Abstract
Gastrointestinal nematode parasites and gastrointestinal protozoan parasites are considered detrimental to the livestock population and manifest production-limiting effects. Small and large ruminants (cattle, buffalo, goats, and sheep) are important components of the rural economy of northern India. However, the epidemiology of gastrointestinal parasites in this agro-climatic region has not been studied extensively. In this study, the prevalence of gastrointestinal parasites was determined in 163 animals, including cattle (n = 86), buffalo (n = 11), goats (n = 48), and sheep (n = 18) from 26 sampling sites by copro-parasitological analysis. The prevalence values of 94.47% and 66.87% were recorded for the nematodes and protozoa, respectively. The group-wise prevalence of gastrointestinal nematode parasites was 95.3%, 90.9%, 93.7%, and 94.4% in cattle, buffalo, goats, and sheep, respectively, whereas for gastrointestinal protozoan parasites, the respective values were 70.9%, 54.5%, 60.4%, and 72.2%. Copromicroscopy revealed ten genera of nematodes—Ascaris, Capillaria, Cooperia, Haemonchus, Nematodirus, Oesophagostomum, Ostertagia, Strongyloides, Trichostrongylus, Trichuris, and one protozoan genus—Eimeria. The prevalence of Trichostrongylus spp. was highest in buffaloes, whereas in cattle, Ascaris spp. were predominant. In both goats and sheep, Haemonchus contortus was found to be predominant. The highest prevalence of gastrointestinal parasites was recorded in the rainy season. These findings indicate the prevalence of gastrointestinal parasites in the ruminant population in this region and necessitate the implementation of preventive and control strategies for effective animal health management.
1. Introduction
Livestock farming remains the backbone of the rural economy in India [1,2,3]. It is recognized as the most important sub-sector of Indian agriculture and supports the basic needs and income of rural households in most parts of the country especially of northern hilly states [2,4,5,6,7]. According to the 20th Livestock Census 2019 report, the total livestock population in India is 536.76 million, of which 95.78% are from rural areas of the country [8]. The total number of cattle, buffaloes, goats, and sheep is 193.46 million, 109.85 million, 148.88 million, and 74.26 million which represent 36.04%, 20.47%, 27.74% and 13.83% of the total livestock population, respectively [8]. In Himachal Pradesh, the total livestock population is 4.41 million and constitutes an essential component of the livelihood of the rural population [8].
Helminth parasites represented by Platyhelminthes and Nemathelminthes are considered a serious health risk for livestock worldwide [9,10,11,12]. These are responsible for distress, debilitating diseases, malnutrition, anemia, stunted growth, and allergic conditions in domesticated livestock and grazing animals [3,13,14,15,16]. Their parasitism results in reduced milk yields, decreased wool production, and low growth in most grazing herds and flocks. Overall, helminth parasites exert a significant impact on animal health, livestock productivity, the income of livestock owners, and food security [12,16]. Helminth parasites manifest their adverse effects on both small and large ruminants. Grazing ruminants are more severely impacted as compared to those kept in animal shed conditions [5,17,18]. The most widely prevalent gastrointestinal nematode parasite genera in domesticated ruminants are Ascaris, Haemonchus, Strongyloides, Trichostrongylus, Cooperia, Ostertagia, Nematodirus, Trichuris, Capillaria, and Oesophagostomum, among others [9,10,19,20,21]. Gastrointestinal protozoan parasites viz. Balantidium, Eimeria, and Cryptosporidium also infect ruminants, resulting in adverse effects on their health, productivity, and reproduction [22,23,24,25,26]. It is generally observed that tropical and sub-tropical conditions, high humidity, damp soil, high temperature, and a cold environment offer optimum conditions for the survival, multiplication, and transmission of gastrointestinal nematode parasites and gastrointestinal protozoan parasites [24]. Usually, these parasitic infestations of domesticated ruminants are seasonal in nature, with a higher frequency in the monsoon season and lower infection rates in the summer [27,28,29]. There is a considerable role of animal husbandry practices and conditions, i.e., animal sheds, floor conditions, crowding, cleaning routines, deworming practices, etc., in the transmission of veterinary parasitosis [10,30]. Further, helminthiases of ruminants are influenced by immunity level, feeding pattern, water supply, grazing habits, humidity, temperature, rainfall, vegetation, husbandry facilities, and deworming practices [17,27,31]. This study was planned to determine the prevalence of gastrointestinal nematode parasites and gastrointestinal protozoan parasites in cattle, buffalo, goats, and sheep domesticated in the mountainous rural region of Himachal Pradesh, India.
2. Materials and Methods
2.1. Study Area
The study was carried out from February 2019 to July 2019 in the Sirmaur district of Himachal Pradesh, situated in the outer Himalayan Shivalik range of northern India (Figure 1). The study involved 26 villages (sampling sites) from five tehsils within district Sirmaur (Table 1).
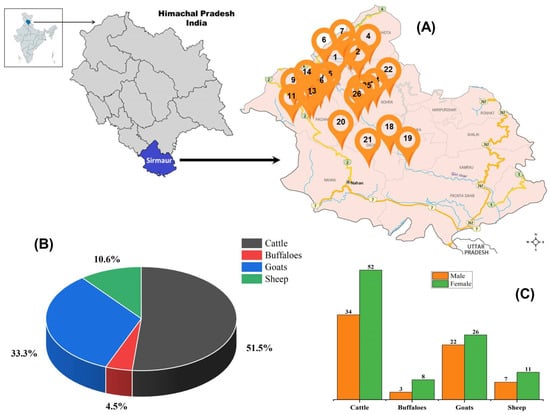
Figure 1.
Geographical location, sampling locations and animals studied. (A) Sampling sites located in five tehsils of rural tropical Himalayan regions of Himachal Pradesh, India. (B) represents the distribution of ruminants in numbers and percentage, and (C) represents the gender-wise distribution of study animals from which fecal samples were collected for parasitological analysis.

Table 1.
Sampling sites located in the Sirmaur district of the northern Indian state of Himachal Pradesh from where fecal samples of domesticated animals were collected and analyzed to determine the prevalence of gastrointestinal nematode parasites and gastrointestinal protozoan parasites.
2.2. Study Animals Selection
The domesticated ruminants viz. cattle (Bos Taurus Linnaeus, 1758), buffalo (Bubalus bubalis Linnaeus, 1758), goat (Capra hircus Linnaeus, 1758), and sheep (Ovis aries Linnaeus, 1758) were selected randomly from the sampling locations as mentioned in Table 1. The study animals were from the following breeds: cattle—Jersey, Sahiwal and Holstein Friesian; buffalo—Murrah; goat—Chegu and Gaddi and sheep—Rampur bushair and Gaddi. During the collection of freshly laid fecal samples, the livestock owners were made aware of the study objectives and provided a printed short questionnaire requesting them to furnish some basic information, i.e., age of animals, gender, breed, animal husbandry practices, farm size, animal shed floor type (cement concrete or soil), feedstuffs, fodders, grazing routines, cleaning and sanitation, and deworming history. The personal details of the livestock owners were kept anonymous and confidential.
2.3. Fecal Sample Collection
Freshly laid fecal samples from the study animals were collected in sterile polyethylene bags or sterile disposable 100 mL containers, labeled, and immediately brought to the laboratory under refrigerated conditions. Samples were processed and analyzed on the same day. However, in the cases where fecal samples could not be analyzed on the same day, they were kept under refrigeration (2–4 °C) until further analysis the next day.
2.4. Qualitative Analysis by the Saturated Salt Flotation Method
Fecal sample processing and microscopic identification of different life stages of gastrointestinal nematode parasites and gastrointestinal protozoan parasites were performed as per standard procedures and veterinary parasitology manuals [9,32,33,34]. A saturated salt solution of sodium chloride (specific gravity: 1.18–1.2) was prepared by suspending 400 g in 1000 mL of distilled water, followed by stirring for 30–60 min. Similarly, a saturated zinc sulphate solution (specific gravity: 1.18) was prepared by mixing 371 g of zinc sulphate in 1000 mL of distilled water and then thorough mixing. The saturated salt solutions were stored at room temperature under ambient conditions until experimental use. Each fecal sample (3.0 g) was thoroughly mixed in a 50 mL saturated salt solution with a glass rod, and the suspension was strained through a tea strainer. The suspension so obtained was gently transferred into 15 mL sterile glass test tubes and placed in a straight position. A clean glass coverslip was placed on the top of the convex surface of the test tube in such a way that the solution was in direct contact with the coverslip. After 20–30 min, the coverslip was carefully removed with forceps and placed on a glass slide for microscopic observations under the 10× and 40× objectives of a bright-field compound light microscope (Dewinter Optical, Inc., New Delhi, India). The nematode eggs and protozoan cysts were photographed with a camera (Leica Microsystems, Switzerland) attached to the microscope, and their dimensions (length and width) were determined by image processing software. Gastrointestinal nematode parasites and gastrointestinal protozoan parasites identified from fecal samples of ruminants on the basis of the presence of eggs, cysts, and characteristic shape, size, coloration, operculum, polar plugs, anterior and posterior ends, presence of blastomeres, and outer sheath.
2.5. Quantitative Analysis of Fecal Samples
The quantitative estimation of eggs per gram (EPG) counts of animal fecal samples was determined by Stoll’s dilution method and the modified Wisconsin sugar flotation method [9,32,34]. Briefly, a 3.0 g fecal sample was suspended in 42 mL of distilled water and thoroughly mixed with a glass rod. The fecal suspension was strained through a tea strainer into a clean beaker and left to stand for 5 min. A drop of the suspension was placed on a clean glass slide, covered with a cover slip, and examined under a bright-field compound light microscope (Dewinter Optical, Inc., New Delhi, India). EPG counts were calculated by the formula: EPG count = Number of eggs counted × 100. In the modified Wisconsin sugar flotation method, a 3.0 g fecal sample was suspended in 10 mL of Sheather’s solution, mixed with a spatula and strained through a tea strainer to remove large debris and feed particles. The suspension was centrifuged at 2000× rpm for 5 min, then test tube was filled with Sheather’s solution to form a meniscus and covered with a glass cover slip. After 5–10 min, the cover slip was removed, placed on a clean glass slide, and observed under a bright-field compound light microscope (Dewinter Optical, Inc., New Delhi, India) at 10× and 40× objectives. Parasite egg counts in fecal samples were calculated as EPG count = number of eggs counted/3. Quantitative estimation of EPG counts in fecal samples of study animals was performed from two sampling sites, i.e., Lana Machher and Bongli Kech, from February 2019 to July 2019.
2.6. Meteorological Data
The data related to mean rainfall, average high temperature, and average low temperature in Sirmaur district during the period from February to July 2019 were retrieved from the websites of the Indian Meteorological Department, Shimla http://weathershimla.nic.in/index.html (accessed on 31 July 2019), and the Accuweather website https://www.accuweather.com/ (accessed on 31 July 2019). The rainfall recorded in the months of February, March, April, May, June, and July was 138.4 mm, 29.6 mm, 54.6 mm, 27.6 mm, 55.0 mm, and 236.4 mm, respectively. The average high temperature ranged from 21.4 to 44.6 °C and the average low temperature was between 10.1 to 27.8 °C during the study period with peak values in June.
2.7. Statistical Analysis
Experiments were performed in triplicate, and the data were expressed as mean ± SD. The results were analyzed using MS-Excel (Microsoft).
3. Results
The copro-parasitological evaluation of the study animals revealed the prevalence of ten genera of gastrointestinal nematodes and one genus of parasitic protozoa. These were identified as Ascaris spp., Capillaria spp., Cooperia spp., Haemonchus contortus (Rudolphi, 1803) Cobb, 1898, Nematodirus spp., Oesophagostomum spp., Ostertagia spp., Strongyloides spp., Trichostrongylus spp., Trichuris spp., and Eimeria spp. (Figure 2). The parasite eggs and oocysts were identified on the basis of distinguishing characteristics such as the presence of polar plugs in the case of Trichuris sp., operculum in the case of Eimeria oocysts, and embryos in eggs in strongyles (Figure 2). The characteristic features of all the identified genera are given in Table 2. The genera-specific prevalence of gastrointestinal nematode parasites in cattle, buffaloes, goats, and sheep is shown in Figure 3 and Figure 4.
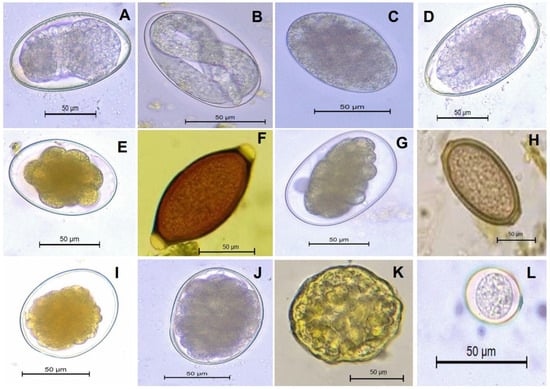
Figure 2.
Morphological characteristics of diagnostic stages (eggs and oocysts) of gastrointestinal nematode parasites and gastrointestinal protozoan parasites observed by coprological analysis of the fecal matter of domesticated ruminants. (A,B) Strongyloides spp., (C) Trichostrongylus spp., (D) Ostertagia spp., (E) Nematodirus spp., (F) Trichuris spp., (G) Haemonchus contortus, (H) Capillaria spp., (I) Cooperia spp., (J) Oesophagostomum spp., (K) Ascaris spp., and (L) Eimeria spp. The fecal samples were observed at 400× magnification after processing and photomicrographs were morphometrically analyzed. Scale bar = 50 μm.

Table 2.
Morphological characteristics and morphometric details of gastrointestinal nematode parasites and gastrointestinal protozoan parasites identified from fecal samples of domesticated ruminants of rural areas of Sirmaur, Himachal Pradesh.
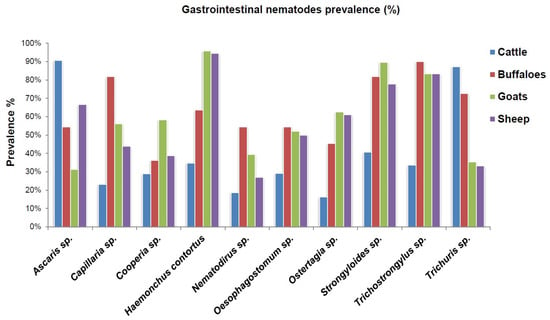
Figure 3.
The copro-parasitological prevalence of gastrointestinal nematode parasites in cattle, buffaloes, goats, and sheep of rural areas of Himachal Pradesh, India.
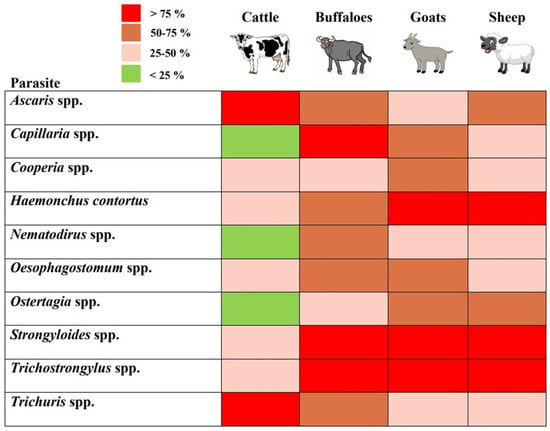
Figure 4.
The heat map showing coprological predominance of gastrointestinal nematode parasites in cattle, buffaloes, goats, and sheep from the rural regions of Sirmaur, Himachal Pradesh. Here, rows represent nematode genera and column represents study animals.
The predominant copro-prevalence of Ascaris spp. (90%) and Trichuris spp. (87%) was observed in cattle (Figure 4). The prevalence of other parasite species ranged between 16.2 and 40.6% (Figure 3). In the case of buffalo, the prominent gastrointestinal nematode parasites were Trichostrongylus spp. (90%), Capillaria spp. (81.8%), Strongyloides spp. (81.8%), and Trichuris spp. (72.7%). As compared to cattle and buffalo, the dominant nematode parasite in the gastrointestinal tracts of goats was Haemonchus contortus, with a 95.8% prevalence. This was followed by Strongyloides spp. (89.5%) and Trichostrongylus spp. (83.3%). However, the prevalence of Ascaris spp. and Trichuris spp. was lower as compared to that of cattle (Figure 3 and Figure 4). Similarly, in sheep, H. contortus was detected in the majority of the animals which corresponds to a 94.4% prevalence. The lowest prevalence was of Nematodirus spp. (27%), whereas that of Ascaris spp. was 66.6% (Figure 3). In total, 154 ruminants (94.47%) showed the prevalence of nematode life stages (eggs and early-stage larvae) in their fecal matter. In comparison, the overall prevalence of gastrointestinal protozoan parasites was 66.87%. As depicted in Figure 5A, the prevalence of gastrointestinal nematode parasites in ruminants ranged between 90.9 and 95.3% with cattle being the highest among all the groups. On the other hand, the gastrointestinal protozoan parasite prevalence was highest in sheep, i.e., 72.2%, followed by cattle (70.9%), goats (60.4%), and buffalo, i.e., 54.5%. Overall, it was observed that the protozoal infestation in ruminants was lesser as compared to gastrointestinal nematode parasites.
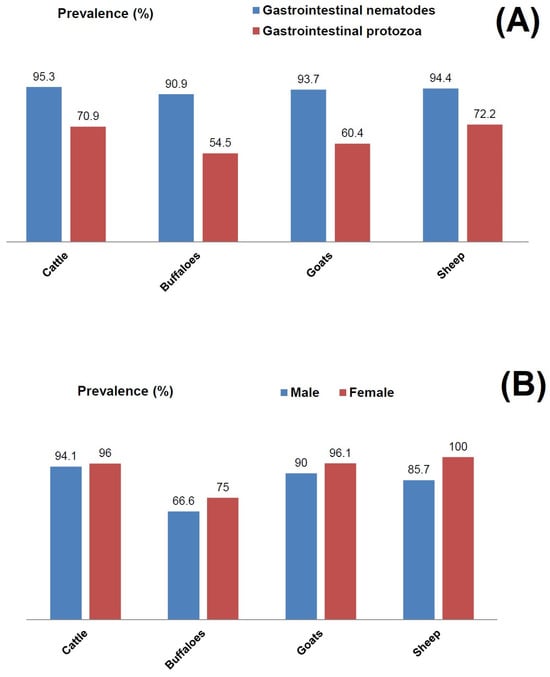
Figure 5.
Prevalence of gastrointestinal nematode parasites and gastrointestinal protozoan parasites in domesticated ruminants. (A) Overall prevalence of gastrointestinal parasites in cattle, buffaloes, goats and sheep. (B) Gender-specific prevalence of gastrointestinal nematode parasites in fecal matters in male and female animals from the rural regions of Himachal Pradesh, India.
Fecal sample analysis of male and female ruminants revealed the presence of parasite eggs and cysts, but to a different extent (Figure 5B). In contrast to cattle, where both male and female animals had a comparable parasite prevalence, there was a higher parasite prevalence in female animals as compared to male counterparts in buffaloes, goats, and sheep. In the case of sheep, 85.7% of male animals had a positive gastrointestinal parasite prevalence, whereas all the female counterparts were positive for the prevalence of parasites (Figure 5B). A similar pattern was also observed with buffaloes and goats. As evident, the highest rainfall was recorded in July during the monsoon season and there were higher EPG counts in the study animals during this period. From the sampling site Bongali Kech, EPG counts in cattle were 27.5 ± 5.7 in February, which gradually increased to 72.5 ± 7.8 in the month of July (Figure 6). A similar increasing trend was observed in the cases of the other three animal groups. EPG counts were highest in the monsoon season, when rainfall and humidity were higher as compared to the winter and dry seasons. From the second sampling site Lana Machher, lower EPG counts were recorded in February and March, whereas 2–3-fold higher counts were observed in July.
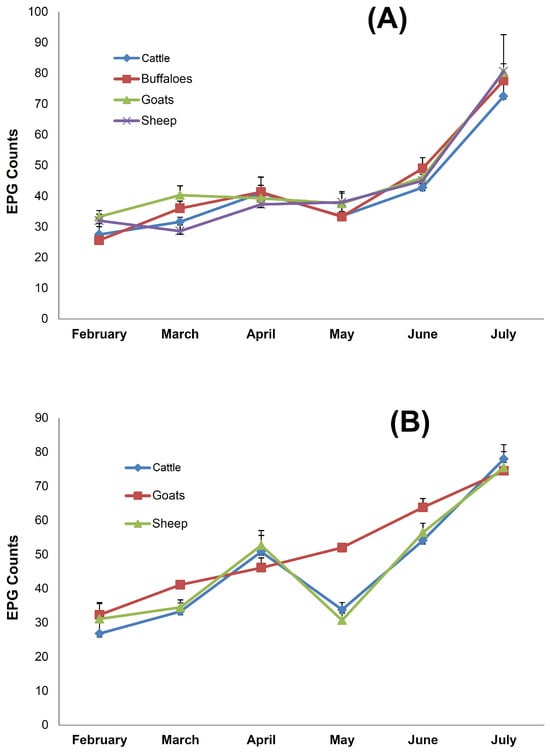
Figure 6.
The correlation between meteorological parameters and eggs per gram (EPG) counts of gastrointestinal nematode parasites in domesticated ruminants. (A) Fecal EPG counts in ruminants from the sampling site Bongali Kech, and (B) fecal EPG counts in ruminants from the sampling site Lana Machher. Data represent the mean ± SD of three independent experiments.
4. Discussion
Livestock farming is indispensable for the sustainability of the rural economy in India, including the northern state of Himachal Pradesh. However, gastrointestinal nematodes and gastrointestinal protozoa in domesticated ruminants are known to exert tremendous economic losses by adversely impacting animal health, productivity, yields, and reproduction [20,25,35,36,37]. Moreover, these infections are often neglected by livestock owners and result in significant economic losses in the long term [20]. The study areas of current investigation fall in the sub-mountain and low hills subtropical agro-climatic zone in the district of Sirmaur [1]. Fecal sample analyses of domesticated ruminants revealed an overall prevalence of 94.47% for gastrointestinal nematode parasites and 66.87% for gastrointestinal protozoan parasites. Ruminants were found to harbor a diverse community of gastrointestinal nematode parasites which include the species of Ascaris, Trichuris, Strongyloides, Trichostrongylus, Haemonchus, Cooperia, Oesophagostomum, Ostertagia, Capillaria, and Nematodirus as detected by coproscopical analysis. In terms of the genera-specific prevalence of gastrointestinal nematode parasites, the experimental data revealed the predominance of Trichostronglylus spp. and Ascaris spp. in buffaloes and cattle, respectively. The other three most dominant parasites in buffaloes were Strongyloides sp., Capillaria spp., and Trichuris spp., whereas Strongyloides spp., Trichuris spp., and Haemonchus contortus were among the top four gastrointestinal nematode parasites in cattle. Jithendran [1] has also reported the predominance of the eggs of Strongyloides spp. in goats and sheep. Similarly, Choudhary et al. [38] observed the highest prevalence of Trichostrongylus spp. in cattle. A meta-analysis of the prevalence of gastrointestinal parasites in Indian buffaloes during the period 2011 to 2018 revealed a 46.67% average prevalence rate which was attributed to their low productivity over the years [39]. In the small ruminants, Haemonchus contortus had the highest prevalence (>94%) in both the goat and sheep populations. The other dominant parasites were Trichostronglylus spp. and Strongyloides spp. In other published reports, H. contortus was found to be predominant in both large and small ruminants’ fecal contents [40,41,42,43]. Similarly, a high prevalence of Haemonchus spp., Trichostrongylus spp., Bunostomum spp., Eimeria spp. and Strongyloides spp. was reported from cattle in Ethiopia [44]. In the Andhra Pradesh state of India, a 39.1% prevalence of gastrointestinal nematodes was recorded in domestic ruminants with a predominance of Haemonchus and Paramphistomum [45]. In the case of gastrointestinal protozoan parasites, only one genus, i.e., Eimeria was identified from small and large ruminants. The incidence of Eimeria spp. to the extent of 11.4% in cattle and 4.7% in buffaloes was reported from the Gujarat state of India [46]. The prevalence of Eimeria spp. was also observed by Cruvinel et al. [47] and León et al. [48].
Our findings are in correlation with earlier reports by Choubisa and Jaroli [23], Singh et al. [49], Singh et al. [50], and Pinilla et al. [51], where the overall parasite prevalence ranged between 82 and 94% in small and large ruminants. Interestingly, Yusof and Isha [52] found an even higher prevalence, i.e., 89.2% of coccidiosis in a goat population of Malaysia. On the other hand, lower prevalence rates, i.e., 62.5% and 67.0% of helminth parasites, were reported by Jena et al. [53] and Shit et al. [54], respectively. Krishnamoorthy et al. [55] reported a high prevalence of gastrointestinal parasites in sheep (65%) and in goats (74%) in India. Their findings also indicated a higher parasite prevalence in goats from Himachal Pradesh. Marskole et al. [56] reported a higher parasite prevalence in cattle (75%) than buffaloes (70.4%). In contrast, Wadhwa et al. [57], Velusamy et al. [58], and Das et al. [43] observed a lower, i.e., 10–55%, prevalence of gastrointestinal nematode parasites and gastrointestinal protozoan parasites in ruminants. Similar reports on parasite prevalence rates are also available in the literature from different geographical regions of the world [36,37,59,60]. It was also observed that the female animals had a slightly higher infestation with gastrointestinal parasites as compared to their male counterparts. As compared to domesticated ruminants, the wild species of these animals usually exhibit a low parasite species diversity but higher intensity due to grazing habits, genetic factors, and variable population density [20]. Some possible explanations for the observed variability in parasite prevalence rates of gastrointestinal nematode parasites and gastrointestinal protozoan parasites in the study animals include geographical factors, agro-climatic conditions, grazing habits, deworming schedules, sampling procedures, procedural variations, and counting methods. Further, the contribution of animals’ immunity, nutritional status, age, gender, and genetic makeup in imparting anti-gastrointestinal parasite resistance cannot be underestimated [20,35]. In previous reports, several species of gastrointestinal nematodes were detected from the ruminants of Himachal Pradesh and adjoining states, namely Bunostomum trigonocephalum, Haemonchus contortus, Ostertagia circumcinata, Oesophagostomum columbianum, Trichostrongylus axei, T. colubriformis, Trichuris ovis, and Strongyloides papillosus [1,61]. Among Eimeria species, E. ninakohlyakimovae was predominantly reported from goat populations [22]. The higher prevalence of gastrointestinal nematode parasites in fecal samples of buffaloes, cattle, goats, and sheep suggests that the agro-climatic conditions in the study area were supportive for parasite reproduction, development, and the survival of eggs and larvae inside water and soil. Another explanation for the higher parasite infestation in the ruminants is the poor animal husbandry practices adopted by the livestock owners in rural areas. The variability in the diversity of gastrointestinal parasites in cattle, buffaloes, goats, and sheep even though living in the same habitats and geographical areas might be due to a combination of environmental factors, host behavior, and host animals’ intrinsic traits [20].
Previous studies carried out in the mountainous regions of northern Indian states revealed the occurrence of gastrointestinal nematode infestations in large and small ruminants. An extensive study by Jithendran [1] found 87.2% and 94.3% gastrointestinal helminths infection levels in cattle and buffaloes from Himachal Pradesh with the predominance of the species belonging to genera Haemonchus, Bunostomum, Mecistocirrus, Strongyloides, Trichostrongylus, and Oesophagostomum. Our study also found a high prevalence of these nematode genera, except Bunostomum and Mecistocirrus which were not detected. In sheep and goats, we have found a very high level of Haemonchus, Strongyloides, and Trichostrongylus infestations. Similar prevalence rates and trends were reported previously by Jithendran [1], except that Bunostomum and Chabertia were not found in fecal samples of small ruminants in the current study. In terms of the season-wise intensity of gastrointestinal parasites, the month of July witnessed peak levels of EPG counts in ruminants which is comparable to the data reported by Jithendran [1] where the highest parasite counts were observed in the period during June to September. Sharma et al. [61] also found an 86.1% prevalence of gastrointestinal nematodes with the highest incidence in the rainy season in Gaddi sheep from Palam valley of Himachal Pradesh. Among nematodes, H. contortus was predominant followed by Ostertagia and Trichuris, but the prevalence of Trichostrongylus and Strongyloides was found to be low as compared to our findings.
Environmental conditions such as rainfall, temperature, and relative humidity are some of the influencing factors that increase parasite loads in infected animals due to the enhanced rate of parasite eggs/larvae development [54,62]. Higher humidity in the monsoon months might be responsible for the enhanced survival, transmission, and development of the eggs and other life stages of gastrointestinal nematode parasites. Our findings are in accordance with reports published by Kemal and Terefe [63] and Patel et al. [64]. As less rainfall was received in the months of March, April, May, and June, correspondingly low EPG counts were recorded as compared to July, where the highest rainfall was witnessed during the period of the study. The influence of meteorological parameters especially temperature and rainfall on the prevalence of gastrointestinal nematode parasites quantitatively in both small and large ruminants was observed in this study. Previous reports by Jithendran [1], Nath et al. [65], and Das et al. [43] also supported our findings. Khajuria et al. [66] reported the lowest EPG counts during winters and the highest during monsoons. Kumar et al. [46] also found the highest prevalence of gastrointestinal nematode parasites in the summer season in both cattle and buffaloes. Similarly, Singh et al. [50] observed the highest parasite EPG counts during the monsoon season, although lower values were observed in summer. The higher prevalence of gastrointestinal nematode parasites during the rainy season might be associated with the appropriate molarities of various salts present in soils, which are a crucial factor for inducing ecdysis [34]. Considering the high morbidity and mortality associated with gastrointestinal parasites in livestock, different anthelmintic measures such as chemical drugs, phytochemicals-based herbal medicines, and nano-encapsulated anthelmintics should be evaluated on priority for an effective and sustainable management of gastrointestinal parasites [5,7,67].
5. Conclusions
This study found a high prevalence of gastrointestinal nematodes and gastrointestinal protozoan parasites in the ruminant population from Himachal Pradesh. Rainfall, humidity, temperature, and animal husbandry conditions, grazing, deworming, etc., were found to exert a considerable influence on the prevalence of these parasites. In order to alleviate the biological and economic impact of gastrointestinal parasites on livestock, an integrated, affordable, and combinatorial strategy based on the regular deworming of animals, improving the quality of animal nutrition, safer animal husbandry methods, improved housing, the use of non-protein nitrogenous additives (carbamide-molasses block), nematophagous fungi, and anthelmintic vaccines need to be implemented to minimize the productivity losses and economic impact of these parasites. The findings of this study will prove helpful during the strategic implementation of veterinary health management practices in this geographical region. Collaborative efforts involving veterinary surgeons, para-veterinary officers, zoologists, and NGOs are required to ensure the control, prevention, and eradication of gastrointestinal parasites from livestock.
Author Contributions
Conceptualization, N.S.; methodology, A.S.; formal analysis, N.S. and A.S.; investigation, N.S. and A.S.; resources, N.S.; writing—original draft preparation, N.S.; writing—review and editing, S.S., S.K., A.U.A., K.P. and J.A.A.; supervision, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
RSP2023R358, King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the Chancellor, Eternal University, Baru Sahib for providing necessary facilities for the research work. The authors would like to acknowledge the support provided by the Researchers Supporting Project Number RSP2023R358, King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jithendran, K.P. Helminth parasites—A constraint in animal health management in Himachal Pradesh. ENVIS Bull. 2000, 8, 1–14. [Google Scholar]
- Sati, V.P. Livestock farming in the Uttarakhand Himalaya, India: Use pattern and potentiality. Curr. Sci. 2016, 111, 1955–1960. [Google Scholar]
- Chand, S.; Narayan, P.; Chaudhary, K.R. Sources of risks in livestock production and their management strategies in northern India. Indian J. Anim. Sci. 2018, 88, 612–619. [Google Scholar]
- Thakur, D.; Sharma, A.K.; Katoch, S.; Chander, M.; Mane, B.G.; Sharma, P. High altitude livestock farming: A participatory appraisal in Himachal Pradesh, India. Indian J. Anim. Sci. 2012, 82, 104–108. [Google Scholar]
- Verma, R.; Sharma, D.K.; Paul, S.; Gururaj, K.; Dige, M.; Saxena, V.K.; Banerjee, P.S. Epidemiology of common gastrointestinal parasitic infections in goats reared in semi-arid region of India. J. Anim. Res. 2018, 8, 39–45. [Google Scholar]
- Nehra, M.; Jain, S. Estimation of renewable biogas energy potential from livestock manure: A case study of India. Bioresour. Technol. Rep. 2023, 22, 101432. [Google Scholar]
- Hamid, L.; Alsayari, A.; Tak, H.; Mir, S.A.; Almoyad, M.A.A.; Wahab, S.; Bader, G.N. An Insight into the Global Problem of Gastrointestinal Helminth Infections amongst Livestock: Does Nanotechnology Provide an Alternative? Agriculture 2023, 13, 1359. [Google Scholar]
- DAHD. 20th Livestock Census-All India Report 2019. Department of Animal Husbandry and Dairying, Ministry of Fisheries, Animal Husbandry and Dairying, Government of India. 2019. Available online: https://dahd.nic.in/sites/default/filess/20thLivestockCensus2019AllIndiaReport.pdf (accessed on 15 July 2023).
- Foreyt, W.J. Veterinary Parasitology Reference Manual, 5th ed.; John Wiley and Sons: New York, NY, USA; Blackwell Publishing: Oxford, UK, 2001. [Google Scholar]
- Biswal, D.K.; Debnath, M.; Kharumnuid, G.; Thongnibah, W.; Tandon, V. Northeast India helminth parasite information database (NEIHPID): Knowledge base for helminth parasites. PLoS ONE 2016, 11, e0157459. [Google Scholar]
- Karshima, S.N.; Maikai, B.V.; Kwaga, J.P. Helminths of veterinary and zoonotic importance in Nigerian ruminants: A 46-year meta-analysis (1970–2016) of their prevalence and distribution. Infect. Dis. Poverty 2018, 7, 52–53. [Google Scholar]
- Strydom, T.; Lavan, R.P.; Torres, S.; Heaney, K. The economic impact of parasitism from nematodes, trematodes and ticks on beef cattle production. Animals 2023, 13, 1599. [Google Scholar]
- Fitzpatrick, J.L. Global food security: The impact of veterinary parasites and parasitologists. Vet. Parasitol. 2013, 195, 233–248. [Google Scholar]
- Lanusse, C.; Canton, C.; Virkel, G.; Alvarez, L.; Costa-Junior, L.; Lifschitz, A. Strategies to optimize the efficacy of anthelmintic drugs in ruminants. Trends Parasitol. 2018, 34, 664–682. [Google Scholar]
- Maurizio, A.; Perrucci, S.; Tamponi, C.; Scala, A.; Cassini, R.; Rinaldi, L.; Bosco, A. Control of gastrointestinal helminths in small ruminants to prevent anthelmintic resistance: The Italian experience. Parasitology, 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Charlier, J.; Hoste, H.; Sotiraki, S. COMBAR—Combatting anthelmintic resistance in ruminants. Parasite 2023, 30, E1. [Google Scholar]
- Jithendran, K.P.; Bhat, T.K. Epidemiology of parasitosis in dairy animals in the North West humid Himalayan region of India with particular reference to gastrointestinal nematodes. Trop. Anim. Health Prod. 1999, 31, 205–214. [Google Scholar]
- Belina, D.; Giri, A.; Mengistu, S.; Eshetu, A. Gastrointestinal nematodes in ruminants: The parasite burden associated risk factors and anthelmintic utilization practices in selected districts of East and Western Hararghe, Ethiopia. J. Vet. Sci. Technol. 2017, 8, 1000433. [Google Scholar]
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. The pathophysiology, ecology and epidemiology of Haemonchus contortus infection in small ruminants. Adv. Parasitol. 2016, 93, 95–143. [Google Scholar]
- Obanda, V.; Maingi, N.; Muchemi, G.; Nganga, C.J.; Angelone, S.; Archie, E.A. Infection dynamics of gastrointestinal helminths in sympatric non-human primates, livestock and wild ruminants in Kenya. PLoS ONE 2019, 14, e0217929. [Google Scholar]
- Mohammedsalih, K.M.; Khalafalla, A.; Bashar, A.; Abakar, A.; Hessain, A.; Juma, F.R.; Coles, G.; Krücken, J.; von Samson-Himmelstjerna, G. Epidemiology of strongyle nematode infections and first report of benzimidazole resistance in Haemonchus contortus in goats in South Darfur State, Sudan. BMC Vet. Res. 2019, 15, 184. [Google Scholar]
- Chartier, C.; Paraud, C. Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin. Res. 2012, 103, 84–92. [Google Scholar]
- Choubisa, S.L.; Jaroli, V.J. Gastrointestinal parasitic infection in diverse species of domestic ruminants inhabiting tribal rural areas of southern Rajasthan, India. J. Parasit. Dis. 2013, 37, 271–275. [Google Scholar]
- Rashid, M.; Rashid, M.I.; Akbar, H.; Ahmad, L.; Hassan, M.A.; Ashraf, K.; Saeed, K.; Gharbi, M. A systematic review on modelling approaches for economic losses studies caused by parasites and their associated diseases in cattle. Parasitology 2019, 146, 129–141. [Google Scholar]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar]
- Lianou, D.T.; Arsenopoulos, K.V.; Michael, C.K.; Papadopoulos, E.; Fthenakis, G.C. Protozoan parasites in adult dairy small ruminants and potential predictors for their presence in faecal samples. Microorganisms 2022, 10, 1931. [Google Scholar]
- Garg, R.; Yadav, C.L.; Kumar, R.R.; Banerjee, P.S.; Vatsya, S.; Godara, R. The epidemiology of fasciolosis in ruminants in different geo-climatic regions of north India. Trop. Anim. Health Prod. 2009, 41, 1695–1700. [Google Scholar]
- Jas, R.; Pandit, S. Seasonal variation in prevalence of gastrointestinal helminthoses in cattle of new alluvial zone of West Bengal, India. Biol. Rhyt. Res. 2017, 48, 631–637. [Google Scholar]
- Getahun, T.K.; Siyoum, T.; Yohannes, A.; Eshete, M. Prevalence of gastrointestinal parasites in dry season on dairy cattle at Holeta Agricultural Research Center Dairy Farm, Ethiopia. J. Vet. Med. Anim. Health 2017, 9, 356–360. [Google Scholar]
- Chattopadhyay, A.K.; Bandyopadhyay, S. Seasonal variations of EPG levels in gastro-intestinal parasitic infection in a Southeast Asian controlled locale: A statistical analysis. Springer Plus 2013, 2, 205–214. [Google Scholar]
- Hussain, L.; Saharia, K.K.; Sheikh, I.U.; Borgohain, A.; Kalita, S.; Ray, M.N.; Bulbul, K.H. Common animal husbandry practices by the farmers of Indo- Bangladesh international border areas of Assam, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3570–3578. [Google Scholar]
- Stoll, N.R. On methods of counting nematode ova in sheep dung. Parasitology 1930, 22, 116–136. [Google Scholar]
- Hendrix, C.M. Diagnostic Veterinary Medicine, 2nd ed.; Mosby Publication: London, UK, 1998; pp. 249–259. [Google Scholar]
- Soulsby, E.J.L. Helminths, Arthropods and Protozoa of Domesticated Animals, 7th ed.; Baillière Tindall: London, UK, 1982. [Google Scholar]
- Hendawy, S.H.M. Immunity to gastrointestinal nematodes in ruminants: Effector cell mechanisms and cytokines. J. Parasit. Dis. 2018, 42, 471–482. [Google Scholar]
- Thorn, C.S.; Maness, R.W.; Hulke, J.M.; Delmore, K.E.; Criscione, C.D. Population genomics of helminth parasites. J. Helminthol. 2023, 97, e29. [Google Scholar]
- Tiele, D.; Sebro, E.; H/Meskel, D.; Mathewos, M. Epidemiology of gastrointestinal parasites of cattle in and around Hosanna Town, Southern Ethiopia. Vet. Med. 2023, 14, 1–9. [Google Scholar]
- Choudhary, P.; Gupta, A.; Singh, V. Epizootiological studies on gastrointestinal helminths and associated risk factors in the dairy animals of internal drainage dry zone of Rajasthan, India. Explor. Anim. Med. Res. 2018, 8, 184–189. [Google Scholar]
- Bhangale, G.N. Gastrointestinal parasites in Indian buffaloes: A meta-analysis of prevalence. J. Vet. Parasitol. 2020, 34, 1–11. [Google Scholar]
- Singh, N.K.; Juyal, P.D. Prevalence of gastro-intestinal parasites in buffalo calves from different agro-climatic zones of Punjab. J. Parasit. Dis. 2013, 38, 367–370. [Google Scholar]
- Varadharajan, A.; Vijayalakshmi, R. Prevalence and seasonal occurrence of gastrointestinal parasites in small ruminants of coastal areas of Tamil Nadu. J. Vet. Parasitol. 2015, 17, 159–160. [Google Scholar]
- Renwal, K.K.; Gupta, A.; Kumar, N.; Pilania, P.K.; Manohar, G.S. Prevalence and risk assessment of gastrointestinal helminthoses in dairy animals of Bikaner, Rajasthan. J. Parasit. Dis. 2017, 41, 557–561. [Google Scholar]
- Das, G.; Kumbhakar, N.K.; Verma, R.; Lata, K.; Saiyam, R.A. Coprological survey of common gastrointestinal parasitic infections in buffaloes in Jabalpur district of Madhya Pradesh, India. J. Entomol. Zool. Stud. 2018, 6, 315–318. [Google Scholar]
- Terfa, W.; Kumsa, B.; Ayana, D.; Maurizio, A.; Tessarin, C.; Cassini, R. Epidemiology of Gastrointestinal Parasites of Cattle in Three Districts in Central Ethiopia. Animals 2023, 13, 285. [Google Scholar]
- Malathi, S.; Shameem, U.; Komali, M. Prevalence of gastrointestinal helminth parasites in domestic ruminants from Srikakulam district, Andhra Pradesh, India. J. Parasit. Dis. 2021, 45, 823–830. [Google Scholar]
- Kumar, B.; Maharana, B.R.; Prasad, A.; Joseph, J.P.; Patel, B.; Patel, J.S. Seasonal incidence of parasitic diseases in bovines of south western Gujarat (Junagadh), India. J. Parasit. Dis. 2016, 40, 1342–1346. [Google Scholar]
- Cruvinel, L.B.; Nicaretta, J.E.; Bastos, T.S.A.; Couto, L.F.M.; Santos, J.B.D.; Zapa, D.M.B.; Cavalcante, A.S.A.; Cruz, B.C.; Borges, D.G.L.; Borges, F.A.; et al. Eimeria species in dairy and beef cattle of different ages in Goias state, Brazil. Braz. J. Vet. Parasitol. 2018, 27, 169–176. [Google Scholar]
- León, J.C.P.; Delgado, N.U.; Florez, A.A. Prevalence of gastrointestinal parasites in cattle and sheep in three municipalities in the Colombian northeastern mountain. Vet. World 2019, 12, 48–54. [Google Scholar]
- Singh, A.K.; Das, G.; Roy, B.; Nath, S.; Naresh, R.; Kumar, S. Prevalence of gastro-intestinal parasitic infections in goat of Madhya Pradesh, India. J. Parasit. Dis. 2015, 39, 716–719. [Google Scholar]
- Singh, E.; Kaur, P.; Singla, L.D.; Bal, M.S. Prevalence of gastrointestinal parasitism in small ruminants in western zone of Punjab, India. Vet. World 2017, 10, 61–66. [Google Scholar]
- Pinilla, J.C.; Florez, P.; Sierra, M.T.; Morales, E.; Sierra, R.; Vásquez, M.C.; Tobon, J.C.; Sánchez, A.; Ortiz, D. Point prevalence of gastrointestinal parasites in double purpose cattle of Rio de Oro and Aguachica municipalities, Cesar state, Colombia. Vet. Parasitol Reg. Stud. Rep. 2018, 12, 26–30. [Google Scholar]
- Yusof, A.M.; Isha, M.L.M. Prevalence of gastrointestinal nematodiasis and coccidiosis in goats from three selected farms in Terengganu, Malaysia. Asian Pac. J. Trop. Biomed. 2016, 6, 735–739. [Google Scholar]
- Jena, A.; Deb, A.R.; Kumari, L.; Biswal, S.S.; Joshi, S.K. Prevalence of Gastrointestinal helminthes among goats in and around Ranchi, Jharkhand, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3506–3513. [Google Scholar]
- Shit, N.; Hajra, D.K.; Baidya, S.; Debbarma, A. Seasonal occurrence of gastrointestinal helminth parasites in cattle and buffaloes in Banuka district, West Bengal, India. Explor. Anim. Med. Res. 2017, 7, 58–63. [Google Scholar]
- Krishnamoorthy, P.; Lakshmi, H.K.; Jacob, S.S.; Suresh, K.P.; Shome, B.R. Scientometric analysis of gastrointestinal parasites prevalence in sheep and goats of India. Acta. Parasitol. 2023, 68, 496–519. [Google Scholar]
- Marskole, P.; Verma, Y.; Dixit, A.K.; Swamy, M. Prevalence and burden of gastrointestinal parasites in cattle and buffaloes in Jabalpur, India. Vet. World 2016, 9, 1214–1217. [Google Scholar]
- Wadhwa, A.; Tanwar, R.K.; Singla, L.D.; Eda, S.; Kumar, N.; Kumar, Y. Prevalence of gastrointestinal helminthes in cattle and buffaloes in Bikaner, Rajasthan, India. Vet. World 2011, 4, 417–419. [Google Scholar]
- Velusamy, R.; Rani, N.; Ponnudurai, G.; Anbarasi, P. Prevalence of intestinal and haemoprotozoan parasites of small ruminants in Tamil Nadu, India. Vet. World 2015, 8, 1205–1209. [Google Scholar]
- Gupta, A.; Dixit, A.K.; Dixit, P.; Mahajan, C. Prevalence of gastrointestinal parasites in cattle and buffaloes in and around Jabalpur, Madhya Pradesh. J. Vet. Parasitol. 2012, 26, 186–188. [Google Scholar]
- Zvinorova, P.I.; Halimani, T.E.; Muchadeyi, F.C.; Matika, O.; Riggio, V.; Dzama, K. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin. Res. 2016, 143, 75–83. [Google Scholar]
- Sharma, D.; Katoch, R.; Agnihotri, R.K. Prevalence of gastrointestinal nematodes in Gaddi sheep of Palam valley, Himachal Pradesh. J. Vet. Parasitol. 2013, 27, 8–11. [Google Scholar]
- Ghafar, A.; Abbas, G.; King, J.; Jacobson, C.; Hughes, K.J.; El-Hage, C.; Beasley, A.; Bauquier, J.; Wilkes, E.J.A.; Hurley, J.; et al. Comparative studies on faecal egg counting techniques used for the detection of gastrointestinal parasites of equines: A systematic review. Curr. Res. Parasitol. Vector Borne Dis. 2021, 1, 100046. [Google Scholar]
- Kemal, J.; Terefe, Y. Prevalence of gastrointestinal parasitism of cattle in Gedebano Gutazer Wolene district, Ethiopia. J. Vet. Med. Anim. Health 2013, 5, 365–370. [Google Scholar]
- Patel, M.J.; Bhatiya, S.I.; Shekh, J.; Makwana, M.D.; Parsani, H.R. Effect of ivermectin on haematological parameters of helminth infested sheep. Life Sci. Leaflets 2017, 91, 41–47. [Google Scholar]
- Nath, S.; Das, G.; Dixit, A.K.; Agrawal, V.; Singh, A.K.; Kumar, S.; Katuri, R.N. Epidemiological studies on gastrointestinal parasites of buffaloes in seven agro-climatic zones of Madhya Pradesh, India. Buffalo Bull. 2016, 35, 355–364. [Google Scholar]
- Khajuria, J.K.; Katoch, R.; Yadav, A.; Godara, R.; Gupta, S.K.; Singh, A. Seasonal prevalence of gastrointestinal helminths in sheep and goats of middle agro-climatic zone of Jammu province. J. Parasit. Dis. 2013, 37, 21–25. [Google Scholar]
- Vercruysse, J.; Charlier, J.; Van Dijk, J.; Morgan, E.R.; Geary, T.; von Samson-Himmelstjerna, G.; Claerebout, E. Control of helminth infection by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).