Long-Term Stability of Harvest Mouse Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
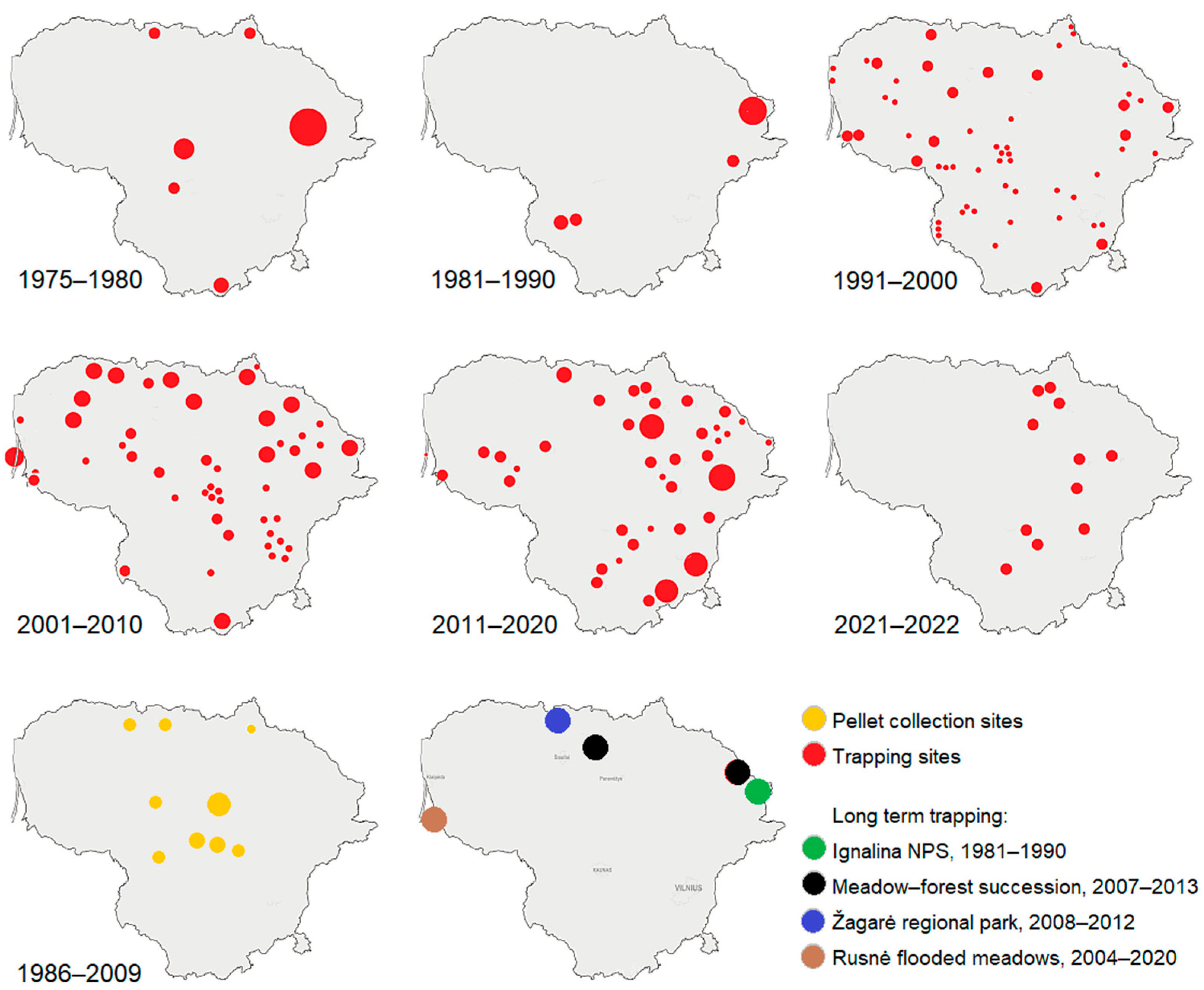
2.2. Data Collection
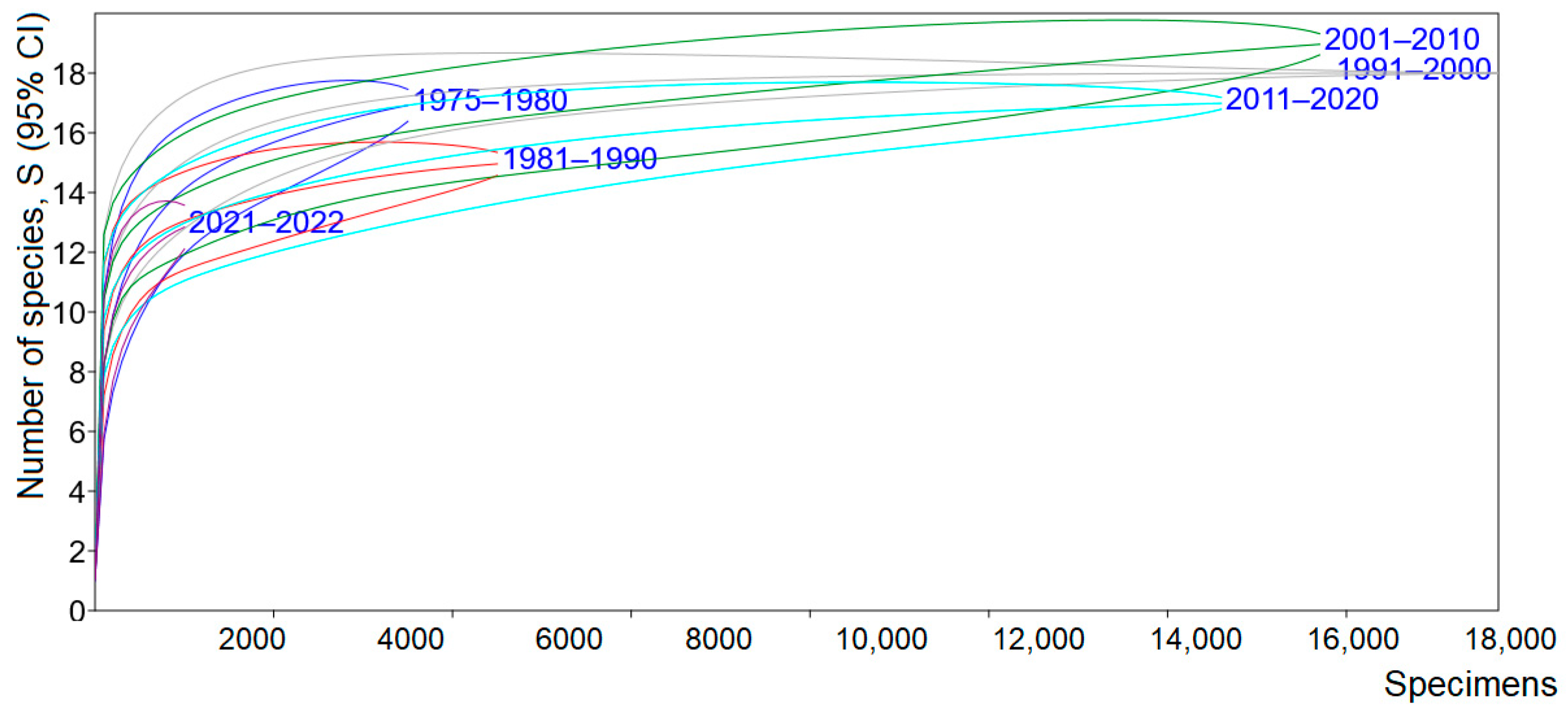
2.3. Data Treatment
3. Results
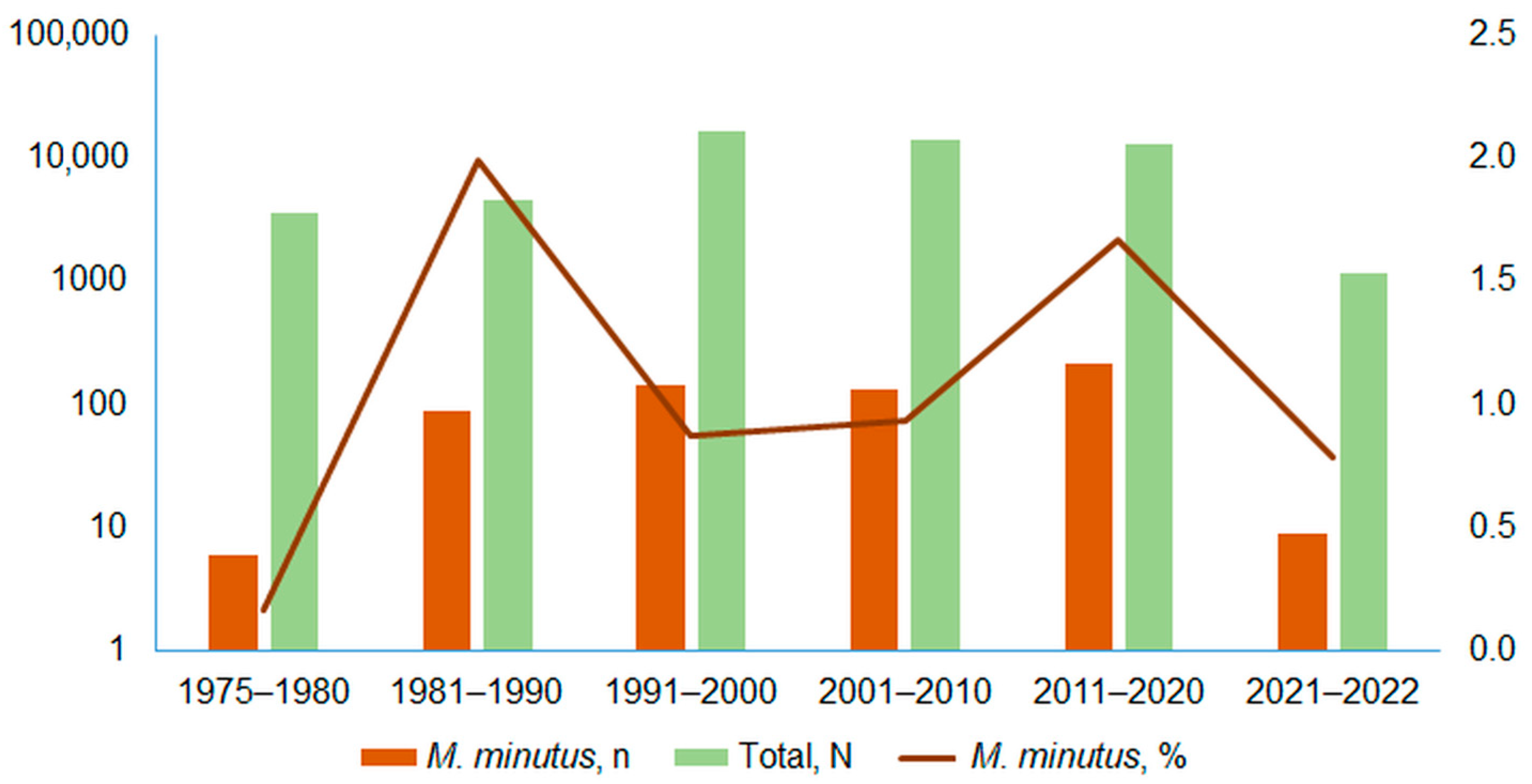
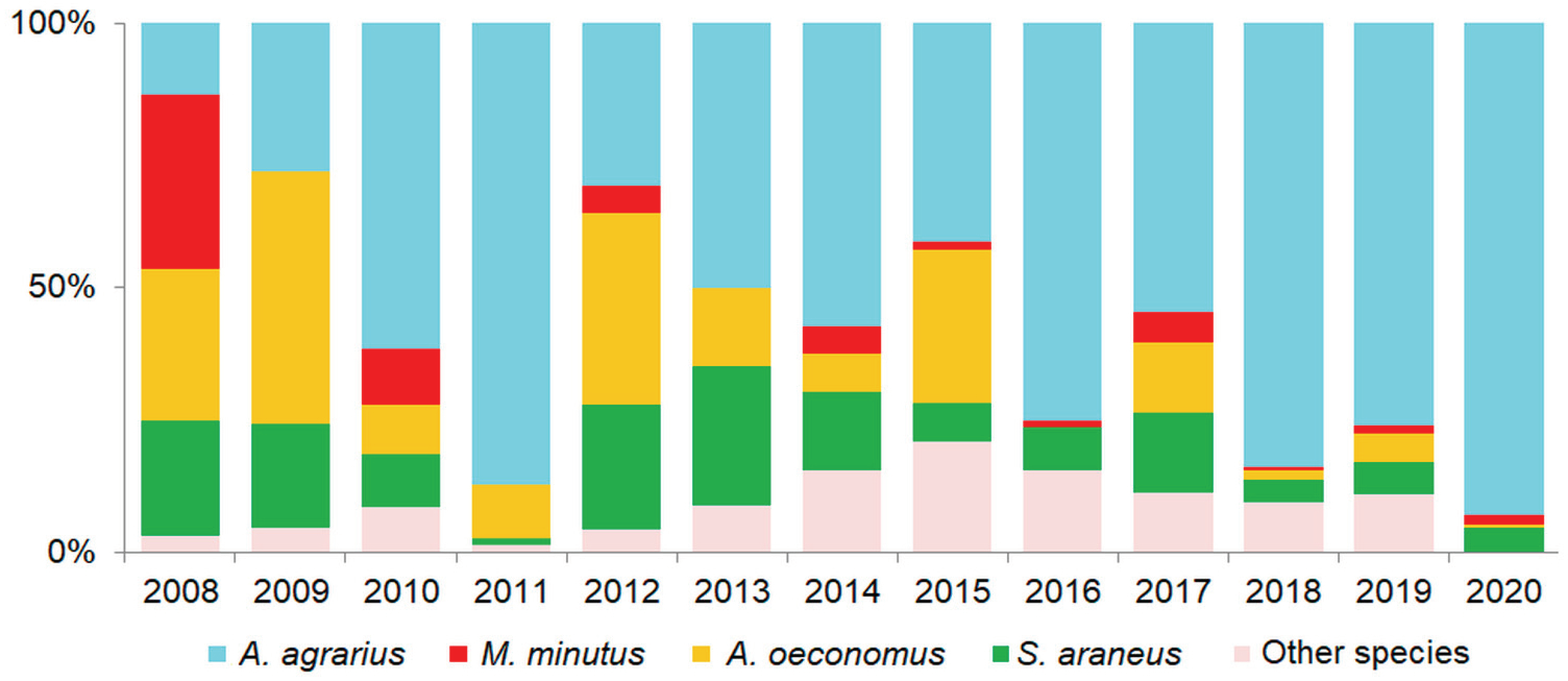
3.1. Harvest Mouse Numbers and Proportion in Small Mammal Communities
3.2. Relative Abundances of Harvest Mouse
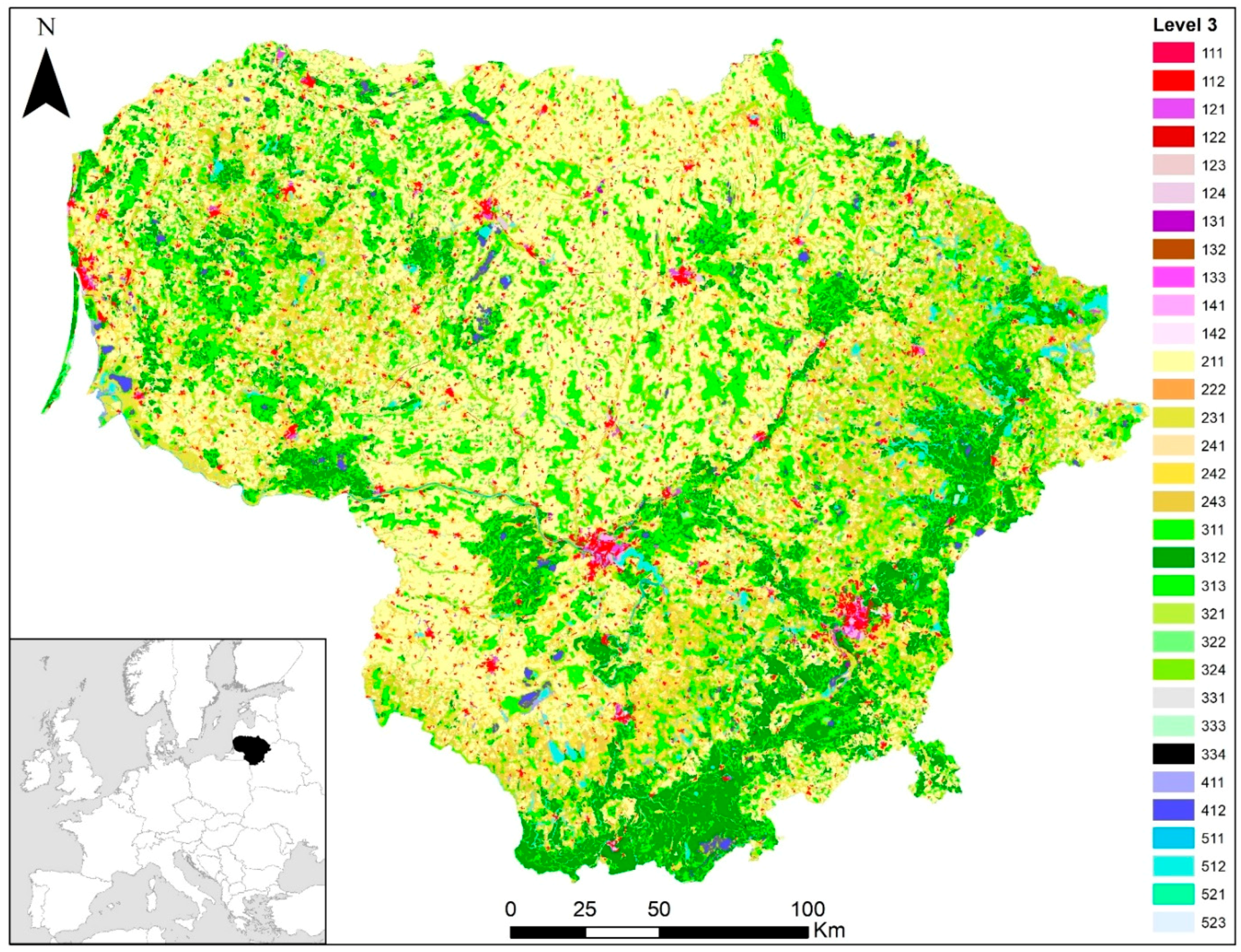
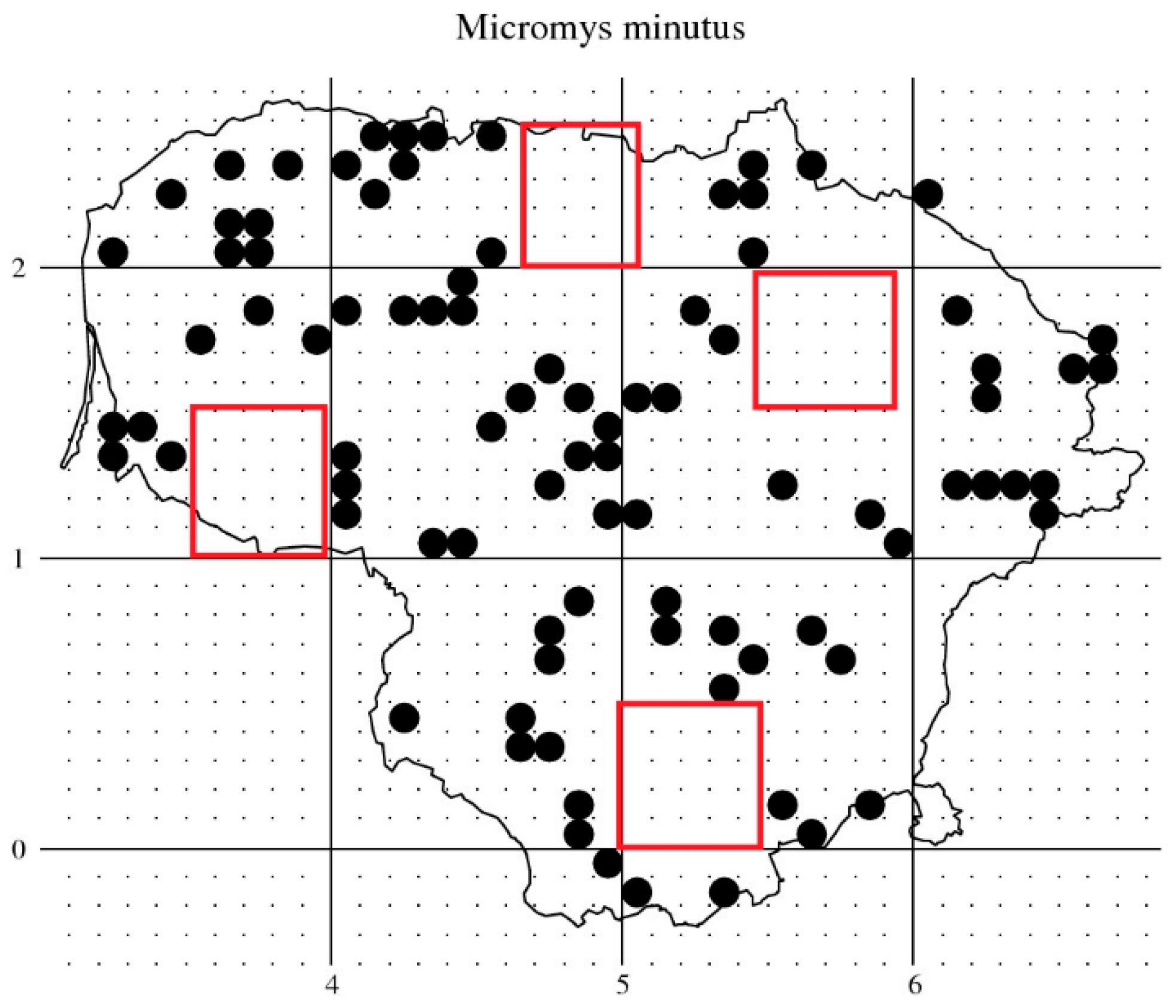
3.3. Harvest Mouse Habitats in Lithuania
3.4. Species Status and Distribution in the Country
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Micromys minutus (Pallas, 1771). Available online: https://www.gbif.org/species/5219833 (accessed on 11 July 2023).
- Spitzenberger, F. Micromys minutus. In The Atlas of European Mammals; Mitchell-Jones, A.J., Amori, G., Bogdanowicz, W., Krystufek, B., Reijnders, P., Eds.; Academic Press: London, UK, 1999; 484p. [Google Scholar]
- van der Kooij, J.; Isaksen, K.; Olsen, K.M. Dvergmus Micromys minutus påvist som ny pattedyrart for Norge. Fauna 2001, 54, 110–120. [Google Scholar]
- Csanády, A. Occurrence of the harvest mouse (Micromys minutus) and the hazel dormouse (Muscardinus avellanarius) in Slovakia based on summer nests (2013–2021). Acta Musei Silesiae. Sci. Nat. 2022, 71, 223–236. [Google Scholar] [CrossRef]
- Kryštufek, B.; Lunde, D.P.; Meinig, H.; Aplin, K.; Batsaikhan, N.; Henttonen, H. Micromys minutus. In The IUCN Red List of Threatened Species 2019: E.T13373A119151882; IUCN: Gland, Switzerland, 2019. [Google Scholar]
- Species—Harvest Mouse. Available online: https://www.mammal.org.uk/species-hub/full-species-hub/discover-mammals/species-harvest-mouse/ (accessed on 1 September 2023).
- Haberl, W.; Kryštufek, B. Spatial distribution and population density of the harvest mouse Micromys minutus in a habitat mosaic at Lake Neusiedl, Austria. Mammalia 2003, 67, 355–365. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Balčiauskienė, L.; Jasiulionis, M. Resource partitioning confirmed by isotopic signatures allows small mammals to share seasonally flooded meadows. Ecol. Evol. 2019, 9, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Trout, R.C. A review of studies on populations of wild Harvest mice (Micromys minutus (Pallas). Mammal Rev. 1978, 8, 143–158. [Google Scholar] [CrossRef]
- Harris, S. History, distribution, status and habitat requirements of the harvest mouse (Micromys minutus) in Britain. Mammal Rev. 1979, 9, 159–171. [Google Scholar] [CrossRef]
- Coomber, F.; Le Marquand, C.; Crawley, D.; Gilford, E.; Kirk, S.; Lloyd, A.; Web, S.; Gurnell, J. The Mammal Society’s National Harvest Mouse Survey: Results from the 2021–2022 Survey Season. Available online: https://www.mammal.org.uk/science-research/harvest-mouse-project/results/ (accessed on 17 September 2023).
- Vecsernyés, F. Autumn habitat selection of the harvest mouse (Micromys minutus Pallas, 1771) in a rural and fragmented landscape. Rev. Suisse Zool. 2020, 126, 111–125. [Google Scholar] [CrossRef]
- Darinot, F.; Le Petitcorps, Q.; Arnal, V.; Coulon, A.; Montgelard, C. Effects of landscape features and flooding on the genetic structure of a small wetland rodent, the harvest mouse (Micromys minutus). Landsc. Ecol. 2021, 36, 1755–1771. [Google Scholar] [CrossRef]
- Mori, E.; Viviano, A.; Mazzotti, S.; Sogliani, D.; Bini, A.; Baratti, M. Unveiling the genetic diversity of declining population of the harvest mouse Micromys minutus in Italy. Diversity 2022, 14, 627. [Google Scholar] [CrossRef]
- Bertolino, S.; Ancillotto, L.; Bartolommei, P.; Colangelo, P.; Capizzi, D.; Mori, E.; Melcore, I.; Paniccia, C.; Amori, G.; Gasperini, S.; et al. It is time to ensure protection for non-protected native Italian small mammals. Hystrix Ital. J. Mammal. 2023. [Google Scholar] [CrossRef]
- Sawabe, K.; Natuhara, Y. Extensive distribution models of the harvest mouse (Micromys minutus) in different landscapes. Global Ecol. Conserv. 2016, 8, 108–115. [Google Scholar] [CrossRef]
- Koskela, P.; Viro, P. The abundance, autumn migration, population structure and body dimensions of the harvest mouse in Northern Finland. Acta Theriol. 1976, 21, 375–387. [Google Scholar] [CrossRef]
- Lanszki, J. Somogyi lápok talajszinten élő emlős faunáinak vizsgálata. Állattani Közlemények 2004, 89, 23–30. [Google Scholar]
- Rowe, F.P.; Taylor, E.J. The numbers of harvest mice (Micromys minutus) in corn-ricks. Proc. Zool. Soc. London 1964, 142, 181–185. [Google Scholar]
- Darinot, F. The harvest mouse (Micromys minutus Pallas, 1771) as prey: A literature review. Folia Zool. 2016, 65, 117–137. [Google Scholar] [CrossRef]
- Bon, M.; Ratti, E.; Sartor, A. Seasonal variations in the diet of the little owl Athene noctua (Scopoli, 1769) in a tilled site on the edge of Venice Lagoon. Boll. Mus. Civ. Stor. Nat. Venezia 2001, 52, 193–212. [Google Scholar]
- Sándor, A.D. The summer diet of barn owl (Tyto alba) (Aves: Strigiformes) in the Southern part of Danube delta biosphere reserve. Acta Zool. Bulgar. 2009, 61, 87–92. [Google Scholar]
- Temme, M. Die Beutetiere aus Gewöllen von Schleiereulen Tyto alba von der Weserinsel Strohauser Plate. Nat. Und Umweltschutz (Zeitschrif Mellumrat) 2006, 5, 5–10. [Google Scholar]
- Romanowski, J.; Altenburg, D.; Zmihorski, M. Seasonal variation in the diet of the little owl, Athene noctua in agricultural landscape of Central Poland. North-West. J. Zool. 2013, 9, 310–318. [Google Scholar]
- Obuch, J. Spatial and temporal diversity of the diet of the tawny owl (Strix aluco). Slovak Rapt. J. 2011, 5, 1–120. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I. Dynamics of Asio otus L., 1758 (Aves: Strigiformes) winter-spring trophic regime in western plain (Romania). Trav. Mus. Natl. Hist. Nat. “Grigore Antipa” 2010, 53, 479–487. [Google Scholar] [CrossRef][Green Version]
- Canova, L. Influence of snow cover on prey selection by long-eared owls Asio otus. Ethol. Ecol. Evol. 1989, 1, 367–372. [Google Scholar] [CrossRef]
- Galeotti, P.; Tavecchia, G.; Bonetti, A. Home-range and habitat use of long-eared owls in open farmland (Po plain, Northern Italy), in relation to prey availability. J. Wildlife Res. 1997, 2, 137–145. [Google Scholar]
- Alivizatos, H.; Goutner, V.; Zogaris, S. Contribution to the study of the diet of four owl species (Aves, Strigiformes) from mainland and island areas of Greece. Belg. J. Zool. 2005, 135, 109–118. [Google Scholar]
- Gotta, A.; Pigozzi, G. Trophic niche of the barn owl and little owl in a rice field habitat in northern Italy. Ital. J. Zool. 1997, 64, 55–59. [Google Scholar] [CrossRef][Green Version]
- Pocora, V.; Popovici, M.; Manci, C.O.; Iorgu, I.S. Feeding of the little owl during nesting season in the Danube delta (Romania). An. Stiint. Univ. Al. I. Cuza 2012, 58, 107–114. [Google Scholar]
- Dickman, C.R. Habitat utilization and diet of the harvest mouse Micromys minutus, in an urban environment. Acta Theriol. 1986, 31, 249–256. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskienė, L. Long-term changes in a small mammal community in a temperate zone meadow subject to seasonal floods and habitat transformation. Integr. Zool. 2022, 17, 443–455. [Google Scholar] [CrossRef]
- Surmacki, A.; Gołdyn, B.; Tryjanowski, P. Location and habitat characteristics of the breeding nests of the harvest mouse (Micromys minutus) in the reed-beds of an intensively used farmland. Mammalia 2005, 69, 5–9. [Google Scholar] [CrossRef]
- Bence, S.L.; Stander, K.; Griffiths, M. Habitat characteristics of harvest mouse nests on arable farmland. Agr. Ecosyst. Environ. 2003, 99, 179–186. [Google Scholar] [CrossRef]
- Čanády, A. Nest dimensions and nest sites of the harvest mouse (Micromys minutus Pallas, 1771) from Slovakia: A case study from field margins. Zool. Ecol. 2013, 23, 253–259. [Google Scholar] [CrossRef]
- Hata, S. Nesting Characteristics of Harvest Mice (Micromys minutus) in Three Types of Japanese Grasslands with Different Inundation Frequencies. Mamm. Study 2011, 36, 49–53. [Google Scholar] [CrossRef]
- Kuroe, M.; Ohori, S.; Takatsuki, S.; Miyashita, T. Nest-site selection by the harvest mouse Micromys minutus in seasonally changing environments. Acta Theriol. 2007, 52, 355–360. [Google Scholar] [CrossRef]
- Juškaitis, R.; Remeisis, R. Harvest mice Micromys minutus and common dormice Muscardinus avellanarius live sympatric in woodland habitat. Acta Theriol. 2007, 52, 349–354. [Google Scholar] [CrossRef]
- Okutsu, K.; Takatsuki, S.; Ishiwaka, R. Food composition of the harvest mouse (Micromys minutus) in a western suburb of Tokyo, Japan, with reference to frugivory and insectivory. Mamm. Study 2012, 37, 155–158. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Skipitytė, R.; Garbaras, A.; Stirkė, V.; Balčiauskienė, L.; Remeikis, V. Isotopic niche of syntopic granivores in commercial orchards and meadows. Animals 2021, 11, 2375. [Google Scholar] [CrossRef]
- Ylönen, H. Spatial avoidance between the bank vole Clethrionomys glareolus and the harvest mouse Micromys minutus: An experimental study. Ann. Zool. Fenn. 1990, 21, 313–320. [Google Scholar]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Stirkė, V.; Garbaras, A.; Skipitytė, R.; Balčiauskienė, L. Stable isotope analysis supports omnivory in bank voles in apple orchards. Agriculture 2022, 12, 1308. [Google Scholar] [CrossRef]
- National Land Service under the Ministry of Agriculture of the Republic of Lithuania. Available online: http://www.nzt.lt/go.php (accessed on 6 September 2023).
- Land Cover Country fact Sheets 2000–2018. Available online: https://www.eea.europa.eu/themes/landuse/land-cover-country-fact-sheets (accessed on 8 September 2023).
- Juknelienė, D.; Kazanavičiūtė, V.; Valčiukienė, J.; Atkocevičienė, V.; Mozgeris, G. Spatiotemporal Patterns of Land-Use Changes in Lithuania. Land 2021, 10, 619. [Google Scholar] [CrossRef]
- CORINE Land Cover. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 10 July 2023).
- Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/country/lithuania/climate-data-historical (accessed on 9 September 2023).
- Balčiauskas, L.; Balčiauskienė, L. Small Mammal Diversity Changes in a Baltic Country, 1975–2021: A Review. Life 2022, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available online: http://OpenEpi.com (accessed on 2 September 2023).
- Fleiss, J.L.; Levin, B.; Paik, M.C. Statistical Methods for Rates and Proportions, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; 800p. [Google Scholar]
- G-Test Calculator. Available online: https://elem.com/~btilly/effective-ab-testing/g-test-calculator.html (accessed on 12 August 2023).
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Ulevičius, A.; Juškaitis, R. Dzūkijos nacionalinio parko žinduoliai (išskyrus šikšnosparnius). Theriol. Litu. 2003, 3, 11–29. [Google Scholar]
- Balčiauskas, L.; Juškaitis, R. Diversity of small mammal communities in Lithuania (1. A review). Acta Zool. Litu. 1997, 7, 29–45. [Google Scholar] [CrossRef]
- Botelho, A.L.M.; D’Andrea, P.S.; Crisóstomo, C.F.; Silveira, M.; Lucio, C.S.; Santos, P.Z.L.; Bonvicino, C.R.; Gentile, R. Evaluating the efficiency of different sampling techniques to survey non-flying small mammals in the Amazon. Mamm. Res. 2023. [Google Scholar] [CrossRef]
- Feldmann, R. Studien zur Autökologie und Fortpflanzungsbiologie der Zwergmaus, Micromys minutus. Abh. Westfal. Mus. Naturkunde Landschaftsverb 1997, 59, 107–115. [Google Scholar]
- Ishiwaka, R.; Kinoshita, Y.; Satou, H.; Kakihara, H.; Masuda, Y. Overwintering in nests on the ground in the harvest mouse. Landscape Ecol. Eng. 2010, 6, 335–342. [Google Scholar] [CrossRef]
- Invazinių Lietuvoje Rūšių Sąrašas. Available online: https://am.lrv.lt/lt/veiklos-sritys-1/gamtos-apsauga/invazines-rusys/invaziniu-lietuvoje-rusiu-sarasas (accessed on 13 September 2023).
- Perrow, M.; Jowitt, A. What future for the harvest mouse? Br. Wildl. 1995, 6, 356–365. [Google Scholar]
- Stirkė, V.; Balčiauskas, L.; Balčiauskienė, L. Common Vole as a Focal Small Mammal Species in Orchards of the Northern Zone. Diversity 2021, 13, 134. [Google Scholar] [CrossRef]
- Kitrytė, N. Diversity of Small Mammal Parasites and Factors Shaping Their Communities. Ph.D. Thesis, Vilnius University, Vilnius, Lithuania, 2022. [Google Scholar]
- Atkočaitis, O. Sodybos pastatuose sugauti smulkieji žinduoliai. Theriol. Litu. 2003, 3, 57–61. [Google Scholar]
- Atkočaitis, O. Sodybos pastatuose sugauti smulkieji žinduoliai: Nauji duomenys. Theriol. Litu. 2005, 5, 76–77. [Google Scholar]
- Juškaitis, R.; Ulevičius, A. Smulkiųjų žinduolių rūšinė sudėtis Vilkaviškio ir Šakių rajonuose. Theriol. Litu. 2003, 3, 39–44. [Google Scholar]
- Juškaitis, R.; Dementavičius, D. Būdos miško pakraštyje (Kaišiadorių rajonas) sugauti smulkieji žinduoliai. Theriol. Litu. 2005, 5, 77–78. [Google Scholar]
- Balčiauskienė, L.; Naruševičius, V. Coincidence of small mammal trapping data with their share in the Tawny Owl diet. Acta Zool. Litu. 2006, 16, 93–101. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Čepukienė, A.; Balčiauskienė, L. Small mammal community response to early meadow–forest succession. For. Ecosyst. 2017, 4, 11. [Google Scholar] [CrossRef]
- Maldžiūnaitė, S.; Mažeikytė, R.; Gruodis, S. Small mammalia on cultivated pastures in the Middle Lithuania (1. Species composition of small mammalia in non-irrigated cultivated pastures). Liet. TSR Moksl. Akad. Darb. 1981, 4, 71–78. [Google Scholar]
- Ancillotto, L.; Viviano, A.; Baratti, M.; Sogliani, D.; Ladurner, E.; Mori, E. Every branch in its niche: Intraspecific variation in habitat suitability of a widely distributed small mammal, the harvest mouse Micromys minutus. Mammal Res. 2023, 68, 575–585. [Google Scholar] [CrossRef]
| Indices | 1961–1990 | 1991–2020 | ||||||
|---|---|---|---|---|---|---|---|---|
| WIN | SPR | SUM | AUT | WIN | SPR | SUM | AUT | |
| OASMT | −5.13 | 5.79 | 16.2 | 7 | −3.1 | 6.97 | 17.34 | 7.56 |
| OSP | 69.98 | 130.97 | 224.3 | 185.74 | 89.22 | 131.43 | 227.92 | 177.62 |
| Year | All Species, N | M. minutus, n | M. minutus, % | M. minutus, % CI |
|---|---|---|---|---|
| 1981 | 361 | 4 | 1.1 | 0.4–2.8 |
| 1981 | 361 | 4 | 1.1 | 0.4–2.8 |
| 1982 | 401 | 6 | 1.5 | 0.7–3.3 |
| 1983 | 261 | 6 | 2.3 | 1.1–4.9 |
| 1984 | 386 | 6 | 1.6 | 0.7–3.4 |
| 1985 | 455 | 14 | 3.1 | 1.8–5.1 |
| 1986 | 224 | 0 | 0 | |
| 1987 | 372 | 1 | 0.3 | 0.1–1.5 |
| 1988 | 357 | 4 | 1.1 | 0.4–2.9 |
| 1989 | 700 | 2 | 0.3 | 0.1–1.0 |
| 1990 | 303 | 4 | 1.3 | 0.5–3.3 |
| Total | 3820 | 47 | 1.2 | 0.9–1.6 |
| Year | All Species, N | M. minutus, n | M. minutus, % | M. minutus, % CI |
|---|---|---|---|---|
| 2007 | 197 | 1 | 0.5 | 0.1–2.8 |
| 2008 | 136 | 0 | ||
| 2010 | 378 | 2 | 0.5 | 0.2–1.9 |
| 2011 | 387 | 1 | 0.3 | 0.05–1.5 |
| 2012 | 284 | 7 | 2.5 | 1.2–5.0 |
| Period | Samples, n | RA, Average ± SE/1000 Trap Days | Maximum RA/1000 Trap Days |
|---|---|---|---|
| 1975–1980 | 6 | 0.25 ± 0.19 | 1.14 |
| 1981–1990 | 11 | 4.01 ± 2.11 | 23.33 |
| 1991–2000 | 111 | 1.82 ± 0.38 | 26.67 |
| 2001–2010 | 175 | 0.78 ± 0.23 | 33.80 |
| 2011–2020 | 278 | 1.24 ± 0.37 | 88.00 |
| 2021–2022 | 87 | 0.72 ± 0.38 | 26.67 |
| Habitat | Samples, n | RA, Average ± SE | Maximum RA |
|---|---|---|---|
| Open sedge | 1 | 13.33 ± 0.00 | 13.33 |
| Meadow | 58 | 4.69 ± 1.73 | 88.00 |
| Wetland | 18 | 2.95 ± 1.13 | 15.00 |
| Agriculture | 1 | 2.86 ± 0.00 | 2.86 |
| Ecotone | 4 | 1.25 ± 1.25 | 5.00 |
| Commensal | 26 | 0.14 ± 0.11 | 2.78 |
| Mixed | 180 | 1.19 ± 0.22 | 23.33 |
| Forest | 113 | 0.71 ± 0.24 | 20.00 |
| Cormorant colony | 31 | 0.45 ± 0.23 | 6.67 |
| Island | 1 | 0 | 0 |
| Shore | 1 | 0 | 0 |
| Period | 1975–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 |
|---|---|---|---|---|---|
| 1981–1990 | 501 | ||||
| 1991–2000 | 1615 | 1769 | |||
| 2001–2010 | 1439 | 2038 | 333,854 | ||
| 2011–2020 | 632 | 26,283 | 3136 | 3816 | |
| 2021–2022 | 1922 | 1478 | 171,997 | 58,371 | 2462 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Balčiauskienė, L. Long-Term Stability of Harvest Mouse Population. Diversity 2023, 15, 1102. https://doi.org/10.3390/d15101102
Balčiauskas L, Balčiauskienė L. Long-Term Stability of Harvest Mouse Population. Diversity. 2023; 15(10):1102. https://doi.org/10.3390/d15101102
Chicago/Turabian StyleBalčiauskas, Linas, and Laima Balčiauskienė. 2023. "Long-Term Stability of Harvest Mouse Population" Diversity 15, no. 10: 1102. https://doi.org/10.3390/d15101102
APA StyleBalčiauskas, L., & Balčiauskienė, L. (2023). Long-Term Stability of Harvest Mouse Population. Diversity, 15(10), 1102. https://doi.org/10.3390/d15101102








