Abstract
This paper presents the effect of water extracts from the leaves of various Mentha spp. on the growth of selected fungi causing the gray decay of wood. The study determined which of the Mentha spp. extracts used had the best effect on inhibiting the development of fungi on various substrates including pine wood. The best results in the complete inhibition of fungi growth on an agar medium were obtained for the M. × piperita ‘Almira’ extract. Biocidal properties were not achieved on wood samples, although it was noticed that at doses of extracts of 600 g/m2 and higher, the growth of fungi in the initial stages of cultivation was clearly inhibited. Chemical substances in the obtained extracts were characterized by gas chromatography. Oxygen monoterpenes were the dominant group of substances, substances belonging to sesquiterpene hydrocarbons, monoterpene hydrocarbons containing oxygen sesquiterpenes, and one substance belonging to non-terpene hydrocarbons were also identified.
1. Introduction
The fungi Trichoderma viride or Chaetomium globosum are widespread in the environment and, under favorable conditions, inhabit wood and wood materials. The enzymatic potential of these organisms means that polymers that build wood tissue such as cellulose, hemicelluloses, and to some extent lignin are broken down into simple compounds that are absorbed by fungal cells. The most effective way to prevent the growth of fungi is chemical impregnation with biocides, which protects the wood against biodegradation caused by biological factors. Although there is a lot of literature data on the use of various phytochemicals as fungicides in the development of biopreservatives [1,2,3], there are few reports on the research and potential use of water mint extracts in wood protection. Considering the safety of using biocides in wood protection, more and more activities are being undertaken to search for effective biocides based on substances of natural origin [4,5,6] and even biological [7,8,9]. It is noted in the literature that research is being undertaken on plant extracts [10,11], essential oils [12,13,14,15], and natural resins [6] to assess the degree of inhibition of the development of wood-decomposing fungi. Studies to evaluate the fungicide effectiveness of compounds of natural origin were conducted by Kwaśniewska-Sip et al. [16,17], Ratajczak et al. [18], and Tomak and Gonultas [19]. The use of five types of essential oils in the protection of wood was investigated by Hussain et al. [20]. The researchers found that neem, ajwain, and cinnamon oils strongly inhibited the growth of the test fungi. Other analyses conducted by Pánek et al. [21] confirmed the effectiveness of 10 tested essential oils against fungi causing wood mold. At the same time, the authors of the research stated that the tested oils may cause slight changes in the color of some types of wood. Plant extracts as environmentally friendly biocides in the protection of wood were also described in the studies by Bahmani and Schmidt [22], Yang and Clausen [23], Chang et. al. [24], Tascioglu et al. [10], or Bhardwaj et al. [25]. The mechanism of the fungicide action of active substances contained in plant cells is well-described in the literature. Various substances and chemical compounds present in the plant may affect changes in the permeability of cell membranes [26], disturbances in the expression of genes involved in the synthesis of membrane proteins, inhibition of enzymes involved in protection against thermal stress, or disorders of ATP synthesis [27]. Hafedh et al. [28] showed that the menthol contained in Mentha longifolia causes changes in the permeability and integrity of the cell membrane.
The type of mentha provides a raw material or substances with properties valued by various industries [29]. Mint leaves are rich in substances that are bactericidal [30,31] and fungicidal [32]. It seems, therefore, that the use of this plant as a phytoncide in wood protection may be promising, therefore it is worth conducting research on the identification of the composition of active substances and the assessment of the biocidal effectiveness of various plant extracts. Moghtader [33], analyzing the composition of the oil obtained from the dried mint herb, found the presence of 23 volatile substances, the dominant concentration of which was occupied by menthol, menthone, and methyl acetate. The content of the essential oil components in the dried mint leaves depends on various factors, both environmental and species-related as well as the harvest time and storage conditions. Ludwiczuk et al. [34] analyzed the composition of essential oils, distinguished five chemotypes from among the 23 species and varieties of mint studied. The dominant components of the oils of individual chemotypes were: menthol/menthone, piperitenone oxide, linalool, carvone, and 3-octanone. Numerous studies have shown that the ingredients contained in mint have antifungal properties [35], and also prevent biofilm formation [36]. The biocidal properties of linalool and linalool oxide against a wide group of mold fungi were tested by Satoh et al. [37]. The strong fungicidal effect of Mentha piperita essential oil against C. albicans and C. dubliniensis was demonstrated by Saharkhiz et al. [38]. Similar observations, but in relation to fungi of the genus Aspergillus sp. were carried out by Škrinjar et al. [39]. Interesting data on the fungicidal activity of M. piperita essential oil in combination with fluconazole, ketoconazole against yeast fungi and dermatophytes was presented by Tullio et al. [40]. The authors of the research indicated that the combination of the oil with fungicides led to the enhancement of the fungicidal effect against yeast-like fungi. The biocidal properties of M. longifolia oil on wood samples against Aspergillus niger, A. flavus, A. fumigatus, Alternaria tenuissima, Colletotrichum gloeosporioides, Fusarium culmorum, Penicillium chrysogenum, Rhizoctonia solani, and Trichoderma harzianum were evaluated by Ali et al. [41]. Similar observations indicating that raw mint may be a potential component of wood protection measures by protecting wood against fouling by fungi harmful to wood were presented by Şen and Yalçın [42], Verma et al. [43] as well as Perveen and Bokahri [44].
Water mint extracts can also be a source of fungicides. Panda et al. [45] showed that water extracts of Mentha arvensis can be used as a natural and safe food preservative that prevents mold growth. The positive effect of such extracts on coli bacteria was also demonstrated by El-Said and Hassan [46]. Undoubtedly, the costs of water extraction are much lower than the costs of extracts obtained by steam distillation or distillation in supercritical CO2 [47,48], which is an important criterion when designing any preservatives including those for wood protection. Reducing the cost of producing biocides while maintaining high efficiency is an important activity for manufacturers of wood protection agents. In addition, attempts to limit the use of chemically active substances in impregnations are conducive to activities related to environmental protection.
The aim of the presented research was to determine the effect of water extracts from the leaves of various mint varieties on the growth of fungi causing the rot decay of wood and to determine the composition of volatile substances contained in the extracts. The obtained knowledge on the effect of mint water extracts on the growth of wood-decaying fungi will allow future research to assess the possibility of using these raw materials for the formulation of wood protection agents. The possibility of the partial replacement of chemical active substances in wood protection agents with substances of natural origin may lead to a reduction in the toxicity of such preparations, making them more environmentally friendly. Demonstrating that mint water extracts can inhibit the growth of fungi on the wood surface would be a promising result, allowing for further research attempts to assess the interaction of such extracts with chemicals commonly used in wood treatment agents. While we can find a large group of studies describing the antifungal properties of various mint extracts in the literature, these studies have mainly concerned the experiments conducted on agar media. The assessment of fungal growth on a porous material such as wood has not been the subject of deeper analyses thus far.
2. Materials and Methods
2.1. Materials
The research material was obtained from eight varieties belonging to four species of mint: peppermint (Mentha × piperita L.)—four cultivars (No. 1, 2, 6, and 7), spearmint (Mentha spicata L.)—two cultivars (No. 3 and 5), apple mint (Mentha rotundifolia (L.) Huds.) (No. 4), and pineapple mint (Mentha suaveolens Ehrh.) (No. 8) (Figure 1).

Figure 1.
Mint plants used in the experiment: No. 1. Mentha × piperita ‘Swiss’, No. 2. M. × piperita ‘Multimentha’, No. 3. M. spicata ‘Morocco’, No. 4. M. rotundifolia, No. 5. M. spicata ‘Crispa’, No. 6. M. × piperita ‘Almira’, No. 7. M. × piperita ‘Granada’, No. 8. M. suaveolens’ Variegata’ (photographed by Marcin Dadasiewicz).
Mint plants were cultivated in the experimental fields of the Research and Science Innovation Center in Wola Zadybska near Lublin (Poland) (51°44′49″ N, 21°50′38″ E) on clay soil of loess origin. The plants were collected in June 2020, being in the 29 BBCH (Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie) stage at that moment. The herb of all mint taxa was cut at a height of about 10 cm above the ground. Immediately after harvesting, the herb was dried by convection in a laboratory drier with forced air 90 circulation at 32 °C for 3 h. After drying, the stems were manually separated and discarded as the least valuable parts of the raw material, and the test extracts were prepared using only the leaves. A total of 10 g of mint leaves were poured separately with 500 mL of hot water and shaken for 24 h on a laboratory shaker (IKA KS 3000 i control, IKA-Werke GmbH & Co.KG, Staufen, Germany). After this time, the prepared extract was separated from the mint leaves and then sterilized using syringe filters (Bionovo, Legnica, Poland). For this purpose, polyethersulfone filters with a pore diameter of 0.22 µm were used. Mint leaves after extraction were washed twice with distilled water and dried at room temperature. The dried mints were stored in a cardboard package for further testing.
2.2. Methods
2.2.1. Assessment of Fungicidal Properties
The influence of eight water extracts obtained from mint leaves on the growth of filamentous fungi was determined on a maltose-agar medium containing 2.5% maltose extract (Biomxima, Lublin, Poland) and 2.5% agar (BD and Company, Franklin Lakes, NJ, USA). Two species of fungi were used: Trichoderma viride Pers., strain A-102 and Chaetomium globosum Kunze, strain A-141 (ATCC 6205), from the pure culture collection of the Department of Wood Science and Wood Preservation SGGW in Warsaw. Appropriate amounts of mint extracts were added to the maltose-agar medium to obtain solutions of 5, 10, 15, 20, 40, and 60% (v/v). The fungi were centrally inoculated into Petri dishes (90 mm). The inoculum size of each fungus was 2–3 mm. The cultivation was carried out in a thermal incubator model Thermolyne Type 42,000 (ThermoFisher Scientific, Waltham, MA, USA) under the conditions of temperature and relative air humidity of 26 ± 2 °C and 63 ± 2%, respectively. The evaluation of the fungicidal effect of the addition of mint extracts on the fungal growth medium was carried out by measuring the diameter of mycelium growth in two perpendicular directions. Measurements were made at 48 h intervals. The tests were concluded on the day of complete overgrowth of the culture medium in the control samples. The control sample was the pure maltose-agar medium without any additives. The final result is given in “mm” as the average of fungi growth, measured in two directions.
The analysis of variance with the use of Snedecor statistics was used to verify the statistical analysis. Statistical inference was performed for the significance level α = 0.05. If the null hypothesis was rejected, Tukey’s test was performed. The statistical hypothesis was as follows: H0: Ø 0.5 = Ø 5 = Ø 10 = Ø 15 = Ø 20 = Ø 40 = Ø 60= ØK, H1: There were at least two means that differed significantly.
2.2.2. Assessment of the Degree of Fouling of the Impregnated Wood Surface
The mint extracts, which showed the most effective inhibition of fungal growth on the maltose-agar medium, were used for the surface impregnation of wood. Pine wood samples (Pinus sylvestris L.) with dimensions of 4 mm × 40 mm × 40 mm (last dimension along the fiber) were used. Wood samples contained only sapwood. The wood density was 400 kg/m3. Before saturation, the wood samples were sterilized in a steam autoclave (SMS, Warsaw, Poland). The sterilization process was carried out at 121 °C for 20 min.
The mint extract was dosed per area of wood (0.0016 m2) in such a way as to obtain the following concentrations of extract in the wood: 100, 200, 300, 400, 600, 800, and 1000 g/m2. To achieve a final wood extract concentration of 100, 200, and 300 g/m2, the starting extract had to be diluted accordingly, yielding dilutions of 25%, 50%, and 75%. Then, 0.64 g of each solution was taken and spread on the surface of the wood samples. To obtain an extract concentration of 400 g/m2, 0.64 g of undiluted extract had to be applied to the wood surface (0.0016 m2). To obtain extract concentrations in wood of 600, 800, and 1000 g/m2, 0.96 g, 1.28 g, and 1.6 g of undiluted extract had to be applied to the wood surface (0.0016 m2), respectively.
The application was carried out as follows: a sterile wood sample was placed on a sterile analytical balance, then the formulation was added using a sterile pipette and spread on one flat surface.
Only one top plane of the sample was secured, after only it was evaluated for mycelial growth progress. The development of mold on wood samples is superficial, at least in the first period of their growth. The duration of the study was so short that it was considered representative of the initial surface growth of mycelium on the wood surface.
The impregnated wood samples were placed on the maltose-agar medium using glass spacers, separating the direct influence of the medium on the wood. The samples were placed on the pads in such a way that the impregnated surface was on the side of the Petri dish cover, which made it possible to analyze the degree of fungal overgrowth of the sample on successive test days. The inoculum of molds T. viride and Ch. globosum was about 2–3 mm in size. The four inoculates were placed at a specific distance opposite the center of each edge of the sample (i.e., at four points in the medium in a cross pattern). This inoculation arrangement allowed us to track differences in the rate of mycelial growth on the upper surface of the sample from each side of the sample. After fungal inoculation on the maltose-agar medium, Petri dishes with wood samples were incubated in an incubator. Cultivation was carried out under temperature and humidity conditions of 25 °C and 66 ± 2%, respectively.
The degree of growth of the wood sample by fungi was determined on the basis of high-resolution photographic photos taken periodically for each tested sample. The growth of fungi on the sample was determined as the percentage of the growth of the sample by the mycelium in relation to the total area of the test sample.
The percentage value overgrowth of samples were determined with an accuracy of up to 5% with the support of image analysis software ImageJ2 (Fiji v.1.52i) [49].
An example of how to analyze the photo and thus how to determine the percentage of mycelial growth on the wood surface in relation to the total area of the wood sample is shown in Figure 2:
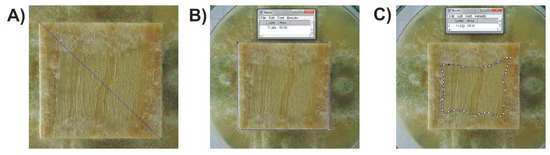
Figure 2.
Method (procedure) for determining the area of mycelium overgrowth on the top surface of a wood sample in ImageJ software.
The image analysis consisted of the following steps:
- (A)
- Determination of the area of the upper surface of the sample as 100%:
Step 1: Indication of sample diagonal [Function; ‘Straight Line’]—Figure 2A.
Step 2: Assigning values to the diagonal [Function: ‘Analyze’, Set Scale; Known distance’(= 14,142)].
Step 3: Verification of the correctness of the determination of the area of the whole sample in % (P100%) [Function: ‘Polygon Selection’; ‘Analyze’; ‘Measure’]—Figure 2B.
- (B)
- Determination of the area of overgrowth of the upper surface of the sample by mycelium:
Step 4: Determination of the % free field (Pf%) [performed similarly to step 3]—Figure 2C.
Step 5: Calculation of the area overgrown in % (Po%), according to Equation (1).
Po% =P100% − Pf%
2.2.3. Growth of Fungi on Plant Material
After extraction, the dried mint herbs were ground in a laboratory knife mill to the form of particles. Particles smaller than 0.3 mm were used for the tests. A total of 1 g of particles were weighed into Petri dishes and sterilized in a steam autoclave. The sterilization process was carried out at 121 °C for 20 min. Then, sterile mints were covered with 10 mL of agar, and after it solidified, the mycelial inoculum of Ch. globosum and T. viride was centrally inoculated. The control sample was the substrate with the mint particles not subjected to water extraction. Fungi growth was defined as the percentage of growth on the surface of the Petri dish relative to the total area of the dish.
2.2.4. GC-MS Analysis
The prepared water samples were evaporated on a vacuum evaporator (Buchi, Zurich, Switzerland, then poured into 10 cm3 ethanol (99.8%), (Chempur, Piekary Śląskie, Poland), and dissolved in an ultrasonic bath (SB3200 DTD, Chemland, Starograd Szczeciński, Poland) for 8 h. Samples after dissolution in ethanol were filtered through 0.43 µm nylon filters (Phenomenx, Torrance, CA, USA). The analysis of components was carried out on a gas Shimadzu chromatograph GC-2010 (Shimadzu, Kyoto, Japan) connected to the GCMS-QP2010 mass spectrometer. Chromatography was used with a capillary column ZB-5MS (Phenomenex, Torrance, CA, USA) with a length of 30 m, a diameter of 0.25 mm, and a deposit of 0.25 µm. Samples were introduced to the column with the AOC-20i autosampler. Samples were analyzed with the help of a dedicated program—GCMS solution Version 2.72 (Shimadzu, Kyoto, Japan), and the peaks were identified with libraries NIST11 and NIST11b.
GC-MS analysis program:
- Temperature: 50 °C maintained for 5 min, increase of 13 °C/min to 200 °C maintained for 5 min, increase of 13 °C/min to 300 °C maintained 10 min;
- Flow: 0.8 mL/min;
- Temperature of the injection: 250 °C;
- Detector voltage: 0.8 kV;
- Carrier gas: helium 5.0 (PGNiG, Warsaw, Poland);
- Injection mode: direct;
- Ion source temperature: 200 °C;
- Interface temperature: 200 °C.
3. Results and Discussion
Analyzing the effect of water extracts of various mint chemotypes on the growth of selected fungi on the maltose-agar medium, it was found that the degree of inhibition of the growth of microorganisms depended on the type of mint and the amount of extracts added to the medium.
It should be noted, however, that the concentration of active substances included in the freshly prepared extracts may be different than in the solidified culture medium, which is the result of the influence of the temperature of the medium on the water extract of mint. However, the practical aspect of the effectiveness of mint water extracts, where the effect of elevated temperature on the extracts is included in the technological process of their acquisition and application, the obtained results provide an initial idea of the expected effects of these extracts both in the conditions of the nutrient test and in the tests on wood.
Table 1 and Table 2 present the results of the evaluation of the effect of mint water extracts on the growth of T. viride and Ch. globosum. The strongest growth-inhibiting properties were obtained for the extract of M. piperita ‘Almira’ (No. 6) to a lesser extent, but also the growth inhibition of the test fungi was clearly caused by water extracts of M. spicata ‘Morocco’ (No. 3), M. spicata ‘Crispa’ (No. 5), and M. suaveolens ‘Variegata’ (No. 8). The growth inhibitory effect of Ch. globosum was clearly larger than T. viride. In addition, due to the rapid growth of T. viride mycelia on the agar medium, the assessment of the growth rate was completed on the fourth, and in some cases, even on the second day of cultivation. The strongest growth-inhibiting properties were observed for the highest concentrations of extracts. On the medium with the highest concentration of M. × piperita ‘Almira’ (No. 6), the growth of the tested fungi was completely inhibited (Figure 3 and Figure 4). A strong change in the color of the medium around the colonies of the fungus Ch. globosum growing on media containing the greatest amount of mint extract was also observed (Figure 3), which may be related to the activation of some physiological processes [50]. Lower concentrations of extracts in microbiological media also markedly inhibited the growth of the fungi. There were no statistically significant differences in the degree of inhibition of the development of the studied fungi on media with different concentrations of extracts of M. × piperita ‘Swiss’ (No. 1), M. rotundifolia (No. 4), and M. × piperita ‘Granada’ (No. 7), and in the case of the fungi Ch. globosum, also different concentrations of infusions from M. × piperita ‘Multimentha’ (No. 2).

Table 1.
Growth diameter of T. viride on a medium containing various amounts of mint extracts.

Table 2.
Growth diameter of Ch. globosum on a medium containing various amounts of mint extracts.
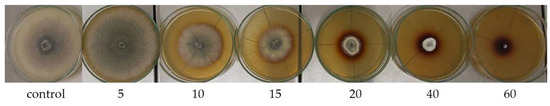
Figure 3.
Growth of Ch. globosum on a malt-agar medium containing various amounts (mL/100 mL) of mint extracts M. × piperita ‘Almira’.

Figure 4.
Growth of T. viride on a malt-agar medium containing various amounts (mL/100 mL) of mint extracts M. × piperita ‘Almira’.
The fungicidal effectiveness of the used mint extracts in practical use as a liquid impregnating wood surfaces has not been confirmed. In the final phase of the study, the same degree of covering of the surface of the control wood samples and the test fungi saturated with the test infusions was observed.
In the case of samples saturated with diluted mint extracts, only some differences in the growth of fungi in the initial phase of the experiment were observed compared to the growth of fungi in the control samples. In most cases, the presence of diluted mint extracts in the treated wood stimulated the development of fungi on the wood.
In the case of the wood samples, the surface of which was saturated with extracts of M. suaveolens ‘Variegata’, a lesser degree of surface fouling by the fungus Ch. globosum, in the first 5 days of the experiment in relation to fouling on a control wood were observed. In the case of wood impregnated with the M. spicata ‘Crispa’ extract, a stimulating effect of treatment was observed, the growth of Ch. globosum. In the case of research on the degree of fouling of T. viride on the wood samples, it should be concluded that the extracts used in the treated wood clearly stimulated the growth of the indicated species. In all of the research variants, the degree of the wood surface cover by T. viride was much stronger than in the control samples treated with water. The slight inhibition of fungal growth was observed in the first 6 days of cultivation on wood samples treated with the liquid with the lowest concentration of M. piperita ‘Almira’.
Figure 5, Figure 6, Figure 7 and Figure 8 show the results of the assessment of fungal development on wood samples containing various concentrations of mint extract. The surface of the wood samples, on which 400 g/m2 of mint extract was applied, intensive fungal growth was observed already in the first days of the abducted research (Figure 5a,b). However, with higher concentrations of extracts, a clear slowdown in fungal growth was observed, especially in the first two days of cultivation. Therefore, it should be concluded that mint extracts, whose concentration in the wood was at least 600 g/m2, have a fungistatic effect at the initial stage of fungal development. However, no fungicide effect was observed in any of the proposed variants of the experiment. At the highest retention of mint extract in wood, 1000 g/m2 (Figure 8a,b), a marked slowdown in the growth of Ch. globosum was observed (Figure 8b). This effect was particularly evident on wood saturated with M. piperita ‘Almira’ mint extract (No. 6). It can therefore be concluded that the M. piperita ‘Almira’ extract had the greatest impact on the development of fungi on wood.
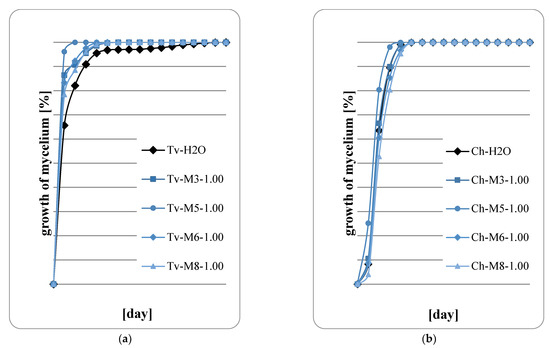
Figure 5.
Fungal growth on wood: (a) T. viride; (b) Ch. globusom (explanation of symbols in the legend: Ch-H20—control, 1.00—sample code indicating the application of 400 g of extract per m2, M3—M. spicata ‘Morocco’, M5—M. spicata ‘Crispa’, M6—M. × piperita ‘Almira’, M8—M. suaveolens’ Variegata’).
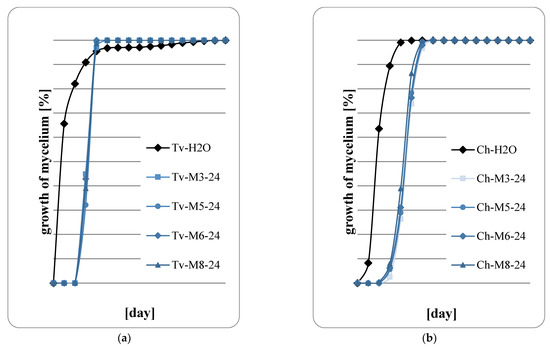
Figure 6.
Fungal growth on wood: (a) T. viride; (b) Ch. globusom (explanation of symbols in the legend: Ch-H20—control, 24—sample code indicating the application of 600 g of extract per m2, M3—M. spicata ‘Morocco’, M5—M. spicata ‘Crispa’, M6—M. × piperita ‘Almira’, M8—M. suaveolens’ Variegata’).
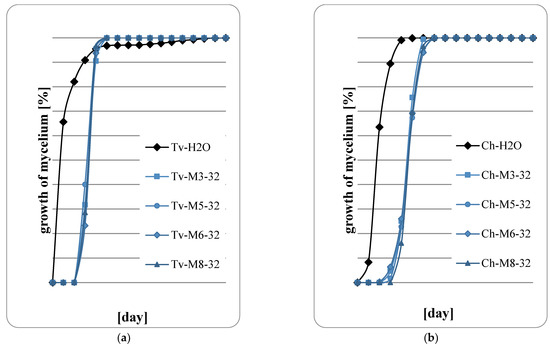
Figure 7.
Fungal growth on wood: (a) T. viride; (b) Ch. globusom (explanation of symbols in the legend: Ch-H20—control, 32—sample code indicating the application of 800 g of extract per m2, M3—M. spicata ‘Morocco’, M5—M. spicata ‘Crispa’, M6—M. × piperita ‘Almira’, M8—M. suaveolens’ Variegata’).
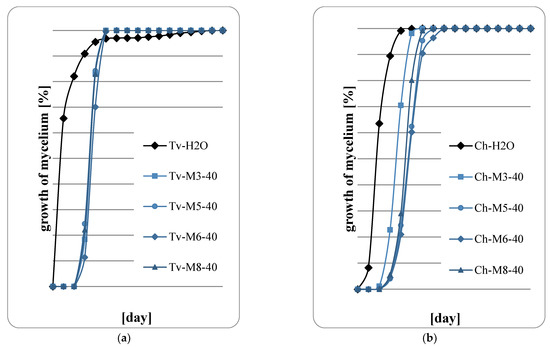
Figure 8.
Fungal growth on wood: (a) T. viride; (b) Ch. globusom (explanation of symbols in the legend: Ch-H20—control, 40—sample code indicating the application of 1000 g of extract per m2, M3—M. spicata ‘Morocco’, M5—M. spicata ‘Crispa’, M6—M. × piperita ‘Almira’, M8—M. suaveolens’ Variegata’).
The fungicidal effects of mint extracts on wood have not been found, however, it has been noticed that at higher doses of extracts, a fungicidal effect appeared in the early stages of fungal growth. Despite the unconfirmed fungicidal effect on wood, it cannot be ruled out that such an effect does not occur. This provides a starting point for searching for ways to enhance the fungicide effect for selected varieties of this plant (especially M. × piperita ‘Almira’) in further research (e.g., by using more concentrated extracts or seeking synergistic effects with other types of plant extracts). An important factor determining the fungicidal effectiveness may be the type of extract, its concentration, type and content of the active ingredient, but also the method of wood impregnation. Ali et al. [51] showed that extracts of M. longifolia L. in the form of essential oils effectively protect wood against Aspergillus niger, A. terreus, and A. flavus. El-Mohamedy [52] confirmed the strong fungicidal effect of M. arvensis L and M. piperita L. Similar observations were observed by Škrinjar et al. [39].
The results of fungal growth on the surface of mint herb before and after water extraction are presented in Figure 9, Figure 10, Figure 11 and Figure 12. In all of the analyzed cases, it was observed that the raw mint material, which was not extracted, slowed down or completely inhibited the growth of fungi. M. × piperita ‘Almira’ mint leaves, which were not extracted, when used as a growth medium, completely inhibited the growth of Chaetomium globosum (Figure 11). Therefore, it should be concluded that this raw material contains substances to which the Ch. globosum fungus is sensitive. Chromatography tests showed that the mint extracts used differed in part of their quantitative composition, and in the M. × piperita ‘Almira’ mint extract, nine chemical substances were identified that were not present in other mints (Table 3). It can therefore be assumed that the identified substances may have a potential inhibitory effect on the growth of the Ch. globosum fungus.
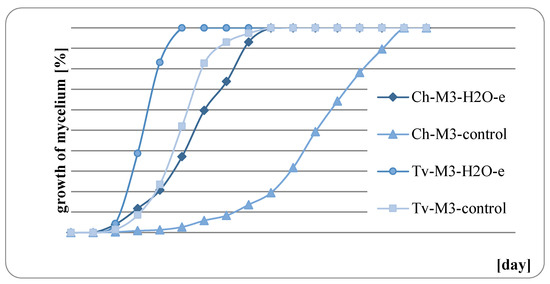
Figure 9.
Growth of fungi on M. spicata ‘Morocco’ with and without extraction.
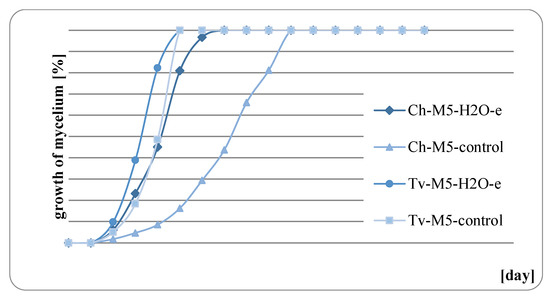
Figure 10.
Growth of fungi on M. spicata ‘Crispa’ with and without extraction.
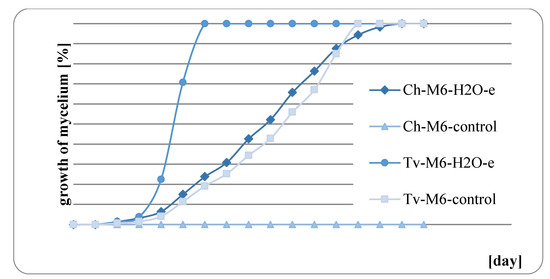
Figure 11.
Growth of fungi on M. × piperita ‘Almira’ with and without extraction.
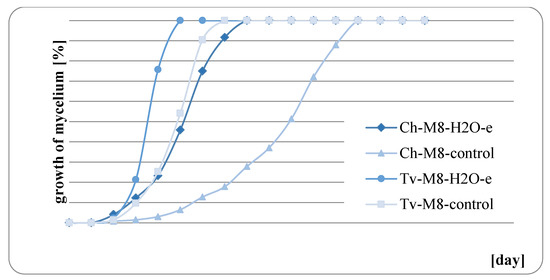
Figure 12.
Growth of fungi on M. suaveolens ‘Variegata’ with and without extraction.

Table 3.
Chemical composition of the water mint extracts.
At the same time, it was found in each of the analyzed cases that the growth of fungi on the medium with the addition of mint herb, previously extracted, was much stronger. It can therefore be concluded that substances with a potential fungistatic or fungicidal effect get into the aqueous solution during the extraction process.
Using the GC-MS technique, the characteristics of the substance composition in the extract water are presented (Figure 13, Table 3). Menthol was the predominant component in the M. spicata ‘Morocco’ and M. spicata ‘Crispa’ extracts. High carvone concentrations were identified in all of the analyzed extracts. Three components ((S)-cis-Verbenol, Isocaryophyllene, .alpha.-Bourbonene) were identified in the M. suaveolens ‘Variegata’ extract, which were not found in the remaining mints. The greatest differences in the chemical composition of the analyzed mints were found for M. × piperita ‘Almira’, in which as many as nine components were identified that were not detected in the other extracts. Similar reports on the composition of the volatile substances of mint herb can be found in the literature. In studies conducted by Mohammadhosseini et al. [53], the dominance of containing oxygen monoterpenes was confirmed in the volatile fraction of oils from M. pulegium L. The qualitative assessment of substances contained in extracts of various mint varieties and the possibility of identifying their antifungal properties on wood samples may allow for the identification of mint varieties with high fungicide potential in the future. The evaluation of fungicidal effectiveness combined with chemical analysis techniques also enable better characterization of the fungicide variability of the different varieties of this plant.
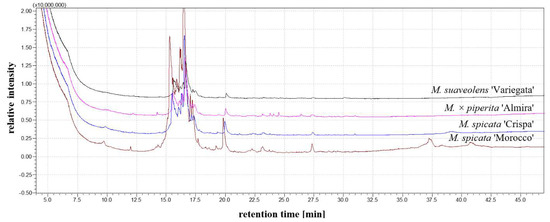
Figure 13.
Mentha spp. chromatogram.
4. Conclusions
The results obtained in this study indicate that water mint extracts inhibit the growth of Trichoderma viride and Chaetomium globosum fungi on an agar medium. Although no fungistatic effect was observed on the pine wood samples, the effect of inhibiting the growth of fungi on the wood surface was visible in the first two or three days after inoculation on the wood samples that were treated with higher concentrations of mint extracts. Among the tested mint extracts, the best results were obtained for the variety M. × piperita ‘Almira’. In this mint variety, nine substances were identified that were not present in the other extracts analyzed. It is likely that these substances are responsible for the complete inhibition of the growth of the Chaetomium globosum fungus on the agar medium with the addition of mint leaves.
It is likely that the use of other wood impregnation methods would result in better fungicide properties. Equally, other types of mint extracts could positively increase the biocidal efficacy, but the properties of aqueous mint extracts should be recognized as widely as possible before practical attempts are made to develop wood preservatives based on such natural substances. It should be borne in mind that extracts from Mentha sp. contain numerous volatile substances, the quantity and quality of which may change during the wood impregnation process and its use. Therefore, the different results obtained in our study for the same mint extract between the in vitro and wood tests may be related to differences in the extract composition.
Even if other authors have achieved a better efficacy of mint extracts, our study contributes due to the fact that mint extracts are not always very effective, which should lead other authors to be cautious about formulating overly optimistic conclusions.
Author Contributions
Conceptualization, I.B.; Methodology, I.B., B.A., D.S. and K.K.; Software, B.A.; Validation, B.A.; Formal analysis, I.B., A.K.-D. and B.A.; Investigation, I.B.; Resources, I.B. and B.A.; Data curation, I.B. and D.S.; Writing—original draft preparation, I.B.; Writing—review and editing, B.A., K.K., D.S., J.Z. and A.K.-D.; Visualization, I.B.; Supervision, I.B.; Project administration, I.B. and B.A.; Funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as biopesticides against the pinewood nematode Bursaphelenchus xylophilus: A Review on essential oils and their volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M. Antifungal Agents in Wood Protection—A Review. Molecules 2022, 27, 6392. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.A.M.; Samsudin, N.; Benbelgacem, F.F.; Hashim, Y.Z.H.-Y.; Salleh, H.M.; Santhanam, J. Phytochemicals with antifungal properties, Cure from nature. Malays. J. Microbiol. 2020, 16, 2231–7538. [Google Scholar] [CrossRef]
- Asamoah, A.; Frimpong-Mensah, K.; Antwi-Boasiako, C. Efficacy of Erythropleumsuaveolens(potrodom) and Distemonanthusbenthamianus (bonsamdua) water extractives on the durability of five Ghanaian less used timber species of varying perviousness and retentiveness. J. Ind. Acad. Wood Sci. 2014, 11, 72–81. [Google Scholar] [CrossRef]
- Betlej, I.; Andres, B.; Krajewski, K. Evaluation of fungicidal effects of post-culture medium of selected mold fungi and bacteria in relation to Basidiomycetes fungi, causing wood destruction. BioResources 2020, 15, 2471–2482. [Google Scholar] [CrossRef]
- Broda, M. Natural compounds for wood protection against fungi—A review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef]
- Abanikannda, J.P.; Adetogun, A.C.; Mukhtar, R.B. Evaluation of honey bee propolis as wood preservative using weight loss. Sci. World J. 2020, 5, 45–47. [Google Scholar]
- Betlej, I.; Andres, B.; Szadkowska, D.; Krajewski, K.; Ościłowska, A. Fungicidal properties of the medium from SCOBY microorganism cultivation in saturated wood against Coniophora puteana fungus. BioResources 2021, 16, 1287–1295. [Google Scholar] [CrossRef]
- Betlej, I.; Krajewski, K. Application of wood destroying fungi in biotechnological processes. Ochr. Przed Koroz. 2012, 55, 32–35. [Google Scholar]
- Tascioglu, C.; Yalçın, M.; Şen, S.; Akcay, C. Antifungal properties of some plant extracts used as wood preservatives. Int. Biodeterior. Biodegrad. 2013, 85, 23–28. [Google Scholar] [CrossRef]
- Sumthong, P.; Romero-González, R.R.; Verpoorte, R. Identification of anti-wood rot compounds in teak (Tectona grandis L.f.) sawdust extract. J. Wood Chem. Technol. 2008, 28, 247–260. [Google Scholar] [CrossRef]
- Chittenden, C.; Singh, T. Antifungal activity of essential oils against wood degrading fungi and their applications as wood preservatives. Int. Wood Prod. J. 2011, 2, 44–48. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Chanotiya, C.S.; Dubey, N.C. Antifungal, antiaflatoxigenic, and insecticidal efficacy of spearmint (Mentha spicata L.) essential oil. Int. Biodeterior. Biodegrad. 2014, 89, 29–36. [Google Scholar] [CrossRef]
- Ngo-Mback, M.N.L.; Famewo, E.B.; Mubarak Ali, D.; Eke, P.; Thajuddin, N.; Afolayan, A.J.; Jazet Dongmo, P.M.; FekamBoyom, F. An investigation of chemical composition and antimicrobial activity of essential oils extracted from Aeollanthus and Plectranthus species. Biocatal. Agric. Biotechnol. 2019, 22, 101412. [Google Scholar] [CrossRef]
- Cheng, S.S.; Lin, C.-Y.; Gu, H.-J.; Chang, S.-T. Antifungal activities and chemical composition of wood and leaf essential oils from Cunnin ghamiakonishii. J. Wood Chem. Technol. 2011, 31, 204–217. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Cofta, G.; Nowak, P.B. Resistance of fungal growth on Scots pine treated with caffeine. Int. Biodeterior. Biodegrad. 2018, 132, 178–184. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Woźniak, M.; Jankowski, W.; Ratajczak, I.; Cofta, G. Chemical changes of wood treated with caffeine. Materials 2021, 14, 497. [Google Scholar] [CrossRef]
- Ratajczak, I.; Woźniak, M.; Kwaśniewska-Sip, P.; Szentner, K.; Cofta, G.; Mazela, B. Chemical characterization of wood treated with a formulation based on propolis, caffeine and organosilanes. Eur. J. Wood Wood Prod. 2018, 76, 775–781. [Google Scholar] [CrossRef]
- Tomak, E.D.; Gonultas, O. The wood preservative potentials of valonia, chestnut, tara and sulphited oak tannins. J. Wood Chem. Technol. 2018, 38, 13–197. [Google Scholar] [CrossRef]
- Hussain, A.; Shrivastav, A.; Jain, S.K. Antifungal activity of essential oils against local wood degrading cellulolytic filamentous fungi. Adv. Biores. 2013, 4, 161–167. [Google Scholar]
- Pánek, M.; Reinprecht, L.; Hulla, M. Ten essential oils for beech wood protection—Efficacy against wood-destroying fungi and moulds, and effect on wood discoloration. BioResources 2014, 9, 5588–5603. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas Cienc. Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Yang, V.W.; Clausen, C.A. Antifungal effect of essential oils on southern yellow pine. Int. Biodeterior. Biodegrad. 2007, 59, 302–306. [Google Scholar] [CrossRef]
- Chang, T.-C.; Chang, S.-T.; Cheng, S.-S. Antioxidant activities of ethanolic extract and lyoniresinol from bark of Zelkova serrata. J. Wood Chem. Technol. 2022, 42, 265–273. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Singla, S.K.; Bhardwaj, R.K. Evaluation of plant extracts as antifungal agents against wood rotting fungi Coriolus versicolor (L.: Fr.) Quelet. J. Ind. Acad. Wood Sci. 2012, 9, 62–65. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Mamone, G.; Ferranti, P.; Ercolini, D.; Mauriello, G. Changes in the proteome of Salmonella enterica serovar Thompson as stress adaptation to sublethal concentrations of thymol. Proteomics 2010, 10, 1040–1049. [Google Scholar] [CrossRef]
- Hafedh, H.; Fethi, B.A.; Mejdi, S.; Emira, N.; Amina, B. Effect of Mentha longifolia L. ssp. longifolia essential oil on the morphology of four pathogenic bacteria visualized by atomic force microscopy. Afr. J. Microbiol. Res. 2010, 4, 1122–1127. [Google Scholar]
- Kiełtyka-Dadasiewicz, A.; Jabłońska-Trypuć, A.; Taraseviciene, Z.; Kubat-Sikorska, A. Characteristics and functional properties of mint’s raw materials. Pol. J. Commod. Sci. 2016, 1, 93–105. [Google Scholar] [CrossRef]
- Shahbazi, Y. Chemical composition and in vitro antibacterial activity of Mentha spicata essential oil against common food-borne pathogenic bacteria. J. Pathog. 2015, 2015, 916305. [Google Scholar] [CrossRef]
- Singh, R.; Shushin, M.A.M.; Belkheir, A. Antibacterial and antioxidant activities of Mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Moghtader, M. In vitro antifungal effects of the essential oil of Mentha piperita L. and its comparison with synthetic menthol on Aspergillus niger. Afr. J. Plant Sci. 2013, 7, 521–527. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Kiełtyka-Dadasiewicz, A.; Sawicki, R.; Golus, J.; Ginalska, G. Essential oils of some Mentha species and cultivars, their chemistry and bacteriostatic activity. Nat. Prod. Commun. 2016, 11, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.A.; Lemos, M.J.; Brito, D.M.C.; Fernandes, M.S.; Castro, R.N.; Souza, S.R. Production and quality of menthol mint essential oil and antifungal and antigerminative activity. Am. J. Plant Sci. 2014, 5, 3311–3318. [Google Scholar] [CrossRef]
- Ejaz, R.; Malik, S.; Ahmad, M.; Ali, H.; Choudhry, S. Anti-biofilm potential of menthol purified from Mentha piperita L. (mint). Biol. Clin. Sci. Res. J. 2020, 2020, 37. [Google Scholar] [CrossRef]
- Satoh, M.; Kusumoto, N.; Matsui, N.; Makino, R.; Hashida, K.; Arai, D.; Iiduka, Y.; Ashitani, T. Antitermitic and antifungal properties of enantiopure linalool and furanoid linalool oxide confirmed in Lindera umbellata var. membranacea. J. Wood Chem. Technol. 2022, 42, 37–45. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Motamedi, M.; Zomorodian, K.; Pakshir, K.; Miri, R.; Hemyari, K. Chemical composition, antifungal and antibiofilm activities of the essential oil of Mentha piperita L. Int. Sch. Res. Not. 2012, 2012, 718645. [Google Scholar] [CrossRef]
- Škrinjar, M.M.; Mandić, A.I.; Mišan, A.Č.; Sakač, M.B.; Šarić, L.Ć.; Zec, M.M. Effect of mint (Mentha piperita L.) and caraway (Carum carvi L.) on the growth of some toxigenic Aspergillus species and aflatoxin B1 production. Zb. Matice Srp. Prir. Nauk. 2009, 116, 131–139. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Scales, D.; Mandras, N. Evaluation of the antifungal activity of Mentha x piperita (Lamiaceae) of pancalieri (Turin, Italy) essential oil and its synergistic interaction with azoles. Molecules 2019, 24, 3148. [Google Scholar] [CrossRef]
- Ali, J.; Hussain, A.; Rehman, S.; Khan, F.A.; Sher, M. Antifungal potential of Mentha piperita leaves and stem extracts against phytopathogenic fungi. Spec. J. Biol. Sci. 2017, 3, 38–43. [Google Scholar]
- Şen, S.; Yalçın, M. Activity of commercial still waters from volatile oils production against wood decay fungi. Maderas Cienc. Tecnol. 2010, 12, 127–133. [Google Scholar] [CrossRef]
- Verma, R.K.; Chaurasia, L.; Kumar, M. Antifungal activity of essential oils against selected building fungi. Indian J. Nat. Prod. Resour. 2011, 2, 448–451. [Google Scholar]
- Perveen, K.; Bokahri, N.A. Management of Alternaria leaf blight in tomato plants by mentha essential oil. Plant Prot. Sci. 2020, 56, 191–196. [Google Scholar] [CrossRef]
- Panda, P.; Aiko, V.; Mehta, A. Effect of aqueous extracts of Mentha arvensis (mint) and Piper betle (betel) on growth and citrinin production from toxigenic Penicillium citrinum. J. Food Sci. Technol. 2015, 55, 3466–3474. [Google Scholar] [CrossRef]
- El-Said, M.A.; Hassan, R.G. Evaluation of the antimicrobial activity of aqueous extract of mint leaves and basil leaves for using in water purification. Egypt J. Appl. Sci. 2021, 36, 41–50. [Google Scholar]
- Prado, J.M.; Leal, P.F.; Meireles, A.A. Comparison of manufacturing cost of thyme extract obtained by supercritical fluid extraction and steam distillation. In Proceedings of the 9th International Symposium on Supercritical Fluids, New trends in Supercritical Fluids: Energy, Materials, Processing, Arcachon, France, 18–20 May 2009. [Google Scholar]
- Veggi, P.C.; Prado, I.M.; Vaz, N.; Prado, J.M.; Meireles, M.A.A. Manufacturing cost of extracts from jackfruit (Artocarpus heterophyllus) leaves obtained via supercritical technology and solvent extraction. In Proceedings of the 9th International Symposium on Supercritical Fluids, New trends in Supercritical Fluids: Energy, Materials, Processing, Arcachon, France, 18–20 May 2009. [Google Scholar]
- Borysiuk, P.; Krajewski, K.; Auriga, A.; Auriga, R.; Betlej, I.; Rybak, K.; Nowacka, M.; Boruszewski, P. PLA Biocomposites: Evaluation of resistance to mold. Polymers 2022, 14, 157. [Google Scholar] [CrossRef]
- Górski, R.; Dorna, H.; Rosińska, A.; Szopińska, D.; Kałużewicz, A. Effects of essential oils on in vitro growth of fungi Cladobotryum dendroides and Mycogone perniciosa infecting button mushroom. Ecol. Chem. Eng. S. 2021, 28, 411–427. [Google Scholar] [CrossRef]
- Ali, H.M.; Abo Elgat, W.A.A.; EL-Hefny, M.; Salem, M.Z.M.; Taha, A.S.; Al Farraj, D.A.; Elshikh, M.S.; Hatamleh, A.A.; Abdel-Salam, E.M. New approach for using of Mentha longifolia L. and Citrus reticulata L. essential oils as wood-biofungicides: GC-MS, SEM, and MNDO quantum chemical studies. Materials 2021, 14, 1361. [Google Scholar] [CrossRef]
- El-Mohamedy, R.S.R. Plant essential oils for controlling plant 9 pathogenic fungi. In Volatiles and Food Security. Role of Volatiles in Agro-Ecosystems; Choudhary, D.K., Sharma, A.K., Agarwal, P., Varma, A., Tuteja, N., Eds.; Springer: Singapore, 2017; pp. 171–198. [Google Scholar]
- Mohammadhosseini, M.; Venditti, A.; Mahdavi, B. Characterization of essential oils and volatiles from the aerial parts of Mentha pulegium L. (Lamiaceae) using microwave-assisted hydrodistillation (MAHD) and headspace solid phase microextraction (HS-SPME) in combination with GC-MS. Nat. Prod. Res. 2021, 30, 338–342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).