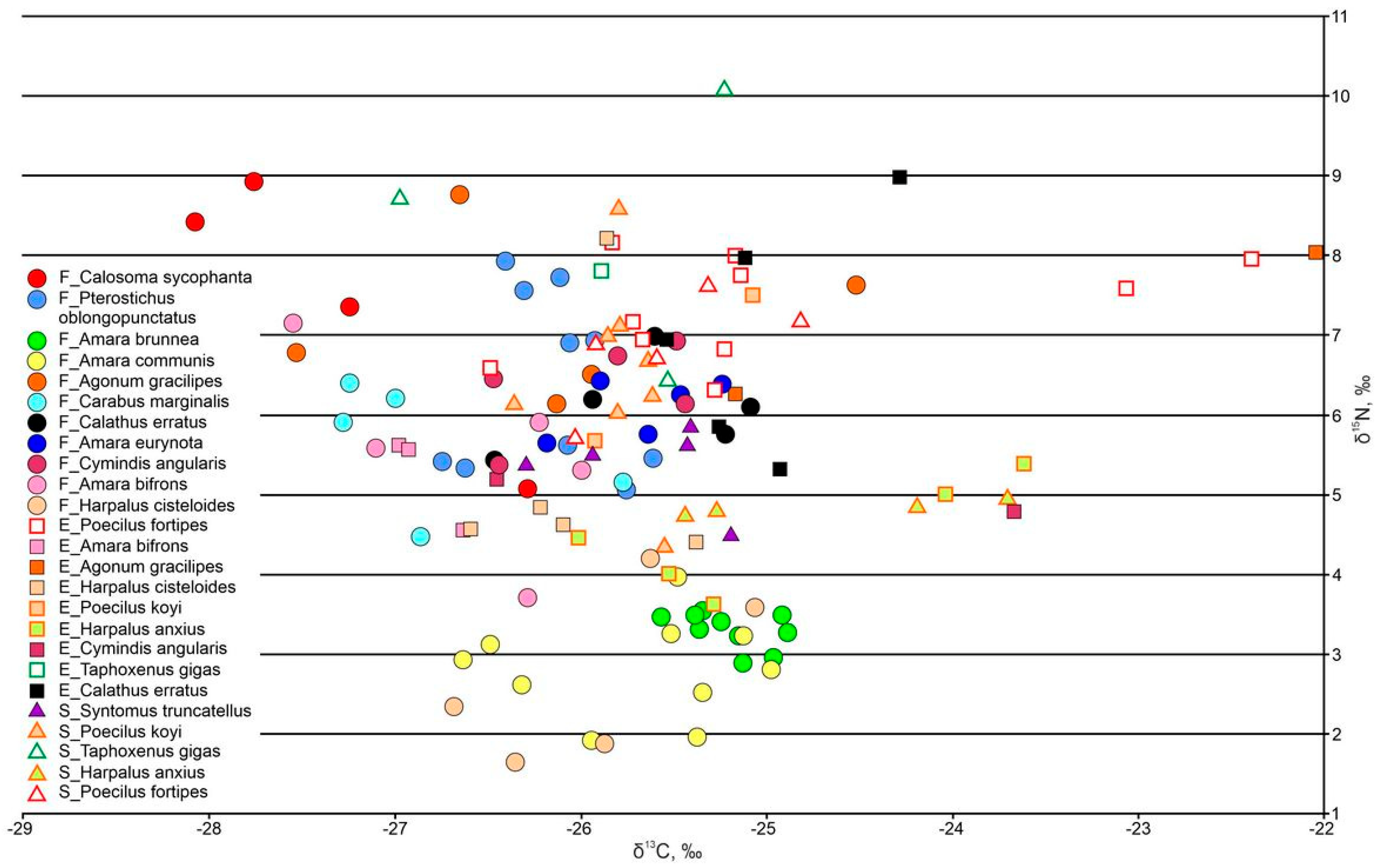
3.3.3. Ground Beetles
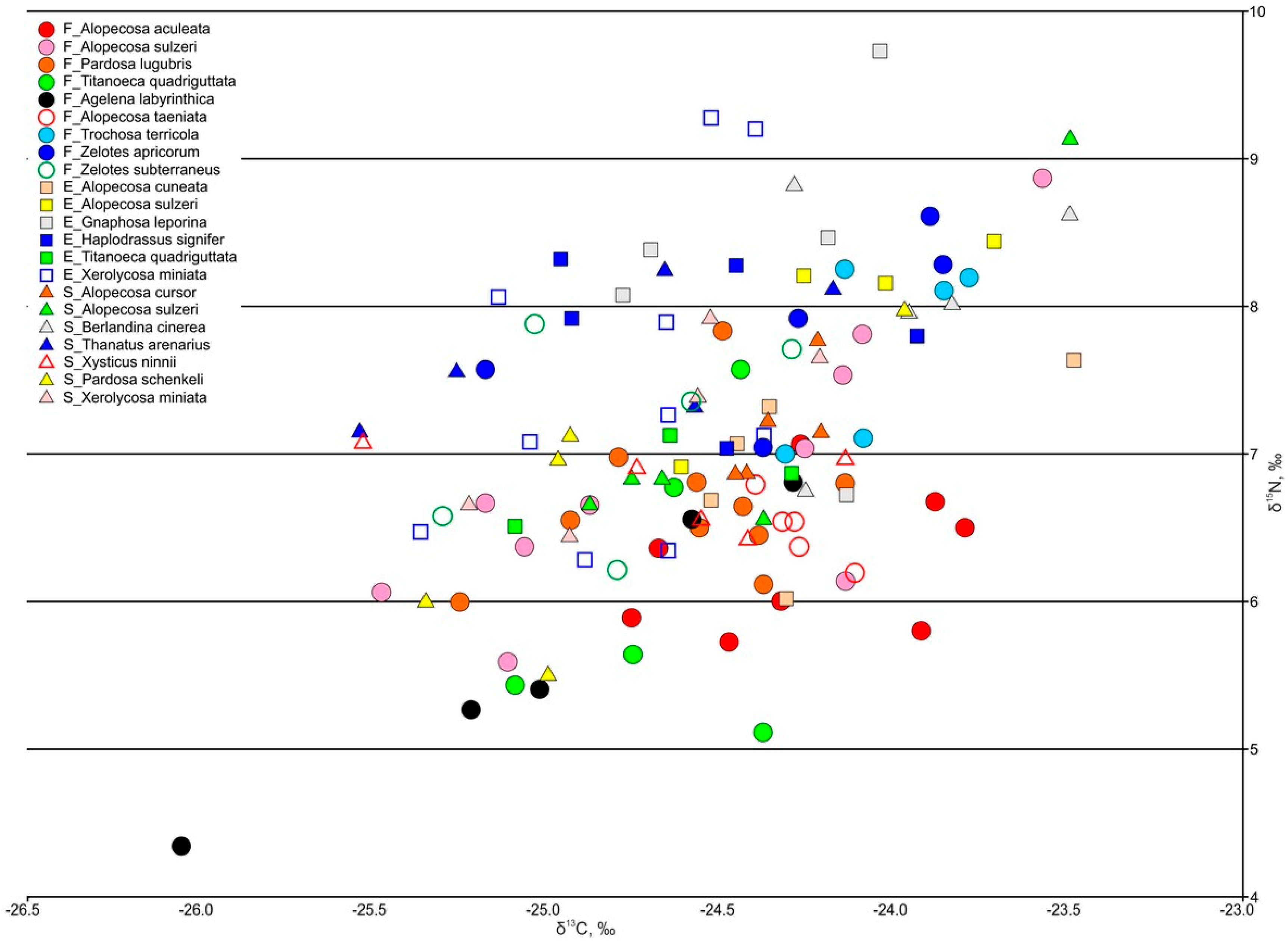
3.3.4. Spiders
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 13C | 15N | |||||
|---|---|---|---|---|---|---|
| F | E | S | F | E | S | |
| Soil | −26.70 ± 0.05 n = 10 | −26.56 ± 0.07 n = 10 | −26.65 ± 0.11 n = 10 | 2.51 ± 0.22 n = 10 | 3.05 ± 0.12 n = 10 | 3.10 ± 0.33 n = 10 |
| Plant litter | −27.58 ± 0.12 n = 10 | −27.73 ± 0.11 n = 10 | −27.63 ± 0.09 n = 10 | 0.12 ± 0.35 n = 10 | −0.01 ± 0.15 n = 10 | −0.57 ± 0.17 n = 10 |
| Plants | ||||||
| Betula pendula | −29.40 ± 0.21 n = 10 | - | - | −0.88 ± 0.22 n = 10 | - | - |
| Populus tremula | −28.83 ± 0.28 n = 10 | - | - | −0.90 ± 0.30 n = 10 | - | - |
| Rosa majalis | −28.10 ± 0.15 n = 10 | - | - | −1.50 ± 0.34 n = 10 | - | - |
| Rubus saxatilis | −27.70 ± 0.13 n = 5 | - | - | −3.68 ± 0.47 n = 5 | - | - |
| Peucedanum morisoni | - | −27.83 ± 0.30 n = 10 | - | - | 0.09 ± 0.27 n = 10 | - |
| Gramineae | - | −27.59 ± 0.29 n = 10 | - | - | −1.12 ± 0.23 n = 10 | - |
| Pedicularis sp. | - | −28.20 ± 0.26 n = 5 | - | - | −1.24 ± 0.36 n = 5 | - |
| Glycyrrhiza glabra | - | −25.54 ± 0.25 n = 5 | −25.85 ± 0.09 n = 5 | - | −1.59 ± 0.11 n = 5 | −1.35 ± 0.11 n = 5 |
| Stipa pennata | - | - | −26.60 ± 0.05 n = 10 | - | - | −0.73 ± 0.26 n = 10 |
| Miscellaneous herbs | - | - | −28.52 ± 0.32 n = 5 | - | - | −0.58 ± 0.07 n = 5 |
| Phytophages | ||||||
| Heteroptera, Miridae | - | - | −25.32 ± 0.22 n = 5 | - | - | 3.12 ± 0.41 n = 5 |
| Coleoptera, Curculionidae | −25.52 ± 0.17 n = 5 | −26.86 ± 0.78 n = 3 | −26.63 ± 0.03 n = 2 | 2.85 ± 0.34 n = 5 | 2.98 ± 0.59 n = 3 | 4.21 ± 0.26 n = 2 |
| Lepidoptera, Lymantria dispar, larva | −28.16 ± 0.32 n = 5 | - | - | 0.38 ± 0.50 n = 5 | - | - |
| Omnivores | ||||||
| Orthoptera, Bicolorana roeselii, larva | −24.21 ± 0.19 n = 5 | −24.34 ± 0.19 n = 3 | −0.29 ± 0.22 n = 5 | 0.86 ± 0.20 n = 3 | ||
| Hymenoptera, Formica aquilonia | −24.93 ± 0.09 n = 5 | −23.84 ± 0.11 n = 5 | −24.22 ± 0.18 n = 5 | 4.20 ± 0.08 n = 5 | 4.99 ± 0.10 n = 5 | 5.98 ± 0.12 n = 5 |
| Omnivorous carabids | ||||||
| Amara brunnea | −25.19 ± 0.07 n = 10 | - | - | 3.29 ± 0.07 n = 10 | - | - |
| Amara communis | −25.71 ± 0.19 n = 10 | - | - | 2.81 ± 0.20 n = 10 | - | - |
| Amara eurynota | −25.68 ± 0.17 n = 5 | - | - | 6.07 ± 0.16 n = 5 | - | - |
| Amara bifrons | −26.62 ± 0.30 n = 5 | −26.84 ± 0.11 n = 3 | - | 5.51 ± 0.55 n = 5 | 5.23 ± 0.35 n = 3 | - |
| Harpalus cisteloides | −25.91 ± 0.28 n = 2 | −26.02 ± 0.20 n = 5 | - | 2.71 ± 0.50 n = 2 | 5.31 ± 0.73 n = 5 | - |
| Harpalus anxius | - | −25.60 ± 0.21 n = 3 | −25.35 ± 0.08 n = 2 | - | 4.01 ± 0.24 n = 3 | 4.78 ± 0.03 n = 2 |
| Saprophages | ||||||
| Coleoptera, Tenebrionidae: Oodescelis polita | −24.91 ± 0.13 n = 10 | −24.86 ± 0.18 n = 5 | −25.68 ± 0.25 n = 5 | 3.65 ± 0.33 n = 10 | 4.42 ± 0.22 n = 5 | 3.90 ± 0.15 n = 5 |
| Coleoptera, Tenebrionidae: Blaps lethifera | - | −25.81 ± 0.28 n = 5 | −25.76 ± 0.39 n = 5 | - | 3.76 ± 0.37 n = 5 | 5.06 ± 0.33 n = 5 |
| Coleoptera, Silphidae: Silpha carinata | −24.46 ± 0.15 n = 5 | - | - | 5.50 ± 0.25 n = 5 | - | - |
| Coleoptera, Silphidae: Nicrophorus sp. | −26.58 ± 0.55 n = 5 | - | - | 9.54 ± 0.73 n = 5 | - | - |
| Predaceous carabids | ||||||
| Pterostichus oblongopunctatus | −26.16 ± 0.11 n = 10 | - | - | 6.39 ± 0.36 n = 10 | - | - |
| Agonum gracilipes | −26.15 ± 0.49 n = 5 | −23.60 ± 1.57 n = 2 | - | 7.15 ± 0.47 n = 5 | 7.13 ± 0.89 n = 2 | - |
| Carabus marginalis | −26.82 ± 0.28 n = 5 | - | - | 5.61 ± 0.36 n = 5 | - | - |
| Calathus erratus | −25.65 ± 0.25 n = 5 | −25.02 ± 0.21 n = 5 | - | 6.07 ± 0.26 n = 5 | 6.99 ± 0.67 n = 5 | - |
| Cymindis angularus | −25.92 ± 0.22 n = 5 | −26.44 n = 1 | - | 6.31 ± 0.27 n = 5 | 5.18 n = 1 | - |
| Poecilus fortipes | - | −24.99 ± 7.31 n = 10 | −25.53 ± 0.22 n = 5 | - | 0.40 ± 0.20 n = 10 | 6.83 ± 0.31 n = 5 |
| Poecilus koyi | - | −25.49 ± 0.43 n = 2 | −25.80 ± 0.09 n = 8 | - | 6.58 ± 0.92 n = 2 | 6.52 ± 0.42 n = 8 |
| Taphoxenus gigas | - | −25.88 n = 1 | −25.91 ± 0.54 n = 3 | - | 7.78 n = 1 | 8.42 ± 1.07 n = 3 |
| Syntomus truncatellus | - | - | −25.65 ± 0.20 n = 5 | - | - | 5.38 ± 0.2 n = 5 |
| Calosoma sycophanta | −27.77 ± 0.53 n = 5 | - | - | 7.70 ± 0.72 n = 5 | - | - |
| Predators: Spiders | ||||||
| Alopecosa aculeata | −24.27 ± 0.13 n = 8 | - | - | 6.25 ± 0.17 n = 8 | - | - |
| Alopecosa sulzeri | −24.59 ± 0.20 n = 10 | −24.15 ± 0.19 n = 4 | −24.44 ± 0.25 n = 5 | 6.87 ± 0.31 n = 10 | 7.93 ± 0.34 n = 4 | 7.22 ± 0.49 n = 5 |
| Alopecosa cuneata | - | −24.23 ± 0.19 n = 5 | - | - | 6.94 ± 0.28 n = 5 | - |
| Alopecosa cursor | - | - | −24.34 ± 0.05 n = 5 | - | - | 7.18 ± 0.16 n = 5 |
| Alopecosa taeniata | −24.28 ± 0.05 n = 5 | - | - | 6.48 ± 0.10 n = 5 | - | - |
| Pardosa lugubris | −24.60 ± 0.10 n = 10 | - | - | 6.67 ± 0.16 n = 10 | - | - |
| Pardosa schenkeli | - | - | −24.84 ± 0.23 n = 5 | - | - | 6.72 ± 0.44 n = 5 |
| Xerolycosa miniata | - | −24.77 ± 0.10 n = 10 | −24.70 ± 0.18 n = 5 | - | 7.50 ± 0.35 n = 10 | 7.22 ± 0.29 n = 5 |
| Trochosa terricola | −24.04 ± 0.10 n = 5 | - | - | 7.73 ± 0.28 n = 5 | - | - |
| Berlandina cinerea | - | - | −23.97 ± 0.15 n = 5 | - | - | 8.04 ± 0.36 n = 5 |
| Gnaphosa leporina | - | −24.37 ± 0.15 n = 5 | - | - | 8.27 ± 0.48 n = 5 | - |
| Haplodrassus signifer | - | −24.55 ± 0.19 n = 5 | - | - | 7.87 ± 0.23 n = 5 | - |
| Zelotes apricorum | −24.32 ± 0.24 n = 5 | - | - | 7.88 ± 0.27 n = 5 | - | - |
| Zelotes subterreaneus | −24.80 ± 0.17 n = 5 | - | - | 7.14 ± 0.32 n = 5 | - | - |
| Agelena labyrinthica | −25.04 ± 0.30 n = 5 | - | - | 5.67 ± 0.45 n = 5 | - | - |
| Titanoeca quadriguttata | −24.66 ± 0.13 n = 5 | −24.68 ± 0.23 n = 3 | - | 6.10 ± 0.46 n = 5 | 6.83 ± 0.18 n = 3 | - |
| Thanatus arenarius | - | - | −24.84 ± 0.25 n = 5 | - | - | 7.69 ± 0.22 n = 5 |
| Xysticus ninnii | - | - | −24.68 ± 0.23 n = 5 | - | - | 6.80 ± 0.13 n = 5 |

References
- Scheu, S. Plants and generalist predators as links between the below-ground and above-ground system. Basic Appl. Ecol. 2001, 2, 3–13. [Google Scholar] [CrossRef]
- Potapov, A.M.; Tiunov, A.V.; Scheu, S. Uncovering trophic positions and food resources of soil animals using bulk natural stable isotope composition. Biol. Rev. 2019, 94, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Korobushkin, D.I.; Gongalsky, K.B.; Tiunov, A.V. Isotopic niche (δ13C and δ15N values) of soil macrofauna in temperate forests. Rapid Commun. Mass Spectrom. 2014, 28, 1303–1311. [Google Scholar] [CrossRef]
- Oelbermann, K.; Scheu, S. Trophic guilds of generalist feeders in soil animal communities as indicated by stable isotope analysis (15N/14N). Bull. Entomol. Res. 2010, 100, 511–520. [Google Scholar] [CrossRef]
- Talarico, F.; Giglio, A.; Pizzolotto, R.; Brandmayr, P. A synthesis of feeding habits and reproduction rhythm in Italian seed-feeding ground beetles (Coleoptera: Carabidae). Eur. J. Entomol. 2016, 113, 325–336. [Google Scholar] [CrossRef]
- Thiele, H.U. Carabid Beetles in Their Environments; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Mader, V.; Diehl, E.; Wolters, V.; Birkhofer, K. Agri-environmental schemes affect the trophic niche size and diet of common carabid species in agricultural landscapes. Ecol. Entomol. 2018, 43, 823–835. [Google Scholar] [CrossRef]
- Lundgren, J.G. Relationships of Natural Enemies and Non-Prey Foods; Springer International: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Zalewski, M.; Dudek, D.; Godeau, J.-F.; Maruszkiewicz, M. Stable isotopic research on ground beetles. Review of methods Baltic. J. Coleopterol. 2012, 12, 91–98. [Google Scholar]
- Okuzaki, Y.; Tayasu, I.; Okuda, N.; Sota, T. Stable isotope analysis indicates trophic differences among forest floor carabids in Japan. Entomologia Experimentalis et Applicata 2010, 135, 263–270. [Google Scholar] [CrossRef]
- Ikeda, H.; Kubota, K.; Kagawa, A.; Sota, T. Diverse diet compositions among harpaline ground beetle species revealed by mixing model analyses of stable isotope ratios. Ecol. Entomol. 2010, 35, 307–316. [Google Scholar] [CrossRef]
- Sasakawa, K.; Ikeda, H.; Kubota, T. Feeding ecology of granivorous carabid larvae: A stable isotope analysis. J. Appl. Entomol. 2010, 134, 116–122. [Google Scholar] [CrossRef]
- Zalewski, M.; Dudek-Godeau, D.; Godeau, J.-F.; Kujawa, K.; Sienkiewicz, P.; Tiunov, A.V.; Ulrich, W. Trophic generalism at the population level in ground beetles (Coleoptera: Carabidae). Can. Entomol. 2016, 148, 284–293. [Google Scholar] [CrossRef]
- Raso, D.; Sint, D.; Mayer, R.; Plangg, S.; Recheis, T.; Brunner, S.; Kaufmann, R.; Traugott, M. Intraguild predation in pioneer predator communities of alpine glacier forelands. Mol. Ecol. 2014, 23, 3744–3754. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, M.; Dudek, D.; Tiunov, A.V.; Godeau, J.-F.; Okuzaki, Y.; Ikeda, H.; Sienkiewicz, P.; Ulrich, W. High niche overlap in the stable isotope space of ground beetles. Ann. Zool. Fennici. 2014, 51, 301–312. [Google Scholar] [CrossRef]
- Den Boer, P.J. Exclusion or coexistence and the taxonomic or ecological relationscip between species. Neth. J. Zool. 1980, 30, 278–306. [Google Scholar] [CrossRef]
- Girard, J.; Baril, A.; Mineau, P.; Fahrig, L. Carbon and nitrogen stable isotope ratios differ among invertebrates from field crops, forage crops, and non-cropped land uses. Ecoscience 2011, 18, 98–109. [Google Scholar] [CrossRef]
- Zuev, A.; Heidemann, K.; Leonov, V.; Schaefer, I.; Scheu, S.; Tanasevitch, A.; Tiunov, A.; Tsurikov, S.; Potapov, A. Different groups of ground-dwelling spiders share similar trophic niches in temperate forests. Ecol. Entomol. 2020, 45, 1346–1356. [Google Scholar] [CrossRef]
- McNabb, D.N.; Halaj, J.; Wise, D.H. Inferring trophic positions of generalist predators and their linkage to the detrital food web in agroecosystems: A stable isotope analysis. Pedobiologia 2001, 45, 289–297. [Google Scholar] [CrossRef]
- Wise, D.H.; Moldenhauer, D.M.; Halaj, J. Using stable isotopes to reveal shifts in prey consumption by generalist predators. Ecol. Appl. 2006, 16, 865–876. [Google Scholar] [CrossRef]
- Berman, D.I.; Mordkovich, V.G. Entomological features of the polar stepes of Yakutia Bulletin of Moscow Society of Naturalists. Biol. Ser. 1979, 84, 39–45. [Google Scholar]
- Crotty, F.V.; Blackshaw, R.P.; Adl, S.M.; Inger, R.; Murray, P.J. Divergence of feeding channels within the soil food web determined by ecosystem type. Ecol. Evol. 2014, 4, 1–13. [Google Scholar] [CrossRef]
- Mordkovich, V.G.; Khudyaev, S.A.; Dudko, R.Y.; Lyubechanskii, I.I. Zoological Indication of Climate Change in the Central Kazakh Steppe Compared to the Middle of the 20th Century Using the Example of Carabid and Tenebrionid Beetles. Contemp. Probl. Ecol. 2020, 13, 443–468. [Google Scholar] [CrossRef]
- Mordkovich, V.G.; Dudko, R.J.; Khudyaev, S.A.; Lyubechanskii, I.I. Changes in the Ground Beetle and Darkling Beetle Communities (Coleoptera: Carabidae, Tenebrionidae) in the Mountain Hollows of the Tuva and Altai Republics over 60 Years: A Trend or a Fluctuation? Contemp. Probl. Ecol. 2022, 15, 579–596. [Google Scholar] [CrossRef]
- Odum, E.P. Basic Ecology; W.B. Saunders: Philadelphia, PA, USA, 1983. [Google Scholar]
- Hyodo, F.; Kohzu, A.; Tayasu, I. Linking aboveground and belowground food webs through carbon and nitrogen stable isotope analyses. Ecol. Res. 2010, 25, 745–756. [Google Scholar] [CrossRef]
- Lyubechanskii, I.I. Carabid beetles community of the typical habitats in southern forest-steppe (West Siberia). Euroasian Entomol. J. 2009, 8, 315–318. [Google Scholar]
- Lyubechanskii, I.I.; Bespalov, A.N. Spatial Heterogeneity of a Ground Beetle (Coleoptera, Carabidae) Population along a Forest-Steppe Transect: Local Level of Consideration. Contemp. Probl. Ecol. 2011, 4, 388–395. [Google Scholar] [CrossRef]
- Lyubechanskii, I.I.; Azarkina, G.N. Ecological Structure of the West Siberian Forest-Steppe Spider Community (Arachnida, Araneae) and Its Comparison with the Ground-Beetle (Coleoptera, Carabidae) Community. Contemp. Probl. Ecol. 2017, 10, 164–177. [Google Scholar] [CrossRef]
- Lyubechanskii, I.I.; Dudko, R.Y.; Tiunov, A.V.; Mordkovich, V.G. Trophic Structure of Ground-Dwelling Insects in the Coastal Zone of a Salt Lake in Southern Siberia Based on the Data of Isotopic Analysis. Arid. Ecosyst. 2015, 5, 222–229. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Tcherkez, G.; Keitel, C.; Cornwell, W.K.; Santiago, L.S.; Knohl, A.; M Barbour, M.M.; Williams, D.G.; Reich, P.B.; Ellsworth, D.S.; et al. Why are non-photosynthetic tissues generally 13C enriched compared with leaves in C3 plants? Review and synthesis of current hypotheses. Funct. Plant Biol. 2009, 36, 199–213. [Google Scholar] [CrossRef]
- Codron, J.; Codron, D.; Lee-Thorp, J.A.; Sponheimer, M.; Bond, W.J.; de Ruiter, D.; Grant, R. Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J. Archaeol. Sci. 2005, 32, 1757–1772. [Google Scholar] [CrossRef]
- Lorenz, W. Systematic List of Extant Ground Beetles of the World (Coleoptera «Geadephaga»: Trachypachidae and Carabidae, inc. Paussinae, Cicindelinae, Rhysodinae), 2nd ed.; Lorenz: Tutzing, Germany, 2005. [Google Scholar]
- Marshall, J.A.; Haes, E.C.M. Grasshoppers and Allied Insects of Great Britain and Ireland; Harley Books: Colchester, UK, 1988. [Google Scholar]
- Tsurikov, S.M.; Goncharov, A.A.; Tiunov, A.V. Intra-body variation and ontogenetic changes in the isotopic composition (13C/12C and 15N/14N) of beetles (Coleoptera). Entomol. Rev. 2015, 95, 326–333. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Brooks, J.R.; Flanagan, L.B.; Buchmann, N.; Ehleringer, J.R. Carbon isotope composition of boreal plants: Functional grouping of life forms. Oecologia 1997, 110, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Tiunov, A.V. Stable isotopes of carbon and nitrogen in soil ecological studies. Biol. Bull. 2007, 34, 395–407. [Google Scholar] [CrossRef]
- Iakovlev, I.K.; Novgorodova, T.A.; Tiunov, A.V.; Reznikova, Z.I. Trophic position and seasonal changes in the diet of the red wood ant Formica aquilonia as indicated by stable isotope analysis. Ecol. Entomol. 2017, 42, 263–272. [Google Scholar] [CrossRef]
- Wilson, A.C.C.; Sternberg, L.d.S.L.; Hurley, K.B. Aphids alter host-plant nitrogen isotope fractionation. Proc. Natl. Acad. Sci. USA 2011, 108, 10220–10224. [Google Scholar] [CrossRef]
- Rozanova, O.L.; Tsurikov, S.M.; Krivosheina, M.G.; Tanasevitch, A.V.; Fedorenko, D.N.; Leonov, V.D.; Timokhov, A.V.; Tiunov, A.V.; Semenina, E.E. The isotopic signature of the “arthropod rain” in a temperate forest. Sci. Rep. 2022, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Reznikova, Z.I. Mezhvidovye Otnosheniya u Muraviev [Interspecific Interactions of Ants]; Nauka: Novosibirsk, USSR, 1983. [Google Scholar]
- Bennett, P.M.; Hobson, K.A. Trophic structure of a boreal forest arthropod community revealed by stable isotope (δ13C, δ15N) analyses. Entomol. Sci. 2009, 12, 17–24. [Google Scholar] [CrossRef]
- Kudrin, A.A.; Tsurikov, S.M.; Tiunov, A.V. Trophic position of microbivorous and predatory soil nematodes in a boreal forest as indicated by stable isotope analysis. Soil Biol. Biochem. 2015, 86, 193–200. [Google Scholar] [CrossRef]
- Kennedy, S.; Lim, J.Y.; Clavel, J.; Krehenwinkel, H.; Gillespie, R.G. Spider webs, stable isotopes and molecular gut content analysis: Multiple lines of evidence support trophic niche differentiation in a community of Hawaiian spiders. Funct. Ecol. 2019, 33, 1722–1733. [Google Scholar] [CrossRef]
- Mayor, J.R.; Schuur, E.A.; Henkel, T.W. Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol. Lett. 2009, 12, 171–183. [Google Scholar] [CrossRef]
- Post, D.M. The long and short of food-chain length. Trends Ecol. Evol. 2002, 17, 269–277. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyubechanskii, I.I.; Bespalov, A.N.; Tiunov, A.V.; Azarkina, G.N.; Dudko, R.Y.; Salisch, L.V.; Mordkovich, V.G. Trophic Structure of the Soil-Dwelling Arthropod Communities at the Border of the Forest and the Steppe in the South of Western Siberia: Isotopic Data. Diversity 2023, 15, 445. https://doi.org/10.3390/d15030445
Lyubechanskii II, Bespalov AN, Tiunov AV, Azarkina GN, Dudko RY, Salisch LV, Mordkovich VG. Trophic Structure of the Soil-Dwelling Arthropod Communities at the Border of the Forest and the Steppe in the South of Western Siberia: Isotopic Data. Diversity. 2023; 15(3):445. https://doi.org/10.3390/d15030445
Chicago/Turabian StyleLyubechanskii, Ilya I., Alexei N. Bespalov, Alexei V. Tiunov, Galina N. Azarkina, Roman Yu. Dudko, Lyudmila V. Salisch, and Vyacheslav G. Mordkovich. 2023. "Trophic Structure of the Soil-Dwelling Arthropod Communities at the Border of the Forest and the Steppe in the South of Western Siberia: Isotopic Data" Diversity 15, no. 3: 445. https://doi.org/10.3390/d15030445
APA StyleLyubechanskii, I. I., Bespalov, A. N., Tiunov, A. V., Azarkina, G. N., Dudko, R. Y., Salisch, L. V., & Mordkovich, V. G. (2023). Trophic Structure of the Soil-Dwelling Arthropod Communities at the Border of the Forest and the Steppe in the South of Western Siberia: Isotopic Data. Diversity, 15(3), 445. https://doi.org/10.3390/d15030445







