Abstract
Generalist mice are key species for the long-term dynamics of fragmented forests due to their dual role as seed dispersers or predators of the dominant trees. Wood mice, Apodemus sylvaticus, usually act as a net predator in woodlots due to higher winter densities and earlier winter reproduction than in forests. Here we analyze the recruitment expectations of young mice born in woodlots in relation to food availability through an index of developmental stability that combined values of fluctuating asymmetry (FA) for six traits of the lower mandibles. FA was measured in young and adult mice caught at the end of the winter in control woodlots, food-supplemented woodlots and in a nearby large forest. Despite low sample sizes (n = 9 for young and n = 74 for adults), FA in young mice born in control woodlots were significantly higher than in those from food-supplemented woodlots and the forest and in all adults. Food limitation in woodlots was thus associated with increased developmental instability of young mice, but it had no effect on adults. Instability likely reduced the survival prospects of young mice through increased mortality, and this should be compensated by yearly recolonization of woodlots by adults from the agricultural matrix in autumn and winter. Future work analyzing mechanisms suggested here but using non-lethal methods will be important to clarify the impacts of FA on the population dynamics of wood mice.
1. Introduction
Habitat loss and fragmentation are the global-change drivers with the strongest negative impacts on biodiversity [1,2,3]. Most research efforts on these drivers have been focused on habitat specialists, as it is accepted that the negative effects of habitat loss should be strongest for species more dependent on the fragmented habitat [1,4,5]. Nonetheless, current views are shifting towards a more comprehensive analysis of community responses to fragmentation, including both habitat specialists and generalists [6]. The latter may be more abundant in disturbed areas [7], modulating key ecological processes within these areas due to their higher abundance and ubiquity [8].
In particular, populations of generalist rodents can affect food webs in fragmented landscapes since they are important prey of predators such as weasels and ermines, Mustela spp.; martens, Martes spp.; foxes, Vulpes vulpes; or cats, Felis spp. [9]. In addition, as seed consumers, they can modulate the recruitment cycle of dominant trees [10,11,12]. In this line, the wood mouse, Apodemus sylvaticus, is widespread in fragmented holm oak, Quercus ilex, woodlands, where it can be an important prey for carnivores and raptors [13,14,15]. Moreover, thanks to its scatter–hoarding foraging behavior, it may be the only seed disperser available for oaks, though it can also act as a net seed predator, imposing strong limitations to tree recruitment [16,17,18]. Both factors are modulated by mice densities, which, in turn, depend on the local habitat quality of woodlots embedded in the agricultural matrix and of nearby forests [19]. Food availability is one of the most important factors affecting quality, modifying the population dynamics of wood mice across the landscape [20] and their foraging behavior [17]. The per capita seed availability can modify their willingness to store seeds or in situ predation, and hence their role as seed dispersers or net predators [16,17,18].
In general, wood mice are thought to tolerate, and even benefit from, forest fragmentation thanks to their ability to exploit food resources available in the matrix in spring and summer, retreating to woodlots after harvest [21,22,23,24]. During autumn–winter, wood mice inhabiting small woodlots may benefit from the enhanced acorn production of holm oak trees and food resources available in the nearby matrix (e.g., seeds from harvested crops) [25,26]. Both factors can explain the enhanced reproductive activity of mice in small fragments and forest edges during autumn [17,25,27], which represents the beginning of the main breeding season in the Mediterranean region [28,29]. However, food-addition experiments have demonstrated nutritional limitations for adult wood mice wintering in small woodlots, which may suffer from lower survival prospects throughout the winter [30]. Moreover, enhanced mouse densities in small woodlots can negatively affect population growth [28], suggesting that earlier reproduction is not necessarily linked to successful recruitment. In this context, evaluating the body condition of young mice inhabiting woodlots could shed some light on the actual condition of populations in fragmented landscapes.
In this work, we aim to evaluate if fragmentation negatively affects the survival prospects of young woody mice and if such effects are mediated by food availability in small woodlots. To this end, we re-analyzed mice material collected in a food-addition experiment carried out to establish whether adult mice populations were food-limited at the end of the winter [30]. To evaluate if food limitation in young mice could affect their survival prospects, we compared the developmental stability of individuals born in supplemented small woodlots with respect to that of controls and large forest fragments, and also the stability of young and adult mice. Given the previously observed food limitation observed in adults wintering in small fragments [30], we expected (a) higher developmental instability of mice born in small woodlots and (b) that food supplementation would decrease the levels of FA of animals to similar values of those found in individuals inhabiting forest habitats. Finally, we expected that differences in FA should be stronger in young individuals than in adults, as the latter could have compensated for higher initial levels of FA during development or incurred in differential mortality [31].
2. Material and Methods
2.1. Study Area and Experimental Design
Field work was carried out near the localities of Quintanar de la Orden–Mota del Cuervo–Los Hinojosos (Toledo–Cuenca provinces; 30S 5080, UTM 43790; Figure 1). The landscape is composed of dry cereals and legume croplands, with scattered small grape and olive groves (Figure 1). The original Holm oak, Quercus ilex, forest covers 28% of the study area only, and it is distributed as fragments of a wide variety of extensions. Holm oaks dominate the tree layer, whereas the understory shrub layer is composed of species in the genera Genista, Asparagus, Rhamnus, and Quercus. Annual crops are harvested in midsummer (June–July), and perennial crops in autumn–winter, whereas the natural vegetation produces fruits in autumn, after the summer drought typical of Mediterranean climates. Hence, food availability in the landscape peaks in autumn rather than in summer, as happens in other temperate climates, due to the effects of drought (see [30] and references therein for a full description).
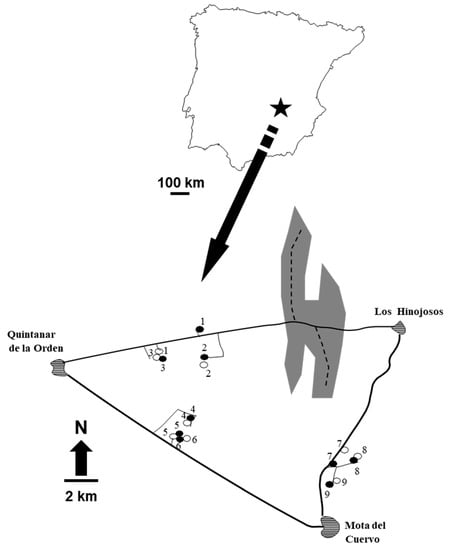
Figure 1.
Study area and sampling design. The upper map shows the location of the study area in the Iberian Peninsula, and the lower sketch the distribution of woodlots (circles: open, control woodlot; closed, food-addition experiment) and the large forest fragment (gray patch). Sampled forest fragments are distributed among the localities of Quintanar de la Orden, Los Hinojosos, and Mota del Cuervo. Continuous lines indicate roads (both paved and unpaved), and broken lines indicate the transects within the forest where traps were located (see Figure 1 in [30] for details).
Wood mice populations exploit the agricultural matrix during spring and summer, but they only occupy woodlots and shrub patches during winter, where they reproduce (see [21,25] and references therein). Mouse densities and reproductive activity increase as forest fragment size decreases [25], despite there being strong evidence for food limitation in fragments due to strong food depletion throughout the winter (see [10] and references therein). Seed depletion is especially strong in the smallest woodlots (<2 ha), because winter abundance of wood mice is larger in the first 60 m of forest edge than in the forest interior [27]. Finally, there is experimental evidence of food limitation by the adult fraction of mice populations breeding in winter in the smallest Holm oak fragments. Females survive better, and males are able to reproduce without losing anti-parasite abilities and potential survival prospects, when food is experimentally supplemented along the winter reproductive season [30].
To distinguish the effects of food supply from other environmental factors associated to fragmentation, we manipulated winter food availability in oak woodlots during January–March 1996 (Figure 1; see [30] for details). Briefly, we selected nine pairs of forest fragments smaller than 2 ha and surrounded by fallow fields. Wood mice are known to use fallows close to the border of fragments (up to c.a. 10 m) to forage from permanent refuges at fragments and forests edges [27], but there is no evidence of use of the agricultural matrix far from forest edges [21]. Fragments within pairs were similar in size, shape, and vegetation structure and were located nearby. For each pair, one of the fragments was selected at random to supply excess food throughout the winter (January and February 1996), leaving the other fragment as a control. We supplemented woodlots with canary (Phalaris canariensis) seeds (32 kg ha−1·month−1), a food that is readily used by wood mice in wild conditions [30]. We established the amount of food supplemented to exceed the winter requirements of expected mice populations, using data on mouse densities gathered in comparable studies, estimates of the winter food requirements of an average mice, and the energy contents of canary seeds; the food added would have maintained 39 times (range 36–49, n = 9) more mice than the numbers caught (see [30] for details). Seeds were hand-scattered homogeneously under tree and shrub cover to prevent use by seed-eating birds and to facilitate use by mice during two visits, one in mid-January and the other in mid-February. We checked whether wood mice used the supplemental food by means of Petri dishes (9 cm diameter) filled with canary seeds set during five days around the new moon of February (19–24 February). Trays were placed under cover to avoid consumption by seed-eating birds and checked for missing seeds, remains of seed coats, and wood mouse droppings that appeared in 20–100% of trays in the nine experimental woodlots [30]. In addition, to compare the effects of food supply in small woodlots with respect to forest habitat, we sampled a nearby large forest (1800 ha), where much lower mice abundance is expected to have enhanced food conditions [25]. We checked acorn availability by counting acorns in the canopies and on the ground below oak canopies in a sample of trees. Only three woodlots had acorns on the ground, and only five had some acorns still attached to the tree canopies out of the 18 selected, and acorn densities were moderate to low as compared to other Holm oak forests, thus indicating low acorn productivity in the study years [30].
We captured all individuals present in the studied woodlots during four nights around the new moon of March 1996 (18–21 March), as well as a sample of the individuals living in the large forest during the same trapping nights. We used snap traps baited with a piece of oiled cotton ribbon. Snap traps kill the trapped individuals at once, reducing suffering. We obtained the necessary permits to perform this study from the corresponding authorities. Traps were set in pairs to avoid saturation. Trapping stations were distributed over the entire woodlot in fragments. In the forest, they were spaced 15 m along line transects running >60 m away from borders [27] (Figure 1). We set six pairs of traps in fragments <1 ha, eight pairs in the larger woodlots, and 100 pairs in two 50-pair transects in the forest. We checked traps early in the morning (06:00–08:00 h GMT) and late in the afternoon (17:00–19:00 h GMT). The individuals caught were placed in plastic bags and transferred daily to the laboratory, where they were sexed, checked for levels of reproductive activity, and measured (body mass to the nearest g with a spring balance and condylobasal length (CBL) to the nearest 0.01 mm with a vernier calliper). We classified mice as young if they were sexually inactive and had a CBL <23.0 mm and a body mass <22 g, and as adults otherwise [25,30]. Skulls were frozen until processing them to extract the lower mandibles. Mandibles were cleaned by boiling them in a water solution of NH4 (10%), followed by manual removal of soft tissues.
2.2. Fluctuating Asymmetry of Lower Mandibles
To assess the developmental stability of wood mice, we used measures of fluctuating asymmetry (FA), which is the deviation with respect to bilateral symmetry due to the inability of an organism to execute its developmental program [31,32]. Hence, it can be used as an indicator of environmental conditions during the development of individuals [31,32,33,34]. Even though the use of FA has been questioned [35,36], our manipulative experiment allowed us to quantify shifts caused by food conditions in small forest fragments. In addition, to test if FA is a reliable proxy of the nutritional status of wood mice from different cohorts, we compared adult and young individuals. We expected higher FA in young than in adult mice if higher FA reduced survival prospects, and no differences otherwise [31].
The left and right sides of each lower mandible were separated at the mandibular symphysis and placed under a 10X binocular magnifying glass. Digital photographs were taken and processed with Motic Plus 2.0 software. To improve the robustness of our findings, we used a multi-trait approach [37]. Six Euclidean distances among pairs of landmark points were measured to the nearest 0.1 micrometer on the photographs of the left and right sides (Figure 2). Landmark points and distances were selected following [38], after removing landmarks for which any available hemimandible was broken. We used the largest number of characters possible to compensate for the very low number of young mice captured [30]. The manipulation and measurement of all mandibles were carried out by the same person (N. Fermín).
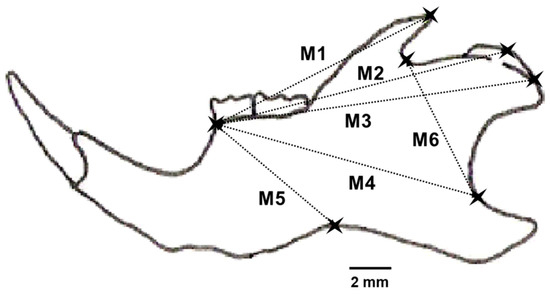
Figure 2.
Drawing of the left lower mandible of Apodemus sylvaticus showing landmarks and the six distances (M1–M6) selected to characterize the individual fluctuating asymmetry in mandible size of the individuals caught (modified from [38]).
Asymmetry was calculated as the absolute difference between the values of each distance between the right and the left lower mandibles. Measurement error was estimated from two repeated non-sequential measures of each character following [39]. The normality of FA distributions values for each trait was analyzed by means of Kolmogorov–Smirnov tests, and the centrality was measured using one-sample t-test against the null hypothesis of zero mean asymmetry [40]. To remove the effects of character size on estimates of FA, absolute values of asymmetry (averaged across the two measurements) were regressed against character size and its squared value [41,42,43]. Standardized residuals of these regressions for each trait were then added to produce a single composite index of asymmetry for each individual mouse (see [41] for a similar approach). Alternative methods based on geometric morphometrics [44] were not considered for consistency with recent work with mice in natural populations [34].
2.3. Statistical Analyses
To evaluate the effects of food supplementation on the condition of wood mice inhabiting fragmented landscapes, we regressed composite values of fluctuating asymmetry against forest type (control woodlots, food-supplemented woodlots, and continuous forest) and mice age (adult vs. young). Linear models were adequate because standardized residuals (and their sums) are always normally distributed [41,42,43,44,45]. Analyses were performed using SPSS 28.0.
3. Results
Overall, we were able to capture 105 individual wood mice; however, in 21 (20%), the snap traps damaged the skulls. Out of the remaining 87, most were adults (74; 89.3%; Appendix A). The distribution of adult captures among forest treatments (28, 29, and 18 caught in control woodlots, food-supplemented woodlots, and the forest, respectively) did not differ from 1/3:1/3:1/3 random expectations (χ22 = 2.96; p = 0.228). The distribution of captures of young mice (3, 3, and 3, respectively) was also uniformly distributed among treatments (χ22 = 0.00; p = 1.000).
Selected traits had measurement errors lower than 0.020% (mean = 0.007, range 0.003–0.012, and n = 12 measurements). The measurement error of signed asymmetries varied between 0.004 and 16.363%, but the repeatability among independent measurements was very high (>0.84; Table 1). The distributions of signed asymmetries did not differ from normality with a mean of zero asymmetry, reflecting fluctuating asymmetries and not anti-symmetry or directional asymmetry [33,40].

Table 1.
Summary statistics for the six traits measuring fluctuating asymmetry in the lower mandible of wood mice (n = 84). Sample sizes for the six mice groups considered (adults in control woodlots, food-supplemented woodlots, and the forest, and young in these three forest categories) were 28, 29, 18, 3, 3, and 3, respectively. See text for details on methods to estimate measurement error and repeatability.
The asymmetry index resulting from the combination of the six fluctuating asymmetries did not differ on average among age groups (F1, 78 = 0.00; p = 0.959) or forest treatments (F2, 78 = 2.97; p = 0.057), but there was a significant treatment x age interaction (F2, 78 = 4.12; p = 0.020). The asymmetry of adults did not differ among treatments (p > 0.427, post hoc DMS tests; Figure 3). The asymmetry of young mice was higher in control woodlots than in food-supplemented woodlots (p = 0.021) and in control woodlots than in forests (p = 0.015), but it did not differ between food-supplemented woodlots and forests (p = 0.908; Figure 3).
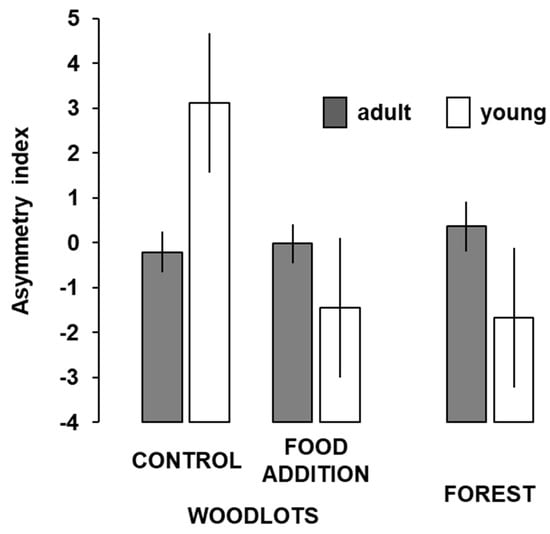
Figure 3.
Mean (±SE) values of the asymmetry index of wood mice, Apodemus sylvaticus, according to age and forest treatment (nadults = 28, 29, and 18; njuvenile= 3, 3, and 3; control, food-supplemented woodlots and forest, respectively).
4. Discussion
The experimental field manipulation of food availability in fragmented forests improved the developmental stability of juvenile mice from small woodlots to similar levels of those found in continuous forests and adults. Differences in fluctuating asymmetry among juvenile mice were an order of magnitude higher than they were for the rest of the individuals (Figure 3). Given the low sample size of young mice populations (nine individuals in total), further work should have been conducted to confirm the result presented here. Nevertheless, juveniles were a minority in our study system (twelve percent of all captures), and hence, obtaining larger sample sizes would have implied killing a high number of individuals. Rather, we tried to extract as much information as possible from the material already available that was obtained in a previous study where killing was necessary to quantify the physiological and parasitological status of trapped mice (30). Despite the study limitations, we believe that our results can shed some light on the effects of fragmentation on wood mice, where positive and negative responses have been found [25,27,30]. Our approach implied a food-addition experiment at landscape scales, and hence, we could link shifts in FA with the changes in the nutritional status of individuals. In addition, the fact that we found strong differences in FA despite our low sample sizes suggests a consistent pattern of fragmentation effects in our study system. In general, low sample sizes tend to inflate type II (not finding and existing effect) rather than type I errors (false effects) [45].
Previous work has shown that fluctuating asymmetry, the estimate we used to measure developmental stability, is associated to the quality and quantity of food available during development in a range of animal species, including humans [31,46,47,48,49]. Juvenile mice showed the highest levels of fluctuating asymmetry in non-supplemented (control) small woodlots. In addition, increased food supplies reduced fluctuating asymmetry to levels similar to those found in forests, where the individual body condition is usually better [25]. Thus, our results extend to juvenile wood mice’s previous evidence of food limitation in adults wintering in Mediterranean fragmented forests [30]. After crop harvesting, wood mice restrict their movements within woodlots, strongly relying on food available within these areas [50,51]. In parallel, the emigration of individuals from croplands [24,51] and the increased reproductive activity of mice [25,30] result in high densities within woodlots. Such circumstances can lead to food depletion in woodlots [52], resulting in impoverished breeding conditions [30], as suggested by the increased FA of young mice from woodlots. Therefore, our results suggest that juveniles born in small woodlots had been more exposed to food limitation during development than in continuous forests, probably due to source monopolization by dominant individuals [53]. Increased FA of young mice from woodlots suggest that newborn individuals may ultimately have lower life prospects. This could explain why populations with high mice densities show lower growth rates, despite their high fecundities [28]. To test this hypothesis, monitoring marked newborn individuals from small woodlots (supplemented and control), as well as from forest areas, should be conducted. Multi-season occupancy models fitted to monitoring data [54] could allow testing whether the patterns of survival of the young mice that were proposed here are true and whether these patterns could explain the population dynamics of wood mice in fragmented oak forests.
In contrast to young mice, the developmental stability of adults did not differ between woodlots and forest areas, and FA of adults was not sensitive to food supplementation. Four main processes could explain this pattern: (a) lagged effects of food conditions in the year previous to the food manipulation; (b) compensatory growth during spring and summer of individuals with high levels of FA [55]; increased mortality of individuals with high asymmetry levels [56,57]; and (d) recolonization of woodlots from adult individuals born in the forest or the agricultural matrix [21,23]. Lagged effects of food condition seemed unlikely. In non-manipulated woodlots, food conditions were similar to those of the previous year. Therefore, under lagged effects, we would have expected differences in FA between non-manipulated woodlots and forest.
Concerning compensatory growth, fluctuating asymmetry has been pointed out as an indicator of environmental stress during the entire growth trajectory of individuals [58], but also it has been suggested to only reflect early ontogenetic stages [59,60] or recent exposure to stressors [61]. In fact, compensatory growth can decrease asymmetry during development due to a left–right negative feedback [31,55,62]. Under such circumstances, the effect of a stressor on FA should fade with time and only reflect the recent growth story [63]. However, the short life span and early reproductive activity of wood mice [64] would make such a compensatory mechanism unlikely, as mice born in fragments during winter will reach adulthood in late spring–early summer, much before food conditions have improved significantly in fragmented oak forests surrounded by cereal croplands [28].
Regarding developmental selection, it would imply higher mortality rates of individuals with higher FA [56]. This is consistent with the idea that developmental instability is usually associated with lower growth rates of individuals and shorter life expectancy [31,65]. Specific mechanisms linking Apodemus sylvaticus mandible asymmetry and fitness are unknown, although higher levels of FA in limb traits of wood mice have been related to increased probabilities of being preyed [66]. Moreover, a consistent positive association of FA levels in bones of extremities and mandible traits have been found in other rodent species [46]. Moreover, since food-mediated survival has been suggested to be an important driver of wood-mice population growth [28], higher mortality rates among more asymmetrical mice would also be expected. Therefore, we believe that an increased mortality of individuals with higher FA is a plausible explanation of the similar values found in adults, as well as the strong differences found in young mice.
Finally, the migration of adult mice born in larger forests or the agricultural matrix to small fragments before harvest could also explain similar levels of FA among adults. In agricultural landscapes, the inter-patch movement of mice has been previously reported [67], and mice can move seasonally among fragments when they exploit the agricultural matrix [21,23,24]. Moreover, home-range expansion before harvest could favor the exchange of individuals between woodlots and the forest. Seasonal fluctuations in home ranges have been widely documented in other study areas (see [51] and references therein), although there is still little information for Mediterranean areas (see [68] and references therein). To evaluate whether the levels of FA found in adults respond to developmental selection, to source–sink dynamics between woodlots and forests, or both, large-scale studies with marked individuals would be needed [29,67]. Mark–recapture studies of newborn individuals could shed some light in this regard. Moreover, multi-season occupancy models (54) could provide information about the role of small woodlots on metapopulation dynamics. In particular, whether small woodlots act as ecological traps [69] despite their high densities and advanced reproduction as compared to forest areas [17,28,30].
5. Conclusions
Our manipulative approach allowed us to quantify the consequences of food limitation on the development of wood mice. We found clear-cut patterns of increased fluctuating asymmetry in young mice born in small woodlots, which were attenuated with food supplementation treatments. This supports previous work reporting that food depletion is an important factor limiting the breeding of wood mice in fragmented landscapes despite their generalist habits [30]. We expected adult mice to show lower values of FA than juveniles, as well as differences between small woodlots and forests in both adult and young mice if populations in fragments and forests were stable and showed little interchange of individuals (see [25]). However, the lack of differences between woodlot types and treatments was surprising. The increased mortality of individuals with high levels of FA and inter-patch migration could explain such patterns. Future work analyzing these two plausible mechanisms will be important to clarify the impacts of FA on the population dynamics of wood mice. Wood mice are keystone modulators of holm oak recruitment [16,17], as well as staple food for predators [13,14,15]. Hence, understanding mechanisms driving wood mice population dynamics in fragmented forest landscapes will be essential to understand, and eventually manage, the ecosystem processes affected by forest fragmentation.
Author Contributions
Investigation, M.D.; Writing—original draft, M.D.; Writing—review & editing, M.D. and T.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is a contribution to the Spanish-funded projects RiskDisp (CGL2009-08430; 2010–2012) and VULGLO (CGL2010e22180-C03e03; 2011–2013); all them have ended long time ago.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The full database is published in Appendix A.
Acknowledgments
C.L. Alonso gave us permission to use the data and materials gathered during his food-addition experiment. N. Fermín prepared the materials and made the measurements, financed by a PFPU grant of the Spanish government. Regional authorities of Castilla-La Mancha provided the permits to trap wood mice.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
The database of measurements (in micrometers) taken from the left and right sides of the lower mandibles of 84 adult and young wood mice caught in three forest treatments (control woodlots, food-addition woodlots, and forest). The two non-consecutive measurements of each of the six traits (M1–M6) are given.
References
- Saunders, D.A.; Hobbs, R.J.; Margules, C.R. Biological consequences of ecosystem fragmentation: A review. Conserv. Biol. 1991, 5, 18–32. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Butchart, S.H.; Walpole, M.; Collen, B.; Van Strien, A.; Scharlemann, J.P.; Almond, R.E.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Gehring, T.M.; Swihart, R.K. Body size, niche breadth, and ecologically scaled responses to habitat fragmentation: Mammalian predators in an agricultural landscape. Biol. Conserv. 2003, 109, 283–295. [Google Scholar] [CrossRef]
- Lanchier, N.; Neuhauser, C. A spatially explicit model for competition among specialists and generalists in a heterogeneous environment. Ann. Appl. Prob. 2006, 16, 1385–1410. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Let. 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Mattos, I.D.; Zimbres, B.; Marinho-Filho, J. Habitat specificity modulates the response of small mammals to habitat fragmentation, loss, and quality in a Neotropical savanna. Front. Ecol. Evol. 2021, 9, 751315. [Google Scholar] [CrossRef]
- Richmond, C.E.; Breitburg, D.L.; Rose, K.A. The role of environmental generalist species in ecosystem function. Ecol. Model. 2005, 188, 279–295. [Google Scholar] [CrossRef]
- Šálek, M.; Kreisinger, J.; Sedláček, F.; Albrecht, T. Do prey densities determine preferences of mammalian predators for habitat edges in an agricultural landscape? Landsc. Urban Plan. 2010, 98, 86–91. [Google Scholar] [CrossRef]
- Manson, R.H.; Ostfeld, R.S.; Canham, C.D. Long-term effects of rodent herbivores on tree invasion dynamics along forest-field edges. Ecology 2001, 82, 3320–3329. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Z.; Wang, Y. Impacts of scatter-hoarding rodents on restoration of oil tea Camellia oleifera in a fragmented forest. For. Ecol. Manag. 2004, 196, 405–412. [Google Scholar] [CrossRef]
- Gómez, J.M.; Schupp, E.W.; Jordano, P. Synzoochory: The ecological and evolutionary relevance of a dual interaction. Biol. Rev. 2019, 94, 874–902. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A.; Freixas, L.; Ribas, A.; Flaquer, C.; Díaz, M. Using scats of a generalist carnivore as a tool to monitor small mammal communities in Mediterranean habitats. Basic Appl. Ecol. 2013, 14, 155–164. [Google Scholar] [CrossRef]
- Torre, I.; Díaz, M. Small mammal abundance in Mediterranean post-fire habitats: a role for predators? Acta Oecol. 2004, 25, 137–142. [Google Scholar] [CrossRef]
- Oró, D.; Sanz-Aguilar, A.; Carbonell, F.; Grajera, J.; Torre, I. Multi-species prey dynamics influence local survival in resident and wintering generalist predators. Oecologia 2021, 197, 437–446. [Google Scholar] [CrossRef]
- Santos, T.; Tellería, J.L. Vertebrate predation on Holm Oak, Quercus ilex, acorns in a fragmented habitat: Effects on seedling recruitment. For. Ecol. Manag. 1997, 98, 181–187. [Google Scholar] [CrossRef]
- Morán-López, T.; Fernández, M.; Alonso, C.L.; Flores, D.; Valladares, F.; Díaz, M. Effects of forest fragmentation on the oak-rodent mutualism. Oikos 2015, 124, 1482–1491. [Google Scholar] [CrossRef]
- Morán-López, T.; Wiegand, T.; Morales, J.M.; Valladares, F.; Díaz, M. Predicting forest management effects on oak—Rodent mutualisms. Oikos 2016, 125, 1445–1457. [Google Scholar] [CrossRef]
- Fuentes-Montemayor, E.; Ferryman, M.; Watts, K.; Macgregor, N.A.; Hambly, N.; Brennan, S.; Coxon, R.; Langridge, H.; Park, K.J. Small mammal responses to long-term large-scale woodland creation: The influence of local and landscape-level attributes. Ecol. Appl. 2020, 30, e02028. [Google Scholar] [CrossRef]
- Macdonald, D.W.; Tew, T.E.; Todd, I.A.; Garner, J.P.; Johnson, P.J. Arable habitat use by wood mice (Apodemus sylvaticus). 3. A farm-scale experiment on the effects of crop rotation. J. Zool. 2000, 250, 313–320. [Google Scholar] [CrossRef]
- Alcántara, M.; Tellería, J.L. Habitat selection of the Wood mouse (Apodemus sylvaticus) in cereal steppes of Central Spain. Z. Säugetierkunde 1991, 56, 347–351. [Google Scholar]
- Fitzgibbon, C.D. Small mammals in farm woodlands: The effects of habitat, isolation and surrounding land-use patterns. J. Appl. Ecol. 1997, 34, 530–539. [Google Scholar] [CrossRef]
- Ouin, A.; Paillat, G.; Butet, A.; Burel, F. Spatial dynamics of wood mouse (Apodemus sylvaticus) in an agricultural landscape under intensive use in the Mont Saint Michel Bay (France). Agric. Ecosyst. Environ. 2000, 78, 159–165. [Google Scholar] [CrossRef]
- Todd, I.A.; Tew, T.E.; Macdonald, D.W. Habitat use of the arable ecosystem by wood mice, Apodemus sylvaticus. 1. Macrohabitat. J. Zool. 2000, 250, 299–303. [Google Scholar] [CrossRef]
- Díaz, M.; Santos, T.; Tellería, J.L. Effects of forest fragmentation on the winter body condition and population parameters of an habitat generalist, the wood mouse Apodemus sylvaticus: A test of hypotheses. Acta Oecol. 1999, 20, 39–49. [Google Scholar] [CrossRef]
- Morán-López, T.; Forner, A.; Flores-Rentería, D.; Díaz, M.; Valladares, F. Some positive effects of the fragmentation of holm oak forests: Attenuation of water stress and enhancement of acorn production. For. Ecol. Manag. 2016, 370, 22–30. [Google Scholar] [CrossRef]
- García, F.J.; Díaz, M.; De Alba, J.M.; Alonso, C.L.; Carbonell, R.; De Carrión, M.L.; Monedero, C.; Santos, T. Edge effects and patterns of winter abundance of wood mice Apodemus sylvaticus in Spanish fragmented forests. Acta Theriol. 1998, 43, 255–262. [Google Scholar] [CrossRef]
- Díaz, M.; Torre, I.; Arrizabalaga, A. Relative roles of density and rainfall on the short-term regulation of Mediterranean wood mouse Apodemus sylvaticus populations. Acta Theriol. 2010, 55, 251–260. [Google Scholar] [CrossRef]
- Rosário, I.T.; Mathias, M.L. Annual weight variation and reproductive cycle of the wood mouse (Apodemus sylvaticus) in a Mediterranean environment. Mammalia 2004, 68, 133–140. [Google Scholar] [CrossRef]
- Díaz, M.; Alonso, C.L. Wood mouse Apodemus sylvaticus winter food supply: Density, condition, breeding, and parasites. Ecology 2003, 84, 2680–2691. [Google Scholar] [CrossRef]
- Møller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability, and Evolution; Oxford University Press: Oxford, UK, 1997; ISBN 0-19-854895-8. [Google Scholar]
- Wayne, R.K.; Modi, W.S.; O’Brien, S.J. Morphological variability and asymmetry in the cheetah (Acinonyx jubatus), a genetically uniform species. Evolution 1986, 40, 78–85. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Shadrina, E.; Vol’pert, Y. Fluctuating asymmetry of craniological features of small mammals as a reflection of heterogeneity of natural populations. Symmetry 2016, 8, 142. [Google Scholar] [CrossRef]
- Floate, K.D.; Coghlin, P.C. No support for fluctuating asymmetry as a biomarker of chemical residues in livestock dung. Can. Entomol. 2010, 142, 354–368. [Google Scholar] [CrossRef]
- Vangestel, C.; Lens, L. Does fluctuating asymmetry constitute a sensitive biomarker of nutritional stress in house sparrows (Passer domesticus)? Ecol. Ind. 2011, 11, 389–394. [Google Scholar] [CrossRef]
- Leung, B.; Forbes, M.R.; Houle, D. Fluctuating asymmetry as a bioindicator of stress: Comparing efficacy of analyses involving multiple traits. Am. Nat. 2000, 155, 101–115. [Google Scholar] [CrossRef]
- Leamy, L. Heritability of directional and fluctuating asymmetry for mandibular characters in random-bred mice. J. Evol. Biol. 1999, 12, 146–155. [Google Scholar] [CrossRef]
- Yezerinac, S.M.; Lougheed, S.C.; Handford, P. Measurement error and morphometric studies: Statistical power and observer experience. Syst. Biol. 1992, 41, 471–482. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Witter, M.S.; Cuthill, I.C. The analysis of fluctuating asymmetry. Anim. Behav. 1994, 48, 986–989. [Google Scholar] [CrossRef]
- Díaz, M.; Pulido, F.J.; Møller, A.P. Herbivore effects on developmental instability and fecundity of holm oaks. Oecologia 2004, 139, 224–234. [Google Scholar] [CrossRef]
- Nachman, G.; Heller, K.E. Fluctuating asymmetry as an index of fitness: Causality or statistical artifact? Oikos 1999, 86, 357–365. [Google Scholar] [CrossRef]
- Møller, A.P.; Gangestad, S.W.; Thornhill, R. Nonlinearity and the importance of fluctuating asymmetry as a predictor of fitness. Oikos 1999, 86, 366–368. [Google Scholar] [CrossRef]
- Klingenberg, C. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; Freeman: New York, NY, USA, 1981; ISBN 0-7167-2411-1. [Google Scholar]
- Leamy, L.J.; Meagher, S.; Taylor, S.; Carroll, L.; Potts, W.K. Size and fluctuating asymmetry of morphometric characters in mice: Their associations with inbreeding and t-haplotype. Evolution 2001, 55, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Developmental stability and ideal despotic distribution of blackbirds in a patchy environment. Oikos 1995, 72, 228–234. [Google Scholar] [CrossRef]
- Parsons, P.A. Fluctuating asymmetry: An epigenetic measure of stress. Biol. Rev. Camb. Phil. Soc. 1990, 65, 131–145. [Google Scholar] [CrossRef]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating asymmetry: Methods, theory, and applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef]
- Tew, T.E.; Macdonald, D.W. The effects of harvest on arable wood mice Apodemus sylvaticus. Biol. Conserv. 1993, 65, 279–283. [Google Scholar] [CrossRef]
- Tattersall, F.H.; Macdonald, D.W.; Hart, B.J.; Manley, W.J.; Feber, R.E. Habitat use by wood mice (Apodemus sylvaticus) in a changeable arable landscape. J. Zool. 2001, 255, 487–494. [Google Scholar] [CrossRef]
- Tellería, J.L.; Santos, T.; Alcántara, M. Abundance and food-searching intensity of wood mice (Apodemus sylvaticus) in fragmented forests. J. Mammal. 1991, 72, 183–187. [Google Scholar] [CrossRef]
- Wolf, M.; Batzli, G.O. Effects of forest edge on populations of white-footed mice Peromyscus leucopus. Ecography 2002, 25, 193–199. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Hines, J.E.; Knutson, M.G.; Franklin, A.B. Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 2003, 84, 2200–2207. [Google Scholar] [CrossRef]
- Swaddle, J.P.; Witter, M.S. On the ontogeny of developmental stability in a stabilized trait. Proc. R. Soc. Lond. B 1997, 264, 329–334. [Google Scholar] [CrossRef]
- Polak, M.; Trivers, R. The science of symmetry in biology. Trends Ecol. Evol. 1994, 9, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Møller, A.P.; Pulido, F.J. Fruit abortion, developmental selection and developmental stability in Quercus ilex. Oecologia 2003, 135, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Hallgrímsson, B. Fluctuating asymmetry in Macaca fascicularis: A study of the etiology of developmental noise. Int. J. Primatol. 1993, 14, 421–443. [Google Scholar] [CrossRef]
- Chippindale, A.K.; Palmer, A.R. Persistence of subtle departures from symmetry over multiple molts in individual brachyuran crabs: Relevance to developmental stability. Genetica 1993, 89, 185–199. [Google Scholar] [CrossRef]
- Gileva, E.A.; Ialkovskaia, L.E.; Borodin, A.V.; Zykov, S.V.; Kshniasev, I.A. Fluctuating asymmetry of craniometric characters in rodents (Mammalia: Rodentia): interspecific and interpopulational comparisons. Zhurnal Obs. Biol. 2007, 68, 221–230. [Google Scholar]
- Kellner, J.R.; Alford, R.A. The ontogeny of fluctuating asymmetry. Am. Nat. 2003, 161, 931–947. [Google Scholar] [CrossRef]
- Hallgrímsson, B.; Miyake, T.; Wilmore, K.; Hall, B.K. Embryological origins of developmental stability: Size, shape and fluctuating asymmetry in prenatal random bred mice. J. Exp. Zool. B 2003, 296, 40–57. [Google Scholar] [CrossRef]
- Knierim, U.; Van Dongen, S.; Forkman, B.; Tuyttens, F.A.M.; Špinka, M.; Campo, J.L.; Weissengruber, G.E. Fluctuating asymmetry as an animal welfare indicator—A review of methodology and validity. Physiol. Behav. 2007, 92, 398–421. [Google Scholar] [CrossRef] [PubMed]
- Torre, I.; Arrizabalaga, A.; Díaz, M. Ratón de campo Apodemus sylvaticus. Galemys 2002, 14, 1–26. [Google Scholar]
- Clarke, G.M. Relationships between developmental stability and fitness: Application for conservation biology. Conserv. Biol. 1995, 9, 18–24. [Google Scholar] [CrossRef]
- Galeotti, P.; Vicario, V. Fluctuating asymmetry in body traits increases predation risks: Tawny owl selection against asymmetric woodmice. Evol. Ecol. 2005, 19, 405–418. [Google Scholar] [CrossRef]
- Tattersall, F.H.; Macdonald, D.W.; Hart, B.J.; Manley, W.J. Balanced dispersal or source–sink–do both models describe wood mice in farmed landscapes? Oikos 2004, 106, 536–550. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Ferreira, D.; Leitão, I.; Santos-Reis, M. Usage patterns of Mediterranean agro-forest habitat components by wood mice Apodemus sylvaticus. Mamm. Biol. 2011, 76, 268–273. [Google Scholar] [CrossRef]
- Hale, R.; Swearer, S.E. Ecological traps: Current evidence and future directions. Proc. R. Soc. B 2016, 283, 20152647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).