Abstract
From a phylogenetic point of view, the Malacopsyllidae family and the Rhopalopsillidae family (comprising Parapsyllinae and Rhopalopsyllinae subfamilies) have been traditionally classified within the Malacopsylloidea superfamily, mostly restricted to South America. The phylogenetic relationships and taxonomic status of Malacopsyllidae and Rhopalopsillidae have never been assessed since no molecular loci of Malacopsyllidae have been sequenced by any authors, and the phylogeny provided so far was not based on any sort of formal quantitative analysis of flea morphology. Based on these precedents, the objective of this study was to carry out a comparative phylogenetic, molecular and morphological study of two different species belonging to each family, Phthiropsylla agenoris (Malacopsylla) and Polygenis (Polygenis) rimatus (Rhopalopsyllidae, Rhopalopsyllinae). In this study, we demonstrated the usefulness of several morphological features as diagnostic characters to differentiate between P. (P.) rimatus and P. agenoris. Using molecular and phylogenetic data, we easily discriminated between the two taxa (P. agenoris and P. (P.) rimatus) by comparing both nuclear and mitochondrial markers. This fact proves the usefulness of ITS2, EF1−α, cox1, cytb and cox2 as molecular diagnostic markers to characterize and identify different Siphonaptera taxa. Additionally, the phylogenetic results confirm, for the first time, the monophyly of the Malacopsyllidae family and suggest a clear paraphyletic position of the Paraspsyllinae subfamily and, consequently, the Rhopalopsyllidae family.
1. Introduction
Fleas, as blood−sucking insects parasitic on wild and domestic birds and mammals, including humans, are important in public health as parasites, intermediate hosts and vectors of pathogens [1]. Phylogenetic relationships among flea taxa based on molecular data were virtually neglected until Whiting et al. [2] published the first comprehensive attempt to reconstruct deep evolutionary relationships for fleas using a formal analysis of character data from four loci: 18S ribosomal DNA, 28S ribosomal DNA, cytochrome c oxidase subunit 1 (cox1) and elongation factor 1−alpha (EF1−α). For this purpose, they used 128 different taxa representing 16 families, 25 subfamilies, 26 tribes and 83 flea genera collected from around the world. Among their findings, they confirmed the monophyly of ten families: Tungidae, Lycopsyllidae, Pygiopsyllidae, Stivaliidae, Stephanocircidae, Rhopalopsyllidae, Chimaeropsyllidae, Pulicidae, Ischnopsyllidae and Ceratophyllidae.
In spite of that, they claimed the necessity to keep providing and re−examining new molecular, phylogenetic and morphological flea data to clarify the systematics of fleas, especially those regarding the Ctenophthalmidae family, which was defined by these authors as a “catch−all group” or an unnatural grouping of fleas that are grossly paraphyletic. In this sense, few papers have been published since 2008 in order to improve and update some taxonomically doubtful points exposed by [2]. While it is true that the recent studies published by [3,4,5] have tried to clarify the taxonomic status of different Ctenophthalmidae taxa, other families of fleas, such as Ancistropsyllidae, Malacopsyllidae and Xiphiopsyllidae, not included in the study published by [2], remain unknown from a phylogenetic point of view.
Malacopsyllidae is a very small family endemic to Argentina, including only two genera, each one with only one species, Malacopsylla grossiventris (Weyenbergh, 1879) [6] and Phthiropsylla agenoris (Rothschild, 1904) [7,8]. These two taxa share similar morphological traits and geographical distributions, with armadillos (Xenarthra, Dasypodidae) (e.g., Chaetophractus villosus (Desmarest, 1904), Dasypus sp. and Zaedyus pichiy (Desmarest, 1904)) as their main hosts. In addition, Malacopsyllidae fleas have also been reported on carnivores (Lycalopex gymnocercus (Fischer, 1814) and Cerdocyon thous (Linnaeus, 1766)) and some caviid rodents (e.g., Microcavia australis (Geoffroy y d’Orbigny, 1833) and Galea musteloides (Meyen, 1832)) [9]. Malacopsyllids are large fleas; engorged females can reach a length of 6.5 mm with an abdominal diameter of 3 mm [8]. These fleas are confined to the ventral dermecos of armadillos, and as this underside is liable to brush against the substrate, malacopsyllids must therefore be able to cling very firmly to the coarse hairs of these hosts [10]. In addition, although laciniae are not heavily serrated, females were observed fixed with their mouthparts to the skin of their hosts, like ticks. Indeed, some of these specimens were observed copulating on the ventral region of their hosts [6,11].
From a phylogenetic point of view, Malacopsyllidae and Rhopalopsillidae families have been traditionally classified within Malacopsylloidea by several authors, a superfamily mostly restricted to South America, with some exceptions [8,12,13]. The Rhopalopsyllidae family comprises 14 genera and about 130 species and subspecies distributed in 2 subfamilies, Parapsyllinae and Rhopalopsyllinae, each of them of monophyletic origin [2]. These fleas mainly have a Neotropical distribution and parasitize birds and mammals, mainly cricetid rodents [2,8,9]. Rhopalopsyllinae comprises eight different genera (Gephyropsylla, Hechtiella, Ayeshaepsylla, Neotropsylla, Polygenis, Rhopalopsyllus, Scolopsyllus and Tiamastus), including species that mainly infest cricetid and octodontid rodents in the Neotropical region [1]. From these, Polygenis is the most important genus because of its wide geographical distribution and a high number of species and subspecies (44 in total). In addition, species of Polygenis were reported related to the maintenance of sylvatic plague among wild rodents [1], as well as associated with Rickettsia felis, the etiologic agent of flea−borne spotted fever [14,15,16].
In spite of that, the phylogenetic relationships and taxonomic status of Malacopsyllidae and Rhopalopsillidae have never been assessed since no molecular loci of Malacopsyllidae were sequenced and assessed by [1] or any other authors. Thus, the taxonomic relationship between these two families should be considered clearly unresolved since the intuitive phylogeny provided in [8,12,13] was not based on a formal quantitative analysis of flea morphology.
Based on these precedents, the objective of this study was to carry out a comparative phylogenetic, molecular and morphological study of two different species belonging to Malacopsyllidae and Rhopalopsyllidae families, P. agenoris and Polygenis (Polygenis) rimatus [17], in order to clarify the taxonomic status and phylogenetic relationships of these families for the first time. In addition, we examined the morphological features and performed the molecular characterization of P. agenoris and P. (P.) rimatus (Jordan, 1932).
2. Materials and Methods
2.1. Collection of Samples
Fleas were recovered from hosts (trapped alive) by using tweezers and stored in 96% ethanol. Three males and four females of Malacopsyllidae fleas were collected from an armadillo identified as Zaedyus pichiy (PPA 693), captured alive, 20 km S Perito Moreno and RN 40, Santa Cruz Province, Argentina, 6−II−2013, coll. Marcela Lareschi.
Four males of Rhopalopsyllidae fleas were collected individually from four different rondent hosts, all of them captured at Parque Provincial Ernesto Tornquist, Sierra de la Ventana, Buenos Aires Province, Argentina, 14−IX−2010 and 21−V−2011, coll. Marcela Lareschi. The rodents were identified as Akodon azarae (Fischer, 1829) (CNP 4333) and Akodon dolores (Thomas, 1916) (CNP3773, ROB 15, and ROB17). Voucher hosts were housed at the Mammals Collection of the Patagonic Nacional Center (CNP; Puerto Madryn, Chubut, Argentina) (CNP4333 and CNP3773). The remaining rodents and the armadillo were released in the same places where they were captured.
2.2. Morphological Study
Fleas were cleared and softened in 10% KOH, dehydrated in an increasing series of ethanol (80–100%), further diaphanized in eugenol, mounted in Canadian balsam to study them under a light microscope and photographed by using a microscope (Olympus BX51) equipped with a Photographic Camera (Olympus DP71, BX51TF, Tokyo, Japan). Morphology was studied by comparing our specimens with the male lectotype of P. agenoris and the male holotype of P. (P.) rimatus, as well as other specimens of the latter species deposited at the Natural History Museum (NHM), London, U.K. Additionally, we followed descriptions and illustrations provided in the original descriptions of the species in [7] and in [8]. The morphological terminology used by these authors was followed. The fleas studied will be deposited at the Department of Entomology of the Museum of La Plata, Argentina.
2.3. Molecular and Phylogenetic Study
We amplified and sequenced five different molecular markers of five specimens of P. agenoris and three specimens of P. (P.) rimatus: nuclear elongation factor 1 alpha (EF1−α), Internal Transcribed Spacer 2 (ITS2) ribosomal DNA (rDNA) and partial cytochrome c oxidase subunits 1 and 2 (cox1 and cox2) and cytochrome b (cytb) mitochondrial (mt) gene fragments. Our study was completed using several sequences retrieved from GenBank, representing 12 families, 26 genera and 41 species of fleas, to set up a molecular matrix of four loci (EF1−α, cox1, cox2 and cytb).
All molecular markers sequenced in the present study were amplified by polymerase chain reaction (PCR) using a thermal cycler (Eppendorf AG; Eppendorf, Hamburg, Germany). The PCR mix, PCR conditions and PCR primers are summarized in the Supporting Information (Table S1). The EF1−α, ITS2, and partial cox1, cox2 and cytb gene sequences obtained from all specimens analyzed were deposited in the GenBank database (Table 1).
The PCR products were checked on SYBR Safe−stained 2% Tris–borate–ethylenediaminetetraacetic acid agarose gels. Bands were eluted and purified from the agarose gel using the QWizard SV Gel and PCR Clean−Up System Kit (Promega, Madison, WI, USA). Once purified, the products were sequenced by Stab Vida (Lisbon, Portugal). To obtain a nucleotide sequence alignment file, the MUSCLE alignment method [18] was used in MEGA, version 10.1.8 [19]. To assess the similarity among all marker sequences of all specimens analyzed in the present study and other flea taxa, the number of base differences per sequence was assessed using the number of differences method in MEGA, version 10.1.8 [19]. For this purpose, we used species and genera belonging to Rhopalopsyllidae (Polygenis pradoi (Wagner, 1937), Polygenis roberti roberti (Jordan, 1939), Polygenis rimatus (Jordan, 1932), Ectinorus sp., Tiamastus cavicola (Weyenbergh, 1881), Rhopalopsyllus australis (Rothschild, 1904), Listronius fortis (Jordan & Rothschild, 1923), Parapsyllus humboldti (Jordan, 1942), Parapsyllus longicornis (Enderlein, 1901) and Tetrapsyllus sp.) and Malacopsyllidae (P. agenoris and M. grossiventris) families available in GenBank.


Table 1.
GenBank accession numbers of ITS2, EF1−α and partial cytb, cox1 and cox2 gene sequences of individuals of Phthiropsylla agenoris and Polygenis rimatus obtained in this study.
Table 1.
GenBank accession numbers of ITS2, EF1−α and partial cytb, cox1 and cox2 gene sequences of individuals of Phthiropsylla agenoris and Polygenis rimatus obtained in this study.
| Species | Sample ID/ Geographical Area | Host | Number of Fleas | Base Pairs (bp) | Accession Number |
|---|---|---|---|---|---|
| ITS2 | |||||
| P. agenoris | PA1−PA5/Santa Cruz, Argentina | Zaedyus pichiy | 5 | 482 | OU706236 |
| P. (P.) rimatus | P1−P3/Buenos Aires, Argentina | Akodon dolores | 3 | 453 | OU706235 |
| Cox1 | |||||
| P. agenoris | PA1−PA5/Santa Cruz, Argentina | Zaedyus pichiy | 5 | 658 | OU706243 |
| P. (P.) rimatus | P1/Buenos Aires, Argentina | Akodon dolores | 1 | 658 | OU706244 |
| P. (P.) rimatus | P2−P3/Buenos Aires, Argentina | Akodon dolores | 2 | 658 | OU706245 |
| Cox2 | |||||
| P. agenoris | PA1/Santa Cruz, Argentina | Zaedyus pichiy | 1 | 729 | OU707013 |
| P. agenoris | PA3/Santa Cruz, Argentina | Zaedyus pichiy | 1 | 729 | OU707015 |
| P. agenoris | PA5/Santa Cruz, Argentina | Zaedyus pichiy | 1 | 729 | OU707016 |
| P. agenoris | PA2, PA4/Santa Cruz, Argentina | Zaedyus pichiy | 2 | 729 | OU707014 |
| P. (P.) rimatus | P1/Buenos Aires, Argentina | Akodondolores | 1 | 739 | OU707017 |
| P. (P.) rimatus | P2−P3/Buenos Aires, Argentina | Akodon dolores | 2 | 739 | OU707018 |
| Cytb | |||||
| P. agenoris | PA1, PA3/Santa Cruz, Argentina | Zaedyus pichiy | 2 | 374 | OU706744 |
| P. agenoris | PA2, PA4−PA5/Santa Cruz, Argentina | Zaedyus pichiy | 3 | 374 | OU706745 |
| P. (P.) rimatus | P1/Buenos Aires, Argentina | Akodon dolores | 1 | 374 | OU706746 |
| P. (P.) rimatus | P2−P3/Buenos Aires, Argentina | Akodon dolores | 2 | 374 | OU706743 |
| EF1−α | |||||
| P. agenoris | PA1, PA3−PA5/Santa Cruz, Argentina | Zaedyus pichiy | 4 | 975 | OU706239 |
| P. agenoris | PA2/Santa Cruz, Argentina | Zaedyus pichiy | 1 | 975 | OU706240 |
| P. (P.) rimatus | P1, P3/Buenos Aires, Argentina | Akodon dolores | 2 | 975 | OU706241 |
| P. (P.) rimatus | P2/Buenos Aires, Argentina | Akodon dolores | 1 | 975 | OU706242 |
The concatenated phylogenetic tree was inferred using nucleotide data and constructed using two methods: maximum likelihood (ML) and Bayesian inference (BI). The maximum likelihood tree was generated using the PHYML package from [20], whereas Bayesian inference was generated using MRBAYES, version 3.2.6 [21]. JMODELTEST [22] was used to determine the best−fit substitution model for the parasite data (EF1−α, cox1, cox2 and cytb). Models of evolution were chosen for subsequent analyses according to the Akaike information criterion [23,24]. To investigate the dataset containing the concatenation of four markers (EF1−α, cox1, cox2 and cytb), analyses based on BI were partitioned by gene, and models for individual genes within partitions were selected by JMODELTEST. For ML inference, best−fit nucleotide substitution models included Transitional Model 2 with gamma−distributed rate variation and a proportion of invariable sites equal to TIM2 + I + G (cox2), Transitional Model 1 with gamma−distributed rate variation and a proportion of invariable sites equal to TIM1 + I + G (cox1) and a general time−reversible model with gamma−distributed rate variation and a proportion of invariable sites equal to GTR + I + G (EF1−α and cytb). Support for the topology was examined using bootstrapping (heuristic option) [25] with 1000 replications to assess the relative reliability of clades. The commands used in MRBAYES, version 3.2.6, for Bayesian inference were nst = 6 with invgamma rates (EF1−α, cox1, cox2 and cytb). For BI, the standard deviation of split frequencies was used to determine whether the number of generations completed was enough; the chain was sampled every 500 generations, and each dataset was run for 10 million generations. The adequacy of sampling and run convergence was assessed using the effective sample size (ESS) diagnostic in Tracer, version 1.6 [26]. Trees from the first million generations were discarded based on the assessment of convergence. Burn−in was determined empirically by examining the log−likelihood values of the chains. The Bayesian posterior probabilities (BPPs) comprise the significance scores for the nodes. The obtained phylogenetic tree was then visualized and edited in Figtree 1.4.4 [27].
Phylogenetic analyses, based on concatenated markers EF1−α, cox1, cox2 and cytb sequences, were carried out using our sequences and those obtained from the GenBank database (see Table S2). The phylogenetic tree was rooted using Panorpa meridionalis (Rambur, 1842) (Mecoptera: Panorpidae) as the outgroup. This choice was based on the combination of morphological and molecular data obtained in previous studies, which provided compelling evidence for a sister−group relationship between Mecoptera and Siphonaptera [2,28].
The ITS2 sequences obtained in this work were exclusively used to molecularly characterize P. agenoris and P. (P.) rimatus fleas isolated from Argentina. Thus, no phylogenetic trees with other Siphonaptera species based on ITS2 sequences were constructed, so this marker was removed from the concatenated dataset. This decision was based on the high length and nucleotide divergence observed among Mecoptera and Siphonaptera ITS2 sequences, together with the absence of other Malacopsyllidae and Rhopalopsyllidae ITS2 sequences available in GenBank.
3. Results
3.1. Morphological Characterization of Fleas
The seven fleas collected from the armadillo (Zaedyus pichiy) were identified as P. agenoris (three males and four females), and those from Akodon species were identified as P. (P.) rimatus (four males), in accordance with morphological features observed in specimens deposited at the NHM, as well as those presented in the literature, as detailed below.
3.1.1. Phthiropsylla agenoris
Description (females and males): Frons rounded, without frontal tubercle; with the lower two setae of the ocular row very short, stout and spiniform; pronotum with rows of long setae and two short blunt pseudo−spines, each side widely separated from each other (Figure 1a); setae on the posterior margin of the fore tibia, not spiniform (Figure 1b); oblique break of mid coxa complete (Figure 1c); hindtarsus with distitarsomeres with five pairs of lateral plantar setae (Figure 1d). Female spermatheca with an oval bulga and a long and narrow hilla. The basis of the hilla appeared to be penetrating the lumen of the bulga (Figure 1e); male terminalia, with telomere rather small and inserted in the posterior part of the basimere. Phallosome and aedeagus as in Figure 1f.
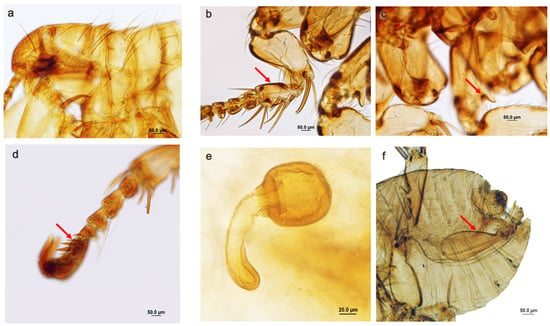
Figure 1.
(a–f) Phthiropsylla agenoris: (a) Details of the head and thorax; (b) details of fore tibia (arrow); (c) oblique break of mid coxa (arrow); (d) hindtarsus with distitarsomeres with 5 pairs of lateral plantar setae (arrow); (e) spermatheca; (f) male terminalia, paramere and aedeagus (arrow).
3.1.2. Polygenis (Polygenis) rimatus
Description (males): Labial palp not reaching apex of fore coxa (Figure 2a); oblique break of mid coxa uncomplete (Figure 2b); acetabular seta below the level of the upper margin of acetabulum; distal arm of ninth sternum laterally with setae extending two−thirds the distance from the apex; angle between distal and proximal arms of the basal part of aedeagal tubus about 90°; coil of aedeagal inner tube making 2.5 turns (Figure 2c). Unfortunately, we could not find female specimens of P. (P.) rimatus in this study in order to compare the main morphological traits between the two taxa.
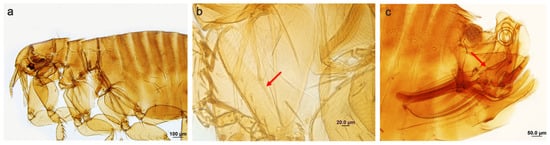
Figure 2.
(a–c) Polygenis (Polygenis) rimatus: anterior general view (a); oblique break of mid coxa (arrow) (b); male terminalia, paramere and aedeagus (arrow) (c).
3.2. Molecular Results
3.2.1. ITS2 Fragment and EF1−α Partial Gene Analysis
The lengths of the ITS2 sequences of P. agenoris and P. (P.) rimatus specimens were 482 and 453 base pairs (bp), respectively, whereas the length of the partial EF1−α gene was 975 bp for all sequences analyzed (Table 1). There were no intraspecific nucleotide differences among any of the ITS2 sequences assessed in this study, whereas a total of 77 different base pairs were observed between P. agenoris and P. (P.) rimatus for the same molecular marker (84.9% nucleotide similarity). The EF1−α intraspecific similarity between P. agenoris and P. (P.) rimatus ranged from 99.8% to 100% and 99.4% to 100%, respectively, observing two different haplotypes for the two species and showing that the interspecific similarity between the two species ranged from 80.4% to 80.7% (Table 2). On the other hand, when we compared our sequences with other partial EF1−α gene sequences from Rhopalopsyllidae and Malacopsyllidae species retrieved from GenBank, we noted that the percentages of similarity ranged from 80 to 90%, except among congeneric Polygenis species, where these values always appeared higher than 90% (Table 2).
3.2.2. Partial cox1, cox2 and cytb mtDNA Gene Analysis
The partial cox1 and cytb mtDNA gene sequences of P. agenoris and P. (P.) rimatus specimens amplified in our study were 658 bp and 374 bp in length, respectively. However, the length of cox2 sequences differed by 10 bp between the two taxa (739 bp for P. (P.) rimatus and 729 bp for P. agenoris) (Table 1). When we compared the nucleotide similarities for these mitochondrial markers among all of the sequences obtained in this work, we noted that the intraspecific similarity ranged from 99% to 100%, whereas, the interspecific similarities between P. agenoris and P. (P.) rimatus were lower (80–85%), with the maximum nucleotide divergence between the two species observed in the cox2 analysis (Table 3, Table 4 and Table 5). In concordance with the nuclear marker analysis, the percentage of similarity among our sequences and other congeneric species retrieved from GenBank always appeared higher than 90%, in contrast to percentages observed among our sequences and those from other specimens belonging to Rhopalopsyllidae and Malacopsyllidae families, which had values lower than 90% (Table 3, Table 4 and Table 5).
3.3. Phylogenetic Results
The concatenated dataset of EF1−α and partial cytb, cox1 and cox2 gene sequences included 2059 aligned sites and 56 taxa, including P. agenoris and P. (P.) rimauts specimens from Argentina and the outgroup (P. meridionalis). The phylogenetic analysis of the concatenated dataset yielded a tree with strongly supported nodes (Figure 3). This analysis showed a well−supported clade comprising all genera and species belonging to Rhopalopsyllidae and Malacopsyllidae families. Within this group, we note three well−defined subclades. The first one included all Malacopsyllidae taxa (P. agenoris and M. grossiventris), clustering phylogenetically close to the second one, which comprised some genera belonging to the Rhopalopsyllidae family (Polygenis sp., Ectinorus sp., Listronius sp. and Parapsyllus sp.) (Figure 3). Finally, Tetrapsyllus thombus and Tetrapsyllus maulinus (Rhopalopsyllidae) set up the third subclade within the Rhopalopsyllidae–Malacopsyllidae group, which appeared phylogenetically separated from the remaining families (Cthenophthalmidae, Pulicidae, Stenoponiidae, Ceratophyllidae, Stephanocircidae, Hystrichopsyllidae, Vermipsyllidae, Pygiopsyllidae and Stivaliidae) (Figure 3).





Table 2.
Intraspecific (*) and interspecific similarities observed among all partial EF1−α gene sequences of nuclear DNA of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
Table 2.
Intraspecific (*) and interspecific similarities observed among all partial EF1−α gene sequences of nuclear DNA of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
| EF1−α | P. agenoris (This Study) OU706239−40 | P. (P.) rimatus (This Study) OU706241−42 | Polygenis rimatus EU336290 | Polygenis pradoi EU336289 | Polygenis roberti roberti KM890524 | Malacopsylla grossiventris KM890469 | Tetrapsyllus sp. KM890506 KM890507 | Ectinorus sp. KM890519 KM890515 KM890512 EU336294 | Listronius fortis KM890511 | Parapsyllus sp. AF423872 EU336266 | Tiamastus cavicola EU336279 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. agenoris (this study) OU706239−40 | 99.8–100 * | ||||||||||
| P. (P.) rimatus (this study) OU706241−42 | 80.4–80.7 | 99.4–100 * | |||||||||
| Polygenis rimatus EU336290 | 80.7–80.9 | 98.0–98.1 | − | ||||||||
| Polygenis pradoi EU336289 | 82.6–82.8 | 90.8–91.0 | 90.0 | − | |||||||
| Polygenis roberti roberti KM890524 | 81.0–81.1 | 97.6–97.7 | 97.4 | 91.4 | − | ||||||
| Malacopsylla_grossiventris KM890469 | 80.6–80.7 | 83.2–83.4 | 83.7 | 83.2 | 83.0 | − | |||||
| Tetrapsyllus sp. KM890506 KM890507 | 81.9–82.5 | 84.0–84.4 | 84.4 | 82.3–85.9 | 84.0–84.7 | 84.2–84.8 | 96.6 | ||||
| Ectinorus sp. KM890519 KM890515 KM890512 EU336294 | 80.0–81.4 | 83.0–85.9 | 83.1–85.8 | 83.2–87.4 | 82.5–85.4 | 86.6–89.7 | 84.4–88.7 | 87.1–90.4 | |||
| Listronius fortis KM890511 | 81.3–81.4 | 86.5–86.6 | 86.6 | 87.3 | 87.1 | 88.0 | 87.3–88.0 | 88.2–90.9 | − | ||
| Parapsyllus sp. AF423872 EU336266 | 82.0–82.6 | 84.5–85.4 | 85.2–85.4 | 85.0–86.4 | 84.9–85.4 | 86.0–88.5 | 85.5–87.1 | 86.4–89.7 | 88.7–89.4 | 90.8 | |
| Tiamastus cavicola EU336279 | 83.0–83.1 | 86.3–86.5 | 86.8 | 90.0 | 87.4 | 84.4 | 85.0–85.9 | 84.5–86.7 | 87.1 | 86.186.3 | − |

Table 3.
Intraspecific (*) and interspecific similarities observed among all partial cox1 mtDNA gene sequences of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
Table 3.
Intraspecific (*) and interspecific similarities observed among all partial cox1 mtDNA gene sequences of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
| Cox1 | P. agenoris (This Study) OU706243 | P. (P.) rimatus (This Study) OU706244−45 | P. agenoris KM891005, KM890899 | Polygenis roberti roberti KM890958 | Malacopsylla_grossiventris KM890898 | Ectinorus sp. KM890943, KM890949 | Rhopalopsyllus australis KM890994 | Listronius fortis KM890945 | Tetrapsyllus sp. KM890937−38 | Parapsyllus humboldti MK104348 |
|---|---|---|---|---|---|---|---|---|---|---|
| P. agenoris (this study) OU706243 | 100 * | |||||||||
| P. (P.) rimatus (this study) OU706244−45 | 83.2–83.5 | 99.8–100 * | ||||||||
| P. agenoris KM891005, KM890899 | 99.2 | 83–83.5 | 99.5 * | |||||||
| Polygenis roberti roberti KM890958 | 83.5 | 93.4–93.6 | 83.7–83.9 | − | ||||||
| Malacopsylla_grossiventris KM890898 | 88.0 | 86.1–86.3 | 88.4–88.6 | 84.6 | − | |||||
| Ectinorus sp. KM890943, KM890949 | 85.6–85.9 | 83.7–83.9 | 85.1–86.1 | 83.0–83.5 | 84.6–86.1 | 84.0 | ||||
| Rhopalopsyllus australis KM890994 | 86.1 | 83.7–83.9 | 85.9 | 83.7 | 86.5 | 80.9–83.9 | − | |||
| Listronius fortis KM890945 | 86.3 | 83.0 | 86.1–86.3 | 83.0 | 84.8 | 83.9–85.6 | 83.2 | − | ||
| Tetrapsyllus sp. KM890937−38 | 83.2–85.6 | 82.8–83.0 | 82.6–85.3 | 80.9–81.1 | 85.4–88.4 | 78.4–84.8 | 81.3–85.1 | 80.9–81.3 | 88.4 | |
| Parapsyllus humboldti MK104348 | 84.8 | 83.7–83.9 | 85.1–85.3 | 83.7 | 88.6 | 84.6–87.7 | 84.6 | 82.6 | 81.9–86.7 | − |

Table 4.
Intraspecific (*) and interspecific similarities observed among all partial ctyb mtDNA gene sequences of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
Table 4.
Intraspecific (*) and interspecific similarities observed among all partial ctyb mtDNA gene sequences of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
| Cytb | P. agenoris (This Study) OU706744−45 | P. (P.) rimatus (This Study) OU706746, OU706743 | P. agenoris KM890590, KM890742 | Polygenis roberti roberti KM890693 | Malacopsylla_grossiventris KM890589 | Ectinorus sp. KM890676, KM890682−83 | Rhopalopsyllus australis KM890729 | Listronius fortis KM890675 | Tetrapsyllus sp. KM890670−71 | Parapsyllus longicornis KM890604 |
|---|---|---|---|---|---|---|---|---|---|---|
| P. agenoris (this study) OU706744−45 | 99.7–100 * | |||||||||
| P. (P.) rimatus (this study) OU706746, OU706743 | 84.0–84.3 | 99.4–100 * | ||||||||
| P. agenoris KM890590, KM890742 | 99.4–99.7 | 84.0 | 100* | |||||||
| Polygenis roberti roberti KM890693 | 82.8–83.1 | 92.3 | 82.8 | − | ||||||
| Malacopsylla_grossiventris KM890589 | 85.2–85.5 | 81.9 | 85.8 | 80.7 | − | |||||
| Ectinorus sp. KM890676, KM890682−83 | 83.4–85.5 | 81.0–83.4 | 84.0–85.8 | 81.6–82.8 | 84.0–86.6 | 84.3–86.1 | ||||
| Rhopalopsyllus australis KM890729 | 82.8–83.1 | 82.5 | 83.4 | 82.8 | 83.7 | 83.4–84.0 | − | |||
| Listronius fortis KM890675 | 83.4–83.7 | 85.5 | 84.0 | 84.6 | 83.4 | 83.4–87.2 | 86.0 | − | ||
| Tetrapsyllus sp. KM890670−71 | 81.9–83.7 | 81.3–82.8 | 82.5–84.0 | 80.1–81.3 | 79.2–81.6 | 81.9–84.3 | 81.9–85.5 | 84.3–85.8 | 91.7 | |
| Parapsyllus longicornis KM890604 | 85.5–85.8 | 85.8 | 86.0 | 83.7 | 89.0 | 86.3–90.0 | 84.6 | 87.5 | 82.8–87.2 | − |

Table 5.
Intraspecific (*) and interspecific similarities observed among all partial cox2 mtDNA gene sequences of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
Table 5.
Intraspecific (*) and interspecific similarities observed among all partial cox2 mtDNA gene sequences of P. agenoris and P. (P.) rimatus (obtained in this study) and different species and genera belonging to Rhopalopsyllidae and Malacopsyllidae families retrieved from GenBank database. Values are given in percentages.
| Cox2 | P. agenoris (This Study) OU707013−16 | P. (P.) rimatus (This Study) OU707017−18 | P. agenoris KM890763 | Polygenis pradoi AF424043 | Polygenis roberti roberti KM890830 | Malacopsylla grossiventris KM890589 | Ectinorus sp. KM890813 KM890816 EU336012 KM890820 | Rhopalopsyllus australis KM890865 | Listronius fortis KM890815 | Tetrapsyllus sp. KM890807−08 | Parapsyllus longicornis EU335985 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. agenoris (this study) OU707013−16 | 99.6–100 * | ||||||||||
| P. (P.) rimatus (this study) OU707017−18 | 80.0–80.8 | 99.6–100 * | |||||||||
| P. agenoris KM890763 | 99.2 | 80.0–80.4 | − | ||||||||
| Polygenis pradoi AF424043 | 79.8–80.2 | 91.6 | 80.2 | − | |||||||
| Polygenis roberti roberti KM890830 | 78.2–78.6 | 90.1 | 78.2 | 91.4 | − | ||||||
| Malacopsylla_grossiventris KM890589 | 85.9–86.3 | 81.1–81.5 | 86.1 | 82.1 | 80.8 | − | |||||
| Ectinorus sp. KM890813 KM890816 EU336012 KM890820 | 82.3–85.2 | 80.2–84.6 | 83.1–84.8 | 80.6–84.6 | 78.6–83.1 | 81.7–85.5 | 85.0–89.0 | ||||
| Rhopalopsyllus australis KM890865 | 82.3–82.7 | 86.5–86.9 | 82.3 | 86.3 | 85.2 | 84.4 | 80.0–86.5 | − | |||
| Listronius fortis KM890815 | 82.5–82.9 | 80.6 | 82.3 | 80.6 | 78.9 | 82.3 | 83.3–85.0 | 82.9 | − | ||
| Tetrapsyllus sp. KM890807−08 | 81.3–81.9 | 78.2–81.5 | 81.3–81.5 | 79.1–79.8 | 79.1–79.3 | 81.3–82.9 | 81.5–84.8 | 81.7–82.9 | 80.0–81.1 | 91.8 | |
| Parapsyllus longicornis EU335985 | 84.4–84.8 | 84.4–84.8 | 84.2 | 83.5 | 84.8 | 84.8 | 89.7–91.8 | 85.5 | 84.8 | 84.0 | − |
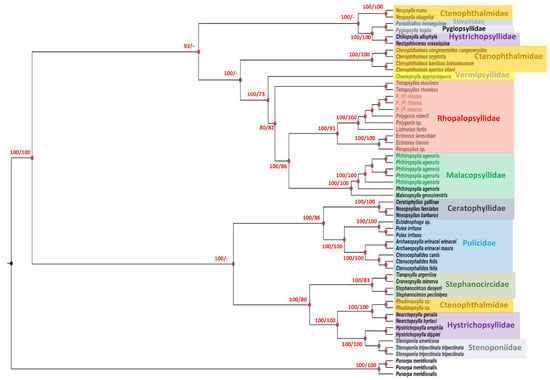
Figure 3.
Phylogenetic tree of P. agenoris (bold and green) and P. (P) rimatus (bold and red) specimens assessed in this study (see Table 1). This analysis was based on concatenated sequences of partial elongation factor 1 alpha (EF1−α) from nuclear DNA and partial cytochrome c oxidase subunit 1 (cox1), cytochrome c oxidase subunit 2 (cox2) and cytochrome b (cytb) genes from mitochondrial DNA inferred using the Bayesian inference (BI) and maximum likelihood (ML) methods and Bayesian topology. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown on the branches (BPP/bootstrap). The Bayesian posterior probabilities (BPPs) are converted to percentages. BPP and bootstrap values lower than 60% are not shown.
4. Discussion
Phthiropsylla agenoris was originally described as Malacopsylla agenoris on the basis of female and male specimens collected from Dasypus sp. in Cruz del Eje, Córdoba Province, Argentina [7]. Originally, the genus Phthiropsylla was described to include only this species and differentiate it from Malacopsylla [29]. Currently, the species, as well as the family, is considered endemic to Argentina. On the other hand, P. (P.) rimatus was originally described as Pulex bohlsi [30] from female specimens collected from the marsupial Didelphis sp. from Sapucay, Paraguay. Subsequently, the species was moved to Rhopalopsyllus bohlsi (Jordan and Rothschild, 1908) [31] based on specimens collected from Paraguay and Argentina, and later specimens previously thought to be R. bohlsi were described as a new species, Rhopalopsyllus rimatus [17]. Afterward, the species was included in the genus Polygenis as P. rimata by [32] and later renamed P. rimatus [33,34,35,36,37]. Linardi and Guimarães [38] proposed two subgenera within the Polygenis genus, Neopolygenis and Polygenis, and the species was renamed to its current name, P. (P.) rimatus. From the original description, new diagnostic features were described in order to differentiate P. (P.) rimatus from other sympatric congeneric species [39,40,41], and interspecific morphological variations in males and females were also provided that differentiate between specimens from Argentina and those from Brazil [42]. The species was also recorded in Paraguay and Peru, but most of the records were from Brazil and Argentina [8].
The morphological analysis conducted for specimens of P. (P.) rimatus assessed in this study agrees with figures and descriptions given by [31], on the basis of a specimen collected from Paraguay, as well as figures and descriptions presented in [43] and the original figures and descriptions given in [8] on the basis of the male paratype collected in Los Ingleses (Buenos Aires Province, Argentina). In the same way, the morphological description of P. agenoris provided in this study agrees with those described in [8] based on male and female paratypes collected in Rio Negro, Argentina.
The geographical origin of the samples analyzed in this study again confirmed the South−American distribution of Malacopsylloidea. In this sense, several authors [8,9,42,44] have reported that Malacopsyllidae and Rhopallopsyllidae are mainly located in the Neotropical region, although they could reach as far north as the southernmost United States, with one notable exception, the genus Parapsyllus, which has adapted to penguins and sea birds (albatrosses, fulmars, petrels, shags, prions and shearwaters) and has a pan−Antarctic distribution, primarily on Antarctica, Southern Hemisphere islands and the southern coastal areas of the southern continents [2].
The phylogenetic relationship between Malacopsyllidae and Rhopallopsyllidae was first mentioned in [44] and then by several authors [8,13,45,46]. As we mention in the Introduction section, these authors included both families within the Malacopsylloidea superfamily based exclusively on morphological features and their geographical distribution. The main morphological characteristic within the Malacopsylloidea group is the absence of ctenidia; however, this character is common in other families of fleas, such as Vermipsyllidae or Coptopsyllidae [47]. Thus, other morphological traits, some of them observed and shown in our study, have acquired an important diagnostic value within the Malacopsylloidea superfamily: the presence of an anterior branch of the tentorium, often joined to a ridge arising from the anterior margin of the eye; hind coxa without spiniform setae on the inner side; tooth at the apex of the outer side of the hind tibia, pointed; distitarsomeres with four or five (exceptionally three) pairs of lateral plantar setae; terga with one or two rows of setae; and only one antesensilial seta on each side [8,44]. In this study, we demonstrate the usefulness of these morphological features, described above, as diagnostic characters in order to differentiate between P. (P.) rimatus and P. agenoris.
On the other hand, this work has tried to assess, for the first time, the phylogenetic relationship between Malacopsyllidae and Rhopallopsyllidae from a molecular point of view. The combination of mitochondrial and nuclear markers as a useful taxonomic and phylogenetic tool has been more than sufficiently proven within the Siphonaptera field. Therefore, this approach has recently been used to clarify the taxonomic status of congeneric species and subspecies [48,49], to gain evolutionary insights on the cat flea, Ctenocephalides felis (Bouché, 1835) [50,51], or even to reconstruct a general Siphonaptera phylogeny [2].
Using molecular data and phylogenetic approaches, we could easily discriminate between the two taxa (P. agenoris and P. (P.) rimatus) by comparing both nuclear and mitochondrial markers. This fact proves the usefulness of ITS2, EF1−α, cox1, cytb and cox2 as molecular diagnostic markers to characterize and identify different Siphonaptera taxa, even when they belong to the same superfamily. In this sense, the molecular divergence observed between the two species appeared quite similar among all molecular markers assessed, always ranging from 80% to 85% nucleotide similarity. This molecular pattern was not expected since nuclear markers such as ITS2 and EF1−α are known to have higher sequence conservation than mitochondrial ones [52,53]. This fact has been widely expressed by several authors, who highlighted the fact that the inheritance properties of mtDNA make it more likely than any single nuclear marker to accurately reflect recent divergence, so it is used to show higher degrees of variability [54]. In addition, the difference observed in the length of ITS2 sequences could also be a useful strategy to discriminate between the two species, even more so when using fleas’ universal and conserved primers, such as those used in this study (see Table S1). Using these primers, if we review the large flea literature, we can note that the sequence lengths of cox1, cox2, cytb and EF1−α remain fixed at 658, 727–739, 374 and 975–976 bp, respectively [2,18,49,55]; however, ITS1 and ITS2 fragments appear quite variable within the Siphonaptera Order. Thus, we can observe sequence lengths of ITS2 ranging from 318 bp in Nosopsyllus barbarous (Jordan and Rothschild, 1912) and Nosopsyllus fasciatus (Bosc, 1800) [56] to 492 bp in different congeneric taxa belonging to Ctenophthalmus sp. [53] for the same nuclear marker. The results of the present study also agree with [57,58], who found different ITS sequence lengths in some flea populations belonging to Tungidae and Pulicidae families. Hence, our results reinforce the idea supported by several authors about the usefulness of ITSs as markers of choice for carrying out phylogenetic studies [59].
Our phylogenetic analysis based on a concatenated dataset was able to discriminate between P. agenoris and P. (P.) rimatus species, again proving the usefulness of the combination of nuclear and mitochondrial markers in order to discriminate among flea taxa belonging to different but closely related families.
The phylogenetic results confirm, for the first time, the monophyly of the Malacopsyllidae family, taking into account that this family consists of only two taxa, P. agenoris (assessed in this study) and M. grossiventris (sequences retrieved from the GenBank database). Therefore, this family could join the remaining 10 families, whose monophyletic origin was confirmed in [2]: Tungidae, Lycopsyllidae, Pygiopsyllidae, Stivaliidae, Stephanocircidae, Rhopalopsyllidae, Chimaeropsyllidae, Pulicidae, Ischnopsyllidae and Ceratophyllidae. Regarding the Rhopalopsyllidae family, we note two well−supported subclades, one of them corresponding to the Parapsyllinae subfamily (Ectinorus sp. and Parapsyllus sp.) and a second one clustering Listronius sp. (Parapsyllinae) and Polygenis spp. (Rhopalopsyllinae). In addition, the phylogenetic position of Tetrapsyllus (Parapsyllinae) remains controversial since this genus clustered separately from the Malacopsyllidae and Rhopalopsyllidae clades. This fact was also observed in the phylogenetic tree provided in [60] and could suggest the clear paraphyletic position of the Paraspsyllinae subfamily and, consequently, of the Rhopalopsyllidae family. For that reason, we suggest the necessity to provide further molecular and phylogenetic analyses based on different taxa belonging to Rhopalopsyllidae, specifically focused on the Tetrapsyllus genus, in order to clarify the taxonomic and phylogenetic position of this family and genus in particular. Recently, Ref. [61] provided an exhaustive morphological key to identify different Tetrapsyllus species, as well as a phylogenetic analysis based on different morphological traits. These authors observed a monophyletic origin for this genus, closely related to Ectinorus sp.; however, no taxonomic studies of Tetrapsyllus sp. based on molecular data have been published so far.
Finally, our phylogenetic analysis confirms the close relationship between Malacopsyllidae and Rhopalopsyllidae within the taxonomy of the Siphonaptera Order, both clustering in the same clade corresponding to the Malacopsylloidea group (BPP/Bootstrap−100/86). Additionally, our phylogenetic tree also agrees with the taxonomy and systematics provided in [12] and [2] since the Vermipsyllidae family appeared as a sister group of Malacopsylloidea, and we reinforce the theory of the paraphyletic origin of Hystrichopsyllidae and Ctenophthalmidae [2].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d15020308/s1, Table S1: PCR mix, primers and conditions used for each molecular marker sequenced in this study. Table S2: List of taxa used in the analysis, including GenBank accession numbers and taxonomic information. References [62,63,64] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, A.Z., M.L. and C.C.; methodology, A.Z. and M.L.; software, A.Z. and M.L.; validation, M.L. and C.C.; formal analysis, A.Z. and M.L.; investigation, A.Z., M.L. and C.C.; resources, M.L. and C.C.; data curation, A.Z. and M.L.; writing—original draft preparation, A.Z. and M.L.; writing—review and editing, A.Z., M.L. and C.C.; visualization, M.L. and C.C.; supervision, M.L. and C.C.; project administration, M.L. and C.C.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the manuscript and Supplementary Materials.
Acknowledgments
The authors thank Ulyses Pardiñas (IDEAus, Argentina), Carlos Galliari (CEPAVE), M. del Rosario Robles (CEPAVE), Pablo Teta (Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Argentina) and Agustín Abba (CEPAVE) for their collaboration in fieldwork; G. Galliari and U. Pariñas for the identification of the hosts; Luis Giambelluca (CEPAVE) for the photographs; M. Laura Morote (CEPAVE) for editing figures; and Theresa Howard and Erica McAlister (NHM) for their assistance to M.L. during her visit to study specimens deposited at the Rothschild Collection at the Natural History Museum (NHM), London. Fieldwork and the visit of M.L. to the NHM were funded by the Universidad Nacional de La Plata and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Linardi, P.M.; Guimarães, L.R. Sifonápteros do Brasil; Museum of Zoology, University of Sao Paulo: São Paulo, Brazil, 2000. [Google Scholar]
- Whiting, M.F.; Whiting, A.S.; Hastriter, M.W.; Dittmar, K. A molecular phylogeny of fleas (Insecta: Siphonaptera): Origins and host associations. Cladistics 2008, 24, 677–707. [Google Scholar]
- Zurita, A.; Callejón, R.; De Rojas, M.; Gómez−López, M.S.; Cutillas, C. Molecular study of Stenoponia tripectinata tripectinata (Siphonaptera: Ctenophthalmidae: Stenoponiinae) from the Canary Islands: Taxonomy and phylogeny. Bull. Entomol. Res. 2015, 104, 704–711. [Google Scholar]
- Zurita, A.; García−Sánchez, A.M.; Cutillas, C. Ctenophthalmus baeticus boisseauorum (Beaucournu, 1968) and Ctenophthalmus apertus allani (Smit, 1955) (Siphonaptera: Ctenophthalmidae) as synonymous taxa: Morphometric, phylogenetic, and molecular characterization. Bull. Entomol. Res. 2020, 110, 663–676. [Google Scholar]
- Zurita, A.; García-Sánchez, A.M.; Cutillas, C. Comparative molecular and morphological study of Stenoponia tripectinata tripectinata (Siphonaptera: Stenoponiidae) from the Canary Islands and Corsica. Bull. Entomol. Res. 2022, 112, 681–690. [Google Scholar]
- Weyenbergh, H. The Argentine fauna—Description d’une puce gigantesque, Pulex grossiventris, m. Boletín Acad. Nac. Cienc. 1879, 3, 188–193. [Google Scholar]
- Rothschild, N.C. Further contributions to the knowledge of the Siphonaptera. Novit. Zool. 1904, 11, 602–653. [Google Scholar]
- Smit, F.G.A.M. An Illustrated Catalogue of the Rothschild fleas (Siphonaptera) in the British Museum (Natural History) 7: Malacopsylloidea (Malacopsyllidae and Rhopalopsyllidae); Oxford University Press: Oxford, UK, 1987; 380p. [Google Scholar]
- Lareschi, M.; Autino, A.; Sanchez, J. A review of the fleas (Insecta− Siphonaptera) from Argentina. Zootaxa 2016, 3, 239–258. [Google Scholar]
- Smit, F.G.A.M. On some adaptative structures in Siphonaptera. Folia Parasitol. 1972, 19, 5–17. [Google Scholar]
- Ezquiaga, M.C.; Lareschi, M. Surface Ultrastructure of the Eggs of Malacopsylla grossiventris and Phthiropsylla agenoris (Siphonaptera: Malacopsyllidae). J. Parasitol. 2012, 98, 1029–1031. [Google Scholar]
- Medvedev, S.G. Morphological basis of the classification of fleas (Siphonaptera). Entomol. Rev. 1994, 73, 30–51. [Google Scholar]
- Lewis, R.E. Notes on the geographical distribution and host preferences in the order Siphonaptera. Part 8. New taxa described between 1984 and 1990, with a current classification of the order. Entomol. Soc. Am. 1993, 30, 239–256. [Google Scholar]
- Horta, M.C.; Labruna, M.B.; Pinter, A.; Linardi, P.M.; Schumaker, T.T. Rickettsia infection in five areas of the state of São Paulo, Brazil. Memórias Inst. Oswaldo Cruz 2007, 102, 793–801. [Google Scholar]
- Peniche−Lara, G.; Dzul−Rosado, K.; Perez−Osorio, C.; Zavala−Castro, J. Rickettsia typhi in rodents and R. felis in fleas in Yucatán as a possible causal agent of undefined febrile cases. Rev. Inst. Med. Trop. São Paulo 2015, 57, 129–132. [Google Scholar]
- Melis, M.; Espinoza−Carniglia, M.; Savchenko, E.; Nava, S.; Lareschi, M. Molecular detection and identification of Rickettsia felis in Polygenis (Siphonaptera, Rhopalopsyllidae, Rhopalopsyllinae) associated with cricetid rodents in a rural area from central Argentina. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100445. [Google Scholar]
- Jordan, K. Notes on Siphonaptera. Novit. Zool. 1932, 38, 291–294. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MrBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar]
- Posada, D. Jmodeltest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar]
- Huelsenbeck, J.P.; Rannala, B. Phylogenetic methods come of age: Testing hypotheses in an evolutionary context. Science 1997, 276, 227–232. [Google Scholar]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar]
- Rambaut, A.; Drummond, A. Tracer v1.6. 2007. Available online: http://beast.bio.ed.ac.uk (accessed on 4 December 2021).
- Rambaut, A.; Drummond, A. FigTree Version 1.4.4. 2018. Available online: https://github.com/rambaut/figtree/releases (accessed on 5 December 2021).
- Whiting, M.F. Mecoptera is paraphyletic: Multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool. Scr. 2002, 31, 93–104. [Google Scholar]
- Wagner, J. Bermerkungen über die Fam. Malacopsyllidae und Beschreibung der neuen Arten. Z. Parasitenk. 1939, 11, 58–67. [Google Scholar]
- Wagner, J. Aphanipterologische Studien. IV. Beschreibung neuer Arten der Gattungen Ceratophyllus, Pulex und Typhlopsylla. Trudy Russk. Ent. Obshch. 1901, 35, 17–29. [Google Scholar]
- Jordan, K.; Rothschild, N.C. Revision of the non−combed eyed Siphonaptera. Parasitology 1908, 1, 1–100. [Google Scholar]
- Guimarães, L.R. Sôbre algumas espécies de pulgas brasileiras. Papéis Avulsos Zool. 1942, 2, 197–203. [Google Scholar]
- Costa Lima, D.A.; Hathaway, C.R. Pulgas. Bibliografía, catálogo e animais por elas sugados. Monogr. Inst. Oswaldo Cruz 1964, 4, 522. [Google Scholar]
- Guimarães, L.R. Sôbre algumas espécies do gènero Polygenis Jordan, 1939 (Pulicidae−Suctoria). Arq. Zool. 1948, 5, 539–552. [Google Scholar]
- Capri, J.J.; Capri, N.A.R. Suctoria. Prim. J. Entom. Argent. 1960, 2, 581–586. [Google Scholar]
- Del Ponte, E. Notas sobre Suctoria argentinos V. Nuevos datos sobre Rhopalopsyllidae, Rhopalopsyllinae. Rev. Soc. Entomol. Argent. 1963, 26, 75–87. [Google Scholar]
- Gomes, A.C. Pulgas colhidas em residências e sobre pequenos animais de algumas áreas do Brasil. Rev. Bras. Malariol. Doenças Trop. 1969, 21, 775–779. [Google Scholar]
- Linardi, P.M.; Guimarães, L.R. Systematic review of genera and subgeneraof Rhopalopsyllinae (Siphonaptera: Rhopalopsyllidae) by phonetic and cladistics methods. J. Med. Entomol. 1993, 30, 161–170. [Google Scholar]
- Linardi, P.M. Utilização de algumas estruturas na caracterização de espécies da ordem Siphonaptera. I. A fratura da mesocoxa na separação de espécies de Polygenis Jordan 1939. Rev. Bras. Entomol. 1981, 25, 27–29. [Google Scholar]
- Linardi, P.M. Utilização de algumas estruturas na caracterização de espécies da ordem Siphonaptera. III. A variabilidade do braço ventral do esternito IX em Polygenis rimatus e suas implicaçoes taxonômicas. Rev. Bras. Entomol. 1984, 28, 261–262. [Google Scholar]
- Hastriter, M.W.; Peterson, N.E. Notes on some fleas (Siphonaptera) from Amazonas and Bahia States, Brazil. Entomol. News 1997, 108, 290–296. [Google Scholar]
- Lareschi, M.; Linardi, P.M. New data on the morphology of Polygenis (Polygenis) rimatus (Jordan) (Siphonaptera: Rhopalopsyllidae). Neotrop. Entomol. 2005, 34, 121−125. [Google Scholar]
- Jordan, K.; Rothschild, N.C. On the genera Rhopalopsyllus and Parapsyllus. Ectoparasites 1923, 1, 320–370. [Google Scholar]
- Baker, C.F. The classification of the Southamerican siphonaptera. Proc. U. S. Natl. Mus. 1905, 29, 121–170. [Google Scholar]
- Medvedev, S.G. Classification of fleas (Order Siphonaptera) and its theoretical foundations. Entomol. Rev. 1998, 78, 1080–1093. [Google Scholar]
- Smit, F.G.A.M. Key to the genera and subgenera of Ceratophyllidae. In Key to the Genera and Subgenera of Ceratophyllidae; Traub, R., Rothschild, M., Haddow, J., Eds.; Academic Press: New York, NY, USA, 1983; pp. 1–37. [Google Scholar]
- Beaucournu, J.C.; Launay, H. Les Puces (Siphonaptera) de France et du BassinMéditerranéen Occidental, Faune de France; Fedération Française des Sociétés des Sciences Naturelles: Paris, France, 1990; Volume 76. [Google Scholar]
- Zurita, A.; Cutillas, C. Combination of nuclear and mitochondrial markers as a useful tool to identify Ctenophthalmus species and subspecies (Siphonaptera: Ctenophthalmidae). Org. Divers. Evol. 2021, 21, 547–559. [Google Scholar]
- Zurita, A.; Rivero, J.; García−Sánchez, A.M.; Callejón, R.; Cutillas, C. Morphological, molecular and phylogenetic characterization of Leptopsylla segnis and Leptopsylla taschenbergi (Siphonaptera). Zool. Scrip. 2022, 51, 741–754. [Google Scholar] [CrossRef]
- Lawrence, A.L.; Webb, C.E.; Clark, N.J.; Halajian, A.; Mihalca, A.D.; Miret, J.; D’Amico, G.; Brown, G.; Kumsa, B.; Modrý, D.; et al. Out-of-Africa, human-mediated dispersal of the common cat flea, Ctenocephalides felis: The hitchhiker’s guide to world domination. Int. J. Parasitol. 2019, 49, 321–336. [Google Scholar]
- Van der Mescht, L.; Matthee, S.; Matthee, C.A. New taxonomic and evolutionary insights relevant to the cat flea, Ctenocephalides felis: A geographic perspective. Mol. Phylogenetics Evol. 2021, 155, 106990. [Google Scholar]
- Friedlander, T.P.; Jerome, C.R.; Mitter, C. Phylogenetic information content of five nuclear gene sequences in animals: Initial assessment of character sets from concordance and divergence studies. Syst. Biol. 1994, 43, 511–525. [Google Scholar]
- Zurita, A.; Callejón, R.; García-Sánchez, Á.M.; Urdapilleta, M.; Lareschi, M.; Cutillas, C. Origin, evolution, phylogeny and taxonomy of Pulex irritans. Med. Vet. Entomol. 2019, 33, 296–311. [Google Scholar]
- Toews, D.P.; Brelsford, A. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar]
- Lawrence, A.L.; Brown, G.K.; Peters, B.; Spielman, D.S.; Morin-Adeline, M.; Slapeta, J. High phylogenetic diversity of the cat flea (Ctenocephalides felis) at two mitochondrial DNAmarkers. Med. Vet. Entomol. 2014, 28, 330–336. [Google Scholar]
- Zurita, A.; Callejón, R.; de Rojas, M.; Cutillas, C. Morphological and molecular study of the genus Nosopsyllus (Siphonaptera: Ceratophyllidae). Nosopsyllus barbarus (Jordan & Rothschild 1912) as a junior synonym of Nosopsyllus fasciatus (Bosc, d’Antic 1800). Insect Syst. Evol. 2018, 49, 81–101. [Google Scholar]
- Vobis, M.; D’Haese, J.; Mehlhorn, H.; Mencke, N.; Blagburn, B.L.; Bond, R.; Denholm, I.; Dryden, M.W.; Payne, P.; Rust, M.K.; et al. Molecular phylogeny of isolates of Ctenocephalides felis and related species based on analysis of ITS1, ITS2 and mitochondrial 16S rDNA sequences and random binding primers. Parasitol. Res. 2004, 94, 219–226. [Google Scholar]
- Ghavami, M.B.; Mirzadeh, H.; Mohammadi, J.; Fazaeli, A. Molecular survey of ITS1 spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol. Res. 2018, 117, 1433–1442. [Google Scholar]
- Calonje, M.; Martín-Bravo, S.; Dobes, C.; Gong, W.; Jordon-Thaden, I.; Kiefer, C.; Kiefer, M.; Paule, J.; Schmickl, R.; Koch, M.A. Non−coding nuclear DNA markers in phylogenetic reconstruction. Plant. Syst. Evol. 2009, 282, 257–280. [Google Scholar]
- Zhu, Q.; Hastriter, M.W.; Whiting, M.F.; Dittmar, K. Fleas (Siphonaptera) are cretaceous, and evolved with Theria. Mol. Phylogenet. Evol. 2015, 90, 129–139. [Google Scholar]
- Berrizbeitia, M.F.L.; Hastriter, M.W.; Barquez, R.M.; Díaz, M.M. Fleas of the genus Tetrapsyllus (Siphonaptera:Rhopalopsyllidae) associated with rodents from Northwestern Argentina. Int. J. Parasitol. Parasites Wildl. 2019, 9, 80–89. [Google Scholar]
- Luchetti, A.; Trentini, M.; Pampiglone, S.; Fiorawanti, M.L.; Mantovani, B. Genetic variability of Tunga penetrans (Siphonaptera, Tungidae) and fleas across South America and Africa. Parasitol. Res. 2007, 100, 593–598. [Google Scholar]
- Dittmar, K.; Whiting, M.F. Genetic and phylogeographic structure of populations of Pulex simulans (Siphonaptera) in Peru inferred from two genes (CytB and CoII). Parasitol. Res. 2003, 91, 55–59. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).