The Curious Case of Fritillaria sonnikovae (Liliaceae) in South Siberia: New Insights into Its Origin and Phylogeny
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution Data and Plant Material Collection
2.2. DNA Isolation, PCR
2.3. Cloning and Sequencing
2.4. Sequence Alignment and Phylogenetic Analysis
3. Results
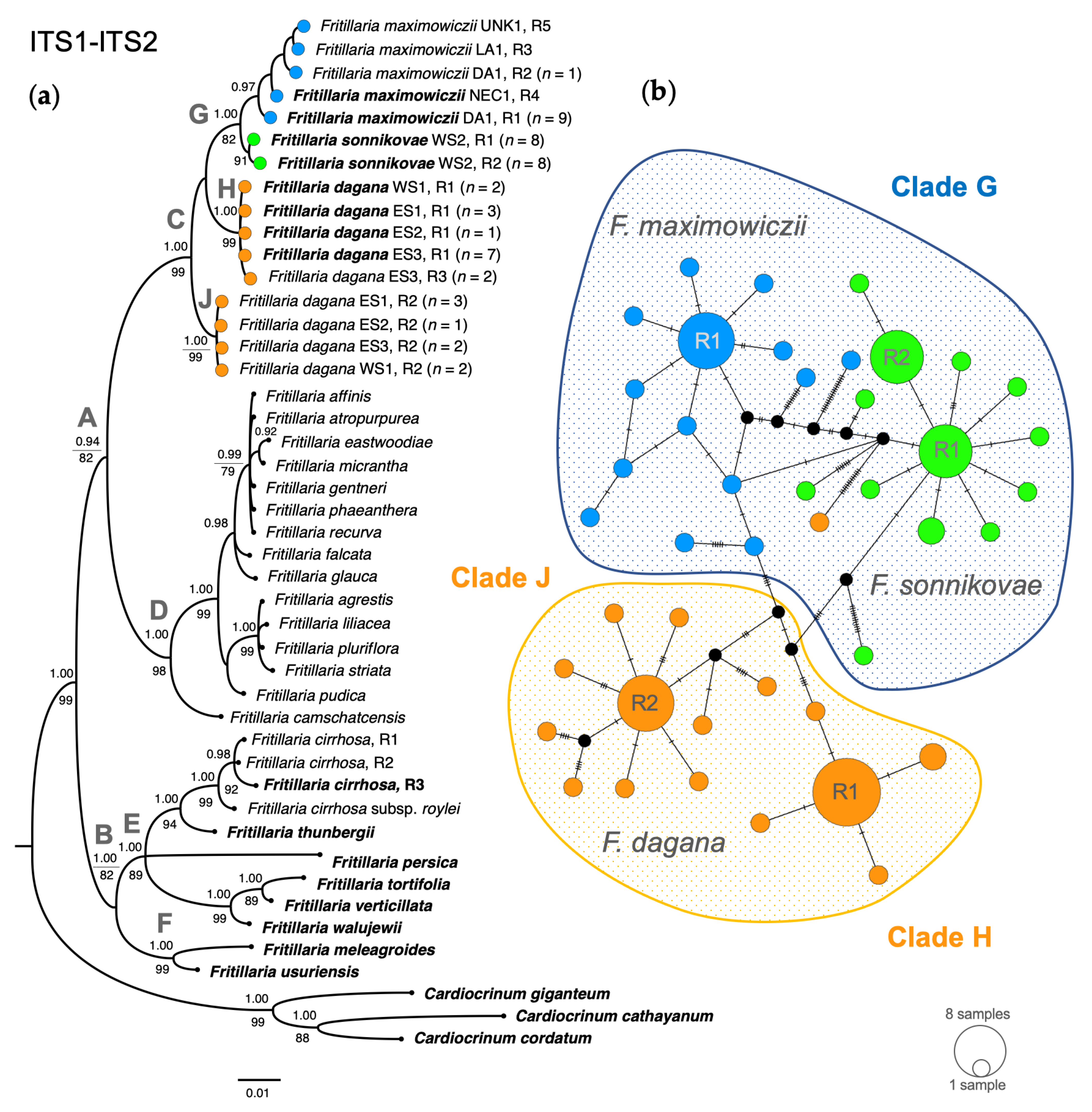
3.1. Phylogenetic Analysis Based on Nuclear DNA
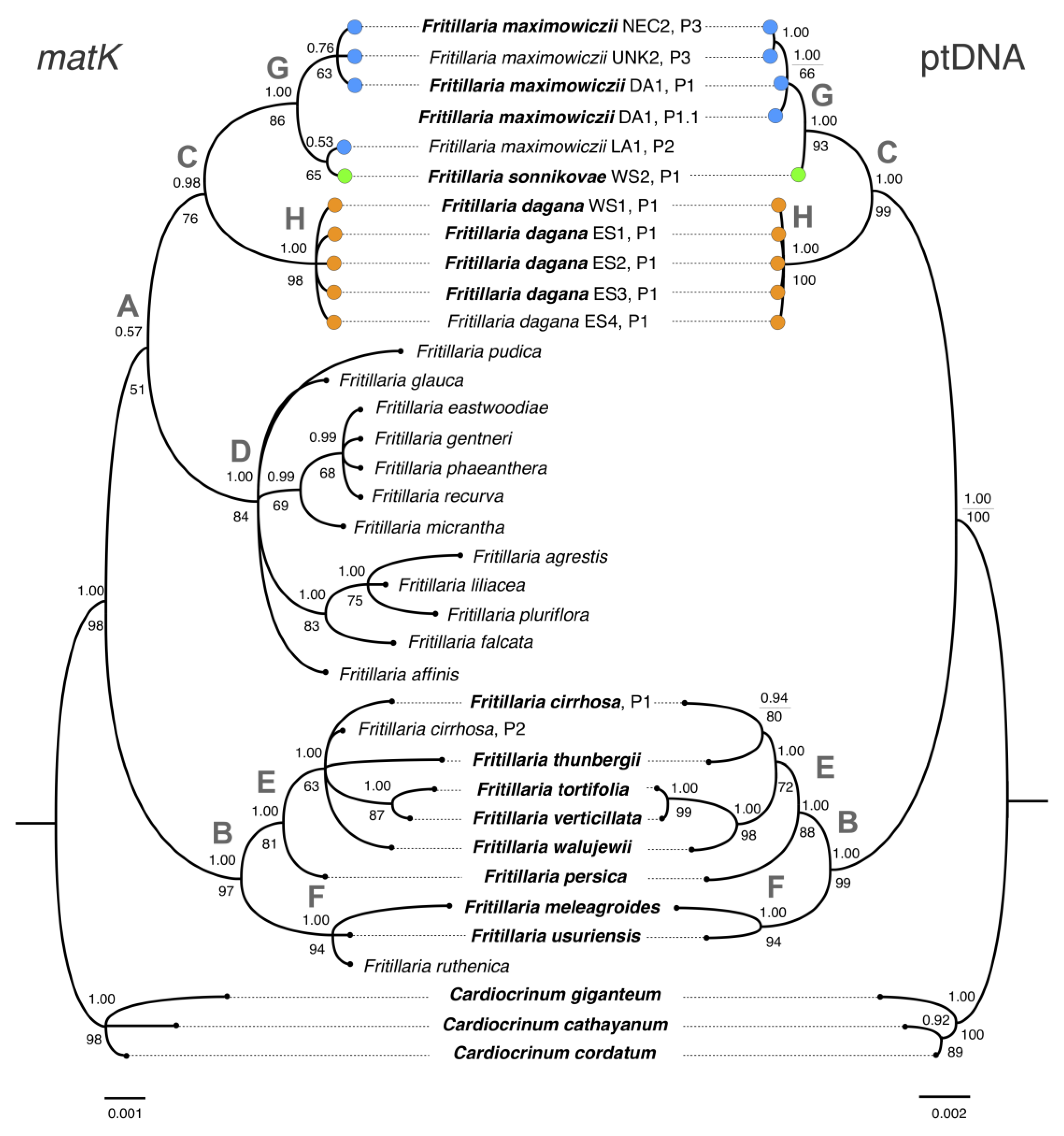
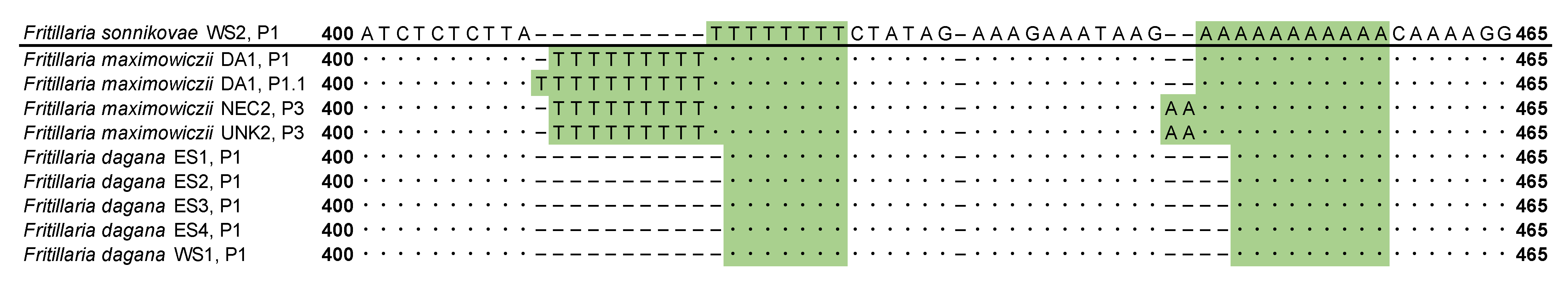
3.2. Phylogenetic Analysis Based on Plastid DNA
3.3. Combined Phylogenetic Analysis
4. Discussion
4.1. Phylogenetic Relationships between F. sonnikovae and Other Fritillaria Species
4.2. Comparative Morphological Features of Three Closely Related Species of Siberian Fritillaria
4.3. The Possible Origin of F. sonnikovae and Diversification of Siberian Taxa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassler, M. World Plants. Synonymic Checklist and Distribution of the World Flora. Version 14.2. Last Update 15 October 2022. Available online: https://www.worldplants.de (accessed on 10 December 2022).
- The Royal Botanic Gardens, Kew. The World Checklist of Vascular Plants (WCVP). In Catalogue of Life Checklist (4.0); Available online: https://doi.org/10.48580/dfpk-4nz (accessed on 10 December 2022).
- Bi, Y.; Zhang, M.; Xue, J.; Dong, R.; Du, Y.; Zhang, X. Chloroplast Genomic Resources for Phylogeny and DNA Barcoding: A Case Study on Fritillaria. Sci. Rep. 2018, 8, 1184. [Google Scholar] [CrossRef] [PubMed]
- Rønsted, N.; Law, S.; Thornton, H.; Fay, M.F.; Chase, M.W. Molecular Phylogenetic Evidence for the Monophyly of Fritillaria and Lilium (Liliaceae; Liliales) and the Infrageneric Classification of Fritillaria. Mol. Phylogenetics Evol. 2005, 35, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Rix, E.M. Fritillaria: A Revised Classification Together with an Updated List of Species; Fritillaria Group of the Alpine Garden Society Press: Pershore, UK, 2001. [Google Scholar]
- Day, P.D.; Berger, M.; Hill, L.; Fay, M.F.; Leitch, A.R.; Leitch, I.J.; Kelly, L.J. Evolutionary Relationships in the Medicinally Important Genus Fritillaria, L. (Liliaceae). Mol. Phylogenetics Evol. 2014, 80, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, L.-Q.; Yu, Y.; Liu, Y.-M.; Xie, D.-F.; Li, J.; He, X.-J.; Zhou, S.-D. Molecular Phylogenetics and Historical Biogeography of the Tribe Lilieae (Liliaceae): Bi-Directional Dispersal between Biodiversity Hotspots in Eurasia. Ann. Bot. 2018, 122, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.P. Molecular Phylogeny and Character Evolution of Fritillaria Subgenus Liliorhiza (Liliaceae). Master’s Thesis, San Diego State University, San Diego, CA, USA, 2014. [Google Scholar]
- Hill, L. Fritillaria Icones. Available online: http://www.fritillariaicones.com (accessed on 10 December 2022).
- Shaulo, D.N.; Erst, A.S. A New Species of Fritillaria L. (Liliaceae) from West Sayan. Turczaninowia 2010, 13, 46–49. [Google Scholar]
- Shaulo, D.N. Flora of the Western Sayan. Turczaninowia 2006, 9, 5–336. (In Russian) [Google Scholar]
- Cherepnin, L.M. Flora Yuzhnoy Chasti Krasnoyarskogo Kraya [Flora of the Southern Part of Krasnoyarskiy Kray]; Krasnoyarsk State Pedagogical Institute: Krasnoyarsk, Russia, 1959; Volume 2, p. 211. (In Russian) [Google Scholar]
- Krasnoborov, I.M. Flora Alpina Montium Sajanesium Occidentalium; Nauka: Novosibirsk, Russia, 1976. (In Russian) [Google Scholar]
- Sandanov, D.V. Developing the Database of Vascular Plants Distribution for Asian Russia. Information Technology in Biodiversity Research. In Proceedings of the III National Conference with International Participation, Dedicated to the 100th Anniversary of the Birth of Russian Academician Pavel Gorchakovskii, Ekaterinburg, Russia, 5–10 October 2020; University for the Humanities: Ekaterinburg, Russia, 2020; pp. 470–472. (In Russian). [Google Scholar]
- Sandanov, D.V. Finding of Fritillaria dagana (Lilliaceae) in Mongolia. Rastit. Mir Aziat. Ross. 2013, 1, 44–46. (In Russian) [Google Scholar]
- Baasanmunkh, S.; Oyuntsetseg, B.; Tsegmed, Z.; Oyundelger, K.; Urgamal, M.; Gantuya, B.; Javzandolgor, C.; Nyambayar, N.; Kosachev, P.; Choi, H.J. Distribution of Vascular Plants in Mongolia—I Part. Mong. J. Biol. Sci. 2022, 20, 3–28. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Protopopova, M.V.; Pavlichenko, V.V. Eranthis Salisb. (Ranunculaceae) in South Siberia: Insights into Phylogeography and Taxonomy. Diversity 2022, 14, 779. [Google Scholar] [CrossRef]
- Utelli, A.; Roy, B.; Baltisberger, M. Molecular and Morphological Analyses of European Aconitum Species (Ranunculaceae). Plant Syst. Evol. 2000, 224, 195–212. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Cuenoud, P.; Savolainen, V.; Chatrou, L.W.; Powell, M.; Grayer, R.J.; Chase, M.W. Molecular Phylogenetics of Caryophyllales Based on Nuclear 18S RDNA and Plastid RbcL, AtpB, and MatK DNA Sequences. Am. J. Bot. 2002, 89, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.S.; Fazekas, A.J.; Kesanakurti, P.R.; Graham, S.W.; Husband, B.C.; Newmaster, S.G.; Percy, D.M.; Hajibabaei, M.; Barrett, S.C.H. Discriminating Plant Species in a Local Temperate Flora Using the RbcL+MatK DNA Barcode. Methods Ecol. Evol. 2011, 2, 333–340. [Google Scholar] [CrossRef]
- Lee, C.-S.; Downie, S.R. Phylogenetic Relationships within Cicuta (Apiaceae Tribe Oenantheae) Inferred from Nuclear RDNA ITS and CpDNA Sequence Data. Can. J. Bot. 2006, 84, 453–468. [Google Scholar] [CrossRef]
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and Diverse Origins of the Polyploid Species. Syst. Bot. 2003, 28, 723–737. [Google Scholar]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA Phylogeny, Reticulate Evolution, and Biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef]
- Lahr, D.J.G.; Katz, L.A. Reducing the Impact of PCR-Mediated Recombination in Molecular Evolution and Environmental Studies Using a New-Generation High-Fidelity DNA Polymerase. Biotechniques 2009, 47, 857–866. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Shinoda, K. ITS DNA Phylogeny and Infrageneric Classification of Genus Lilium; Vegetable and Ornamental Crops Laboratory, Hokkaido National Agricultural Experiment Station: Sapporo, Japan, 1998. [Google Scholar]
- Lu, R.-S.; Li, P.; Qiu, Y.-X. The Complete Chloroplast Genomes of Three Cardiocrinum (Liliaceae) Species: Comparative Genomic and Phylogenetic Analyses. Front. Plant Sci. 2017, 7, 2054. [Google Scholar] [CrossRef]
- Yang, L.Q.; He, X.J.; Zhou, S.D. Molecular Phylogeny and Biogeography of the Tribe Lilieae (Liliaceae S. Str.) Focusing on the Chinese Species; College of Life Science, Sichuan University: Sichuan, China, 2015. [Google Scholar]
- Gao, Y.-D.; Hohenegger, M.; Harris, A.; Zhou, S.-D.; He, X.-J.; Wan, J. A New Species in the Genus Nomocharis Franchet (Liliaceae): Evidence That Brings the Genus Nomocharis into Lilium. Plant Syst. Evol. 2012, 298, 69–85. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Song, J.; Xu, H.; Xu, J.; Zhu, Y.; Li, X.; Gao, H.; Dong, L.; Qian, J.; et al. High-Accuracy de Novo Assembly and SNP Detection of Chloroplast Genomes Using a SMRT Circular Consensus Sequencing Strategy. New Phytol. 2014, 204, 1041–1049. [Google Scholar] [CrossRef]
- Zhang, J. Identify Original Species of Four Fritillaria Species and a Primary Discussion on Cultivation Situation; College of Pharmacy and Chemistry, Dali University: Dali, China, 2020. [Google Scholar]
- Chen, Q.; Wu, X.; Zhang, D. Comparison of the Abilities of Universal, Super, and Specific DNA Barcodes to Discriminate among the Original Species of Fritillariae cirrhosae Bulbus and Its Adulterants. PLoS ONE 2020, 15, e0229181. [Google Scholar] [CrossRef]
- Gochar, M.; Goyal, P.; Yusuf, M.; Gupta, S. GenBank Direct Submission. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT539156.1 (accessed on 10 December 2022).
- Chen, Q.; Hu, H.; Zhang, D. DNA Barcoding and Phylogenomic Analysis of the Genus Fritillaria in China Based on Complete Chloroplast Genomes. Front. Plant Sci. 2022, 13, 764255. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, Y.; Liu, Y.-M.; Xie, D.-F.; He, X.-J.; Zhou, S.-D. Comparative Chloroplast Genomics of Fritillaria (Liliaceae), Inferences for Phylogenetic Relationships between Fritillaria and Lilium and Plastome Evolution. Plants 2020, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, H.; Wei, X.; Wei, J.; Liu, H.; Li, X.; Fan, C.; Zhang, B.; Qi, Y. Study on the Phylogenetic Relationship of Fritillaria L; From Xinjiang; Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine: Beijing, China, 2018. [Google Scholar]
- Li, Y.; Zhang, Z.; Lv, G. The Complete Chloroplast Genome of Fritillaria Yuminensis, a Rare and Endangered Species Endemic to China. Mitochondrial DNA Part B 2017, 2, 913–914. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of the Human-Ape Splitting by a Molecular Clock of Mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary Trees from DNA Sequences: A Maximum Likelihood Approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of Protein Molecules; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 21–132. [Google Scholar]
- Rambaut, A. FigTree: Tree Figure Drawing Tool, Version 1.4.3. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 July 2022).
- Templeton, A.R.; Crandall, K.A.; Sing, C.F. A Cladistic Analysis of Phenotypic Associations with Haplotypes Inferred from Restriction Endonuclease Mapping and DNA Sequence Data. III. Cladogram Estimation. Genetics 1992, 132, 619–633. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-Feature Software for Haplotype Network Construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Ribosomal RNA Genes in Plants: Variability in Copy Number and in the Intergenic Spacer. Plant Mol. Biol. 1987, 9, 509–520. [Google Scholar] [CrossRef]
- Losina-Losinskaja, A.S. Fritillaria L. In Flora of the USSR, vol. 4; Komarov, V.L., Ed.; Academy of Sciences of the USSR: Leningrad, USSR, 1935; pp. 302–320. (In Russian) [Google Scholar]
- Vlasova, S.N. Fritillaria L. In Flora Sibiriae. Araceae-Orchidaceae; Malyschev, L.I., Peschkova, G.A., Eds.; Nauka: Novosibirsk, USSR, 1987; pp. 99–101. (In Russian) [Google Scholar]
- Barkalov, V.Y. Fritillaria L. In Plantae Vasculares Orientis Extremi Sovietici, Vol. 2; Charkevicz, S.S., Ed.; Nauka: Leningrad, USSR, 1987; pp. 370–373. (In Russian) [Google Scholar]
- Xinqi, C.; Mordak, H. Fritillaria maximowiczii Freyn. In Flora of China, vol. 24 (Flagellariaceae–Marantaceae); Wu, Z.-Y., Raven, P.H., Deyuan, H., Eds.; Science Press and Missouri Botanical Garden Press: Beijing, China; St. Louis, MO, USA, 2000; p. 133. [Google Scholar]
- Leonova, T.V.; Barsukova, I.N.; Ankipovich, E.S. Some Aspects of the Population Biology Study of Fritillaria sonnikovae Schaulo et A. Erst (Liliaceae) on the Territory of the Western Sayan. Vestn. KRASGAU Her. Krasn. State Agric. Univ. 2016, 2, 3–7. (In Russian) [Google Scholar]
- Gileva, M.V. Fritillaria maximowiczii Freyn. In Red Data Book of Zabaikalsky Krai. Plants; OOO Dom Mira: Novosibirsk, Russia, 2017; pp. 48–49. (In Russian) [Google Scholar]
- Sandanov, D.V.; Gileva, M.V. Fritillaria Dagana Turcz. In Red Data Book of Zabaikalsky Krai. Plants; OOO Dom Mira: Novosibirsk, Russia, 2017; pp. 46–47. (In Russian) [Google Scholar]
- Bartolucci, F.; Caparelli, K.F.; Peruzzi, L. Biometric Study of Fritillaria montana Hoppe ex W.D.J. Koch s.l. (Liliaceae) Shows a Single Polymorphic Species, with no Infraspecific Taxa. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2009, 143, 516–527. [Google Scholar] [CrossRef]
- Chimitov, D. Fritillaria Dagana Turcz. Available online: https://www.gbif.org/occurrence/3873414270 (accessed on 30 December 2022).
- Arkhipov, V. Fritillaria Maximowiczii Freyn. Available online: https://www.gbif.org/occurrence/3889051817 (accessed on 30 December 2022).
- Bel’mas, L. Fritillaria sonnikovae Shaulo & Erst. Available online: https://www.plantarium.ru/page/image/id/598372.html (accessed on 30 December 2022).
- Day, P.D. Studies in the Genus Fritillaria L. (Liliaceae). Ph.D. Thesis, Queen Mary, University of London, London, UK, 2018. [Google Scholar]
- Mucciarelli, M.; Ferrazzini, D.; Belletti, P. Genetic Variability and Population Divergence in the Rare Fritillaria Tubiformis Subsp. Moggridgei Rix (Liliaceae) as Revealed by RAPD Analysis. PLoS ONE 2014, 9, e101967. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.I. Northern Hemisphere Plant Disjunctions: A Window on Tertiary Land Bridges and Climate Change? Ann. Bot. 2006, 98, 465–472. [Google Scholar] [CrossRef]
- Alter, S.P. K Istorii Formirovaniya Doliny Eniseya [to the History of the Yenisey Valley Formation]. Dokl. Inst. Geogr. Sib. I Dal’nego Vost. Rep. Inst. Geogr. Sib. Far East 1965, 8, 38–44. (In Russian) [Google Scholar]
- Sandanov, D.V. Modern Approaches to Modeling Plant Diversity and Spatial Distribution of Plant Species: Implication Prospects in Russia. Vestn. Tomsk. Gos. Univ. Biol. 2019, 46, 82–114. (In Russian) [Google Scholar] [CrossRef]
- Polozhii, A.V.; Krapivkina, E.D. Relikty Tretichnyh Shirokolistvennyh Lesov vo Flore Sibiri [Relics of Tertiary Deciduous Forests in the Flora of Siberia]; Tomsk University Press: Tomsk, USSR, 1985. (In Russian) [Google Scholar]
- Belov, A.V.; Bezrukova, E.V.; Sokolova, L.P.; Abzayeva, A.A.; Letunova, P.P.; Fisher, E.E.; Orlova, L.A. Vegetation of the Baikal Region as an Indicator of Global and Regional Changes in Natural Conditions of North Asia in the Late Cainozoic. Geogr. Nat. Resour. 2006, 6, 5–18. (In Russian) [Google Scholar]
- Krestov, P.V.; Barkalov, V.Y.; Omelko, A.M.; Yakubov, V.V.; Nakamura, Y.; Sato, K. Relic Vegetation Complexes in the Modern Refugia of Northeast Asia. Komar. Chtenia V. L. Komar. Meml. Lect. 2009, 56, 5–63. (In Russian) [Google Scholar]
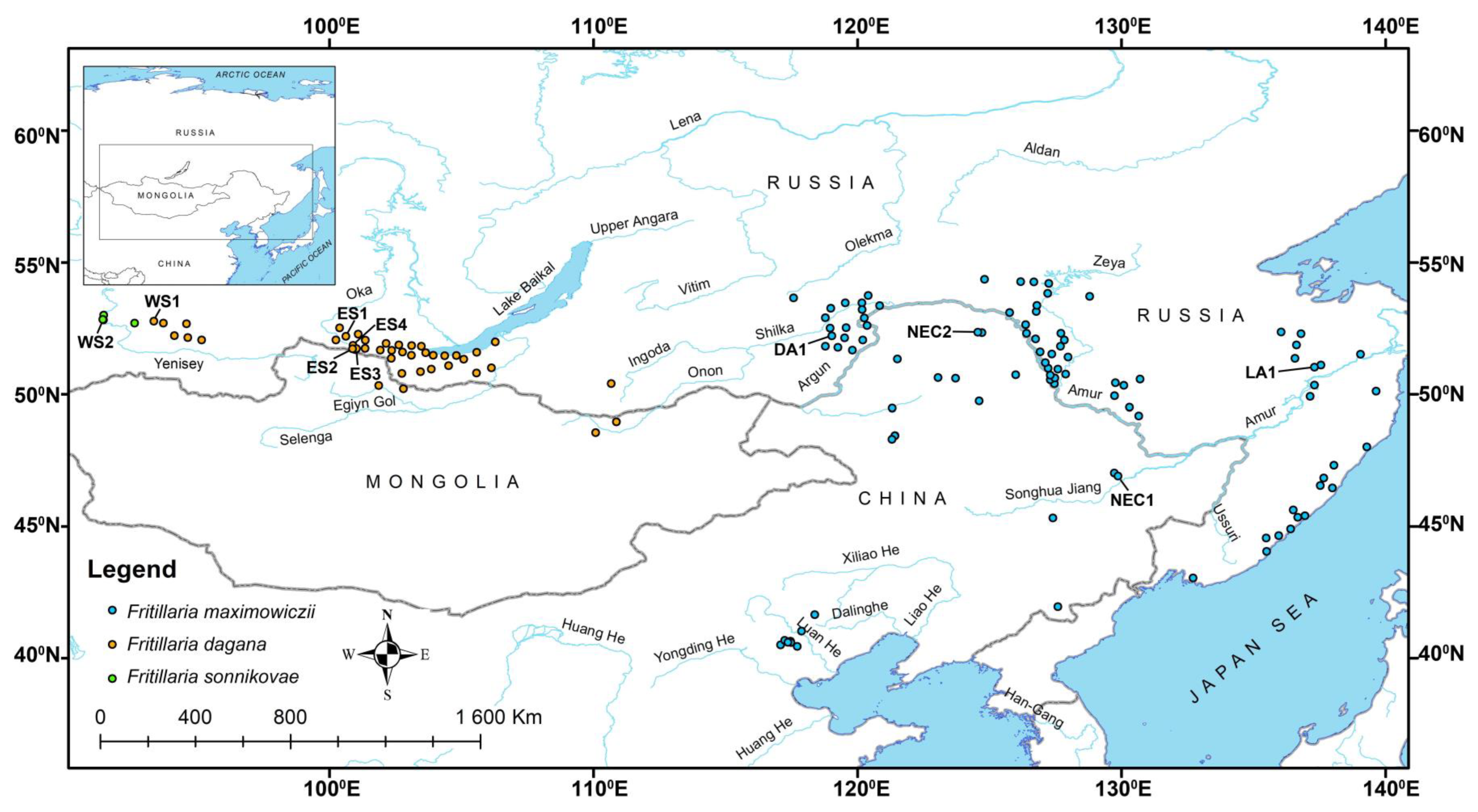



| Locality Abbr. | Herbarium Voucher Information | Coordinates, Altitude 1 |
|---|---|---|
| ES1 | Fritillaria dagana Turcz. Russia, the Republic of Buryatia, Tunkinskiy Raion, the Eastern Sayan Mts, near Mondy settlement., 11 July 1992, Shvetsova (UUH004118). | Unknown |
| ES2 | Fritillaria dagana Turcz. Russia, the Republic of Buryatia, Tunkinskiy Raion, the Eastern Sayan Mts, near Mondy settlement., 7 July 2003, Yu.A. Rupyshev (UUH004121). | Unknown |
| ES3 | Fritillaria dagana Turcz. Russia, the Republic of Buryatia, Tunkinskiy Raion, the Eastern Sayan Mts, near Mondy settlement., 23 June 2021, D. Sandanov (UUH019871). | 51.70000° N, 100.96719° E, 1612 m alt. |
| ES4 | Fritillaria dagana Turcz. Russia, the Republic of Buryatia, Tunkinskiy Raion, the Eastern Sayan Mts, near Mondy settlement., 20 July 1991, Yu. Rupyshev (UUH004123). | Unknown |
| WS1 | Fritillaria dagana Turcz. Russia, Krassnoyarskiy Kray, Ermakovskiy Raion, the Western Sayan Mts., Ergaki Nature Park., Tushkanchik Mt., 14 June 2019, N. Stepanov (KRSU T52012). | 52.766371° N, 93.350444° E, 1080 m alt. |
| WS2 | Fritillaria sonnikovae Shaulo & Erst Russia, Krassnoyarskiy Kray, Schushennskiy Raion, the Western Sayan Mts., near Maina reservoir, 20 May 2010, A.S. Erst, Yu.N. Danylov, A.E. Sonnikova; isotypes (NS0000192). | 52.52° N, 91.26° E, 765 m alt. |
| DA1 | Fritillaria maximowiczii Freyn Russia, Zabaykalsiy Kray, Gazimuro-Zavodskoy Raion, Argun Dahuria, near Kurleya settlement., 11 June 2022, D. Sandanov (UUH019872). | 52.21448° N, 119.02705° E, 635 m alt. |
| Species Name Used in This Study | Locality 1 | Haplotype | GenBank Accession Numbers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ribotype | Plastotype | Ref. | |||||||
| ITS | matK | rps16 | trnH-psbA | ||||||
| Cardiocrinum giganteum (Wall.) Makino | – | – | – | AF092515.1 | – | KX528334.1 | KX528334.1 | KX528334.1 | [27,28] |
| Cardiocrinum cathayanum (E.H.Wilson) Stearn | – | – | – | KP712016.1 | – | KX575836.1 | KX575836.1 | KX575836.1 | [28,29] |
| Cardiocrinum cordatum (Thunb.) Makino | – | – | – | KP712019.1 | – | KX575837.1 | KX575837.1 | KX575837.1 | [7,28] |
| Fritillaria affinis (Schult. & Schult.f.) Sealy | – | – | – | AY616710.1 | – | LM993042.1 | – | – | [4,6] |
| Fritillaria agrestis Greene | – | – | – | MW025089.1 | – | LM993043.1 | – | – | [6,8] |
| Fritillaria atropurpurea Nutt. | – | – | – | MW025090.1 | – | – | – | – | [8] |
| Fritillaria camschatcensis (L.) Ker Gawl. | – | – | – | MW025094.1 | – | – | – | – | [8] |
| Fritillaria cirrhosa D.Don | – | – | R1 | HM045469.1 | P1 | KF769143.1 | KF769143.1 | KF769143.1 | [30,31] |
| Fritillaria cirrhosa D.Don | – | – | R2 | KP711997.1 | P2 | MT806755 | – | – | [29,32] |
| Fritillaria cirrhosa D.Don | – | – | R3 | MH588409.1 | – | – | – | – | [33] |
| Fritillaria cirrhosa subsp. roylei (Hook.) Ali | – | – | – | MT539156.1 | – | – | – | – | [34] |
| Fritillaria dagana Turcz. | ES1 | H1 | R1 | OQ244464 | P1 | OQ267631 | OQ267639 | OQ267647 | this study |
| Fritillaria dagana Turcz. | ES1 | – | R2 | OQ244465 | – | – | – | – | this study |
| Fritillaria dagana Turcz. | ES2 | H1 | R1 | OQ244466 | P1 | OQ267632 | OQ267640 | OQ267648 | this study |
| Fritillaria dagana Turcz. | ES2 | – | R2 | OQ244467 | – | – | – | – | this study |
| Fritillaria dagana Turcz. | ES3 | H1 | R1 | OQ244468 | P1 | OQ267633 | OQ267641 | OQ267649 | this study |
| Fritillaria dagana Turcz. | ES3 | – | R2 | OQ244469 | – | – | – | – | this study |
| Fritillaria dagana Turcz. | ES3 | – | R3 | OQ244470 | – | – | – | – | this study |
| Fritillaria dagana Turcz. | ES4 | – | – | – | P1 | OQ267634 | OQ267642 | OQ267650 | this study |
| Fritillaria dagana Turcz. | WS1 | H1 | R1 | OQ244471 | P1 | OQ267635 | OQ267643 | OQ267651 | this study |
| Fritillaria dagana Turcz. | WS1 | – | R2 | OQ244472 | – | – | – | – | this study |
| Fritillaria eastwoodiae R.M.Macfarl. | – | – | – | MW025096.1 | – | LM993068.1 | – | – | [6,8] |
| Fritillaria falcata (Jeps.) D.E.Beetle | – | – | – | AY616720.1 | – | AY624436.1 | – | – | [4] |
| Fritillaria gentneri Gilkey | – | – | – | AY616721.1 | – | AY624437.1 | – | – | [4] |
| Fritillaria glauca Greene | – | – | – | AY616723.1 | – | AY624439.1 | – | – | [4] |
| Fritillaria liliacea Lindl. | – | – | – | MW025102.1 | – | LM993087.1 | – | – | [6,8] |
| Fritillaria maximowiczii Freyn | DA1 | H1 | R1 | OQ244473 | P1 | OQ267636 | OQ267644 | OQ267652 | this study |
| Fritillaria maximowiczii Freyn | DA1 | H2 | P1.1 | OQ267637 | OQ267645 | OQ267653 | this study | ||
| Fritillaria maximowiczii Freyn | DA1 | – | R2 | OQ244474 | – | – | – | – | this study |
| Fritillaria maximowiczii Freyn | LA1 | – | R3 | AY616729.1 | P2 | AY624444.1 | – | – | [4] |
| Fritillaria maximowiczii Freyn | NEC1 | H3 | R4 | MG525328.1 | – | – | – | – | [7] |
| Fritillaria maximowiczii Freyn | NEC2 | H3 | – | – | P1 | MN810992.1 | MN810992.1 | MN810992.1 | [35] |
| Fritillaria maximowiczii Freyn | UNK1 | – | R5 | HM045471.1 | – | – | – | – | [30] 2 |
| Fritillaria maximowiczii Freyn | UNK2 | – | – | – | P1 | MK258138.1 | MK258138.1 | MK258138.1 | [36] |
| Fritillaria meleagroides Patrin ex Schult.f. | – | – | – | MG946144.1 | – | MF947710.1 | MF947710.1 | MF947710.1 | [3,37] |
| Fritillaria micrantha A.Heller | – | – | – | AY616732.1 | – | LM993091.1 | – | – | [4,6] |
| Fritillaria persica L. | – | – | – | AY616736.1 | – | MF947709.1 | MF947709.1 | MF947709.1 | [3,4] |
| Fritillaria phaeanthera Purdy | – | – | – | AY616737.1 | – | AY624452.1 | – | – | [4] |
| Fritillaria pluriflora Torr. ex Benth. | – | – | – | MW025109.1 | – | LM993104.1 | – | – | [6,8] |
| Fritillaria pudica (Pursh) Spreng. | – | – | – | MW025110.1 | – | LM993107.1 | – | – | [6,8] |
| Fritillaria recurva Benth. | – | – | – | MW025113.1 | – | AY624455.1 | – | – | [4,8] |
| Fritillaria ruthenica Wikstr. | – | – | – | – | – | LM993110.1 | – | – | [6] |
| Fritillaria sonnikovae Shaulo & Erst | WS2 | H1 | R1 | OQ244475 | P1 | OQ267638 | OQ267646 | OQ267654 | this study |
| Fritillaria sonnikovae Shaulo & Erst | WS2 | H2 | R2 | OQ244476 | this study | ||||
| Fritillaria striata Eastw. | – | – | – | AY616743.1 | – | – | – | – | [4] |
| Fritillaria thunbergii Miq. | – | – | – | MH588428.1 | – | MH244914.1 | MH244914.1 | MH244914.1 | [33] |
| Fritillaria tortifolia X.Z.Duan & X.J.Zheng | – | – | – | MG946151.1 | – | MN810987.1 | MN810987.1 | MN810987.1 | [35,37] |
| Fritillaria usuriensis Maxim. | – | – | – | MH588434.1 | – | MH593369.1 | MH593369.1 | MH593369.1 | [33] |
| Fritillaria verticillata Willd. | – | – | – | KP712007.1 | – | MG211823 | MG211823 | MG211823 | [29,38] |
| Fritillaria walujewii Regel | – | – | – | KP712008.1 | – | MN810990.1 | MN810990.1 | MN810990.1 | [29,35] |
| Feature | F. dagana [49,50] | F. maximowiczii [51] | F. sonnikovae [10] |
|---|---|---|---|
| Plant height, cm | 20–35 | Up to 45 | 26–52 |
| Stem coloration | Spotted | Non-spotted | Non-spotted |
| Leaves number (bracts excluded) | 2–5 | 2–6 | 3–7 |
| Whorls number, possition on the stem | 1, in the middle | 1–2 1 (few), above the middle | 1–2, above the middle |
| Bracts number | 1 | 1–2 2 | 1–2 |
| Leaves shape | Oblong-(ovate)-lanceolate | Linear-lanceolate, linear 2 | Linear-lanceolate, linear |
| Leaves length, mm | 80 | 50–90 | 70–120 |
| Leaves width, mm | 15 | 4–9 | 3–13 |
| Outer perianth color | Brown-purple 3 | Purple 3 | Light greenish yellow with translucent checkerboard or linear patterns |
| Inner perianth color | Yellowish with a checkerboard patterns, mottled | Yellowish with a checkerboard patterns, mottled | Bright yellow with purple speckles |
| Tepals length, mm | Up to 40 | 32–45 | 40–65 |
| Tepals width, mm | 13 | 10–15 | 11–16 |
| Tepals shape | Oblong-obovate | Oblong-elliptical, margin erose | Oblong-elliptical, margin erose |
| Nectaries shape | triangular | oval | oval |
| Capsule length, mm | 15 | 15–25 | 10 |
| Capsule shape | With oblong narrow wings | With wide wings blunted at the end | With wide wings blunted at the end |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protopopova, M.; Sandanov, D.; Pavlichenko, V.; Selyutina, I.; Stepanov, N. The Curious Case of Fritillaria sonnikovae (Liliaceae) in South Siberia: New Insights into Its Origin and Phylogeny. Diversity 2023, 15, 193. https://doi.org/10.3390/d15020193
Protopopova M, Sandanov D, Pavlichenko V, Selyutina I, Stepanov N. The Curious Case of Fritillaria sonnikovae (Liliaceae) in South Siberia: New Insights into Its Origin and Phylogeny. Diversity. 2023; 15(2):193. https://doi.org/10.3390/d15020193
Chicago/Turabian StyleProtopopova, Marina, Denis Sandanov, Vasiliy Pavlichenko, Inessa Selyutina, and Nikolay Stepanov. 2023. "The Curious Case of Fritillaria sonnikovae (Liliaceae) in South Siberia: New Insights into Its Origin and Phylogeny" Diversity 15, no. 2: 193. https://doi.org/10.3390/d15020193
APA StyleProtopopova, M., Sandanov, D., Pavlichenko, V., Selyutina, I., & Stepanov, N. (2023). The Curious Case of Fritillaria sonnikovae (Liliaceae) in South Siberia: New Insights into Its Origin and Phylogeny. Diversity, 15(2), 193. https://doi.org/10.3390/d15020193









