Density Estimates and Habitat Preferences of Two Sympatric Bird Species as Potential Bioindicators of Tropical Forest Alterations
Abstract
1. Introduction
2. Materials and Methods
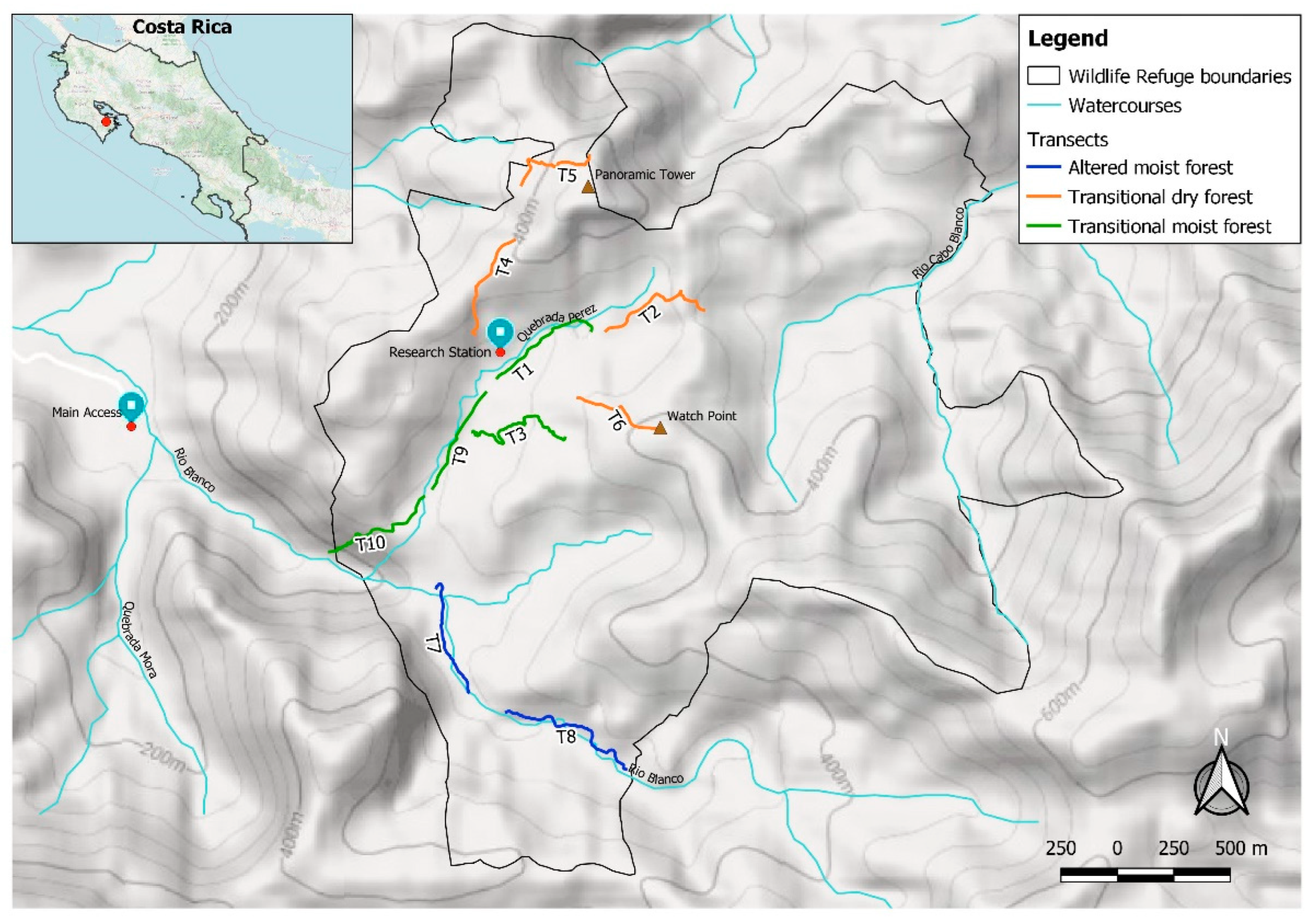
2.1. Study Area
2.2. Data Collection
2.3. Analyses
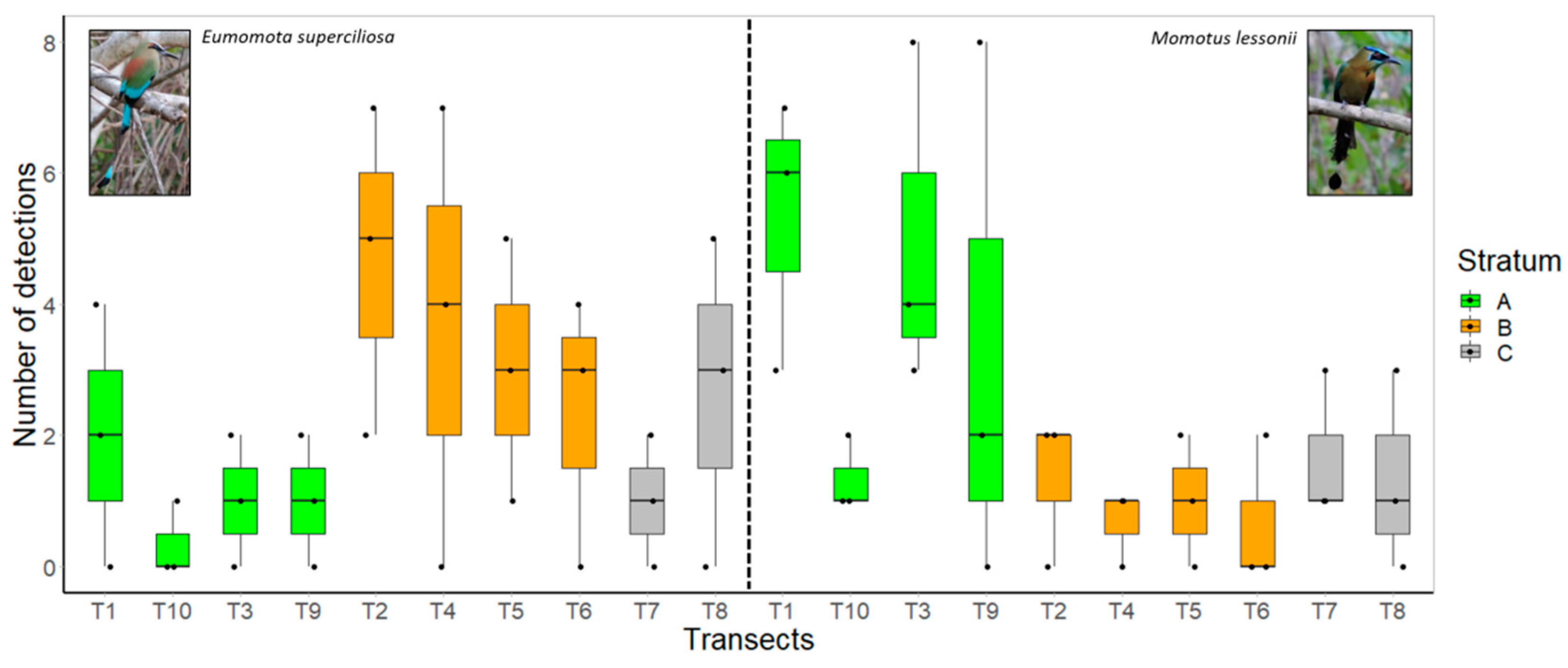
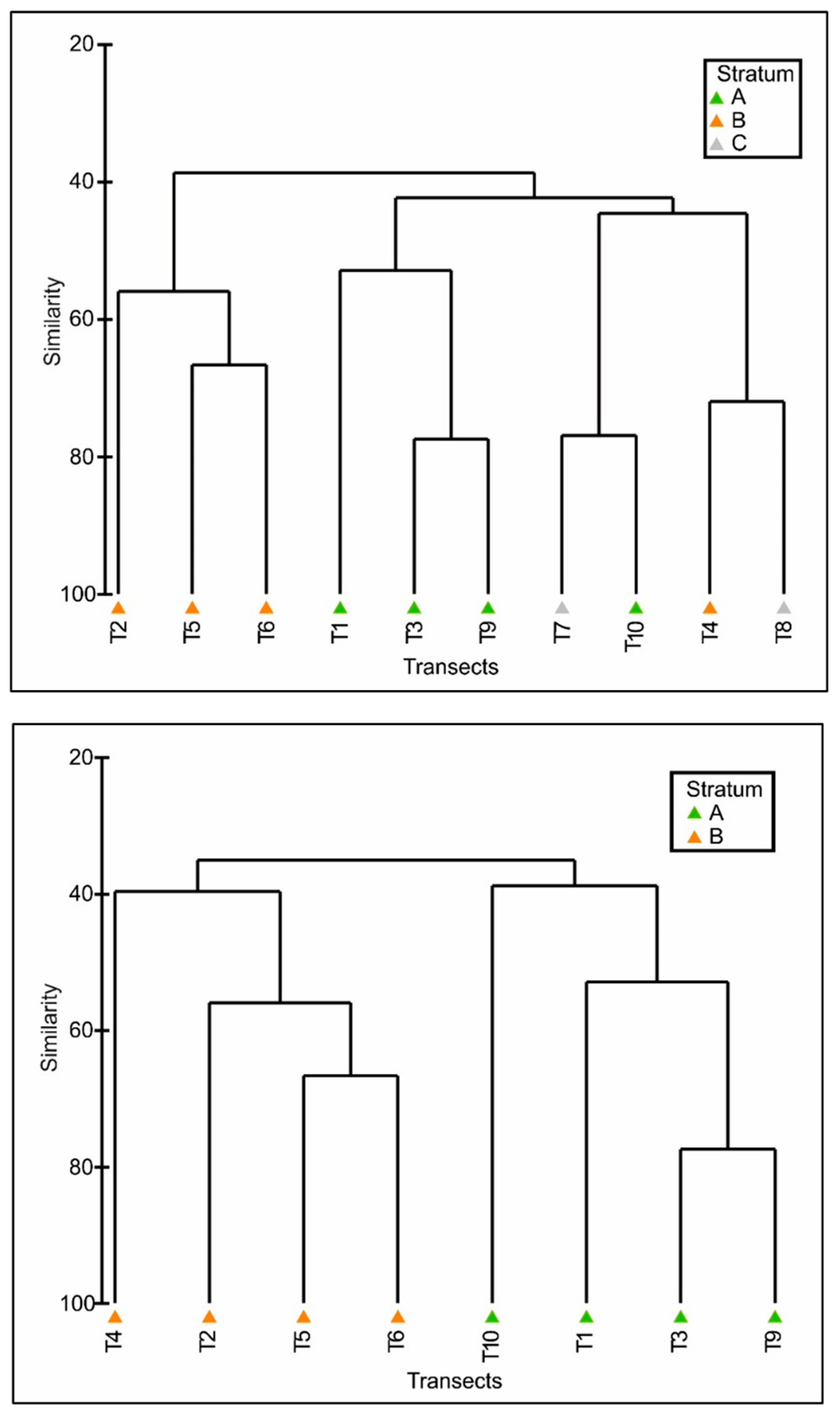
3. Results
Density Estimates and Relative Abundance
| Nr. | Species | Analysis | Model | k | w | n | ΔAIC | KSt GoF | CvMt GoF | p(χ2) | p | ESW | D ± SE | %CV | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | E. superciliosa | MCDS | Half Normal + Cosine | 3 | 210 | 60 | 0.00 | 0.59 | 0.60 < p ≤ 0.70 | 0.37 | 0.36 | 76.35 | 22.78 ± 5.70 | 25.01 | 13.43–38.64 |
| 2 | E. superciliosa | MCDS | Hazard Rate + Cosine | 5 | 210 | 60 | 1.09 | 0.51 | 0.40 < p ≤ 0.50 | 0.14 | 0.40 | 84.07 | 20.69 ± 5.09 | 24.60 | 12.26–34.91 |
| 3 | E. superciliosa | CDS | Half Normal + Cosine | 3 | 210 | 60 | 3.49 | 0.45 | 0.60 < p ≤ 0.70 | 0.43 | 0.41 | 86.27 | 20.16 ± 5.59 | 27.72 | 11.44–35.54 |
| 4 | M. lessonii | CDS | Hazard Rate | 2 | 210 | 65 | 0.00 | 0.64 | 0.50 < p ≤ 0.60 | 0.31 | 0.40 | 83.73 | 22.50 ± 6.50 | 28.88 | 12.38–40.91 |
| 5 | M. lessonii | MCDS | Hazard Rate | 3 | 210 | 65 | 0.09 | 0.60 | 0.70 < p ≤ 0.80 | 0.23 | 0.42 | 88.39 | 21.32 ± 5.65 | 26.49 | 12.09–37.57 |
| 6 | M. lessonii | MCDS | Half Normal | 2 | 210 | 65 | 1.13 | 0.41 | 0.40 < p ≤ 0.50 | 0.16 | 0.43 | 89.29 | 21.10 ± 5.57 | 26.41 | 11.98–37.17 |
4. Discussion
| Transect ID | Habitat | Length (km) | No. of Detections E. superciliosa | No. of Detections M. lessonii | KAI | Mean KAI/Transect | KAI | Mean KAI/Transect |
|---|---|---|---|---|---|---|---|---|
| E. superciliosa | E. superciliosa | M. lessonii | M. lessonii | |||||
| T1 | Moist forest | 0.55 | 0 | 6 | 0 | 3.64 | 10.91 | 9.70 |
| T1 | Moist forest | 0.55 | 4 | 7 | 7.27 | 12.73 | ||
| T1 | Moist forest | 0.55 | 2 | 3 | 3.64 | 5.45 | ||
| T3 | Moist forest | 0.73 | 1 | 3 | 1.37 | 1.37 | 4.11 | 6.85 |
| T3 | Moist forest | 0.73 | 0 | 4 | 0 | 5.48 | ||
| T3 | Moist forest | 0.73 | 2 | 8 | 2.74 | 10.96 | ||
| T9 | Moist forest | 0.5 | 0 | 0 | 0 | 2.00 | 0 | 6.67 |
| T9 | Moist forest | 0.5 | 1 | 2 | 2 | 4 | ||
| T9 | Moist forest | 0.5 | 2 | 8 | 4 | 16 | ||
| T10 | Moist forest | 0.64 | 0 | 1 | 0 | 0.52 | 1.56 | 2.08 |
| T10 | Moist forest | 0.64 | 1 | 1 | 1.56 | 1.56 | ||
| T10 | Moist forest | 0.64 | 0 | 2 | 0 | 3.12 | ||
| Mean | 1.88 | 6.32 | ||||||
| T2 | Dry forest | 0.6 | 7 | 2 | 11.67 | 7.78 | 3.33 | 2.22 |
| T2 | Dry forest | 0.6 | 5 | 0 | 8.33 | 0 | ||
| T2 | Dry forest | 0.6 | 2 | 2 | 3.33 | 3.33 | ||
| T4 | Dry forest | 0.53 | 0 | 1 | 0 | 6.92 | 1.89 | 1.26 |
| T4 | Dry forest | 0.53 | 4 | 0 | 7.55 | 0 | ||
| T4 | Dry forest | 0.53 | 7 | 1 | 13.21 | 1.89 | ||
| T5 | Dry forest | 0.47 | 5 | 0 | 10.64 | 6.38 | 0 | 2.13 |
| T5 | Dry forest | 0.47 | 1 | 2 | 2.13 | 4.25 | ||
| T5 | Dry forest | 0.47 | 3 | 1 | 6.38 | 2.13 | ||
| T6 | Dry forest | 0.43 | 4 | 0 | 9.3 | 5.43 | 0 | 1.55 |
| T6 | Dry forest | 0.43 | 3 | 2 | 6.98 | 4.65 | ||
| T6 | Dry forest | 0.43 | 0 | 0 | 0 | 0 | ||
| Mean | 6.63 | 1.79 |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Putz, F.E.; Blate, G.M.; Redford, K.H.; Fimbel, R.; Robinson, J. Tropical forest management and conservation of biodiversity: An overview. Conserv. Biol. 2001, 15, 7–20. [Google Scholar] [CrossRef]
- Kohlmann, B. Biodiversity conservation in Costa Rica-An animal and plant biodiversity atlas. In Research in Biodiversity-Models and Applications; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Calvo-Alvarado, J.; McLennan, B.; Sánchez-Azofeifa, A.; Garvin, T. Deforestation and Forest Restoration in Guanacaste, Costa Rica: Putting Conservation Policies in Context. For. Ecol. Manag. 2009, 258, 931–940. [Google Scholar] [CrossRef]
- Zahawi, R.A.; Duran, G.; Kormann, U. Sixty-Seven Years of Land-Use Change in Southern Costa Rica. PLoS ONE 2015, 10, e0143554. [Google Scholar] [CrossRef]
- Blake, J.G.; Loiselle, B.A. Bird Assemblages in Second-Growth and Old-Growth Forests, Costa Rica: Perspectives from Mist Nets and Point Counts. Auk 2001, 118, 304–326. [Google Scholar] [CrossRef]
- Casas, G.; Darski, B.; Ferreira, P.M.; Kindel, A.; Müller, S.C. Habitat Structure Influences the Diversity, Richness and Composition of Bird Assemblages in Successional Atlantic Rain Forests. Trop. Conserv. Sci. 2016, 9, 503–524. [Google Scholar] [CrossRef]
- Allen, L. The Application of Biodiversity indicators to Infer Ecosystem Health in Regenerating Tropical Forest. Doctoral Dissertation, University of Glasgow, Glasgow, UK, 2019. [Google Scholar]
- Gill, F.; Donsker, D.; Rasmussen, P. IOC World Bird List (V12. 2). 2022. Available online: https://www.worldbirdnames.org/new/ (accessed on 5 November 2022).
- Snow, D.W. Family Momotidae (Motmots). Handb. Birds World 2001, 6, 264–285. [Google Scholar]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Motmots (Momotidae), version 1.0. In Birds of the World 2022; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2022. [Google Scholar] [CrossRef]
- Gillespie, T.W. Life History Characteristics and Rarity of Woody Plants in Tropical Dry Forest Fragments of Central America. J. Trop. Ecol. 1999, 15, 637–649. [Google Scholar] [CrossRef]
- Huettmann, F. Central American Biodiversity: Conservation, Ecology, and a Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Janzen, D.H. Tropical Dry Forest: The Most Endangered Mayor Tropical Ecosystem. In Biodiversity; National Academy Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Powers, J.S.; Becknell, J.M.; Irving, J.; Perez-Aviles, D. Diversity and Structure of Regenerating Tropical Dry Forests in Costa Rica: Geographic Patterns and Environmental Drivers. For. Ecol. Manag. 2009, 258, 959–970. [Google Scholar] [CrossRef]
- Stiles, F.G. A Review of the Genus Momotus (Coraciiformes: Momotidae) in Northern South America and Adjacent Areas: Una Revisión Del Género Momotus (Coraciiformes: Momotidae) En El Norte de Sudamérica y Áreas Adyacentes. Ornitol. Colomb. 2009, 8, 29–75. [Google Scholar]
- Becker, J.J. A Fossil Motmot (Aves: Momotidae) from the Late Miocene of Florida. Condor 1986, 88, 478–482. [Google Scholar] [CrossRef]
- Chapman, F.M.; Watkins, H. The Distribution of the Motmots of the Genus Momotus; American Museum of Natural History: New York, NY, USA, 1923. [Google Scholar]
- De Pietri, V.L.; Mourer-Chauviré, C.; Menkveld-Gfeller, U.; Meyer, C.A.; Costeur, L. An Assessment of the Cenozoic Avifauna of Switzerland, with a Description of Two Fossil Owls (Aves, Strigiformes). Swiss J. Geosci. 2013, 106, 187–197. [Google Scholar] [CrossRef]
- McCullough, J.M.; Moyle, R.G.; Smith, B.T.; Andersen, M.J. A Laurasian Origin for a Pantropical Bird Radiation Is Supported by Genomic and Fossil Data (Aves: Coraciiformes). Proc. R. Soc. B 2019, 286, 20190122. [Google Scholar] [CrossRef]
- Olson, S.L. Oligocene Fossils Bearing on the Origins of the Todidae and the Momotidae (Aves: Coraciiformes). In Collected Papers in Avian Paleontology Honoring the 90th Birthday of Alexander Wetmore; Smithsonian Institution Press: Washington, DC, USA, 1976. [Google Scholar]
- Smith, S.M. Innate Recognition of Coral Snake Pattern by a Possible Avian Predator. Science 1975, 187, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.C. Rates of Molecular Evolution and Their Application to Neotropical Avian Biogeography; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2004. [Google Scholar]
- Korzun, L.P.; Érard, C.; Gasc, J.-P. Morphofunctional Study of the Bill and Hyoid Apparatus of Momotus momota (Aves, Coraciiformes, Momotidae): Implications for Omnivorous Feeding Adaptation in Motmots. Comptes Rendus Biol. 2004, 327, 319–333. [Google Scholar] [CrossRef]
- Pascotto, M.C.; Donatelli, R.J. Cranial Osteology in Momotidae (Aves: Coraciiformes). J. Morphol. 2003, 258, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.G. Lack of Assortative Mating for Tail, Body Size, or Condition in the Elaborate Monomorphic Turquoise-Browed Motmot (Eumomota superciliosa). Auk 2008, 125, 11–19. [Google Scholar] [CrossRef]
- Murphy, T.G. Predator-Elicited Visual Signal: Why the Turquoise-Browed Motmot Wag-Displays Its Racketed Tail. Behav. Ecol. 2006, 17, 547–553. [Google Scholar] [CrossRef]
- Murphy, T. Lack of Melanized Keratin and Barbs That Fall off: How the Racketed Tail of the Turquoise-Browed Motmot Eumomota superciliosa Is Formed. J. Avian Biol. 2007, 38, 139–143. [Google Scholar] [CrossRef]
- Murphy, T.G. Display of an Inedible Prop as a Signal of Aggression? Adaptive Significance of Leaf-Display by the Turquoise-Browed Motmot, Eumomota Superciliosa. Ethology 2008, 114, 16–21. [Google Scholar] [CrossRef]
- Murphy, T.G. Tail-Racket Removal Increases Hematocrit in Male Turquoise-Browed Motmots (Eumomota superciliosa). J. Ornithol. 2010, 151, 241–245. [Google Scholar] [CrossRef]
- Murphy, T.G.; Pham, T.T. Condition and Brightness of Structural Blue-Green: Motmot Tail-Racket Brightness Is Related to Speed of Feather Growth in Males, but Not in Females. Biol. J. Linn. Soc. 2012, 106, 673–681. [Google Scholar] [CrossRef]
- Nishikawa, E. The Wag-Display of the Blue-Crowned Motmot (Momotus momota) as a Predator-Directed Signal. Undergraduate Honors Thesis, University of Colorado Boulder, Boulder, CO, USA, 2011. [Google Scholar]
- Tubaro, P.L.; Mahler, B.; Lijtmaer, D.A. Patterns of Rectrix Rachis Modification in Pintails and the Evolution of Sexually Selected Traits. Biol. J. Linn. Soc. 2005, 86, 477–485. [Google Scholar] [CrossRef]
- Martin, R.F.; Scott, P.E.; Martin, M.W. Mate Fidelity and Breeding-Site Specificity of the Turquoise-Browed Motmot. Condor 1989, 91, 217–219. [Google Scholar] [CrossRef]
- Scott, P.E.; Martin, R.F. Reproduction of the Turquoise-Browed Motmot at Archaeological Ruins in Yucatan. Biotropica 1983, 8–14. [Google Scholar] [CrossRef]
- Scott, P.E.; Martin, R.F. Clutch Size and Fledging Success in the Turquoise-Browed Motmot. Auk 1986, 103, 8–13. [Google Scholar] [CrossRef]
- Skutch, A.F. Life History of the Turquoise-Browed Motmot. Auk 1947, 64, 201–217. [Google Scholar] [CrossRef]
- Skutch, A.F. Life History of the Blue-Diademed Motmot Momotus momota. IBIS 1964, 106, 321–332. [Google Scholar] [CrossRef]
- Chacón-Madrigal, E.; Barrantes, G. Blue-Crowned Motmot (Momotus momota) Predation on a Long-Tongued Bat (Glossophaginae). Wilson Bull. 2004, 116, 108–110. [Google Scholar] [CrossRef]
- García-C, J.M.; Zahawi, R.A. Predation by a Blue-Crowned Motmot (Momotus momota) on a Hummingbird. Wilson J. Ornithol. 2006, 118, 261–263. [Google Scholar] [CrossRef]
- Remsen, J.V., Jr.; Hyde, M.A.; Chapman, A. The Diets of Neotropical Trogons, Motmots, Barbets and Toucans. Condor 1993, 95, 178–192. [Google Scholar] [CrossRef]
- Sandoval-Comte, A.; Degante-González, A.P.; Santiago-Alarcon, D. Predation of White Anole (Anolis laeviventris) by Blue-Crowned Motmot (Momotus momota) in a Montane Forest Reserve in Veracruz, Mexico. Herpetol. Notes 2014, 7, 721–722. [Google Scholar]
- Solano-Ugalde, A.; Arcos-Torres, A. Nocturnal Foraging Observations of the Blue-Crowned Motmot (Momotus momota) in San José, Costa Rica. Wilson J. Ornithol. 2008, 120, 653–654. [Google Scholar] [CrossRef]
- Thurber, W.A.; Komar, O. Turquoise-Browed Motmot (Eumomota superciliosa) Feeds by Artificial Light. Wilson Bull. 2002, 114, 525–526. [Google Scholar] [CrossRef]
- Orejuela, J.E. Niche Relationships between Turquoise-Browed and Blue-Crowned Motmots in the Yucatan Peninsula, Mexico. Wilson Bull. 1980, 9, 229–244. [Google Scholar]
- Orejuela, J.E. Comparative Biology of Turquoise-Browed Motmot and Blue-Crowned Motmot in the Yucatan Peninsula Mexico. Living Bird 1977, 16, 193–208. [Google Scholar]
- Anciães, M.; Peterson, A.T. Climate Change Effects on Neotropical Manakin Diversity Based on Ecological Niche Modeling. Condor 2006, 108, 778–791. [Google Scholar] [CrossRef]
- Langen, T.A.; Berg, E.C. What Determines the Timing and Duration of the Nesting Season for a Tropical Dry Forest Bird, the White-Throated Magpie-Jay (Calocitta formosa)? Wilson J. Ornithol. 2016, 128, 32–42. [Google Scholar] [CrossRef]
- Wolfe, J.D.; Ralph, C.J.; Elizondo, P. Changes in the Apparent Survival of a Tropical Bird in Response to the El Niño Southern Oscillation in Mature and Young Forest in Costa Rica. Oecologia 2015, 178, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Dal Zotto, M.; Romeo, G.; Aguilar, L.A.M.; Sonetti, D.; Pederzoli, A. The Avian Community of the Karen Mogensen Reserve, a Wealth of Biodiversity within the Poorly Investigated and Threatened Environments of Northwestern Costa Rica. ZooKeys 2017, 722, 101–135. [Google Scholar] [CrossRef] [PubMed]
- Lombroso, L.; Teggi, S.; Despini, F.; Costanzini, S. Annuario Delle Osservazioni Meteoclimatiche Dell’anno 2018 Dell’Osservatorio Geofisico Di Modena: L’Osservatorio Restaurato. Atti Soc. Nat. Mat. Modena 2019, 150, 5–34. [Google Scholar]
- Griscom, H.P.; Ashton, M.S. Restoration of Dry Tropical Forests in Central America: A Review of Pattern and Process. For. Ecol. Manag. 2011, 261, 1564–1579. [Google Scholar] [CrossRef]
- Lugo, A.E.; Brown, S.L.; Dodson, R.; Smith, T.S.; Shugart, H.H. The Holdridge Life Zones of the Conterminous United States in Relation to Ecosystem Mapping. J. Biogeogr. 1999, 26, 1025–1038. [Google Scholar] [CrossRef]
- Gregory, R.D.; Gibbons, D.W.; Donald, P.F. Bird Census and Survey Techniques. In Bird Ecology and Conservation; Oxford University Press: Oxford, UK, 2004; pp. 17–56. [Google Scholar]
- Bibby, C.J.; Jones, M.; Marsden, S. Bird Surveys; Expedition Advisory Centre: London, UK, 1998. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 0-521-84462-2. [Google Scholar]
- Vorisek, P.; Klvanova, A.; Wotton, S.; Gregory, R. A Best Practice Guide for Wild Bird Monitoring Schemes; CSO/RSPB; JAVA: Třeboň, Czech Republic, 2008. [Google Scholar]
- Broekema, I.; Overdyck, O. Distance Sampling to Estimate Densities of Four Native Forest Bird Species during Multispecies Surveys. N. Z. J. Ecol. 2012, 36, 1. [Google Scholar]
- Buckland, S.T.; Marsden, S.J.; Green, R.E. Estimating Bird Abundance: Making Methods Work. Bird Conserv. Int. 2008, 18, S91–S108. [Google Scholar] [CrossRef]
- Buckland, S.T.; Rexstad, E.A.; Marques, T.A.; Oedekoven, C.S. Distance Sampling: Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2015; Volume 431. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L. Distance Sampling: Estimate Abundance of Biological Populations; Chapman and Hall: London, UK, 1993; University of St. Andrews; p. 448. [Google Scholar]
- Cassey, P.; Craig, J.L.; McArdle, B.H.; Barraclough, R.K. Distance Sampling Techniques Compared for a New Zealand Endemic Passerine (Philesturnus carunculatus rufusater). N. Z. J. Ecol. 2007, 31, 223–231. [Google Scholar]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.; Marques, T.A.; Burnham, K.P. Distance Software: Design and Analysis of Distance Sampling Surveys for Estimating Population Size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gale, G.A.; Round, P.D.; Pierce, A.J.; Nimnuan, S.; Pattanavibool, A.; Brockelman, W.Y. A Field Test of Distance Sampling Methods for a Tropical Forest Bird Community. Auk 2009, 126, 439–448. [Google Scholar] [CrossRef]
- Gottschalk, T.K.; Huettmann, F. Comparison of Distance Sampling and Territory Mapping Methods for Birds in Four Different Habitats. J. Ornithol. 2011, 152, 421–429. [Google Scholar] [CrossRef]
- Oostra, V.; Gomes, L.G.; Nijman, V. Implications of Deforestation for the Abundance of Restricted-Range Bird Species in a Costa Rican Cloud-Forest. Bird Conserv. Int. 2008, 18, 11–19. [Google Scholar] [CrossRef]
- Bibby, C.J.; Hill, D.A.; Burgess, N.D.; Mustoe, S. Bird Census Technique; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Gale, G.A.; Thongaree, S. Density Estimates of Nine Hornbill Species in a Lowland Forest Site in Southern Thailand. Bird Conserv. Int. 2006, 16, 57–69. [Google Scholar] [CrossRef]
- Shaw, P.; Shewry, M. Population Density and Habitat Associations of Restricted-Range Bird Species at Ruhija, Bwindi Impenetrable Forest, Uganda. Bird Conserv. Int. 2001, 11, 161–174. [Google Scholar] [CrossRef]
- Woog, F.; Renner, S.C.; Fjeldsa, J. Tips for Bird Surveys and Censuses in Countries without Existing Monitoring Schemes. ABC Taxa 2010, 8, 558–586. [Google Scholar]
- Marques, T.A.; Thomas, L.; Fancy, S.G.; Buckland, S.T. Improving Estimates of Bird Density Using Multiple-Covariate Distance Sampling. Auk 2007, 124, 1229–1243. [Google Scholar] [CrossRef]
- de Gabriel Hernando, M.; Roa, I.; Fernández-Gil, J.; Juan, J.; Fuertes, B.; Reguera, B.; Revilla, E. Trends in Weather Conditions Favor Generalist over Specialist Species in Rear-Edge Alpine Bird Communities. Ecosphere 2022, 13, e3953. [Google Scholar] [CrossRef]
- Thiollay, J.-M. Structure, Density and Rarity in an Amazonian Rainforest Bird Community. J. Trop. Ecol. 1994, 10, 449–481. [Google Scholar] [CrossRef]
- Terborgh, J.; Robinson, S.K.; Parker III, T.A.; Munn, C.A.; Pierpont, N. Structure and Organization of an Amazonian Forest Bird Community. Ecol. Monogr. 1990, 60, 213–238. [Google Scholar] [CrossRef]
- Johnson, E.I.; Stouffer, P.C.; Vargas, C.F. Diversity, Biomass, and Trophic Structure of a Central Amazonian Rainforest Bird Community. Rev. Bras. Ornitol. 2011, 19, 1–16. [Google Scholar]
- Robinson, S.K.; Terborgh, J. Bird Community Dynamics along Primary Successional Gradients of an Amazonian Whitewater River. Ornithol. Monogr. 1997, 48, 641–672. [Google Scholar] [CrossRef]
- Ribon, R.; Marini, M.Â. Small Territory Sizes and High Densities of Insectivorous Birds in an Atlantic Forest Secondary Fragment, Brazil. Rev. Bras. Ornitol. 2016, 24, 303–313. [Google Scholar] [CrossRef]
- Robinson, W.D.; Brawn, J.D. Forest bird community structure in central Panama: Influence of spatial scale and biogeography. Ecol. Monogr. 2000, 70, 209–235. [Google Scholar] [CrossRef]
- Miller, B.W.; Miller, C.M. New Information on the Status and Distribution of the Keel-Billed Motmot Electron carinatum in Belize, Central America. Cotinga 1996, 6, 61–64. [Google Scholar]
- Renner, S.C. The Blue-Throated Motmot (Aspatha gularis) in the Central Cloud Forests of Guatemala: An Indicator for Primary Forest? Boletín Soc. Antiq. Ornitol. 2005, 15, 16–25. [Google Scholar]
- Murphy, P.G.; Lugo, A.E. Ecology of Tropical Dry Forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- Sánchez-Azofeifa, G.A.; Kalacska, M.; do Espírito-Santo, M.M.; Fernandes, G.W.; Schnitzer, S. Tropical Dry Forest Succession and the Contribution of Lianas to Wood Area Index (WAI). For. Ecol. Manag. 2009, 258, 941–948. [Google Scholar] [CrossRef]
- Sanchez-Azofeifa, A.; Powers, J.S.; Fernandes, G.W.; Quesada, M. Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Garrigues, R.; Dean, R. The Birds of Costa Rica; Cornell University Press: Ithaca, NY, USA, 2014. [Google Scholar]
- Stiles, F.G.; Skutch, A.F. Guide to the Birds of Costa Rica; Cornell University Press: Ithaca, NY, USA, 1989. [Google Scholar]
- Visco, D.M.; Michel, N.L.; Boyle, W.A.; Sigel, B.J.; Woltmann, S.; Sherry, T.W. Patterns and Causes of Understory Bird Declines in Human-Disturbed Tropical Forest Landscapes: A Case Study from Central America. Biol. Conserv. 2015, 191, 117–129. [Google Scholar] [CrossRef]
- Chapman, C.A.; Chapman, L.J. Density and Growth Rate of Some Tropical Dry Forest Trees: Comparisons between Successional Forest Types. Bull. Torrey Bot. Club 1990, 117, 226–231. [Google Scholar] [CrossRef]
- Fagan, M.E.; DeFries, R.S.; Sesnie, S.E.; Arroyo, J.P.; Walker, W.; Soto, C.; Chazdon, R.L.; Sanchun, A. Land Cover Dynamics Following a Deforestation Ban in Northern Costa Rica. Environ. Res. Lett. 2013, 8, 034017. [Google Scholar] [CrossRef]
- Gibbs, H.K.; Ruesch, A.S.; Achard, F.; Clayton, M.K.; Holmgren, P.; Ramankutty, N.; Foley, J.A. Tropical Forests Were the Primary Sources of New Agricultural Land in the 1980s and 1990s. Proc. Natl. Acad. Sci. USA 2010, 107, 16732–16737. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Jetz, W. Avian Distributions under Climate Change: Towards Improved Projections. J. Exp. Biol. 2010, 213, 862–869. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, D.; Fonda, F.; Monti, F.; Dal Zotto, M. Density Estimates and Habitat Preferences of Two Sympatric Bird Species as Potential Bioindicators of Tropical Forest Alterations. Diversity 2023, 15, 208. https://doi.org/10.3390/d15020208
Lopez D, Fonda F, Monti F, Dal Zotto M. Density Estimates and Habitat Preferences of Two Sympatric Bird Species as Potential Bioindicators of Tropical Forest Alterations. Diversity. 2023; 15(2):208. https://doi.org/10.3390/d15020208
Chicago/Turabian StyleLopez, Dayron, Federica Fonda, Flavio Monti, and Matteo Dal Zotto. 2023. "Density Estimates and Habitat Preferences of Two Sympatric Bird Species as Potential Bioindicators of Tropical Forest Alterations" Diversity 15, no. 2: 208. https://doi.org/10.3390/d15020208
APA StyleLopez, D., Fonda, F., Monti, F., & Dal Zotto, M. (2023). Density Estimates and Habitat Preferences of Two Sympatric Bird Species as Potential Bioindicators of Tropical Forest Alterations. Diversity, 15(2), 208. https://doi.org/10.3390/d15020208








