Abstract
Naturally occurring benzophenones represent a relatively small group of plant metabolites with narrow distribution, mainly in members of Clusiaceae, Gentianaceae, Hypericaceae, Polygalaceae, Myrtaceae, etc.; however, there were reports of several compounds derived from microorganisms belonging to the Aspergillaceae and Valsaceae families and propolis. Benzophenones exhibit many biological activities, such as antioxidant, anti-inflammatory, cytotoxic, antimicrobial, etc. Few reviews on benzophenones that have appeared in the literature were focused on their prenylated derivatives. Summarized information on structural diversity, distribution, and biological activities of simple oxygenated naturally occurring benzophenones and their glycosides has not been found in the literature. Until 2000, only benzophenone C-glycosides were known to occur in nature. Since then, many O-glycosides have been isolated, structurally, and biologically characterized. This review covers the years from 1850 to 2023 and was compiled using databases such as Chemical Abstracts, Scopus, Google Scholar, PubMed, and ResearchGate. Based on their degree of oxidation, 210 chemical structures of benzophenone derivatives and glycosides were grouped into six categories. In addition, in one group of 40 miscellaneous benzophenones, where one or several protons are replaced by a methyl, alcohol, carboxyl, or acyl group, glycosidic forms with such an aglycone and dimeric compounds with xanthone was included. Simple oxygenated benzophenones and their glycosides were found in 77 plant genera belonging to 44 families. The allergy-associated bezophenone-1, benzophenone-2 and benzophenone-3 have limited distribution across natural sources. A wide range of biological activities (antioxidant, anti-inflammatory, cytotoxic, antitumor, cytoprotective, antimicrobial, MAO-A, antiarthritic, anticholinesterase, anti-atherosclerotic, laxative, etc.) of simple oxygenated benzophenones and their glycosides that appeared in the literature were discussed.
1. Introduction
Naturally occurring benzophenones are considered compounds derived from diphenylmethanone (Figure 1), in which one or several protons are replaced by hydroxyl, alkyl, alkyloxy groups, or halogen atoms. So far, unsubstituted benzophenone has been found in nature only in Iris adriatica [1] and Hemidesmus indicus [2].
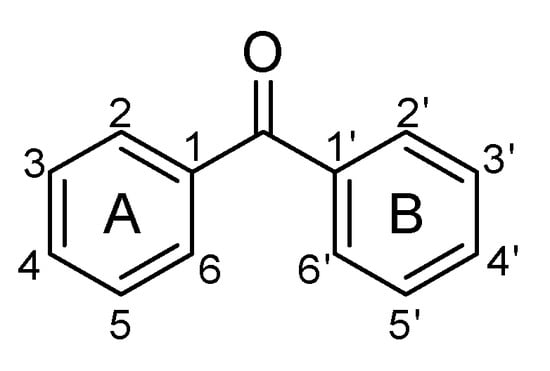
Figure 1.
The structure of unsubstituted benzophenone.
Despite the large number of isolated compounds over the past 50 years, the few published reviews focused almost entirely on prenylated benzophenones [3,4,5]. Singh and Bharate reviewed naturally occurring benzophenones with a phloroglucinol ring up to December 2005 [6]. Up to now, a systematic review of the existing naturally occurring simple oxygenated benzophenones and their glycosides has not been performed. This review compiles references from 1850 to 2023 using databases like Chemical Abstracts, Scopus, Google Scholar, PubMed, and ResearchGate. The main goal of this review is to summarize the available information on structural diversity of simple oxygenated benzophenones. A consensus classification of this group of compounds is also lacking. If the biogenesis of xanthones is considered, it can be seen that ring A and the attached CO group are produced by the shikimate pathway, while ring B is formed in the acetate–malonate pathway [7], yielding an oxygenated benzophenone intermediate, which subsequently undergoes molecular dehydration to give xanthone. Furthermore, it has been reported that the transformation can be accomplished from a benzophenone glycoside by elimination of the glycosidic moiety and subsequent dehydration [8]. Therefore, simple oxygenated benzophenones and their glycosides are precursors in the biogenesis of xanthones. Thus, the classification for the biosynthetically related xanthones provided by Mandal et al. [9] is used in this review. Special attention is given to benzophenone C- and O-glycosides. The second goal of this review is to summarize the published information on the distribution of simple oxygenated benzophenones and their glycosides. Some synthetic hydroxy and methoxy benzophenones are heavily used in sunscreens and in personal care cosmetic products and both associated with allergy and photoallergy [10]. This review tries to answer which of these compounds are found in natural sources. This review’s third task is to summarize the biological properties of this class of naturally occurring compounds.
2. Structural Diversity of Simply Oxygenated Benzophenones
2.1. Monooxygenated Benzophenones
Only two naturally occurring benzophenones (Figure 2), which have a hydroxyl or methoxy group in para position to carbonyl function, were reported in the literature. p-Hydroxybenzophenone 1 was isolated from 1,2-dichloroethylene extract of Talauma mexicana (DC.) G.Don leaves [11] and p-methoxybenzophenone 2 was found as a component of the essential oil of Costus speciosus (J.Koenig) Sm. [12].
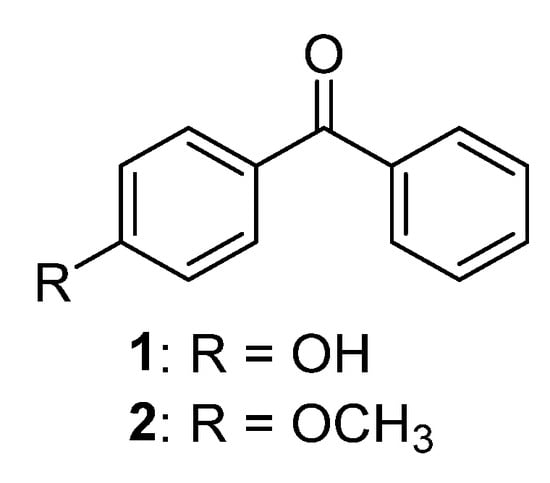
Figure 2.
The structures of monooxygenated benzophenones.
2.2. Dioxygenated Bezophenones
The literature survey showed three compounds, 3–5 (Figure 3), isolated from the endophytic moss Polytrichastrum formosum (Hedw.) G.L. Smith [13], while 6 was found in Pogonatum inflexum (Lindb.) Sande Lac. [14]. The nitrogen-containing derivatives of 2,4-dihydrobenzophenone 7 and 8 were isolated from the ethanol extract of the moss Polytrichum commune Hedw. [15]. Compounds 9–11 were found in Polytrichastrum formosum [13,16] and compound 12 was detected in Pogonatum inflexum [14]. All of these compounds possess 2,4-dioxygenation pattern only in one of the phenyl rings. O-glycosidic forms of the deoxygenated benzophenones have not been discovered so far, but there was a report of a C-glycoside, namely hyperinone 13, isolated from Hypericum styphelioides A.Rich. [17].
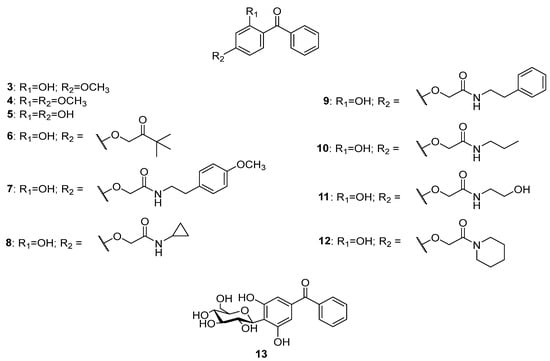
Figure 3.
The structures of dioxygenated benzophenones.
2.3. Trioxygenated Bezophenones
The first reports of isolated trioxygenated simple benzophenones date from nearly a century and a half ago, when Jobst and Hesse began to study the composition of Coto and Paracoto barks. Although the origin of the two barks remains still unknown, it can be safely assumed that they belong to the Lauraceae family [18]. The first isolated compound was cotoin 14 (Figure 4), which was obtained from a diethyl ether extract of Coto bark [19,20]. Hydrocotoine 15 and methylhydrocotoine 16 were subsequently isolated from an alcoholic extract of Paracoto bark [20]. More than a decade later, thanks to the outstanding work of Ciamiciani and Silber, the structures of these compounds were finally established [21,22,23,24]. 2,4,6-Trihydroxybenzophenone 17 and 2,4-dihydroxy-6-methoxybenzophenone 18 were found to be major components in the methanol extract of leaves and stems of Helichrysum triplinerve DC. [25].
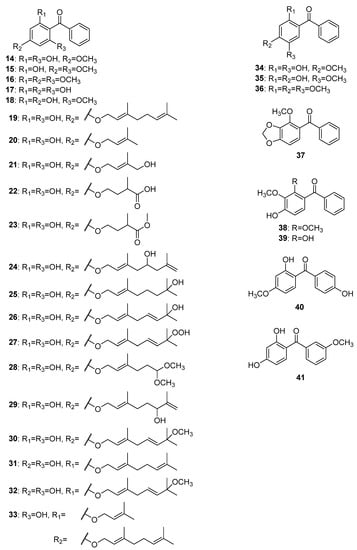
Figure 4.
The structures of hydroxy, methoxy, and alkyloxy derivatives of trioxygenated benzophenones.
Most of the simply oxygenated naturally occurring benzophenones with 2,4,6-oxygenation patterns were alkyloxy derivatives. Sixteen compounds from this group have been reported to date, fourteen of which have been found in species of the genus Hypericum. The first alkyloxy benzophenone 19 was discovered in 1978 by Bohlmann and Suwita in the root of Leontonyx squarrosus DC and aerial parts of L. spathulatus Less. [26]. Eleven years later, four more compounds, 20–23, belonging to this group were isolated from Helichrysum asperum (Thunb.) Hilliard and B.L.Burtt [27]. Phytochemical studies of the aerial parts of Hypericum sampsonii Hance led to the isolation of seven new compounds, 24, 25 [28]; sampbenzophenones D-G (26–29) [29]; and 33 [30]. The compounds hyperinokone 30, elegaphenone 31, and hyperinagasone 32 were isolated from H. nokoense Ohwi [31], H. elegans Steph. ex Willd. [32] and H. nagasawae Hayata [33], respectively. In 1968, the isolation of a trioxygenated benzophenone cearoin 34 from extracts of Dalbergia cearensis Ducke and D. miscolobium Benth. was reported [34]. It was the first natural benzophenone with 2,4,5-trioxygenation pattern. In addition, two compounds with the same oxygenation pattern, 35 and 36, were isolated from D. odorifera T. C. Chen [35] and Securidaca longipedunculata Fresen [36], respectively. Two benzophenones, 37 and 38, possessing 2,3,4-trioxygenation patterns were isolated from S. inappendiculata Hassk [37]. In addition, compound 39 with the same oxygenation was isolated from the dichloromethane extract of S. diversifolia (L.) Blake [38]. The only two trioxygenated benzophenones with oxygenation on both rings were 40 and 41 isolated from Tolpis webbii Sch. Bip. [39] and Securidaca longepedunculata Fresen. [36], respectively.
Fourteen glycosides of trioxygenated benzophenones (Figure 5) mostly sharing a common 2,4,6-trioxygenation pattern have been reported to date. The first tryoxygenated C-glycoside malaferin A 42 was isolated from an ethanolic extract of twigs and leaves of Malania oleifera Chun and S.K.Lee [40], while a di-C-glycoside arrilanin G 43 was obtained from an ethanolic extract of the stem bark of Polygala rillate Buch.-Ham. ex D.Don [41]. 2,4,6-Trihydroxybenzophenone 17 was the most common aglycon of the O-glycosides of the of trioxygenated benzophenones. Garcimangosone D 44 was its first O-glycoside isolated from the ethanol extract of Garcinia mangostana L. fruits [42]. Furthermore, four 6-O-biosides of 17 including tricornoside A 46 (from Polygala tricornis Gagnep.) [43], pyrafortunoside B 47, and C 48 (from Pyracantha fortuneana (Maxim.) H. Li) [44] as well as piperwallioside A 49 (from Piper wallichii (Miq.) Hand.-Mazz.) [45] were isolated from the respective species. Two 4-O-glycosides of 17 (50 and 51) were isolated from the ethanol extract of the Psidium guajava L. leaves [46,47]. The phytochemical investigations of Polygala tenuifolia Willd. led to the isolation of three O-glycosides of 2,4-dihydroxy-6-methoxybenzophenone 18, namely tenuiside B 52 and C 45 [48] as well as the O-bioside tenuiphenone A 53 [49]. In addition, a O-bioside 54 of 4-hydroxy-2,6-dimethoxybenzophenone was found in P. sibirica var. megalopha Franch. [50]. An O-glycoside pruniflonone B 55 having an unusual aglycone (3,4,5-trihydroxybenzophenone) was isolated from the methanol extract of Cratoxylum formosum (Jack) Dyer roots [51].
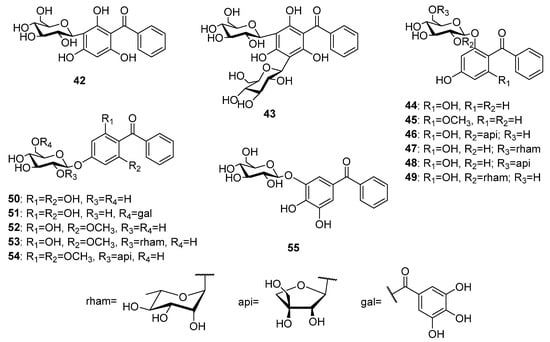
Figure 5.
The structures of C- and O-glycosides of trioxygenated benzophenones.
2.4. Tetraoxygenated Bezophenones
This group includes eighteen hydroxy and methoxy tetraoxygenated benzophenones (Figure 6). Only three compounds (56–58) have substituents on ring A but not in B and share a common 2,3,4,5-tetraoxygenation pattern. Compounds 56 and 57 were isolated from the wood of Machaerium scleroxylon Tul. [52,53], while 58 was found in the roots of Securidaca longipedunculata [54]. Four compounds (59–62) share a common phloroglucinol oxygenation pattern in ring A and a monooxygenation in ring B. The first occurrence in nature of compounds 59–62 was reported for Morus alba L. [55], Aniba duckei Kosterm. [56], Gentiana lutea L. [57], and for Allanblackia floribunda Oliv. [58], respectively. Compounds 63–65 possess a rare 2,4,5-trioxynation pattern of ring A and structurally differ by the position of the hydroxyl group in ring B. The former two compounds, 64 and 65, were isolated from the heartwood of Dalbergia melanoxylon Guill. and Perr. [59,60] while the latter 66 was found in D. cochinchinensis Pierre [61]. Compounds 66–69 shared a common pyrogallol-like ring A and monooxygenated ring B. These compounds were reported as the first to occur in biogenetically different plant species: 66 in Securidaca diversifolia [38]; 67 in Anemarrhena asphodeloides Bunge [62]; 68 in Oroxylum indicum (L.) (L.) Benth. ex Kurz [63]; and 69 in Garcinia mangostana [64]. Compounds 70–73 possess di-oxygenation on both rings and were found in Garcinia cantleyana Whit. var. cantleyana [65], Hypericum lanceolatum Lam. [66], Garcinia eugenifolia Wall. ex T. Anderson [67], and Syzygium polyanthum (Wight) Walp. [68], respectively.
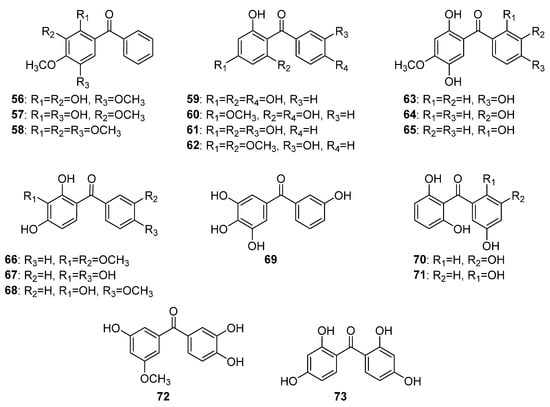
Figure 6.
The structures of hydroxy and methoxy tetraoxygenated benzophenones.
To date, 24 C-glycosides of tetraoxygenated benzophenones (Figure 7) have been isolated from the plant sources. The aglycone of most of these compounds is iriflophenone 59. The first occurrence of iriflofenone-3-C-β-D-glucoside 74 was reported for the leaf extracts of the ferns Hypodematium fauriei (Kodama) Tagawa and H. crenatum (Forssk.) Kuhn [69]. Two phytochemical studies on mango tree bark and leaves led to the isolation of the galloyl esters 75–77 [70,71] and one p-hydroxybenzoyl ester 78 [71] of 74. Further galloyl and p-hydroxybenzoyl esters 79–84, as well as an ester of 3,4-dihydroxy-5-methoxybenzoic acid 85, were isolated from the ethanol extract of the leaves of Mangifera indica [72,73]. A mixed C,O-diglycoside 86 was isolated from water extract of aerial parts of Cyclopia genistoides (L.) R.Br. [74]. A di-C-glycoside 87 of iriflophenone 59 was isolated from the leaves extract of Aquilaria sinensis (Lour.) Spreng. [75]. Five C-glycosides 88–92 of 2,4′,6-trihydoxy-4-methoxybenzophenone 60 were found in leaves of Mangifera indica [72,73]. The only C-galactosyl benzophenone 93 known up to now was isolated from the leaves of the mango tree [76]. The only C-glycoside of 2,6,3′-trihydroxy-4-methoxybenzophenone rhodanthenone C 94 was isolated from a methanolic extract of the aerial parts of Gentiana rhodantha Franch. ex Hemsl. [77]. The di-C-glucosides 95 and 96 were isolated from Polygala tenuifolia [49] and P. glomerata Lour. (P. chinensis L.) [78], respectively. Di-C glucoside pseuduvarioside 97 was isolated from the leaves extract of Pseuduvaria fragrans Y.C.F.Su, Chaowasku and R.M.K.Saunders [79].
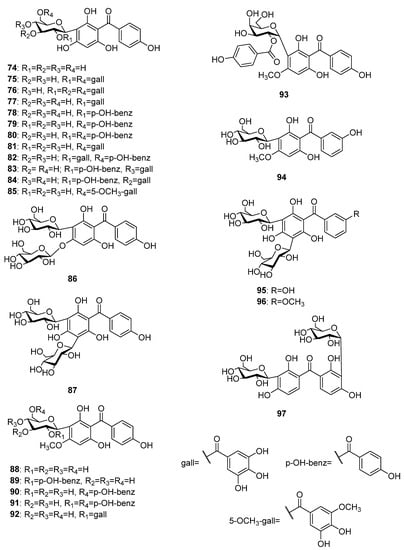
Figure 7.
The structures of C-glycosides of tetraoxygenated benzophenones.
Some C-glycosyl tetraoxygenated benzophenones (Figure 8) form rare spirocyclic systems. The first compound from this group was aquilarinoside A 98, which was isolated from hydroethanolic extract of Aquilaria sinensis leaves [80]. Compound 99, similar to 98 but with β-configuration of fructofuranose, was isolated from hydroethanolic extract of mango tree leaves [76]. In addition, 100 and 101 were found in leaf extract from the later plant [81,82]. Compounds 102 and 103 were isolated from the later plant source differed from 99 and 101, respectively, by the cyclization type of fructose unit [76].
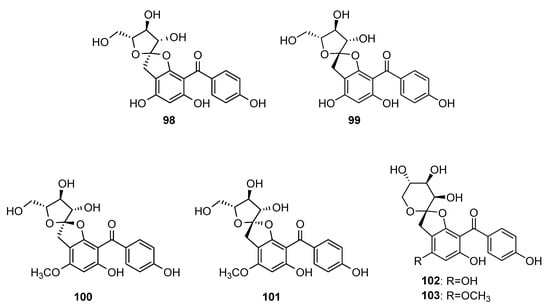
Figure 8.
The structures of spirocyclic C-glycosides of tetraoxygenated benzophenones.
More than twenty O-glycosides of tetraoxygenated benzophenones (Figure 9) have been isolated, so far. Most of these compounds (104–121) share iriflophenone 59 as their aglycone. The first O-glycoside of iriflophenone 104 was isolated from Coleogyne ramosissima Torr. [83]. Three acylated derivatives, 105–107, of 104 were found in the leaves of Planchonella obovata (R.Br.) Pierre [84]. Iriflophenone-2-O-α-L-rhamnoside 108 and its acylated derivatives 109–112 were reported for Aquilaria sinensis leaves and flower buds [75,85,86]. The methyl ester 113 of the later compound was isolated from the pericarps of A. yunnanensis S. C. Huang [87]. The presence of five biosides 114–118 of iriflophenone 59 in A. sinensis was established [85,88]. In addition, a 2-O-β-D-xylopyranoside 119 and a 2-O-α-L-arabinopyranoside 120 of 59 were reported for A. sinensis [86] and Mangifera indica [76], respectively. The only glycoside 121 with sugar connected to position 4 of iriflophenone was found in Davallia solida (G.Forst.) Sw. rhizomes [89]. Three compounds, 122–124, possess 2,4′,6-trihydroxy-4-methoxybenzophenone 60 as aglycone. The former compound 122 was isolated from the aerial parts of Gnidia involucrata Steud. ex A.Rich. [90]. The latter two compounds 123 and 124 were reported for nut shell of Phaleria macrocarpa (Scheff.) Boerl. fruits [91] and for roots of Vangueria agrestis (Schweinf. ex Hiern) Lantz [92], respectively. Mahkoside A 125 was isolated from the ethanolic extract of Phlaleria macrocarpa pits [93]. The tetraoxygenated benzophenone 61 was an aglycone of three glycosides, 126–128, that were isolated from Gentiana rhodantha Franch. ex Hemsl. [77], G. verna L. subsp. pontica (Soltok.) Hayek [94] and Garcinia mangostana fruit pericarp [95], respectively. The tetraoxygenated benzophenone O-glycosides 129–132 that possess unique aglycones were found in Gentiana verna subsp. pontica [94], Tripterospermum japonicum (Siebold and Zucc.) Maxim. [96], Cratoxylum formosum [97], and Anemarrhena asphodeloides [98], respectively.
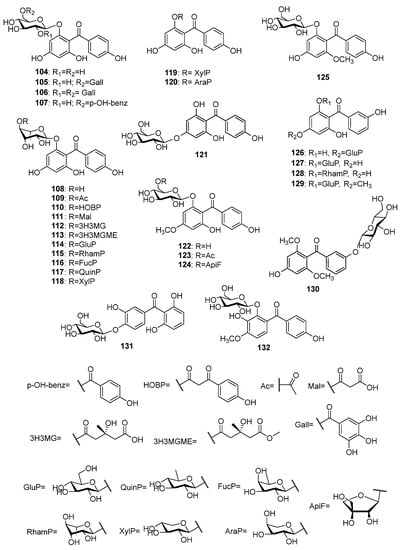
Figure 9.
The structures of O-glycosides of tetraoxygenated benzophenones.
2.5. Pentaoxygenated Benzophenones
More than 20 non-glycosylated (aglycones) naturally occurring pentaoxygenated benzophenones (Figure 10) representing seven oxygenation patterns have been reported, so far. Maclurin 133 was the firstly found pentaoxygenated benzophenone possessing 2,3′,4,4′,6-pentaoxygenation pattern. It was isolated in 1850 by Wagner from Maclura tinctoria (L.) Steud. [99,100] but its structure was finally established in 1895 by Ciamician and Silber [101]. Methoxy derivatives 134–138 of maclurin were found in Garcinia subelliptica Merr. [102], G. multiflora Champ. ex Benth. [103], G. mangostana [104], G. livingstonei T.Anderson [105], and G. mangostana [106], respectively. Compounds 139 and 140 were the only methylenedioxy-benzophenones with 2,3′,4,4′,6-pentaoxygenation pattern originally isolated from Aniba pseudocoto bark [20,22]. Compounds 141 and 142 were found in Garcinia subelliptica [107] and G. smeathmannii (Planch. and Triana) Oliv. [108], respectively, and shared a common 2,2′,3′,4,6-pentaoxygenation pattern. Compounds 143–148, possessing a common 2,3′,4,5′,6-pentaoxygenation pattern, were originally isolated from G. pedunculata Roxb. ex Buch.-Ham. [109], G. mangostana [106], G. hombroniana Pierre [110], G. speciosa Wall. [111], Hypericum annulatum Moris [8], and H. sampsonii [112], respectively. The rare compounds 149 and 150 share common ring A and differ in ring B; both were isolated from Dalbergia melanoxylon [59,113]. Compounds 151–153 possess a common 3,3′,4,5,5′-pentaoxygenation pattern. The former 151 and 152 were isolated from the twigs of Garcinia cantleyana var. cantleyana [65] while the later 153 was found in the pericarp of mangosteen (G. mangostana) [114]. The sole compound 154 with 2,3,3′,4,4′-pentaoxygenation pattern was isolated from the Securidaca diversifolia roots [38].
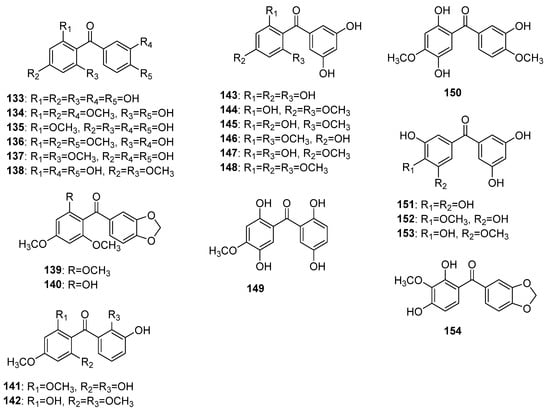
Figure 10.
The structures of hydroxy and methoxy pentaoxygenated benzophenones.
More than fifteen C-glycosylated derivatives of the pentaoxygenated benzophenones (Figure 11) have been found in plants, so far. Most of these compounds possess a 2,3′,4,4′,6-pentaoxygenation pattern. The first report for naturally occurring C-glycosylated pentaoxygenated benzophenones dated from 1984 when Tanaka and coworkers isolated maclurin-3-C-β-D-glucoside 155 along with its galloyl and p-hydroxybenzoyl esters 156–159 from the Mangifera indica leaves [70]. Further galloyl esters 160 and 161 [71] as well as methoxy derivatives 162 [81] and 163 [72] of 155 were found in the same plant source. Additional methoxy derivatives 164–166 of 155 were isolated from the aerial parts of Polygala telephioides Willd. [115,116]. The only di-C-glycoside 167 and C,O-glycoside 168 of pentaoxygenated benzophenones were isolated from an ethanolic extract of P. glomerata [78]. Compound 169 is the only benzophenone C-glycoside with 2,3′,4′5′,6-pentaoxygenation pattern that was isolated from the aerial parts of Hypericum humifusum L. ssp. austral Rouy et Foue [117]. C-glycosyl benzophenones 170 and 171 share common ring A with 2,3,5,6-oxygenation patters and differ in the hydroxyl place in ring B. The former was isolated from Gnidia involucrata [90] while the later was found in Polygala telephioides [115].
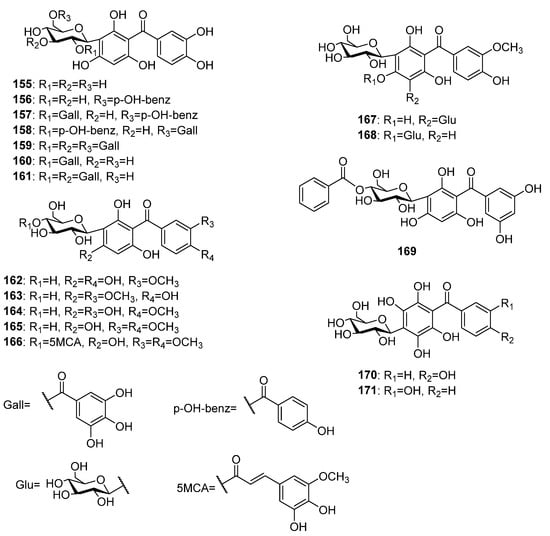
Figure 11.
The structures of C-glycosides of pentaoxygenated benzophenones.
Over 30 O-glycosides of pentaoxygenated benzophenones (Figure 12) have been reported to occur in plant species, so far. Contrary to C-glycosides, only six O-glycosides with 2,3′,4,4′,6-pentaoxygenation patterns 172–177 have been encountered. Maclurin-6-O-β-D-glucopyranoside 172 was simultaneously isolated from Gentiana verna subsp. pontica [94] and from G. rhodantha [77]. Compounds 173, 174, and 177 were isolated from Dobinea delavayi (Baill.) Baill. roots [118,119], while 175 and 176 were found in aerial parts of Polygala tenuifolia [48] and Garcinia livingstonei [105], respectively. The vast majority of O-glycosides (178–205) possess aglycones with 2,3′,4,5′,6-pentaoxygenation pattern and glycosylation at position 6(2), 4 or 3′(5′). The arabinofuranosides 178 and 179 were isolated from the aerial parts of Hypericum annulatum [120], while 180–183 were found in H. thasium Griseb. [121,122]. The 6(2)-O-rhamosides 187 and 188 were isolated from H. elegans [123] and H. pseudopetiolatum Keller, respectively. In addition, compounds 189–191 were also found in later species [124]. Further acylated rhamosides 192 and 193 of 143 were isolated from the aerial parts of H. seniawinii Maxim. [125]. The 6(2)-O-rhamnosides 194–196 of 145 were found in aerial parts of H. wightianum Wall. ex Wight et Arn. [126]. Only three O-xylopyranosides 197–199 of penataoxygenated benzophenones were originally isolated from H. thasium [121], while only two O-arabinopyranosides 200 and 201 were found in H. humifusum [117]. Compounds 202 and 203 isolated from the later plant [117] possess glycosylation at position 4, while 204 and 205 were found in H. sampsonii [112] and H. ellipticum Hook. [127], respectively, the sugar was attached at position 5′(3′) position. The compounds 206 and 207 share rare 2,2′,4,5′,6-pentaoxygenation patterns that were isolated from H. annulatum [8] and Garcinia mangostana [128], respectively.
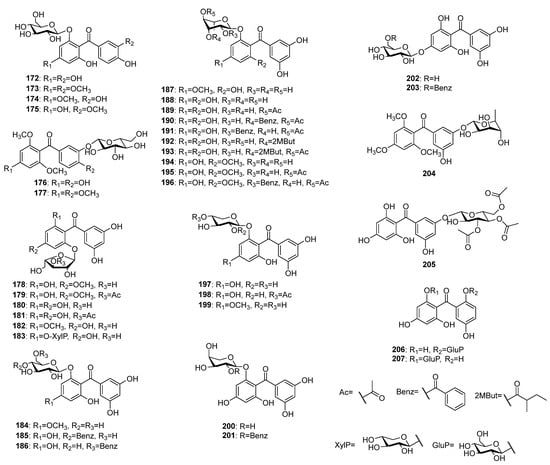
Figure 12.
The structures of O-glycosides of pentaoxygenated benzophenones.
2.6. Hexaxygenated Benzophenones
Hexaxygenated benzophenones were rarely found in nature. To date, only three compounds, 208–210 (Figure 13), have been isolated from Garcinia mangostana [128,129].
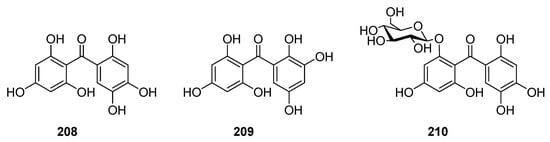
Figure 13.
The structures of hexaoxygenated benzophenones.
2.7. Uncategorized and Miscellaneous Benzophenones
In addition to the compounds shown so far, there are data on benzophenone derivatives (Figure 14) in which one or several protons are replaced by a methyl, alcohol, carboxyl, or acyl group, and dimeric compounds with xanthone have also been found. Some glycosides of this class benzophenones have been detected, as well. The first records of this type of benzophenone appeared in 1963 when 6-hydroxy-2,4-dimethoxy-3-methylbenzophenone 211 and its isomer 2-hydroxy-4,6-dimethoxy-3-methylbenzophenone 212 were isolated from the essential oil of Leptospermum luehmannii F.M.Bailey [130]. Eighteen years later, bis(2-hydroxy-4,6-dimethoxy-3-methylphenyl)methanone 213 was found in a benzene extract of the wood of Qualea lubouriauana [131].
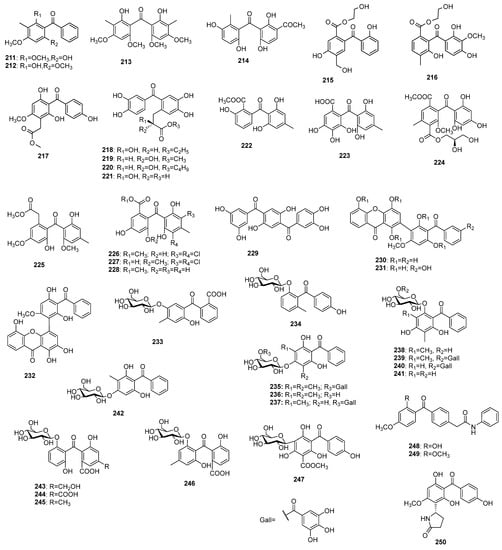
Figure 14.
The structures of uncategorized and miscellaneous benzophenones.
For the first time, beishouwubenzophenone 214 was isolated from root tubers of Cynanchum auriculatum Royle ex Wight [132]. Morintrifolin A 215 and B 216 were obtained from hydromethanolic extract of Morinda citrifolia L. roots [133]. Compound 217 was found in an extract of the Anemarrhena asphodeloides roots [134]. The adducts of 3,3′,4,4′-trihydroxybenzophenone, and 2-hydroxypropionic acid 218–221 were isolated from Ranunculus ssp. The former three compounds were found in R. ternatus DC. [135,136], while the later was obtained from R. muricatus Moench [137]. The benzophenones 222–228 have been isolated from fungi mostly belonging to Ascomycota group. Nidulalin B 222 was found in a dichloromethane extract of Emericella nidulans var. lata (Thom and Raper) Subram. [138], while cytosporaphenone A 223 was obtained from an ethyl acetate extract of Cytospora rhizophorae Kohlm. and E. Kohlm. [139]. Wentiphenone A 224 was discovered in the ethyl acetate extract of Aspergillus wentii Wehmer [140], while 225 was isolated from the fermentation broth of A. fumigatus Fresen. SWZ01 [141]. A halogenated benzophenone 226 was discovered in an extract of A. flavipes PJ03-11 [142], while 227 and 228 were found in A. terreus Thom [143] and A. wentii [144], respectively. Garcihombrianone 229, which was found in the roots of Garcinia hombroniana Pierre [145], can be viewed as two benzophenone molecules sharing a common benzene nucleus. Three benzophenone-xanthone dimers 230–232 were isolated from roots of G. dulcis (Roxb.) Kurz [146]. Benzophenone O-glucosides 233 and 234 were found in Mitracarpus villosus (Sw.) DC. [147] and Dobinea delavayi [119]. Seven benzophenone O-glucosides 235–241, some of which were galloyl esters, were isolated from the leaves or fruits of Psidium guajava [47,148,149,150], while 242 was found in P. littorale Raddi [151]. Compounds 243 and 244 were isolated from Cassia senna var. angustifolia L. [152], while 245 and 246 were found in C. abbreviata Oliv. [153]. The only C-glycoside 247 of this class of miscellaneous benzophenones was isolated from Aquilaria sinensis [154]. Two N-containing benzophenones 248 and 249 were found in the moss Pogonatum spinulosum Mitt. [155], while 250 was detected in Anemarrhena asphodeloides [62].
3. Distribution of Naturally Occurring Simply Oxygenated Benzophenones
The distribution of the benzophenones from Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13 and Figure 14 is provided in Table 1. Up to now, simple oxygenated benzophenones and their glycosides have been established in 77 plant genera distributed in 44 families, from which the family Corallinaceae belong to the division Rhodophyta; the family Polytrichaceae to division Bryophyta; the families Davalliaceae, Dryopteridaceae, and Hypodematiaceae to the division Polypodiophyta; and 36 families from the division Magnoliophyta. The simply oxygenated benzophenones are isolated from various plant organs, mainly from aerial parts, leaves, roots, rhizomes, barks, and wood of angiosperms. They are less commonly found in stems, twigs, flowers, and fruits of angiosperms; the thallus of mosses and leaves; and rhizomes of ferns. There are only a few cases in which the compound from this group is obtained using essential oils or a thallus of algae. Of all 250 reported compounds, the largest number of representatives 51 were isolated from the Hypericaceae family, followed by 43 representatives from Anacardiaceae, 36 from Clusiaceae, 24 from Polygalaceae, 22 from Thymelaeaceae, and 18 from Fabaceae. In addition, there are data on the isolation of one compound from Nepalese propolis and seven from microorganisms belonging to the Aspergillaceae and Valsaceae families.

Table 1.
Distribution of naturally occurring simply oxygenated benzophenones.
It should also be noted that the least numerous groups of simply oxygenated benzophenones are those with mono- and hexaoxygenation. The former group includes only two representatives isolated from Talauma mexicana (Magnoliaceae) and Costus speciosus (Costaceae), while the later consists of three representatives obtained from Garcinia mangostana (Clusiaceae). Dioxygenated benzophenones number 11 compounds, one of which was isolated from Hypericum styphelioides (Hypericaceae), while the remaining 10 were found in mosses from Polytrichaceae. Trioxygenated benzophenones comprise 42 compounds detected in species of Clusiaceae (1 compound), Olacaceae (1), Piperaceae (1), Fabaceae (2), Rosaceae (2), Myrtaceae (2), Lauraceae (3), Asteraceae (8), Hypericaceae (11), and Polygalaceae (11). The most numerous groups are those of pentaoxygenated and tetraoxygenated benzophenones. The former group consists of 75 representatives found in members of Gentianaceae (1), Moraceae (1), Thymelaeaceae (1), Lauraceae (2), Fabaceae (2), Polygalaceae (8), Anacardiaceae (12), Clusiaceae (16), and Hypericaceae (32) while the latter include 77 compounds isolated from species of Annonaceae (1), Rosaceae (1), Davalliaceae (1), Rubiaceae (1), Myrtaceae (1), Bignoniaceae (1), Lauraceae (1), Moraceae (1), Hypodematiaceae (2), Asparagaceae (2), Hypericaceae (2), Sapotaceae (3), Polygalaceae (4), Clusiaceae (5), Gentianaceae (5), Fabaceae (6), Thymelaeaceae (17), and Anacardiaceae (23).
The allergy-associated benzophenones 3 (bezophenone-3 or BP-3), 5 (benzophenone-1 or BP-1), and 73 (benzophenone-2 or BP-2) [10] have limited distribution across natural sources. The former two compounds were found only in Polytrichastrum formosum while the later was detected only in Syzygium polyanthum. The origin of these compounds is uncertain: whether they are a result of biosynthesis or environmental pollution.
4. Biological Properties of Naturally Occurring Simply Oxygenated Benzophenones
Biological and pharmacological studies on simple oxidized benzophenones and their glycosides have established various activities, among which the most prominent are antioxidant, anti-inflammatory, cytotoxic, and antimicrobial. Additionally, their influence on α-glucosidase, fatty acid, and triglyceride metabolism has also been reported. Up to the beginning of 2023, 250 compounds have been isolated or detected, of which ca 150 have been pharmacologically tested, which is 60% of all.
4.1. Antioxidant Activities
4.1.1. DPPH Radical Scavenging Activity
The results of in vitro radical scavenging activity assays using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical showed moderate to strong activity for 31 [205,209,250], 55 [51], 61 [169], 133 [103], 137 [51,105], 145 [169], 147, 206 [205,209,250], 208, and 210 [129]. Low or very low radical-scavenging activity was found for 44 [176], 59 [76], 78, 79 [81], 87 [251], 93 [76], 98, 120 [81], 142 [108], 162, 163 [81], 176 [105], 178, 179, 184 [205,209,250], 225 [141], 238, and 241 [232].
4.1.2. ABTS Radical Scavenging Activity
The radical scavenging activity of the benzophenones was confirmed in vitro using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) radical cation-based assays. The tested compounds possessed moderate to strong radical scavenging activities. Compound 13 showed moderate activity (1.93 mM TE) compared to the activity of the reference antioxidant compounds quercetin (3.33 mM TE) and rutin (2.78 mM TE) [17]. Annulatophenone 147 (91.7%) and its O-glycosides annulatophenonoside 178 (93.35%) and acetylannulatophenonoside 179 (85.9%) possessed significantly strong ABTS radical scavenging activity, which is comparable to that of ascorbic acid (96.2%) [209]. The results of another study showed that neoannulatophenonoside 184 (IC50 = 0.25 µM) showed the highest ABTS activity, while hypericophenonoside 206 (IC50 = 41.52 µM), elegaphenonoside 187 (IC50 = 87.17 µM), and elegaphenone 31 (IC50 = 379.85 µM) possessed moderate to lower activity compared to hyperoside (IC50 = 6.61 µM) and BHT (IC50 = 0.08 µM) [250]. Moreover, compounds 145 (IC50 = 4.92 µM), 135 (IC50 = 4.60 µM), and 61 (IC50 = 9.9 µM) displayed higher inhibition of ABTS•+ than Trolox (IC50 = 10.9 µM) [169]. The results showed that iriflophenone-3-C-glucoside 74 (1.04 µM TE) scavenged ABTS•+ radicals [252], while 142 (IC50 > 100 µg/mL) possessed lower activity [108]. Additionally, guavinoside D 241 (IC50 = 29.27 µg/mL) and guavinoside E 238 (IC50 = 32.97 µg/mL) displayed moderate activity compared to vitamin C (IC50 = 8.53 µg/mL) [232].
4.1.3. FRAP (Ferric Reducing Antioxidant Power) Assay
FRAP activities of compounds annulatophenonoside 178, acetylannulatophenonoside 179, annulatophenone 147 (0.1 mM) were compared to BHT and Vit C at the same concentration. In contrast to both glycosides, only their aglycone 147 possessed moderate activity (6.9 mM TE) [209]. Furthermore, compound 31 displayed (942.16 µM TE) stronger FRAP activity compared to the control hyperoside (421.75 µM TE) [250]. Additionally, iriflophenone 3-C-β-D-glucoside 74 (1.2 mmol Fe2+/g) and iriflophenone-3,5-C-β-D-diglucoside 87 (0.2 mmol Fe2+/g) showed lower FRAP activity as compared to Trolox (8.0 mmol Fe2+/g) [251].
4.1.4. Inhibition of Lipid Peroxidation
The inhibition of lipid peroxidation of compounds (0.1 mM) compounds 31, 206, 184, and 187 were determined in a linoleic acid system using the ferric thiocyanate (FTC) method. Only neoannulatophenonoside 184 inhibited the oxidation of linoleic acid for five days. All tested benzophenones demonstrated lower antioxidant activity compared to BHT [250]. The antioxidant activity 4-geranyloxy-2,6-dihydroxybenzophenone 19 (70%) was assessed by monitoring the fluorescence decay of Fe2+-induced oxidation of a model liposome system [207], while compound 205 exhibited 61% inhibition of Fe2+-induced lipid peroxidation by using large unilamellar vesicles (LUVs) [127]. Additionally, 151 inhibited the copper-mediated oxidation of LDL (low-density lipoprotein) with IC50 = 3.6 µM, which was comparable to that of the positive control probucol (0.6 µM) [65].
4.2. Antiallergic Activity
In the mast cell degranulation experiment, cearoin 34 displayed significant inhibitory effects on the release of β-glucuronidase (IC50 = 17.9 µM) and histamine (IC50 = 16.3 µM) from rat mast cells compared to the positive control mepacrine (IC50 = 22.3 µM for β-glucuronidase and IC50 = 14.7 µM for histamine). The results suggested that 34 could be a promising antiallergic agent [253].
4.3. Immunosuppressive Activity
Compounds 100 and 101 estimated a good inhibition of concanavalin A-induced spleen cell proliferation in a concentration-dependent manner (10 to 40 μM). At a concentration of 40 μM, 100 (31.92% ± 3.84) and 101 (31.67% ± 3.43) had a similar immunosuppressive activity lower than the activity of positive control dexamethasone (56.99% ± 4.22) at the same concentration [82].
4.4. Anti-Inflammatory Activity
Rat neutrophil degranulation showed the significant inhibitory ability of cearoin 34 on the release of β-glucuronidase and lysozyme with IC50 values of 7.9 µM and 11.7 µM, respectively, compared to a positive control of trifluoperazine (IC50 = 16.9 µM for β-glucuronidase and 12.8 µM for lysozyme) [253]. Moreover, 34 showed significant concentration-dependent inhibition of NO (nitric oxide) production in lipopolysaccharide (LSP)-activated macrophage-like J774.1 cells with better IC50 = 15.4 µM value than the positive control NG- monomethyl-L-arginine (L-NMMA) (IC50 = 27.1 µM) [249]. The anti-inflammatory activities were also investigated by measuring NO production in LSP-induced RAW 264.7 cells. The results showed that 50 (10 µmol/L) [46], 34 (IC50 = 11.7 μM) [254], 148 (IC50 = 2.40 µM), 204 (IC50 = 2.29 µM), 188 (IC50 = 2.00 µM) compared to the positive control cadamonin (IC50 = 1.41 µM) [112], and 44 (IC50 = 17.23 µM, compared to indomethacin IC50 = 15.20 µM) [255] possessed significant inhibitory activity against NO production. Additionally, the results showed that iriflophenone-2-O-α-L-rhamnoside 108 were able to reduce significantly NO production in RAW 264.7 cells stimulated by LPS/IFN-ɣ at low concentration (6.25 μg/mL) [243] and possessed IC50 = 19.22 μM in LPS-treated RAW 264.7 cells [222]. Some of the tested benzophenones showed moderate NO inhibitory activities on LSP-induced RAW 264.7 macrophage cells such as hydrocotoin 15 with an inhibition rate of 38.12% [256] and compounds 60, 78, 79, 88, 98, 120, 122, 162, at concentrations of 50 and 100 μM [81]. Additionally, low NO inhibitory activities on LSP induced RAW 264.7 macrophage cells displayed benzophenones aquilarinenside E 109 (200 μg/mL), 74 (10, 25 and 200 μg/mL) [243,251], 19 (IC50 = 23.41 µM), 33 (IC50 = 6.23 µM) [30], 30 (IC50 = 30.05 µM) [31], 32 (IC50 = 26.14 µM) [33], 192, 193 (IC50 > 20 µM) [125], 93 (IC50 > 100 µM), 59 (IC50 = 58.72 µM) [76], 95 (16.97%) [256], and 113 (IC50 = 95.4 µM) [87]. The anti-neuroinflammatory activity of 186 was evaluated by determining its ability to inhibit the production of NO in LPS-activated BV-2 microglial cells. The results showed that 186 displayed significant anti-neuroinflammatory activity (IC50 = 0.61 µM) [211]. Garcimangosone D 44 also exhibited high anti-inflammatory activities in LPS-stimulated BV-2 microglial cells (IC50 = 14.52 μM) and LPS-stimulated THP-1 macrophage cells (IC50 = 19.14 μM) compared to indomethacin (IC50 = 17.64 μM and IC50 = 19.37 μM), respectively [255]. Furthermore, iriflophenone 59 (IC50 = 52.59 µM) and aquilarinoside A 98 (IC50 = 89.92 µM) showed significant inhibitory activity against neutrophil respiratory burst stimulated by PMA [80], while hydrocotoin 15 (IC50 = 11.03 µM) showed strong inhibitory effects on PGE2 production in RAW 264.7 macrophage cells [256]. In addition, the cyclooxygenase assay of 31 estimated a 13% inhibition of the COX-1 enzyme and 48% for COX-2 at a concentration of 25 µg/mL [207].
4.5. Estrogenic Activity
Compounds 108 (IC50 = 630 μM) and 59 (IC50 = 700 μM) showed significant binding abilities to the estrogen receptor (ERα) at 1 mM concentration compared to positive control of 17β-estradiol (IC50 = 18 nM). Furthermore, 108 and 59 exhibited 80% and 68% inhibition, respectively, of labeled estradiol binding to Erα at 1 mM [248].
4.6. Cytotoxic and Antitumor Activities
4.6.1. Cytotoxicity on Human Cancer Cell Lines
Cytotoxicity assays performed on cell lines HD-MY-Z (Hodgkin’s lymphoma), K-562 (chronic myeloid leukemia), KE-37 (T-cell leukemia), CCRF-CEM (acute lymphoblastic leukemia), HL-60 (myeloid leukemia), and Raji cells (non-Hodgkin’s lymphoma) showed significant antiproliferative activity of elegaphenone 31 with IC50 values of 15.9 µM (HD-MY-Z), 13.9 µM (K-562), 16.9 µM (KE-37) [32], and 108 with IC50 = 8.9 μM (HL-60) [222]. Furthermore, hyperinagazone 32 exhibited IC50 = 29.05 μM on CCRF-CEM [33], hyperinokone 30 showed IC50 = 36.44, while 19 had IC50 = 27.04 [31]. In addition, a short-term in vitro assay with 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced Epstein–Barr virus early antigen (EBV-EA) activation in Raji cells showed a significant inhibitory effect of maclurin 133, compared to a positive control of the strong antitumor promoter glycyrrhetic acid (enoxolone) [168]. Assay performed on the MCF-7 cell line (breast adenocarcinoma) showed significant cytotoxicity of 19 with an IC50 value of 4.8 µg/mL [180]. Very low cytotoxicity on the MCF-7 cell line showed benzophenones 15 (IC50 > 200 µM) [119], 145 (inhibition < 10% at 20 µM) [169], 146 (IC50 = 119.3 μg/mL) [175], sampbenzophenones D–G 26–29 (IC50 > 40 µM) [29], 7 (IC50 > 100 µM), 8 (IC50 > 100 µM) [15], 172 (IC50 > 100 µM), 127 (IC50 > 100 µM), 143 (IC50 > 100 µM), and 44 (IC50 > 100 µM) [257]. Weak cytotoxicity against MDA-MB-231 cells was reported for garcimangosone D 44 (IC50 = 67.20 ± 1.19 µM) [255], hyperprzeone A 19 (IC50 = 123.87 µM), and 71 (IC50 > 200µM) [210]. Another study showed that 135 (ED50 = 2.56 µM) and 145 (ED50 = 6.91 µM) displayed significant cytotoxic activity against BCA-1 cell line (human breast cancer) [111]. Additionally, treatment of MCF-7 and T-47D (infiltrating ductal carcinoma of the breast) cell lines with 59 was reported to result in increased cell proliferation in a concentration-dependent manner with EqE10 values of 0.7 µM and 4.9 µM for MCF-7 and T-47D cell lines, respectively [213]. Melanoxoin 150 showed high cytotoxicity against Ca9-22 (gingival carcinoma), with an IC50 value of 0.46 μg/mL [201]. An analysis performed on the HCT-116 cell line (colon cancer with a mutation in codon 13 of the ras-protooncogene) showed a significant cytotoxicity of 31 (IC50 = 8.2 µg/mL) [207], 208 (IC50 = 1.9 μM), and 210 (IC50 = 1.8 μM) [129]. The results also showed that compounds 239 (IC50 = 60 µM) and 235 (IC50 = 80.3 µM) inhibited HCT-116 cells growth up to 81.4% and 66.2% at dose of 100 μM, respectively [258]. Low cytotoxicity on the HCT-116 cell line was reported for 248, 249 (IC50 > 50 μM) [155], 7, 8 [15], 9, 10 [16], 44, 172, 127, 143 with IC50 values > 100 μM [257]. Furthermore, very low cytotoxic activity against the HT-29 (colorectal adenocarcinoma with epithelial morphology) cell line possessed garcimangozone D 44 (IC50 > 150 μM) [176]. Moreover, moderate to low activity against cellular Col-2 line (human colon cancer) was reported for compounds 135 (ED50 = 11.76 µM) and 145 (ED50 = 20.85 µM) compared to the positive control ellipticine (ED50 = 2.43 µM) [111]. Assays performed on HepG2 cell line (liver cancer) showed high cytotoxicity for 12 [15], 11 (IC50 = 38.14 μM) [13], 250 (IC50 = 153.1 nM), 217 (IC50 = 3161 nM), and 67 (IC50 = 1764 nM). Additionally, the last four compounds were compared to the positive control 5-fluorouracil (IC50 = 5659 nM) [62]. Moderate cytotoxicity against HepG2 cell line displayed 145 (IC50 = 9.81 µM) and 138 (IC50 = 19.41 µM) compared to the positive control sorafenib (IC50 = 2.66 µM) and doxorubicin (IC50 = 3.07 µM). [106]. Furthermore, very low cytotoxic activity possessed benzophenones 174, 234, 121 [119], 248 (IC50 > 50 μM), 249 (IC50 > 50 μM) [155], and 44, 172, 127, 143 [257]. Furthermore, 250 (IC50 = 180.6 nM), 217 (IC50 = 6250 nM), 96 (IC50 = 1182 nM), and 67 (IC50 = 2274 nM) showed significant cytotoxicity against Hep3B (liver cancer with epithelial morphology) cells compared to the positive control 5-fluorouracil (IC50 = 51709 nM) [62]. Additionally, compound 71 showed moderate cytotoxic against SMMC-7721 cells, with IC50 values of 46.91 µM compared to the positive control cisplatin (IC50 = 12.49 µM) [210]. A significant and moderate cytotoxic effect against KB (human oral nasopharyngeal carcinoma) cells was reported for 135 (ED50 = 2.02 µM) and 145 (ED50 = 14.12 µM), respectively, compared to the positive control ellipticine (ED50 = 2.19 µM) [111]. Cytotoxicity assays performed on AGS (gastric adenocarcinoma) and SGC-7901 (human gastric carcinoma) cell lines showed an IC50 value for 31 was 12.4 µg/mL (AGS) [207], for 241 and 238 were >10 µg/mL (SGC-7901) [232] and 44 (IC50 = 107.73 µM) (SGC-7901) compared to cisplatin (IC50 = 15.74 µM) [255]. The cytotoxicity study of 145 on U2OS (human osteosarcoma with epithelial morphology) cell line demonstrated 27% cell death at 72 h [169]. Benzophenone 210 possessed a significant cytotoxic potential towards A549 (human alveolar basal epithelial cell adenocarcinoma) (IC50 = 1.4 μM), while 208 exhibited moderate activity with IC50 = 2.3 μM in comparison to doxorubicin (IC50 = 0.6 μM) [129]. Compounds 135 (ED50 = 5.68 µM) and 145 (ED50 = 12.34 µM) showed a significant and moderate cytotoxic potential, respectively, against Lu-1 (human lung cancer) cells compared to ellipticinec (ED50 = 2.11 µM) [111]. Benzophenone 19 (4.4 µg/mL) demonstrated significant cytotoxic activities towards H460 (non-small cell lung cancer) [180], while alternative study showed IC50 = 52.13 µM. In the later research compound 71 (IC50 = 97.13 µM) showed low cytotoxicity against the same cell line compared to cisplatin (IC50 = 8.78 µM) [210]. Garcimangosone D 44 displayed strong cytotoxic activity against A-375 cell line (human malignant melanoma) with an IC50 value of 24.67 µM compared the positive control cisplatin (IC50 = 6.61 µM) [255]. Additionally, 19 (IC50 = 100.21 µM) and 71 (IC50 = 63.67 µM) showed weak cytotoxic activity against A375 [210]. Cytotoxicity assays performed against cell lines SHSY-5Y (neuroblastoma), IMR-32 (neuroblastoma with rare areas of organoid differentiation) demonstrated IC50 values of 16.83 µM (IMR-32) for compound 32 compared to doxorubicin (IC50 = 0.037 µM) [33] and IC50 values of 25.54 µM and 69.36 µM (SHSY-5Y) for 19 and 71, respectively, compared to cisplatin (IC50 = 4.00 µM) [210]. In addition, 44 was found to possess high and selective cytotoxicity (IC50 = 27.70 µM) against SHSY-5Y cells compared to cisplatin (IC50 = 5.14 µM) [255]. Benzophenone 145 (at 20 µM) was reported to cause 49% cell death after 72 h treatment in DBTRG (human glioblastoma) cell line [169], while 205 inhibited the proliferation of SF-268 (human brain glioblastoma/astrocytoma) cell line by 73% at a concentration of 25 μg/mL [127]. Cytotoxicity of 19 was reported to be high against SF-268 cells at a concentration of 2.0 µg/mL [180]. Garcimangosone D 44 was reported to exhibit cytotoxicity against the SiHa (human cervical squamous cell carcinoma) cell line with an IC50 value of 64.83 µM compared to a positive control cisplatin (IC50 = 9.87 µM) [255]. The cytotoxicity test against PANC-1 cell line (pancreatic carcinoma with epithelial morphology) showed that pogonatone C 248 possessed high activity with an IC50 value of 9.2 µM, while pogonatone D 249 had moderate activity with an IC50 of 28.3 µM compared to vinblastine (IC50 = 2.7 µM) [155].
4.6.2. Cytotoxicity on Animal Cell Lines and Models
The brine shrimp lethality test (BSL) showed high toxicity for maclurin 133 (LD50 = 43.1 µM) [103]. Benzophenone O-glucoside 122 demonstrated cytotoxicity in a dose-dependent manner with an IC50 value of 83 μg/mL against the NS-1 (mouse myeloma) cell line [245] and inhibitory ability with an IC50 of 5.1 μg/mL against L1210 (mouse leukemia) cell line [259]. Aquilaside B 111 and C 112 were found to show moderate cytotoxicity against SK-MEL (mammalian amelanotic cutaneous melanoma) cells with an IC50 value of 17.0 and 12.0 μg/mL, respectively, compared to doxorubicin (IC50 = 1.6 μg/mL) [86]. Furthermore, the assay against ASK (rat glioma cell) showed that 135 also possessed moderate cytotoxic activity with an ED50 value of 9.95 µM compared to ellipticine [111].
4.7. Cytoprotective Effects
The benzophenones 147, 178, 179, 184, and 206 were tested for hepatoprotective effect against carbon tetrachloride toxicity in isolated rat hepatocytes. The results indicated that 147, 178, and 179 showed weaker toxic effects compared to CCl4 and in combination exhibited statistically significant protection against the toxic agent [260]. In addition, the benzophenone O-glycosides 178, 179, 184, and 206 exhibited cytoprotective effects in a model of epirubicin-induced cellular toxicity in K-562 cells. The cytoprotective potential was concentration-dependent and more pronounced at the higher concentration of 25 µM with IC50 values of 1.45–3.51 µM. Furthermore, benzophenones 178, 179, and 184 showed an ability to ameliorate epirubicin-induced anti-clonogenic effects on bone marrow cell colony-forming units in vitro [205]. It was found that compounds 43, 95, 96, 167, and 168 at a concentration of 10−5 mol/mL showed hepatoprotective activity against D-galactosamine-induced toxicity in WB- F344 rat hepatic epithelial stem-like cells [78]. Furthermore, guavinoside B 235 was reported to exhibit significant activity on acetaminophen (APAP)-induced liver injury in vitro and in vivo. In vitro, at a concentration of 30 μM, 235 significantly reduced intracellular ROS levels in HepG2 cells. Additionally, in vivo pretreatment with compound 235 (100 mg/kg/day) significantly alleviated hepatocyte infiltration and necrosis in C57BL/6 mice, improved serum and liver biochemical parameters such as ALT, AST, SOD, GSH, ROS, MDA as well as TNF-α levels [261]. It was reported that iriflofenone-3,5-C-β-diglucoside 87 at a very low concentration (1 ng/mL) showed protective activity (81.77% cell viability) on cultured P19-derived neurons compared to the control condition (52.81% cell viability). The results also showed that compound 87 could significantly increase the number of neurites (3.77 branches) and stimulate the outgrowth of the cultured neurons (64.62 µm) compared with the control (number of neurites: 1.71 branches; length: 40.97 µm) [262]. The protective effects of hyperwightin E 196 and petiolin G 189 against corticosterone-induced PC12 cell injury were assessed. The tested benzophenones exhibited noticeable neuroprotection at 10 μM. They significantly increased the cell survival rate from 58.57% (for the model) to 67.89% and 66.23%, respectively, compared to positive control desipramine (cell viability of 72.33%) [126].
4.8. Antimicrobial Activity
4.8.1. Antibacterial Activity
The benzophenones 19 (MIC = 12.5 µg/mL) [180] and 31 (MIC = 5–12.5 µM) [263] were reported to have inhibitory activity against Gram-positive Staphylococcus aureus. Iriflofenone-3-C-β-D-glucoside 74 (MIC = 62.5 µg/mL) also exhibited strong antibacterial activity against S. aureus in comparison to cefixime, a well-known antibiotic (MIC = 62.5 µg/mL) [157]. Moderate antibacterial activities possessed mitraphenone A 14 (MIC = 50 µM) compared to moxifloxacin [147] and cearoin 34 (with 15 mm inhibitory zone) compared to a positive control imipenem (with 30 mm inhibitory zone) [230]. Weak antibacterial activity against S. aureus was reported for 142 (MIC = 128 µg/mL) [108], 44 (MIC = 250 µg/mL), 241 (MIC = 400 µg/mL) [150], 60 (MIC = 1800 µg/mL), 122 (MIC = 1800 µg/mL) [264], and melannoin 63 (MIC = 3.1 mg/mL) [60]. Weak antibacterial activity against Bacillus subtilis was also reported for 74 (MIC = 125 µg/mL) [157], 34 (with 12 mm inhibitory zone) [230], 60 (MIC = 1800 µg/mL), and 122 (MIC = 1800 µg/mL) [264]. Elegaphenone 31 showed good antibacterial activity against Enterococcus faecalis (MIC = 7.5 µM) and E. rivorum (MIC = 12.5 µM) [263]. Mitraphenone A 233 [147] and garcimangosone 44 [176] showed moderate and weak antibacterial activity against E. faecium with MIC = 50 μM and MIC > 128 μg/mL, respectively. Significant antibacterial activity against Mycobacterium tuberculosis was found for 219 (MIC = 41.67 µg/mL) compared to rifampicin as a reference drug (MIC = 2.08 µg/mL). Additionally, the combination (1:1) of 219 and gallic acid (MIC = 20.83 µg/mL) was better than that of 219 alone [136]. Furthermore, the results also showed that 233 (MIC > 50 μM) [147] possessed moderate activity, while 220 (MIC = 266.67 µg/mL) exhibited weak antibacterial activity against M. tuberculosis [136]. Additionally, 19 was reported to exhibit significant activity at a concentration of 12.5 µg/mL against M. smegmatis [180]. Iriflofenone-3-C-β-D-glucoside 74 (MIC = 62.5 µg/mL) exhibited strong antibacterial activity against Gram-negative bacteria Escherichia coli in comparison to cefixime (MIC = 62.5 µg/mL) [157]. Weak and very weak antibacterial activity against E. coli was reported for garcimangosone D 44 (MIC = 650 µg/mL), guajaphenone A 241 (MIC = 900 µg/mL) [150], 60 (MIC = 900 µg/mL), and 122 (MIC = 900 µg/mL) [264]. A moderate antibacterial activity against Pseudomonas aeruginosa was determined for cearoin 34 (16 mm inhibitory zone) compared to a standard drug imipenem (with 24 mm inhibitory zone) [230]. In addition, it was found that elegaphenone 31 enhanced the elimination of intracellular P. aeruginosa in macrophages exposed to subinhibitory concentrations of the fluoroquinolone antibiotic norfloxacin [263]. Furthermore, 37 (4 mm inhibitory zone) showed weak antibacterial activity compared to gentamicin (15 mm inhibitory zone) [237]. In addition, very weak activity against P. putida was found for 60 and 122 with an MIC value of 900 µg/mL [264]. Cearoin 34 exhibited antibacterial activity against Salmonella typhi with an inhibitory zone of 18 mm compared to a standard drug imipenem (25 mm) [230]. To date, antibacterial activity against Klebsiella pneumoniae has been reported only for iriflofenone-3-C-β-D-glucoside 74, which showed significant activity with an MIC value of 31.1 µg/mL comparable to the well-known antibiotic cefixime (MIC = 31.1 µg/mL) [157].
4.8.2. Antimycotic Activity
Significant antimycotic activity against Aspergillus fumigatus has been reported for 37 with an inhibitory zone of 11 mm compared to ketoconazole (inhibitory zone of 16 mm) [237]. Compounds 242 (MIC = 25 µg/mL) and 237 (MIC = 25 µg/mL) exhibited better antimycotic activity against Candida glabrata than the positive control fluconazole (MIC = 50 µg/mL). The results also showed that compound 50 (MIC = 50 µg/mL) showed the same antimycotic activity as the positive control. Furthermore, compound 242 (MIC = 25 µg/mL) showed better antimycotic activity against C. krusei than fluconazole (MIC = 50 µg/mL), while the other compounds, 50 and 237, possessed the same activity as the positive control [151]. Additionally, compound 124 demonstrated a weak inhibitory activity (29%) on Cryptococcus neoformans [92].
4.8.3. Antiviral Activity
Benzophenone 66 showed selective and moderate inhibitory activity in vitro against the proliferation of Herpes simplex type 1 (HSV-1) with an IC50 value of 4 µg/mL compared to a positive control pirodavir (IC50 = 0.085 µg/mL) [38]. Compound 143 exhibited very high anti-HIV activity against syncytium formation in the syncytium inhibition assay (EC50 = 68.88 µM, SI > 1.59) and in the reverse transcriptase assay (IC50 = 271.75 µM) [111]. Furthermore, garcimangosone D 44 exhibited moderate anti-HIV-1 activity with EC50 values of 18.06 μg/mL and TI (therapy index) > 11.07, while malaferin A 42 showed weak bioactivity with EC50 = 131.70 μg/mL and TI > 1.52 [40]. In addition, foliamangiferoside A4 92 showed a moderate inhibitory activity against influenza NA (neuraminidase) from pandemic A/RI/5+/1957 H2N2 influenza A virus (47.4%) and coxsackie B3 virus 3C protease (53.8%) at a concentration of 100 µM [265].
4.8.4. Antiparasitic Activity
It was found that compound 122 exhibited considerable growth-suppressing effect against Trypanosoma brucei with IC50 values of 22.3 µM [92], while cearoin 34 has very weak activity against Leishmania major (IC50 > 100 µM) [193]. In addition, 38 (IC50 = 18.6 µM) and 58 (IC50 = 28.8 µM) showed ex vivo antiplasmodial activities [54].
4.8.5. Antimalarial Activity
The antimalarial activity of 174 was assessed as weak with an inhibition value of 29.8%, relative to the positive control of chloroquine diphosphate (95.1%) [119].
4.9. Metabolic Syndrome
4.9.1. Antidiabetic Activity
- α-Glucosidase inhibitory activity
It was found that compounds 227 (IC50 = 0.042 mM) and 228 (IC50 = 0.199 mM) showed stronger α-glucosidase inhibitory activity than positive control acarbose (IC50 = 0.685 mM) [142]. The results also showed that aquilarisinin 114 (IC50 = 151.6 µg/mL) exhibited a strong inhibitory effect against α-glucosidase, which was about twofold that of acarbose (IC50 = 372.6 µg/mL) [88]. The benzophenone 106 showed 91.4% inhibition activity at 10 µg/mL against the α-glucosidase from Bacillus stearothermophilus compared to acarbose (inhibition = 70.5% at 40 ng/mL) [84], while compounds 155 and 86 exhibited significant α-glucosidase inhibitory activity of 54% and 43%, respectively (at 200 µM), against an enzyme mixture extracted from rat intestinal acetone powder [74]. Furthermore, inhibitory effect against α-glucosidase of compounds iriflofenone-2-O-α-L-rhamnoside 108 (IC50 = 143.7 µg/mL) and iriflofenone-3-C-β-D-glucopyranoside 74 (IC50 = 165.1 µg/mL) was about twofold of that of acarbose (IC50 = 372.0 µg/mL), while iriflofenone-3,5-C-β-diglucoside 87 (273.6 µg/mL) showed inhibitory effect almost the same as the positive control [88]. In addition, strong to moderate α-glucosidase inhibitory activity compared to acarbose (IC50 = 185.25 μM) possessed benzophenones 162 (IC50 = 284.93 μM), 78 (IC50 = 415.79 μM), 120 (IC50 = 520.94 µM), 60 (IC50 = 657.23 µM), and 79 (IC50 = 834.66 µM) [81].
- 2.
- α-Amylase inhibitory activity
Garcimangophenone A 209 (IC50 = 12.2 µM) and B 207 (IC50 = 9.3 µM) showed significant α-amylase inhibitory activity compared with acarbose (IC50 = 6.4 µM) [128]. Additionally, the results also showed that garcimangophenone C 128 exhibited very strong α-amylase inhibition with IC50 of 12.9 µM compared to acarbose (IC50 = 6.7 µM) [95].
- 3.
- The efficiency of glucose uptake
The assay for glucose uptake stimulatory activity conducted in rat skeletal muscle L6 cells reported that iriflofenone-3-C-β-D-glucoside 74 (100 μg/mL) and iriflofenone-3,5-C-β-diglucoside 87 (100 μg/mL) showed a glucose uptake enhancement of 234.5% and 119.9%, respectively, when compared with the blank control (100% uptake). The positive control insulin (0.5 μM) showed 146.6% enhancement [159].
4.9.2. Antihyperlipidemic Activity
At a dose of 30 µM, compounds 74, 78–81, 88, and 155 [72], as well as 84 (10 µM) and 85 (10 µM), significantly suppressed triglyceride (TG) and free fatty acid (FFA) accumulation in 3T3-L1 adipocytes [73]. Based on the AMP-activated protein kinase (AMPK) signaling pathway, several of the abovementioned benzophenones were found to increase the AMPK enzyme expression and downregulate lipogenic enzyme gene expression such as SREBP1c (sterol regulatory element-binding protein 1c), FAS (fatty acid synthase), and HSL (hormone-sensitive lipase). The results showed that compared with the control group, AMPK gene expression of compounds 58, 74, 78, 88, 122, 155, 163 [72], 82–85, 91, and 92 [73] were significantly increased. All of these compounds significantly suppressed the SREBP1c level [72,73]. Furthermore, compounds 52, 56–59, 84, 85, 135 [72], and 61–63 [73] significantly downregulated HSL gene expression. It was found that 74, 78, 88, 155 [72], 82–85, and 92 [73] significantly reduced FAS gene expression. Additionally, compounds 69 and 143 exhibited better inhibitory activity against FAS than the known FAS inhibitor EGCG (epigallocatechin gallate) (IC50 = 51.97 µM), returning lower IC50 values of 14.76 µM and 8.59 µM, respectively [64].
4.9.3. Antihypertensive Activity
It was reported that maclurin-6-O-β-D-glucopyranoside 172 significantly alleviated the exaggerated vasoconstriction of metabolic syndrome (MetS) aortae and at the same time showed significant vasodilation of PE (phenylephrine) precontracted aortae. To further illustrate the mechanism of action, the observed vasodilation was completely blocked by the nitric oxide (NO) synthase inhibitor Nω-nitro-L-arginine methyl ester hydrochloride and inhibited by guanylate cyclase inhibitor methylene blue. The results showed that vasodilation was not affected by the potassium channel blocker, tetraethylammonium, or the cyclooxygenase inhibitor; indomethacin and 172 stimulated NO generation from isolated aortae to levels comparable with acetylcholine. Furthermore, 172 inhibited reactive oxygen species generation in MetS aortae. In conclusion, it could be said that maclurin-6-O-β-D-glucopyranoside (172) ameliorated the exaggerated vasoconstriction in MetS aortae through the vasodilatation-NO generation mechanism [171].
4.10. Inhibitory Activities on Platelet Aggregation
Until now, only three compounds possessed inhibitory potential against platelet aggregation induced by one, two, or three inducers such as arachidonic acid (AA), adenosine diphosphate (ADP), and collagen at 100 µg/mL in human whole blood in vitro. Among the tested compounds, only 152 exhibited strong inhibitory activity against platelet aggregation induced by AA (IC50 = 53.6 µM), ADP (IC50 = 125.7 µM), and collagen (IC50 = 178.6 µM). The results also showed that benzophenone 152 was the only compound effective against collagen-induced platelet aggregation. Compound 151 demonstrated the strongest inhibition on platelet aggregation induced by ADP with an IC50 value of 34.7 µM. Additionally, 70 showed selective inhibitory activity on platelet aggregation induced by AA and ADP with IC50 values of 91.1 µM and 69.5 µM, respectively [65].
4.11. MAO-A Inhibition Activity
It was found that compounds 129 and 172 exhibited significant inhibitory activity against MAO-A with an IC50 value of 31.3 μM and 41 μM, respectively, compared to the positive control clorgyline (IC50 = 0.08 µM) [266]. A weak MAO-A inhibitory effect was found for compounds 180 (IC50 = 111.2 μM), 182 (IC50 = 310.3 μM), and 183 (IC50 = 726.0 µM). Clorgyline was again used as positive control MAO-A inhibitor with an IC50 value of 0.5 µM [122].
4.12. Antiarthritic Activity
Studies on proapoptotic activity on TNF-α-stimulated synovial cells isolated from patients with rheumatoid arthritis demonstrated that iriflofenone 3-C-β-glucoside 74 possessed 71% efficacy, measured as the percent of apoptotic cells [187].
4.13. Anticholinesterase Activity
Elegaphenone 31 was found to possess moderate inhibitory activity against acetylcholinesterase with IC50 value of 192.19 μM, against a positive control of galantamine hydrobromide (IC50 = 0.43 μM) [250].
4.14. Anti-Atherosclerotic Activity
It was reported that compound 37 (0.40 mM) exhibited inhibitory activity of 54% against hACAT-1 (human acyl-CoA: cholesterol acyltransferase) compared to the positive control oleic acid anilide (57% inhibition at 0.3 µM concentration) [224].
4.15. Laxative Effect
The laxative effect of iriflofenone-2-O-α-L-rhamnoside 108 was estimated in vivo in male ddY mice (aged 7–10 weeks and 20–35 g body weight) after administration of an oral dose of 1000 mg/kg. The results showed that benzophenone 108 exhibited slow-acting laxative activity, as its effect appears 6–8 h after administration and does not cause subsequent diarrhea [75].
4.16. Negative Effects of Naturally Occurring Simply Oxygenated Benzophenones on Human and Animal Health and the Environment
However, of the variety and number of benzophenone products of natural and synthetic origin, only a small part of them, in particular those possessing a hydroxyl or methoxy group in the para position to the carbonyl function, a model of 2,4-dioxygenation and rarer model of 2,2′4,4′-tetraoxygenation, are among the most tested for negative effects on humans and the environment. This is due to the fact that these groups of compounds are among the most commonly used UV absorbing agents, mainly due to their large molar extinction coefficients in both the UVA and UVB ranges. 2-Hydroxy-4-methoxybenzophenone (benzophenone-3 or BP-3) 3 is known to have been used for many years as a UV filter in sunscreen cosmetics [267]. On the other hand, 3 has been shown to be metabolized to three intermediates in rats, including 2,4-dihydroxybenzophenone (benzophenone-1 or BP-1) 5, which is formed by demethylation [268]. The latter is also used as a UV filter in sun protection products. Exposure to elevated levels of 5 may be associated with increased chances of endometriosis diagnosis in women [269], and it has also been reported to have significant uterotrophic effects to increase the growth of BG-1 human cancer cells on ovaries and to show high affinity in binding to hERα (human estrogen receptor α), while 3 showed only a slight estrogenic effect in the assay with MCF-7 human cancer cells and pS2 protein [270]. Compound 5 was also reported to exhibit greater inhibitory activity against human KGN 3β-HSD (3β-hydroxysteroid dehydrogenase) with an IC50 = 5.66 μM, than BP-3 (5.84 μM) [271]. In addition, in vitro yeast-based reporter assay found that compound 5 has strong estrogenic (EC50 = 1.29 µM), weak antiandrogenic (IC50 > 30 µM), and mushroom tyrosinase inhibitory activity (IC50 > 50 µM), while 3 has weak estrogenic (EC50 > 30 µM), antiandrogenic (IC50 > 30 µM), mushroom tyrosinase inhibitory (IC50 > 50 µM), and moderate antiprogestrogenic activity (IC50 = 11.81 µM). Furthermore, 2,2′,4,4′-tetrahydroxybenzophenone (benzophenone-2 or BP-2) 73 exhibits weak estrogenic activity (EC50 = 17.29 µM) and significant inhibitory activity against mushroom tyrosinase (IC50 = 19.7 µM) [272]. A number of studies have been conducted to evaluate the estrogenic and toxic effects of benzophenone UV filters in fish. It was established that compound 3 at a concentration of 2.4–312 μg/L affected genes involved in steroidogenesis and hormonal pathways in zebrafish at different developmental stages [273]. Furthermore, acute toxicity tests with medaka larvae showed that at 96 h exposure, compounds 3 (LC50 = 4.10 μM) and 5 (LC50 = 2.22 μM) exhibited significantly higher toxicity than 73 (LC50 = 18.43 μM). The latter at an ecologically relevant concentration (5–50 nM) did not alter locomotion and oxidative stress responses of larvae from 24 h to 7-day exposure, while 3 even at 5 nM induced hypoactivity or changed fish swimming angles [272]. In addition, adverse effects on algae (Scenedesmus vacuolatus and Desmodesmus subspicatus), freshwater flea (Daphnia magna), and planarian (Dugesia japonica) altered endocrine signaling gene expression, ultraspiracle in aquatic invertebrate (Chironomus riparius), and reduced reproductive performance in Japanese medaka (Oryzias latipes) after exposure to 620 μg/L for 21 days have been reported for compound 3 [270].
5. Biogenetic Significance of Simply Oxygenated Benzophenones
Simply oxygenated benzophenones are active secondary metabolites and are closely related to xanthones as their immediate biosynthetic precursors. Biogenetic relationship between benzophenones and xanthones were discussed in several reviews. The intermediate benzophenones were built by acetate and shikimate biosynthetic pathways. Further intermolecular reaction led to formation of a xanthone. The mechanisms of last formation step could involve four possible pathways [7,274,275,276]. The literature data showed that maclurin 133 is an intermediate in the biosynthesis of macluraxanthone [277], 1,3,5,6- and 1,3,6,7-tetraoxyxanthones from the families Guttiferae and Moraceae [179]. Furthermore, after feeding Anemarrhena asphodeloides with radiolabeled 1,3,6,7-tetrahydroxyxanthone and 133, only the latter was efficiently incorporated into mangiferin and isomangiferin, indicating that C-glucosylation of mangiferin and isomangiferin occurs prior to xanthone core formation [278]. Additionally, the study of xanthones from Gentiana lutea with the inclusion of 14C- and 3H-labeled compounds demonstrated that compound 61 (2,3′,4,6-tetrahydroxybenzophenone) is a precursor of gentisein [279]. A study on Hypericum annulatum showed that an acid and enzymatic hydrolysis of hypericophenonoside 206 led directly to the formation of 1,3,7-trihydroxyxanthone (gentisein). This finding favored the hypothesis that some xanthones could be formed in plants by dehydration of 2,2′-dihydroxybenzophenones, and the intermediate precursors might be benzophenone with O-glycosilation ortho to the carbonyl function [8]. In general, the formation of the benzophenone (C13) skeleton is catalyzed by benzophenone synthase. In cell cultures of Hypericum androsaemum, this enzyme stepwise condenses one molecule of benzoyl-CoA with three molecules of malonyl-CoA to provide a tetraketide intermediate, which is cyclized by an intramolecular Claisen condensation to 17 (2,4,6-trihydroxybenzophenone). The subsequent 3′-hydroxylation is catalyzed by cytochrome P450 monooxygenase [280]. In cell cultures of Centaurium erythraea, the preferred starting substrate for benzophenone synthase is 3-hydroxybenzoyl-CoA, immediately producing compound 61 [202]. In addition, it should be noted that in fungi, biogenetic benzophenone can be formed from two separate units, each separately derived from acetate and malonate pathways, or by an anthrone and/or anthraquinone intermediate derived from a single C16 polyketide chain [281].
6. Conclusions
This review includes more than 280 references to naturally occurring simple oxygenated benzophenones covering the years from 1850 up to 2023 and was compiled using databases such as Chemical Abstracts, Scopus, Google Scholar, PubMed, and ResearchGate. This article discusses the structural diversity, distribution, and biological activities of natural simple oxidized benzophenones and their glycosides.
Two hundred and fifty chemical structures belonging to seven groups of benzophenone derivatives and their glycosides (mono-oxidized, di-oxidized, tri-oxidized, tetra-oxidized, penta-oxidized, hexa-oxidized, and others that cannot be classified into any of the first six) were established.
Naturally occurring benzophenones represent a relatively small group of plant metabolites with narrow distribution. Up to now, simple oxygenated benzophenones and their glycosides have been established in 77 plant genera distributed in 44 families. Of all 250 reported compounds, the largest number of representatives 51 was isolated from the Hypericaceae family, followed by 43 representatives from Anacardiaceae, 36 from Clusiaceae, 24 from Polygalaceae, 22 from Thymelaeaceae, and 18 from Fabaceae. The allergy associated bezophenone-1, benzophenone-2, and benzophenone-3 have limited distribution across natural sources.
A wide range of pharmacological activities of simple oxygenated benzophenones and their glycosides such as antioxidant activities; anti-inflammatory activity; cytotoxic, antitumor, and cytoprotective activities; antimicrobial activity; MAO-A inhibition activity; antiarthritic activity; anticholinesterase activity; anti-atherosclerotic activity; laxative effect; metabolic syndrome; etc., that appeared in the literature were discussed as well.
The authors hope that this review will draw the attention of scientists of simple oxygenated benzophenones and their glycosides and stimulate them to continue with phytochemical, pharmacological, and chemotaxonomical studies that could lead to the discovery of compounds that can serve as models for the synthesis of a new pharmacological agent as well as help to solve certain taxonomical issues.
Author Contributions
Conceptualization, P.T.N.; methodology, P.T.N.; software, T.M. and P.T.N.; validation, Z.K.-N. and P.T.N.; formal analysis, Z.K.-N.; investigation, T.M.; resources, P.T.N.; data curation, Z.K-N. and T.M.; writing—original draft preparation, T.M.; writing—review and editing, P.T.N. and Z.K.-N.; visualization, T.M.; supervision, P.T.N.; project administration, Z.K.-N.; funding acquisition, P.T.N. All authors have read and agreed to the published version of the manuscript.
Funding
The funding was provided by the European Union project NextGenerationEU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project no. BG-RRP-2.004-0004-C01.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bukvički, D.; Novaković, M.; Ab Ghani, N.; Marin, P.D.; Asakawa, Y. Secondary Metabolites from Endemic Species Iris Adriatica Trinajstić Ex Mitić (Iridaceae). Nat. Prod. Res. 2018, 32, 1849–1852. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Mukherjee, A.; Pandey, D.K.; Ray, P.; Dey, A. Indian Sarsaparilla (Hemidesmus Indicus): Recent Progress in Research on Ethnobotany, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2020, 254, 112609. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Rubio, O.; Piccinelli, A.L.; Rastrelli, L. Chemistry and Biological Activity of Polyisoprenylated Benzophenone Derivatives. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 32, pp. 671–720. ISBN 1572-5995. [Google Scholar]
- Acuna, M.U.; Jancovski, N.; Kennelly, J.E. Polyisoprenylated Benzophenones from Clusiaceae: Potential Drugs and Lead Compounds. Curr. Top. Med. Chem. 2009, 9, 1560–1580. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Long, C.; Kennelly, E.J. Structural Diversity and Bioactivities of Natural Benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174. [Google Scholar] [CrossRef]
- Singh, P.I.; Bharate, S.B. Phloroglucinol Compounds of Natural Origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Sultanbawa, M.U.S. Xanthonoids of Tropical Plants. Tetrahedron 1980, 36, 1465–1506. [Google Scholar] [CrossRef]
- Kitanov, G.M.; Nedialkov, P.T. Benzophenone O-Glucoside, a Biogenic Precursor of 1,3,7-Trioxygenated Xanthones in Hypericum annulatum. Phytochemistry 2001, 57, 1237–1243. [Google Scholar] [CrossRef]
- Mandal, S.; Das, P.C.; Joshi, P.C. Naturally Occurring Xanthones from Terrestrial Flora. J. Indian Chem. Soc. 1992, 69, 611–636. [Google Scholar]
- Heurung, A.R.; Raju, S.I.; Warshaw, E.M. Benzophenones. Dermatitis 2014, 25, 3–10. [Google Scholar] [CrossRef]
- Pallares, E.S.; Hector, M.G. Study of Yoloxochitl. Arch. Inst. Cardiol. Mex. 1947, 17, 833–849. [Google Scholar]
- Sharma, M.L.; Nigam, M.C.; Hande, K.L. Essential Oil of Costus speciosus. Perfum. Essent. Oil Rec. 1963, 54, 579–580. [Google Scholar]
- Liu, L.-N.; Zhang, X.-W.; Hou, F.-J.; Duan, X.-H.; Zhao, J.-C.; Chen, Y.-L. Benzophenones from Endohydric Moss Polytrichastrum Formosum and Their Cytotoxic Activities. Chin. Tradit. Herb. Drugs 2022, 53, 667–670. [Google Scholar]
- Duan, X.-H.; He, P.; Qin, M.; Li, L.; Pei, L.; Zhao, J.-C. A New Benzophenone from Endohydric Moss Pogonatum Inflexum. Chin. Tradit. Herb. Drugs 2019, 50, 1291–1293. [Google Scholar] [CrossRef]
- Duan, X.-H.; Zhao, J.-C.; Li, L.; Pei, L.; He, P.; Wang, R. Two New Benzophenones from Endohydric Moss Polytrichum Commune. Nat. Prod. Res. 2019, 33, 2750–2754. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-H.; Zhang, X.-W.; Qin, M.; He, P.; Pei, L.; Zhao, J.-C.; Chen, Y.-L. Two New Benzophenones from the Endohydric Moss Polytrichastrum formosum. Nat. Prod. Commun. 2021, 16, 1934578X211002623. [Google Scholar] [CrossRef]
- Gamiotea-Turro, D.; Cuesta-Rubio, O.; Prieto-González, S.; De Simone, F.; Passi, S.; Rastrelli, L. Antioxidative Constituents from the Leaves of Hypericum styphelioides. J. Nat. Prod. 2004, 67, 869–871. [Google Scholar] [CrossRef]
- Rusby, H.H. New Species of Trees of Medical Interest from Bolivia. Bull. Torrey Bot. Club 1922, 49, 259–264. [Google Scholar] [CrossRef]
- Jobst, J. Ueber Coto-Rinden Und Deren Krystallisirbare Bestandtheile. Berichte Der Dtsch. Chem. Ges. 1876, 9, 1633–1634. [Google Scholar] [CrossRef]
- Jobst, J.; Hesse, O. Ueber Die Cotorinden Und Ihre Charakteristischen Bestandtheile. Justus Liebigs Ann. Der Chem. 1879, 199, 17–96. [Google Scholar] [CrossRef]
- Ciamician, G. Ueber Die Constitution Des Cotoïns. Berichte Der Dtsch. Chem. Ges. 1894, 27, 409–426. [Google Scholar] [CrossRef]
- Ciamician, G.; Silber, P. Ueber Das Hydrocotoïn, Einen Bestandtheil Der Cotorinde. Berichte Der Dtsch. Chem. Ges. 1891, 24, 299–301. [Google Scholar] [CrossRef]
- Ciamician, G.; Silber, P. Synthese Des Benzophloroglucintrimethyläther. (Methylhydrocotoïn Oder Benzoylhydrocoton). Berichte Der Dtsch. Chem. Ges. 1894, 27, 1497–1501. [Google Scholar] [CrossRef]
- Ciamician, G.; Silber, P. Ueber Die Constitution Einiger in Der Paracotorinde Enthaltenen Bestandtheile. Berichte Der Dtsch. Chem. Ges. 1892, 25, 1119–1138. [Google Scholar] [CrossRef]
- Randriaminahy, M.; Proksch, P.; Witte, L.; Wray, V. Lipophilic Phenolic Constituents from Helichrysum Species Endemic to Madagascar. Z. Für Naturforschung C 1992, 47, 10–16. [Google Scholar] [CrossRef]
- Bohlmann, F.; Suwita, A. Neue Phloroglucin-Derivate Aus Leontonyx-Arten Sowie Weitere Verbindungen Aus Vertretern Der Tribus Inuleae. Phytochemistry 1978, 17, 1929–1934. [Google Scholar] [CrossRef]
- Jakupovic, J.; Zdero, C.; Grenz, M.; Tsichritzis, F.; Lehmann, L.; Hashemi-Nejad, S.M.; Bohlmann, F. Twenty-One Acylphloroglucinol Derivatives and Further Constituents from South African Helichrysum Species. Phytochemistry 1989, 28, 1119–1131. [Google Scholar] [CrossRef]
- Don, M.-J.; Huang, Y.-J.; Huang, R.-L.; Lin, Y.-L. New Phenolic Principles from Hypericum sampsonii. Chem. Pharm. Bull. 2004, 52, 866–869. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Tan, D.; Li, D.; Guo, Y.; Wei, G.; Zhang, J.; Wang, J.; Luo, Z.; Xue, Y.; et al. Sampbenzophenones A–G, Prenylated Benzoylphloroglucinol Derivatives from Hypericum sampsonii. RSC Adv. 2016, 6, 86710–86716. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chang, T.-C.; Wu, Y.-J.; Chen, Y.; Chen, J.-J. Benzophenone and Benzoylphloroglucinol Derivatives from Hypericum Sampsonii with Anti-Inflammatory Mechanism of Otogirinin A. Molecules 2020, 25, 4463. [Google Scholar] [CrossRef]
- Wu, F.-S.; Hung, C.-J.; Lin, C.-L.; Huang, H.-Y.; Kuo, Y.-H.; Chang, T.-H.; Chen, C.-L.; Sung, P.-J.; Cheng, M.-J.; Kuo, C.-W.; et al. A New Benzophenone and Bioactive Constituents of Hypericum nokoense. Chem. Nat. Compd. 2021, 57, 645–649. [Google Scholar] [CrossRef]
- Nedialkov, P.T.; Zheleva-Dimitrova, D.; Momekov, G.; Karlov, K.; Girreser, U.; Kitanov, G.M. Elegaphenone and 7-Epi-Clusianone, the Major Cytotoxic Constituents of Hypericum elegans. Nat. Prod. Res. 2011, 25, 1743–1750. [Google Scholar] [CrossRef]
- Wu, F.-S.; Wang, I.-C.; Liaw, C.-C.; Huang, H.-Y.; Chang, T.-H.; Chen, C.-L.; Sung, P.-J.; Cheng, M.-J.; Kuo, C.-W.; Chen, J.-J. New Benzophenone and Bioactive Constituents from Hypericum Nagasawae. Chem. Nat. Compd. 2022, 58, 833–838. [Google Scholar] [CrossRef]
- Ollis, W.D. Proceedings of the 6th Annual Symposium of the Plant Phenolics Group of North America, 1966; Mabry, T.J., Alston, R.E., Runeckles, V.C., Eds.; Recent Advances in Phytochemistry; Appleton–Century–Crofts: New York, NY, USA, 1968; Volume 1. [Google Scholar]
- Wang, W.; Weng, X.; Cheng, D. Antioxidant Activities of Natural Phenolic Components from Dalbergia odorifera T. Chen. Food Chem. 2000, 71, 45–49. [Google Scholar] [CrossRef]
- Dibwe, D.F.; Awale, S.; Kadota, S.; Morita, H.; Tezuka, Y. Heptaoxygenated Xanthones as Anti-Austerity Agents from Securidaca longepedunculata. Bioorganic Med. Chem. 2013, 21, 7663–7668. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.-Y.; Wang, Z.-M.; Li, Z.-Q.; Xu, X.-J. Three New Compounds from Securidaca inappendiculata. Helv. Chim. Acta 2005, 88, 2771–2776. [Google Scholar] [CrossRef]
- Casu, L.; Solinas, M.N.; Saba, A.R.; Cottiglia, F.; Caboni, P.; Floris, C.; Laconi, S.; Pompei, R.; Leonti, M. Benzophenones from the Roots of the Popoluca Amerindian Medicinal Plant Securidaca Diversifolia (L.) S.F. Blake. Phytochem. Lett. 2010, 3, 226–229. [Google Scholar] [CrossRef]
- Triana, J.; López, M.; Pérez, F.J.; Platas, J.G.; Estévez, F.; León, J.F.; Hernández, J.C.; Brouard, I.; Bermejo, J. Chemical Constituents of Tolpis Species. Fitoterapia 2009, 80, 437–441. [Google Scholar] [CrossRef]
- Wu, X.-D.; Cheng, J.-T.; He, J.; Zhang, X.-J.; Dong, L.-B.; Gong, X.; Song, L.-D.; Zheng, Y.-T.; Peng, L.-Y.; Zhao, Q.-S. Benzophenone Glycosides and Epicatechin Derivatives from Malania Oleifera. Fitoterapia 2012, 83, 1068–1071. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Ouyang, M.-A.; Yang, C.-R. Oligosaccharide Esters and Phenol Compounds from Polygala Arillata. Acta Bot. Yunnan. 2000, 22, 482–494. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Chen, C.-C.; Chen, Y.-J.; Huang, R.-L.; Shieh, B.-J. Three Xanthones and a Benzophenone from Garcinia mangostana. J. Nat. Prod. 2001, 64, 903–906. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Tu, P.-F. Xanthone O-Glycosides and Benzophenone O-Glycosides from the Roots of Polygala tricornis. J. Nat. Prod. 2005, 68, 1802–1804. [Google Scholar] [CrossRef]
- Dai, Y.; He, X.-J.; Zhou, G.-X.; Kurihara, H.; Ye, W.-C.; Yao, X.-S. Acylphloroglucinol Glycosides from the Fruits of Pyracantha fortuneana. J. Asian Nat. Prod. Res. 2008, 10, 111–117. [Google Scholar] [CrossRef]
- Shi, Y.-N.; Shi, Y.-M.; Yang, L.; Li, X.-C.; Zhao, J.-H.; Qu, Y.; Zhu, H.-T.; Wang, D.; Cheng, R.-R.; Yang, C.-R.; et al. Lignans and Aromatic Glycosides from Piper wallichii and Their Antithrombotic Activities. J. Ethnopharmacol. 2015, 162, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.Z.; Yang, J.Z.; Li, C.J.; Zhang, D.M. A New Benzophenone Glycoside from the Leaves of Psidium Guajava L. Chin. Chem. Lett. 2011, 22, 178–180. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Ishii, R.; Kobiyama, K.; Kitanaka, S. New Benzophenone and Quercetin Galloyl Glycosides from Psidium guajava L. J. Nat. Med. 2010, 64, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.-X.; Wang, S.; Zeng, K.-W.; Tu, P.-F.; Jiang, Y. Inhibitory Constituents from the Aerial Parts of Polygala Tenuifolia on LPS-Induced NO Production in BV2 Microglia Cells. Bioorganic Med. Chem. Lett. 2013, 23, 5904–5908. [Google Scholar] [CrossRef]
- Jiang, Y.; Tu, P. Four New Phenones from the Cortexes of Polygala tenuifolia. Chem. Pharm. Bull. 2005, 53, 1164–1166. [Google Scholar] [CrossRef][Green Version]
- Zhou, L.-Y.; Wang, J.-M.; Huang, Y.-J.; Yu, X.-H.; Lu, B.; Hua, Y. Two New Glycosides Isolated from Polygala sibirica L. Var. megalopha Fr. Phytochem. Lett. 2016, 16, 174–177. [Google Scholar] [CrossRef]
- An, H.; Thanh, L.N.; Khanh, L.Q.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Lee, H.H.; Turk, A.; Lee, K.Y.; Hwang, B.Y.; et al. Characterization of Antioxidant and α-Glucosidase Inhibitory Compounds of Cratoxylum formosum ssp. pruniflorum and Optimization of Extraction Condition. Antioxidants 2023, 12, 511. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Fineberg, M.; Salignac De Souza Guimaraes, I.; Taveira Magalhaes, M.; Ollis, W.D.; Eyton, W.B. The chemistry of the Brazilian leguminosae. VII. The Constituents of Machaerium scleroxylon. An. Acad. Bras. Cienc. 1964, 36, 33–34. [Google Scholar]
- Eyton, W.B.; Ollis, W.D.; Fineberg, M.; Gottlieb, O.R.; Salignac de Souza Guimarães, I.; Taveira Magalhães, M. The Neoflavanoid Group of Natural Products—II: The Examination of Machaerium Scleroxylon and Some Biogenetic Proposal Regarding the Neoflavanoids. Tetrahedron 1965, 21, 2697–2705. [Google Scholar] [CrossRef]
- Ochora, D.O.; Kakudidi, E.; Namukobe, J.; Heydenreich, M.; Coghi, P.; Yang, L.J.; Mwakio, E.W.; Andagalu, B.; Roth, A.; Akala, H.M.; et al. A New Benzophenone, and the Antiplasmodial Activities of the Constituents of Securidaca longipedunculata Fresen (Polygalaceae). Nat. Prod. Res. 2022, 36, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Spada, A.; Cameroni, R.; Bernabei, M.T. The Pigments of Morus Alba. Gazz. Chim. Ital. 1956, 86, 46–55. [Google Scholar]
- De Barros Corrêa, D.; Gottlieb, O.R. Duckein, an Alkaloid from Aniba Duckei. Phytochemistry 1975, 14, 271–272. [Google Scholar] [CrossRef]
- Atkinson, J.E.; Gupta, P.; Lewis, J.R. Some Phenolic Constituents of Gentiana Lutea. Tetrahedron 1969, 25, 1507–1511. [Google Scholar] [CrossRef]
- Locksley, H.D.; Murray, I.G. Extractives from Guttiferae. Part XIX. The Isolation and Structure of Two Benzophenones, Six Xanthones and Two Biflavonoids from the Heartwood of Allanblackia floribunda Oliver. J. Chem. Soc. C 1971, 1332–1340. [Google Scholar] [CrossRef]
- Donnelly, D.M.X.; O’Reilly, J.; Whalley, W.B. Neoflavanoids of Dalbergia melanoxylon. Phytochemistry 1975, 14, 2287–2290. [Google Scholar] [CrossRef]
- Lin, S.; Liu, R.-H.; Ma, G.-Q.; Mei, D.-Y.; Shao, F.; Chen, L.-Y. Two New Compounds from the Heartwood of Dalbergia Melanoxylon. Nat. Prod. Res. 2020, 34, 2794–2801. [Google Scholar] [CrossRef]
- Pathak, V.; Shirota, O.; Sekita, S.; Hirayama, Y.; Hakamata, Y.; Hayashi, T.; Yanagawa, T.; Satake, M. Antiandrogenic Phenolic Constituents from Dalbergia cochinchinensis. Phytochemistry 1997, 46, 1219–1223. [Google Scholar] [CrossRef]
- Wu, D.-L.; Liao, Z.-D.; Chen, F.-F.; Zhang, W.; Ren, Y.-S.; Wang, C.-C.; Chen, X.-X.; Peng, D.-Y.; Kong, L.-Y. Benzophenones from Anemarrhena Asphodeloides Bge. Exhibit Anticancer Activity in HepG2 Cells via the NF-ΚB Signaling Pathway. Molecules 2019, 24, 2246. [Google Scholar] [CrossRef]
- Smitha, C.; Udayan, P. GC-MS and HR-LCMS Fingerprinting of Various Parts of Oroxylum Indicum (L.) Vent. A Comparative Phytochemical Study Based on Plant Part Substitution Approach. J. Pharmacogn. Phytochem. 2020, 9, 1817–1824. [Google Scholar]
- Jiang, H.Z.; Quan, X.F.; Tian, W.X.; Hu, J.M.; Wang, P.C.; Huang, S.Z.; Cheng, Z.Q.; Liang, W.J.; Zhou, J.; Ma, X.F.; et al. Fatty Acid Synthase Inhibitors of Phenolic Constituents Isolated from Garcinia Mangostana. Bioorganic Med. Chem. Lett. 2010, 20, 6045–6047. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Saputri, F.C. Benzophenones and Xanthones from Garcinia Cantleyana Var. Cantleyana and Their Inhibitory Activities on Human Low-Density Lipoprotein Oxidation and Platelet Aggregation. Phytochemistry 2012, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wabo, H.K.; Kowa, T.K.; Lonfouo, A.H.N.; Tchinda, A.T.; Tane, P.; Kikuchi, H.; Frédérich, M.; Oshima, Y. Phenolic Compounds and Terpenoids from Hypericum lanceolatum. Rec. Nat. Prod. 2012, 6, 94–100. [Google Scholar]
- Mian, J.V.Y.; Lian, E.G.C.; Aspollah, S.M.; Hin, T.-Y.Y.; Yen, K.H.; Yok, C.M.K. Benzophenone Constituents from the Roots of Garcinia eugenifolia. Res. J. Chem. Environ. 2012, 16, 36–39. [Google Scholar]
- Azlini, I.; Erlena, N.A.A.R.; Muhammad, N.O.; Wan, A.N.W.A. Antihypertensive Assay-Guided Fractionation of Syzygium Polyanthum Leaves and Phenolics Profile Analysis Using LCQTOF/ MS. Pharmacogn. J. 2020, 12, 1670–1692. [Google Scholar] [CrossRef]
- Murakami, T.; Tanaka, N.; Wada, H.; Saiki, Y.; Chen, C.-M. Chemical and Chemotaxonomical Studies on Filices. LXIII. Yakugaku Zasshi 1986, 106, 378–382. [Google Scholar] [CrossRef]
- Tanaka, T.; Sueyasu, T.; Nonaka, G.; Nishioka, I. Tannins and Related Compounds. XXI. Isolation and Characterization of Galloyl and p-Hydroxybenzoyl Esters of Benzophenone and Xanthone C-Glucosides from Mangifera indica L. Chem. Pharm. Bull. 1984, 32, 2676–2686. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, Q.; Ge, D.; Li, Y.; Wang, X.; Chen, Q.; Gao, X.; Wang, T. Identification of Benzophenone C-Glucosides from Mango Tree Leaves and Their Inhibitory Effect on Triglyceride Accumulation in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2011, 59, 11526–11533. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, L.; Ge, D.; Liu, X.; Liu, E.; Wu, C.; Gao, X.; Wang, T. Isolation, Structural Elucidation, MS Profiling, and Evaluation of Triglyceride Accumulation Inhibitory Effects of Benzophenone C-Glucosides from Leaves of Mangifera indica L. J. Agric. Food Chem. 2013, 61, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Beelders, T.; Brand, D.J.; de Beer, D.; Malherbe, C.J.; Mazibuko, S.E.; Muller, C.J.F.; Joubert, E. Benzophenone C- and O-Glucosides from Cyclopia Genistoides (Honeybush) Inhibit Mammalian α-Glucosidase. J. Nat. Prod. 2014, 77, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Ise, Y.; Morimoto, N.; Shimazawa, M.; Ichihashi, K.; Ohyama, M.; Iinuma, M. Laxative Effect of Agarwood Leaves and Its Mechanism. Biosci. Biotechnol. Biochem. 2008, 72, 335–345. [Google Scholar] [CrossRef]
- Pan, J.; Yi, X.; Zhang, S.; Cheng, J.; Wang, Y.; Liu, C.; He, X. Bioactive Phenolics from Mango Leaves (Mangifera indica L.). Ind. Crops Prod. 2018, 111, 400–406. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, M.; Wang, D.; Yang, C.-R.; Zhang, Y.-J. Phenolic Compounds from the Whole Plants of Gentiana rhodantha (Gentianaceae). Chem. Biodivers. 2011, 8, 1891–1900. [Google Scholar] [CrossRef]
- Li, C.-J.; Zhang, D.-M.; Yu, S.-S. Benzophenone C-Glucosides from Polygala glomerata Lour. J. Asian Nat. Prod. Res. 2008, 10, 293–297. [Google Scholar] [CrossRef]
- Panidthananon, W.; Chaowasku, T.; Sritularak, B.; Likhitwitayawuid, K. A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria Fragrans and Their α-Glucosidase Inhibitory Activity. Molecules 2018, 23, 1600. [Google Scholar] [CrossRef]
- Qi, J.; Lu, J.-J.; Liu, J.-H.; Yu, B.-Y. Flavonoid and a Rare Benzophenone Glycoside from the Leaves of Aquilaria sinensis. Chem. Pharm. Bull. 2009, 57, 134–137. [Google Scholar] [CrossRef]
- Pan, J.; Yi, X.; Wang, Y.; Chen, G.; He, X. Benzophenones from Mango Leaves Exhibit α-Glucosidase and NO Inhibitory Activities. J. Agric. Food Chem. 2016, 64, 7475–7480. [Google Scholar] [CrossRef]
- Gu, C.; Yang, M.; Zhou, Z.; Khan, A.; Cao, J.; Cheng, G. Purification and Characterization of Four Benzophenone Derivatives from Mangifera Indica L. Leaves and Their Antioxidant, Immunosuppressive and α-Glucosidase Inhibitory Activities. J. Funct. Foods 2019, 52, 709–714. [Google Scholar] [CrossRef]
- Ito, H.; Nishitani, E.; Konoshima, T.; Takasaki, M.; Kozuka, M.; Yoshida, T. Flavonoid and Benzophenone Glycosides from Coleogyne ramosissima. Phytochemistry 2000, 54, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-S.; Tseng, C.-C.; Chen, C.-K. Three New Benzophenone Glucosides from the Leaves of Planchonella Obovata. Helv. Chim. Acta 2010, 93, 522–529. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Xia, F.; Wang, K.-Y.; Chen, J.-M.; Tu, P.-F. Five New Benzophenone Glycosides from the Leaves of Aquilaria sinensis (Lour.) Gilg. Chin. Chem. Lett. 2014, 25, 1573–1576. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, J.; Wang, M.; Khan, S.I.; Zhai, C.; Xu, Q.; Huang, J.; Peng, C.; Xiong, G.; Wang, W.; et al. Benzophenone Glycosides from the Flower Buds of Aquilaria sinensis. Fitoterapia 2017, 121, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Y.-F.; Huo, H.-X.; Guan, P.-W.; Wang, C.-C.; Yao, H.-N.; Zhao, Y.-F.; Tu, P.-F.; Li, J. Benzophenone Glycosides from the Pericarps of Aquilaria Yunnanensis S. C. Huang. Nat. Prod. Res. 2020, 34, 2030–2036. [Google Scholar] [CrossRef]
- Feng, J.; Yang, X.-W.; Wang, R.-F. Bio-Assay Guided Isolation and Identification of α-Glucosidase Inhibitors from the Leaves of Aquilaria Sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef]
- Rancon, S.; Chaboud, A.; Darbour, N.; Comte, G.; Bayet, C.; Simon, P.-N.; Raynaud, J.; Di Pietro, A.; Cabalion, P.; Barron, D. Natural and Synthetic Benzophenones: Interaction with the Cytosolic Binding Domain of P-Glycoprotein. Phytochemistry 2001, 57, 553–557. [Google Scholar] [CrossRef]
- Ferrari, J.; Terreaux, C.; Sahpaz, S.; Msonthi, J.D.; Wolfender, J.-L.; Hostettmann, K. Benzophenone Glycosides from Gnidia involucrata. Phytochemistry 2000, 54, 883–889. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, Q.-H.; Zhao, W.; Zhang, X.; Zhang, Q.; Bi, Y.-F.; Zhang, Y.-B. Isolation, Characterization and Cytotoxic Activity of Benzophenone Glucopyranosides from Mahkota Dewa (Phaleria macrocarpa (Scheff.) Boerl). Bioorganic Med. Chem. Lett. 2012, 22, 6862–6866. [Google Scholar] [CrossRef]
- Osman, A.G.; Ali, Z.; Fantoukh, O.; Raman, V.; Kamdem, R.S.T.; Khan, I. Glycosides of Ursane-Type Triterpenoid, Benzophenone, and Iridoid from Vangueria Agrestis (Fadogia Agrestis) and Their Anti-Infective Activities. Nat. Prod. Res. 2020, 34, 683–691. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Xu, X.-J.; Liu, H.-M. Chemical Constituents from Mahkota Dewa. J. Asian Nat. Prod. Res. 2006, 8, 119–123. [Google Scholar] [CrossRef]
- Kaya, D.; Yalçın, F.N.; Bedir, E.; Çalış, İ.; Steinhauser, L.; Albert, K.; Ersöz, T. New Benzophenone Glucosides from the Aerial Parts of Gentiana Verna L. Subsp. Pontica (Soltok.) Hayek. Phytochem. Lett. 2011, 4, 459–461. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. Garcixanthone E and Garcimangophenone C: New Metabolites from Garcinia Mangostana and Their Cytotoxic and Alpha Amylase Inhibitory Potential. Life 2022, 12, 1875. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Kijima, K. An Iridoid Gentiobioside, a Benzophenone Glucoside and Acylated Flavone C-Glycosides from Tripterospermum japonicum (SIEB. et ZUCC.) MAXIM. Chem. Pharm. Bull. 2001, 49, 699–702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duan, Y.; Dai, Y.; Wang, G.; Chen, H.; Gao, H.; Chen, J.; Yao, X.; Zhang, X. Xanthone and Benzophenone Glycosides from the Stems of Cratoxylum formosum ssp. pruniflorum. Chem. Pharm. Bull. 2011, 59, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kim, S.B.; Ahn, J.H.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Inhibitory Activity of Benzophenones from Anemarrhena asphodeloides on Pancreatic Lipase. Nat. Prod. Commun. 2013, 8, 1934578X1300800419. [Google Scholar] [CrossRef]
- Wagner, R. Ueber Die Farbstoffe Des Gelbholzes (Morus Tinctoria). J. Für Prakt. Chem. 1850, 51, 82–106. [Google Scholar] [CrossRef]
- Hlasiwetz, H.; Pfaundler, L. Ueber Das Morin Und Die Moringerbsäure. Justus Liebigs Ann. Der Chem. 1863, 127, 351–361. [Google Scholar] [CrossRef]
- Ciamician, G.; Silber, P. Ueber Die Constitution Des Maclurins Und Phloretins. Berichte Der Dtsch. Chem. Ges. 1895, 28, 1393–1398. [Google Scholar] [CrossRef][Green Version]
- Minami, H.; Kinoshita, M.; Fukuyama, Y.; Kodama, M.; Yoshizawa, T.; Sugiura, M.; Nakagawa, K.; Tago, H. Antioxidant Xanthones from Garcinia subelliptica. Phytochemistry 1994, 36, 501–506. [Google Scholar] [CrossRef]
- Chiang, Y.-M.; Kuo, Y.-H.; Oota, S.; Fukuyama, Y. Xanthones and Benzophenones from the Stems of Garcinia multiflora. J. Nat. Prod. 2003, 66, 1070–1073. [Google Scholar] [CrossRef]
- Nguyen, L.H.D.; Venkatraman, G.; Sim, K.Y.; Harrison, L.J. Xanthones and Benzophenones from Garcinia Griffithii and Garcinia Mangostana. Phytochemistry 2005, 66, 1718–1723. [Google Scholar] [CrossRef]
- Muriithi, E.; Bojase-Moleta, G.; Majinda, R.R.T. Benzophenone Derivatives from Garcinia Livingstonei and Their Antioxidant Activities. Phytochem. Lett. 2016, 18, 29–34. [Google Scholar] [CrossRef]
- Choodej, S.; Koopklang, K.; Raksat, A.; Chuaypen, N.; Pudhom, K. Bioactive Xanthones, Benzophenones and Biphenyls from Mangosteen Root with Potential Anti-Migration against Hepatocellular carcinoma Cells. Sci. Rep. 2022, 12, 8605. [Google Scholar] [CrossRef]
- Minami, H.; Hamaguchi, K.; Kubo, M.; Fukuyama, Y. A Benzophenone and a Xanthone from Garcinia subelliptica. Phytochemistry 1998, 49, 1783–1785. [Google Scholar] [CrossRef]
- Fouotsa, H.; Lannang, A.M.; Dzoyem, J.P.; Tatsimo, S.J.N.; Neumann, B.; Mbazoa, C.D.; Razakarivony, A.A.; Nkengfack, A.E.; Eloff, J.N.; Sewald, N. Antibacterial and Antioxidant Xanthones and Benzophenone from Garcinia smeathmannii. Planta Medica 2015, 81, 594–599. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Sarma, M.R.; Venkataraman, K.; Yemul, S.S. A Benzophenone and Xanthone with Unusual Hydroxylation Patterns from the Heartwood of Garcinia pedunculata. Phytochemistry 1974, 13, 1241–1244. [Google Scholar] [CrossRef]
- Nargis, J.; Wong, K.-C.; Khairuddin, M.; Chantrapromma, S.; Fun, H.-K. (2,4-Dihydroxy-6-Methoxyphenyl)(3,5-Dihydroxyphenyl)Methanone Monohydrate. Acta Crystallogr. Sect. E 2011, 67, o2717–o2718. [Google Scholar] [CrossRef]
- Pailee, P.; Kuhakarn, C.; Sangsuwan, C.; Hongthong, S.; Piyachaturawat, P.; Suksen, K.; Jariyawat, S.; Akkarawongsapat, R.; Limthongkul, J.; Napaswad, C.; et al. Anti-HIV and Cytotoxic Biphenyls, Benzophenones and Xanthones from Stems, Leaves and Twigs of Garcinia speciosa. Phytochemistry 2018, 147, 68–79. [Google Scholar] [CrossRef]
- Nguyen Viet, D.; Le Ba, V.; Nguyen Duy, T.; Pham Thi, V.A.; Tran Thi, H.; Le Canh, V.C.; Bach Long, G.; Kim, Y.H.; Tuan Anh, H.L. Bioactive Compounds from the Aerial Parts of Hypericum sampsonii. Nat. Prod. Res. 2021, 35, 646–648. [Google Scholar] [CrossRef]
- Wang, M.; Ma, G.; Shao, F.; Liu, R.; Chen, L.; Liu, Y.; Yang, L.; Meng, X. Neoflavonoids from the Heartwood of Dalbergia melanoxylon. Nat. Prod. Res. 2022, 36, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Ninomiya, K.; Tagashira, Y.; Maejima, K.; Yoshida, T.; Amakura, Y. Polyphenolic Constituents of the Pericarp of Mangosteen (Garcinia mangostana L.). J. Agric. Food Chem. 2015, 63, 7670–7674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-C.; Nohara, T. Benzophenone C-Glucosides from Polygala telephioides. Chem. Pharm. Bull. 2000, 48, 1354–1355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, T.-J.; Shi, X.-C.; Jia, C.-X. Telephenone D, A New Benzophenone C-Glycoside from Polygala telephioides. Chin. J. Nat. Med. 2010, 8, 9–11. [Google Scholar] [CrossRef]
- Rouis, Z.; Abid, N.; Aouni, M.; Faiella, L.; Dal Piaz, F.; De Tommasi, N.; Braca, A. Benzophenone Glycosides from Hypericum Humifusum ssp. austral. J. Nat. Prod. 2013, 76, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.-Q.; Yang, D.; Ma, Q.-Y.; Yi, X.-H.; Zhou, J.; Zhao, Y.-X. A New Benzophenone from Dobinea delavayi. Chem. Nat. Compd. 2013, 49, 46–48. [Google Scholar] [CrossRef]
- Wu, X.-R.; Lang, L.-J.; Shen, Y.; Dong, X.; Xiao, C.-J.; Jiang, B. Four New Phenolic Glycosides from Dobinea delavayi. Nat. Prod. Res. 2023, 37, 1146–1153. [Google Scholar] [CrossRef]
- Nedialkov, P.T.; Kitanov, G.M. Two Benzophenone O-Arabinosides and a Chromone from Hypericum annulatum. Phytochemistry 2002, 59, 867–871. [Google Scholar] [CrossRef]
- Demirkiran, O.; Ahmed Mesaik, M.; Beynek, H.; Abbaskhan, A.; Iqbal Choudhary, M. Cellular Reactive Oxygen Species Inhibitory Constituents of Hypericum thasium Griseb. Phytochemistry 2009, 70, 244–249. [Google Scholar] [CrossRef]
- Demirkiran, O. Three New Benzophenone Glycosides with MAO-A Inhibitory Activity from Hypericum thasium Griseb. Phytochem. Lett. 2012, 5, 700–704. [Google Scholar] [CrossRef]
- Nedialkov, P.T.; Zheleva-Dimitrova, D.; Girreser, U.; Kitanov, G.M. Benzophenone O-Glycosides from Hypericum elegans. Nat. Prod. Res. 2009, 23, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kubota, T.; Kashiwada, Y.; Takaishi, Y.; Kobayashi, J. Petiolins F—I, Benzophenone Rhamnosides from Hypericum pseudopetiolatum var. kiusianum. Chem. Pharm. Bull. 2009, 57, 1171–1173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, J.; Hu, B.; Qian, M.; Zhang, J.; Wu, L. Benzophenone Rhamnosides and Chromones from Hypericum Seniawinii Maxim. Molecules 2022, 27, 7056. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Z.-M.; Wang, Y.; Li, R.-S.; Wang, F.; Wang, K. Phenolic Constituents with Neuroprotective Activities from Hypericum wightianum. Phytochemistry 2019, 165, 112049. [Google Scholar] [CrossRef] [PubMed]
- Petrunak, E.; Kester, A.C.; Liu, Y.; Bowen-Forbes, C.S.; Nair, M.G.; Henry, G.E. New Benzophenone O-Glucoside from Hypericum ellipticum. Nat. Prod. Commun. 2009, 4, 1934578X0900400412. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Mohamed, G.A.; Fahmy, U.A.; Eid, B.G.; Ahmed, O.A.; Al-Rabia, M.W.; Khedr, A.I.; Nasrullah, M.Z.; Ibrahim, S.R. New Alpha-Amylase Inhibitory Metabolites from Pericarps of Garcinia mangostana. Life 2022, 12, 384. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. New Benzophenones and a Dihydroflavanonol from Garcinia Mangostana Pericarps and Their Antioxidant and Cytotoxic Activities. Phytochem. Lett. 2020, 39, 43–48. [Google Scholar] [CrossRef]
- Powell, V.; Sutherland, M. Substituted Benzophenones from Leptospermum luehmannii (F. M. Bailey). Aust. J. Chem. 1963, 16, 282–284. [Google Scholar] [CrossRef]
- de Corréa, D.B.; Guerra, L.F.B.; Gottlieb, O.R.; Maia, J.G.S. C-Methyl Phenolics from Qualea Species. Phytochemistry 1981, 20, 305–307. [Google Scholar] [CrossRef]
- Gong, S.; Liu, C.; Liu, S.; Du, Y.; Kang, W.; Dong, X. Studies on constituents of the Chinese traditional drug baishouwu (Cynanchum auriculatum Royle ex Wight). Yao Xue Xue Bao 1988, 23, 276–280. [Google Scholar]
- Deng, Y.; Chin, Y.-W.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Anthraquinones with Quinone Reductase-Inducing Activity and Benzophenones from Morinda citrifolia (Noni) Roots. J. Nat. Prod. 2007, 70, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-D.; Xu, F.-Q.; Wu, D.-L.; Zhang, W.; Huang, Q. A new benzophenone isolated from fibrous roots of Anemarrhena asphodeloides. Zhongguo Zhong Yao Za Zhi 2019, 44, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Deng, K.Z.; Gao, W.Y.; Guo, Y.Q.; Zhang, T.J. A Novel Alkenoic Acid Ester and a New Benzophenone from Ranunculus ternatus. Chin. Chem. Lett. 2007, 18, 1364–1366. [Google Scholar] [CrossRef]
- Deng, K.-Z.; Xiong, Y.; Zhou, B.; Guan, Y.-M.; Luo, Y.-M. Chemical Constituents from the Roots of Ranunculus Ternatus and Their Inhibitory Effects on Mycobacterium tuberculosis. Molecules 2013, 18, 11859–11865. [Google Scholar] [CrossRef]
- Wu, B.-L.; Zou, H.-L.; Qin, F.-M.; Li, H.-Y.; Zhou, G.-X. New Ent-Kaurane-Type Diterpene Glycosides and Benzophenone from Ranunculus muricatus Linn. Molecules 2015, 20, 22445–22453. [Google Scholar] [CrossRef]
- Kawahara, N.; Sekita, S.; Satake, M.; Udagawa, S.I.; Kawai, K.I. Structures of a New Dihydroxanthone Derivative, Nidulalin A, and a New Benzophenone Derivative, Nidulalin B, from Emericella nidulans. Chem. Pharm. Bull. 1994, 42, 1720–1723. [Google Scholar] [CrossRef]
- Liu, H.-X.; Tan, H.-B.; Liu, Y.; Chen, Y.-C.; Li, S.-N.; Sun, Z.-H.; Li, H.-H.; Qiu, S.-X.; Zhang, W.-M. Three New Highly-Oxygenated Metabolites from the Endophytic Fungus Cytospora rhizophorae A761. Fitoterapia 2017, 117, 1–5. [Google Scholar] [CrossRef]
- Form, I.C.; Bonus, M.; Gohlke, H.; Lin, W.; Daletos, G.; Proksch, P. Xanthone, Benzophenone and Bianthrone Derivatives from the Hypersaline Lake-Derived Fungus Aspergillus wentii. Bioorganic Med. Chem. 2019, 27, 115005. [Google Scholar] [CrossRef]
- Liu, B.; Chen, N.; Chen, Y.; Shen, J.; Xu, Y.; Ji, Y. A New Benzophenone with Biological Activities Purified from Aspergillus fumigatus SWZ01. Nat. Prod. Res. 2021, 35, 5710–5719. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Feng, B.-M.; Zhao, Y.-Q.; Sun, Y.; Liu, B.; Liu, F.; Chen, G.; Bai, J.; Hua, H.-M.; Wang, H.-F.; et al. Polyketide Butenolide, Diphenyl Ether, and Benzophenone Derivatives from the Fungus Aspergillus flavipes PJ03-11. Bioorganic Med. Chem. Lett. 2016, 26, 346–350. [Google Scholar] [CrossRef]
- Liao, W.-Y.; Shen, C.-N.; Lin, L.-H.; Yang, Y.-L.; Han, H.-Y.; Chen, J.-W.; Kuo, S.-C.; Wu, S.-H.; Liaw, C.-C. Asperjinone, a Nor-Neolignan, and Terrein, a Suppressor of ABCG2-Expressing Breast Cancer Cells, from Thermophilic aspergillus Terreus. J. Nat. Prod. 2012, 75, 630–635. [Google Scholar] [CrossRef]
- Assante, G.; Camarda, L.; Nasini, G. Secondary Mould Metabolites. IX. Structure of a New Bianthrone and of Three New Secoanthraquinones from Asperguillus wentii Wehmer. Gazz. Chim. Ital. 1980, 110, 629–631. [Google Scholar]
- Salleh, W.M.N.H.W.; On, S.; Ahmad, F.; Sirat, H.M.; Taher, M.; Sarker, S.D.; Nahar, L. A New Xanthone and a New Benzophenone from the Roots of Garcinia hombroniana. Phytochem. Lett. 2020, 35, 216–219. [Google Scholar] [CrossRef]
- Iinuma, M.; Tosa, H.; Ito, T.; Tanaka, T.; Riswan, S. Three New Benzophenone-Xanthone Dimers from the Root of Garcinia dulcis. Chem. Pharm. Bull. 1996, 44, 1744–1747. [Google Scholar] [CrossRef][Green Version]
- Ngwoke, K.G.; Orame, N.; Liu, S.; Okoye, F.B.C.; Daletos, G.; Proksch, P. A New Benzophenone Glycoside from the Leaves of Mitracarpus villosus. Nat. Prod. Res. 2017, 31, 2354–2360. [Google Scholar] [CrossRef]
- Shu, J.; Chou, G.; Wang, Z. Two New Benzophenone Glycosides from the Fruit of Psidium guajava L. Fitoterapia 2010, 81, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-J.; Matsuta, T.; Kanazawa, T.; Chang, K.-J.; Park, C.-H.; Onjo, M. Phenolic Compounds from the Leaves of Psidium Guajava. I. Hydrolysable Tannins and Benzophenone glycosides. Chem. Nat. Compd. 2011, 47, 632. [Google Scholar] [CrossRef]
- Ukwueze, S.E.; Osadebe, P.O.; Okoye, F.B.C. A New Antibacterial Benzophenone Glycoside from Psidium guajava (Linn.) Leaves. Nat. Prod. Res. 2015, 29, 1728–1734. [Google Scholar] [CrossRef]
- Shu, J.-C.; Peng, C.-Y.; Liu, J.-Q.; Zhang, R. New Benzophenone and Diphenylmethane Glycosides from Psidium Littorale. Chem. Nat. Compd. 2015, 51, 865–869. [Google Scholar] [CrossRef]
- Terreaux, C.; Wang, Q.; Ioset, J.-R.; Ndjoko, K. Grimminger, Wolf; Hostettmann, Kurt Complete LC/MS Analysis of a Tinnevelli Senna Pod Extract and Subsequent Isolation and Identification of Two New Benzophenone glucosides. Planta Medica 2002, 68, 349–354. [Google Scholar] [CrossRef]
- Munsimbwe, L.; Suganuma, K.; Ishikawa, Y.; Choongo, K.; Kikuchi, T.; Shirakura, I.; Murata, T. Benzophenone Glucosides and B-Type Proanthocyanidin Dimers from Zambian Cassia Abbreviata and Their Trypanocidal Activities. J. Nat. Prod. 2022, 85, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, E.; Li, Y.; Wu, Q.; Tian, W.; Liu, K.; Niu, Y.; Wang, D.; Liu, J.-G.; Hu, Y. Iriflophenone Glycosides from Aquilaria sinensis. Chem. Nat. Compd. 2016, 52, 834–837. [Google Scholar] [CrossRef]
- Duan, W.-B.; Peng, A.-T.; Yuan, S.-N.; Wang, S.-N.; Li, B.-W.; Duan, X.-H. Two New Benzophenones from the Moss Pogonatum spinulosum. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dixit, D.; Reddy, C.R.K. Non-Targeted Secondary Metabolite Profile Study for Deciphering the Cosmeceutical Potential of Red Marine Macro Alga Jania Rubens—An LCMS-Based Approach. Cosmetics 2017, 4, 45. [Google Scholar] [CrossRef]
- Ishaque, M.; Bibi, Y.; Ayoubi, S.A.; Masood, S.; Nisa, S.; Qayyum, A. Iriflophenone-3-C-β-d Glucopyranoside from Dryopteris Ramosa (Hope) C. Chr. with Promising Future as Natural Antibiotic for Gastrointestinal Tract Infections. Antibiotics 2021, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ge, D.-D.; Zhang, Y.; Liu, E.-W.; Wang, T.; Hu, L.-M. Chemical Constituents of Mangifera indica Leaves (I). Chin. Tradit. Herb. Drugs 2011, 42, 428–431. [Google Scholar]
- San, H.T.; Chaowasku, T.; Khine, H.E.E.; Chaotham, C.; Rodsiri, R.; Sritularak, B.; Buraphaka, H.; Putalun, W.; Likhitwitayawuid, K. Chemical Constituents of Huberantha Jenkinsii Leaves and Their Glucose Uptake Stimulatory, Anti-Adipogenic, and Neuroprotective Activities. Chem. Nat. Compd. 2022, 58, 1146–1149. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Sommit, J.; Kasai, R.; Otsuka, H.; Yamasaki, K. Chemical Constituents of Thai Medicinal Plant, Polyalthia cerasoides. Nat. Med. 2002, 56, 268–271. [Google Scholar]
- Sun, Y.; Lin, H.; Wang, J.; Hu, J.; Liu, Z.; Gao, A. An Application of High-Speed Counter-Current Chromatography for Separation and Purification of Bungeiside-A, Bungeiside-B and Baishouwubenzophenone from Cynanchum bungei Decne. Phytochem. Anal. 2011, 22, 526–531. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Shen, Y.-M.; He, H.-P.; Mu, Q.-Z.; Hao, X.-J. Antifungal Agent and Other Constituents from Cynanchum otophyllum. Nat. Prod. Res. 2007, 21, 203–210. [Google Scholar] [CrossRef]
- Bian, J.; Xu, S.; Huang, S.; Wang, Z. Study on the Chemical Constituents of Anemarrhena asphodeloides Bge. Shenyuang Yaoke Daxue Xuebao 1996, 13, 34–40. [Google Scholar]
- Cai, J.; Xin, H.; Cheng, L.; Fu, Y.; Jiang, D.; Feng, J.; Fu, Q.; Jin, Y.; Liang, X. Preparative Separation of the Polar Part from the Rhizomes of Anemarrhena Asphodeloides Using a Hydrophilic C18 Stationary Phase. J. Chromatogr. B 2017, 1063, 149–155. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alsaggaf, A.H.A.; Ahmed, N. LC-QTOF-MS Analysis of Phenolics and Saponins Extracted from Aloe Vera Leaves via Microwave Technology in Optimal Condition. S. Afr. J. Bot. 2021, 139, 362–373. [Google Scholar] [CrossRef]
- Li, X.-Z.; Cheng, L.-Z.; Yan, Y.-M.; Liu, B.-H.; Cheng, Y.-X. SIRT1 Inhibitory Compounds from the Roots of Codonopsis pilosula. J. Asian Nat. Prod. Res. 2019, 21, 25–32. [Google Scholar] [CrossRef]
- Laraoui, H.; Haba, H.; Long, C.; Benkhaled, M. A New Flavanone Sulfonate and Other Phenolic Compounds from Fumana montana. Biochem. Syst. Ecol. 2019, 86, 103927. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Miyamoto, Y.; Onoda, S.; Rao, K.S.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Furukawa, H. Polyprenylated Benzophenones from Garcinia Assigu and Their Potential Cancer Chemopreventive Activities. J. Nat. Prod. 2003, 66, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Jamila, N.; Khairuddean, M.; Yaacob, N.S.; Kamal, N.N.S.N.M.; Osman, H.; Khan, S.N.; Khan, N. Cytotoxic Benzophenone and Triterpene from Garcinia hombroniana. Bioorganic Chem. 2014, 54, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.T.T.; Cuong, P.V.; Tra, N.T.; Anh, L.T.T.; Cham, B.T.; Son, N.T. Chemical Constituents from Methanolic Extract of Garcinia Mackeaniana Leaves and Their Antioxidant Activity. Vietnam J. Sci. Technol. 2020, 58, 411–418. [Google Scholar] [CrossRef]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Phenolics from Garcinia Mangostana Alleviate Exaggerated Vasoconstriction in Metabolic Syndrome through Direct Vasodilatation and Nitric Oxide Generation. BMC Complement. Altern. Med. 2016, 16, 359. [Google Scholar] [CrossRef]
- Holloway, D.M.; Scheinmann, F. Phenolic Compounds from the Heartwood of Garcinia mangostana. Phytochemistry 1975, 14, 2517–2518. [Google Scholar] [CrossRef]
- See, I.; Ee, G.C.; Teh, S.S.; Kadir, A.A.; Daud, S. Two New Chemical Constituents from the Stem Bark of Garcinia mangostana. Molecules 2014, 19, 7308–7316. [Google Scholar] [CrossRef] [PubMed]
- Ohno, R.; Moroishi, N.; Sugawa, H.; Maejima, K.; Saigusa, M.; Yamanaka, M.; Nagai, M.; Yoshimura, M.; Amakura, Y.; Nagai, R. Mangosteen Pericarp Extract Inhibits the Formation of Pentosidine and Ameliorates Skin Elasticity. J. Clin. Biochem. Nutr. 2015, 57, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Darwati, D.; Safitri, A.N.; Ambardhani, N.; Mayanti, T.; Nurlelasari, N.; Kurnia, D. Effectiveness and Anticancer Activity of a Novel Phenolic Compound from Garcinia Porrecta Against the MCF-7 Breast Cancer Cell Line In Vitro and In Silico. Drug Des. Dev. Ther. 2021, 15, 3523–3533. [Google Scholar] [CrossRef] [PubMed]
- Messi, B.B.; Ho, R.; Meli Lannang, A.; Cressend, D.; Perron, K.; Nkengfack, A.E.; Carrupt, P.-A.; Hostettmann, K.; Cuendet, M. Isolation and Biological Activity of Compounds from Garcinia preussii. Pharm. Biol. 2014, 52, 706–711. [Google Scholar] [CrossRef]
- Merza, J.; Aumond, M.-C.; Rondeau, D.; Dumontet, V.; Le Ray, A.-M.; Séraphin, D.; Richomme, P. Prenylated Xanthones and Tocotrienols from Garcinia virgata. Phytochemistry 2004, 65, 2915–2920. [Google Scholar] [CrossRef]
- Baslas, R.K.; Kumar, P. Isolation and Characterization of Biflavanone and Xanthones in the Fruits of Garcinia xanthochymus. Acta Cienc. Indica Chem. 1981, 7, 31–34. [Google Scholar]
- Locksley, H.D.; Moore, I.; Scheinmann, F. Extractives from Guttiferae—VI: The Significance of Maclurin in Xanthone biosynthesis. Tetrahedron 1967, 23, 2229–2234. [Google Scholar] [CrossRef]
- Pecchio, M.; Solís, P.N.; López-Pérez, J.L.; Vásquez, Y.; Rodríguez, N.; Olmedo, D.; Correa, M.; San Feliciano, A.; Gupta, M.P. Cytotoxic and Antimicrobial Benzophenones from the Leaves of Tovomita longifolia. J. Nat. Prod. 2006, 69, 410–413. [Google Scholar] [CrossRef]
- Nierenstein, M.; Webster, T.A. Weiße Mangrove von Der Westküste Afrikas. Chem. Zent. 1908, 2, 80. [Google Scholar]
- Xiong, Y.; Du, C.; Duan, Y.; Yuan, C.; Huang, L.; Gu, W.; Hao, X. Chemical Constituents and Pharmacological Activities of Sedum Aizoon Form Guizhou Province. Chin. Tradit. Herb. Drugs 2019, 50, 5404–5410. [Google Scholar] [CrossRef]
- Ahmad, M.; Muhammad, N.; Ahmad, M.; Arif Lodhi, M.; Mahjabeen; Jehan, N.; Khan, Z.; Ranjit, R.; Shaheen, F.; Iqbal Choudhary, M. Urease Inhibitor from Datisca cannabina Linn. J. Enzym. Inhib. Med. Chem. 2008, 23, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xue, J.; Xu, S.-X.; Zhang, R.-Q. Chemical Constituents of the Leaves of Diospyros Kaki and Their Cytotoxic Effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Nierenstein, M. On the Presence of Maclurin in the Sapwood of the Cutch-Producing Acacias. J. Indian Chem. Soc 1931, 8, 143–145. [Google Scholar]
- Silva, T.S.; Gomes, J.M.; Camilla, P.; Maria, F.; Marcelo, S.; Edeltrudes, O.; Josean, F.T. Evaluation of Antimicrobial Activity of Extract, Fractions and Isolated Substances from Calliandra umbellifera Benth. Lat. Am. J. Pharm. 2013, 32, 1408–1411. [Google Scholar]
- Kokotkiewicz, A.; Luczkiewicz, M.; Pawlowska, J.; Luczkiewicz, P.; Sowinski, P.; Witkowski, J.; Bryl, E.; Bucinski, A. Isolation of Xanthone and Benzophenone Derivatives from Cyclopia genistoides (L.) Vent. (Honeybush) and Their pro-Apoptotic Activity on Synoviocytes from Patients with Rheumatoid Arthritis. Fitoterapia 2013, 90, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Roza, O.; Martins, A.; Hohmann, J.; Lai, W.-C.; Eloff, J.; Chang, F.-R.; Csupor, D. Flavonoids from Cyclopia genistoides and Their Xanthine Oxidase Inhibitory Activity. Planta Medica 2016, 82, 1274–1278. [Google Scholar] [CrossRef]
- Walters, N.A.; de Beer, D.; de Villiers, A.; Walczak, B.; Joubert, E. Genotypic Variation in Phenolic Composition of Cyclopia Pubescens (Honeybush Tea) Seedling Plants. J. Food Compos. Anal. 2019, 78, 129–137. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Luczkiewicz, M.; Sowinski, P.; Glod, D.; Gorynski, K.; Bucinski, A. Isolation and Structure Elucidation of Phenolic Compounds from Cyclopia subternata Vogel (honeybush) Intact Plant and In Vitro Cultures. Food Chem. 2012, 133, 1373–1382. [Google Scholar] [CrossRef]
- Ollis, W.D. The Neoflavanoids, a New Class of Natural Products. Experientia 1966, 22, 777–783. [Google Scholar] [CrossRef]
- Rao, P.R.; Narayanan, M.C.; Gopalakrishnan, S.M.; Shanmugam, N.N. Two New Isoflavonoids from the Roots of Dalbergia congesta (Grah). J. Asian Nat. Prod. Res. 2006, 8, 143–148. [Google Scholar] [CrossRef]
- Mori-Yasumoto, K.; Hashimoto, Y.; Agatsuma, Y.; Fuchino, H.; Yasumoto, K.; Shirota, O.; Satake, M.; Sekita, S. Leishmanicidal Phenolic Compounds Derived from Dalbergia cultrata. Nat. Prod. Res. 2021, 35, 4907–4915. [Google Scholar] [CrossRef]
- Donnelly, D.M.X.; Criodain, T.O.; O’Sullivan, M. Dalbergia Species: XV. Dalcriodain, a Binary Neoflavanoid. Proc. R. Ir. Academy. Sect. B Biol. Geol. Chem. Sci. 1983, 83B, 39–48. [Google Scholar]
- Chan, S.-C.; Chang, Y.-S.; Kuo, S.-C. Neoflavonoids from Dalbergia odorifera. Phytochemistry 1997, 46, 947–949. [Google Scholar] [CrossRef]
- An, R.-B.; Jeong, G.-S.; Kim, Y.-C. Flavonoids from the Heartwood of Dalbergia Odorifera and Their Protective Effect on Glutamate-Induced Oxidative Injury in HT22 Cells. Chem. Pharm. Bull. 2008, 56, 1722–1724. [Google Scholar] [CrossRef] [PubMed]
- Muangnoicharoen, N.; Frahm, A.W. Neoflavanoids of Dalbergia Parviflora. Phytochemistry 1982, 21, 767–772. [Google Scholar] [CrossRef]
- Kumar, P.; Kushwaha, P.; Khedgikar, V.; Gautam, J.; Choudhary, D.; Singh, D.; Trivedi, R.; Maurya, R. Neoflavonoids as Potential Osteogenic Agents from Dalbergia sissoo Heartwood. Bioorganic Med. Chem. Lett. 2014, 24, 2664–2668. [Google Scholar] [CrossRef]
- Khera, U.; Chibber, S. Chemical Constituents of Dalbergia Volubilis. Isolation of Cearoin and (+)-Medicarpin. Indian J. Chem. Sect. B 1978, 16, 78–79. [Google Scholar]
- Wu, S.-F.; Hwang, T.-L.; Chen, S.-L.; Wu, C.-C.; Ohkoshi, E.; Lee, K.-H.; Chang, F.-R.; Wu, Y.-C. Bioactive Components from the Heartwood of Pterocarpus santalinus. Bioorganic Med. Chem. Lett. 2011, 21, 5630–5632. [Google Scholar] [CrossRef]
- Wu, S.-F.; Chang, F.-R.; Wang, S.-Y.; Hwang, T.-L.; Lee, C.-L.; Chen, S.-L.; Wu, C.-C.; Wu, Y.-C. Anti-Inflammatory and Cytotoxic Neoflavonoids and Benzofurans from Pterocarpus santalinus. J. Nat. Prod. 2011, 74, 989–996. [Google Scholar] [CrossRef]
- Beerhues, L. Benzophenone Synthase from Cultured Cells of Centaurium erythraea. FEBS Lett. 1996, 383, 264–266. [Google Scholar] [CrossRef]
- Kumar, V.; Sood, H.; Chauhan, R.S. Detection of Intermediates through High-Resolution Mass Spectrometry for Constructing Biosynthetic Pathways for Major Chemical Constituents in a Medicinally Important Herb, Swertia Chirayita. Nat. Prod. Res. 2015, 29, 1449–1455. [Google Scholar] [CrossRef]
- Schmidt, W.; Beerhues, L. Alternative Pathways of Xanthone Biosynthesis in Cell Cultures of Hypericum androsaemum L. FEBS Lett. 1997, 420, 143–146. [Google Scholar] [CrossRef]
- Momekov, G.; Nedialkov, P.T.; Kitanov, G.M.; Zh Zheleva-Dimitrova, D.; Tzanova, T.; Girreser, U.; Karaivanova, M. Cytoprotective Effects of 5 Benzophenones and a Xanthone from Hypericum Annulatum in Models of Epirubicin-Induced Cytotoxicity: SAR-Analysis and Mechanistic Investigations. Med. Chem. 2006, 2, 377–384. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Li, Y.; Li, K.-Z.; Peng, L.-Y.; He, J.; Wang, K.; Pan, Z.-H.; Cheng, X.; Li, M.-M.; Zhao, Q.-S.; et al. Spirocyclic Acylphloroglucinol Derivatives from Hypericum beanii. Chem. Pharm. Bull. 2011, 59, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.E.; Campbell, M.S.; Zelinsky, A.A.; Liu, Y.; Bowen-Forbes, C.S.; Li, L.; Nair, M.G.; Rowley, D.C.; Seeram, N.P. Bioactive Acylphloroglucinols from Hypericum densiflorum. Phytother. Res. 2009, 23, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.-T.; An, Z.; Tang, D.; Peng, G.-R.; Cao, C.-Y.; Xu, Y.-Z.; Li, C.-H.; Liu, P.-L.; Jiang, Z.-M.; Gao, J.-M. Hyperelatosides A–E, Biphenyl Ether Glycosides from Hypericum Elatoides, with Neurotrophic Activity. RSC Adv. 2018, 8, 26646–26655. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Kitanov, G. Benzophenones and Flavonoids from Hypericum Maculatum and Their Antioxidant Activities. Nat. Prod. Res. 2012, 26, 1576–1583. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Chen, Q.; Li, N. Hyperprzeone A, a New Benzophenone with Cytotoxicity from Hypericum przewalskii Maxim. Nat. Prod. Res. 2021, 35, 4960–4968. [Google Scholar] [CrossRef]
- Xie, J.-Y.; Jin, Q.; Gao, J.-M.; Zong, S.-C.; Yan, X.-T. Two New Benzophenone Glycosides from the Aerial Parts of Hypericum przewalskii. Nat. Prod. Res. 2022, 36, 3520–3528. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Yin, F.; Hu, L.-H.; Lu, P. Sulfonated Xanthones from Hypericum Sampsonii. Phytochemistry 2004, 65, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Monthakantirat, O.; De-Eknamkul, W.; Umehara, K.; Yoshinaga, Y.; Miyase, T.; Warashina, T.; Noguchi, H. Phenolic Constituents of the Rhizomes of the Thai Medicinal Plant Belamcanda Chinensis with Proliferative Activity for Two Breast Cancer Cell Lines. J. Nat. Prod. 2005, 68, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.-Y.; Zhu, Y.; Shu, P.; Qin, X.-Y.; Wu, G.; Wang, Q.; Qin, M.-J. Phenolic Metabolite Profiles and Antioxidants Assay of Three Iridaceae Medicinal Plants for Traditional Chinese Medicine “She-Gan” by on-Line HPLC–DAD Coupled with Chemiluminescence (CL) and ESI-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2014, 98, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Morita, N.; Kondo, Y.; Takemoto, T. Studies on Constituents of Iris Genus Plants. IV. The Constituents of Iris florentina L. (2). Chem Pharm Bull 1973, 21, 2323–2328. [Google Scholar] [CrossRef]
- Dhar, K.L.; Kalla, A.K. 2,4,6,4′-Tetrahydroxybenzophenone in Iris germanica. Phytochemistry 1974, 13, 2894. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Gašić, U.M.; Pešić, M.B.; Stanojević, S.P.; Barać, M.B.; Mačukanović-Jocić, M.P.; Avramov, S.N.; Tešić, Ž.L. Phytochemical Analysis and Total Antioxidant Capacity of Rhizome, Above-Ground Vegetative Parts and Flower of Three Iris Species. Chem. Biodivers. 2019, 16, e1800565. [Google Scholar] [CrossRef] [PubMed]
- Лужанин, В.Г.; Уэйли, А.; Пoнкратoва, А.О.; Жoхoва, Е.В.; Зингалюк, М.А.; Пряхина, Н.И. Касатик мoлoчнo-белый (Iris lactea Pall.)-перспективный истoчник биoлoгически активных веществ. Химия Растительнoгo Сырья 2021, 5–17. [Google Scholar] [CrossRef]
- Roger, B.; Jeannot, V.; Fernandez, X.; Cerantola, S.; Chahboun, J. Characterisation and Quantification of Flavonoids in Iris Germanica L. and Iris pallida Lam. Resinoids from Morocco. Phytochem. Anal. 2012, 23, 450–455. [Google Scholar] [CrossRef]
- Purev, O.; Purevsuren, C.; Narantuya, S.; Lkhagvasuren, S.; Mizukami, H.; Nagatsu, A. New Isoflavones and Flavanol from Iris potaninii. Chem. Pharm. Bull. 2002, 50, 1367–1369. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Wang, H.; Dong, X.-F.; Zhao, C.-Q. Chemical Constituents from Iris Scariosa and Iris halophila var. Sogdiana. Chin. Tradit. Herb. Drugs 2013, 44, 1371–1375. [Google Scholar]
- Tung, N.H.; Hung, L.Q.; Van Oanh, H.; Huong, D.T.L.; Thuong, P.T.; Long, D.D.; Hai, N.T. Bioactive Phenolic Compounds from the Roots of Danshen (Salvia miltiorrhiza). Nat. Prod. Commun. 2018, 13, 1934578X1801301018. [Google Scholar] [CrossRef]
- Gottlieb, O.R.; Mors, W.B. The Chemistry of Rosewood. II. Isolation and Identification of Cotoin and Pinocembrin. J. Am. Chem. Soc. 1958, 80, 2263–2265. [Google Scholar] [CrossRef]
- Song, M.-C.; Nigussie, F.; Jeong, T.-S.; Lee, C.-Y.; Regassa, F.; Markos, T.; Baek, N.-I. Phenolic Compounds from the Roots of Lindera fruticosa. J. Nat. Prod. 2006, 69, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-C.; Nigussie, F.; Yang, H.-J.; Baek, N.-I. A New Benzophenone from Lindera fruticosa. Bull. Korean Chem. Soc. 2007, 28, 1209–1210. [Google Scholar] [CrossRef]
- Seil, H.A. Composition of Nectandra Coto, Rusby Nov. Preliminary Report. J. Am. Pharm. Assoc. (1912) 1922, 11, 904–906. [Google Scholar] [CrossRef]
- Olalere, O.A.; Gan, C.-Y.; Akintomiwa, O.E.; Adeyi, O.; Adeyi, A. Optimisation of Microwave-Assisted Extraction and Functional Elucidation of Bioactive Compounds from Cola Nitida Pod. Phytochem. Anal. 2021, 32, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Nierenstein, M. Identity of Laguncurin, Kino-Yellow and Maclurin. Quart. J. Pharm. Pharmacol. 1943, 16, 11–12. [Google Scholar]
- Tian, L.-W.; Xu, M.; Li, Y.; Li, X.-Y.; Wang, D.; Zhu, H.-T.; Yang, C.-R.; Zhang, Y.-J. Phenolic Compounds from the Branches of Eucalyptus maideni. Chem. Biodivers. 2012, 9, 123–130. [Google Scholar] [CrossRef]
- Shaheen, F.; Ahmad, M.; Nahar Khan, S.; Samreen Hussain, S.; Anjum, S.; Tashkhodjaev, B.; Turgunov, K.; Sultankhodzhaev, M.N.; Choudhary, M.I. Atta-ur-Rahman New α-Glucosidase Inhibitors and Antibacterial Compounds from Myrtus communis L. Eur. J. Org. Chem. 2006, 2006, 2371–2377. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Li, R.; Li, L.; Wang, N. Study on Anti-Oxidative Components from Leaves of Psidium guajava. Chin. Tradit. Herb. Drugs 2010, 41, 1593–1597. [Google Scholar]
- Feng, X.; Wang, Z.; Meng, D.; Li, X. Cytotoxic and Antioxidant Constituents from the Leaves of Psidium guajava. Bioorganic Med. Chem. Lett. 2015, 25, 2193–2198. [Google Scholar] [CrossRef]
- Ding, L.; Zuo, Q.; Li, D.; Feng, X.; Gao, X.; Zhao, F.; Qiu, F. A New Phenone from the Roots of Paeonia suffruticosa Andrews. Nat. Prod. Res. 2017, 31, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Giambanelli, E.; Gómez-Caravaca, A.M.; Ruiz-Torralba, A.; Guerra-Hernández, E.J.; Figueroa-Hurtado, J.G.; García-Villanova, B.; Verardo, V. New Advances in the Determination of Free and Bound Phenolic Compounds of Banana Passion Fruit Pulp (Passiflora tripartita, Var. Mollissima (Kunth) L.H. Bailey) and Their In Vitro Antioxidant and Hypoglycemic Capacities. Antioxidants 2020, 9, 628. [Google Scholar] [CrossRef]
- Mane, M.P.; Patil, R.S.; Magdum, A.B.; Kakade, S.S.; Patil, D.N.; Nimbalkar, M.S. Chemo-Profiling by UPLC-QTOF MS Analysis and in Vitro Assessment of Anti-Inflammatory Activity of Field Milkwort (Polygala arvensis Willd.). S. Afr. J. Bot. 2022, 149, 49–59. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Zhang, S.-Y.; Guo, Q.; Chai, X.-Y.; Jiang, Y.; Peng-Fei, Y.U. Chemical Investigation of the Roots of Polygala sibirica L. Chin. J. Nat. Med. 2013, 12, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.C.; Moshi, M.J.; Sempombe, J.; Nkunya, M.H.H. (4-Methoxy-Benzo[1,3]Dioxol-5-Yl)-Phenylmethanone: An Antibacterial Benzophenone from Securidaca Longepedunculata. Afr. J. Tradit Complement Altern Med. 2006, 3, 80–86. [Google Scholar] [CrossRef][Green Version]
- Green, M.W.; King, C.G.; Beal, G.D. Constituents in Cascara Sagrada Extract. 3. The Lipids and Glycosides. J. Am. Pharm. Assoc. 1938, 27, 95–100. [Google Scholar] [CrossRef]
- Duangsodsri, T.; Villain, L.; Vestalys, I.R.; Michalet, S.; Abdallah, C.; Breitler, J.-C.; Bordeaux, M.; Villegas, A.M.; Raherimandimby, M.; Legendre, L.; et al. 5-CQA and Mangiferin, Two Leaf Biomarkers of Adaptation to Full Sun or Shade Conditions in Coffea arabica L. Metabolites 2020, 10, 383. [Google Scholar] [CrossRef]
- Ma, Q.; Xie, H.; Jiang, Y.; Wei, X. Phenolics and Sesquiterpenes from Litchi pericarp. J. Funct. Food. 2014, 9, 156–161. [Google Scholar] [CrossRef]
- Yue, W.; Sun, W.; Rao, R.S.P.; Ye, N.; Yang, Z.; Chen, M. Non-Targeted Metabolomics Reveals Distinct Chemical Compositions among Different Grades of Bai Mudan White Tea. Food Chem. 2019, 277, 289–297. [Google Scholar] [CrossRef]
- Ito, T.; Kakino, M.; Tazawa, S.; Oyama, M.; Maruyama, H.; Araki, Y.; Hara, H.; Iinuma, M. Identification of Phenolic Compounds in Aquilaria Crassna Leaves via Liquid Chromatography-Electrospray Ionization Mass Spectroscopy. Food Sci. Technol. Res. 2012, 18, 259–262. [Google Scholar] [CrossRef]
- Eissa, M.A.; Hashim, Y.Z.H.-Y.; Abdul Azziz, S.S.; Salleh, H.M.; Isa, M.L.M.; Abd Warif, N.M.; Abdullah, F.; Ramadan, E.; El-Kersh, D.M. Phytochemical Constituents of Aquilaria Malaccensis Leaf Extract and Their Anti-Inflammatory Activity against LPS/IFN-γ-Stimulated RAW 264.7 Cell Line. ACS Omega 2022, 7, 15637–15646. [Google Scholar] [CrossRef] [PubMed]
- Susilawati, S.; Matsjeh, S.; Pranowo, H.D.; Anwar, C. Antioxidant Activity of 2,6,4′-Trihydroxy-4-Methoxy Benzophenone from Ethyl Acetate Extract of Leaves of Mahkota Dewa (Phaleria macrocarpa (Scheff.) Boerl.). Indo. J. Chem. 2011, 11, 180–185. [Google Scholar] [CrossRef]
- Hartati, W.M.S.; Mubarika, S.; Gandjar, I.G.; Hamann, M.T.; Rao, K.V.; Wahyuono, S. Phalerin, a New Benzophenoic Glucoside Isolated from the Methanolic Extract of Mahkota Dewa [Phaleria macrocarpa (Scheff). Boerl.] Leaves. Indones. J. Pharm. 2005, 16, 51–57. [Google Scholar]
- Oshimi, S.; Zaima, K.; Matsuno, Y.; Hirasawa, Y.; Iizuka, T.; Studiawan, H.; Indrayanto, G.; Zaini, N.C.; Morita, H. Studies on the Constituents from the Fruits of Phaleria macrocarpa. J. Nat. Med. 2008, 62, 207–210. [Google Scholar] [CrossRef]
- Tambunan, R.M.; Simanjuntak, P. Determination of Chemical Structure of Antioxidant Compound Benzophenon Glycoside from N-Butanol Extract of the Fruits of Mahkota Dewa [Phaleria Macrocarpa (Scheff) Boerl.]. Indones. J. Pharm. 2006, 17, 184–189. [Google Scholar]
- Kitalong, C.; El-Halawany, A.M.; El-Dine, R.; Chao-mei, M.; Hattori, M. Phenolics from Phaleria Nisidai with Estrogenic Activity. Rec. Nat. Prod. 2012, 6, 296–300. [Google Scholar]
- Awale, S.; Shrestha, S.P.; Tezuka, Y.; Ueda, J.; Matsushige, K.; Kadota, S. Neoflavonoids and Related Constituents from Nepalese Propolis and Their Nitric Oxide Production Inhibitory Activity. J. Nat. Prod. 2005, 68, 858–864. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Momekov, G. Benzophenones from Hypericum Elegans with Antioxidant and Acetylcholinesterase Inhibitory Potential. Pharmacogn. Mag. 2013, 9, S1. [Google Scholar] [CrossRef]
- Wongwad, E.; Pingyod, C.; Saesong, T.; Waranuch, N.; Wisuitiprot, W.; Sritularak, B.; Temkitthawon, P.; Ingkaninan, K. Assessment of the Bioactive Components, Antioxidant, Antiglycation and Anti-Inflammatory Properties of Aquilaria Crassna Pierre Ex Lecomte Leaves. Ind. Crops Prod. 2019, 138, 111448. [Google Scholar] [CrossRef]
- Malherbe, C.J.; Willenburg, E.; de Beer, D.; Bonnet, S.L.; van der Westhuizen, J.H.; Joubert, E. Iriflophenone-3-C-Glucoside from Cyclopia Genistoides: Isolation and Quantitative Comparison of Antioxidant Capacity with Mangiferin and Isomangiferin Using on-Line HPLC Antioxidant Assays. J. Chromatogr. B 2014, 951–952, 164–171. [Google Scholar] [CrossRef]
- Chan, S.-C.; Chang, Y.-S.; Wang, J.-P.; Chen, S.-C.; Kuo, S.-C. Three New Flavonoids and Antiallergic, Anti-Inflammatory Constituents from the Heartwood of Dalbergia odorifera. Planta Medica 2007, 64, 153–158. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Ohsawa, K.; Ishikawa, T.; Nakamura, F.; Ueda, F.; Narukawa, Y.; Kiuchi, F.; Tamura, H.; Tago, K.; Kasahara, T. Inhibitory Effects of Flavonoids Extracted from Nepalese Propolis on the LPS Signaling Pathway. Int. Immunopharmacol. 2016, 40, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Di, L.; Zhang, Y.; Li, N. Chemical Constituents with Cytotoxic and Anti-Inflammatory Activity in Hypericum Sampsonii and the Antitumor Potential under the View of Cancer-Related Inflammation. J. Ethnopharmacol. 2020, 259, 112948. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-R.; Yoon, Y.-S.; Hong, J.-P.; Kim, J.-M.; Lee, K.-T.; Jang, D.S. Chemical Constituents of the Roots of Polygala tenuifolia and Their Anti-Inflammatory Effects. Plants 2022, 11, 3307. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Al-Abd, A.M.; El-halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New Xanthones and Cytotoxic Constituents from Garcinia Mangostana Fruit Hulls against Human Hepatocellular, Breast, and Colorectal Cancer Cell Lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ouyang, W.; Pan, C.; Gao, Z.; Han, Y.; Song, M.; Feng, K.; Xiao, H.; Cao, Y. Identification of a New Benzophenone from Psidium guajava L. Leaves and Its Antineoplastic Effects on Human Colon Cancer Cells. Food Funct. 2019, 10, 4189–4198. [Google Scholar] [CrossRef] [PubMed]
- Winarno, H.; Katrin, W.E. Benzophenone Glucoside Isolated from the Ethyl Acetate Extract of the Bark of Mahkota Dewa [Phaleria macrocarpa (Scheff.) Boerl.] and Its Inhibitory Activity on Leukemia L1210 Cell Line. Indones. J. Chem. 2009, 9, 142–145. [Google Scholar] [CrossRef]
- Mitcheva, M.; Kondeva, M.; Vitcheva, V.; Nedialkov, P.; Kitanov, G. Effect of Benzophenones from Hypericum Annulatum on Carbon Tetrachloride-Induced Toxicity in Freshly Isolated Rat Hepatocytes. Redox Rep. 2006, 11, 3–8. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Li, D.; Ma, H.; Mu, Y.; Huang, X.; Li, L. Guavinoside B from Psidium Guajava Alleviates Acetaminophen-Induced Liver Injury via Regulating the Nrf2 and JNK Signaling Pathways. Food Funct. 2020, 11, 8297–8308. [Google Scholar] [CrossRef]
- Supasuteekul, C.; Tadtong, S.; Putalun, W.; Tanaka, H.; Likhitwitayawuid, K.; Tengamnuay, P.; Sritularak, B. Neuritogenic and Neuroprotective Constituents from Aquilaria Crassna Leaves. J. Food Biochem. 2017, 41, e12365. [Google Scholar] [CrossRef]
- Zhao, W.; Cross, A.R.; Crowe-McAuliffe, C.; Weigert-Munoz, A.; Csatary, E.E.; Solinski, A.E.; Krysiak, J.; Goldberg, J.B.; Wilson, D.N.; Medina, E.; et al. The Natural Product Elegaphenone Potentiates Antibiotic Effects against Pseudomonas aeruginosa. Angew. Chem. Int. Ed. 2019, 58, 8581–8584. [Google Scholar] [CrossRef]
- Othman, S.N.A.M.; Sarker, S.D.; Talukdar, A.D.; Ningthoujam, S.S.; Khamis, S.; Basar, N. Chemical Constituents and Antibacterial Activitiy of Phaleria macrocarpa (Scheff.) Boerl. Int. J. Pharm. Sci. Res. 2014, 5, 3157–3162. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Bayoumi, S.A.H.; Chen, C.; Vavricka, C.J.; Li, L.; Malik, A.; Dai, H.; Song, F.; Wang, L.; Zhang, J.; et al. Benzophenone C-Glucosides and Gallotannins from Mango Tree Stem Bark with Broad-Spectrum Anti-Viral Activity. Bioorganic Med. Chem. 2014, 22, 2236–2243. [Google Scholar] [CrossRef]
- Kaya, D.; Jäger, A.K.; Yalçın, F.N.; Ersöz, T. MAO-A Inhibition Profiles of Some Benzophenone Glucosides from Gentiana verna subsp. Pontica. Nat. Prod. Commun. 2014, 9, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, I.; Chisvert, A.; León, Z.; Salvador, A. Determination of Hydroxylated Benzophenone UV Filters in Sea Water Samples by Dispersive Liquid–Liquid Microextraction Followed by Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2010, 1217, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-K.; Sarma, S.N.; Kim, Y.-J.; Ryu, J.-C. Toxicokinetics and Metabolisms of Benzophenone-Type UV Filters in Rats. Toxicology 2008, 248, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kunisue, T.; Chen, Z.; Buck Louis, G.M.; Sundaram, R.; Hediger, M.L.; Sun, L.; Kannan, K. Urinary Concentrations of Benzophenone-Type UV Filters in U.S. Women and Their Association with Endometriosis. Environ. Sci. Technol. 2012, 46, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K. Occurrences, Toxicities, and Ecological Risks of Benzophenone-3, a Common Component of Organic Sunscreen Products: A Mini-Review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef]
- Wang, M.; Yu, Y.; Tang, Y.; Pan, C.; Fei, Q.; Hu, Z.; Li, H.; Zhu, Y.; Wang, Y.; Ge, R. Benzophenone-1 and -2 UV-Filters Potently Inhibit Human, Rat, and Mouse Gonadal 3β-Hydroxysteroid Dehydrogenases: Structure-Activity Relationship and in Silico Docking Analysis. J. Steroid Biochem. Mol. Biol. 2023, 230, 106279. [Google Scholar] [CrossRef]
- Thia, E.; Chou, P.-H.; Chen, P.-J. In Vitro and In Vivo Screening for Environmentally Friendly Benzophenone-Type UV Filters with Beneficial Tyrosinase Inhibition Activity. Water Res. 2020, 185, 116208. [Google Scholar] [CrossRef]
- Blüthgen, N.; Zucchi, S.; Fent, K. Effects of the UV Filter Benzophenone-3 (Oxybenzone) at Low Concentrations in Zebrafish (Danio Rerio). Toxicol. Appl. Pharmacol. 2012, 263, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, I.; Locksley, H.D.; Scheinmann, F. Xanthones in Higher Plants: Biogenetic Proposals and a Chemotaxonomic Survey. Phytochemistry 1969, 8, 2013–2025. [Google Scholar] [CrossRef]
- Bennett, G.J.; Lee, H.-H. Xanthones from Guttiferae. Phytochemistry 1989, 28, 967–998. [Google Scholar] [CrossRef]
- Peres, V.; Nagem, T.J. Trioxygenated Naturally Occurring Xanthones. Phytochemistry 1997, 44, 191–214. [Google Scholar] [CrossRef]
- Wolfrom, M.L.; Komitsky, F., Jr.; Fraenkel, G.; Looker, J.H.; Dickey, E.E.; McWain, P.; Thompson, A.; Mundell, P.M.; Windrath, O.M. Osage Orange Pigments. XIV. The Structure of Macluraxanthone1a-c. J. Org. Chem. 1964, 29, 692–697. [Google Scholar] [CrossRef]
- Franz, G.; Grün, M. Chemistry, Occurrence and Biosynthesis of C-Glycosyl Compounds in Plants. Planta Medica 1983, 47, 131–140. [Google Scholar] [CrossRef]
- Atkinson, J.E.; Gupta, P.; Lewis, J.R. Benzophenone Participation in Xanthone Biosynthesis (Gentianaceae). Chem. Commun. 1968, 1386–1387. [Google Scholar] [CrossRef]
- Liu, B.; Falkenstein-Paul, H.; Schmidt, W.; Beerhues, L. Benzophenone Synthase and Chalcone Synthase from Hypericum Androsaemum Cell Cultures: CDNA Cloning, Functional Expression, and Site-Directed Mutagenesis of Two Polyketide Synthases. Plant J. 2003, 34, 847–855. [Google Scholar] [CrossRef]
- Birch, A.J.; Baldas, J.; Hlubucek, J.R.; Simpson, T.J.; Westerman, P.W. Biosynthesis of the Fungal Xanthone Ravenelin. J. Chem. Soc. Perkin Trans. 1 1976, 898–904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).