Potential Spread of Desert Locust Schistocerca gregagia (Orthoptera: Acrididae) under Climate Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
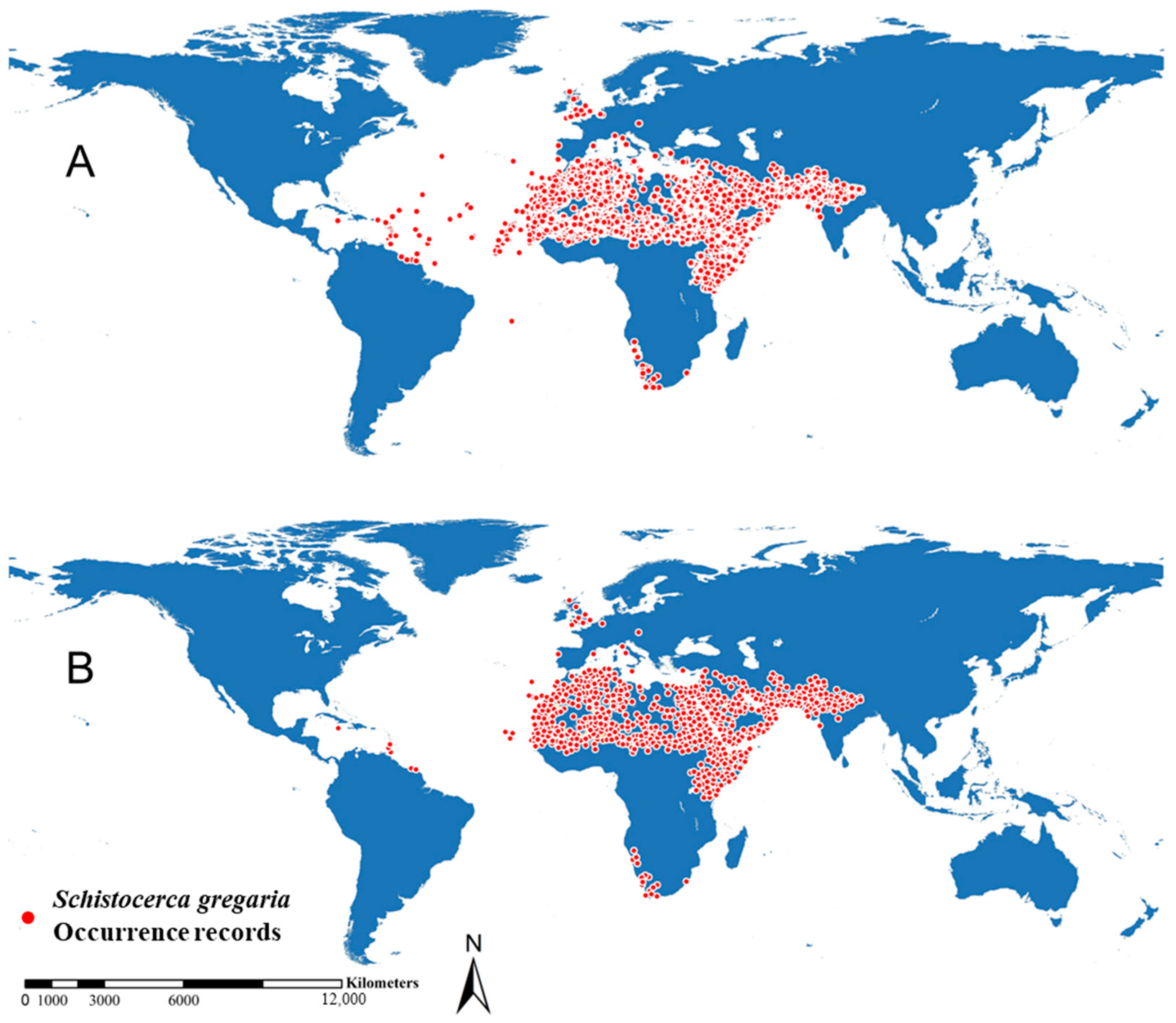
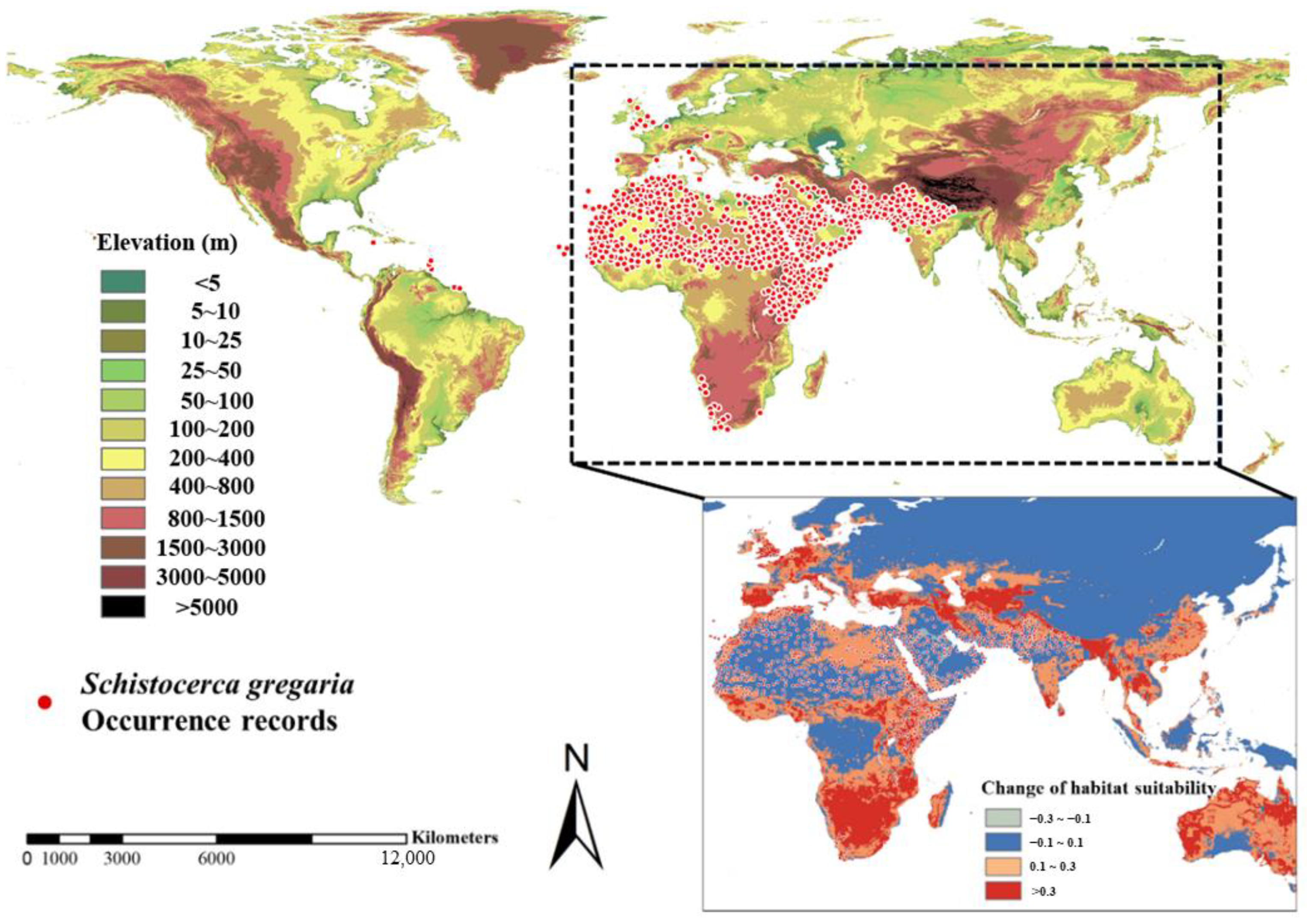
2.1. Global Occurrence Records of Desert Locust
2.2. Climate, Land Use, and Topographical Factors
2.3. Species Distribution Model
2.4. Niche Model
3. Results
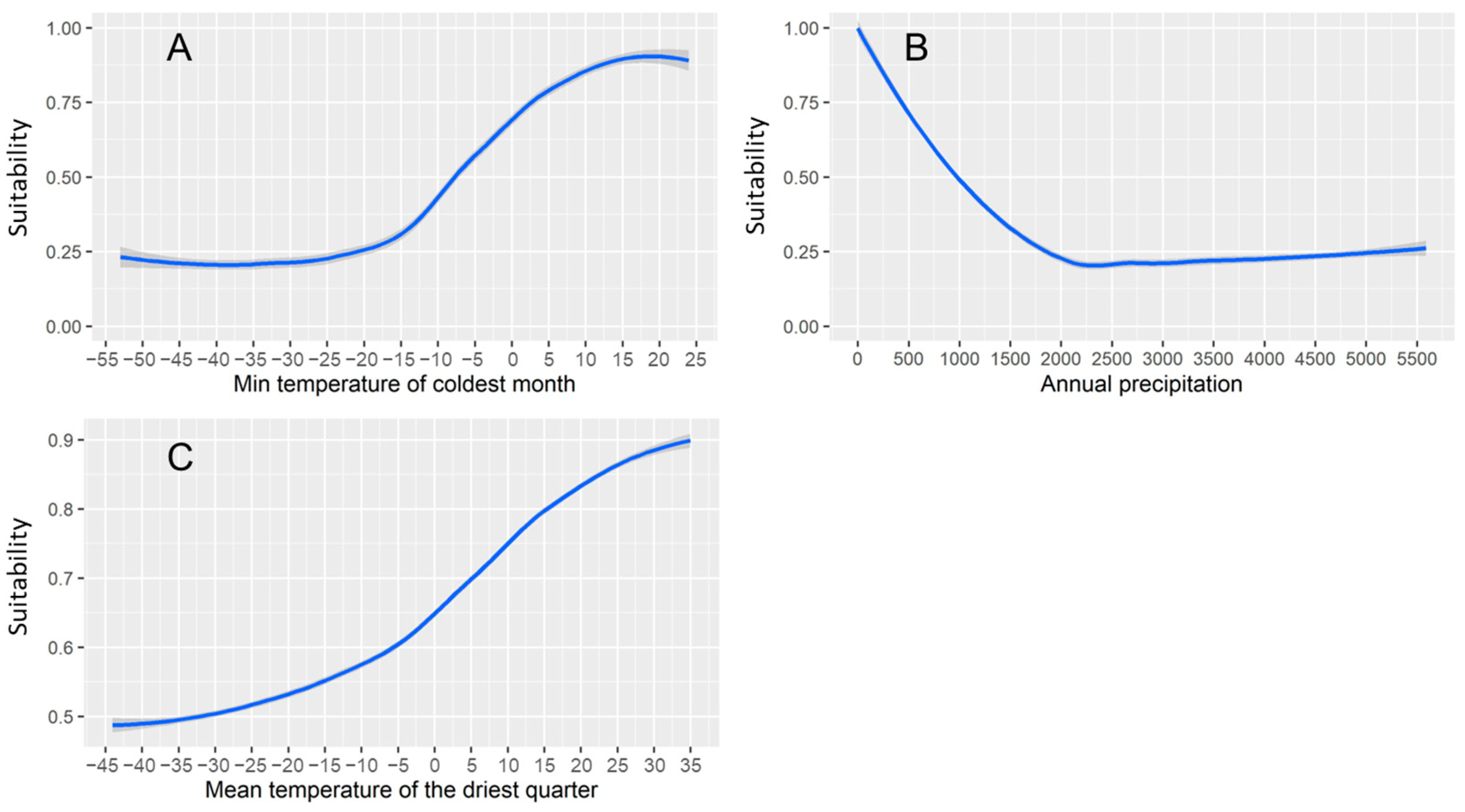
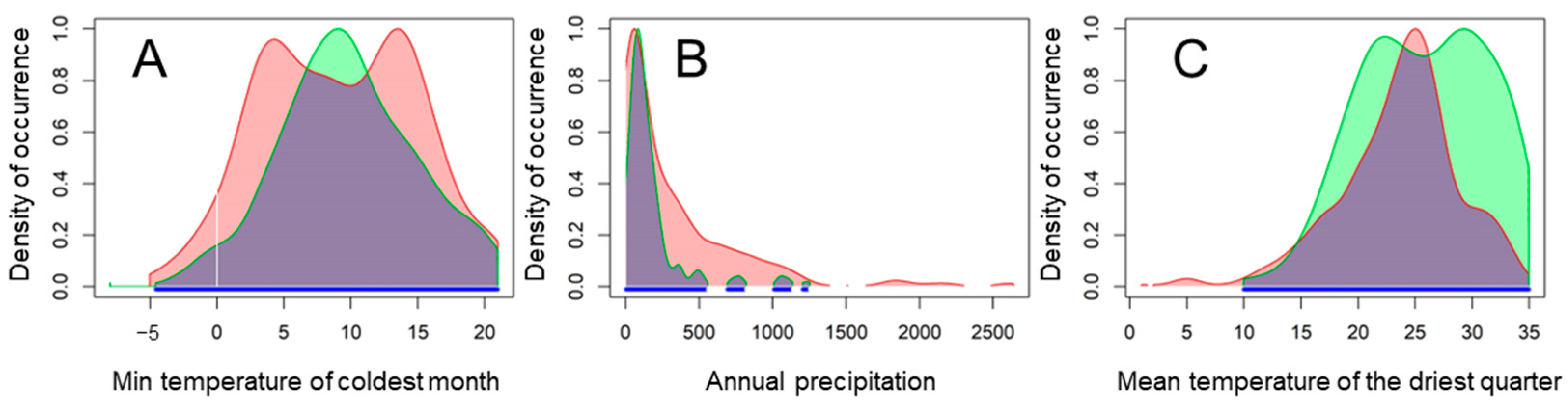
3.1. Factors Shaping Desert Locust Distribution and Response Curves of Three Main Predictors
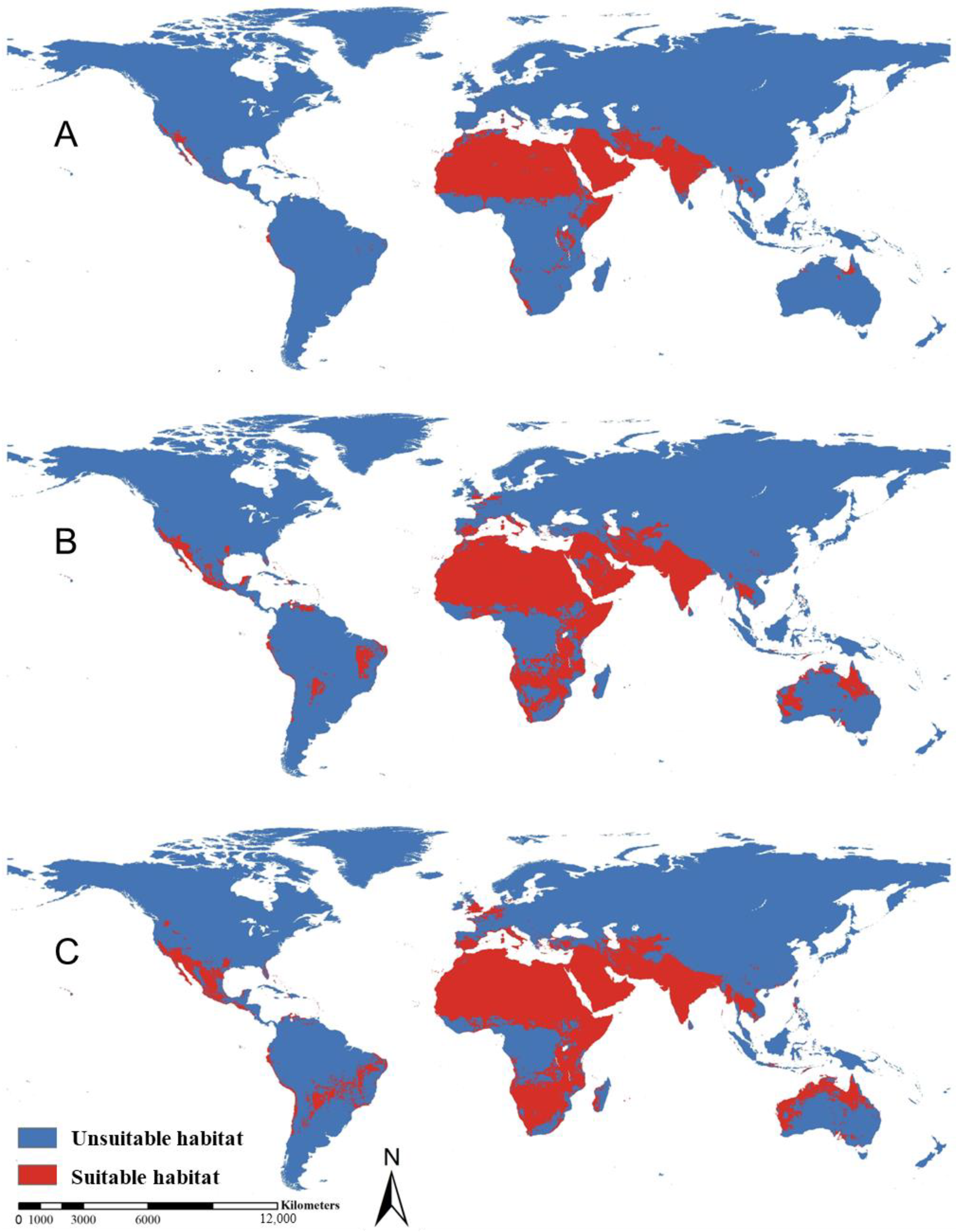
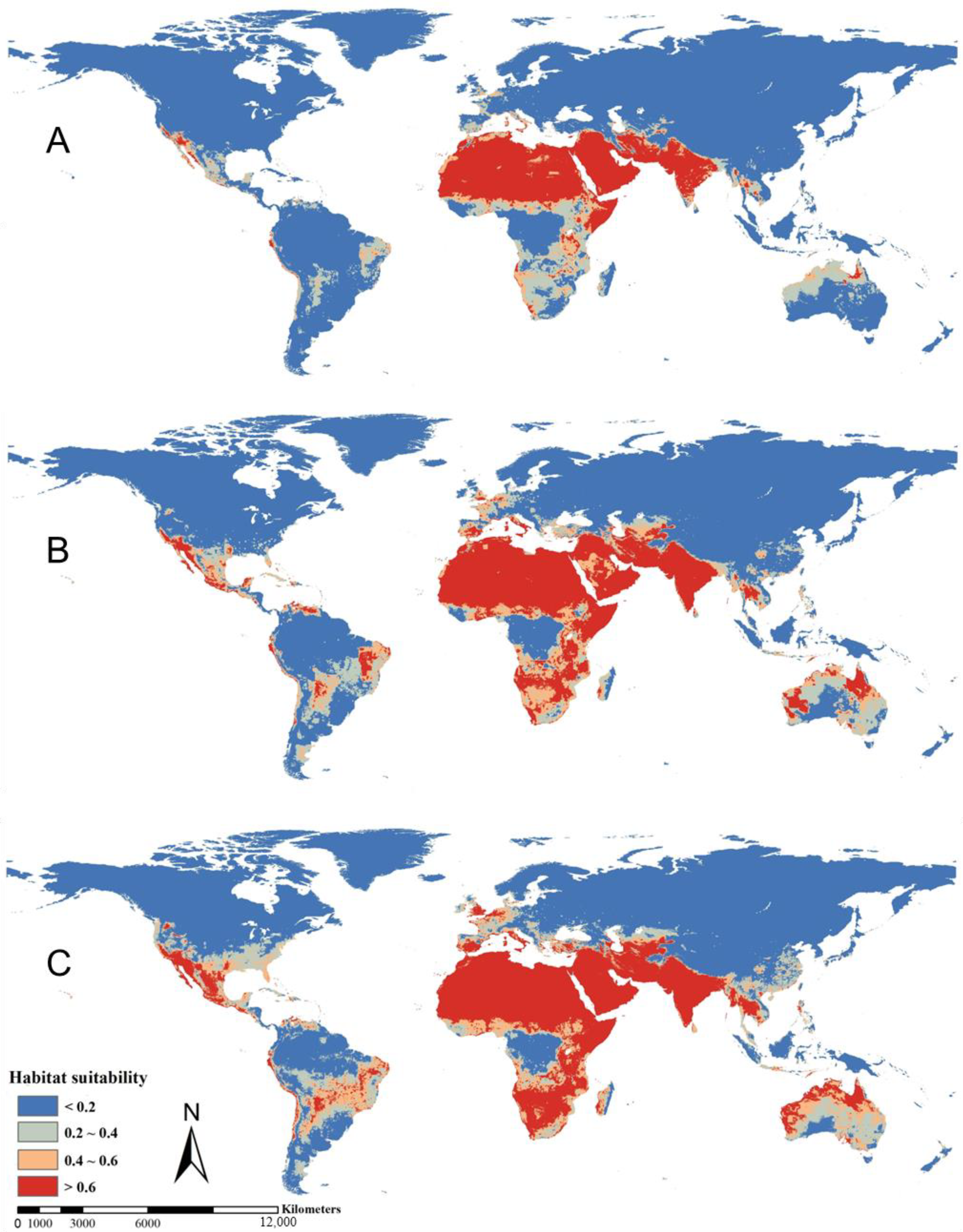
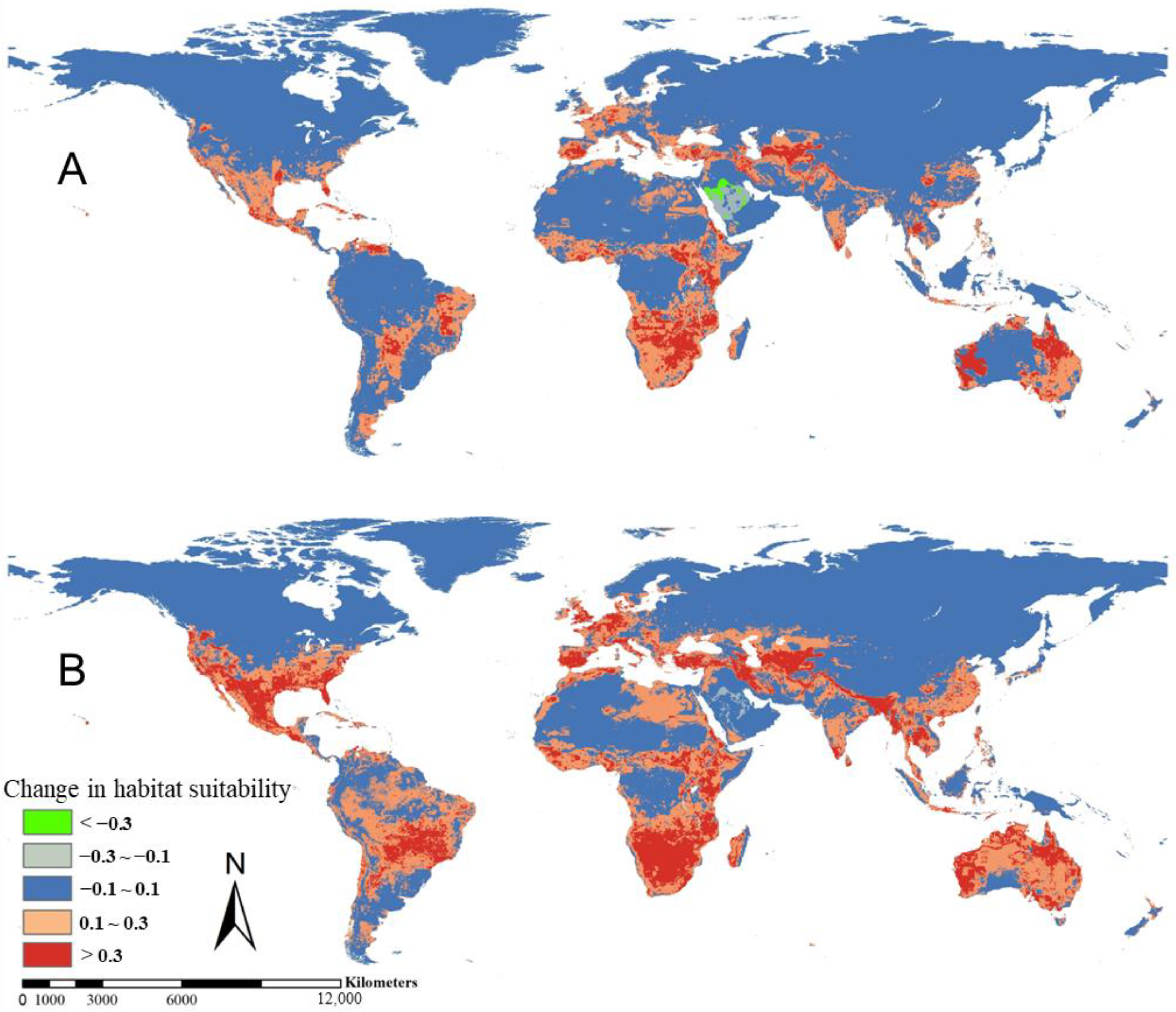
3.2. Suitable Distribution Desert Locust Area and Future Trend under Global Climate Change Scenarios
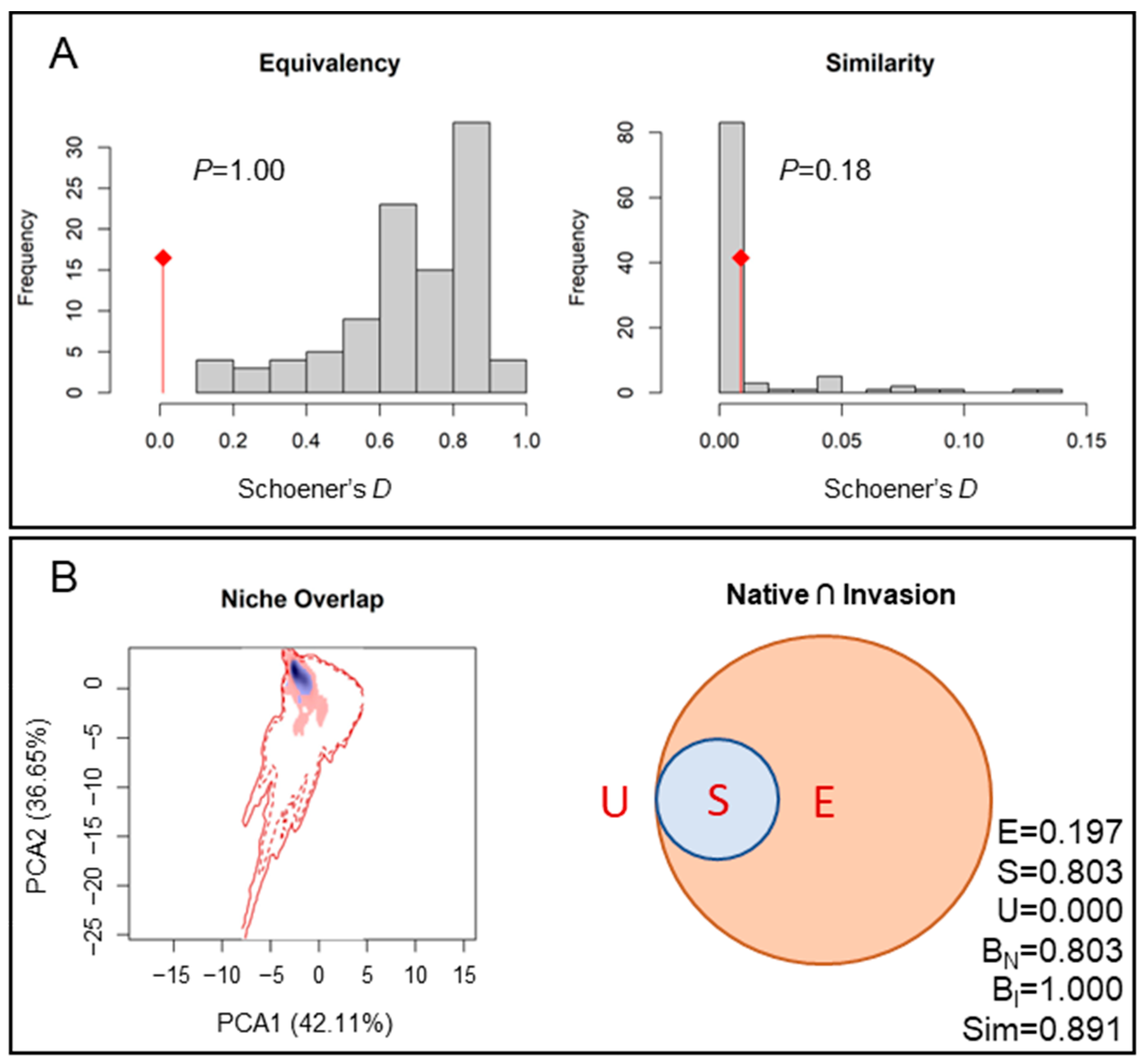
3.3. Desert Locust Niche under Climate Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021, 10, e261. [Google Scholar] [CrossRef]
- Wheeler, T.; Von Braun, J. Climate change impacts on global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Suwal, B.; Kayastha, P.; Suwal, G.; Khanal, D. Desert locust invasion in Nepal and possible management strategies: A review. J. Agric. Food Res. 2021, 5, 100166. [Google Scholar] [CrossRef]
- FAO. Desert Locust. Available online: https://www.fao.org/locusts/en/ (accessed on 20 April 2022).
- Meynard, C.N.; Lecoq, M.; Chapuis, M.P.; Piou, C. On the relative role of climate change and management in the current desert locust outbreak in East Africa. Glob. Chang. Biol. 2020, 26, 3753–3755. [Google Scholar] [CrossRef]
- Saha, A.; Rahman, S.; Alam, S. Modeling current and future potential distributions of desert locust Schistocerca gregaria (Forskål) under climate change scenarios using MaxEnt. J. Asia Pac. Biodivers. 2021, 14, 399–409. [Google Scholar] [CrossRef]
- Harrison, J.F. Gregarious locusts down-regulate muscular catabolic capacities yet fly far. Proc. Natl. Acad. Sci. USA 2022, 119, e2122086119. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global warming promotes biological invasion of a honey bee pest. Glob. Chang. Biol. 2019, 25, 3642–3655. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, J.L. Modelling desert locust presences using 32-year soil moisture data on a large-scale. Ecol. Indic. 2020, 117, 106655. [Google Scholar] [CrossRef]
- Kimathi, E.; Tonnang, H.E.; Subramanian, S.; Cressman, K.; Abdel-Rahman, E.M.; Tesfayohannes, M.; Niassy, S.; Torto, B.; Dubois, T.; Tanga, C.M.; et al. Prediction of breeding regions for the desert locust Schistocerca gregaria in East Africa. Sci. Rep. 2020, 10, 11937. [Google Scholar] [CrossRef]
- Guan, J.; Li, M.; Ju, X.; Lin, J.; Wu, J.; Zheng, J. The potential habitat of desert locusts is contracting: Predictions under climate change scenarios. PeerJ 2021, 9, e12311. [Google Scholar] [CrossRef]
- Liu, J.; Lecoq, M.; Zhang, L. Desert Locust Stopped by Tibetan Highlands during the 2020 Upsurge. Agronomy 2021, 11, 2287. [Google Scholar] [CrossRef]
- Christina, M.; Limbada, F.; Atlan, A. Climatic niche shift of an invasive shrub (Ulex europaeus): A global scale comparison in native and introduced regions. J. Plant Ecol. 2020, 13, 42–50. [Google Scholar] [CrossRef]
- Meynard, C.N.; Gay, P.E.; Lecoq, M.; Foucart, A.; Piou, C.; Chapuis, M.P. Climate-driven geographic distribution of the desert locust during recession periods: Subspecies’ niche differentiation and relative risks under scenarios of climate change. Glob. Chang. Biol. 2017, 23, 4739–4749. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.A.; Baraibar, M.; Mwangi, K.K.; Artan, G. Climate change and locust outbreak in East Africa. Nat. Clim. Chang. 2020, 10, 584–585. [Google Scholar] [CrossRef]
- Gong, X.; Chen, Y.J.; Wang, T.; Jiang, X.F.; Hu, X.K.; Feng, J.M. Double-edged effects of climate change on plant invasions: Ecological niche modeling global distributions of two invasive alien plants. Sci. Total Environ. 2020, 740, 139933. [Google Scholar] [CrossRef] [PubMed]
- Piou, C.; Bacar, M.E.H.J.; Ebbe, M.A.O.B.; Chihrane, J.; Ghaout, S.; Cisse, S.; Lecoq, M.; Halima, T.B. Mapping the spatiotemporal distributions of the desert locust in Mauritania and Morocco to improve preventive management. Basic Appl. Ecol. 2017, 25, 37–47. [Google Scholar] [CrossRef]
- Yang, R.J.; Gong, X.; Hu, X.K.; Hu, Y.W.; Feng, J.M. Global cultivation of wheat crops induces considerable shifts in the range and niche of species relative to their wild progenitors. Environ. Res. Commun. 2021, 3, 115012. [Google Scholar] [CrossRef]
- Rolland, J.; Silvestro, D.; Schluter, D.; Guisan, A.; Broennimann, O.; Salamin, N. The impact of endothermy on the climatic niche evolution and the distribution of vertebrate diversity. Nat. Ecol. Evol. 2018, 2, 459–464. [Google Scholar] [CrossRef]
- Polaina, E.; Soultan, A.; Part, T.; Recio, M.R. The future of invasive terrestrial vertebrates in Europe under climate and land-use change. Environ. Res. Lett. 2021, 16, 044004. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- McSweeney, C.F.; Jones, R.G.; Lee, R.W.; Rowell, D.P. Selecting CMIP5 GCMs for downscaling over multiple regions. Clim. Dynam. 2015, 44, 3237–3260. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araujo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Liu, T.M.; Wang, J.M.; Hu, X.K.; Feng, J.M. Land-use change drives present and future distributions of Fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sci. Total Environ. 2020, 706, 135872. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Peng, W.X.; Ma, N.L.; Zhang, D.Q.; Zhou, Q.; Yue, X.C.; Khoo, S.C.; Yang, H.; Guan, R.R.; Chen, H.L.; Zhang, X.F.; et al. A review of historical and recent locust outbreaks: Links to global warming, food security and mitigation strategies. Environ. Res. 2020, 191, 110046. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Suzuki, T.; Tanaka, S. Synchrony in the hatching of eggs in the desert locust Schistocerca gregaria (Orthoptera: Acrididae): Egg condition influences hatching time in the laboratory and under simulated field temperatures. Appl. Entomol. Zool. 2017, 52, 599–604. [Google Scholar] [CrossRef]
- Waldner, F.; Ebbe, M.A.B.; Cressman, K.; Defourny, P. Operational Monitoring of the Desert Locust Habitat with Earth Ob-servation: An Assessment. ISPRS Int. J. Geo-Inf. 2015, 4, 2379–2400. [Google Scholar] [CrossRef]
- Lazar, M.; Piou, C.; Doumandji-Mitiche, B.; Lecoq, M. Importance of solitarious desert locust population dynamics: Lessons from historical survey data in Algeria. Entomol. Exp. Appl. 2016, 161, 168–180. [Google Scholar] [CrossRef]
- Matthews, G.A. New Technology for Desert Locust Control. Agronomy 2021, 11, 1052. [Google Scholar] [CrossRef]
- Rosenberg, J.; Burt, P. Windborne displacements of desert locusts from Africa to the Caribbean and south America. Aerobiologia 1999, 15, 167–175. [Google Scholar] [CrossRef]
| Category | Predictor | Importance |
|---|---|---|
| Climatic predictors | Minimum temperature of coldest month | 0.285 |
| Annual precipitation | 0.237 | |
| Mean temperature of driest quarter | 0.113 | |
| Maximum temperature of warmest month | 0.038 | |
| Precipitation of driest quarter | 0.029 | |
| Precipitation seasonality | 0.028 | |
| Mean diurnal range | 0.011 | |
| Precipitation of coldest quarter | 0.009 | |
| Land use predictors | Fraction of potentially nonforested secondary land | 0.070 |
| Fraction of nonforested primary land | 0.048 | |
| Fraction of rangeland | 0.036 | |
| Fraction of forested primary land | 0.027 | |
| Fraction of urban land | 0.026 | |
| Fraction of managed pasture | 0.016 | |
| Fraction of cropland | 0.014 | |
| Fraction of potentially forested secondary land | 0.011 | |
| Topographical predictors | Elevation | 0.022 |
| Slope | 0.011 | |
| Aspect | 0.008 |
| Continents | Current | RCP2.6 | RCP8.5 | Difference between Current and RCP2.6 | Difference between Current and RCP8.5 |
|---|---|---|---|---|---|
| Africa | 12.98 | 17.99 | 19.37 | 5.01 | 6.39 |
| Asia | 7.13 | 8.41 | 10.12 | 1.28 | 2.99 |
| North America | 0.35 | 1.15 | 1.96 | 0.80 | 1.61 |
| Australia | 0.16 | 1.60 | 2.24 | 1.44 | 2.08 |
| South America | 0.13 | 1.25 | 1.77 | 1.12 | 1.64 |
| Europe | 0.13 | 0.68 | 1.13 | 0.55 | 1.00 |
| Total | 20.88 | 31.08 | 36.59 | 10.2 | 15.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Feng, J.; Zong, D.; Zhou, J.; Hu, X.; Wang, B.; Wang, T. Potential Spread of Desert Locust Schistocerca gregagia (Orthoptera: Acrididae) under Climate Change Scenarios. Diversity 2023, 15, 1038. https://doi.org/10.3390/d15101038
Tang Q, Feng J, Zong D, Zhou J, Hu X, Wang B, Wang T. Potential Spread of Desert Locust Schistocerca gregagia (Orthoptera: Acrididae) under Climate Change Scenarios. Diversity. 2023; 15(10):1038. https://doi.org/10.3390/d15101038
Chicago/Turabian StyleTang, Qianhong, Jianmeng Feng, Donglin Zong, Jing Zhou, Xiaokang Hu, Bingru Wang, and Tao Wang. 2023. "Potential Spread of Desert Locust Schistocerca gregagia (Orthoptera: Acrididae) under Climate Change Scenarios" Diversity 15, no. 10: 1038. https://doi.org/10.3390/d15101038
APA StyleTang, Q., Feng, J., Zong, D., Zhou, J., Hu, X., Wang, B., & Wang, T. (2023). Potential Spread of Desert Locust Schistocerca gregagia (Orthoptera: Acrididae) under Climate Change Scenarios. Diversity, 15(10), 1038. https://doi.org/10.3390/d15101038






