The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review
Abstract
1. Introduction
2. Desert Plants Responses to Drought Stress
3. Drought Stress Arbitrated Influences in Plants Stimulated by AMF
| Stress | Observed Responses/Mechanisms with AMF Association | References |
|---|---|---|
| Drought | Increased sodium, potassium, catalase (CAT), peroxidases (POD), ascorbate peroxidase (APX), superoxide dismutase (SOD) by Glomus intraradices on Calotropis procera Ait. This improves the nutritive element concentration and antioxidant enzyme activity to decrease oxidative damage. | [92] |
| Drought | Improved leaf relative water content, photosynthetic energy use efficiency, specific leaf area in Cynophalla flexuosa L. This increases tolerance to recurring drought stress leading to high photosynthetic area. | [93] |
| Drought | Increased essential oil content, and oil yield and decreased malondialdehyde (MDA), hydrogen peroxide, catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), glutathione peroxidases (GPX) and improved nutrient concentration, plant biomass and essential oil content and glomalin related soil proteins (GRSP) in Pelargonium graveolense (L.) Herit. | [86] |
| Drought | Improved nitrogen metabolism by positively regulating nitrate and nitrite reductase activity, increased antioxidant enzyme activity, ascorbic acid contents, and reduction in glutathione level. This resulted in significant amelioration of oxidative damage to plant membranes by restricting the excess generation of reactive oxygen species (ROS). Greater content of proline, glucose, and total soluble protein such as hydrogen peroxide. Boosted phosphorous metabolism by increasing alkaline and acid phosphatase enzyme activity in Ephedra foliata. | [94] |
| Drought | Improvement in antioxidant system reducing hydrogen peroxide accumulation and lipid peroxidation. Increased Indole Acetic Acid (IAA) promoting growth. Increases root morphology (length, surface area and volume) in Panicum turgidum. | [95] |
| Drought | Increased levels of phenols, the activities of both peroxidase (POD) and polyphenolxydases after AMF treatment and increased leaf number and leaf area index in Phoenix dactylifera. | [96] |
| Drought | Increased contents of glomalin-related soil protein (GRSP) and increased soil structure and phosphorus content in Medicago sativa. | [97] |
| Drought | Increased catalase (CAT), superoxide dismutase (SOD), photosynthetic rate, stomatal conductance and intrinsic water use efficiency in Leymus chinesis and increased catalase activity and photosynthetic rate in Hemarthria altissima. | [50] |
| Drought | Increased c DNAs, named HaPIP1 water channel proteins, in Helianthemum almeriense. | [98] |
| Drought | Increased drought impact and increased turgor potential and mineral uptake of potassium, nitrogen, zinc, and iron in Olea europaea. | [73] |
| Drought | Increased catalase (CAT), ascorbate peroxidase (APX), increased endogenous level of cis-12-oxophytodienoc acid, jasmonic acid and 12-OH-JA; regulates stomatal conductance, lipid peroxidation, hydrogen peroxide in shoot and root of Digitaria eriantha. | [99] |
| Salinity | Increased shoot and root dry mass, stomatal conductance, soluble sugars, free alpha amino acid, sodium and potassium uptake in Aeluropus littoralis. | [100] |
| Salinity | Improved root and shoot biomass and phosphorus, zinc, and copper content in Acacia nilotica. | [101] |
| Salinity | Increased seedling weight, water content and phosphorus and nitrogen in Leymus chinensis. | [102] |
| Drought | Increased superoxide dismutase (SOD) and total peroxidase (POX) activity in Phillyrea angustifolia. | [103] |
| Drought | Increased plant growth, phosphorus uptake in Salsola laricina. | [104] |
| Stress | Observed Responses/Mechanism in Relation to Dark Septate Endophytes | References |
|---|---|---|
| Drought | Improved root dry weight, NPQ (non-photochemical quenching values, qP (photochemical quenching values), increased secondary metabolites such as polyphenols, flavonoids, anthocyanins and enhanced enzymatic activities related to secondary metabolism in sorghum seedlings. | [105] |
| Drought | Improved plant growth, antioxidant enzyme activity and root development in Artemisia ordosica. | [106] |
| Drought | Increased root biomass. Increase in potassium, calcium content in root of Ammopiptanthus mongolicus by some DSE species. | [107] |
| Drought | Improved root biomass, total biomass, nutrient concentration and antioxidant enzyme activities in Hedysarum scoparium by some DSE strains | [50] |
| Drought | Increase in proline, chlorophyll content, antioxidant enzymatic activities and growth parameters in Seidlitzia rosmarinus, Zygophyllum eichwaldii and Haloxylon ammodendron. | [25] |
| Drought | Increased plant growth, photosynthetic parameters and P uptake in Lolium perenne. | [108] |
| Drought | Increase in plant height, stem girth, leaf characteristics, biomass and proline accumulation in Chrysanthemum indicum. | [109] |
| Drought | Increased accumulation of soluble sugars, decrease in MDA (malondialdehyde) and degradation of chlorophyll in leaves in Alhagi sparsifolia. | [110] |
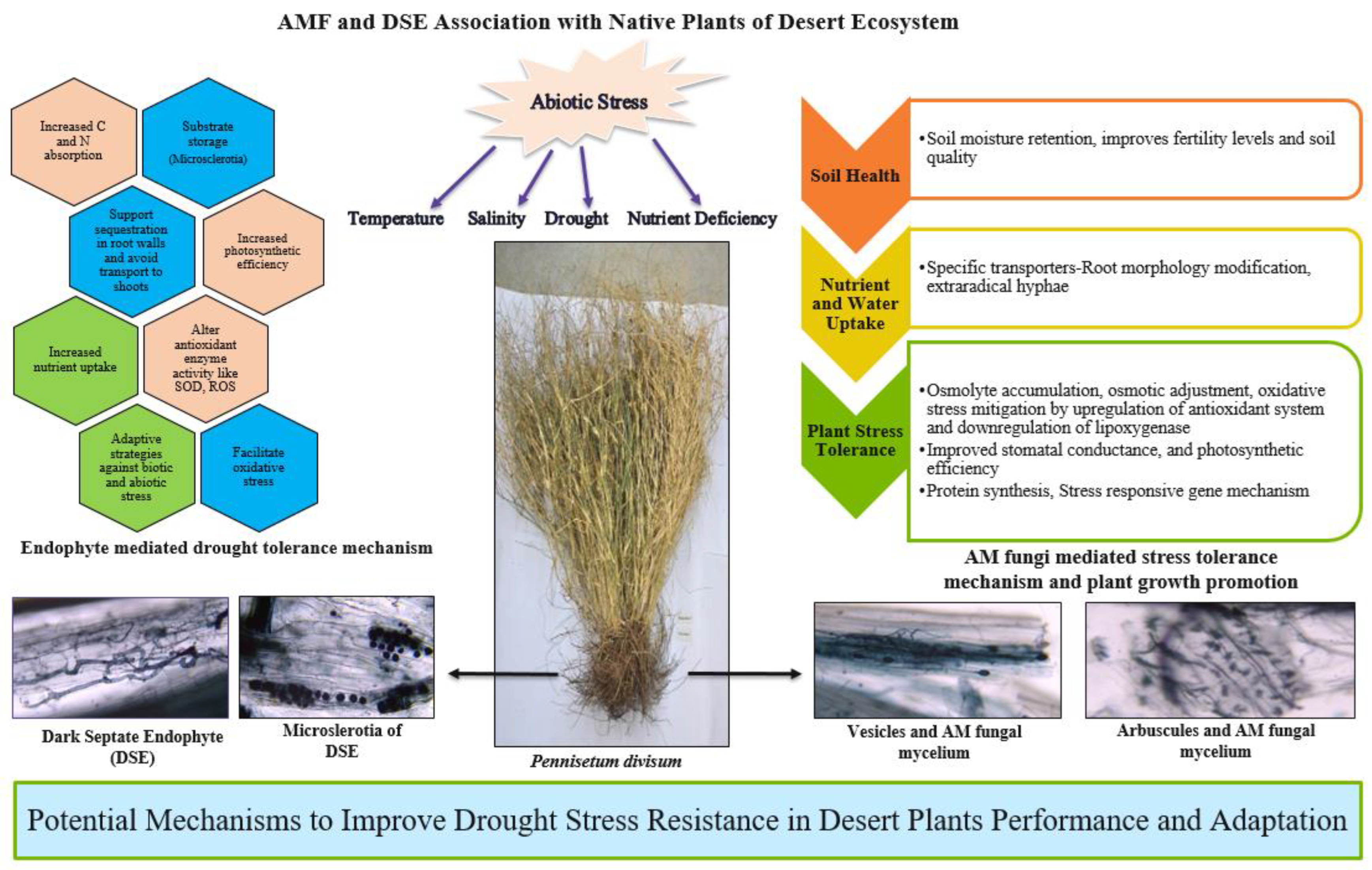
4. Role of Arbuscular Mycorrhizal Fungi in the Desert Ecosystem
5. Dark Septate Endophytes (DSEs) and Drought Stress Tolerance in Desert Plants
6. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naumann, G.; Alfieri, L.; Wyser, K.; Mentaschi, L.; Betts, R.A.; Carrao, H. Global changes in drought conditions under different levels of warming. Geophys. Res. Lett. 2018, 45, 3285–3296. [Google Scholar] [CrossRef]
- National Drought Mitigation Center. 2022. Available online: https://drought.unl.edu/Education/DroughtBasics.aspx (accessed on 4 July 2022).
- Marchin, R.M.; Ossola, A.; Leishman, M.R.; Ellsworth, D.S. A Simple Method for Simulating Drought Effects on Plants. Front. Plant Sci. 2020, 10, 1715. [Google Scholar] [CrossRef] [PubMed]
- Wehner, M.F.; Arnold, J.R.; Knutson, T.; Kunkel, K.E.; LeGrande, A.N. Droughts, floods, and wildfires. In Climate Science Special Report: Fourth National Climate Assessment; Wuebbles, D.J., Fahey, D.W., Hibbard, K.A., Dokken, D.J., Stewart, B.C., Maycock, T.K., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2017; Volume I, pp. 231–256. Available online: https://science2017globalchange.gov/chapter/8/ (accessed on 4 July 2022). [CrossRef]
- Šťovíček, A.; Kim, M.; Or, D.; Gillor, O. Microbial community response to hydration-desiccation cycles in desert soil. Sci. Rep. 2017, 6, 45735. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Dean, J.; Maza, F.; Morel, I.; Pulgar, R.; González, M. Microbial communities from arid environments on a global scale. A systematic review. Biol. Res. 2020, 53, 29. [Google Scholar] [CrossRef] [PubMed]
- Madouh, T.A. Development and Utilization of Desert Forages for Sustainable Livestock Production under Kuwait Conditions (FA078C); Final Report; KISR 11626; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2013. [Google Scholar]
- Jabbari, H.; Akbari, G.A.; Sima, N.A.K.K.; Rad, A.H.S.; Alahdadi, I.; Hamed, A.; Shariatpanahi, M.E. Relationships between seedling establishment and soil moisture content for winter and spring rapeseed genotypes. Ind. Crops Prod. 2013, 49, 177–187. [Google Scholar] [CrossRef]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas enhance drought tolerance of trifoliate orange by enhancing activities and gene expression of antioxidant enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd Allah, E.F.; Wu, Q.S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 23, 809473. [Google Scholar] [CrossRef] [PubMed]
- Tallapragada, P.; Bagyaraj, D.J. The role of arbuscular mycorrhizal fungi in salt and drought stresses. In Microbes for Plant Stress Management; New India Publishing Agency: New Delhi, India, 2017; pp. 183–204. [Google Scholar]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi—From ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Makhalanyane, T.P.; Valverde, A.; Gunnigle, E.; Frossard, A.; Ramond, J.-B.; Don, A.; Cowan, D.A. Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 2015, 39, 203–221. [Google Scholar] [CrossRef]
- Bechtold, U. Plant Life in Extreme Environments: How Do You Improve Drought Tolerance? Front. Plant Sci. 2018, 9, 543. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Madouh, T.A. Eco-Physiological Responses of Native Desert Plant Species to Drought and Nutritional Levels: Case of Kuwait. Front. Environ. Sci. 2022, 10, 785517. [Google Scholar] [CrossRef]
- Killingbeck, K.T. Nutrient resorption in desert shrubs. Rev. Chil. Hist. Nat. 1993, 66, 345–355. [Google Scholar]
- Lü, X.T.; Freschet, G.T.; Flynn, D.F.B.; Han, X. Plasticity in leaf and stem nutrient resorption proficiency potentially reinforces plant–soil feedbacks and microscale heterogeneity in a semi-arid grassland. J. Ecol. 2012, 100, 144–150. [Google Scholar] [CrossRef]
- Han, W.; Tang, L.; Chen, Y.; Fang, J. Relationship between the relative limitation and resorption efficiency of nitrogen vs phosphorus in woody plants. PLoS ONE 2013, 8, e83366. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; He, W.; An, L.; Xu, S. Nutrient resorption or accumulation of desert plants with contrasting sodium regulation strategies. Sci. Rep. 2017, 7, 17035. [Google Scholar] [CrossRef] [PubMed]
- Denaxa, N.K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going underground: Root traits as drivers of ecosystem processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Byregowda, R.; Prasad, S.R.; Oelmüller, R.; Nataraja, K.N.; Prasanna Kumar, M.K. Is Endophytic Colonization of Host Plants a Method of Alleviating Drought Stress? Conceptualizing the Hidden World of Endophytes. Int. J. Mol. Sci. 2022, 23, 9194. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Farooq, S.; Hasan, W.; Ul-Allah, S.; Tanveer, M.; Farooq, M.; Nawaz, A. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–166. [Google Scholar] [CrossRef]
- Todaka, D.; Zhao, Y.; Yoshida, T.; Kudo, M.; Kidokoro, S.; Mizoi, J.; Kodaira, K.S.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; et al. Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plant J. 2017, 90, 61–78. [Google Scholar] [CrossRef]
- Hameed, A.; Wu, Q.-S.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Lone, A.A. Role of AM fungi in alleviating drought stress in plants. In Use of Microbes for Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef]
- Asrar, A.-W.; Elhindi, K.M. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2011, 18, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Abdel-Fattah, G.M.; Eman, F.M.; Abd El_Aziz, M.H.; Shohr, A.E. Arbuscular mycorrhizal fungi and spermine alleviate the adverse effects of salinity stress on electrolyte leakage and productivity of wheat plants. Phyton 2011, 51, 261–276. [Google Scholar]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities-a review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wu, W.; Rasul, F.; Hassan, M.; Huang, K.; Awan, M.I.; Albishi, T.S.; Arshad, M.; Hu, Q.; Huang, G.; et al. Trehalose induced drought tolerance in plants: Physiological and molecular responses. Not. Bot. Horti Agrobot. Cluj. Napoca. 2022, 50, 12584. [Google Scholar] [CrossRef]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Chattha, B.; Soufan, W.; Hassan, M.U.; Rahman, M.A.; Brestic, M.; Zivcak, M.; et al. Mitigation of salinity induced oxidative damage, growth and yield reduction in fine rice by sugarcane press-mud application. Front. Plant Sci. 2022, 13, 840900. [Google Scholar] [CrossRef]
- Bogati, K.; Walczak, M. The impact of drought stress on soil microbial community, enzyme activities and plants. Agronomy 2022, 12, 189. [Google Scholar] [CrossRef]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The Critical Role of Arbuscular Mycorrhizal Fungi to Improve Drought Tolerance and Nitrogen Use Efficiency in Crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Akashi, K.; Yoshimura, K.; Nanasato, Y.; Takahara, K.; Munekage, Y.; Yokota, A. Wild plant resources for studying molecular mechanisms of drought/strong light stress tolerance. Plant Biotechnol. J. 2008, 25, 257–263. [Google Scholar] [CrossRef]
- Gibson, A.C. Structure-Function Relations of Warm Desert Plants. In Adaptations of Desert Organisms; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Holmes, M.G.; Keiller, D.R. Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: A comparison of a range of species. Plant Cell Environ. 2002, 25, 85–93. [Google Scholar] [CrossRef]
- Chávez, R.O.; Clevers, J.G.P.W.; Herold, M.; Acevedo, E.; Ortiz, M. Assessing Water Stress of Desert Tamarugo Trees Using in situ Data and Very High Spatial Resolution Remote Sensing. Remote Sens. 2013, 5, 5064–5088. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsäläinen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A. Assessing the response of plant flavonoids to UV radiation: An overview of appropriate techniques. Phytochem. Rev. 2015, 14, 273–297. [Google Scholar] [CrossRef]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Norton, M.R.; Malinowski, D.P.; Volaire, F. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agron. Sustain. Dev. 2016, 36, 29. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; Volume 1, pp. 25–55. [Google Scholar] [CrossRef]
- Salam, E.A.; Alatar, A.; El-Sheikh, M.A. Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 2017, 25, 1772–1780. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.; Ahmad, P.; Zhang, L. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought Stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress—A meta-analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Quoreshi, A.M.; Suleiman, M.K.; Kumar, V.; Anisul Islam, M.; Ramadan, A.; Al-Othman, A.R.A.; Al-Mulla, L.; Jacob, S.; Manuvel, A.J.; Sivadasan, M.T.; et al. Investigation of Soil Microbial Communities and Vegetation for Baseline Database Development at Selected Sites in Kuwait Desert (FA076C); Final Report; KISR 14865; Kuwait Institute for Scientific Research: Kuwait City, Kuwait, 2018. [Google Scholar]
- Schüßler, A.; Schwarzott, D.; Walker, C. A new fungal phy-lum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Atul-Nayyar, A.; Hamel, C.; Hanson, K.; Germida, J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 2009, 19, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Rodrigues, B.F.; Harris, P.J. The Ecology of Arbuscular Mycorrhizal Fungi. CRC Crit. Rev. Plant Sci. 2013, 32, 1–20. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Smith, S.E.; Reed, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Harrison, M.J.; Dewbre, G.R.; Liu, J. A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. Plant Cell 2002, 14, 2413–2429. [Google Scholar] [CrossRef]
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.B.; Xu, G.; Jakobsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.K.; Javot, H.; Deewatthanawong, P.; Torres-Jerez, I.; Tang, Y.; Blancaflor, E.B.; Udvardi, M.K.; Harrison, M.J. Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Sieh, D.; Watanabe, M.; Devers, E.A.; Brueckner, F.; Hoefgen, R.; Krajinski, F. The arbuscular mycorrhizal symbiosis influences sulfur starvation responses of Medicago truncatula. New Phytol. 2013, 197, 606–616. [Google Scholar] [CrossRef]
- Giovannetti, M.; Tolosano, M.; Volpe, V.; Kopriva, S.; Bonfante, P. Identification and functional characterization of a sulfate transporter induced by both sulfur starvation and mycorrhiza formation in Lotus japonicus. New Phytol. 2014, 204, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Veresoglou, S.D.; Leifheit, E.F.; Rillig, M.C. Arbuscular mycorrhizal influence on zinc nutrition in crop plants: A meta-analysis. Soil Biol. Biochem. 2014, 69, 123–131. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—A meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Zou, Y.N.; Srivastava, A.K.; Wu, Q.S. Glomalin: A potential soil conditioner for perennial fruits. Int. J. Agric. Biol. 2016, 18, 293–297. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef]
- Hamedani, N.G.; Gholamhoseini, M.; Bazrafshan, F.; Habibzadeh, F.; Amiri, B. Yield, irrigation water productivity and nutrient uptake of arbuscular mycorrhiza inoculated sesame under drought stress conditions. Agric. Water Manag. 2022, 266, 107569. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Arbuscular Mycorrhizal Fungi and Tolerance of Drought Stress in Plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer: Singapore, 2017. [Google Scholar]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Sci. Hort. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Liu, J.; Guo, C.; Chen, Z.L.; He, J.D.; Zou, Y.N. Mycorrhizal inoculation modulates root morphology and root phytohormone responses in trifoliate orange under drought stress. Emir. J. Food Agric. 2016, 28, 251–256. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant 2018, 40, 81. [Google Scholar] [CrossRef]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of arbuscular mycorrhizal fungi on plant growth and performance: Importance in biotic and abiotic stressed regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Alqarawi, A.A.; Hashem, A.; Abd_Allah, E.F.; Alshahrani, T.S.; Huqail, A.A. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol. Hung. 2014, 65, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Perez-Tornero, O.; Morte, A. Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the root stock salt tolerance. J. Plant Physiol. 2014, 171, 76–85. [Google Scholar] [CrossRef]
- Rilling, M.C. Arbuscular Mycorrhizae and Terrestrial Ecosystem Processes. Ecol. Lett. 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Simard, S.; Austin, M. The Role of Mycorrhizas in Forest Soil Stability with Climate Change. In Climate Change and Variability; Simard, S., Ed.; IntechOpen: London, UK, 2010. [Google Scholar]
- Augé, R.M.; Saxton, A.M.; Toler, H.D. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2014, 25, 13–24. [Google Scholar] [CrossRef]
- Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mona, S.A.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Wu, H.H.; Zou, Y.N.; Rahman, M.M.; Ni, Q.D.; Wu, Q.S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef]
- Wu, Z.; McGrouther, K.; Huang, J.; Wu, P.; Wu, W.; Wang, H. Decomposition and the contribution of glomalin-related soil protein (GRSP) in heavy metal sequestration: Field experiment. Soil Biol. Biochem. 2014, 68, 283–290. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Yokota, A.; Takahara, K.; Akashi, K. Water stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Rao, K.V.M., Raghavendra, A.S., Reddy, K.J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 15–40. [Google Scholar]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular Responses to Drought Stress; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Bahadur, A.; Jin, Z.; Jiang, S.; Chai, Y.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Arbuscular mycorrhizal spores distribution across different ecosystems of Qinghai Tibetan Plateau. Pak. J. Bot. 2019, 51, 1481–1492. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Wang, Y.M.; Xu, S.X.; Lan, Z.J.; Mickan, B.S.; Zhang, X.L.; Wang, F.Y. Arbuscular Mycorrhizal Fungi Enhance Plant Diversity, Density and Productivity of Spring Ephemeral Community in Desert Ecosystem. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 301–307. [Google Scholar] [CrossRef]
- Qiao, Y.; Bai, Y.; Zhang, Y.; She, W.; Lai, Z.; Qin, S. Arbuscular mycorrhizal fungi shape the adaptive strategy of plants by mediating nutrient acquisition in a shrub-dominated community in the Mu Us Desert. Plant Soil. 2019, 443, 549–564. [Google Scholar] [CrossRef]
- Bahmani, M.; Naghdi, R.; Kartoolinejad, D. Milkweed seedlings tolerance against water stress: Comparison of inoculations with Rhizophagus irregularis and Pseudomonas putida. Environ. Technol. Innov. 2018, 10, 111–121. [Google Scholar] [CrossRef]
- Barros, V.; Frosi, G.; Santos, M.; Ramos, D.G.; Falcão, H.M.; Santos, M.G. Arbuscular mycorrhizal fungi improve photosynthetic energy use efficiency and decrease foliar construction cost under recurrent water deficit in woody evergreen species. Plant Physiol. Biochem. 2018, 127, 469–477. [Google Scholar] [CrossRef]
- Al-Arjani, A.B.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra faliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Abd_Allah, E.F.; Tabassum, B.; Alqarawi, A.A.; Safar, T.; Alshahrani, J.A.; Hashem, A. Physiological markers mitigate drought stress in Panicum turgidum Forssk. By arbuscular mycorrhizal fungi. Pak. J. Bot. 2019, 51, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Ye, J.S.; Li, T.; Hu, Y.J.; Hao, Z.P.; Gao, Y.Z.; Wang, Y.S.; Chen, B. Influences of AM fungi on plant growth and water-stable soil aggregates under drought stresses. Acta Ecol. Sin. 2013, 33, 1080–1090. [Google Scholar] [CrossRef]
- Navarro-Ródenas, A.; Bárzana, G.; Nicolás, E.; Carra, A.; Schubert, A.; Morte, A. Expression analysis of aquaporins from desert truffle mycorrhizal symbiosis reveals a fine-tuned regulation under drought. Mol. Plant-Microbe Interact. 2013, 26, 1068–1078. [Google Scholar] [CrossRef]
- Pedranzani, H.; RodrãGuez-Rivera, M.; Gutiérrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef]
- Hajiboland, R.; Dashtebani, F.; Aliasgharzad, N. Physiological responses of halophytic C4 grass, Aeluropus littoralis to salinity and arbuscular mycorrhizal fungi colonization. Photosynthetica 2015, 53, 572–584. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica, to salt stress by arbuscular mycorrhiza, Glomus fasciculatum, may be partly related to elevated K/Na ratios in root and shoot tissues. Microbiol. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lizarazo, J.C.; Moreno-Fonseca, L.P. Mechanisms for tolerance to water-deficit stress in plants inoculated with arbuscular mycorrhizal fungi. A review. Agron. Columbiana 2016, 34, 179–189. [Google Scholar] [CrossRef]
- Nouri, E.; Matinizadeh, M.; Moshki, A.; Zolfaghari, A.; Rajaei, S.; Janoušková, M. Arbuscular mycorrhizal fungi benefit drought-stressed Salsola laricina. Plant Ecol. 2020, 221, 683–694. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, M.; Shi, Z.; Gao, J.; Wang, X. Biodiversity and Variations of Arbuscular Mycorrhizal Fungi Associated with Roots along Elevations in Mt. Taibai of China. Diversity 2022, 14, 626. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, M.; Ye, Q.; Gao, H.; He, X. Improved Tolerance of Artemisia ordosica to Drought Stress via Dark Septate Endophyte (DSE) Symbiosis. J. Fungi. 2022, 8, 730. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Hou, L.; Ren, Y.; Wang, S.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 7896. [Google Scholar] [CrossRef]
- Moghaddam, M.S.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef]
- Li, F.; Duan, T.; Li, Y. Effects of the fungal endophyte Epichloë festucae var. lolii on growth and physiological responses of perennial ryegrass cv. fairway to combined drought and pathogen stresses. Microorganisms 2020, 8, 1917. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xin, K.; Liu, H.; Cheng, J.; Shen, X.; Wang, Y.; Zhang, L. Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci. Rep. 2017, 7, 41564. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H. Desert Plants and Mycorrhizae (A mini-review). J. Pure Appl. Microbiol. 2009, 3, 457–466. Available online: https://www.academia.edu/923186/Desert_Plants_and_Mycorrhizae_A_mini_review_?auto=citations&from=cover_page (accessed on 15 August 2022).
- Rilling, M.C.; Mummey, D.L. Mycorrhiza and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Host response to osmotic stresses: Stomatal behaviour and water use efficiency of arbuscular mycorrhizal plants. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 239–256. [Google Scholar] [CrossRef]
- Alsharif, W.; Saad, M.M.; Hirt, H. Desert Microbes for Boosting Sustainable Agriculture in Extreme Environments. Front. Microbiol. 2020, 11, 1666. [Google Scholar] [CrossRef] [PubMed]
- Al-Yahya’ei, M.N.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of Southern Arabia. Mycorrhiza 2011, 21, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Vasar, M.; Davison, J.; Sepp, S.-K.; Öpik, M.; Moora, M.; Koorem, K.; Meng, Y.; Oja, J.; Akhmetzhanova, A.A.; Al-Quraishy, S.; et al. Arbuscular Mycorrhizal Fungal Communities in the Soils of Desert Habitats. Microorganisms 2021, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Bever, J.D. Dynamics within mutualism and the maintenance of diversity: Inference from a model of interguild frequency dependence. Ecol. Lett. 1999, 2, 52–61. [Google Scholar] [CrossRef]
- Alrajhei, K.; Saleh, I.; Abu-Dieyeh, M.H. Biodiversity of arbuscular mycorrhizal fungi in plant roots and rhizosphere soil from different arid land environment of Qatar. Plant Direct. 2022, 6, e369. [Google Scholar] [CrossRef]
- Klironomos, J.; Zobel, M.; Tibbett, M.; Stock, W.D.; Rillig, M.C.; Parrent, J.L.; Moora, M.; Koch, A.M.; Facelli, J.M.; Facelli, E.; et al. Forces that structure plant communities: Quantifying the importance of the mycorrhizal symbiosis. New Phytol. 2011, 189, 366–370. [Google Scholar] [CrossRef] [PubMed]
- González-Teuber, M.; Vilo, C.; Bascuñán-Godoy, L. Molecular characterization of endophytic fungi associated with the roots of Chenopodium quinoa inhabiting the Atacama Desert. Genom. Data 2017, 11, 109–112. [Google Scholar] [CrossRef]
- Jumpponen, A. Dark septate endophytes–are they mycorrhizal? Mycorrhiza 2001, 11, 207–211. [Google Scholar] [CrossRef]
- Knapp, D.G.; Pintye, A.; Kovács, G.M. The Dark Side Is Not Fastidious–Dark Septate Endophytic Fungi of Native and Invasive Plants of Semiarid Sandy Areas. PLoS ONE 2012, 7, e32570. [Google Scholar] [CrossRef]
- Hou, L.; He, X.; Li, X.; Wang, S.; Zhao, L. Species composition and colonization of dark septate endophytes are affected by host plant species and soil depth in the Mu Us sandland, northwest China. Fungal Ecol. 2019, 39, 276–284. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ye, Q.; Xu, M.; He, X. Application of Desert DSEs to Nonhost Plants: Potential to Promote Growth and Alleviate Drought Stress of Wheat Seedlings. Agriculture 2022, 12, 1539. [Google Scholar] [CrossRef]
- Jumpponen, A.R.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Li, T.; Liu, M.J.; Zhang, X.T.; Zhang, H.B.; Sha, T.; Zhao, Z.W. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci. Total Environ. 2011, 409, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Ruotsalainen, A.L.; Kauppinen, M.; Wäli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes, mutualism from by-products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Zuo, Y.; Su, F.; He, X.; Li, M. Colonization by dark septate endophytes improves the growth of Hedysarum scoparium under multiple inoculum levels. Symbiosis 2020, 82, 201–214. [Google Scholar] [CrossRef]
- Shi, Z.; Mickan, B.; Feng, G.; Chen, Y. Arbuscular mycorrhizal fungi improved plant growth and nutrient acquisition of desert ephemeral Plantago minuta under variable soil water conditions. J. Arid. Land 2015, 7, 414–420. [Google Scholar] [CrossRef]
- González-Teuber, M.; Urzúa, A.; Plaza, P.; Bascuñán-Godoy, L. Effects of root endophytic fungi on response of Chenopodium quinoa to drought stress. Plant Ecol. 2018, 219, 231–240. [Google Scholar] [CrossRef]
- Santos, S.G.; Silva, P.R.; Garcia, A.C.; Zilli, J.É.; Berbara, R.L. Dark septate endophyte decreases stress on rice plants. Braz. J. Microbiol. 2017, 48, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ni, Q.; Zou, Y.; Wu, Q.; Huang, Y. Preliminary study on the mechanism of AMF in enhancing the drought tolerance of plants. J. Fungal Res. 2017, 15, 8–13. [Google Scholar]
- Xie, W.; Hao, Z.; Zhou, X.; Jiang, X.; Xu, L.; Wu, S.; Zhao, A.; Zhang, X.; Chen, B. Arbuscular mycorrhiza facilitates the accumulation of glycyrrhizin and liquiritin in Glycyrrhiza uralensis under drought stress. Mycorrhiza 2018, 28, 285–300. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.; Urquiaga, S.; Santa-Catarina, C.; Schultz, N.; da Silva Araújo, E.; de Carvalho Balieiro, F.; Xavier, G.R.; Zilli, J.E. Dark septate endophytic fungi increase green manure-N-15 recovery efficiency, N contents, and micronutrients in rice grains. Front. Plant Sci. 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- Malicka, M.; Magurno, F.; Piotrowska-Seget, Z. Plant association with dark septate endophytes: When the going gets tough (and stressful), the tough fungi get going. Chemosphere 2022, 302, 134830. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Cornelissen, J.H.; Wang, Y.; Wu, C.; He, Y.; Ou, J.; Tan, Q.; Xia, T.; Kang, L.; Guo, Y.; et al. AM fungi alleviate phosphorus limitation and enhance nutrient competitiveness of invasive plants via mycorrhizal networks in Karst areas. Front. Ecol. Evol. 2020, 8, 125. [Google Scholar] [CrossRef]
- Yu, T.; Nassuth, A.; Peterson, R.L. Characterization of the interaction between the dark septate fungus Phialocephala fortinii and Asparagus officinalis roots. Can. J. Microbiol. 2001, 47, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Narisawa, K. The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 2017, 28, 1–10. [Google Scholar] [CrossRef]
- Berthelot, C.; Blaudez, D.; Leyval, C. Differential growth promotion of poplar and birch inoculated with three dark septate endophytes in two trace element-contaminated soils. Int. J. Phytoremediat. 2017, 19, 1118–1125. [Google Scholar] [CrossRef]
- Jin, H.Q.; Liu, H.B.; Xie, Y.Y.; Zhang, Y.G.; Xu, Q.Q.; Mao, L.J.; Li, X.J.; Chen, J.; Lin, F.C.; Zhang, C.L. Effect of the dark septate endophytic fungus Acrocalymma vagum on heavy metal content in tobacco leaves. Symbiosis 2018, 74, 89–95. [Google Scholar] [CrossRef]
- Santos, M.; Cesanelli, I.; Diánez, F.; Sánchez-Montesinos, B.; Moreno-Gavíra, A. Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. J. Fungi. 2021, 7, 939. [Google Scholar] [CrossRef]
- He, C.; Zeng, Q.; Chen, Y.; Chen, C.; Wang, W.; Hou, J.; Li, X. Colonization by dark septate endophytes improves the growth and rhizosphere soil microbiome of licorice plants under different water treatments. Appl. Soil Ecol. 2021, 166, 103993. [Google Scholar] [CrossRef]
- Zuo, Y.; Hu, Q.; Liu, J.; He, X. Relationship of root dark septate endophytes and soil factors to plant species and seasonal variation in extremely arid desert in Northwest China. Appl. Soil Ecol. 2022, 175, 104454. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madouh, T.A.; Quoreshi, A.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391. https://doi.org/10.3390/d15030391
Madouh TA, Quoreshi AM. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity. 2023; 15(3):391. https://doi.org/10.3390/d15030391
Chicago/Turabian StyleMadouh, Tareq A., and Ali M. Quoreshi. 2023. "The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review" Diversity 15, no. 3: 391. https://doi.org/10.3390/d15030391
APA StyleMadouh, T. A., & Quoreshi, A. M. (2023). The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity, 15(3), 391. https://doi.org/10.3390/d15030391






