Diversity of Zooplankton in the Rice Fields in Suphan Buri Province, Thailand, with a New Record of Cyclopoid Copepod
Abstract
1. Introduction
2. Materials and Methods
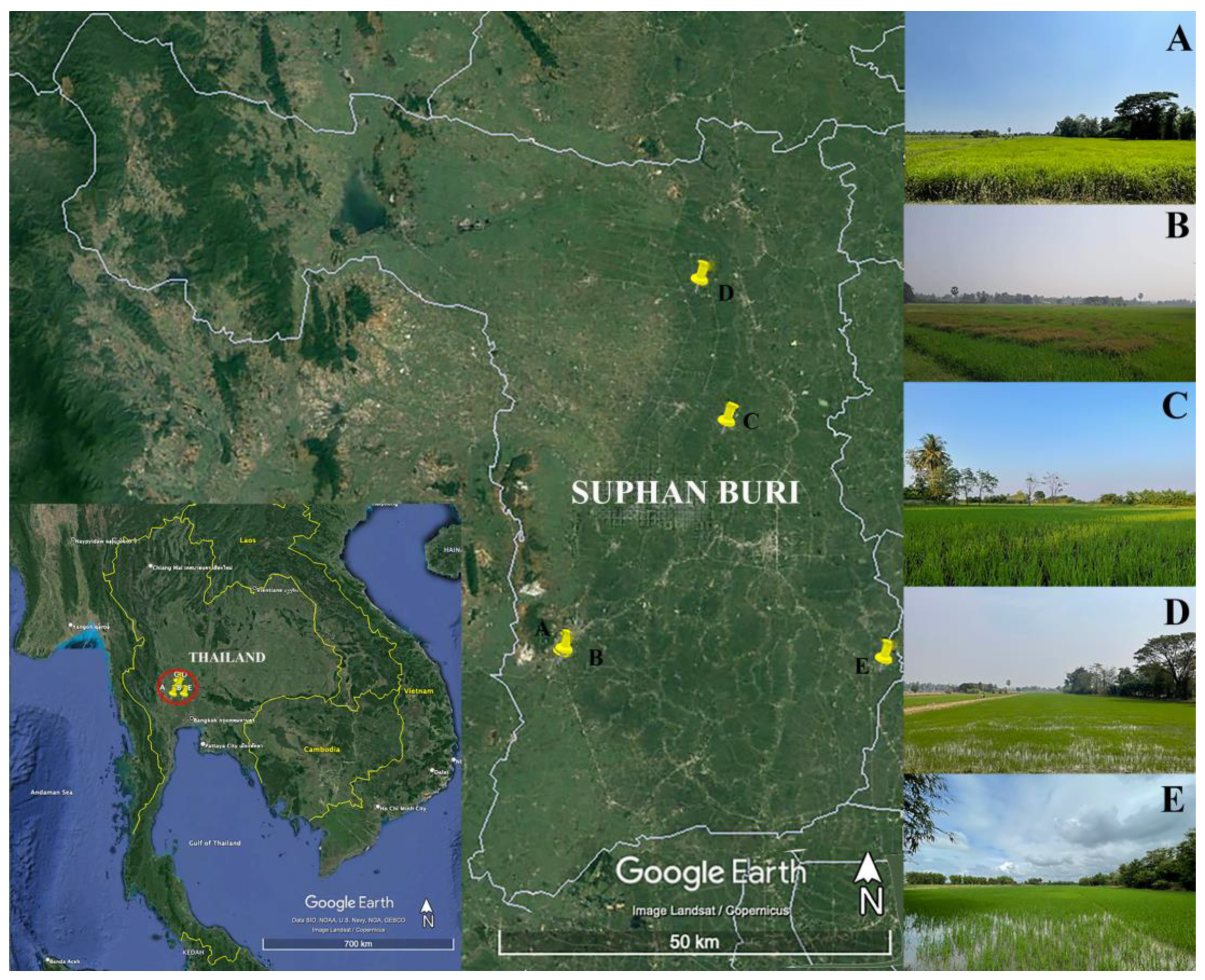
2.1. Study Area
2.2. Sample Collection, Species Identification, and Count
2.3. Data Analyses
3. Results
3.1. Diversity and Distribution of Zooplankton
3.2. New Record of Mesocyclops kayi Holynska & Brown, 2003 in Thailand
3.2.1. Short Description
3.2.2. Remark
3.2.3. Ecological Distribution
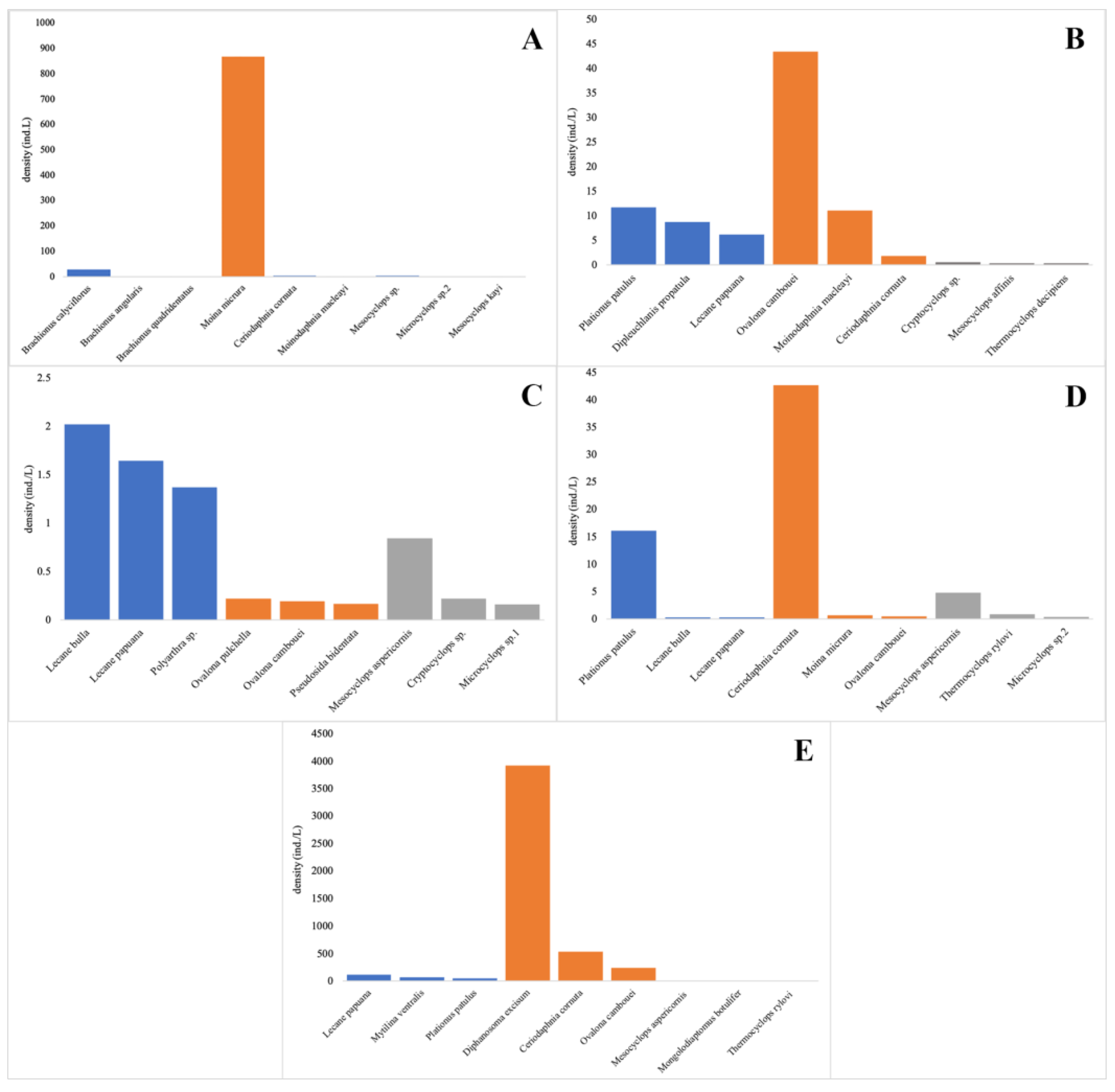
3.3. Density, Relative Abundance, and Dominant Species
3.4. Similarity of Species Composition between the Present Rice Fields and Those in Previous Studies in Thailand
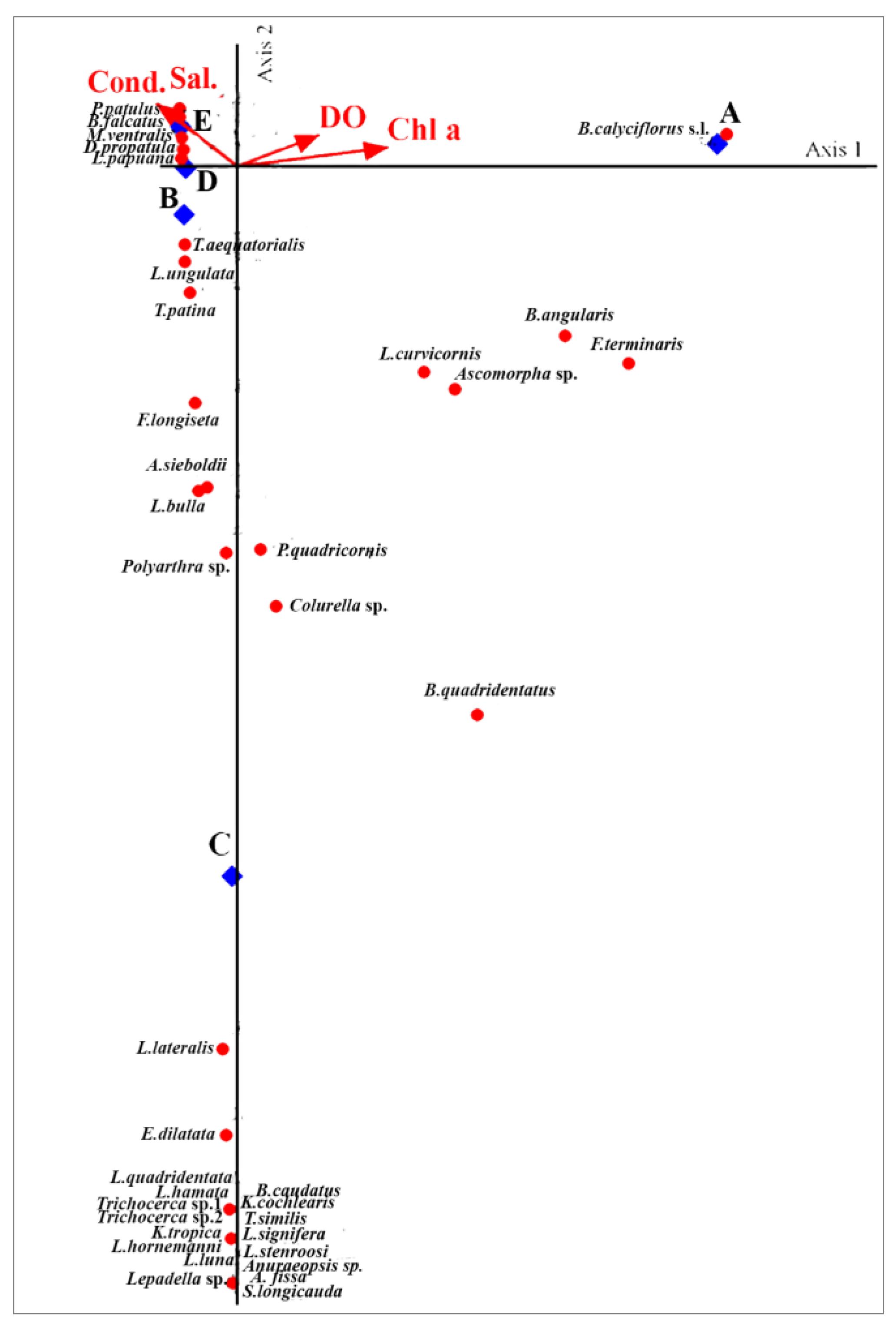
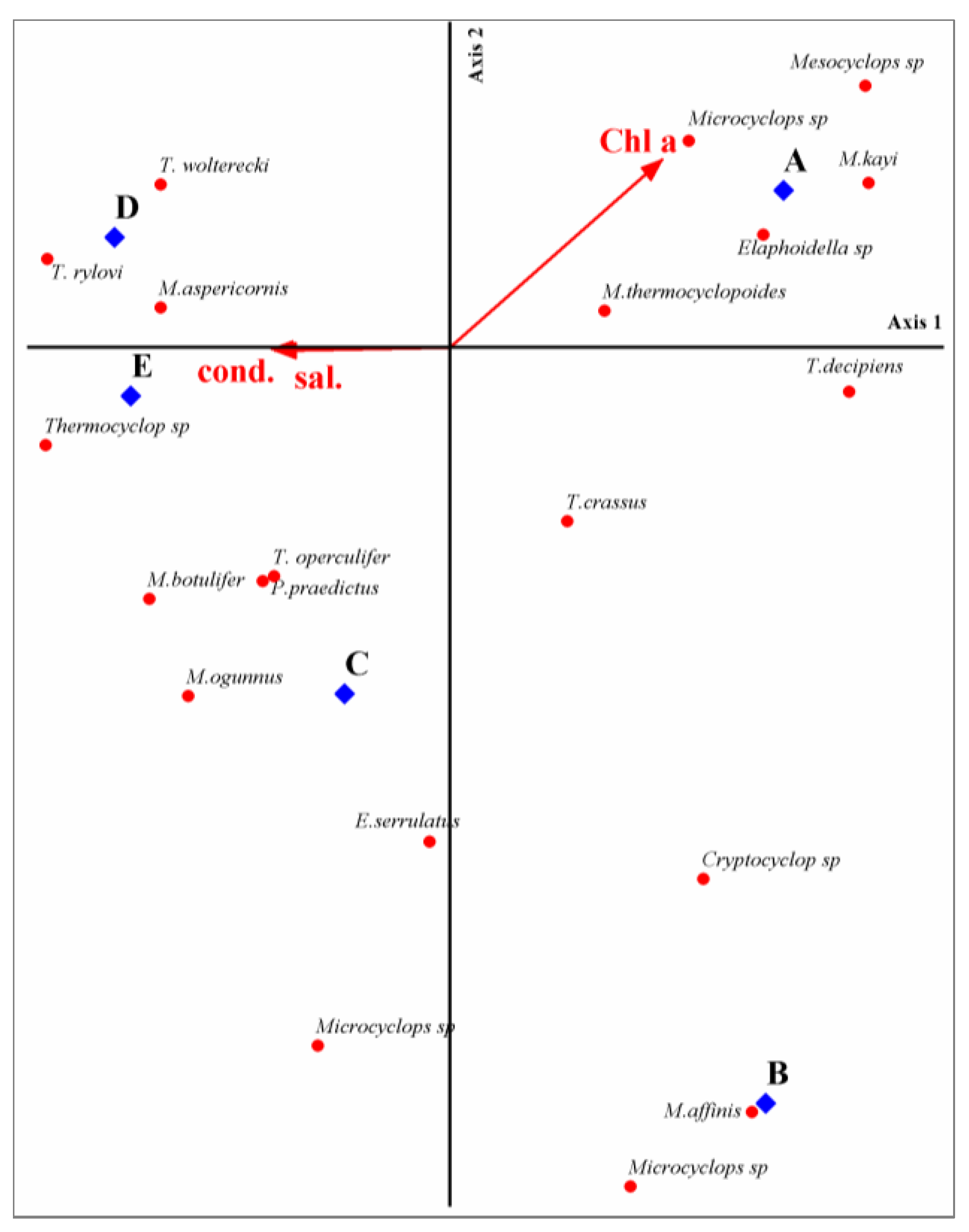
3.5. Species–Environment Associations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edirisinghe, J.P.; Bambaradeniya, C.N.B. Rice fields: An ecosystem rich in biodiversity. J. Nat. Sci. Found. Sri Lanka 2006, 34, 57–59. [Google Scholar] [CrossRef]
- Watanabe, T. Paddy Fields as Artificial and Temporal Wetland. In Irrigation in Agroecosystems; Ondrašek, G., Ed.; IntechOpen, Inggris: London, UK, 2018; pp. 143–157. [Google Scholar] [CrossRef]
- Bambaradeniya, C.; Edirisinghe, J.; De Silva, D.; Gunatilleke, C.; Ranawana, K.; Wijekoon, S. Biodiversity associated with an irrigated rice agroecosystem in Sri Lanka. Biodivers. Conserv. 2004, 13, 1715–1753. [Google Scholar] [CrossRef]
- Maiphae, S.; Limbut, W.; Choikaew, P.; Pechrat, P. The Cladocera (Ctenopoda and Anomopoda) in rice fields during a crop cycle at Nakhon Si Thammarat province, southern Thailand. Crustaceana 2010, 83, 1469–1482. Available online: https://www.jstor.org/stable/29779479 (accessed on 1 September 2023). [CrossRef]
- Maiphae, S.; Janpriang, P. The Cladocera in rice fields in Songkhla Province. KKU Sci. J. 2009, 37, 305–313. [Google Scholar]
- Chittapun, S.; Pholpunthin, P.; Sanoamuang, L. Diversity and composition of zooplankton in rice fields during a crop cycle at Pathum Thani province, Thailand. Songklanakarin J. Sci. Technol. 2009, 31, 261–267. [Google Scholar]
- Romero, N.; Attademo, A.M.; Reno, U.; Regaldo, L.; Repetti, M.R.; Lajmanovich, R.; Gagneten, A.M. Analysis of the zooplanktonic community in rice fields during a crop cycle in agroecological versus conventional management. Limnetica 2022, 41, 107–120. [Google Scholar] [CrossRef]
- Frison, E.A.; Cherfas, J.; Hodgkin, T. Agricultural Biodiversity Is Essential for a Sustainable Improvementin Food and Nutrition Security. Sustainability 2011, 3, 238–253. [Google Scholar] [CrossRef]
- Kremen, C.; Miles, A. Ecosystem services in biologically diversified versus conventional farming systems: Benefits, externalities, and trade-offs. Ecol. Soc. 2012, 17, 40. [Google Scholar] [CrossRef]
- Plangklang, N.; Athibai, S. Species composition of rotifers, cladocerans and copepods in an organic rice field in saline area, Nakhon Ratchasima province. Wichcha J. NSTRU 2021, 40, 14–28. (In Thai) [Google Scholar]
- Plangklang, N.; Athibai, S. Comparisons of zooplankton community structure between with- and without-pesticide applications on rice fields. Diversity 2021, 13, 644. [Google Scholar] [CrossRef]
- Saetang, T.; Sanoamuang, L.; Maiphae, S. A new species of genus Tropodiaptomus Kiefer, 1932 (Crustacea: Copepoda: Calanoida: Diaptomidae) from Thailand. J. Nat. Hist. 2020, 54, 2297–2322. [Google Scholar] [CrossRef]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters III: Euchlanidae, Mytilinidae and Trichotriidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1989, 113, 85–114. [Google Scholar]
- Koste, W.; Shiel, R.J. Rotifera from Australian inland waters V. Lecanidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. 1990, 114, 1–36. [Google Scholar]
- Michaloudi, E.; Papakostas, S.; Stamou, G.; Neděla, V.; Tihlaříková, E.; Zhang, W.; Declerck, S.A.J. Reverse taxonomy applied to the Brachionus calyciflorus cryptic species complex: Morphometric analysis confirms species delimitations revealed by molecular phylogenetic analysis and allows the (re)description of four species. PLoS ONE 2018, 13, e0203168. [Google Scholar] [CrossRef] [PubMed]
- Nogrady, T.; Pourriot, R.; Segers, H. Rotifera: Notommatidae and Scaridiidae. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H.J.F., Nogrady, T., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1995; pp. 1–248. [Google Scholar]
- Segers, H. Annotated checklist of the rotifers (Phylum rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar] [CrossRef]
- Shiel, R.J.; Koste, W. Rotifera from Australian inland waters. VIII: Trichocercidae (Monogononta). Trans. R. Soc. S. Aust. 1992, 116, 1–27. [Google Scholar]
- Idris, B.A.G. Freshwater Zooplankton of Malaysia (Crustacea: Cladocera); Perenbit University Press: Pertanian, Malaysia, 1983; pp. 1–153. [Google Scholar]
- Korovchinsky, N.M. Sididae and Holopediidae (Crustacea: Daphniiformes). In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; SPB Academic Publishing: The Hague, The Netherlands, 1992; Volume 3, pp. 1–82. [Google Scholar]
- Smirnov, N.N. The Macrothricidae of the world. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H.J.F., Ed.; SPB Academic Publishing: Amsterdam, The Netherlands, 1992; pp. 1–143. [Google Scholar]
- Smirnov, N.N. Cladocera: The Chydorinae and Sayciinae (Chydoridae) of the world. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Dumont, H.J.F., Ed.; SPB Academic Publishing: Amsterdam, The Netherlands, 1996; pp. 1–197. [Google Scholar]
- Hołyńska, M.; Brown, M. Three new species of Mesocyclops GO Sars, 1914 (Copepoda, Cyclopoida) from Australia and Burma, with comments on the Mesocyclops fauna of Australia. Crustaceana 2003, 75, 1301–1334. [Google Scholar] [CrossRef]
- Hołyńska, M.; Reid, J.W.; Ueda, H. Genus Mesocyclops Sars, 1914. In Copepoda: Cyclopoida Genera Mesocyclops and Thermocyclops, Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Ueda, H., Reid, J.W., Dumont, H.J.F., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 12–213. [Google Scholar]
- Mirabdullayev, I.M.; Reid, J.W.; Ueda, H. Genus Thermocyclops Kiefer, 1927. In Copepoda: Cyclopoida Genera Mesocyclops and Thermocyclops, Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Ueda, H., Reid, J.W., Dumont, H.J.F., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 214–302. [Google Scholar]
- Sanoamuang, L. Freshwater Zooplankton: Calanoid Copepods in Thailand, 1st ed.; Klangnanatham Publishers: Khon Kaen, Thailand, 2002; pp. 1–159. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD. Multivariate Analysis of Ecological Data; Version 7; MjM Software Design: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Saetang, T.; Maiphae, S. Diversity of the genus Tropodiaptomus Kiefer, 1932 (Crustacea, Copepoda, Calanoida, Diaptomidae) in Thailand, with the description of two new species. Zoosyst. Evol. 2023, 99, 399–422. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Dabseepai, P. Diversity, distribution, and habitat occurrence of the diaptomid copepods (Crustacea: Copepoda: Diaptomidae) in freshwater ecosystems of Thailand. Water 2021, 13, 2381. [Google Scholar] [CrossRef]
- Watiroyram, S.; Koompoot, K. Elaphoidella stygobiotica (Copepoda: Harpacticoida: Canthocamptidae), a new species of cave-dwelling copepod from western Thailand. Raffles Bull. Zool. 2023, 71, 496–510. [Google Scholar] [CrossRef]
- Choi, G.; Do, M.S.; Son, S.J.; Nam, H.K. Effect of different management techniques on bird taxonomic groups on rice fields in the Republic of Korea. Sci. Rep. 2021, 11, 22347. [Google Scholar] [CrossRef] [PubMed]
- Athibai, S.; Segers, H.; Sanoamuang, L. Diversity and distribution of Brachionidae (Rotifera) in Thailand, with a key to the species. J. Limnol. 2013, 72, 345–360. [Google Scholar] [CrossRef]
- Pardo, M.J.; Scott-Frías, J.; Soto, L.M.; Stamou, G.; Michaloudi, E.; Torres, R.; González, E.; López, C. Rotifers (Rotifera: Monogononta) Associated with Littoral Macrophyte Habitats in Flooded Neotropical Ponds: A Qualitative Study. Diversity 2023, 15, 590. [Google Scholar] [CrossRef]
- Lynch, M. The Evolution of Cladoceran Life Histories. Q. Rev. Biol. 1980, 55, 23–42. [Google Scholar] [CrossRef]
- Murugan, N. Egg production, development and growth in Moina micrura Kurz (1874) (Cladocera: Moinidae). Freshw. Biol. 1975, 5, 245–250. [Google Scholar] [CrossRef]
- Murugan, N. Dynamics of a population of Ceriodsphnia cornuta Sars (Crustacea: Cladocera) from a seasonal pond in Madurai. Proc. Indian Acad. Sci. Anim. Sci. 1990, 99, 151–161. [Google Scholar] [CrossRef]
- Nogrady, T.; Wallace, R.L.; Snell, T.W. Rotifera Volume 1: Biology, Ecology and Systematics. In Guide to the Identification of the Microinvertebrates of the Continental Waters of the Word; Dumont, H.J.F., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1993; pp. 1–142. [Google Scholar]
- Fussmann, G. The importance of crustacean zooplankton in structuring rotifer and phytoplankton communities; an enclosure study. J. Plankton Res. 1996, 18, 1897–1915. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Athibai, S. A new species of Neodiaptomus (Copepoda, Diaptomidae) from temporary waters in northeast Thailand. Hydrobiologia 2002, 489, 71–82. [Google Scholar] [CrossRef]
- Alekseev, V.R.; Sanoamuang, L. Biodiversity of cyclopoid copepods in Thailand with a description of Afrocyclops henrii sp. n. Arthropoda Sel. 2006, 15, 277–290. [Google Scholar]
- Proongkiat, K.; Sanoamuang, L. Description of Neodiaptomus siamensis, a new diaptomid copepod (Copepoda, Calanoida) from temporary pools in northern Thailand. Crustaceana 2008, 81, 177–189. [Google Scholar] [CrossRef]
- Saetang, T. Species Diversity of Freshwater Copepods (Calanoid, Cyclopoid and Harpacticoid) in Thale-Noi, Phatthalung Province. Master’s Thesis, Prince Songkla University, Songkhla, Thailand, 2015; pp. 1–109. (In Thai). [Google Scholar]
- Choedchim, W.; Van Damme, K.; Maiphae, S. Spatial and temporal variation of Cladocera in a tropical shallow lake. Ann. Limnol. Int. J. Limnol. 2017, 53, 233–252. [Google Scholar] [CrossRef]
- Frisch, D.; Green, A.J. Copepods come in first: Rapid colonization of new temporary ponds. Fundam. Appl. Limnol. 2007, 168, 289–297. [Google Scholar] [CrossRef]
- Marten, G.G.; Nguyen, M.; Ngo, G. Copepod predation on Anopheles quadrimaculatus larvae in rice fields. J. Vector Ecol. 2000, 25, 1–6. [Google Scholar] [PubMed]
- Snell, T.W.; Johnston, R.K.; Gribble, K.E.; Welch, D.B.M. Rotifers as experimentaltools for investigating aging. Invertebr. Reprod. Dev. 2015, 59 (Suppl. 1), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.S.S.; Nandini, S.; Gulati, R.D. Life history strategies of cladocerans: Comparisons of tropical and temperate taxa. Hydrobiologia 2005, 542, 315–333. [Google Scholar] [CrossRef]
- Phong, T.V.; Tuno, N.; Kawada, H.; Takagi, M. Comparative evaluation of fecundity and survivorship of six copepod (Copepoda: Cyclopidae) species: In relation to selection of candidate biological control agents against Aedes aegypti. J. Am. Mosq. Control Assoc. 2008, 24, 61–69. [Google Scholar] [CrossRef]
- Starkweather, P.L.; Gilbert, J.J. Feeding in the rotifer Brachionus calyciflorus. II. Effect of food density on feeding rates using Euglena glutinis. J. Oecologia 1977, 28, 133–139. [Google Scholar] [CrossRef]
- Park, H.G.; Lee, K.W.; Cho, S.H.; Kim, H.S.; Jung, M.M.; Kim, H.S. High density culture of the freshwater rotifer, Brachionus calyciflorus. Hydrobiologia 2001, 446, 369–374. [Google Scholar] [CrossRef]
- Halabowski, D.; Bielanska-Grajner, I.; Lewin, I. Effect of underground salty mine water on the rotifer communities in the Bolina River (Upper Silesia, Southern Poland). Knowl. Manag. Aquat. Ecosyst. 2019, 420, 31. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Vila-Gispert, A.; Quintana, X.D.; Au, V.H.; Nguyen, T.P.; Vu, N.U. Effects of salinity on species composition of zooplankton on Hau River, Mekong Delta, Vietnam. Ann. Limnol. Int. J. Limnol. 2020, 56, 20. [Google Scholar] [CrossRef]
- Bielańska-Grajner, I.; Cudak, A. Effects of Salinity on Species Diversity of Rotifers in Anthropogenic Water Bodies. Pol. J. Environ. Stud. 2014, 23, 27–34. [Google Scholar]
- Sa-Ardrit, P.; Beamish, F.W.H. Cladocera diversity, abundance and habitat in a western Thailand stream. Aquat. Ecol. 2005, 39, 353–365. [Google Scholar] [CrossRef]
- Sa-Ardrit, P.; Pholpunthin, P.; Segers, H. A checklist of the freshwater rotifer fauna of Thailand (Rotifera, Monogononta, Bdelloidea). J. Limnol. 2013, 72, 361–375. [Google Scholar] [CrossRef][Green Version]
- Plangklang, N.; Athibai, S. Viability of zooplankton resting eggs in rice field sediment after pesticide applications. Biodivers. Data J. 2023, 11, e106418. [Google Scholar] [CrossRef]



| Rice Field | Sampling Period | General Characteristics | Environmental Factors | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Cond. (μS cm−1) | TDS (mg L−1) | Sal. (psu) | DO (mg L−1) | Chl a (μg L−1) | Phyco- Cyanin (μg L−1) | pH | |||
| A | February–March 2023 | Organic rice fields, irrigation water twice during rice plantations, and weeds were present. | 28.5–31.1 | 403.2–406.2 | 235–247 | 0.17–0.18 | 2.55–17.22 | 5.16–79.33 | 0.11–3.45 | 7.5–7.6 |
| B | March–April 2023 | Organic rice field, irrigation water, managed weeds, and no drying period during rice cultivation | 28.5–33.2 | 283.8–440 | 173–253 | 0.13–0.18 | 2.63–12.07 | 3.17–5.12 | 0.12–0.81 | 3.9–7.5 |
| C | November 2022–February 2023 | Chemical rice field, irrigation water, managed weeds, water level was 30–40 cm, no dried period during rice cultivation | 22.2–28.7 | 200.3–345.7 | 129–237 | 0.09–0.17 | 2.25–7.48 | 3.74–15.72 | 0.07–0.8 | 7.6–7.8 |
| D | November 2022 | Chemical rice field, irrigation water, long drying period, water level of about 20 cm, switch to growing Crotalaria juncea during the off-season. | 28.26 | 465.5 | 285 | 0.21 | 2.17 | 21.3 | 0.76 | 7.3 |
| E | March–April 2023 | Chemical rice field, roadside, irrigation water, water level was 20–30 cm, 3 months of rice cultivation whereas others are 4 months, flooding before rice plantation period every year. | 31.8–36.1 | 1176.5–1449.9 | 77–676 | 0.21–0.58 | 7.54–7.54 | 5.31–17.39 | 0.17–17.39 | 7.0–7.3 |
| Species | |
|---|---|
| Rotifers | Cladocerans |
| 1. Anuraeopsis fissa Gosse, 1851 | 1. Anthalona harti Van Damme, Sinev & Dumont, 2011 |
| 2. Anuraeopsis sp. | 2. Bosminopsis detersi Richard, 1895 |
| 3. Ascomorpha sp. | 3. Ceriodaphnia cornuta Sars, 1885 |
| 4. Asplanchna sieboldii (Leydig, 1854) | 4. Chydorus eurynotus Sars, 1901 |
| 5. Asplanchna sp. | 5. Coronatella monacantha (Sars, 1901) |
| 6. Brachionus angularis Gosse, 1851 | 6. Coronatella rectangula (Sars, 1861) |
| 7. Brachionus budapestinensis Daday, 1885 ** | 7. Diaphanosoma excisum Sars, 1885 |
| 8. Brachionus calyciflorus s.l. | 8. Guernella raphaelis Richard, 1892 |
| 9. Brachionus caudatus Barrois and Daday, 1894 | 9. Kurzia longirostris (Daday, 1898) |
| 10. Brachionus falcatus Zacharias, 1898 | 10. Leydigia acanthocercoides (Fischer, 1854) |
| 11. Brachionus lyratus Shephard, 1911 ** | 11. Macrothrix spinosa King, 1853 |
| 12. Brachionus quadridentatus Hermann, 1783 | 12. Moina micrura Kurz, 1875 |
| 13. Colurella sp. | 13. Moinodaphnia macleayi King, 1853 |
| 14. Conochilus sp. | 14. Ovalona cambouei (Guerne & Richard, 1983) |
| 15. Dipleuchlanis propatula (Gosse, 1886) | 15. Ovalona pulchella (King, 1853) |
| 16. Euchlanis dilatata Ehrenberg, 1832 | 16. Pseudosida bidentata Herrick, 1884 |
| 17. Filinia longiseta (Ehrenberg, 1834) | 17. Scapholeberis kingi Sars, 1888 |
| 18. Filinia opoliensis (Zacharias, 1898) | 18. Simocephalus heilongigensis Shi & Shi, 1994 |
| 19. Filinia terminalis (Plate, 1886) ** | Copepods |
| 20. Keratella cochlearis (Gosse, 1851) | 1. Mongolodiaptomus botulifer (Kiefer, 1974) |
| 21. Keratella tropica (Apstein, 1907) | 2. Phyllodiaptomus christineae Dumont, Ranga Reddy & Sanoamuang, 1996 ** |
| 22. Lecane aculeata (Jakubski, 1912) | 3. Phyllodiaptomus praedictus Dumont & Ranga Reddy, 1994 |
| 23. Lecane bulla (Gosse, 1851) | 4. Pseudodiaptomus sp. |
| 24. Lecane chinesensis Zhuge & Koste 1996 ** | 5. Cryptocyclops sp. |
| 25. Lecane closterocerca (Schmarda, 1859) | 6. Ectocyclops sp. |
| 26. Lecane curvicornis (Murray, 1913) | 7. Eucyclops serrulatus (Fischer, 1851) ** |
| 27. Lecane hornemanni (Ehrenberg, 1834) ** | 8. Eucyclops sp. |
| 28. Lecane hamata (Stokes, 1896) | 9. Mesocyclops affinis van de Velde, 1987 |
| 29. Lecane lateralis Sharma, 1978 | 10. Mesocyclops aspericornis (Daday, 1906) |
| 30. Lecane luna (Müller, 1776) | 11. Mesocyclops ogunnus Onabamiro, 1957 |
| 31. Lecane papuana (Murray, 1913) | 12. Mesocyclops thermocyclopoides Harada, 1931 |
| 32. Lecane quadridentata (Ehrenberg, 1830) | 13. Mesocyclops kayi Holynska & Brown, 2003 *,** |
| 33. Lecane signifera (Jennings, 1896) | 14. Mesocyclops sp. |
| 34. Lecane stenroosi (Meissner, 1908) | 15. Microcyclops sp. 1 |
| 35. Lecane unguitata (Fadeev, 1926) | 16. Microcyclops sp. 2 |
| 36. Lecane ungulata (Gosse, 1887) | 17. Microcyclops sp. 3 |
| 37. Lepadella dactyliseta (Stenroos, 1898) ** | 18. Microcyclops sp. 4 |
| 38. Lepadella sp. | 19. Paracyclops affinis (Sars G.O., 1863) ** |
| 39. Manfredium sp. | 20. Thermocyclops crassus (Fischer, 1853) ** |
| 40. Mytilina ventralis (Ehrenberg, 1830) | 21. Thermocyclops decipiens Kiefer, 1929 |
| 41. Platyias quadricornis (Ehrenberg, 1832) | 22. Thermocyclops operculifer Kiefer, 1930 ** |
| 42. Plationus patulus (Müller, 1786) | 23. Thermocyclops taihokuensis Harada, 1931 ** |
| 43. Polyarthra sp. | 24. Thermocyclops rylovi (Smirnov, 1928) ** |
| 44. Scaridium longicauda (Müller, 1786) ** | 25. Thermocyclops vermifer Lindberg, 1935 ** |
| 45. Testudinella incisa (Ternetz, 1892) | 26. Thermocyclops wolterecki Kiefer, 1938 ** |
| 46. Testudinella patina (Hermann, 1783) | 27. Thermocyclops sp. 1 |
| 47. Trichocerca similis (Wierzejski, 1893) | 28. Thermocyclops sp. 2 |
| 48. Trichocerca sp. 1 | 29. Elaphoidella sp. |
| 49. Trichocerca sp. 2 | 30. Onychocamptus vitiospinulosa (Shen & Tai, 1963) ** |
| 50. Trichocerca sp. 3 | |
| 51. Trichotria tetractis (Ehrenberg, 1830) ** | |
| 52. Trochosphaera aequatorialis Semper, 1872 | |
| Rice Fields | B | C | D | E |
|---|---|---|---|---|
| A | 0.7 | 0.38 | 0.75 | 0.61 |
| B | - | 0.52 | 0.72 | 0.85 |
| C | - | - | 0.31 | 0.49 |
| D | - | - | - | 0.62 |
| E | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiphae, S.; Saetang, T.; Jantawong, N.; Wongkamhaeng, K.; Piyasaengthong, N. Diversity of Zooplankton in the Rice Fields in Suphan Buri Province, Thailand, with a New Record of Cyclopoid Copepod. Diversity 2023, 15, 1054. https://doi.org/10.3390/d15101054
Maiphae S, Saetang T, Jantawong N, Wongkamhaeng K, Piyasaengthong N. Diversity of Zooplankton in the Rice Fields in Suphan Buri Province, Thailand, with a New Record of Cyclopoid Copepod. Diversity. 2023; 15(10):1054. https://doi.org/10.3390/d15101054
Chicago/Turabian StyleMaiphae, Supiyanit, Thanida Saetang, Natthida Jantawong, Koraon Wongkamhaeng, and Narisara Piyasaengthong. 2023. "Diversity of Zooplankton in the Rice Fields in Suphan Buri Province, Thailand, with a New Record of Cyclopoid Copepod" Diversity 15, no. 10: 1054. https://doi.org/10.3390/d15101054
APA StyleMaiphae, S., Saetang, T., Jantawong, N., Wongkamhaeng, K., & Piyasaengthong, N. (2023). Diversity of Zooplankton in the Rice Fields in Suphan Buri Province, Thailand, with a New Record of Cyclopoid Copepod. Diversity, 15(10), 1054. https://doi.org/10.3390/d15101054






