The European Ground Squirrel’s Genetic Diversity in Its Ancestral Land: Landscape Insights and Conservation Implications
Abstract
1. Introduction
2. Material and Methods
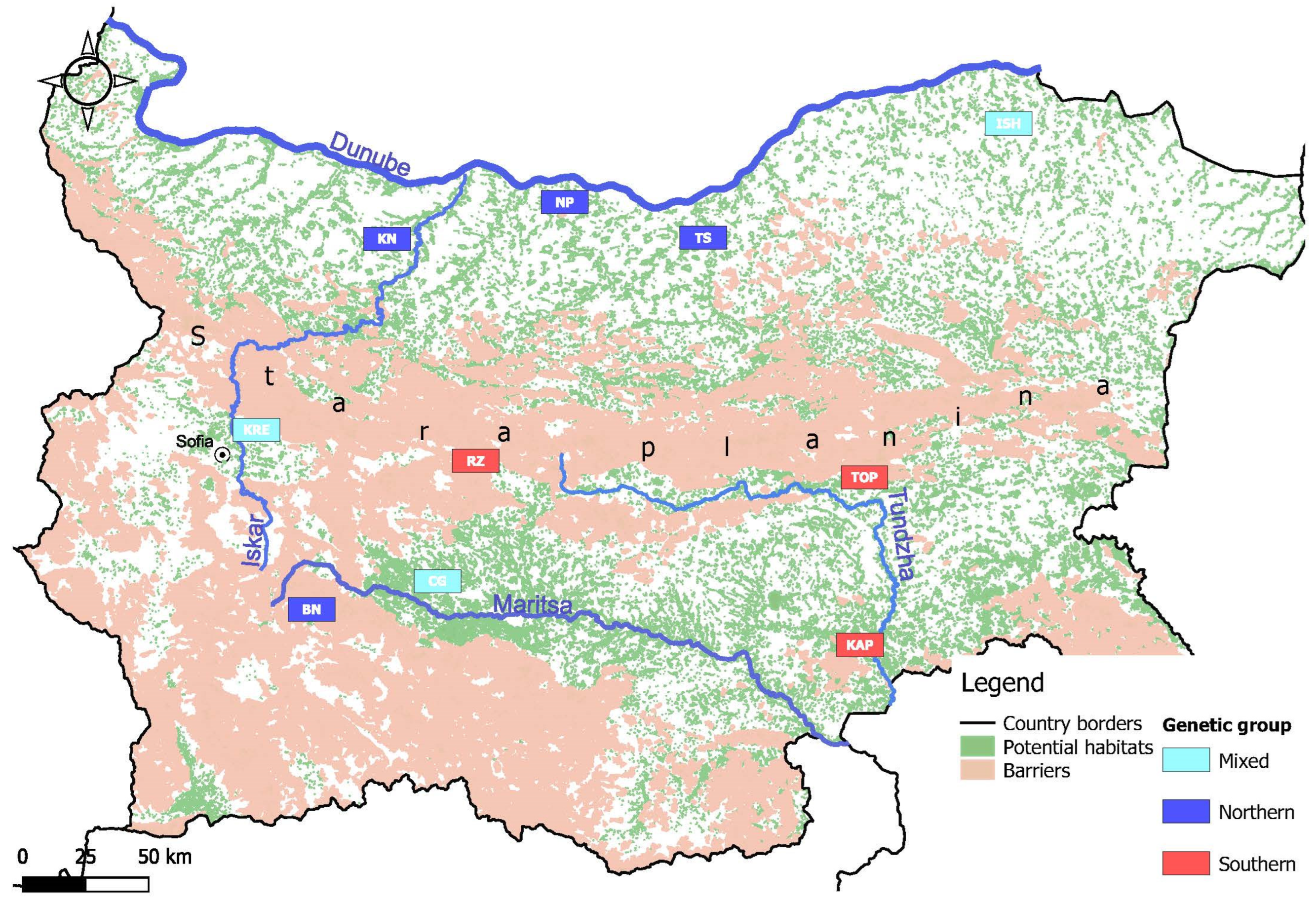
2.1. Study Area, Sampling and Dataset
2.2. Intra-Population Analysis, Spatial Genetic Structure and Gene Flow
3. Results
3.1. Genetic Diversity
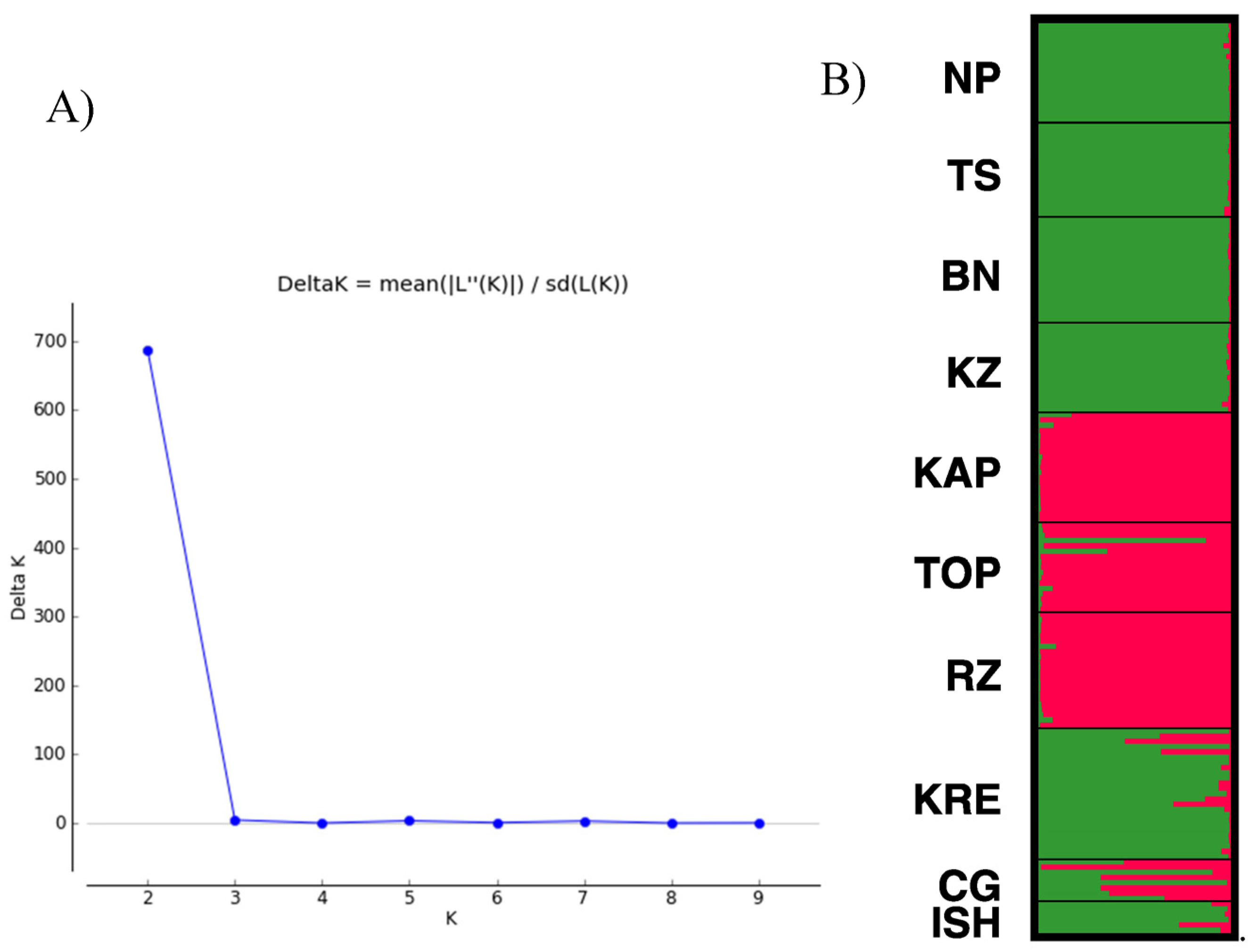
3.2. Spatial Clustering
4. Discussion
5. Conservation Implication and Role of the EGS in the Ecosystem
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collen, B.; Loh, J.; Whitmee, S.; McRAE, L.; Amin, R.; Baillie, J.E.M. Monitoring Change in Vertebrate Abundance: The Living Planet Index. Conserv. Biol. 2009, 23, 317–327. [Google Scholar] [CrossRef]
- Mackay, R. The Atlas of Endangered Species; Routledge: London, UK, 2014. [Google Scholar] [CrossRef]
- Sartorello, Y.; Pastorino, A.; Bogliani, G.; Ghidotti, S.; Viterbi, R.; Cerrato, C. The Impact of Pastoral Activities on Animal Biodiversity in Europe: A Systematic Review and Meta-Analysis. J. Nat. Conserv. 2020, 56, 125863. [Google Scholar] [CrossRef]
- Smulders, M.J.M.; Snoek, L.B.; Booy, G.; Vosman, B. Complete Loss of MHC Genetic Diversity in the Common Hamster (Cricetus cricetus) Population in The Netherlands. Consequences for Conservation Strategies. Conserv. Genet. 2003, 4, 441–451. [Google Scholar] [CrossRef]
- Neumann, K.; Jansman, H.; Kayser, A.; Maak, S.; Gattermann, R. Multiple Bottlenecks in Threatened Western European Populations of the Common Hamster Cricetus cricetus (L.). Conserv. Genet. 2004, 5, 181–193. [Google Scholar] [CrossRef]
- Biedrzycka, A.; Konopiński, M.K. Genetic Variability and the Effect of Habitat Fragmentation in Spotted Suslik Spermophilus suslicus Populations from Two Different Regions. Conserv. Genet. 2008, 9, 1211–1221. [Google Scholar] [CrossRef]
- Canestrelli, D.; Bisconti, R.; Sacco, F.; Nascetti, G. What Triggers the Rising of an Intraspecific Biodiversity Hotspot? Hints from the Agile Frog. Sci. Rep. 2014, 4, 5042. [Google Scholar] [CrossRef]
- Neaves, L.E.; Zenger, K.R.; Prince, R.I.; Eldridge, M.D.; Cooper, D.W. Landscape Discontinuities Influence Gene Flow and Genetic Structure in a Large, Vagile Australian Mammal, Macropus fuliginosus. Mol. Ecol. 2009, 18, 3363–3378. [Google Scholar] [CrossRef]
- Taylor, A.C.; Walker, F.M.; Goldingay, R.L.; Ball, T.; Van Der Ree, R. Degree of Landscape Fragmentation Influences Genetic Isolation among Populations of a Gliding Mammal. PLoS ONE 2011, 6, e26651. [Google Scholar] [CrossRef]
- Goldingay, R.L.; Harrisson, K.A.; Taylor, A.C.; Ball, T.M.; Sharpe, D.J.; Taylor, B.D. Fine-Scale Genetic Response to Landscape Change in a Gliding Mammal. PLoS ONE 2013, 8, e80383. [Google Scholar] [CrossRef]
- Brown, N.L.; Peacock, M.M.; Ritchie, M.E. Genetic Variation and Population Structure in a Threatened Species, the Utah Prairie Dog Cynomys parvidens: The Use of Genetic Data to Inform Conservation Actions. Ecol. Evol. 2016, 6, 426–446. [Google Scholar] [CrossRef]
- Freeland, J.R. Molecular Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2020; p. 384. ISBN 978-1-119-42615-8. [Google Scholar]
- Manel, S.; Holderegger, R. Ten Years of Landscape Genetics. Trends Ecol. Evol. 2013, 28, 614–621. [Google Scholar] [CrossRef]
- Segelbacher, G.; Cushman, S.A.; Epperson, B.K.; Fortin, M.-J.; Francois, O.; Hardy, O.J.; Holderegger, R.; Taberlet, P.; Waits, L.P.; Manel, S. Applications of Landscape Genetics in Conservation Biology: Concepts and Challenges. Conserv. Genet. 2010, 11, 375–385. [Google Scholar] [CrossRef]
- Hedrick, P.W. A Standardized Genetic Differentiation Measure. Evolution 2005, 59, 1633–1638. [Google Scholar] [CrossRef]
- McEachern, M.B.; Van Vuren, D.H.; Floyd, C.H.; May, B.; Eadie, J.M. Bottlenecks and Rescue Effects in a Fluctuating Population of Golden-Mantled Ground Squirrels (Spermophilus lateralis). Conserv. Genet. 2011, 12, 285–296. [Google Scholar] [CrossRef]
- Garner, A.; Rachlow, J.L.; Waits, L.P. Genetic Diversity and Population Divergence in Fragmented Habitats: Conservation of Idaho Ground Squirrels. Conserv. Genet. 2005, 6, 759–774. [Google Scholar] [CrossRef]
- Luikart, G.; Amish, S.J.; Winnie, J.; Beja-Pereira, A.; Godinho, R.; Allendorf, F.W.; Harris, R.B. High Connectivity among Argali Sheep from Afghanistan and Adjacent Countries: Inferences from Neutral and Candidate Gene Microsatellites. Conserv. Genet. 2011, 12, 921–931. [Google Scholar] [CrossRef]
- Helgen, K.M.; Cole, F.R.; Helgen, L.E.; Wilson, D.E. Generic Revision in the Holarctic Ground Squirrel Genus Spermophilus. J. Mammal. 2009, 90, 270–305. [Google Scholar] [CrossRef]
- Koshev, Y.S. Distribution and Status of the European Ground Squirrel (Spermophilus citellus) in Bulgaria. Lynx Ser. Nova 2008, 39, 251–261. [Google Scholar]
- Koshev, Y. Action Plan for the Conservation of the European Ground Squirrel (Spermophilus citellus) in Bulgaria 2022–2031; MoEW, BSPB: Sofia, Bulgaria, 2022. [Google Scholar]
- Říčanová, Š.; Koshev, Y.; Říčan, O.; Ćosić, N.; Ćirović, D.; Sedláček, F.; Bryja, J. Multilocus Phylogeography of the European Ground Squirrel: Cryptic Interglacial Refugia of Continental Climate in Europe. Mol. Ecol. 2013, 22, 4256–4269. [Google Scholar] [CrossRef]
- Hegyeli, Z. Spermophilus citellus. In The IUCN Red List of Threatened Species; 2020; p. E.T20472A91282380. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T20472A91282380.en (accessed on 30 September 2022).
- Janák, M.; Marhoul, P.; Matějů, J. Action Plan for the Conservation of the European Ground Squirrel Spermophilus citellus in the European Union; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Rammou, D.-L.; Kavroudakis, D.; Youlatos, D. Distribution, Population Size, and Habitat Characteristics of the Endangered European Ground Squirrel (Spermophilus citellus, Rodentia, Mammalia) in Its Southernmost Range. Sustainability 2021, 13, 8411. [Google Scholar] [CrossRef]
- Stefanov, V. European Ground Squirrel (Spermophilus citellus Linnaeus, 1776). In Red Data Book of the Republic of Bulgaria. Part 2. Animals; Golemansky, V., Peev, D., Eds.; BAS & MOEW: Sofia, Bulgaria, 2015; p. 232. [Google Scholar]
- Ćosić, N.; Říčanová, Š.; Bryja, J.; Penezić, A.; Ćirović, D. Do Rivers and Human-Induced Habitat Fragmentation Affect Genetic Diversity and Population Structure of the European Ground Squirrel at the Edge of Its Pannonian Range? Conserv. Genet. 2013, 14, 345–354. [Google Scholar] [CrossRef]
- Gedeon, C.I.; Hoffmann, I.E.; Váczi, O.; Knauer, F.; Ben Slimen, H.; Stefanović, M.; Lehoczky, É.; Laborczi, A.; Suchentrunk, F. The Role of Landscape History in Determining Allelic Richness of European Ground Squirrels (Spermophilus citellus) in Central Europe. Hystrix 2017, 28, 240–246. [Google Scholar] [CrossRef]
- Altbacker, V.; Czabán, D.; Németh, A.; Laczkó, L.; Altbäcker, V.; Cserkész, T.; Koroknai, V.; Sós, E.; Fidlóczky, Z.; Sramkó, G. Providing Finer Resolution of a Part of a Picture-the Detailed Molecular Phylogeography of European Ground Squirrel in Hungary. In Proceedings of the 6th European Ground Squirrel Meeting, Belgrade, Serbia, 4–6 November 2016. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, a Program to Estimate and Test Gene Diversities and Fixation Indices (Version 2.9.3). 2001. Available online: http://www./unil.ch/izea/softwares/fstat.html (accessed on 10 January 2016).
- Belkhir, K. GENETIX 4.05, Logiciel Sous Windows TM Pour La Génétique Des Populations. 2004. Available online: http://www.genetix.univ-montp2.fr/genetix/genetix.html (accessed on 10 February 2016).
- Chapuis, M.-P.; Estoup, A. Microsatellite Null Alleles and Estimation of Population Differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [CrossRef]
- Dempster, A.P.; Laird, N.M.; Rubin, D.B. Maximum Likelihood from Incomplete Data via the EM Algorithm. J. R. Stat. Soc. Ser. B 1977, 39, 1–22. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Luikart, G. Description and Power Analysis of Two Tests for Detecting Recent Population Bottlenecks from Allele Frequency Data. Genetics 1996, 144, 2001–2014. [Google Scholar] [CrossRef]
- Luikart, G.; Cornuet, J.-M. Empirical Evaluation of a Test for Identifying Recently Bottlenecked Populations from Allele Frequency Data. Conserv. Biol. 1998, 12, 228–237. [Google Scholar] [CrossRef]
- Weir, B.S. Genetic Data Analysis II: Methods for Discrete Population Genetic Data; Sinauer Associates, Inc.: Sunderland, MA, USA, 1996. [Google Scholar]
- Mantel, N. The Detection of Disease Clustering and a Generalized Regression Approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (Version 3.0): An Integrated Software Package for Population Genetics Data Analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of Population Structure Using Multilocus Genotype Data: Linked Loci and Correlated Allele Frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Corander, J.; Waldmann, P.; Sillanpää, M.J. Bayesian Analysis of Genetic Differentiation between Populations. Genetics 2003, 163, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Wen, W.; Falush, D. Documentation for STRUCTURE Software; Version 2; Department of Human Genetics University of Chicago: Chicago, IL, USA, 2003. [Google Scholar]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A Cluster Matching and Permutation Program for Dealing with Label Switching and Multimodality in Analysis of Population Structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.A. DISTRUCT: A Program for the Graphical Display of Population Structure. Mol. Ecol. Notes 2004, 4, 137–138. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of Molecular Variance Inferred from Metric Distances among DNA Haplotypes: Application to Human Mitochondrial DNA Restriction Data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Hulová, Š.; Sedláček, F. Population Genetic Structure of the European Ground Squirrel in the Czech Republic. Conserv. Genet. 2008, 9, 615–625. [Google Scholar] [CrossRef]
- Di Marco, M.; Santini, L.; Visconti, P.; Mortelliti, A.; Boitani, L.; Rondinini, C. Using Habitat Suitability Models to Scale up Population Persistence Targets. Hystrix 2016, 27. [Google Scholar] [CrossRef]
- Říčanová, Š.; Bryja, J.; Cosson, J.F.; Gedeon, C.; Choleva, L.; Ambros, M.; Sedláček, F. Depleted Genetic Variation of the European Ground Squirrel in Central Europe in Both Microsatellites and the Major Histocompatibility Complex Gene: Implications for Conservation. Conserv. Genet. 2011, 12, 1115–1129. [Google Scholar] [CrossRef]
- Ben Slimen, H.; Gedeon, C.I.; Hoffmann, I.E.; Suchentrunk, F. Dwindling Genetic Diversity in European Ground Squirrels? Mamm. Biol. 2012, 77, 13–21. [Google Scholar] [CrossRef]
- Fortelius, M. Neogene of the Old World Database of Fossil Mammals (NOW) 2011, University of Helsinki. Available online: http://www.helsinki.fi/science/now/ (accessed on 10 January 2016).
- Popova, L.V.; Maul, L.C.; Zagorodniuk, I.V.; Veklych, Y.M.; Shydlovskiy, P.S.; Pogodina, N.V.; Bondar, K.M.; Strukova, T.V.; Parfitt, S.A. ‘Good Fences Make Good Neighbours’: Concepts and Records of Range Dynamics in Ground Squirrels and Geographical Barriers in the Pleistocene of the Circum-Black Sea Area. Quat. Int. 2019, 509, 103–120. [Google Scholar] [CrossRef]
- Sebe, K.; Csillag, G.; Pazonyi, P.; Ruszkiczay-Rüdiger, Z. Quaternary Evolution of the River Danube in the Central Pannonian Basin and Its Possible Role as an Ecological Barrier to the Dispersal of Ground Squirrels. Hist. Biol. 2021, 33, 116–135. [Google Scholar] [CrossRef]
- Kopralev, I.; Yordanova, M.; Mladenov, C. Geography of Bulgaria; ForKom: Sofia, Bulgaria, 2002; p. 760. [Google Scholar]
- Kajtoch, Ł.; Cieślak, E.; Varga, Z.; Paul, W.; Mazur, M.A.; Sramkó, G.; Kubisz, D. Phylogeographic Patterns of Steppe Species in Eastern Central Europe: A Review and the Implications for Conservation. Biodivers. Conserv. 2016, 25, 2309–2339. [Google Scholar] [CrossRef]
- Fet, V.; Popov, A. Biogeography and Ecology of Bulgaria; Springer: Berlin/Heidelberg, Germany, 2007; Volume 82. [Google Scholar] [CrossRef]
- Peshev, T.; Peshev, D.; Popov, V. Mammalia. In Fauna Bulgarica; Marin Drinov: Sofia, Bulgaria, 2004; Volume 27. [Google Scholar]
- Vanlerberghe, F.; Boursot, P.; Catalan, J.; Gerasimov, S.; Bonhomme, F.; Botev, B.A.; Thaler, L. Analyse Génétique de La Zone d’hybridation Entre Les Deux Sous-Espèces de Souris Mus musculus Domesticus et Mus Musculus Musculus En Bulgarie. Genome 1988, 30, 427–437. [Google Scholar] [CrossRef]
- Koshev, Y.; Popov, V. General Report Target Species: 1335. European Ground Squirrel (Spermophilus citellus). In Specific Position 4: Mapping and Determining the Conservation Status of Mammals, Excluding Bats; MoEW: Sofia, Bulgaria, 2013; Available online: https://natura2000.egov.bg/EsriBg.Natura.Public.Web.App/Home/Reports?reportType=Mammals (accessed on 30 September 2022).
- Koshev, Y. Distribution, Isolation and Recent Status of European Ground Squirrel (Spermophilus citellus L.) in Pazardzhik District, Bulgaria. Annu. Shumen Univ. “Konstantin Preslavsky” Fac. Nat. Sci. 2009, 19, 97–109. [Google Scholar]
- Tonkov, S.; Bozilova, E.; Possnert, G. Lateglacial to Holocene Vegetation Development in the Central Rila Mountains, Bulgaria. Holocene 2016, 26, 17–28. [Google Scholar] [CrossRef]
- Golemansky, V.G.; Koshev, Y.S. Systematic and Ecological Survey on Coccidians (Apicomplexa: Eucoccidida) in European Ground Squirrel (Spermophilus citellus L.) (Rodentia: Sciuridae) from Bulgaria. Acta Zool. Bulg. 2009, 61, 143–150. [Google Scholar]
- Koshev, Y. Ecological and Ethological Characterization of European Ground Squirrel (Spermophilus citellus L.) in Model Colonies in Bulgaria. Ph.D. Thesis, IBER-BAS, Sofia, Bulgaria, 2012; p. 30. [Google Scholar]
- Ramos-Lara, N.; Koprowski, J.L.; Kryštufek, B.; Hoffmann, I.E. Spermophilus citellus (Rodentia: Sciuridae). Mamm. Species 2014, 913, 71–87. [Google Scholar] [CrossRef]
- Koshev, Y.; Genov, P. New Record of Steppe Polecat (Mustela Eversmanni Lesson, 1827) in Northwestern Bulgaria. Hist. Nat. Bulg. 2008, 19, 183–184. [Google Scholar]
- Demerdzhiev, D.; Boev, Z.; Dobrev, D.; Nedyalkov, N.; Petrov, T. Does Temporal and Spatial Diet Alteration Lead to Successful Adaptation of the Eastern Imperial Eagle, a Top Predator? Diversity 2022, 14, 1000. [Google Scholar] [CrossRef]
- Demerdzhiev, D.; Angelov, I.; Dobrev, D. Foraging Patterns of Non-Territorial Eastern Imperial Eagle (Aquila heliaca): A Case of Successful Adaptation. Diversity 2022, 14, 1060. [Google Scholar] [CrossRef]
- Matějů, J.; Říčanová, S.; Ambros, M.; Kala, B.; Hapl, E.; Matějů, K. Reintroductions of the European Ground Squirrel (Spermophilus citellus) in Central Europe (Rodentia: Sciuridae). Lynx Ser. Nova 2010, 41, 175–191. [Google Scholar]
- Koshev, Y.; Kachamakova, M.; Arangelov, S.; Ragyov, D. Translocations of European Ground Squirrel (Spermophilus citellus) along Altitudinal Gradient in Bulgaria—An Overview. Nat. Conserv. 2019, 35, 63–95. [Google Scholar] [CrossRef]
- Demerdzhiev, D.; Dobrev, D.; Popgeorgiev, G.; Stoychev, S. Landscape Alteration Affects the Demography of an Endangered Avian Predator by Reducing the Habitat Quality. Avian Res. 2022, 13, 100030. [Google Scholar] [CrossRef]


| N | S | p | |
|---|---|---|---|
| AR | 3.927 | 4.597 | 0.12880 |
| Ho | 0.541 | 0.587 | 0.32040 |
| He | 0.646 | 0.722 | 0.21167 |
| FIS | 0.162 | 0.187 | 0.83180 |
| FST | 0.179 | 0.174 | 0.89720 |
| Rel | 0.273 | 0.262 | 0.87313 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshev, Y.; Říčanová, Š.; Kachamakova, M.; Říčan, O. The European Ground Squirrel’s Genetic Diversity in Its Ancestral Land: Landscape Insights and Conservation Implications. Diversity 2023, 15, 365. https://doi.org/10.3390/d15030365
Koshev Y, Říčanová Š, Kachamakova M, Říčan O. The European Ground Squirrel’s Genetic Diversity in Its Ancestral Land: Landscape Insights and Conservation Implications. Diversity. 2023; 15(3):365. https://doi.org/10.3390/d15030365
Chicago/Turabian StyleKoshev, Yordan, Štěpánka Říčanová, Maria Kachamakova, and Oldřich Říčan. 2023. "The European Ground Squirrel’s Genetic Diversity in Its Ancestral Land: Landscape Insights and Conservation Implications" Diversity 15, no. 3: 365. https://doi.org/10.3390/d15030365
APA StyleKoshev, Y., Říčanová, Š., Kachamakova, M., & Říčan, O. (2023). The European Ground Squirrel’s Genetic Diversity in Its Ancestral Land: Landscape Insights and Conservation Implications. Diversity, 15(3), 365. https://doi.org/10.3390/d15030365








