Abstract
This research provides an extensive analysis of the biodiversity and distribution patterns of copepod crustaceans associated with octocoral species. A comprehensive dataset comprising 966 records pertaining to 233 copepod species, encompassing 54 genera, 18 families, and 3 orders, was compiled from 92 scientific papers published between 1858 and 2023, and updated as open data to GBIF. These copepods were found to be closely associated with 183 octocoral species, representing 72 genera and 28 families. The analysis revealed a total of 393 distinct interspecific associations between copepods, classified under the orders Cyclopoida, Harpacticoida, and Siphonostomatoida, and diverse octocorals. Approximately 60% of these associations were reported only once in the literature, which poses challenges to assessing the level of host specificity among the majority of copepod species linked with octocorals. Notably, over 91% of the recorded copepod species were found at depths not exceeding 30 m, with only four copepod species reported at greater depths surpassing 500 m. The presence of these symbiotic copepods was documented across 215 sampling sites situated within 8 of the 12 defined marine ecoregions, with particular attention to the Western Indo-Pacific, Central Indo-Pacific, and Temperate Northern regions. Despite the comprehensive examination of available data, this study highlights substantial gaps in our comprehension of copepod crustacean diversity and distribution in association with octocorals. Moreover, crucial information concerning symbiotic copepods is conspicuously absent for approximately 94% of potential octocoral host species. These disparities emphasize the imperative need for further scientific inquiry to unveil the intricacies of symbiotic relationships and to contribute to a more holistic understanding of copepod–octocoral associations.
1. Introduction
Copepoda (Crustacea) are diminutive crustaceans renowned for their remarkable diversity and pivotal roles in aquatic ecosystems [1,2,3]. They adapted to thrive in an array of environments and often established commensal and parasitic associations across a broad spectrum of animal taxa [1,4,5,6,7]. This intrinsic capability to forge intimate bonds with an extensive repertoire of animal taxa has contributed to the extraordinary morphological diversity observed within copepods, an outcome of their colonization of diverse host groups. Nevertheless, despite persistent endeavors to elucidate the phylogenetic underpinnings of copepods and the evolutionary trajectories governing their symbiotic liaisons with various organismal assemblages [6,8,9], a multitude of questions remains unresolved or encircled by controversy [10,11]. Much of this predicament is rooted in the paucity or absence of comprehensive molecular and other empirical datasets encompassing numerous copepod taxa participating in symbiotic associations with diverse invertebrates [3,12].
Extensive investigations have been conducted into the diversity of ecto- and endo-symbiotic copepods affiliated with Cnidaria, particularly within hexacorals and octocorals [2,13,14]. The wealth of published data, including our own yet-to-be-published findings, unveils an exceptionally high and comparatively underexplored diversity of copepods cohabiting with cnidarians and other invertebrate cohorts such as sponges and echinoderms, among others. Nevertheless, the extant knowledge concerning these copepods dwelling amidst cnidarians predominantly comprises taxonomic characterizations, occurrence records, depth-related information, host nomenclature, and several check lists [13,15,16,17,18]. The compiled data allude to the potential occurrence of numerous instances of host switching and a multifarious spectrum of geographical distribution patterns and host specificity among copepods that form associations with cnidarians and other invertebrates [12,13,19,20].
The class Octocorallia (Cnidaria: Anthozoa), characterized by its considerable size and diversity, thrives across marine ecosystems spanning from tropical shallow waters to abyssal depths, contributing significantly to the provision of habitats for a myriad of marine single-cell and multicellular organisms [21,22,23,24,25]. Despite their ecological importance, both octocorals and their symbiotic counterparts have garnered relatively less scientific scrutiny when juxtaposed with reef-building scleractinian corals. The limited understanding of octocoral species diversity, a predicament shared with numerous other invertebrate groups, can primarily be ascribed to the scarcity of taxonomists and the existence of numerous cryptic or yet-undescribed species [12,26]. Currently, a tally of approximately 3500 validated octocoral species exists; however, it is posited that this figure merely accounts for 30% of the total species that await formal taxonomic delineation. Nevertheless, recent investigations have underscored the pivotal role played by Octocorallia in shallow and deep-water ecosystems, shed light on the adverse repercussions of shallow and deep-water fishing, and bolstered biomaterials science research, while also unveiling disease outbreaks that impact octocoral populations [22,25,27].
Octocorals like scleractinians are susceptible to diseases, although their study in this regard remains relatively limited [28,29,30]. These diseases may manifest as discoloration, tissue impairment, lesions, or atypical growth patterns within coral colonies. The etiologies of octocoral diseases are multifarious, encompassing microbial pathogens, environmental stressors, shifts in water quality, or interactions with other organisms. Notably, the presence of gall-forming and other symbiotic copepods on octocorals raises pertinent queries concerning the potential implication of copepods in the genesis and transmission of coral diseases. An exemplar of significance is the pervasive multifocal purple spots observed in the Caribbean Sea fan Gorgonia ventalina, an occurrence recently attributed to the presence of gall-inducing lamippid copepods [24,28,31,32]. This accentuates the conceivable role of ostensibly parasitic copepods in the realm of coral diseases, thereby beckoning further investigations into their interactions and ramifications for coral health.
The primary objective of this research paper is to undertake a pioneering endeavor in collating and scrutinizing all extant records pertaining to the association between copepods and octocorals. Given the dispersed nature of these data and the conceivable significance of these minuscule symbionts in the context of corals and coral communities, this endeavor aspires to enhance our comprehension of the intricate interplay between copepods and octocorals. Through this initiative, we aim to furnish insights into the breadth of diversity and the contemporary state of knowledge regarding these relationships, while also envisaging prospects and potential avenues for further exploration in this domain.
2. Materials and Methods
To compile the requisite information, we conducted a comprehensive review of all 92 identified papers, which provide descriptions and/or document records of copepods associated with octocoral corals (Table 1, Table A1 and Table S1). Subsequently, we integrated these data into an original database utilizing Microsoft Access software. (Version 16.0) The database “Global diversity and distributions of symbiotic copepod crustaceans living on octocorallians” is structured around five primary tables: Host Taxonomy, Host Synonymy, Symbiont Taxonomy, Symbiont Synonymy, and Symbiont Descriptions. These tables are intricately linked through the Records table. Within the database, each entry encompasses comprehensive details regarding the taxonomy of both the host and its symbiont, and these details are cross-referenced with unique identifiers for each taxon as listed in the World Register of Marine Species (WoRMS database) [33].

The dataset employed for the analysis features 62 columns filled with metadata and details relevant to taxonomy, habitat features, and associations with host species, as detailed in Table A2. For the purpose of elucidating the methodologies employed in the collection of these records. These collection techniques encompass a spectrum of approaches, including SCUBA diving, bottom trawling, utilization of Remotely Operated Vehicles (ROVs), dredging operations, snorkeling, and manual hand sampling. Sampling locations, including geographical names and coordinates, sampling depths, and dates, have been incorporated into the dataset entries, conforming to Darwin Core standards [119]. This meticulous approach ensures a comprehensive and standardized representation of crucial contextual information associated with each record, thereby facilitating a deeper understanding and improved interoperability.
The classification of oceanic ecoregions aligns with the methodology advocated by Spalding et al. [120]. To visualize and generate plots, we employed RStudio version 1.2.5001, harnessing the capabilities of various packages such as tidyverse [121], dplyr [122], ggplot2 [123], ggExtra [124], ggpubr [125], gridExtra [126], magrittr [127], maps [128], stringr [129], and RColorBrewer [130]. Additionally, all graphical representations were crafted using Adobe Photoshop CC.
2.1. Dataset Description
The dataset is organized following the Darwin Core Standard [119]. Each row within the dataset represents a record of a copepod taxon obtained from various samples, as documented in the literature. The columns within the dataset encompass both the original and revised taxon names, supplementary taxonomic details, as well as data regarding the geographical location, environmental parameters, and the source of the data.
Object name: Global diversity and distributions of symbiotic copepod crustaceans living on octocorallians.
Occurrence dataset: https://doi.org/10.15468/msp4n8 (accessed on 1 October 2023).
GBIF:
Character encoding: UTF-8
Format name: csv
Format version: 1.5
Distribution: https://www.gbif.org/dataset/be8f0b51-2030-4402-80e9-01095875de64 (DOI: doi.org/10.15468/msp4n8)
Date of creation: 11 November 2020
Date of last revision: 2 October 2023
Date of publication: 3 October 2023
Update policy: The dataset in GBIF is updated as additional data are accumulated.
Language: English
Licence of use: Access and use are free to any user (CC-BY 4.0). The authors would appreciate users providing a link to the original dataset (GBIF: https://www.gbif.org/dataset/be8f0b51-2030-4402-80e9-01095875de) or citing the present paper when using the data in research projects.
Metadata language: English
2.2. Management Details
Project title: Global diversity and distributions of symbiotic copepod crustaceans living on octocorallians.
Temporal coverage: The present dataset includes all the records of copepods published in the literature between 1858 and 2023.
Record basis: Literature records
2.3. Geographic Coverage
Geographical Scope: World Ocean. The information is georeferenced using WGS 84 standards. Where coordinates were provided in the source, they were retained. When only a sampling site description was available, coordinates were determined to the highest degree of precision possible, and any uncertainty was noted in a separate column. In certain instances, there was no georeferenced information.
Geographical Subcategories: The World Ocean.
Sampling Approach: The overarching approach was to acquire all the published records of copepods known across the entirety of the World Ocean.
Habitat Classification: Details on habitat types were extracted from the source literature and are represented as originally denoted. There was no effort made to standardize the habitat classifications.
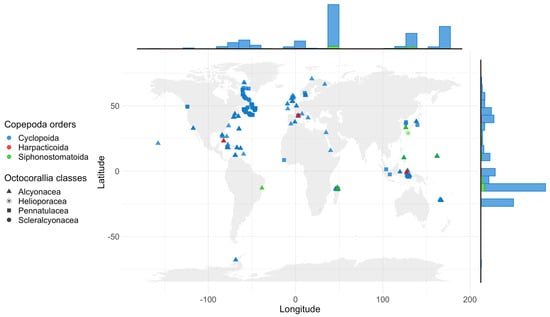
Biogeographical Regions: Following the categorization by Spalding et al. [120], the dataset encompasses 8 of 12 biogeographical domains: Arctic, Central Indo-Pacific, Eastern Indo-Pacific, Southern Ocean, Temperate Northern Atlantic, Temperate Northern Pacific, Tropical Atlantic, and Western Indo-Pacific.
Countries: Barbados, Bermuda, Bonaire, Brazil, Canada, Bahamas, Cuba, Curaçao, Eritrea, France, Greenland, Iceland, Indonesia, Ireland, Israel, Italy, Jamaica, Japan, Madagascar, Marshall Islands, Mayotte, New Caledonia, Norway, Philippines, Puerto Rico, Republic of Korea, Russia, Saba, Saint Martin, Singapore, Sint Eustatius, Spain, Sweden, United Kingdom, USA.
Verification of Geographic Data: Coordinate reliability was evaluated using Google Maps to confirm the accuracy of the provided locations. This process involved verifying the format of geographic coordinates, ensuring that the coordinates fell within the appropriate regional boundaries, and checking for any irregular ASCII symbols in the dataset.
2.4. Literature Review
General Overview: The data regarding copepod living on octocorals discoveries are sourced from articles published in scientific publications.
Literature Search Methods: A comprehensive literature search was performed using academic search engines Google Scholar, Scopus, and Web of Science. Various keywords were utilized to refine the search and target specific organism pairs, such as Copepoda, copepods, copepod crustaceans, Octocorallia, octocorals, Alcyonacea, Gorgoniidae, sea pens, and gorgonians. Each identified publication underwent a thorough examination to extract relevant information, and any supplementary references cited within these publications were also meticulously reviewed and assessed.
Compilation of Literature: The 92 identified references contain information on copepods, at least at the family level (Table 1).
Quality Assurance for Literary Data: The search for additional literature concluded when no further references could be identified in the bibliographies of the analyzed papers.
2.5. Taxonomic Coverage
General Overview: The dataset is exclusively comprised of crustaceans from the subclass Copepoda, which serve as symbionts, and those from the class Octocorallia, which act as hosts.
Taxonomic Levels: Information in the dataset spans entries with taxonomic classification ranging from the subspecies to the order level.
Taxonomic Approaches: The accuracy and validity of taxonomic names mentioned in the published literature were verified using the World Register of Marine Species (WoRMS database) [33]. Names that were marked as “accepted” were retained in the “Records” table. In cases where a name had undergone a taxonomic change, the proposed alternative with an “accepted” status in the WoRMS database was adopted in the “Records” table. The original name mentioned in the initial article was recorded as a synonym in either the “Symbiont synonyms” or “Host synonyms” table, as appropriate. Only names that held the “accepted” status in the WoRMS database were included in the GBIF dataset, and synonyms were excluded from the dataset.
Quality Assurance for Taxonomic Data: The verification and updating of nomenclature were performed by cross-referencing the data with the WoRMS database.
3. Results
The dataset regarding copepods inhabiting octocorals in the World Ocean is derived from an extensive analysis of scholarly articles published between 1858 and 2023. Remarkably, the rate of publication was rather low until the 1960s, with an average of two articles per decade. Subsequently, there was a notable increase in reported research during the 1960s–1970s and 2000s–2010s, with 12, 18, 17, and 11 articles published during these respective decades (Figure 1). This dataset has been curated and made accessible via the GBIF website (https://doi.org/10.15468/msp4n8). Within this dataset, a total of 966 occurrence records have been documented, with a remarkable 961 records (constituting 99.5%) accompanied by georeferenced coordinates, ensuring precise spatial referencing.
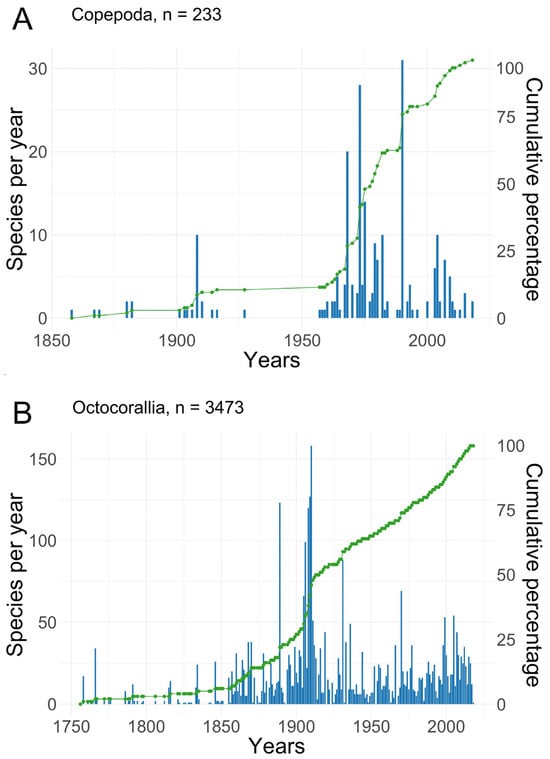
Figure 1.
Numbers of new species and cumulative percentage (green line) of known species of (A) octocorals and associated with them (B) symbiotic copepods described published over time. Based on the WoRMS database [33].
The compiled database encompasses 966 entries, providing a comprehensive overview of the symbiotic associations between copepods and octocorals (Table 2, Table A1 and Table S1). These entries include data on 236 copepod species, spanning 54 genera and 18 families within the orders Cyclopoida, Harpacticoida, and Siphonostomatoida (Figure 2). These copepods exhibit diverse forms of association, ranging from residing in host tissues, galls, and the digestive tract to residing on the surfaces of 183 octocoral species, representing 72 genera and 28 families.

Table 2.
Numbers of Copepoda taxa known and recorded on octocorals *.
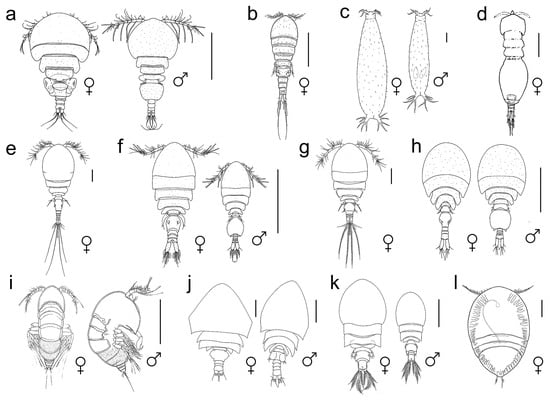
Figure 2.
Habitus of copepod crustaceans living on octocorals: (a)—Panjakus auriculatus (Anchimolgidae), dorsal view, scale bar 0.5 mm [44]; (b)—Hippomolgus latipes (Clausidiidae), dorsal view, scale bar 0.5 mm [112]; (c)—Enalcyonium digitigerum (Lamippidae), dorsal view, scale bar 0.1 mm [91]; (d)—Paranotodelphys procax (Notodelphyidae), dorsal view, scale bar 0.2 mm [116]; (e)—Tubiporicola inflatus (Pseudanthessiidae), dorsal view, 0.5 mm [117]; (f)—Paramolgus litophyticus (Rhynchomolgidae), dorsal view, 0.5 mm [44]; (g)—Eupolymniphilus brevicaudatus (Sabelliphilidae), dorsal view, 0.2 mm [117]; (h)—Forhania philippinensis (Thamnomolgidae), dorsal view, 0.4 mm [56]; (i)—Parategastes conexus (Tegastidae), dorsal view, 0.2 mm [40]; (j)—Cryptopontius phyllogorgius (Artotrogidae), dorsal view, 0.2 mm [98]; (k)—Orecturus finitimus (Asterocheridae), dorsal view, 0.3 mm [95]; (l)—Entomopsyllus takara (Entomolepididae), dorsal view, 0.2 mm [98] (a–h)—Cyclopoida, (i)—Harpacticoida, (j–l)—Siphonostomatoida.
The analysis shows that 955 entries underwent species-level identification of copepods discovered in association with octocorals. Moreover, eight entries were ascribed to taxa categorized at the genus level, while an additional six entries were linked to taxa positioned at the family level within the taxonomic hierarchy. The data indicate that precise identification at the species level was applied to 912 entries, encompassing a diverse spectrum of 183 distinct species. Furthermore, 51 entries were intricately connected with taxa classified at the genus level, and a solitary entry was affiliated with a taxon categorized at the order level.
A total of 74 copepod species and 53 coral species had undergone changes in their species or generic names since their description in the original papers. Two genera (Alcyonicola and Metaxymolgus) of copepods and nine genera of corals have been synonymized with other genera as junior synonyms since their description. Taking account of these taxonomic and nomenclatorial changes is essential when evaluating data from primary sources. Failing to accommodate these changes can significantly skew the results of the analyzed data, potentially resulting in flawed conclusions and misinterpretations.
The primary methods employed for collecting copepod–octocoral association data are available for 423 records, encompassing 44% of the entire dataset. These include SCUBA diving (30% of cases), bottom trawling (9.5% of cases), and the use of Remotely Operated Vehicles (ROVs) (1% of cases). Other collection techniques, such as dredging, snorkeling, and hand sampling were also used, each account for less than one percent of all records. Additionally, in 15 cases (1.5% of cases), multiple collection methods were utilized, making precise classification challenging.
Additionally, insights into the methods employed for detecting copepods on their hosts are documented in 334 instances, accounting for 33.5% of the dataset’s records. The sampling methods encompass rinsing procedures employing ethanol or formalin solutions in conjunction with seawater, as well as the dissection of galls and host tissues for thorough examination and analysis. The predominant method for detecting copepods involved rinsing (30.6% of cases), with the majority of rinsing procedures employing a 5% ethanol solution (28% of cases), while others used 10% or 4% formalin in seawater. Only six instances mentioned opening of galls, and four instances described the process of dissecting hosts. Some records also documented the utilization of multiple detection methods.
Among the copepod species, 91% were classified under Cyclopoida, 8% under Harpacticoida, and 10% under Siphonostomatoida (Table 3). Among copepods, the most frequently encountered families in the samples were the mainly ectosymbiotic Rhynchomolgidae (686 records and 146 species) and the gall-inducing and endoparasitic Lamippidae (209 records and 54 species), along with the ectosymbiotic siphonostomatoid copepods of the family Asterocheridae (37 records and 15 species) (Figure 3 and Table 3, Table 4 and Table 5). Notably, both families of Cyclopoida were previously categorized within the order Poecilostomatoida, and the decision to merge Poecilostomatoida into Cyclopoida as a junior synonym remains a topic of ongoing discussion [11]. It is noteworthy that the mention of representatives of the family Corallovexiidae on these corals is considered possibly misclassified as Lamippidae [63]. Only two species were identified as Harpacticoida. Harpacticoids are represented by Amphiascus pallidus, residing on Eunicella singularis of the family Gorgoniidae, and Parategastes conexus, found on Plexaurella grisea of the family Plexauridae [40,131].

Table 3.
Octocorallia families in relation to copepods.
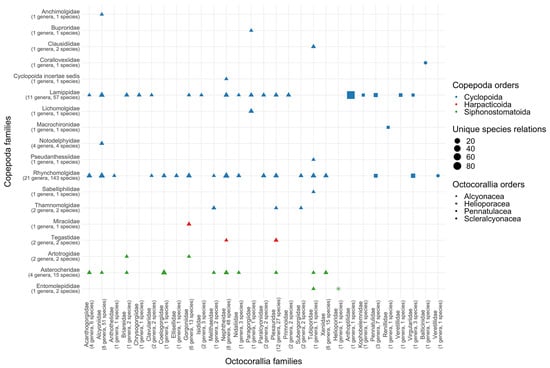
Figure 3.
Number of records per association of symbiotic copepod families with octocoralian families. Size of figure means number of records. Color of figure means order of copepods.

Table 4.
The families of Copepoda in relation to Octocorallia.

Table 5.
The distribution of symbiotic copepods and their hosts in the ecoregions *.
Of the 167 host species, 71.7% belonged to Alcyonacea, 6% to Pennatulacea, 0.9% to Scleralcyonacea, and 0.4% to Helioporacea. The octocoral families Pennatulidae, Alcyoniidae, Nephtheidae, and Plexauridae are the most extensively studied (Table 3). There are no recorded observations of symbiotic copepods associated with 94% of potential octocoral host species.
Data on the diversity of copepods, coral symbionts, are mainly represented by data on copepods collected at depths of up to 30 m (705 out of 966 records) (Figure 4, Table A1 and Table S1). The use of deep-sea submersibles led to the discovery of copepods at depths of more than 250 m. Ten species from deep-water corals were reported in eight publications [34,35,36,60,61,62,63,132]. Among deep-sea copepods, representatives of the family Lamippidae stand out, which were found at depths of more than 1000 m and have a strongly modified morphology [18]. Data on symbiotic copepods living on deep-sea octocorals are fragmentary and allow us to state their existence, but do not allow us to assess the diversity of symbionts of deep-sea Octocorallia.
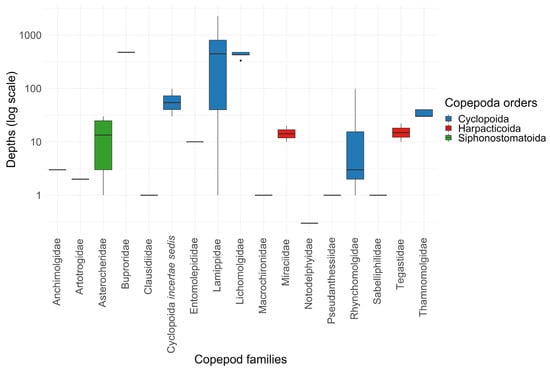
Figure 4.
Distribution of symbiotic copepods associated with octocorals by depth (see also Table 5, Table A1 and Table S1). The horizontal line within each box represents the median of the dataset. The box defines the interquartile range, covering the 25th to 75th percentiles. Whiskers extending from each box show the minimum and maximum data values. Data points appearing outside of these whiskers are identified as outliers.
A comprehensive inventory has documented a total of 393 distinct interspecies interactions involving copepods and various octocorals. Remarkably, a significant majority, approximately 60%, of these unique associations are supported by isolated recorded instances (Table 5). These recorded associations encompass copepods originating from three distinct orders: Cyclopoida, Siphonostomatoida, and Harpacticoida, engaged in symbiotic relationships with octocorals representing four different orders, specifically Alcyonacea, Helioporacea, Pennatulacea, and Scleralcyonacea (Table 3 and Table 4). Notably, copepods from all three orders have been identified in association with octocorals belonging to the order Alcyonacea. Additionally, Cyclopoids have been observed in interactions with both Pennatulacea and Scleralcyonacea, while Siphonostomatoida have been documented in association with Helioporacea (Figure 3). It is essential to highlight those interactions involving Cyclopoida and Pennatulacea, as well as those involving Siphonostomatoida and Scleralcyonacea, are sparsely documented, with only two and one instances recorded, respectively. The prevalence of numerous isolated records of these unique symbiotic associations underscores the limited extent of research concerning copepod symbiosis, rendering them unsuitable for analyzing host specificity and distribution patterns at this juncture.
Despite the inherent limitations in the available dataset, it is evident that copepods exhibit a notable density and diversity within individual octocoral colonies. A compelling example comes from the Molucca Islands, where a remarkable assemblage of 830 specimens of Colobomolgus bandensis was extracted from a solitary colony of Sinularia polydactyla [56]. Furthermore, the data also reveal that up to nine copepod species can coexist within a single host species colony (as detailed in Table 4). Within a sample obtained from a colony of Litophyton cupressiformis, five copepod species were identified, namely Paramolgus nephtheanus, P. prominulus, P. accinctus, Metaxymolgus lumarius, and M. aculeatus [42]. These and other observations strongly suggest that copepods associated with octocorals likely utilize various microhabitats provided by the coral, where the coral colony serves as both their habitat and a potential food source.
The analysis of data concerning the global geographic distribution of copepods associated with octocorals reveals that the available information is relatively fragmented and concentrated in 215 locations within eight of the 12 ecoregions found in the World Ocean (Figure 5 and Table 5, Table A1 and Table S1). Remarkably, certain regions, such as the Mediterranean coast of France, the northern part of Madagascar, and Curaçao, have been subject to more extensive research efforts. The Western Indo-Pacific, Central Indo-Pacific, and Temperate North Atlantic regions exhibit the highest numbers of sampling sites and recorded data, with 58 localities and 358 records, 51 localities and 328 records, and 50 localities and 104 records, respectively. However, it is important to note that no published records are available from vast territories, including the Tropical East Pacific, Temperate South America, Temperate South Africa, and Temperate Australia ecoregions, highlighting significant gaps in our knowledge of copepod–octocoral associations in these areas.
4. Discussion
The historical trajectory of research pertaining to the diversity of copepods associated with corals can be bifurcated into two distinct phases. The inaugural phase commenced in 1858 with the initial documentation of the gall-inducing endosymbiont Lamippe rubra residing on the sea pen Pennatula rubra [80]. During this period, investigations into copepods were primarily centered around species inhabiting coral galls, predominantly collected through trawling expeditions. Over the course of this two-century epoch, 26 instances of symbiotic copepods were identified prior to the early 1960s (Figure 1), marking the advent of the second phase.
The second phase, which endures to the present day, ushered in the utilization of SCUBA diving within scientific research and the refinement of methodologies for capturing loosely associated symbionts. This transformative shift resulted in the recording and description of a substantial array of copepod species (comprising 158 species, encompassing 588 out of 966 records) inhabiting diverse shallow-water octocorals at depths of up to 30 m. Approximately 60% of copepod species discovered in association with octocorals amounting to 144 species from shallow tropical octocorals have been described by Arthur Humes and his coauthors [132]. Furthermore, the integration of both manned and remotely operated underwater vehicles in deep-sea biodiversity research unveiled the presence of copepods dwelling within deep-sea octocorals at depths exceeding 250 and 1000 m [17]. These insights underscore the existence of considerable biodiversity among symbiotic copepods and other invertebrates inhabiting the depths of the ocean, a realm that remains underexplored.
Evidently, the exploration of copepod diversity in association with octocorals significantly lags behind the research endeavors focused on their host organisms. The protracted decline in research activity pertaining to the description of novel taxa can, in our assessment, be attributed to a diminishing pool of specialists and a dearth of integrated research endeavors encompassing the biodiversity of corals and copepods, culminating in the characterization of new taxa (Figure 1). Another indicator of this trend could be data obtained from studying the molecular diversity of copepods and corals from various marine communities [11].
The data indicate that octocorals provide a wide range of microhabitats for both ecto- and endosymbiotic copepods, and there are reports of various copepod species coexisting within a single colony. However, quantifying the density of copepods of a specific species associated with a particular octocoral colony is often challenging due to the microscopic size of copepods (Figure 2). Additionally, in the case of gall-inducing copepods, the presence of galls makes it difficult to accurately determine symbiont density without dissection. As a result, information regarding the co-occurrence of different copepod species on a single octocoral colony is exceedingly scarce, hindering a comprehensive analysis of copepod species cohabitation.
Copepods belonging to the large family Rhynchomolgidae, which comprises cyclopoid copepods, have been extensively documented in symbiotic relationships with scleractinian corals and various other invertebrates [2,133]. In stark contrast, the gall-inducing Lamippidae, another family within the cyclopoid copepod group, exhibit obligate symbiosis exclusively with octocorals [17,18,64,79]. The discoveries of siphonostomatoid copepods from the vast family Asterocheridae are particularly intriguing due to their association with a diverse range of host organisms, encompassing both ectosymbiotic and endosymbiotic species, as well as gall-inducing ones [133,134]. The identification of harpacticoid copepods belonging to the Tegastidae family residing on octocorals is of significant interest, given that these copepods have previously been observed in symbiotic relationships with other shallow-water cnidarians and in deep-sea chemosynthetic environments [40,135,136]. All these diverse findings provide insights into the intricate yet insufficiently explored evolutionary history of copepods associated with octocorals (Figure 3 and Figure 4 and Table 3 and Table 4). Further, more rigorous research on copepods associated with corals is expected to uncover interesting cases of adaptations and instances of transitions from one host group to another.
The absence of published records from vast territories such as the Tropical East Pacific, Temperate South America, Temperate South Africa, and Temperate Australia ecoregions may be due to a lack of infrastructure, such as research stations, like it has been described in other groups of aquatic invertebrates [137,138], creating a shortage of specialists with expertise in microscopic copepods [139] (Figure 5, Table 1, Table 5, Table A1 and Table S1). The prevalence of copepods symbiotic with octocorals in the tropical Indo-Pacific region could be partially attributed to greater research activity in this area and the relatively high diversity of shallow-water alcyonaceans.
5. Conclusions
The existing data highlight the significant gap in knowledge regarding the extensive copepod diversity associated with octocorals worldwide, with approximately 94% of potential octocoral host species lacking sufficient data. Based on the available morphological data, it is conceivable that the number of copepod species with potential associations with octocorals may exceed 4400 species. This estimation, however, should be regarded as provisional and subject to modification, particularly in the light of potential advancements stemming from molecular analysis techniques. Furthermore, the refinement of this estimate may also depend on a more comprehensive investigation into the host specificity, a facet that remains inadequately explored thus far. As research progresses, incorporating molecular methodologies and delving deeper into the intricacies of copepod–octocoral interactions, we can anticipate a more precise quantification of copepod diversity within this ecological context.
Many aspects of copepod feeding behaviors and their potential influence on octocorals warrant further investigation. To unravel the intricacies of copepod–octocoral relationships, additional research is imperative. The examination of corals and their associated fauna is crucial for assessing the ecological significance of both shallow and deep-water communities and for providing scientifically substantiated recommendations for sustainable habitat management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15111140/s1, Table S1: Octocorals as hosts of copepod crustaceans.
Author Contributions
Conceptualization and methodology, V.N.I. and D.F.; software, O.A.K.; validation, O.A.K., D.Y.G. and X.C.; formal analysis, O.A.K., D.Y.G. and X.C.; investigation, O.A.K., D.Y.G. and X.C.; data curation, V.N.I. and O.A.K.; writing—original draft preparation, O.A.K. and D.Y.G.; writing—review and editing, V.N.I. and D.F.; visualization, O.A.K. and D.Y.G.; supervision, V.N.I.; project administration, V.N.I. and O.A.K.; funding acquisition, V.N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation Grant No. 22-24-00365.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in doi.org/10.15468/msp4n8 (accessed on 1 October 2023).
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Copepod crustaceans recorded as associated with octocorals (see also Table S1. Octocorals as hosts of copepod crustaceans).
Table A1.
Copepod crustaceans recorded as associated with octocorals (see also Table S1. Octocorals as hosts of copepod crustaceans).
| Copepod | Host Species: Valid Name (and as in Original Record) | Host Abbreviation * | Site Abbreviation ** | Depth (m) | Reference |
|---|---|---|---|---|---|
| Cyclopoida | |||||
| Buproridae | |||||
| Buprorus sp. | Paragorgia arborea (Linnaeus, 1758) | Parg | CA | 475; 477 | [62] |
| Cyclopoida incertae sedis | |||||
| Ruthra humesi Kim, 2003 | Stereonephthya inordinata Tixier-Durivault, 1970 | Nep | NC | 30 | [53] |
| Anchimolgidae | |||||
| Panjakus auriculatus Humes, Dojiri, 1979 | Lobophytum crassum von Marenzeller, 1886 | Alc | ID | 3 | [44] |
| Clausidiidae | |||||
| Hippomolgus cognatus Humes, Ho, 1967 | Tubipora musica Linnaeus, 1758 | Tub | MG | 1 | [112] |
| Hippomolgus cognatus Humes, Ho, 1967 | Tubipora musica Linnaeus, 1758 | Tub | YT | 1 | [112] |
| Hippomolgus latipes Humes, Ho, 1967 | Tubipora musica Linnaeus, 1758 | Tub | YT | 1 | [112] |
| Lamippidae | |||||
| Enalcyonium affinis (Zulueta, 1908) (=Lamippe affinis Zulueta, 1908) | Eunicella verrucosa (Pallas, 1766) (=Gorgonia verrucosa Pallas) | Gor | FR | [74] | |
| Enalcyonium albidum (Zulueta, 1908) (as Lamippe albida Zulueta, 1908) | Pteroeides griseum (Linnaeus, 1767) | Pen | FR | [67,74] | |
| Enalcyonium alcyonii (Joliet, 1882) (=Lamippe alcyonii Joliet, 1882) | Paralcyonium spinulosum (Delle Chiaje, 1822) | Parl | [69] | ||
| Enalcyonium auriculatum Kim, 2004 | Lobophytum schoedei Moser, 1919 | Alc | NC | 1 | [54] |
| Enalcyonium bullatum Kim, 2004 | Siphonogorgia variabilis (Hickson, 1903) | Nid | NC | 30 | [54] |
| Enalcyonium caledonensis Kim, 2004 | Lobophytum schoedei Moser, 1919 | Alc | NC | 1 | [54] |
| Enalcyonium capillatum Kim, 2004 | Rumphella antipathes (Linnaeus, 1758) | Gor | NC | 1 | [54] |
| Enalcyonium carrikeri Dudley, 1973 | Gersemia rubiformis (Ehrenberg, 1834) | Nep | US | 25–28.4; 29; 35 | [84] |
| Enalcyonium ceramensis Kim, 2007 | Rumphella aggregata (Nutting, 1910) | Gor | ID | 10 | [47] |
| Enalcyonium ciliatum Stock, 1972 | Dendronephthya hemprichi Klunzinger, 1877 | Nep | ER; IL | 3 | [108] |
| Enalcyonium circulatum Kim, 2007 | Muricella sp. | Aca | ID | 2 | [47] |
| Enalcyonium concinnum (Humes, 1957) (=Lamippe concinna Humes, 1957) | Virgularia schultzei Kükenthal, 1910 | Vir | SL | 5 | [92] |
| Enalcyonium confusum Stock, 1988 | Alcyonium acaule Marion, 1878 | Alc | FR | 10 | [73] |
| Enalcyonium confusum Stock, 1988 | Alcyonium palmatum Pallas, 1766 | Alc | FR | 60; 80 | [73] |
| Enalcyonium digitigerum Ho, 1984 | Bellonella rigida Putter, 1900 | Alc | JP | [91] | |
| Enalcyonium euniceae Stock, 1973 | Eunicea mammosa Lamouroux, 1816 (=Eunicea (Eunicea) mammosa Lamouroux) | Ple | PR | 3 | [85] |
| Enalcyonium forbesi (T. Scott, 1901) | Alcyonium digitatum Linnaeus, 1758 | Alc | FR; GB; IE | 20 | [73,77,86,87] |
| Enalcyonium forbesi (T. Scott, 1901) | Chrysogorgia flexilis (Wright, Studer, 1889) | Chr | ID | [57] | |
| Enalcyonium grandisetigerum Kim, 2009 | Dendronephthya cirsium Kükenthal, 1905 Dendronephthya cirsium (Kükenthal, 1905) | Nep | MG | [117] | |
| Enalcyonium heegaardi Bouligand, 1960 | Gersemia rubiformis (Ehrenberg, 1834) | Nep | GL | 2258 | [34] |
| Enalcyonium humesi Kim, 2004 | Lobophytum schoedei Moser, 1919 | Alc | NC | 1 | [54] |
| Enalcyonium kohsiangi Uyeno, 2015 | Pteroeides griseum (Linnaeus, 1767) | Pen | SG | 10.3; 10.6 | [57] |
| Enalcyonium lobophyti Kim, 2004 | Lobophytum schoedei Moser, 1919 | Alc | NC | 1 | [54] |
| Enalcyonium nudum Stock, 1973 | Plexaura homomalla (Esper, 1794) (=Plexaura homomalla f. homomalla Esper, 1794) | Ple | PR | 3 | [85] |
| Enalcyonium olssoni (Zulueta, 1908) | Alcyonium sp. | Alc | SE | [81] | |
| Enalcyonium olssoni (Zulueta, 1908) | Alcyonium digitatum Linnaeus, 1758 | Alc | SE | [70] | |
| Enalcyonium olssoni (Zulueta, 1908) | Primnoa resedaeformis (Gunnerus, 1763) | Pri | US | 334–367; 432; 476 | [62] |
| Enalcyonium pusillum (Zulueta, 1908) (=Lamippe pusilla Zulueta, 1908) | Gorgonia sarmentosa Esper, 1789 (=Gorgonella sarmentosa (Lamarck)) | Gor | FR | [74] | |
| Enalcyonium pusillum (Zulueta, 1908) | Leptogorgia sarmentosa (Esper, 1789) | Gor | FR | 40; 200 | [66] |
| Enalcyonium ramosum Stock, 1973 | Plexaura homomalla (Esper, 1794) (=Plexaura homomalla f. homomalla Esper, 1794) | Ple | PR | 3 | [85] |
| Enalcyonium robustum Kim, 2009 | Dendronephthya regia Verseveldt, 1968 | Nep | MG | [117] | |
| Enalcyonium rubicundum Olsson, 1869 | Alcyonium acaule Marion, 1878 | Alc | FR | 40; 200 | [66] |
| Enalcyonium rubicundum Olsson, 1869 | Alcyonium sp. | Alc | SE | [81] | |
| Enalcyonium rubicundum Olsson, 1869 (as Alcyonicola fusiformis Scott T., Scott A., 1895) | Alcyonium digitatum Linnaeus, 1758 | Alc | GB | [70,73,86,88] | |
| Enalcyonium rubicundum Olsson, 1869 (=Lamippe rubicunda (Olsson, 1869)) | Alcyonium palmatum Pallas, 1766 | Alc | FR | [74] | |
| Enalcyonium rubicundum Olsson, 1869 | Pennatula rubra (Ellis, 1761) | Pen | SE | [70] | |
| Enalcyonium scorpio Stock, 1973 | Leptogorgia sarmentosa (Esper, 1789) | Gor | US | 5 | [85] |
| Enalcyonium setigerum (Zulueta, 1908) (=Lamippe setigera Zulueta, 1908) | Alcyonium coralloides (Pallas, 1766) (=Sympodium coralloides (Pallas)) | Alc | FR | [74] | |
| Enalcyonium setigerum (Zulueta, 1908) | Paramuricea clavata (Risso, 1826) (as Muricea chamaeleon Koch, 1882; Paramuricea chamaeleon (Koch, 1887)) | Ple | FR | 40–200 | [66,82] |
| Enalcyonium sp. | Plexaurella nutans (Duchassaing, Michelotti, 1860) | Ple | CU | 20 | [102] |
| Enalcyonium sympodii (Zulueta, 1910) (=Lamippe sympodii Zulueta, 1910) | Leptogorgia sarmentosa (Esper, 1789) (=Sympodium coralloides (Pallas)) | Gor | FR | [75] | |
| Enalcyonium variicauda Stock, 1973 | Briareum asbestinum (Pallas, 1766) | Bri | PR | 1; 4; 6–8 | [85] |
| Gorgonophilus canadensis Buhl-Mortensen, Mortensen, 2004 | Paragorgia arborea (Linnaeus, 1758) | Parg | CA; GL | 445; 475; 520; 560 | [36] |
| Isidicola antarctica Gravier, 1914 | Primnoisis (Delicatisis) formosa Gravier, 1913 | Isi | AQ | 254 | [61] |
| Isidicola antarctica Gravier, 1914 | Primnoisis (Delicatisis) gracilis (Gravier, 1913) | Isi | AQ | 254 | [61] |
| Lamippe bouligandi Laubier, 1972 | Anthoptilum grandiflorum (Verrill, 1879) | Antp | CA; GL; IS | 90; 98; 136; 600; 1210; 1347 | [35,63] |
| Lamippe proteus Claparède, 1867 | Alcyonium digitatum Linnaeus, 1758 | Alc | GB; IT | [77,87] | |
| Lamippe proteus Claparède, 1867 | Alcyonium sp. | Alc | GB | [87] | |
| Lamippe pteroidis Zulueta, 1910 | Pteroeides griseum (Linnaeus, 1767) | Pen | FR | [75] | |
| Lamippe rubra Bruzelius, 1858 | Gersemia rubiformis (Ehrenberg, 1834) | Nep | FR | [70] | |
| Lamippe rubra Bruzelius, 1858 | Pennatula phosphorea Linnaeus, 1758 | Pen | FR; NO; SE | [67,70,80] | |
| Lamippe rubra decolor Zulueta, 1908 | Pennatula phosphorea Linnaeus, 1758 | Pen | FR | [74] | |
| Lamippe sp. | Chrysogorgia flexilis (Wright, Studer, 1889) | Chr | ID | [57,58] | |
| Lamippella acanellae Grygier, 1983 | Acanella arbuscula (Johnson, 1862) | Isi | FR | 1010 | [68] |
| Lamippella delamarei Bouligand, 1965 | Kophobelemnon stelliferum (Müller, 1776) | Kop | FR | [67] | |
| Lamippella faurei Bouligand, Delamare Deboutteville, 1959 | Alcyonium coralloides (Pallas, 1766) (=Parerythropodium coralloides (Pallas, 1766)) | Alc | FR | 0; 2,2 | [65] |
| Lamippella faurei Bouligand, Delamare Deboutteville, 1959 | Alcyonium palmatum Pallas, 1766 | Alc | FR | 0; 2,2 | [65] |
| Lamippella faurei Bouligand, Delamare Deboutteville, 1959 | Eunicella verrucosa (Pallas, 1766) | Gor | FR | 0; 2,2 | [65] |
| Lamippella faurei Bouligand, Delamare Deboutteville, 1959 | Rolandia coralloides de Lacaze Duthiers, 1900 | Cla | FR | [65] | |
| Lamippella faurei Bouligand, Delamare Deboutteville, 1959 | Swiftia rosea (Grieg, 1887) | Ple | SE | 40 | [81] |
| Lamippina aciculifera (Zulueta, 1908) (as Lamippe brementi Zulueta, 1910) | Alcyonium coralloides (Pallas, 1766) (as Parerythropodium coralloides (Pallas, 1766); Sympodium coralloides (Pallas)) | Alc | FR | [65,75] | |
| Lamippina aciculifera (Zulueta, 1908) | Alcyonium palmatum Pallas, 1766 | Alc | FR | [65,74] | |
| Lamippina aequalis Stock, 1973 | Antillogorgia acerosa (Pallas, 1766) (=Pseudopterogorgia acerosa (Pallas, 1766)) | Gor | CW | 3; 4 | [85] |
| Lamippina aequalis Stock, 1973 | Antillogorgia sp. (=Pseudopterogorgia Kükenthal, 1919) | Gor | CW | 3 | [85] |
| Lamippina laubieri Bouligand, 1960 | Leptogorgia sarmentosa (Esper, 1789) | Gor | FR | 40; 200 | [66] |
| Lamippula chattoni (Zulueta, 1908) (as Enalcyonium chaltoni; Lamippe chattoni Zulueta, 1908) | Pennatula phosphorea Linnaeus, 1758 | Pen | FR | [67,74] | |
| Lamippula duthiersi (Joliet, 1882) (as Lamippe duthiersi Joliet, 1882) | Paralcyonium spinulosum (Delle Chiaje, 1822) (Paralcyonium elegans Milne Edwards, 1857) | Parl | FR | [69,74] | |
| Lamippula pallida (Zulueta, 1908) | Pteroeides griseum (Linnaeus, 1767) | Pen | [65] | ||
| Lamippula pallida (Zulueta, 1908) (as Lamippe pallida Zulueta, 1908) | Veretillum cynomorium (Pallas, 1766) | Ver | FR | [67,74] | |
| Lamippula parva (Zulueta, 1908) (as Lamippe parva Zulueta, 1908) | Paramuricea clavata (Risso, 1826) (as Muricea chamaeleon Koch, 1882) | Ple | FR | [74,82] | |
| Linaresia bouligandi Stock, 1979 | Placogorgia sp. | Ple | US | 73; 78 | [107] |
| Linaresia magna Grygier, 1980 | Placogorgia sp. | Ple | US | 366 | [60] |
| Linaresia mammillifera Zulueta, 1908 | Paramuricea clavata (Risso, 1826) (=Muricea chamaeleon Koch, 1882) | Ple | FR | [74,82] | |
| Magnippe caputmedusae Stock, 1978 | Thesea citrina Deichmann, 1936 | Ple | US | 54.9 | [106] |
| Magnippe caputmedusae Stock, 1978 | Thesea parviflora Deichmann, 1936 | Ple | US | 73.2 | [106] |
| Magnippe caputmedusae Stock, 1978 | Thesea rugosa Deichmann, 1936 | Ple | US | 54.9 | [106] |
| Ptilosarcoma athyrmata Williams, Anchaluisa, Boyko, McDaniel, 2018 | Ptilosarcus gurneyi (Gray, 1860) | Pen | CA | 5; 10 | [89] |
| Sphaerippe caligicola Grygier, 1980 | Callogorgia sp. | Pri | BS | 366 | [60] |
| Sphaerippe sp. | Gorgonia ventalina Linnaeus, 1758 | Gor | NL-BQ3 | 2; 21 | [31] |
| Lichomolgidae | |||||
| Lichomolgidae | Paragorgia arborea (Linnaeus, 1758) | Parg | CA | 332; 426; 446; 475; 477 | [62] |
| Macrochironidae | |||||
| Macrochiron sargassi Sars G.O., 1916 | Renilla reniformis (Pallas, 1766) | Ren | MF | 1 | [52] |
| Notodelphyidae | |||||
| Bysone operculatus Stock, Humes, 1970 | Rhytisma fuscum (Thomson, Henderson, 1906) | Alc | MG | 0.3 | [116] |
| Demoixys affinis Stock, Humes, 1970 | Rhytisma fuscum (Thomson, Henderson, 1906) | Alc | MG | 0.3 | [116] |
| Paranotodelphys procax Stock, Humes, 1970 | Rhytisma fuscum (Thomson, Henderson, 1906) | Alc | MG | 0.3; 0.6 | [116] |
| Thoracodelphys uniseta Stock, Humes, 1970 | Rhytisma fuscum (Thomson, Henderson, 1906) | Alc | MG | 0.5 | [116] |
| Pseudanthessiidae | |||||
| Tubiporicola inflatus Kim, 2009 | Tubipora musica Linnaeus, 1758 | Tub | MG | 1 | [117] |
| Rhynchomolgidae | |||||
| Acanthomolgus aequiseta Stock, 1975 | Muricea laxa Verrill, 1864 | Ple | CW | 34; 41 | [97] |
| Acanthomolgus affinis Stock, 1975 | Eunicea flexuosa (Lamouroux, 1821) (=Plexaura flexuosa Lamouroux, 1821) | Ple | CW | 2 | [97] |
| Acanthomolgus affinis Stock, 1975 | Plexaura homomalla (Esper, 1794) | Ple | CW | 3 | [97] |
| Acanthomolgus affinis Stock, 1975 | Plexaura sp. | Ple | CU | [104] | |
| Acanthomolgus ambonensis Kim, 2007 | Litophyton striatum (Kükenthal, 1903) | Nep | ID | 3 | [47] |
| Acanthomolgus arctatipes Humes, 1974 | Echinogorgia sassapo (Esper, 1791) | Ple | MG | 10; 13; 25 | [109] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Acanthogorgia aspera Pourtalès, 1867 | Aca | MG | 4; 8; 20; 23; 24; 40 | [52,109] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Acanthogorgia sp. | Aca | ID | 25 | [43] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Anthogorgia sp. (=Acalycigorgia Kükenthal, Gorzawsky, 1908) | Aca | ID | 10 | [43] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Muricella rubra robusta Thomson and Simpson | Aca | MG | 10; 15 | [109] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Muricella sp. | Aca | ID; PH | 10; 40 | [43] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Rumphella antipathes (Linnaeus, 1758) | Gor | NC | 2 | [43] |
| Acanthomolgus astrictus Humes, Stock, 1973 | Villogorgia intricata (Gray, 1870) | Ple | PH | 30 | [43] |
| Acanthomolgus bandaensis Kim, 2007 | ID | 25 | [47] | ||
| Acanthomolgus bayeri Humes, 1973 | Pseudoplexaura porosa (Houttuyn, 1772) | Ple | BM; GB | 1; 3 | [49,96] |
| Acanthomolgus bayeri Humes, 1973 | Pseudoplexaura sp. | Ple | CU | [101] | |
| Acanthomolgus bilobipes Humes, Stock, 1973 | Antillogorgia acerosa (Pallas, 1766) | Gor | BB; CW; JM | 3; 4 | [52,97] |
| Acanthomolgus bilobipes Humes, Stock, 1973 | Antillogorgia acerosa var. elastica Bielschowsky, 1929 (=Antillogorgia elastica Bielschowsky, 1929) | Gor | PR | [52] | |
| Acanthomolgus boholensis Humes, 1990 | Dendronephthya pütteri Kükenthal, 1905 (=Dendronephthya puetteri) | Nep | PH | 40 | [50] |
| Acanthomolgus brevifurca Humes, 1990 | Siphonogorgia variabilis (Hickson, 1903) | Nid | ID | 10 | [50] |
| Acanthomolgus combinatus Humes, 1974 | Echinogorgia sassapo (Esper, 1791) | Ple | MG | 10; 13; 25 | [109] |
| Acanthomolgus combinatus Humes, 1974 | Echinogorgia sp. | Ple | ID | 10 | [43] |
| Acanthomolgus cuneipes (Humes, Ho, 1968) | Dendronephthya mucronata (Pütter, 1900) | Nep | MG | 1 | [52] |
| Acanthomolgus cuneipes (Humes, Ho, 1968) (=Lichomolgus cuneipes (Humes, Ho, 1968)) | Stereonephthya acaulis Verseveldt, 1968 | Nep | MG | 1; 2; 10 | [113] |
| Acanthomolgus dionyx Stock, 1975 | Antillogorgia americana (Gmelin, 1791) (=Pseudopterogorgia americana (Gmelin, 1791)) | Gor | CW | 4 | [97] |
| Acanthomolgus dispadactylus Kim, 2007 | Dendronephthya grandiflora Henderson, 1909 | Nep | ID | 10 | [47] |
| Acanthomolgus eminulus Humes, Lewbel, 1977 | Muricea californica Aurivillius, 1931 | Ple | US | 20 | [95] |
| Acanthomolgus exilipes (Humes, Ho, 1968) | Dendronephthya grandiflora Henderson, 1909 | Nep | ID | 10 | [47] |
| Acanthomolgus exilipes (Humes, Ho, 1968) (=Lichomolgus exilipes (Humes, Ho, 1968)) | Dendronephthya koellikeri Kükenthal, 1905 | Nep | MG | 8 | [113] |
| Acanthomolgus exilipes (Humes, Ho, 1968) (=Lichomolgus exilipes (Humes, Ho, 1968)) | Dendronephthya mucronata (Pütter, 1900) | Nep | ID; MG; NC | 25; 1; 1,5; 3; 4; 10; 24; 25 | [50,52,55,113] |
| Acanthomolgus exilipes (Humes, Ho, 1968) (=Lichomolgus exilipes (Humes, Ho, 1968)) | Dendronephthya regia Verseveldt, 1968 | Nep | MG | 23–26; 40 | [52,113] |
| Acanthomolgus exilipes (Humes, Ho, 1968) | Dendronephthya sp. | Nep | MG | 27 | [52] |
| Acanthomolgus exilipes (Humes, Ho, 1968) | Dendronephthya speciosa Kükenthal, 1905 | Nep | MG | 17; 24 | [52] |
| Acanthomolgus exilipes (Humes, Ho, 1968) (=Lichomolgus exilipes (Humes, Ho, 1968)) | Dendronephthya stocki Verseveldt, 1968 | Nep | MG | 25; 40 | [52,113] |
| Acanthomolgus exilipes (Humes, Ho, 1968) | Stereonephthya cordylophora Verseveldt, 1973 | Nep | MG | 24 | [52] |
| Acanthomolgus fissisetiger (Humes, Ho, 1968) (=Lichomolgus fissisetiger (Humes, Ho, 1968)) | Lemnalia elegans (May, 1899) | Nep | MG | 1 | [113] |
| Acanthomolgus fissisetiger (Humes, Ho, 1968) | Lemnalia humesi Verseveldt, 1969 | Nep | MG | 10 | [52] |
| Acanthomolgus fissisetiger (Humes, Ho, 1968) (=Lichomolgus fissisetiger (Humes, Ho, 1968)) | Stereonephthya acaulis Verseveldt, 1968 | Nep | MG | 1; 2; 10; 15 | [52,113] |
| Acanthomolgus fissisetiger (Humes, Ho, 1968) (=Lichomolgus fissisetiger (Humes, Ho, 1968)) | Stereonephthya papyracea Kükenthal, 1905 | Nep | MG | 6 | [113] |
| Acanthomolgus gentilis (Humes, Ho, 1968) (=Lichomolgus gentilis (Humes, Ho, 1968)) | Dendronephthya koellikeri Kükenthal, 1905 | Nep | MG | 8 | [113] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Dendronephthya lokobeensis Verseveldt, 1973 | Nep | MG | 4 | [52] |
| Acanthomolgus gentilis (Humes, Ho, 1968) (=Lichomolgus gentilis (Humes, Ho, 1968)) | Dendronephthya mucronata (Pütter, 1900) | Nep | MG; NC | 1; 1,5; 2; 3; 4; 10; 20; 24 | [52,55,113] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Dendronephthya sp. | Nep | MG | 27 | [52] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Dendronephthya speciosa Kükenthal, 1905 | Nep | MG | 17; 22 | [52] |
| Acanthomolgus gentilis (Humes, Ho, 1968) (=Lichomolgus gentilis (Humes, Ho, 1968)) | Dendronephthya stocki Verseveldt, 1968 | Nep | MG | 20 | [113] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Siphonogorgia variabilis (Hickson, 1903) | Nid | NC | 30 | [53] |
| Acanthomolgus gentilis (Humes, Ho, 1968) (=Lichomolgus gentilis (Humes, Ho, 1968)) | Stereonephthya acaulis Verseveldt, 1968 | Nep | MG | 1; 2; 10; 20 | [113] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Stereonephthya acaulis Verseveldt, 1968 | Nep | MG | 2; 4; 8 | [52] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Stereonephthya acaulis Verseveldt, 1968 | Nep | MG | 17 | [52] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Stereonephthya cordylophora Verseveldt, 1973 | Nep | MG | 24 | [52] |
| Acanthomolgus gentilis (Humes, Ho, 1968) (=Lichomolgus gentilis (Humes, Ho, 1968)) | Stereonephthya papyracea Kükenthal, 1905 | Nep | MG | 6 | [113] |
| Acanthomolgus gentilis (Humes, Ho, 1968) | Umbellulifera striata (Thomson, Henderson, 1905) | Nep | MG | 17 | [52] |
| Acanthomolgus gomumuensis Kim, 2007 | Dendronephthya grandiflora Henderson, 1909 | Nep | ID | 10 | [47] |
| Acanthomolgus gorgoniae Humes, 1973 | Gorgonia ventalina Linnaeus, 1758 | Gor | BM; BQ; CW | 2; 3 | [49,96,97] |
| Acanthomolgus hales Humes, Stock, 1973 | Solenocaulon tortuosum Gray, 1862 | Antt | MG | 18 | [109] |
| Acanthomolgus hians (Humes, Ho, 1968) (=Lichomolgus hians (Humes, Ho, 1968)) | Siphonogorgia pendula Studer, 1889 | Nid | MG | 10; 12; 20 | [114] |
| Acanthomolgus hians (Humes, Ho, 1968) | Siphonogorgia pichoni Verseveldt, 1971 | Nid | MG | 17; 25 | [52] |
| Acanthomolgus intermedius Stock, 1975 | Eunicea laciniata Duchassaing, Michelotti, 1860 | Ple | CW | 6 | [97] |
| Acanthomolgus intermedius Stock, 1975 | Muricea sp. | Ple | CU | [101] | |
| Acanthomolgus longidactylus Stock, 1975 | Eunicea flexuosa (Lamouroux, 1821) (=Plexaura flexuosa Lamouroux, 1821) | Ple | CW | 3 | [97] |
| Acanthomolgus longifurca Stock, 1975 | Eunicea tourneforti Milne Edwards, Haime, 1857 | Ple | CW | 3 | [97] |
| Acanthomolgus longispinifer (Humes, Ho, 1968) | Dendronephthya sp. | Nep | ID | 17 | [47] |
| Acanthomolgus longispinifer (Humes, Ho, 1968) (=Lichomolgus longispinifer (Humes, Ho, 1968)) | Siphonogorgia pendula Studer, 1889 | Nid | MG | 10; 12; 20 | [114] |
| Acanthomolgus longispinifer (Humes, Ho, 1968) | Siphonogorgia pichoni Verseveldt, 1971 | Nid | MG | 17; 25 | [52] |
| Acanthomolgus mononyx Stock, 1975 | Eunicea clavigera Bayer, 1961 | Ple | CW | 22; 24; 33; 40; 41 | [97] |
| Acanthomolgus mopsellae Humes, 1974 | Melithaea rubeola (Wright, Studer, 1889) (=Mopsella rubeola (Wright, Studer, 1889)) | Mel | MG | 3 | [109] |
| Acanthomolgus muriceanus Humes, 1973 | Eunicea flexuosa (Lamouroux, 1821) (=Plexaura flexuosa Lamouroux, 1821) | Ple | GB | 1; 2 | [96] |
| Acanthomolgus muriceanus Humes, 1973 | Muricea atlantica (Kükenthal, 1911) | Ple | BM | 3 | [49,97] |
| Acanthomolgus plantei Humes, Stock, 1973 | Umbellulifera striata (Thomson, Henderson, 1905) | Nep | MG | 17; 47 | [52] |
| Acanthomolgus seticornis Stock, 1975 | Plexaurella dichotoma (Esper, 1791) | Ple | MF | 3 | [97] |
| Acanthomolgus telestophilus (Humes, Ho, 1968) | Coelogorgia palmosa Milne Edwards, Haime, 1857 | Coe | MG | 1; 2 | [1] |
| Acanthomolgus telestophilus (Humes, Ho, 1968) | Telesto (Carijoa) arborea Wright, Studer, 1889 | Cla | MG | 4 | [114] |
| Acanthomolgus tenuispinatus Kim, 2009 | Litophyton striatum (Kükenthal, 1903) | Nep | MG | 25 | [117] |
| Acanthomolgus triangulipes Stock, 1975 | Gorgonia mariae Bayer, 1961 | Gor | CU | 20 | [103] |
| Acanthomolgus triangulipes Stock, 1975 | Gorgonia ventalina Linnaeus, 1758 | Gor | BQ; CW; MF | 2; 3 | [97] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) | Dendronephthya cirsium Kükenthal, 1905 | Nep | MG | 35 | [52] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) (=Lichomolgus varirostratus (Humes, Ho, 1968)) | Dendronephthya koellikeri Kükenthal, 1905 | Nep | MG | 8 | [113] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) | Dendronephthya lokobeensis Verseveldt, 1973 | Nep | MG | 15 | [52] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) (=Lichomolgus varirostratus (Humes, Ho, 1968)) | Dendronephthya mucronata (Pütter, 1900) | Nep | MG; NC | 1; 1,5; 2; 4; 10; 20; 24; 25 | [52,55,114] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) (=Lichomolgus varirostratus (Humes, Ho, 1968)) | Dendronephthya regia Verseveldt, 1968 | Nep | MG | 25; 40 | [52,113] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) | Dendronephthya sp. | Nep | MG | 27 | [52] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) | Dendronephthya speciosa Kükenthal, 1905 | Nep | MG | 10 | [52] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) (=Lichomolgus varirostratus (Humes, Ho, 1968)) | Dendronephthya stocki Verseveldt, 1968 | Nep | MG | 20; 25; 40 | [52,113] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) | Siphonogorgia variabilis (Hickson, 1903) | Nid | NC | 30 | [53] |
| Acanthomolgus varirostratus (Humes, Ho, 1968) | Stereonephthya cordylophora Verseveldt, 1973 | Nep | MG | 24 | [52] |
| Acanthomolgus verrucipes Humes, 1973 | Eunicea calyculata (Ellis, Solander, 1786) | Ple | BM | 1 | [49] |
| Acanthomolgus verseveldti (Humes, Ho, 1968) (=Lichomolgus verseveldti (Humes, Ho, 1968)) | Heteroxenia elisabethae Kölliker, 1874 | Xen | MG; YT | 1 | [114] |
| Acanthomolgus verseveldti (Humes, Ho, 1968) | Heteroxenia fuscescens (Ehrenberg, 1834) | Xen | MG | 20 | [52] |
| Acanthomolgus verseveldti (Humes, Ho, 1968) | Xenia lepida Verseveldt, 1971 | Xen | MG | 10 | [52] |
| Alcyonomolgus bicrenatus (Humes, 1982) (=Anisomolgus bicrenatus Humes, 1982) | Sarcophyton ehrenbergi v. Marenzeller, 1886 | Alc | NC | 1 | [39] |
| Alcyonomolgus dissimilis (Humes, 1982) | Lobophytum depressum Tixier-Durivault, 1966 | Alc | MG | 25 | [50] |
| Alcyonomolgus dissimilis (Humes, 1982) (=Anisomolgus dissimilis (Humes, 1982)) | Sarcophyton ehrenbergi v. Marenzeller, 1886 | Alc | MG | 25 | [39] |
| Alcyonomolgus incisus (Humes, Ho, 1968) | Sarcophyton ehrenbergi v. Marenzeller, 1886 | Alc | ID; MG | 0.5; 3; 4 | [39,52,115] |
| Alcyonomolgus insolens (Humes, Ho, 1968) (=Lichomolgus insolens (Humes, Ho, 1968)) | Lobophytum crassum von Marenzeller, 1886 | Alc | MG; NC | 1; 2 | [50,55,115] |
| Alcyonomolgus insolens (Humes, Ho, 1968) | Lobophytum crebriplicatum von Marenzeller, 1886 | Alc | NC | 2; 3 | [55] |
| Alcyonomolgus insolens (Humes, Ho, 1968) | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | NC | 0.5; 1; 2; 4 | [50] |
| Alcyonomolgus lumellifer Humes, 1990 | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | MG; NC | 0.5; 17 | [50] |
| Alcyonomolgus petalophorus (Humes, 1982) (=Anisomolgus petalophorus (Humes, 1982)) | Sarcophyton ehrenbergi v. Marenzeller, 1886 | Alc | NC | 3 | [39] |
| Alcyonomolgus relativus (Humes, 1982) (=Anisomolgus relativus (Humes, 1982)) | Sarcophyton ehrenbergi v. Marenzeller, 1886 | Alc | ID; NC | 1; 3 | [39] |
| Alcyonomolgus sarcophyticus (Humes, 1982) (=Anisomolgus sarcophyticus (Humes, 1982)) | Sarcophyton cornispiculatum Verseveldt, 1971 | Alc | MG | 17 | [39] |
| Alcyonomolgus sarcophyticus (Humes, 1982) (=Anisomolgus sarcophyticus (Humes, 1982)) | Sarcophyton elegans Moser, 1919 | Alc | NC | 1; 2 | [39] |
| Alcyonomolgus sarcophyticus (Humes, 1982) (=Anisomolgus sarcophyticus (Humes, 1982)) | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | ID; MG | 2; 3; 5 | [39] |
| Alcyonomolgus sarcophyticus (Humes, 1982) (=Anisomolgus sarcophyticus (Humes, 1982)) | Sarcophyton trocheliophorum von Marenzeller, 1886 | Alc | NC | 2 | [39] |
| Anisomolgus ensifer Humes, 1982 (=Anisomolgus ensiferus (Humes, 1982)) | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | NC | 1 | [39] |
| Anisomolgus goniodes Humes, 1982 | Sarcophyton trocheliophorum von Marenzeller, 1886 | Alc | NC | 2 | [39] |
| Anisomolgus limbatus Humes, Dojiri, 1979 | Lobophytum crassum von Marenzeller, 1886 | Alc | ID | 3 | [44] |
| Anisomolgus protentus (Humes, Frost, 1964) | Sarcophyton elegans Moser, 1919 | Alc | NC | 1 | [55] |
| Anisomolgus protentus (Humes, Frost, 1964) | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | ID; MG | 1; 2; 3; 4; 10; 17 | [39,52] |
| Anisomolgus protentus (Humes, Frost, 1964) (=Lichomolgus protentus (Humes, Frost, 1964)) | Sarcophyton sp. | Alc | MG | 3 | [111] |
| Anisomolgus protentus (Humes, Frost, 1964) | Sarcophyton trocheliophorum von Marenzeller, 1886 | Alc | NC | 2 | [39] |
| Anisomolgus pterolobatus Humes, 1982 | Sarcophyton crassum Tixier-Durivault, 1946 | Alc | NC | 1.5 | [39] |
| Anisomolgus pterolobatus Humes, 1982 | Sarcophyton elegans Moser, 1919 | Alc | NC | 1; 2 | [39] |
| Anisomolgus pterolobatus Humes, 1982 | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | ID | 5; 10 | [39] |
| Ascetomolgus plicatus Humes, Stock, 1972 | Studeriotes semperi (Studer, 1888) | Parl | MG | 17 | [52] |
| Colobomolgus bandensis Humes, 1990 | Sinularia polydactyla (Ehrenberg, 1834) | Alc | ID | 2; 3 | [50] |
| Colobomolgus cristatus (Humes, Ho, 1968) | Sinularia firma Tixier-Durivault, 1970 | Alc | NC | 3; 4 | [50] |
| Colobomolgus cristatus (Humes, Ho, 1968) (=Lichomolgus cristatus (Humes, Ho)) | Sinularia leptoclados (Ehrenberg, 1834) | Alc | MG; NC | 1; 2; 10; 15; 20 | [50,52,115] |
| Colobomolgus dentipes (Thompson I.C., Scott A., 1903) | Sinularia firma Tixier-Durivault, 1970 | Alc | NC | 3 | [50] |
| Colobomolgus dentipes (Thompson I.C., Scott A., 1903) | Sinularia humesi Verseveldt, 1968 | Alc | MG | 2; 13; 18 | [52,115] |
| Colobomolgus dentipes (Thompson I.C., Scott A., 1903) | Sinularia polydactyla (Ehrenberg, 1834) | Alc | NC | 2 | [55] |
| Colobomolgus epaxius Humes, 1990 | Sinularia firma Tixier-Durivault, 1970 | Alc | NC | 3 | [50] |
| Colobomolgus laboutei Humes, Stock, 1973) | Sinularia leptoclados (Ehrenberg, 1834) | Alc | MG | 1; 20 | [52] |
| Contomolgus lokobeensis Humes, Stock, 1973 | Dendronephthya stocki Verseveldt, 1968 | Nep | MG | 25 | [52] |
| Contomolgus lokobeensis Humes, Stock, 1973 | Studeriotes semperi (Studer, 1888) | Parl | MG | 17; 18 | [50,52] |
| Critomolgus antennulus Humes, 1990 | Cladiella humesi Verseveldt, 1974 | Alc | NC | 2 | [53] |
| Critomolgus antennulus Humes, 1990 | Cladiella pachyclados (Klunzinger, 1877) | Alc | NC | 0.5; 1; 2 | [50] |
| Critomolgus bulbipes (Stock, Kleeton, 1963) | Alcyonium acaule Marion, 1878 | Alc | FR | 10; 12 | [72] |
| Critomolgus bulbipes (Stock, Kleeton, 1963) | Alcyonium coralloides (Pallas, 1766) | Alc | ES; FR | 20; 23; 26 | [72] |
| Critomolgus cladiellae Humes, 1990 | Cladiella humesi Verseveldt, 1974 | Alc | NC | 2 | [53] |
| Critomolgus cladiellae Humes, 1990 | Cladiella pachyclados (Klunzinger, 1877) | Alc | NC | 0.5; 1; 2 | [50] |
| Critomolgus foxi (Gurney, 1927) | Cladiella humesi Verseveldt, 1974 | Alc | NC | 2 | [50] |
| Critomolgus foxi (Gurney, 1927) | Cladiella krempfi (Hickson, 1919) | Alc | MG | 1 | [115] |
| Critomolgus foxi (Gurney, 1927) (=Doridicola foxi (Gurney, 1927)) | Cladiella laciniosa (Tixier-Durivault, 1944) | Alc | MG | 2 | [52] |
| Critomolgus foxi (Gurney, 1927) (=Doridicola foxi (Gurney, 1927)) | Cladiella latissima (Tixier-Durivault, 1944) | Alc | MG | 1; 18 | [52] |
| Critomolgus foxi (Gurney, 1927) | Cladiella pachyclados (Klunzinger, 1877) | Alc | ID; NC | 0.5; 2; 10 | [50] |
| Critomolgus foxi (Gurney, 1927) (=Doridicola foxi (Gurney, 1927)) | Cladiella sphaerophora (Ehrenberg, 1834) | Alc | MG | 1 | [52] |
| Critomolgus linguifer Kim, 2003 | Cladiella humesi Verseveldt, 1974 | Alc | NC | 2 | [53] |
| Critomolgus orectopus Humes, 1990 | Cladiella pachyclados (Klunzinger, 1877) | Alc | NC | 0.5; 1; 2 | [50] |
| Critomolgus orectopus Humes, 1990 | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | NC | 0.5; 1 | [50] |
| Critomolgus pteropadus (Humes, 1978) (=Doridicola pteropadus Humes, 1978) | Pteroeides oblongum Gray, 1860 | Pen | MG | 17 | [110] |
| Critomolgus virgulariae (Humes, 1978) (=Doridicola virgulariae Humes, 1978) | Virgularia juncea (Pallas, 1766) | Vir | MG | 17; 18; 34 | [110] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Litophyton amentaceum (Studer, 1894) | Nep | MG | 2; 13 | [52] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Lichomolgus aculeatus (Humes, Ho, 1968)) | Litophyton arboreum Forskål, 1775 | Nep | MG | 3 | [113] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Litophyton bumastum (Verseveldt, 1973) | Nep | MG | 8 | [52] |
| Doridicola aculeatus (Humes, Ho, 1968) | Litophyton chabrolii (Andouin, 1828) | Nep | ID | 2 | [38] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Lichomolgus aculeatus (Humes, Ho, 1968)) | Litophyton crassum (Kükenthal, 1903) | Nep | MG | 2 | [113] |
| Doridicola aculeatus (Humes, Ho, 1968) | Litophyton cupressiformis (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Litophyton filamentosum (Verseveldt, 1973) | Nep | MG | 23 | [52] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Litophyton lanternarium (Verseveldt, 1973) | Nep | MG | 15 | [52] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Lichomolgus aculeatus (Humes, Ho, 1968)) | Litophyton savignyi (Ehrenberg, 1834) | Nep | ID; MG | 3; 8; 10 | [38,113] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Lichomolgus aculeatus (Humes, Ho, 1968)) | Litophyton sphaerophorum (Kükenthal, 1903) | Nep | ID; MG | 2; 3 | [38,113] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Litophyton striatum (Kükenthal, 1903) | Nep | ID; MG; YT | 1; 3; 22; 25 | [38,52] |
| Doridicola aculeatus (Humes, Ho, 1968) | Litophyton viridis (May, 1899) | Nep | ID | 3; 10 | [45] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Stereonephthya nosybearia Verseveldt, 1973 | Nep | MG | 10 | [52] |
| Doridicola aculeatus (Humes, Ho, 1968) (=Metaxymolgus aculeatus (Humes, Ho, 1968)) | Stereonephthya scaphis Verseveldt, 1973 | Nep | MG | 25 | [52] |
| Doridicola antheliae (Humes, Stock, 1973) (=Metaxymolgus antheliae (Humes, Stock, 1973)) | Anthelia glauca Lamarck, 1816 | Xen | MG | 8; 12 | [50,52] |
| Doridicola antheliae (Humes, Stock, 1973) (=Metaxymolgus antheliae (Humes, Stock, 1973)) | Anthelia ternatana (Schenk, 1896) | Xen | MG | 18 | [52] |
| Doridicola botulosus (Stock, Kleeton, 1963) | Eunicella singularis (Esper, 1791) | Gor | ES; FR | 10; 25; 30 | [72,83] |
| Doridicola botulosus (Stock, Kleeton, 1963) | Paramuricea clavata (Risso, 1826) | Ple | ES | 20; 23 | [72] |
| Doridicola capnellae Humes, 1990 | Capnella imbricata (Quoy, Gaimard, 1833) | Nep | ID | 10 | [50] |
| Doridicola cincinnatus (Humes, 1975) | Cladiella humesi Verseveldt, 1974 | Alc | NC | 3 | [50] |
| Doridicola cincinnatus (Humes, 1975) (=Metaxymolgus cincinnatus Humes, 1975) | Cladiella pachyclados (Klunzinger, 1877) | Alc | NC | 0.5; 1; 2 | [55] |
| Doridicola cincinnatus (Humes, 1975) | Cladiella rotundata Tixier-Durivault, 1970 | Alc | NC | [50] | |
| Doridicola cincinnatus (Humes, 1975) | Cladiella similis (Tixier-Durivault, 1944) | Alc | NC | 2 | [50] |
| Doridicola cincinnatus (Humes, 1975) | Cladiella sphaerophora (Ehrenberg, 1834) | Alc | NC | 0.2 | [50] |
| Doridicola cinctus (Humes, Stock, 1973) (=Metaxymolgus cinctus Humes, Stock, 1973) | Psammogorgia ramosa Kiikenthal | Ple | MG | 2; 12; 15 | [52,109] |
| Doridicola cinctus (Humes, Stock, 1973) | Rumphella antipathes (Linnaeus, 1758) | Gor | NC | 1; 2 | [43] |
| Doridicola comai Conradi, Megina, López-González, 2004 | Paramuricea clavata (Risso, 1826) | Ple | ES; GB | 20; 25; 30 | [83] |
| Doridicola comparatus (Humes, 1975) (=Metaxymolgus comparatus (Humes, 1975)) | Xenia membranacea Schenk, 1896 | Xen | NC | 15 cm | [55] |
| Doridicola hetaericus (Humes, Ho, 1968) (=Lichomolgus hetaericus (Humes, Ho, 1968)) | Cladiella krempfi (Hickson, 1919) | Alc | MG | 1 | [115] |
| Doridicola hetaericus (Humes, Ho, 1968) (=Metaxymolgus hetaericus (Humes, Ho, 1968)) | Cladiella laciniosa (Tixier-Durivault, 1944) | Alc | MG | 2 | [52] |
| Doridicola hetaericus (Humes, Ho, 1968) (=Lichomolgus hetaericus (Humes, Ho, 1968)) | Cladiella pachyclados (Klunzinger, 1877) | Alc | MG | 1 | [115] |
| Doridicola indistinctus Ho, Ivanenko, 2013 | Gersemia fruticosa (Sars, 1860) | Nep | RU | 24 | [37] |
| Doridicola lumarius (Humes, 1980) (=Metaxymolgus lumarius (Humes, 1980)) | Litophyton cupressiformis (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Doridicola lumarius (Humes, 1980) (=Metaxymolgus lumarius (Humes, 1980)) | Litophyton striatum (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Doridicola mimicus (Humes, 1975) | Cladiella humesi Verseveldt, 1974 | Alc | NC | 2 | [53] |
| Doridicola mimicus (Humes, 1975) (=Metaxymolgus mimicus (Humes, 1975)) | Cladiella pachyclados (Klunzinger, 1877) | Alc | NC | 0.5; 1; 2 | [55] |
| Doridicola parvicaudatus Kim, 2003 | Stereonephthya inordinata Tixier-Durivault, 1970 | Nep | NC | 30 | [53] |
| Doridicola patulus (Humes, 1958) (=Metaxymolgus patulus (Humes, 1958)) | Sinularia mayi Lüttschwager, 1915 | Alc | MG | 20 | [52] |
| Doridicola petalopus Humes, 1990 | Heteroxenia sp. | Xen | NC | 0.5 | [50] |
| Doridicola petalopus Humes, 1990 | Xenia Lamarck, 1816 | Xen | ID | 3 | [50] |
| Doridicola praelongipes (Humes, 1975) (=Metaxymolgus praelongipes (Humes, 1975)) | Xenia membranacea Schenk, 1896 | Xen | NC | 15 cm | [55] |
| Doridicola praelongipes (Humes, 1975) | Xenia viridis Schenk, 1896 | Xen | ID | 3 | [50] |
| Doridicola rostripes Humes, 1990 | Heteroxenia sp. | Xen | NC | 0.5 | [50] |
| Doridicola rostripes Humes, 1990 | Xenia Lamarck, 1816 | Xen | ID | 3 | [50] |
| Doridicola rumphellae Humes, 1993 | Rumphella antipathes (Linnaeus, 1758) | Gor | NC | 1; 2 | [43] |
| Doridicola senticauda Humes, 1990 | Paralemnalia thyrsoides (Ehrenberg, 1834) | Nep | NC | 3 | [50] |
| Doridicola singularipes (Humes, Ho, 1968) (=Metaxymolgus singularipes (Humes, Ho, 1968)) | Alcyonium sp. | Alc | MG | 1 | [52] |
| Doridicola singularipes (Humes, Ho, 1968) | Rhytisma fulvum (Forskål, 1775) (=Parerythropodium fulvum obtusispiculatum Verseveldt) | Alc | MG | 0.2; 0.5 | [50] |
| Doridicola singularipes (Humes, Ho, 1968) (=Lichomolgus singularipes (Humes, Ho, 1968); Metaxymolgus singularipes (Humes, Ho, 1968)) | Rhytisma rubiginosum (Verseveldt, 1968) | Alc | MG | 1; 2 | [52,115] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia africana (May, 1899) | Nep | MG; YT | 2; 12 | [52,113] |
| Doridicola spinulifer (Humes, Frost, 1964) | Lemnalia amabilis Tixier-Durivault, 1966 | Nep | YT | 3 | [113] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia cervicornis (May, 1898) | Nep | MG | 20 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia crassicaulis Verseveldt, 1969 | Nep | MG | 20 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia digitata (May, 1898) | Nep | MG | 2; 17 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) | Lemnalia elegans (May, 1899) | Nep | MG; NC | 0.15; 3 | [55,113] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia flava (May, 1898) | Nep | MG; YT | 1; 1.5; 2 | [52,113] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia longiramus Verseveldt, 1969 | Nep | MG | 12 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia madagascarensis Verseveldt, 1969 | Nep | MG | 24 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Lichomolgus spinulifer (Humes, Frost, 1964)) | Lemnalia sp. | Nep | MG | 1 | [111] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Lemnalia tenuis Verseveldt, 1969 | Nep | MG | 50 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Paralemnalia clavata Verseveldt, 1969 | Nep | MG | 2 | [52] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Paralemnalia thyrsoides (Ehrenberg, 1834) | Nep | ID; MG; NC | 3; 10; 12; 18; 20 | [50,52,55,113] |
| Doridicola spinulifer (Humes, Frost, 1964) (=Metaxymolgus spinulifer (Humes, Frost, 1964)) | Sinularia polydactyla (Ehrenberg, 1834) | Alc | MG | 2 | [52] |
| Doridicola vulcanius Humes, 1990 | Paralemnalia thyrsoides (Ehrenberg, 1834) | Nep | ID | 3 | [50] |
| Mecra ellipsaria Humes, 1980 | Litophyton sphaerophorum (Kükenthal, 1903) | Nep | ID | 2 | [38] |
| Meringomolgus devotus Humes, Stock, 1973 | Sinularia leptoclados (Ehrenberg, 1834) | Alc | MG | 1 | [52] |
| Meringomolgus facetus Humes, Stock, 1973 | Sinularia minima Verseveldt, 1971 | Alc | MG | 15 | [52] |
| Meringomolgus facetus Humes, Stock, 1973 | Sinularia polydactyla (Ehrenberg, 1834) | Alc | MG | 2; 12 | [52] |
| Meringomolgus hamatus Humes, Stock, 1973 | Sinularia humesi Verseveldt, 1968 | Alc | MG | 13; 18 | [52] |
| Meringomolgus hamatus Humes, Stock, 1973 | Sinularia leptoclados (Ehrenberg, 1834) | Alc | MG; NC | 1; 2; 15; 20 | [42,52] |
| Meringomolgus hamatus Humes, Stock, 1973 | Sinularia maxima Verseveldt, 1971 | Alc | MG | 1 | [52] |
| Monomolgus unihastatus Humes, Frost, 1964 | Rhytisma fulvum (Forskål, 1775) (=Parerythropodium fulvum (Forskål, 1775)) | Alc | MG | 1 | [52] |
| Notoxynus mundus Humes, 1975 | Xenia membranacea Schenk, 1896 | Xen | NC | 0.15 | [55] |
| Paradoridicola adelphus (Humes, Ho, 1968) (=Lichomolgus adelphus Humes, Ho, 1968) | Sinularia pedunculata Tixier-Durivault, 1945 | Alc | YT | 3 | [115] |
| Paradoridicola adelphus (Humes, Ho, 1968) (=Lichomolgus adelphus Humes, Ho, 1968) | Sinularia polydactyla (Ehrenberg, 1834) | Alc | MG; MH; NC | 0.2; 0.5; 1; 2 15 | [52,55,115] |
| Paradoridicola adelphus (Humes, Ho, 1968) (=Lichomolgus adelphus Humes, Ho, 1968) | Sinularia whiteleggei Lüttschwager, 1914 | Alc | MG | 2 | [115] |
| Paradoridicola angularis Humes, 1990 | Klyxum flaccidum (Tixier-Durivault, 1966) (=Alcyonium flaccidum Tixier-Durivault, 1966) | Alc | MG | 12; 20 | [50] |
| Paradoridicola angularis Humes, 1990 | Klyxum molle (Thomson, Dean, 1931) (=Alcyonium molle Thomson, Dean, 1931) | Alc | ID | 3 | [50] |
| Paradoridicola angularis Humes, 1990 | Klyxum simplex (Thomson, Dean, 1931) (=Alcyonium simplex Thomson, Dean, 1931) | Alc | NC | 2 | [50] |
| Paradoridicola angularis Humes, 1990 | Klyxum utinomii (Verseveldt, 1971) (=Alcyonium utinomii Verseveldt, 1971) | Alc | MG | 12 | [50] |
| Paradoridicola contiguus Humes, 1990 | Sinularia flexibilis (Quoy, Gaimard, 1833) | Alc | ID | 3; 4 | [50] |
| Paradoridicola drepanophorus Humes, 1990 | Klyxum flaccidum (Tixier-Durivault, 1966) (=Alcyonium flaccidum Tixier-Durivault, 1966) | Alc | MG | 12; 20 | [50] |
| Paradoridicola drepanophorus Humes, 1990 | Klyxum molle (Thomson, Dean, 1931) (=Alcyonium molle Thomson, Dean, 1931) | Alc | ID | 3 | [50] |
| Paradoridicola drepanophorus Humes, 1990 | Klyxum simplex (Thomson, Dean, 1931) (=Alcyonium simplex Thomson, Dean, 1931) | Alc | NC | 2 | [50] |
| Paradoridicola glabripes (Humes, Ho, 1968) | Ovabunda macrospiculata (Gohar, 1940) (=Xenia macrospiculata Gohar, 1940) | Xen | MG | 20 | [52] |
| Paradoridicola glabripes (Humes, Ho, 1968) | Xenia umbellata Lamarck, 1816 | Xen | MG | 1 | [115] |
| Paradoridicola glabripes (Humes, Ho, 1968) | Xenia viridis Schenk, 1896 | Xen | MG | [52] | |
| Paradoridicola hystricosus Humes, 1990 | Sinularia gravis Tixier-Durivault, 1970 | Alc | NC | 1 | [50] |
| Paradoridicola simulator Humes, 1990 | Klyxum simplex (Thomson, Dean, 1931) (=Alcyonium simplex Thomson, Dean, 1931) | Alc | NC | 0.5; 2 | [50] |
| Paradoridicola sinulariae Humes, Stock, 1973 | Sinularia arborea Verseveldt, 1971 | Alc | MG | 2; 12; 13; 23 | [52] |
| Paradoridicola sinulariae Humes, Stock, 1973 | Sinularia flexibilis (Quoy, Gaimard, 1833) | Alc | NC | 3 | [55] |
| Paradoridicola sinularianus Humes, 1990 | Sinularia gravis Tixier-Durivault, 1970 | Alc | NC | 1 | [50] |
| Paradoridicola sinularianus Humes, 1990 | Sinularia nanolobata Verseveldt, 1977 | Alc | ID | 2 | [50] |
| Paradoridicola spinulatus Humes, 1982 | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | ID | 5; 10 | [39] |
| Paradoridicola squamiger (Humes, Frost, 1964) | Sinularia ceramensis Verseveldt, 1977 | Alc | ID | 2 | [50] |
| Paradoridicola squamiger (Humes, Frost, 1964) (=Lichomolgus squamiger Humes, Frost, 1964) | Sinularia polydactyla (Ehrenberg, 1834) | Alc | MG; NC | 20 cm; 0.5; 1; 2; 15 | [52,55,111] |
| Paradoridicola squamiger (Humes, Frost, 1964) | Sinularia whiteleggei Lüttschwager, 1914 | Alc | MG | 2 | [115] |
| Paradoridicola triquetrus (Humes, Ho, 1968) (=Lichomolgus triquetrus Humes, Ho, 1968) | Anthelia gracilis (May, 1898) | Xen | MG | 0.5 | [114] |
| Paradoridicola virgulifer Humes, 1990 | Sinularia polydactyla (Ehrenberg, 1834) | Alc | ID | 2; 3 | [50] |
| Paramolgus abruptus Humes, 1990 | Lobophytum crassum von Marenzeller, 1886 | Alc | MG | 25 | [50] |
| Paramolgus accinctus Humes, 1980 | Litophyton cupressiformis (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Paramolgus accinctus Humes, 1980 | Litophyton savignyi (Ehrenberg, 1834) | Nep | ID | 3 | [38] |
| Paramolgus accinctus Humes, 1980 | Litophyton sphaerophorum (Kükenthal, 1903) | Nep | ID | 2 | [38] |
| Paramolgus accinctus Humes, 1980 | Litophyton striatum (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Paramolgus accinctus Humes, 1980 | Litophyton viridis (May, 1899) | Nep | ID | 3 | [45] |
| Paramolgus alcyoniicus Humes, 1990 | Klyxum legitimum (Tixier-Durivault, 1970) | Alc | NC | 2; 30 | [50] |
| Paramolgus alcyoniicus Humes, 1990 | Klyxum simplex (Thomson, Dean, 1931) (=Alcyonium simplex Thomson, Dean, 1931) | Alc | NC | 0.5; 2 | [50] |
| Paramolgus centor Humes, 1990 | Paralemnalia thyrsoides (Ehrenberg, 1834) | Nep | ID; NC | 3; 10 | [50] |
| Paramolgus clavatus (Humes, Ho, 1968) (=Lichomolgus clavatus Humes, Ho, 1968) | Coelogorgia palmosa Milne Edwards, Haime, 1857 | Coe | MG | 1; 2 | [114] |
| Paramolgus clavatus (Humes, Ho, 1968) | Lemnalia cervicornis (May, 1898) | Nep | MG | 20 | [52] |
| Paramolgus clavatus (Humes, Ho, 1968) | Lemnalia crassicaulis Verseveldt, 1969 | Nep | MG | 20 | [52] |
| Paramolgus clavatus (Humes, Ho, 1968) | Lemnalia longiramus Verseveldt, 1969 | Nep | MG | 12 | [52] |
| Paramolgus clavatus (Humes, Ho, 1968) | Sinularia polydactyla (Ehrenberg, 1834) | Alc | NC | 2 | [53] |
| Paramolgus clavatus (Humes, Ho, 1968) | Stereonephthya inordinata Tixier-Durivault, 1970 | Nep | NC | 30 | [55] |
| Paramolgus congruus Humes, 1990 | Rhytisma fulvum (Forskål, 1775) (=Parerythropodium fulvum obtusispiculatum Verseveldt) | Alc | MG | 0.15; 0.2; 0.5; 1 | [50] |
| Paramolgus congruus Humes, 1990 | Rhytisma fuscum (Thomson, Henderson, 1906) (=Parerythropodium fulvum fuscum (Thomson, Henderson, 1906)) | Alc | MG | 0.15; 0.2; 0.5; 1 | [50] |
| Paramolgus dapsilis Humes, 1993 | Annella reticulata (Ellis, Solander, 1786) (=Suberogorgia reticulata) | Sub | ID; PH | 10; 30 | [43] |
| Paramolgus ellisellae Humes, 1974 | Ctenocella ramosa (Simpson, 1910) (=Ellisella ramosa (Simpson, 1910)) | Ell | MG | 24; 25 | [109] |
| Paramolgus eniwetokensis Humes, 1973 | Lobophytum crassum von Marenzeller, 1886 | Alc | NC | [55] | |
| Paramolgus eniwetokensis Humes, 1973 | Lobophytum crebriplicatum von Marenzeller, 1886 | Alc | NC | 2; 3 | [55] |
| Paramolgus eniwetokensis Humes, 1973 | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | MH; NC | 0.5; 1; 2; 3; 5 | [49,50] |
| Paramolgus extendens Humes, Dojiri, 1979 | Cespitularia multipinnata (Quoy, Gaimard, 1833) | Xen | ID | 5 | [46] |
| Paramolgus galeatus Kim, 2003 | Sarcophyton ehrenbergi v. Marenzeller, 1886 | Alc | NC | [53] | |
| Paramolgus inconstans Humes, Dojiri, 1979 | Lobophytum crassum von Marenzeller, 1886 | Alc | ID | 2 | [44] |
| Paramolgus inconstans Humes, Dojiri, 1979 | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | NC | 2 | [50] |
| Paramolgus litophyticus Humes, Dojiri, 1979 | Litophyton viridis (May, 1899) | Nep | ID | 10 | [45] |
| Paramolgus modicus Humes, 1990 | Lobophytum latilobatum Verseveldt, 1971 | Alc | MG | 1 | [50] |
| Paramolgus nephthaenus Humes, 1980 | Litophyton chabrolii (Andouin, 1828) | Nep | ID | 2 | [38] |
| Paramolgus nephthaenus Humes, 1980 | Litophyton cupressiformis (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Paramolgus nephthaenus Humes, 1980 | Litophyton savignyi (Ehrenberg, 1834) | Nep | ID | 3 | [38] |
| Paramolgus nephthaenus Humes, 1980 | Litophyton sphaerophorum (Kükenthal, 1903) | Nep | ID | 2 | [38] |
| Paramolgus nephthaenus Humes, 1980 | Litophyton striatum (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Paramolgus nephthaenus Humes, 1980 | Stereonephthya inordinata Tixier-Durivault, 1970 | Nep | NC | 30 | [53] |
| Paramolgus ostentus Humes, 1973 | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | MH | 2 | [49] |
| Paramolgus pollicaris Humes, Dojiri, 1979 | Cespitularia multipinnata (Quoy, Gaimard, 1833) | Xen | ID | 5 | [46] |
| Paramolgus promiculus Humes, 1980 | Litophyton cupressiformis (Kükenthal, 1903) | Nep | ID | 3 | [38] |
| Paramolgus promiculus Humes, 1980 (=Paramolgus prominulus Humes, 1980) | Litophyton savignyi (Ehrenberg, 1834) (=Nephthea aberrans (Verseveldt, 1968)) | Nep | ID; NC | 3; 30 | [38] |
| Paramolgus promiculus Humes, 1980 | Litophyton sphaerophorum (Kükenthal, 1903) | Nep | ID | 2 | [38] |
| Paramolgus promiculus Humes, 1980 | Litophyton viridis (May, 1899) | Nep | ID | 3; 10 | [45] |
| Paramolgus promiculus Humes, 1980 | Stereonephthya inordinata Tixier-Durivault, 1970 | Nep | NC | 30 | [53] |
| Paramolgus quadrangulus Humes, 1990 | Sinularia brassica May, 1898 (=Sinularia dura (Pratt, 1903)) | Alc | ID; NC | 2; 3; 10 | [50] |
| Paramolgus resectus Humes, Dojiri, 1979 | Litophyton viridis (May, 1899) | Nep | ID | 3 | [45] |
| Paramolgus spathophorus (Humes, Ho, 1968) | Lobophytum crebriplicatum von Marenzeller, 1886 | Alc | NC | 2; 3 | [55] |
| Paramolgus spathophorus (Humes, Ho, 1968) | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | MG; NC | 0.5; 1; 2; 4; 17 | [50] |
| Paramolgus spathophorus (Humes, Ho, 1968) | Sarcophyton ehrenbergi v. Marenzeller, 1886 (=Sarcophyton acutangulum (v. Marenzeller, 1886)) | Alc | MG; NC | 3; 4; 25 | [39,52] |
| Paramolgus spathophorus (Humes, Ho, 1968) | Sarcophyton elegans Moser, 1919 | Alc | NC | 1; 2 | [39] |
| Paramolgus spathophorus (Humes, Ho, 1968) | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | MG; NC | 0.5; 1; 3; 17 | [39,52,115] |
| Paramolgus spathophorus (Humes, Ho, 1968) | Sarcophyton stolidotum Verseveldt, 1971 | Alc | MG | 17 | [39] |
| Paramolgus subincisus Humes, 1990 | Heteroxenia sp. | Xen | NC | [50] | |
| Paramolgus subincisus Humes, 1990 | Xenia Lamarck, 1816 | Xen | ID | 3 | [50] |
| Paramolgus timendus Humes, 1990 | Klyxum molle (Thomson, Dean, 1931) (=Alcyonium molle Thomson, Dean, 1931) | Alc | ID | 3 | [50] |
| Paramolgus timendus Humes, 1990 | Klyxum simplex (Thomson, Dean, 1931) (=Alcyonium simplex Thomson, Dean, 1931) | Alc | NC | 0.5; 2 | [50] |
| Paredromolgus decorus (Humes, Frost, 1964) | Cladiella humesi Verseveldt, 1974 | Alc | NC | 2 | [50] |
| Paredromolgus decorus (Humes, Frost, 1964) | Cladiella laciniosa (Tixier-Durivault, 1944) | Alc | MG | 2 | [50] |
| Paredromolgus decorus (Humes, Frost, 1964) | Cladiella latissima (Tixier-Durivault, 1944) | Alc | MG | 1; 18 | [52] |
| Paredromolgus decorus (Humes, Frost, 1964) | Cladiella rotundata Tixier-Durivault, 1970 | Alc | NC | 5 | [50] |
| Paredromolgus decorus (Humes, Frost, 1964) | Cladiella sphaerophora (Ehrenberg, 1834) | Alc | MG | 1 | [52] |
| Pennatulicola piscatorius Itoh, Kim, 2015 | Pteroeides sp. | Pen | JP | 15 | [78] |
| Pennatulicola pteroidis (Della Valle, 1880) | Pteroeides griseum (Bohadsch, 1761) (=Pteroides spinulosus) | Pen | [140] | ||
| Pennatulicola pterophilus (Stock, 1962) | Pteroeides sp. | Pen | ID | [48] | |
| Pennatulicola pterophilus (Stock, 1962) | Pteroeides sagamiense Moroff, 1902 | Pen | MG | 18 | [110] |
| Pennatulicola robustclavus Uyeno, 2015 | Pteroeides sp. | Pen | SG | 0; 6.2; 10.3; 10.6; 12.9 | [57] |
| Pennatulicola serratipes (Ummerkutty, 1962) | Pteroeides esperi Herklots, 1858 | Pen | [141] | ||
| Perosyna indonesica Humes, 1982 | Sarcophyton glaucum (Quoy, Gaimard, 1833) | Alc | ID | 5 | [39] |
| Plesiomolgus conjunctus (Humes, Ho, 1967) (=Lichomolgus conjunctus Humes, Ho, 1967) | Tubipora musica Linnaeus, 1758 | Tub | MG; YT | 15 cm; 1; 2 | [112] |
| Plesiomolgus organicus (Humes, Ho, 1967) (=Lichomolgus organicus Humes, Ho, 1967) | Tubipora musica Linnaeus, 1758 | Tub | MG; YT | 15 cm; 1; 2 | [112] |
| Telestacicola angoti Humes, Stock, 1973 | Annella reticulata (Ellis, Solander, 1786) | Sub | MG | 8 | [109] |
| Telestacicola angoti Humes, Stock, 1973 | Coelogorgia palmosa Milne Edwards, Haime, 1857 | Coe | MG | 1; 3 | [1] |
| Telestacicola angoti Humes, Stock, 1973 | Subergorgia suberosa (Pallas, 1766) | Sub | MG | 17 | [109] |
| Telestacicola lobophyti Humes, 1990 | Lobophytum pauciflorum (Ehrenberg, 1834) | Alc | MG | 17 | [50] |
| Zamolgus acanthodes Humes, Stock, 1973 | Sinularia arborea Verseveldt, 1971 | Alc | MG | 2; 12; 13; 23 | [52] |
| Zamolgus cracens Humes, Dojiri, 1979 | Cespitularia multipinnata (Quoy, Gaimard, 1833) | Xen | ID | 5 | [46] |
| Zamolgus tridens Humes, Stock, 1973 | Caementabunda simplex (Thomson, Dean, 1931) | Xen | MG | [52] | |
| Sabelliphilidae | |||||
| Eupolymniphilus brevicaudatus Kim, 2009 | Tubipora musica Linnaeus, 1758 | Tub | MG | 1 | [117] |
| Thamnomolgidae | |||||
| Forhania philippinensis Humes, 1990 | Annella reticulata (Ellis, Solander, 1786) (=Subergorgia reticulata (Ellis, Solander, 1786)) | Sub | PH | 30 | [42] |
| Forhania philippinensis Humes, 1990 | Melithaea rubeola (Wright, Studer, 1889) (=Acabaria rubeola (Wright, Studer, 1889)) | Mel | PH | 40 | [42] |
| Forhania philippinensis Humes, 1990 | Villogorgia intricata (Gray, 1870) | Ple | PH | 30 | [43] |
| Thamnomolgus nodulus Humes, 1990 | Villogorgia intricata (Gray, 1870) | Ple | PH | 30 | [43] |
| Siphonostomatoida | |||||
| Artotrogidae | |||||
| Metapontius walteri Johnsson, Neves, 2005 | Briareum violaceum (Quoy, Gaimard, 1833) | Bri | MH | 2 | [59] |
| Asterocheridae | |||||
| Asterocheres indivisus Kim, 2010 | Cespitularia erecta Macfadyen, 1936 | Xen | MG | 12; 24 | [118] |
| Asterocheres nudicoxus Kim, 2010 | Tubipora musica Linnaeus, 1758 | Tub | MG | 1 | [118] |
| Orecturus ampulus Humes, 1996 | Siphonogorgia variabilis (Hickson, 1903) | Nid | NC | 30 | [51] |
| Orecturus antillensis Varela, 2011 | Eunicea mammosa Lamouroux, 1816 | Ple | CU | 3 | [105] |
| Orecturus excavatus (Humes, 1989) (=Acontiophorus excavatus Humes, 1989) | Dendronephthya koellikeri Kükenthal, 1905 | Nep | ID | 10; 25 | [41] |
| Orecturus excavatus (Humes, 1989) (=Acontiophorus excavatus Humes, 1989) | Dendronephthya mucronata (Pütter, 1900) | Nep | MG | 8; 25 | [41] |
| Orecturus excavatus (Humes, 1989) (=Acontiophorus excavatus Humes, 1989) | Dendronephthya sp. | Nep | ID; PH | 17; 30 | [1,41] |
| Orecturus excavatus (Humes, 1989) | Siphonogorgia pichoni Verseveldt, 1971 | Nid | MG | 20 | [1] |
| Orecturus finitimus Humes, 1993 | Acanthogorgia sp. | Aca | ID | 17; 25 | [43] |
| Orecturus finitimus Humes, 1993 | Villogorgia intricata (Gray, 1870) | Ple | PH | 30 | [43] |
| Orecturus forticulus Humes, 1993 | Melithaea ochracea (Linnaeus, 1758) (=Melitodes ochracea (Linnaeus)) | Mel | ID | 3 | [43] |
| Orecturus grandisetiger Humes, 1992 | Acanthogorgia sp. | Aca | ID | 17; 25 | [43] |
| Orecturus grandisetiger Humes, 1992 | Stereonephthya cordylophora Verseveldt, 1973 | Nep | MG | 24 | [1] |
| Orecturus longicaudatus Kim, Song, 2003 | Calicogorgia granulosa Kükenthal, Gorzawsky, 1908 | Aca | KR | 10 | [94] |
| Orecturus ortizi Varela, Lalana, 2007 | Briareum asbestinum (Pallas, 1766) | Bri | CU | [100] | |
| Orecturus sakalavicus Humes, 1994 | Coelogorgia palmosa Milne Edwards, Haime, 1857 | Coe | MG; YT | 1; 2; 3; 8; 15; 18 | [1] |
| Orecturus similis Kim, Song, 2003 | Dendronephthya sp. | Nep | KR | [94] | |
| Parasteropontius latus (Humes, 1992) (=Asteropontius latus Humes, 1992) | Villogorgia intricata (Gray, 1870) | Ple | PH | 30 | [43] |
| Acontiophorus armatus Brady, 1880 | Alcyonium digitatum Linnaeus, 1758 | Alc | IE | 10 | [77] |
| Asterocheres tubiporae Kim, 2004 | Tubipora musica Linnaeus, 1758 | Tub | MG | 1 | [93] |
| Entomolepididae | |||||
| Entomopsyllus stocki Kim, 2004 | Tubipora musica Linnaeus, 1758 | Tub | [93] | ||
| Entomopsyllus takara Uyeno, Johnsson, 2018 | Heliopora coerulea (Pallas, 1766) | Hel | JP | 10 | [92] |
| Harpacticoida | |||||
| Tegastidae | |||||
| Parategastes conexus Humes, 1984 | Stereonephthya ulicoides Thomson, Dean, 1931 | Nep | ID | 10 | [40] |
* Octocoral family: Aca—Acanthogorgiidae, Alc—Alcyoniidae, Antp—Anthoptilidae, Antt—Anthothelidae, Bri—Briareidae, Chr—Chrysogorgiidae, Cla—Clavulariidae, Coe—Coelogorgiidae, Ell—Ellisellidae, Gor—Gorgoniidae, Hel—Helioporidae, Isi—Isididae, Kop—Kophobelemnidae, Mel—Melithaeidae, Nep—Nephtheidae, Nid—Nidaliidae, Parg—Paragorgiidae, Parl—Paralcyoniidae, Pen—Pennatulidae, Ple—Plexauridae, Pri—Primnoidae, Ren—Renillidae, Sub—Subergorgiidae, Tub—Tubiporidae, Ver—Veretillidae, Vir—Virgulariidae, Xen—Xeniidae. ** Sites: AQ—Antarctica, BB—Barbados, BM—Bermuda, BQ—Bonaire, St. Eustatius and Saba, BS—Bahamas, CA—Canada, CU—Cuba, CW—Curacao, ER—Eritrea, ES—Spain, FR—France, GB—Great Britain, GL—Greenland, ID—Indonesia, IE—Ireland, IL—Israel, IS—Iceland, IT—Italy, JM—Jamaica, JP—Japan, KR—Republic of Korea, MF—Saint Martin (Fr.), MG—Madagascar, MH—Marshall Islands, NC—New Caledonia, NL-BQ3—Sint Eustatius, NO—Norway, PH—Philippines, PR—Puerto Rico, RU—Russia, SE—Sweden, SG—Singapore, SL—Sierra Leone, US—USA, YT—Mayott.

Table A2.
Description of the dataset with specific information relative to column names, description, units, and attribute type.
Table A2.
Description of the dataset with specific information relative to column names, description, units, and attribute type.
| Attribute | Column_Name | Description | Units | Attribute_Type |
|---|---|---|---|---|
| Record number | rID | Unique number corresponding to specific occurrence | Integer | |
| Record ID | recordID | A structured code incorporating a concise article reference, region and country observation identifiers, shorthand for the location coordinates, and specific abbreviations for the symbiont and host families, complemented by a distinct number. | Text | |
| Aphia ID of symbiont | aphiaID_Symbiont | Unique number for taxon from WoRMS database | Integer | |
| Kingdom of symbiont | kingdom_Symbiont | Taxonomic rank below Domain | Text | |
| Phylum of symbiont | phylum_Symbiont | Taxonomic rank below Kingdom | Text | |
| Class of symbiont | class_Symbiont | Taxonomic rank below Phylum | Text | |
| Order of symbiont | order_Symbiont | Taxonomic rank below Class | Text | |
| Family of symbiont | family_Symbiont | Taxonomic rank below Order | Text | |
| Genus of symbiont | genus_Symbiont | Taxonomic rank below Family and first element in the Latin binomial name | Text | |
| Specific epithet of symbiont | specificEpithet_Symbiont | Second element in the Latin binomial name | Text | |
| Scientific name authorship of symbiont | scientificNameAuthorship_Symbiont | Third element in the Latin binomial name | Text | |
| Symbiont ID | symbiontID | Reviewed species name | Text | |
| Taxon rank of symbiont | taxonRank_Symbiont | Taxonomic rank information (e.g., genus, species) | Text | |
| Taxonomic status of symbiont | taxonomicStatus_Symbiont | Taxonomic status information (e.g., accepted, unaccepted) | Text | |
| Link of symbiont | link_Symbiont | Link to taxon in WoRMS database | Text | |
| Female Body Length | femaleLength | The length of the female specimen, measured from head to tail | µm | Text |
| Female Body Weight | femaleWeight | The total weight of the female specimen | µm | Text |
| Male Body Length | maleLength | The length of the male specimen, measured from head to tail | µm | Text |
| Male Body Weight | maleWeight | The total weight of the male specimen | µm | Text |
| Aphia ID of host | aphiaID_Host | Unique number for taxon from WoRMS database [33] | Integer | |
| Kingdom of host | kingdom_Host | Taxonomic rank below Domain | Text | |
| Phylum of host | phylum_Host | Taxonomic rank below Kingdom | Text | |
| Class of host | class_Host | Taxonomic rank below Phylum | Text | |
| Order of host | order_Host | Taxonomic rank below Class | Text | |
| Family of host | family_Host | Taxonomic rank below Order | Text | |
| Genus of host | genus_Host | Taxonomic rank below Family and first element in the Latin binomial name | Text | |
| Specific epithet of host | specificEpithet_Host | Second element in the Latin binomial name | Text | |
| Scientific name authorship of host | scientificNameAuthorship_Host | Third element in the Latin binomial name | Text | |
| Host ID | hostID | Reviewed species name | Text | |
| Taxon rank of host | taxonRank_Host | Taxonomic rank information (e.g., genus, species) | Text | |
| Taxonomic status of host | taxonomicStatus_Host | Taxonomic status information (e.g., accepted, unaccepted) | Text | |
| Link of host | link_Host | Link to taxon in WoRMS database | Text | |
| Site ID | siteID | Unique number for locality | Text | |
| Region code | regionCode | Unique number for region | Text | |
| Region | region | Division of the World Ocean [120] | Text | |
| Ocean | ocean | The name of the ocean in which the locality occurs. | Text | |
| Water body | waterBody | The name of the water body in which the locality occurs. | Text | |
| Island | island | The name of the island near which the locality occurs. | Text | |
| Country | country | The name of the country in which the locality occurs. | Text | |
| Country code | countryCode | The standard code (ISO 3166-1-alpha-2) for the country in which the locality occurs. | Text | |
| Locality | locality | Particular area where the taxon was found | Text | |
| Exact Location Description | verbatimLocalition | A comprehensive description of the location from the original article | Text | |
| Geocoordinates | geocoordinates | A combined representation of both latitude and longitude | Degrees Minutes Seconds (DMS) | Text |
| Latitude | latitude | Coordinate that specifies the N–S position of a point on the Earth surface | Degrees Minutes Seconds (DMS) | Text |
| Longitude | longitude | Coordinate that specifies the E–W position of a point on the Earth surface | Degrees Minutes Seconds (DMS) | Text |
| Decimal geocoordinates | decimalGeocoordinates | A combined representation of both latitude and longitude | Decimal degrees, WGS84 | Numeric |
| Decimal latitude | decimalLatitude | Coordinate that specifies the N–S position of a point on the Earth surface | Decimal degrees, WGS84 | Numeric |
| Decimal longitude | decimalLongitude | Coordinate that specifies the E–W position of a point on the Earth surface | Decimal degrees, WGS84 | Numeric |
| Coordinate uncertainty | coordinateUncertaintyInMeters | The horizontal distance from the given decimal latitude and longitude describing the smallest circle containing the whole of the Location. | m | Integer |
| Minimum depth | minimumDepthInMeters | Vertical distance under sea level | m | Integer |
| Maximum depth | maximumDepthInMeters | Vertical distance under sea level | m | Integer |
| Collecting method | collectingMethod | The method of taking sample | Text | |
| Finding method | findingMethod | The method of finding copepods in sample | Text | |
| Type of association | note | Describes the nature of the interaction. | Text | |
| Host interaction site | locationAtHost | The general location or site on the host where the copepod interacts or resides. | Text | |
| Detailed host interaction site | fullLocationAtHost | A more specific or detailed description of the copepod’s interaction site on the host. | Text | |
| Copepod nutritional source | copepodNutritionSourse | The primary source from which the copepod derives its nutrition. | Text | |
| Event date | eventDate | Date of sampling. | Date | |
| Year | year | The four-digit year in which the Occurence recorded. Format: yyyy. | Integer | |
| Month | month | The ordinal month in which the Occurence recorded. Format: mm. | Integer | |
| Article ID | articleID | Short reference | Text | |
| Reference | reference | Text |
References
- Humes, A.G. Copepoda associated with octocorals in Northwestern Madagascar, including Orecturus sakalavicus n. sp. from the telestacean Coelogorgia palmosa. Trans. Am. Microsc. Soc. 1994, 113, 117–126. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Halsey, S.H. An Introduction to Copepod Diversity; Ray Society: London, UK, 2004. [Google Scholar]
- Bron, J.E.; Frisch, D.; Goetze, E.; Johnson, S.C.; Lee, C.E.; Wyngaard, G.A. Observing copepods through a genomic lens. Fron-Tiers Zool. 2011, 8, 22. [Google Scholar] [CrossRef]
- Gotto, R.V. The association of copepods with marine invertebrates. Adv. Mar. Biol. 1979, 1, 1–109. [Google Scholar]
- Kabata, Z. Parasitic Copepoda of British Fishes; Ray Society: London, UK, 1979; p. 468. [Google Scholar]
- Huys, R.; Boxshall, G.A. Copepod Evolution; Ray Society: London, UK, 1991. [Google Scholar]
- Ho, J.S. Why do symbiotic copepods matter? Hydrobiologia 2001, 453, 1–7. [Google Scholar] [CrossRef]
- Ho, J.S. Copepod phylogeny: A reconsideration of Huys, Boxshall’s “parsimony versus homology”. Hydrobiologia 1994, 292–293, 31–39. [Google Scholar] [CrossRef]
- Ferrari, F.D.; Ivanenko, V.N.; Dahms, H.-U. Body architecture and relationships among basal copepods. J. Crustac. Biol. 2010, 30, 465–477. [Google Scholar] [CrossRef]
- Mikhailov, K.V.; Ivanenko, V.N. Lack of reproducibility of molecular phylogenetic analysis of Cyclopoida. Mol. Phylogenet. Evol. 2019, 139, 106574. [Google Scholar] [CrossRef]
- Mikhailov, K.V.; Ivanenko, V.N. Low support values and lack of reproducibility of molecular phylogenetic analysis of Copepoda orders. Arthrop. Sel. 2021, 30, 39–42. [Google Scholar] [CrossRef]
- Ivanenko, V.N.; Hoeksema, B.W.; Mudrova, S.V.; Nikitin, M.A.; Martínez, A.; Rimskaya-Korsakova, N.N.; Berumen, M.L.; Fontaneto, D. Lack of host specificity of copepod crustaceans associated with mushroom corals in the Red Sea. Mol. Phylogenet. Evol. 2018, 127, 770–780. [Google Scholar] [CrossRef]
- Humes, A.G. Cnidarians and copepods: A success story. Trans. Am. Microsc. Soc. 1985, 104, 313–320. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Mayfield, A.B.; Meng, P.J.; Dai, C.F.; Huys, R. Copepods associated with scleractinian corals: A worldwide checklist and a case study of their impact on the reef-building coral Pocillopora damicornis (Linnaeus, 1758) (Pocilloporidae). Zootaxa 2016, 4174, 291–345. [Google Scholar] [CrossRef] [PubMed]
- Humes, A.G. How many copepods. In Ecology and Morphology of Copepods: Proceedings of the Fifth International Conference on Copepoda, Developments in Hydrobiology, Baltimore, ML, USA, 6–13 June 1993; Ferrari, F.D., Bradley, B.P., Eds.; Springer: London, UK, 1994; Volume 292/293, pp. 1–7. [Google Scholar]
- Humes, A.G. Copepoda associated with scleractinian corals on the Great Barrier Reef, northeastern Australia, with a key to the genera of the Lichomolgidae. J. Nat. Hist. 1991, 25, 1171–1231. [Google Scholar] [CrossRef]
- Korzhavina, O.A.; Hoeksema, B.W.; Ivanenko, V.N. A review of Caribbean Copepoda associated with reef-dwelling cnidarians, echinoderms, and sponges. Contrib. Zool. 2019, 88, 297–349. [Google Scholar] [CrossRef]
- Korzhavina, O.A.; Reimer, J.D.; Ehrlich, H.; Ivanenko, V.N. Global diversity and distribution of lamippidae copepods symbiotic on octocorallia. Symbiosis 2021, 83, 265–277. [Google Scholar] [CrossRef]
- Yeom, J.; Nikitin, M.A.; Ivanenko, V.N.; Lee, W. A new minute ectosymbiotic harpacticoid copepod living on the sea cucumber Eupentacta fraudatrix in the East/Japan Sea. PeerJ 2018, 6, e4979. [Google Scholar] [CrossRef]
- Bernot, J.P.; Boxshall, G.A.; Crandall, K.A. A synthesis tree of the Copepoda: Integrating phylogenetic and taxonomic data reveals multiple origins of parasitism. PeerJ 2021, 9, e12034. [Google Scholar] [CrossRef] [PubMed]
- van Oppen, M.J.H.; Mieog, J.C.; Sanchez, C.A.; Fabricius, K.E. Diversity of algal endosymbionts (Zooxanthellae) in octocorals: The roles of geography and host relationships. Mol. Ecol. 2005, 14, 2403–2417. [Google Scholar] [CrossRef]
- Watling, L.; France, S.C.; Pante, E.; Simpson, A. Biology of deep-water octocorals. Adv. Mar. Biol. 2011, 60, 41–122. [Google Scholar]
- van de Water, J.A.J.M.; Allemand, D.; Ferrier-Pagès, C. Host-microbe interactions in octocoral holobionts—Recent advances and perspectives. Microbiome 2018, 6, 64. [Google Scholar] [CrossRef]
- Shelyakin, P.V.; Garushyants, S.K.; Nikitin, M.A.; Mudrova, S.V.; Berumen, M.; Speksnijder, A.G.; Ivanenko, V.N. Microbiomes of gall-inducing copepod crustaceans from the corals Stylophora pistillata (Scleractinia) and Gorgonia ventalina (Alcyonacea). Sci. Rep. 2018, 8, 11563. [Google Scholar] [CrossRef]
- Lau, Y.W.; Poliseno, A.; Kushida, Y.; Quéré, G.; Reimer, J.D. The classification, diversity, and ecology of shallow water octocorals. In Encyclopedia of the World’s Biomes; Goldstein, M., DellaSala, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–15. [Google Scholar]
- Giangrande, A. Biodiversity, conservation, and the taxonomic impediment. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 451–459. [Google Scholar] [CrossRef]
- Ehrlich, H. Marine Biological Materials of Invertebrate Origin; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Weil, E.; Rogers, C.S.; Croquer, A. Octocoral diseases in a changing ocean. In Marine Animal Forests; Rossi, S., Bramanti, L., Gori, A., Orejas Saco del Valle, C., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Weil, E. Disease Problems. In Mesophotic Coral Ecosystems. Coral Reefs of the World; Loya, Y., Puglise, K., Bridge, T., Eds.; Springer: Cham, Switzerland, 2019; Volume 12. [Google Scholar]
- Kupfner Johnson, S.; Hallock, P. A review of symbiotic gorgonian research in the western Atlantic and Caribbean with recommendations for future work. Coral Reefs 2020, 39, 239–258. [Google Scholar] [CrossRef]
- Ivanenko, V.N.; Nikitin, M.A.; Hoeksema, B.W. Multiple purple spots in the Caribbean sea fan Gorgonia ventalina caused by parasitic copepods at St. Eustatius, Dutch Caribbean. Mar. Biodivers. 2017, 47, 79–80. [Google Scholar] [CrossRef]
- Tracy, A.M.; Weil, E.; Burge, C.A. Ecological Factors Mediate Immunity and Parasitic Co-Infection in Sea Fan Octocorals. Front. Immunol. 2021, 11, 608066. [Google Scholar] [CrossRef] [PubMed]
- World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org/ (accessed on 15 July 2022). [CrossRef]
- Heegaard, P. Notes on parasitic copepods. Vidensk. Medd. Dan. Naturhist. Foren. 1949, 111, 235–245. [Google Scholar]
- Laubier, L. Lamippe (Lamippe) bouligandi sp. nov., Copépode parasite d’Octocoralliaire de la Mer du Labrador’. Crustaceana 1972, 22, 285–293. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Mortensen, P.B. Gorgonophilus canadensis n. gen., n. sp. (Copepoda: Lamippidae), a gall forming endoparasite in the octocoral Paragorgia arborea (L., 1758) from the Northwest Atlantic. Symbiosis 2004, 37, 155–168. [Google Scholar]
- Ho, J.S.; Ivanenko, V.N. Doridicola indistinctus n. sp. (Copepoda: Poecilostomatoida: Rhynchomolgidae) associated with the soft coral Gersemia fruticosa Sars (Octocorallia: Alcyonacea: Nephtheidae) from the White Sea. Syst. Parasitol. 2013, 85, 235–241. [Google Scholar] [CrossRef]
- Humes, A.G. Copepoda (Cyclopoida, Lichomolgidae) associated with the alcyonacean Nephthea in the Moluccas. Hydrobiologia 1980, 68, 49–71. [Google Scholar] [CrossRef]
- Humes, A.G. Copepoda (Poecilostomatoida, Lichomolgidae) associated with alcyonacean genus Sarcophyton in the Indo-Pacific. Publ. Seto Mar. Biol. Lab. 1982, 27, 25–76. [Google Scholar] [CrossRef]
- Humes, A.G. Harpacticoid copepods associated with cnidarians in the tropical Pacific Ocean. Zool. Scripta. 1984, 13, 209–221. [Google Scholar] [CrossRef]
- Humes, A.G. Acontiophorus excavatus, a new species (Copepoda: Siphonostomatoida) associated with the soft coral Dendronephthya (Alcyonacea) in the Indo-Pacific. Proc. Biol. Soc. Wash. 1989, 102, 916–923. [Google Scholar]
- Humes, A.G. Sabelliphilid copepods (Poecilostomatoida) associated with cnidarians in the Philippines. Bull. Mar. Sci. 1990, 47, 581–597. [Google Scholar]
- Humes, A.G. Copepoda associated with gorgonaceans (Cnidaria) in the Indo-Pacific. Bull. Mar. Sci. 1993, 53, 1078–1098. [Google Scholar]
- Humes, A.G.; Dojiri, M. Poecilostome copepods (Cyclopoida, Lichomolgidae) from the alcyonacean Lobophytum crassum in the Moluccas. Bull. Mar. Sci. 1979, 29, 554–571. [Google Scholar]
- Humes, A.G.; Dojiri, M. Poecilostome copepods (Lichomolgidae) associated with the alcyonacean Litophyton in the Moluccas. Trans. Am. Microsc. Soc. 1979, 98, 337–352. [Google Scholar] [CrossRef]
- Humes, A.G.; Dojiri, M. Poecilostome copepods (Lichomolgidae) from the alcyonacean coral Cespitularia multipinnata in the Moluccas. Proc. Biol. Soc. Wash. 1979, 92, 51–69. [Google Scholar]
- Kim, I.H. Copepods (Crustacea) associated with marine invertebrates from the Moluccas. Korean J. Syst. Zool. Spec. Issue 2007, 6, 1–126. [Google Scholar]
- Stock, J.H. Lichomolgus pterophilus n. sp., a cyclopoid copepod associated with the East Indian sea-pen Pteroeides. Beaufortia 1962, 9, 155–163. [Google Scholar]
- Humes, A.G. Cyclopoid copepods of the genus Acanthomolgus (Lichomolgidae) associated with gorgonians in Bermuda. J. Nat. Hist. 1973, 7, 85–115. [Google Scholar] [CrossRef]
- Humes, A.G. Synopsis of lichomolgid copepods (Poecilostomatoida) associated with soft corals (Alcyonacea) in the tropical Indo-Pacific. Zool. Verh. Leiden. 1990, 266, 1–201. [Google Scholar]
- Humes, A.G. Orecturus amplus, a new species (Copepoda: Siphonostomatoida: Asterocheridae) from an alcyonacean in New Caledonia. Proc. Biol. Soc. Wash. 1996, 109, 112–117. [Google Scholar]
- Humes, A.G.; Stock, J.H. A revision of the family Lichomolgidae Kossmann, 1877, cyclopoid copepods mainly associated with marine invertebrates. Smithson. Contrib. Zool. 1973, 127, 1–368. [Google Scholar] [CrossRef]
- Kim, I.H. Copepods (Crustacea) associated with marine invertebrates from New Caledonia. Anim. Syst. Evol. Divers. 2003, 4, 1–167. [Google Scholar]
- Kim, I.-H. Six new species of Enalcyonium (Copepoda, Cyclopoida, Lamippidae) parasitic in octocorals from New Caledonia. Korean J. Syst. Zool. 2004, 20, 141–154. [Google Scholar]
- Humes, A.G. Cyclopoid copepods (Lichomolgidae) associated with alcyonaceans in New Caledonia. Smithson. Contrib. Zool. 1975, 191, 1–27. [Google Scholar] [CrossRef]
- Uyeno, D. Two new species of symbiotic copepods from sea pens (Anthozoa: Octocorallia: Pennatulacea) collected in the Johor Straits, Singapore. Raffles Bull. Zool. Suppl. 2015, 31, 143–151. [Google Scholar]
- Versluys, J. Die Gorgoniden der Siboga-Expeditie. I. Die Chrysogorgiidae. Siboga-Exped. Monogr. 1902, 7, 1–120. [Google Scholar]
- Versluys, J. Die Gorgoniden der Siboga-Expeditie. II. Die Primnoidae. Siboga-Exped. Monogr. 1906, 1, 1–178. [Google Scholar]
- Johnsson, R.; Neves, E. A revision of Metapontius (Siphonostomatoida: Artotrogidae) with the description of a new species associated with an octocoral from Eniwetok Atoll, Marshall Islands (USA). Zootaxa 2005, 1035, 51–59. [Google Scholar] [CrossRef]
- Grygier, M.J. Two new lamippid copepods parasitic on gorgonians from Hawaii and the Bahamas. Proc. Biol. Soc. Wash. 1980, 93, 662–673. [Google Scholar]
- Gravier, C. Isidicola antarctica, Crustacé parasite de quelques Isidae de l’Antarctique sud-américaine. In Deuxième Expédition Antarctique Francaise (1908–1910); Masson: Paris, France, 1914; pp. 99–110. [Google Scholar]
- Buhl-Mortensen, L.; Mortensen, P.B. Crustaceans associated with the deep-water gorgonian corals Paragorgia arborea (L., 1758) and Primnoa resedaeformis (Gunn., 1763). J. Nat. Hist. 2004, 38, 1233–1247. [Google Scholar] [CrossRef]
- Baillon, S.; Hamel, J.-F.; Mercier, A. Diversity, Distribution and nature of faunal associations with deep-sea pennatulacean corals in the northwest Atlantic. PLoS ONE 2014, 9, e111519. [Google Scholar] [CrossRef] [PubMed]
- Penney, H.D.; Baillon, S.; Hamel, J.F.; Pête, J.; Mercier, A. Morphology and biology of the endoparasitic copepod Lamippe bouligandi from the bathyal sea pen Anthoptilum grandiflorum. Symbiosis 2021, 85, 233–248. [Google Scholar] [CrossRef]
- Bouligand, Y.; Delamare Deboutteville, C.L. Lamippella faurei n.g., n.sp. Considérations morphologiques sur la famille des Lamippides, Copépodes parasites des Octocarallaires. Comptesrendus L’acad. Des. Sci. 1959, 249, 1807–1809. [Google Scholar]
- Bouligand, Y. Notes sur la famille des Lamippidae, première partie. Crustaceana 1960, 1, 258–278. [Google Scholar] [CrossRef]
- Bouligand, Y. Notes sur la famille des Lamippidae, 3e partie. Crustaceana 1965, 8, 1–24. [Google Scholar] [CrossRef]
- Grygier, M.J. An endoparasitic lamippid copepod in Acanella from the North Atlantic. Crustaceana 1983, 45, 176–182. [Google Scholar] [CrossRef]
- Joliet, L. Observations sur quelques Crustacés de la Méditerranée. Sur une troisième espèce du genre Lamippe, Lamippe duthiersii, parasite du Paralcyonium elegans. Arch. Zool. Exp. Gén. 1882, 10, 101–111. [Google Scholar]
- Olsson, P. Nova genera parasitantia Copepodorum et Platyelminthium. Lunds Univ. Arsskr. 1869, 6, 1–6. [Google Scholar]
- Soyer, J. Copépodes Harpacticoïdes de Banyuls-sur-Mer 2. Paramphiascopsis pallidus (Sars), Espèce Nouvelle Pour la Méditerranée. Vie Milieu 1963, 14, 571–578. [Google Scholar]
- Stock, J.; Kleeton, G. Copépodes associés aux invertébrés des côtes du Roussillon 2. Lichomolgidae ectoassociés d’octocoralliaires. Vie Milieu 1963, 245–261. [Google Scholar]
- Stock, J.H. Lamippidae (Copepoda: Siphonostomatoida) parasitic in Alcyonium. J. Mar. Biol. Assoc. UK 1988, 68, 351–359. [Google Scholar] [CrossRef]
- de Zulueta, A. Note préliminaire sur la famille des Lamippidae. Copépodes parasites des Alcyonnaires. Arch. Zool. Exp. Gén. 1908, 9, 1–30. [Google Scholar]
- de Zulueta, A. Deuxième note sur la famille des Lamippidae. Copépodes parasites des Alcyonnaires. Arch. Zool. Exp. Gén. 1910, 6, 137–148. [Google Scholar]
- Holmes, J.M.C. Crustacean records from Lough Hyne (Ine), Co. Cork, Ireland: Part VI. Bull.-Ir. Biogeogr. Soc. 1996, 19, 139–147. [Google Scholar]
- Claparède, E. Sur un crustacé parasite de la Lobularia digitata Delle Chiaje. Ann. Sci. Nat. Zool. Paléontol. 1867, 8, 23–28. [Google Scholar]
- Itoh, H.; Kim, I.H. A New Species of Pennatulicola Humes and Stock (Copepoda: Cyclopoida: Rhynchomolgidae) Associated with a Pennatulacean from Tokyo Bay, Japan. Species Divers. 2015, 20, 59–65. [Google Scholar] [CrossRef][Green Version]
- Buhl-Mortensen, L.; Neuhaus, J.; Williams, J.D. Gorgonophilus canadensis (Copepoda: Lamippidae) a parasite in the octocoral Paragorgia arborea—Relation to host, reproduction, and morphology. Symbiosis 2022, 87, 189–199. [Google Scholar] [CrossRef]
- Bruzelius, R. Om en i Pennatula rubra lefvande parasit. Öfvers. Kongl. Vetensk. Akad. Förh. 1858, 15, 181–185. [Google Scholar]
- Bresciani, J.; Lützen, J. Parasitic copepods from the west coast of Sweden including some new or little known species. Vidensk. Medd. Dan. Naturhist. Foren. 1962, 124, 367–408. [Google Scholar]
- Bouligand, Y.; Delamare Deboutteville, C.L. Le dimorphisme sexuel de Linaresia mammillifera Zulueta 1908, Copépode parasite de l’Octocoralliaire Muricea chamaeleon von Koch. CR Acad. Sci. 1959, 248, 286–288. [Google Scholar]
- Conradi Barrena, M.; Megina Martínez, C.; López González, P.J. Sibling species of copepods in association with Mediterranean gorgonians. Sci. Mar. 2004, 68, 370–375. [Google Scholar]
- Dudley, P.L. Enalcyonium carrikeri, a new species of lamippid copepod from Alcyonium carneum Agassiz in New England. Crustaceana 1973, 25, 75–87. [Google Scholar] [CrossRef]
- Stock, J.H. Copepoda of the family Lamippidae from the western Atlantic and the Caribbean. Stud. Fauna Curaçao Carib. Isl. 1973, 43, 22–41. [Google Scholar]
- Scott, T. Additions to the fauna of the Firth of Forth. Ann. Rep. Fish. Board. Scotl. 1896, 14, 158–166. [Google Scholar]
- Scott, T. Notes on gatherings of Crustacea, collected for the most part by the fishery steamer “Garland” and the steam trawler “St. Andrew” of Aberdeen, and examined during the year 1900. Ann. Rep. Fish. Board Scotl. 1901, 19, 235–281. [Google Scholar]
- Scott, T.; Scott, A. On some new and rare British Copepoda. J. Nat. Hist. 1895, 16, 353–362. [Google Scholar] [CrossRef][Green Version]
- Williams, J.D.; Anchaluisa, B.; Boyko, C.B.; McDaniel, N. Description of a new endoparasitic copepod genus and species (Lamippidae) that induces gall formation in leaves of the sea pen Ptilosarcus gurneyi (Octocorallia) from British Columbia. Mar. Biodivers 2018, 48, 1325–1335. [Google Scholar] [CrossRef]
- Ho, J.S. Copepoda associated with sponges, cnidarians, and tunicates of the Sea of Japan. Rep. Sado Mar. Biol. Stn. Niigata Univ. 1984, 14, 23–61. [Google Scholar]
- Uyeno, D.; Johnsson, R. Two new species of Siphonostomatoida (Copepoda) found on cnidarians in Tokara Islands, Southern Japan. J. Nat. Hist. 2018, 52, 2639–2652. [Google Scholar] [CrossRef]
- Humes, A.G. Lamippe concinna sp. n., a copepod parasitic in a West African pennatulid coelenterate. Parasitology 1957, 47, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H. Two new species of siphonostomatoid copepods (Crustacea) associated with the stoloniferan coral Tubipora musica (Linnaeus) from Madagascar. Korean J. Biol. Sci. 2004, 8, 187–196. [Google Scholar] [CrossRef][Green Version]
- Kim, I.H.; Im Song, J. Two new species of Orecturus (Copepoda: Siphonostomatoida: Asterocheridae) associated with octocorals from Korea. Anim. Syst. Evol. Divers. 2003, 19, 177–188. [Google Scholar]
- Humes, A.G.; Lewbel, G.S. Cyclopoid copepods of the genus Acanthomolgus (Lichomolgidae) associated with a gorgonian in California. Trans. Am. Microsc. Soc. 1977, 7, 1–12. [Google Scholar] [CrossRef]
- Humes, A.G. Cyclopoid copepods (Lichomolgidae) from octocorals at Eniwetok Atoll. Beaufortia 1973, 21, 135–151. [Google Scholar]
- Stock, J.H. On twelve species of the genus Acanthomolgus (Copepoda Cyclopoida: Lichomolgidae) associated with West Indian octocorals. Bijdr. Dierkd. 1975, 45, 237–269. [Google Scholar] [CrossRef]
- Ehrlich, H.; Etnoyer, P.; Litvinov, S.D.; Olennikova, M.M.; Domaschke, H.; Farias, A.; Neves, E.G.; Johnsson, R. Two new species of Cryptopontius Giesbrecht, 1899 (Copepoda, Siphonostomatoida, Artotrogidae) associated with invertebrates from Northeastern Brazil. Zootaxa 2020, 4810, 481–494. [Google Scholar]
- Varela, C. Dos nuevas especies de Asterocheres Boeck, 1860 (Copepoda: Siphonostomatoida) de Cuba. Novit. Caribaea 2010, 3, 36–43. [Google Scholar] [CrossRef]
- Varela, C.; Lalana, R. Especie nueva de Orecturus (Crustacea: Copepoda) para Cuba. Solenodon 2007, 6, 15–19. [Google Scholar]
- Varela, C.; Ortiz, M.; Lalana, R. Nuevos registros de copépodos asociados a invertebrados marinos (Poecilostomatoidea: Lichomolgoidea), en aguas cubanas. Rev. Investig. Mar. 2003, 24, 25–256. [Google Scholar]
- Varela, C.; Ortiz, M.; Lalana, R. Especie nueva de Asteropontius (Copepoda: Siphonostomatoida) para Cuba. Solenodon 2005, 5, 6–9. [Google Scholar]
- Varela, C.; Castellanos, S.; Hernández, L. Registros nuevos de invertebrados (Cnidaria y Crustacea) para Cuba. Cocuyo 2008, 17, 12–14. [Google Scholar]
- Varela, C. Especie nueva de Hermannella (Crustacea: Copepoda), con dos nuevos registros de copépodos para Cuba. Solenodon Rev. Antillana Zool. Taxon. 2011, 9, 1–7. [Google Scholar]
- Varela, C. Una nueva especie de Orecturus Humes, 1992 (Copepoda: Siphonostomatoida: Asterocheridae) de Cuba. Rev. Cienc. Marin. Cost. 2011, 3, 91–97. [Google Scholar] [CrossRef]
- Stock, J.H. Magnippe caputmedusae n. gen., n. sp. (Copepoda: Lamippidae), a highly transformed endoparasite in octocorals of the genus Thesea from the Gulf of Mexico. Mem. Hourglass Cruises 1978, 3, 1–11. [Google Scholar]
- Stock, J.H. A new species of Linaresia (Copepoda: Lamippidae) endoparasitic in the octocoral Placogorgia from the Gulf of Mexico. Mem. Hourglass Cruises 1979, 5, 1–7. [Google Scholar]
- Stock, J.H. A new species of Lamippidae (Crustacea, Copepoda) from the Red Sea. Beaufortia 1972, 19, 193–196. [Google Scholar]
- Humes, A.G. Cyclopoid copepods (Lichomolgidae) from gorgonaceans in Madagascar. Proc. Biol. Soc. Wash. 1974, 87, 411–438. [Google Scholar]
- Humes, A.G. Lichomolgid copepods (Cyclopoida), with two new species of Doridicola, from sea pens (Pennatulacea) in Madagascar. Trans. Am. Microsc. Soc. 1978, 97, 524–539. [Google Scholar] [CrossRef]
- Humes, A.G.; Frost, B.W. New lichomolgid copepods (Cyclopoida) associated with alcyonarians and madreporarians in Madagascar. Cah. ORSTOM Océanographie 1964, 6, 131–212. [Google Scholar]
- Humes, A.G.; Ho, J.S. New cyclopoid copepods associated with the alcyonarian coral Tubipora musica (Linnaeus) in Madagascar. Proc. USA Natl. Mus. 1967, 121, 2–24. [Google Scholar] [CrossRef][Green Version]
- Humes, A.G.; Ho, J.S. Cyclopoid copepods of the genus Lichomolgus associated with octocorals of the families Xeniidae, Nidaliidae, and Telestidae in Madagascar. Proc. Biol. Soc. Wash. 1968, 81, 693–750. [Google Scholar]
- Humes, A.G.; Ho, J.S. Cyclopoid copepods of the genus Lichomolgus associated with octocorals of the family Alcyoniidae in Madagascar. Proc. Biol. Soc. Wash. 1968, 81, 635–692. [Google Scholar]
- Humes, A.G.; Ho, J.S. Cyclopoid copepods of the genus Lichomolgus associated with octocorals of the family Nephtheidae in Madagascar. Proc. USA Natl. Mus. 1968, 125, 1–41. [Google Scholar] [CrossRef][Green Version]
- Stock, J.H.; Humes, A.G. On four new notodelphyid copepods, associated with an octocoral Parerythropodium fulvum (Forskål). Madagascar. Zool. Anz. 1970, 184, 193–212. [Google Scholar]
- Kim, I.H. Poecilostome copepods (Crustacea: Cyclopoida) associated with marine invertebrates from tropical waters. Korean J. Syst. Zool. Spec. Issue 2009, 7, 1–90. [Google Scholar]
- Kim, I.H. Siphonostomatoid Copepoda (Crustacea) associated with invertebrates from tropical waters. Korean J. Syst. Zool. Spec. Issue 2010, 8, 1–176. [Google Scholar]
- Wieczorek, J.; Bloom, D.; Guralnick, R.; Blum, S.; Döring, M.; Giovanni, R.; Robertson, T.; Vieglais, D. Darwin Core: An Evolving Community-Developed Biodiversity Data Standard. PLoS ONE 2012, 7, e29715. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Robertson, J. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Wickham, H.; Wickham, M.H. Package Tidyverse. Easily Install and Load the ‘Tidyverse’. Available online: http://tidyverse.tidyverse.org/ (accessed on 1 October 2023).
- Wickham, H.; Francois, R. Dplyr: A Grammar of Data Manipulation, R. Package Version 2021; Available online: https://CRAN.Rproject.org/package=dplyr (accessed on 1 October 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Attali, D.; Baker, C. Ggextra: Add Marginal Histograms to ‘Ggplot2’, and More ‘Ggplot2’ Enhancements. Available online: https://cran.r-project.org/package=ggExtra (accessed on 1 October 2023).
- Kassambara, A. Ggpubr: ‘Ggplot2’ BASED publication Ready Plots. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 1 October 2023).
- Auguie, B.; Antonov, A.; Auguie, M.B. Package ‘gridExtra’. In Miscellaneous Functions for “Grid” Graphics. 2017. Available online: https://cran.r-project.org/web/packages/gridExtra/gridExtra.pdf (accessed on 1 October 2023).
- Bache, S.M.; Wickham, H.; Henry, L.; Henry, M.L. Package ‘Magrittr’. In R. Package Version. 2022. Available online: https://cran.r-project.org/web/packages/magrittr/magrittr.pdf (accessed on 1 October 2023).
- Becker, R.A.; Wilks, A.R.; Brownrigg, R.; Minka, T.P.; Deckmyn, A. Maps: Draw Geographical Maps. Available online: https://cran.r-project.org/web/packages/maps/index.html (accessed on 1 October 2023).
- Wickham, H. Stringr: Modern, consistent string processing. R J. 2010, 2, 38–40. [Google Scholar] [CrossRef]
- Neuwirth, E.; Neuwirth, M.E. Package ‘RColorBrewer’. In ColorBrewer Palettes. 2014. Available online: https://cran.r-project.org/web/packages/RColorBrewer/RColorBrewer.pdf (accessed on 1 October 2023).
- Huys, R. Harpacticoid copepods—Their symbiotic associations and biogenic substrata: A review. Zootaxa 2016, 4174, 448–729. [Google Scholar] [CrossRef] [PubMed]
- Huys, R.; Boxshall, G. An appreciation of the contribution of Arthur Humes to copepod systematics. J. Crustac. Biol. 2001, 21, 13–27. [Google Scholar] [CrossRef][Green Version]
- Humes, A.G.; Boxshall, G.A. A revision of the lichomolgoid complex (Copepoda: Poecilostomatoida), with the recognition of six new families. J. Nat. Hist. 1996, 30, 175–227. [Google Scholar] [CrossRef]
- Ivanenko, V.N.; Defaye, D. A new genus and species of the family Asterocheridae (Copepoda: Siphonostomatoida) from the East Equatorial Atlantic (Angola margin). Crustaceana 2004, 77, 1131–1144. [Google Scholar]
- Ivanenko, V.N.; Corgosinho, P.H.C.; Ferrari, F.; Sarradin, P.-M.; Sarrazin, J. Microhabitat distribution of Smacigastes micheli (Copepoda: Harpacticoida: Tegastidae) from deep-sea hydrothermal vents at the Mid-Atlantic Ridge, 37° N (Lucky Strike), with a morphological description of its nauplius. Mar. Ecol. 2012, 33, 246–256. [Google Scholar] [CrossRef]
- Ivanenko, V.N.; Defaye, D. A new and primitive genus and species of deep-sea Tegastidae (Crustacea, Copepoda, Harpacticoida) from the Mid-Atlantic Ridge, 37° N (Azores Triple Junction, Lucky Strike). Cah. Biol. Mar. 2004, 45, 255–268. [Google Scholar]
- Fontaneto, D.; Barbosa, A.M.; Segers, H.; Pautasso, M. The ‘rotiferologist’ effect and other global correlates of species richness in monogonont rotifers. Ecography 2012, 35, 174–182. [Google Scholar] [CrossRef]
- Rubio-López, I.; Pardos, F.; Fontaneto, D.; Martínez, A.; García-Gómez, G. Biases and distribution patterns in hard-bodied microscopic animals (Acari: Halacaridae). Size does not matter, but generalism and sampling effort do. Divers. Distrib. 2023, 29, 821–833. [Google Scholar] [CrossRef]
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.-T. The silent extinction of species and taxonomists—An appeal to science policymakers and legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- Della Valle, A. Sui coriceidi parassiti, e sull’anatomia del gen. Lichomolgus. Mittheilungen aus der Zoologischen Station. zu Neapel 1880, 2, 83–106. [Google Scholar]
- Ummerkutty, A.N.P. Studies on Indian copepods 5. On eleven new species of marine cyclopoid copepods from the south-east coast of India. J. Mar. Biol. Assoc. India 1961, 3, 19–69. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).