Caulerpa cylindracea Spread on Deep Rhodolith Beds Can Be Influenced by the Morphostructural Composition of the Bed
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Image Collection and Analysis
2.3. Statistical Analysis
3. Results
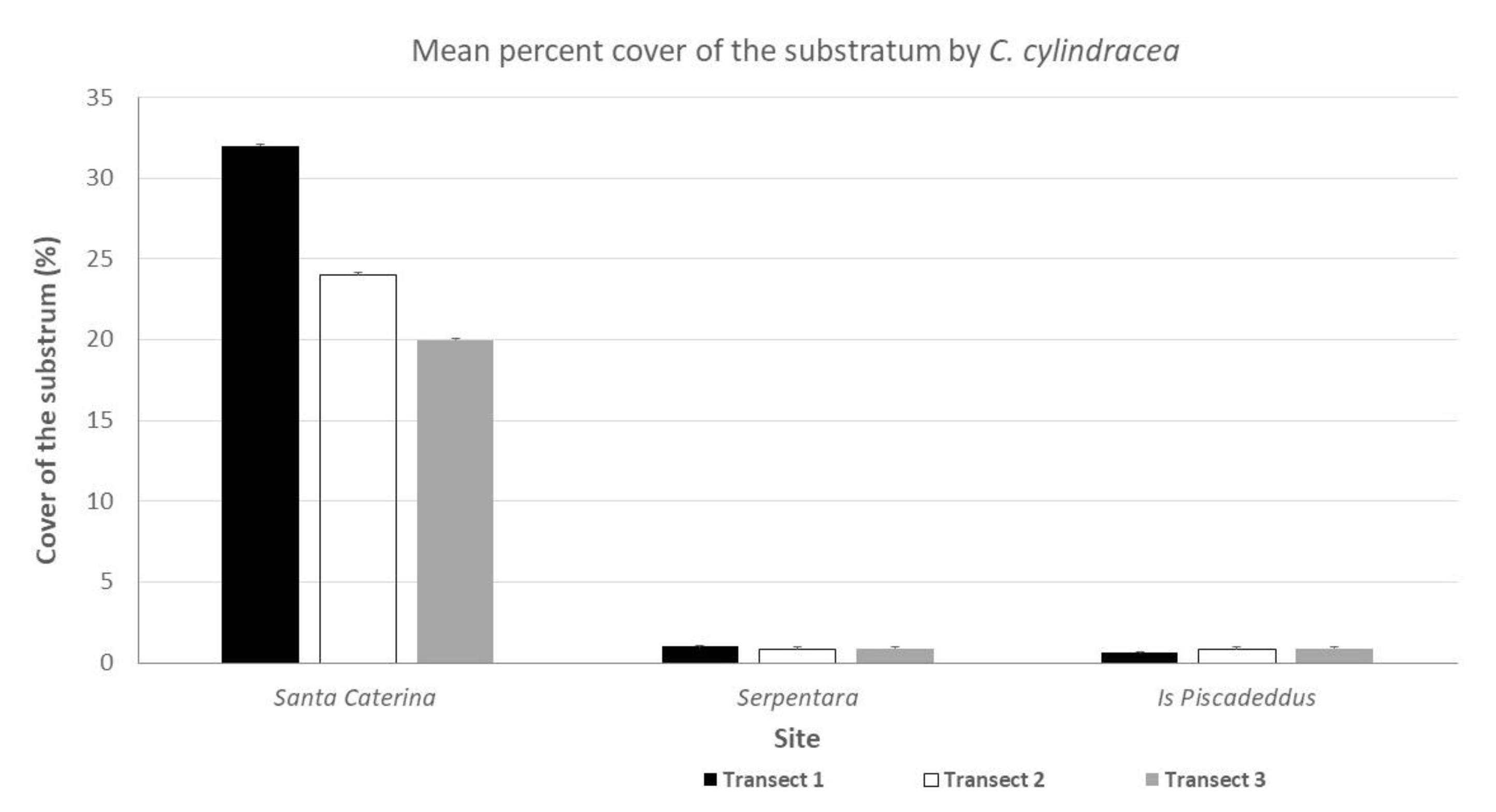
3.1. C. cylindracea Cover
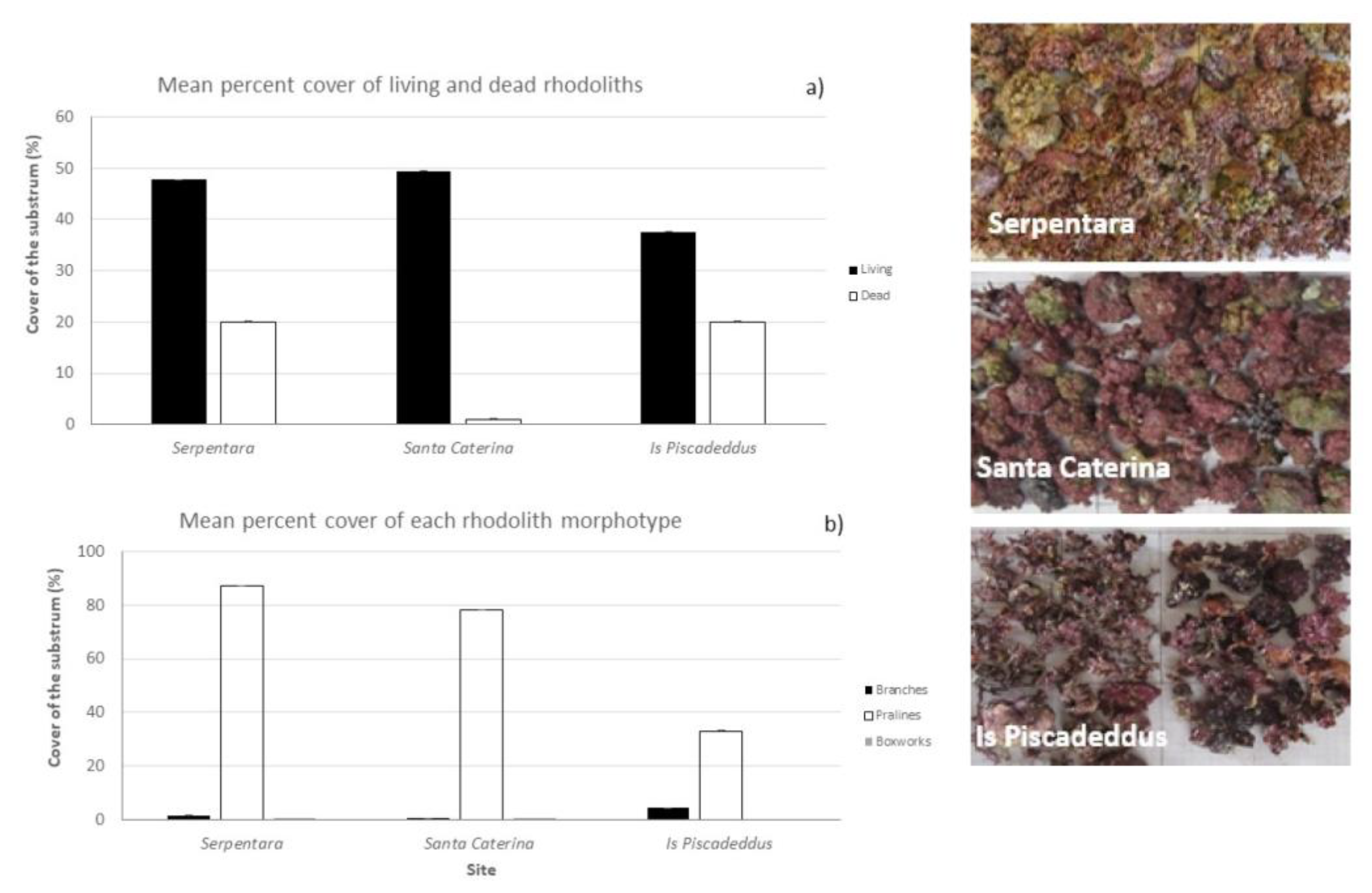
3.2. Rhodolith Cover
3.3. Correlation Rhodoliths—C. cylindracea Cover and Abundance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratana-Arporn, P.; Chirapart, A. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulata. Agric. Nat. Resour. 2006, 40, 75–83. [Google Scholar]
- Klein, J.; Verlaque, M. The Caulerpa racemosa invasion: A critical review. Mar. Poll. Bull. 2008, 56, 205–225. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase, World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2023. [Google Scholar]
- Verlaque, M.; Durand, C.; Huisman, J.M.; Boudouresque, C.F.; Le Parco, Y. On the identity and origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta). Eur. J. Phycol. 2003, 38, 325–339. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Ceccherelli, G.; Cinelli, F. Interactive effect of sedimentation and Caulerpa racemosa var. cylindracea invasion on macroalgal assemblages in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2005, 64, 467–474. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Poll. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Cinelli, F. Evaluation of benthic macroalgal invasion in a harbour area of the western Mediterranean Sea. Eur. J. Phycol. 2003, 38, 223–231. [Google Scholar] [CrossRef]
- Argyrou, M.; Demetropoulos, A.; Hadjichristophorou, M. Expansion of the macroalga Caulerpa racemosa and changes in softbottom macrofaunal assemblages in Moni bay, Cyprus. Oceanol. Acta 1999, 22, 517–528. [Google Scholar] [CrossRef]
- Gubbay, S.; Sanders, N.; Haynes, T.; Janssen, J.A.M.; Rodwell, J.R.; Nieto, A.; García Criado, M.S.; Beal, S.; Borg, J.M.; Kennedy, M.D.; et al. European Red List of Habitats. Part 1: Marine Habitats; European Union: Brussels, Belgium, 2016. [Google Scholar] [CrossRef]
- Basso, D. Carbonate production by calcareous red algae and global change. Geodiversitas 2012, 34, 13–33. [Google Scholar] [CrossRef]
- Bosellini, A.; Ginsburg, R.N. Form and internal structure of recent algal nodules (rhodolites) from Bermuda. J. Geol. 1971, 79, 669–682. [Google Scholar] [CrossRef]
- Basso, D.; Babbini, L.; Ramos-Esplá, A.A.; Salomidi, M. Mediterranean rhodolith beds. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodriguez, R., Ed.; Springer: Berlin, Germany, 2017; pp. 281–298. [Google Scholar]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith bed diversity in the Gulf of California: The importance of rhodolith structure and consequences of disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Basso, D.; Babbini, L.; Kaleb, S.; Bracchi, V.; Falace, A. Monitoring deep Mediterranean rhodolith beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 549–561. [Google Scholar] [CrossRef]
- Aguilar, R.; Pastor, X.; de la Torriente, A.; García, S. Deep-sea coralligenous beds observed with ROV on four seamounts in the Western Mediterranean. In Proceedings of the 1st Mediterranean Symposium on the Conservation of the Coralligenous and Other Calcareous Bio-Concretions, Tabarka, Tunisia, 15–16 January 2009; pp. 148–150. [Google Scholar]
- Bracchi, V.A.; Caronni, S.; Meroni, A.N.; Burguett, E.G.; Atzori, F.; Cadoni, N.; Marchese, F.; Basso, D. Morphostructural Characterization of the Heterogeneous Rhodolith Bed at the Marine Protected Area “Capo Carbonara”(Italy) and Hydrodynamics. Diversity 2022, 14, 51. [Google Scholar] [CrossRef]
- Bosence, D.W.J. Description and classification of rhodoliths (rhodoids, rhodolites). In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 217–224. [Google Scholar] [CrossRef]
- Foster, M.S.; Riosmena-Rodríguez, R.; Steller, D.L.; Woelkerling, W.J. Living rhodolith beds in the Gulf of California and their implications for paleoenvironmental interpretation. Geol. Soc. Am. Bull. 1997, 318, 127–139. [Google Scholar] [CrossRef]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Carvalho, V.F.; Assis, J.; Serrao, E.A.; Nunes, J.M.; Anderson, A.B.; Batista, M.B.; Barufi, J.B.; Silva, J.; Pereira, S.M.B.; Horta, P.A. Environmental drivers of rhodolith beds and epiphytes community along the South Western Atlantic coast. Mar. Environ. Res. 2020, 154, 104827. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Cortés, J. Caulerpa sertularioides, a green alga spreading aggressively over coral reef communities in Culebra Bay, North Pacific of Costa Rica. Coral Reefs 2005, 24, 10. [Google Scholar] [CrossRef]
- Sergio, F.; Newton, I.A.N.; Marchesi, L.; Pedrini, P. Ecologically justified charisma: Preservation of top predators delivers biodiversity conservation. J. Appl. Ecol. 2006, 43, 1049–1055. [Google Scholar] [CrossRef]
- Colautti, R.I.; MacIsaac, H.J. A neutral terminology to define ‘invasive’ species. Divers. Distrib. 2004, 10, 135–141. [Google Scholar] [CrossRef]
- Elton, C.S. The reasons for Conservation. In The Ecology of Invasions by Animals and Plants; Springer: Boston, MA, USA, 1958; pp. 143–153. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Chimienti, G.; Rizzo, L.; Kaleb, S.; Falace, A.; Fraschetti, S.; Giosa, F.D.; Tursi, A.; Barbone, E.; Ungaro, N.; Mastrototaro, F. Rhodolith Beds Heterogeneity along the Apulian Continental Shelf (Mediterranean Sea). J. Mar. Sci. Eng. 2020, 8, 813. [Google Scholar] [CrossRef]
- Orrù, P.; Cocco, A.; Panizza, V. Subaqueous geomorphological investigations from Capo Boi to Is Cappuccinus (south-eastern Sardinia). Mem. Descr. Carta Geol. d’Ital. LII 1994, 70, 163–176. [Google Scholar]
- Dethier, M.N.; Graham, E.S.; Cohen, S.; Tera, L.M. Visual versus random-point percent cover estimations: Objective is not always better. Mar. Ecol. Prog. Ser. 1993, 96, 93. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Meth. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Gattuso, J.P. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Chang. Biol. 2009, 15, 2089–2100. [Google Scholar] [CrossRef]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Basso, D.; Nalin, R.; Nelson, C.S. Shallow-water Sporolithon rhodoliths from north island (New Zealand). Palaios 2009, 24, 92–103. [Google Scholar] [CrossRef]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar] [CrossRef]
- Underwood, A.J.; Chapman, M.G. GMAV 5; Institute of Marine Ecology: Sydney, Australia; University of Sydney: Sydney, Australia, 2008. [Google Scholar]
- Piazzi, L.; Balata, D.; Bulleri, F.; Gennaro, P.; Ceccherelli, G. The invasion of Caulerpa cylindracea in the Mediterranean: The known, the unknown and the knowable. Mar. Biol. 2016, 163, 161. [Google Scholar] [CrossRef]
- McCoy, S.J.; Kamenos, N.A. Coralline algae (Rhodophyta) in a changing world: Integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 2015, 51, 6–24. [Google Scholar] [CrossRef]
- McConnico, L.A.; Carmona, G.H.; Morales, J.S.M.; Rodríguez, R.R. Temporal variation in seaweed and invertebrate assemblages in shallow rhodolith beds of Baja California Sur, México. Aquat. Bot. 2017, 139, 37–47. [Google Scholar] [CrossRef]
- Danovaro, R.; Dell’Anno, A.; Fabiano, M.; Pusceddu, A.; Tselepides, A. Deep-sea ecosystem response to climate changes: The eastern Mediterranean case study. Trends Ecol. Evol. 2001, 16, 505–510. [Google Scholar] [CrossRef]
- Samperio-Ramos, G.; Olsen, Y.S.; Tomas, F.; Marbà, N. Ecophysiological responses of three Mediterranean invasive seaweeds (Acrothamnion preissii, Lophocladia lallemandii and Caulerpa cylindracea) to experimental warming. Mar. Poll. Bull. 2015, 96, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Cerrano, C.; Bastari, A.; Calcinai, B.; Di Camillo, C.; Pica, D.; Puce, S.; Valisano, L.; Torsani, F. Temperate mesophotic ecosystems: Gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. Eur. Zool. J. 2019, 86, 370–388. [Google Scholar] [CrossRef]
- Ramus, J.; Beale, S.I.; Mauzerall, D. Correlation of changes in pigment content with photosynthetic capacity of seaweeds as a function of water depth. Mar. Biol. 1976, 37, 231–238. [Google Scholar] [CrossRef]
- Williams, S.L.; Dennison, W.C. Light availability and diurnal growth of a green macroalga (Caulerpa cupressoides) and a seagrass (Halophila decipiens). Mar. Biol. 1990, 106, 437–443. [Google Scholar] [CrossRef]
- Montefalcone, M.; Morri, C.; Parravicini, V.; Bianchi, C.N. A tale of two invaders: Divergent spreading kinetics of the alien green algae Caulerpa taxifolia and Caulerpa cylindracea. Biol. Invasions 2015, 17, 2717–2728. [Google Scholar] [CrossRef]
- Bernardeau-Esteller, J.; Ruiz, J.M.; Tomas, F.; Sandoval-Gil, J.M.; Marín-Guirao, L. Photoacclimation of Caulerpa cylindracea: Light as a limiting factor in the invasion of native Mediterranean seagrass meadows. J. Exp. Mar. Biol. Ecol. 2015, 465, 130–141. [Google Scholar] [CrossRef]
- Rendina, F.; Ferrigno, F.; Appolloni, L.; Donnarumma, L.; Sandulli, R.; Fulvio, G. Anthropic pressure due to lost fishing gears and marine litter on different rhodolith beds off the Campania Coast (Tyrrhenian Sea, Italy). Ecol. Quest. 2020, 31, 41–51. [Google Scholar] [CrossRef]
- Rendina, F.; Donnarumma, L.; Appolloni, L.; Bruno, R.; Ferrigno, F.; Di Stefano, F.; Russo, G. First Description of a Rhodolith Bed off the Island of Capri and its Associated Benthic Fauna. Biol. Mar. Mediterr. 2017, 24, 126–127. [Google Scholar]
- Del Río, J.; Ramos, D.A.; Sánchez-Tocino, L.; Peñas, J.; Braga, J.C. The punta de la Mona rhodolith bed: Shallow-water Mediterranean rhodoliths (Almuñecar, Granada, Southern Spain). Front. Earth Sci. 2022, 10, 884685. [Google Scholar] [CrossRef]
- Capiomont, A.; Breugnot, E.; den Haan, M.; Meinesz, A. Phenology of a deep-water population of Caulerpa racemosa var. cylindracea in the northwestern Mediterranean Sea. Bot. Mar. 2005, 48, 80–83. [Google Scholar] [CrossRef]
- Pacciardi, L.; De Biasi, A.M.; Piazzi, L. Effects of Caulerpa racemosa invasion on soft-bottom assemblages in the Western Mediterranean Sea. Biol. Invasions 2011, 13, 2677–2690. [Google Scholar] [CrossRef]
- Klein, J.C.; Verlaque, M. Macroalgal assemblages of disturbed coastal detritic bottoms subject to invasive species. Estuar. Coast. Shelf Sci. 2009, 82, 461–468. [Google Scholar] [CrossRef]
- Ceccherelli, G.; Pinna, S.; Cusseddu, V.; Bulleri, F. The role of disturbance in promoting the spread of the invasive seaweed Caulerpa racemosa in seagrass meadows. Biol. Invasions 2014, 16, 2737–2745. [Google Scholar] [CrossRef]
- Caronni, S.; Calabretti, C.; Delaria, M.A.; Bernardi, G.; Navone, A.; Occhipinti-Ambrogi, A.; Panzalis, P.; Ceccherelli, G. Consumer depletion alters seagrass resistance to an invasive macroalga. PLoS ONE 2015, 10, e0115858. [Google Scholar] [CrossRef]
- Airoldi, L. Roles of disturbance, sediment stress, and substratum retention on spatial dominance in algal turf. Ecology 1998, 79, 2759–2770. [Google Scholar] [CrossRef]
- Uyà, M.; Bulleri, F.; Gribben, P.E. Propagules are not all equal: Traits of vegetative fragments and disturbance regulate invasion success. Ecology 2018, 99, 957–965. [Google Scholar] [CrossRef] [PubMed]


| ANOVA (a) | |||||||
|---|---|---|---|---|---|---|---|
| Health Status | Morphotype | ||||||
| Source | df | MS | F | P | MS | F | P |
| Site | 2 | 1114.5060 | 1.20 | 0.3359 | 722.5744 | 22.19 | 0.0000 |
| Status | 1 | 4309.0139 | 45.02 | 0.0000 | 12,706.1219 | 390.26 | 0.0000 |
| Site × Status | 2 | 372.4735 | 3.89 | 0.0498 | 870.2610 | 26.73 | 0.0000 |
| Residual | 12 | 95.7169 | 32.5582 | ||||
| Total | 17 | ||||||
| Cochran’s Test = 0.7124 | Cochran’s Test = 0.7124 | ||||||
| SNK Site × Status | SNK Site × Morphotype (b) | ||||||
| Site | Status | Status | Site | Site | Morphotype | Morphotype | Site |
| SC | Liv. > Dead | L | SC = Se = IP | SC | Br = Bw < Pr | Br | SC = Se = IP |
| Se | Liv. > Dead | D | SC = IP > Se | Se | Br = Bw < Pr | Pr | SC = Se < IP |
| IP | Liv. = Dead | IP | Br = Bw < Pr | Bw | SC = Se = IP | ||
| E.S. = 0.2345 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caronni, S.; Bracchi, V.A.; Atzori, F.; Citterio, S.; Cadoni, N.; Gentili, R.; Montagnani, C.; Quaglini, L.A.; Basso, D. Caulerpa cylindracea Spread on Deep Rhodolith Beds Can Be Influenced by the Morphostructural Composition of the Bed. Diversity 2023, 15, 349. https://doi.org/10.3390/d15030349
Caronni S, Bracchi VA, Atzori F, Citterio S, Cadoni N, Gentili R, Montagnani C, Quaglini LA, Basso D. Caulerpa cylindracea Spread on Deep Rhodolith Beds Can Be Influenced by the Morphostructural Composition of the Bed. Diversity. 2023; 15(3):349. https://doi.org/10.3390/d15030349
Chicago/Turabian StyleCaronni, Sarah, Valentina Alice Bracchi, Fabrizio Atzori, Sandra Citterio, Nicoletta Cadoni, Rodolfo Gentili, Chiara Montagnani, Lara Assunta Quaglini, and Daniela Basso. 2023. "Caulerpa cylindracea Spread on Deep Rhodolith Beds Can Be Influenced by the Morphostructural Composition of the Bed" Diversity 15, no. 3: 349. https://doi.org/10.3390/d15030349
APA StyleCaronni, S., Bracchi, V. A., Atzori, F., Citterio, S., Cadoni, N., Gentili, R., Montagnani, C., Quaglini, L. A., & Basso, D. (2023). Caulerpa cylindracea Spread on Deep Rhodolith Beds Can Be Influenced by the Morphostructural Composition of the Bed. Diversity, 15(3), 349. https://doi.org/10.3390/d15030349








