Use of Human Dominated Landscape as Connectivity Corridors among Fragmented Habitats for Wild Asian Elephants (Elephas maximus) in the Eastern Part of Thailand
Abstract
1. Introduction
2. Materials and Methods
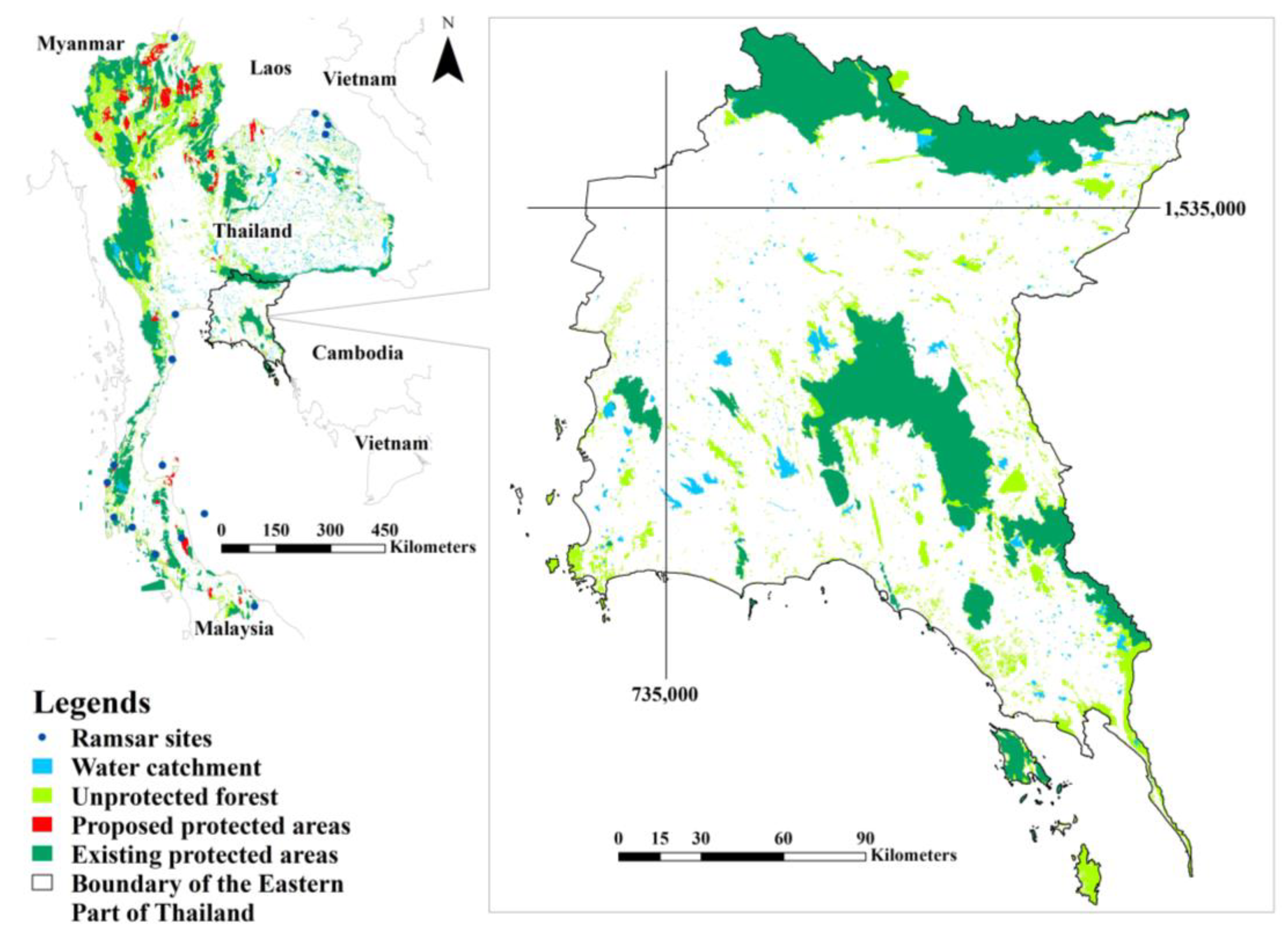
2.1. Study Area
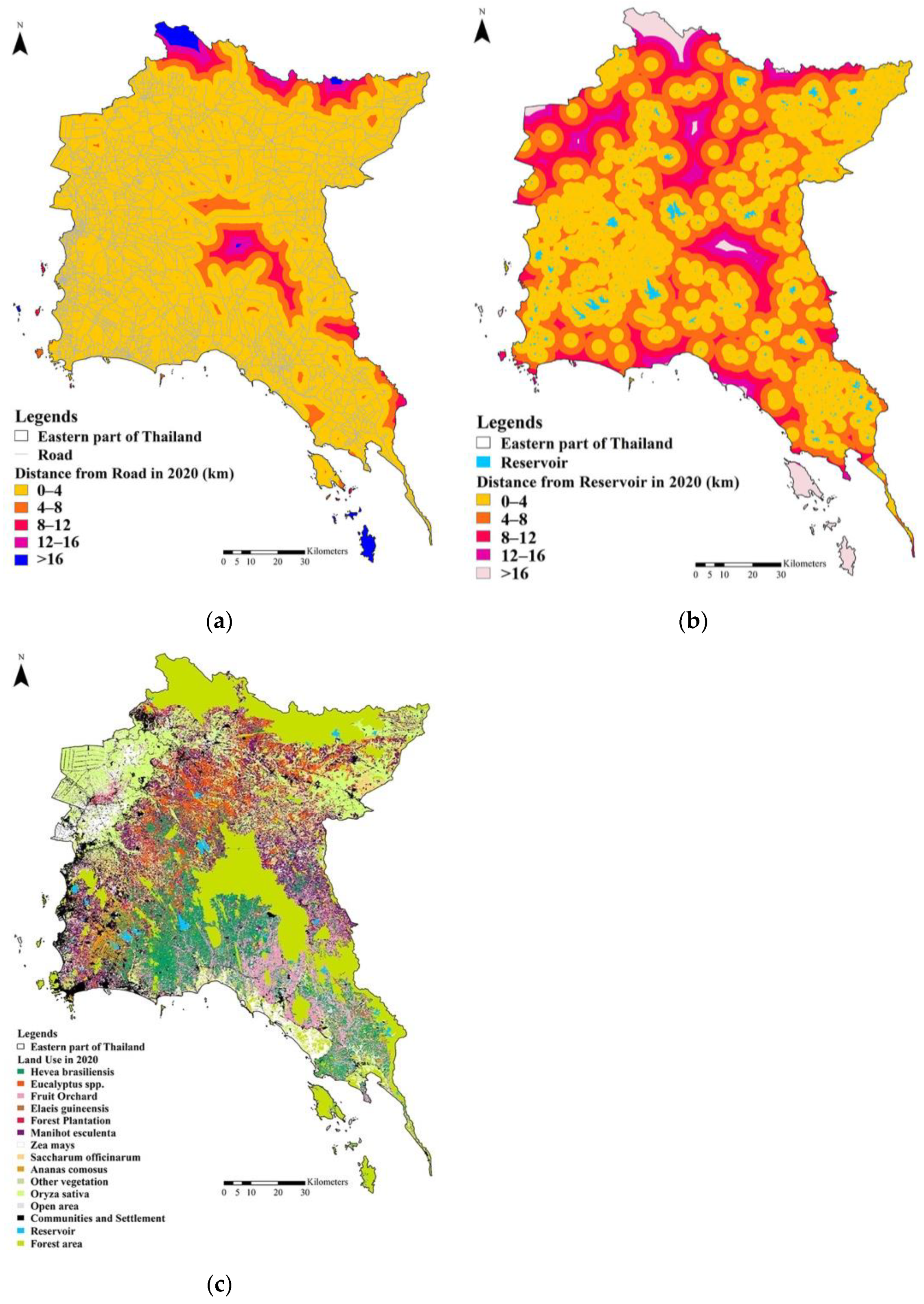
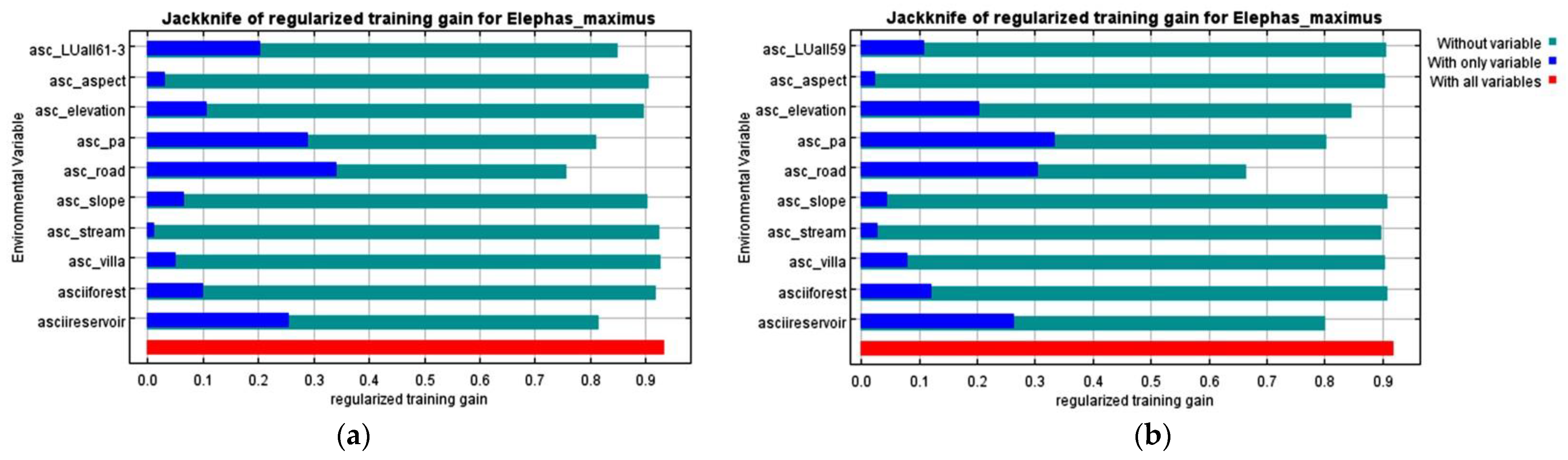
2.2. Environmental Predictor Variables
2.3. Species Distribution Modeling and Corridor Design
2.4. Corridor Design
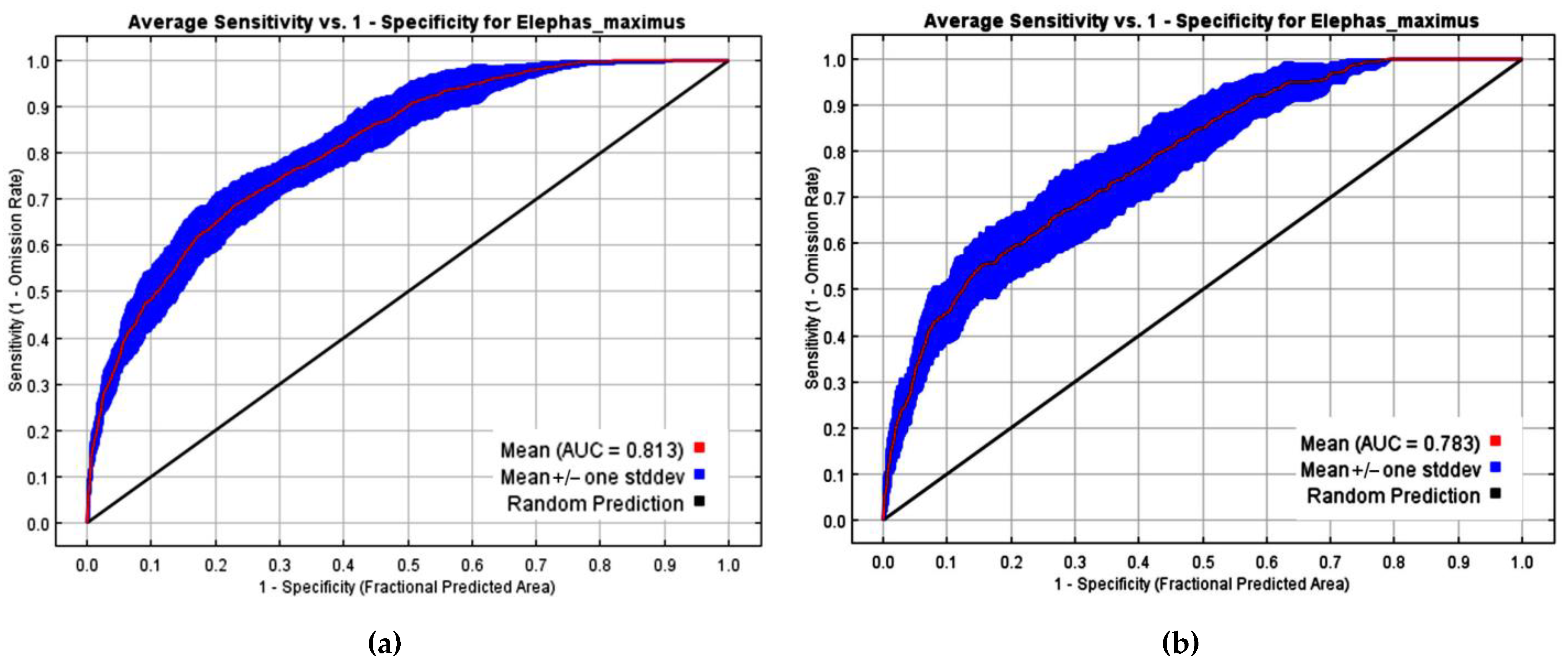
2.5. Data Analysis
3. Results
3.1. Land Use Change in the Eastern Part of Thailand in 2020
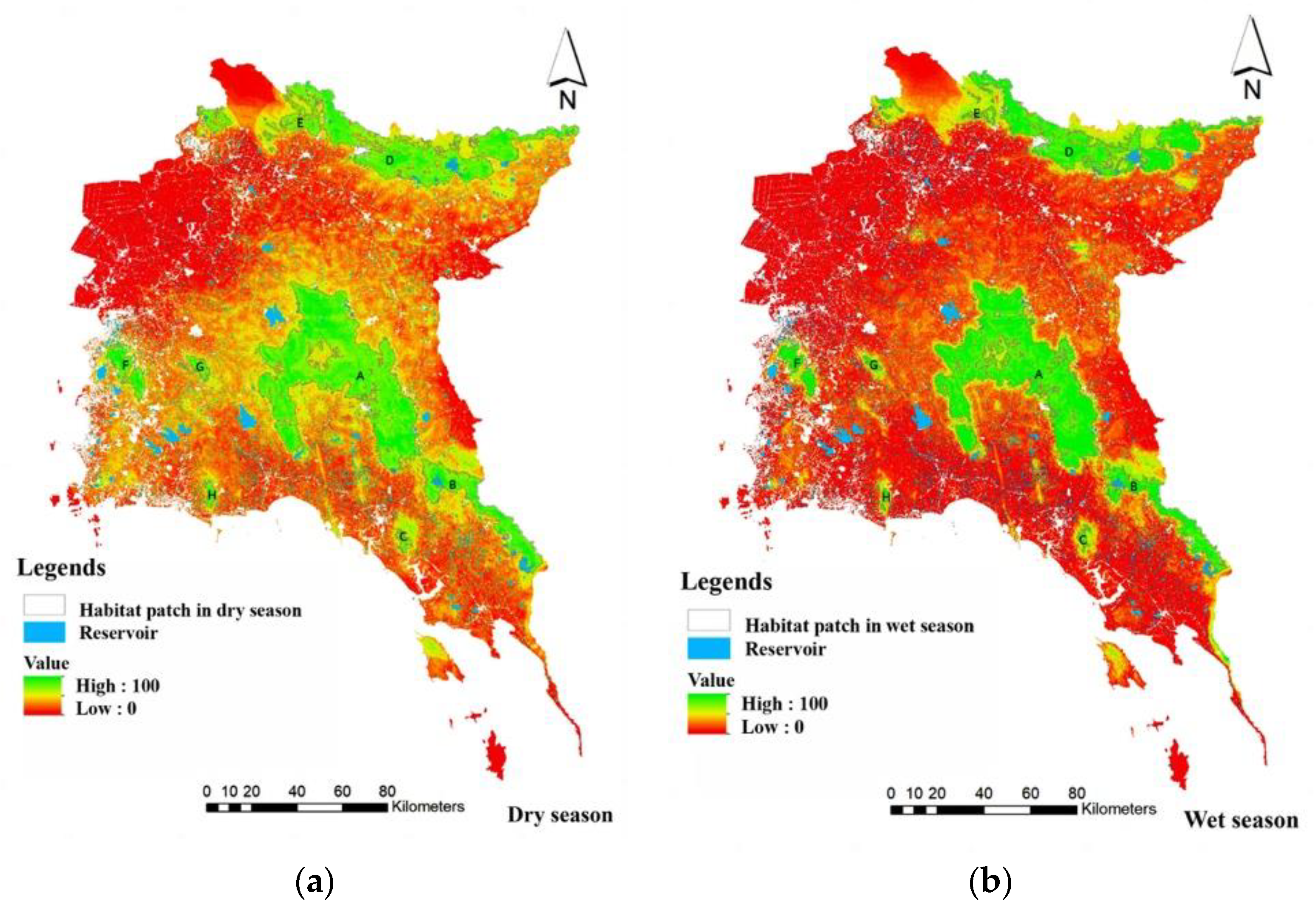
3.2. The Risk of Human–Elephant Conflicts in the Eastern Part of Thailand
3.3. The Human-Elephant Conflict in the Eastern Part of Thailand
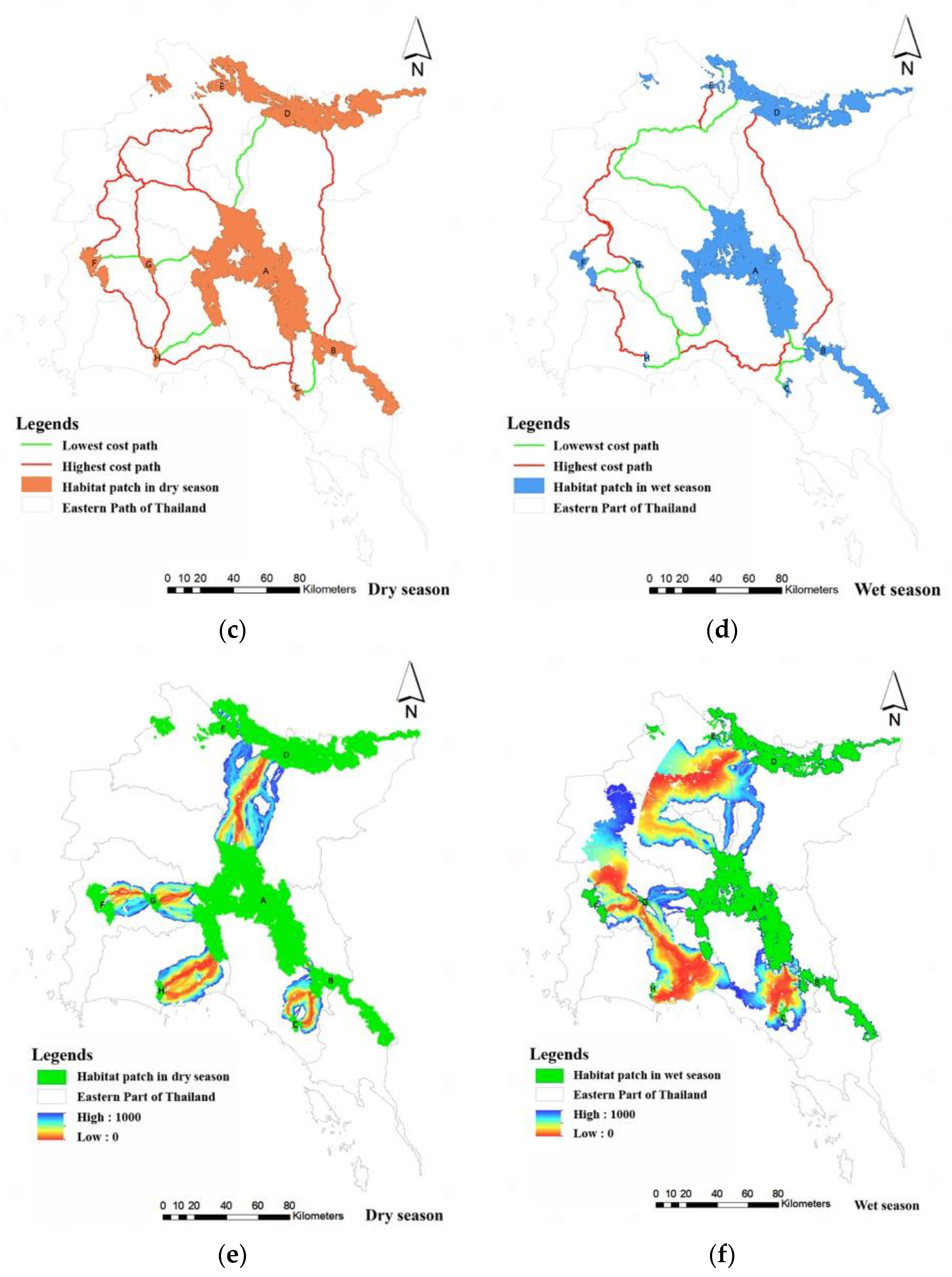
3.4. Habitat Corridor of Wild Elephants
3.5. Landscape Corridor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaiyarat, R.; Youngpoy, N.; Prempree, P. Wild Asian elephant (Elephas maximus) population in Salakpra Wildlife Sanctuary, Thailand. Endanger. Species Res. 2015, 29, 95–102. [Google Scholar] [CrossRef]
- Neupane, D.; Kwon, Y.; Risch, T.S.; Williams, A.C.; Johnson, R.L. Habitat use by Asian elephants: Context matters. Global Ecol. Conserv. 2019, 17, e00570. [Google Scholar] [CrossRef]
- Htet, N.N.P.; Chaiyarat, R.; Thongthip, N.; Anuracpreeda, P.; Youngpoy, N.; Chompoopong, P. Population and distribution of wild Asian elephants (Elephas maximus) in Phu Khieo Wildlife Sanctuary, Thailand. PeerJ 2021, 29, e11896. [Google Scholar] [CrossRef] [PubMed]
- Leimgruber, P.; Gagnon, J.B.; Wemmer, C.; Kelly, D.S.; Songer, M.A.; Selig, E.R. Fragmentation of Asia’s remaining wildlands: Implications for Asian elephant conservation. Anim. Conserv. 2003, 6, 347–359. [Google Scholar] [CrossRef]
- Shannon, G.; Matthews, W.S.; Page, B.R.; Parker, G.E.; Smith, R.J. The effects of artificial water availability on large herbivore ranging patterns in savanna habitats: A new approach based on modelling elephant path distributions. Divers. Distrib. 2009, 15, 776–783. [Google Scholar] [CrossRef]
- Montez, D.; Leng, A. Status of Asian elephant and Human—Elephant conflict (HEC) in Asia: A brief and updated review. J. Nat. Appl. Res. 2021, 1, 28–35. [Google Scholar]
- Ram, A.K.; Mondol, S.; Subedi, N.; Lamichhane, B.R.; Baral, H.S.; Natarajan, L.; Amin, R.; Pandav, B. Patterns and determinants of elephant attacks on humans in Nepal. Ecol. Evol. 2021, 11, 11639–11650. [Google Scholar] [CrossRef]
- Kitratporn, N.; Takeuchi, W. Spatiotemporal distribution of Human-elephant Conflict in eastern Thailand: A model–based assessment using news reports and remotely sensed data. Remote Sens. 2020, 12, 90. [Google Scholar] [CrossRef]
- Mumby, H.S.; Plotnik, J.M. Taking the elephants’ perspective: Remembering elephant behavior, cognition and ecology in human-elephant conflict mitigation. Front. Ecol. Evol. 2018, 6, 122. [Google Scholar] [CrossRef]
- Bal, P.; Nath, C.; Nanaya, K.; Kushalappa, C.; Garcia, C. Elephants also like coffee: Trends and drivers of human–elephant conflicts in coffee agroforestry landscapes of Kodagu, Western Ghats, India. Environ. Manag. 2011, 47, 789–801. [Google Scholar] [CrossRef]
- Neupane, D.; Johnson, R.L.; Risch, T.S. Temporal and spatial patterns of human–elephant conflict in Nepal. In Proceedings of the International Elephant & Rhino Conservation & Research Symposium, Pittsburgh Zoo & PPG Aquarium, Pittsburgh, PA, USA, 26–30 August 2013. [Google Scholar]
- Cook, R.M.; Parrini, F.; Henley, M.D. Elephant movement patterns in relation to human inhabitants in and around the Great Limpopo Transfrontier Park. Koedoe. Afr. Protect. Area Conserv. Sci. 2015, 57, 1–7. [Google Scholar]
- Goswami, V.R.; Medhi, K.; Nichols, J.D.; Oli, M.K. Mechanistic understanding of human–wildlife conflict through a novel application of dynamic occupancy models. Conserv. Biol. 2015, 29, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Davies, T.E.; Hazarika, N.; Zimmermann, A. Understanding spatial and temporal patterns of human–elephant conflict in Assam, India. Oryx 2015, 49, 140–149. [Google Scholar] [CrossRef]
- Wettasin, M.; Chaiyarat, R.; Yongpoy, N.; Cheachean, N.; Sukmasuang, R.; Tanhan, P. Environmental factors influenced the distribution of wild Asian elephant (Elephas maximus) in rural areas of the Eastern Economic Corridor, Thailand. 2022; In preparation. [Google Scholar]
- Mimet, A.; Clauzel, C.; Foltête, J.-C. Locating wildlife crossings for multispecies connectivity across linear infrastructures. Landsc. Ecol. 2016, 31, 1955–1973. [Google Scholar] [CrossRef]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.; Di Marco, M.; Rondinini, C.; Boitani, L. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef]
- Alkemade, R.; van Oorschot, M.; Miles, L.; Nellemann, C.; Bakkenes, M.; Ten Brink, B. GLOBIO3: A framework to investigate options for reducing global terrestrial biodiversity loss. Ecosystem 2009, 12, 374e390. [Google Scholar] [CrossRef]
- Williams, C.; Tiwari, S.K.; Goswami, V.R.; de Silva, S.; Kumar, A.; Baskaran, N.; Yoganand, K.; Menon, V. Elephas maximus (Asian Elephant). IUCN Red List Threat. Species 2020: E.T7140A45818198. Available online: https://asesg.org/PDFfiles/Asian%20Elephant%20Red%20List%20Assessment%202020.pdf (accessed on 20 December 2020).
- Campos-Arceiz, A.; Larrinaga, A.R.; Weerasinghe, U.R.; Takatsuki, S.; Pastorini, J.; Leimgruber, P.; Fernando, P.; Santamaría, L. Behavior rather than diet mediates seasonal differences in seed dispersal by Asian elephants. Ecology 2008, 89, 2684–2691. [Google Scholar] [CrossRef]
- Fritz, H. Long-term field studies of elephants: Understanding the ecology and conservation of a long-lived ecosystem engineer. J. Mammal. 2017, 98, 603–611. [Google Scholar] [CrossRef]
- Sekar, N.; Lee, C.; Sukumar, R. Functional non redundancy of elephants in a disturbed tropical forest. Conserv. Biol. 2017, 31, 1152–1162. [Google Scholar] [CrossRef]
- Poulsen, J.R.; Rosin, C.; Meier, A.; Mills, E.; Nuñez, C.L.; Koerner, S.; Blanchard, E.; Callejas, J.; Moore, S.; Sowers, M. Ecological consequences of forest elephant declines for Afrotropical forests. Conserv. Biol. 2018, 32, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, R. A brief review of the status, distribution and biology of wild Asian elephants Elephas maximus. Int. Zoo Yearb. 2006, 40, 1–8. [Google Scholar] [CrossRef]
- Vinitpornsawan, S.; Bunchornratana, K.; Pukhrua, A.; Panyawiwatanakul, R. Population and age structure of wild Asian elephant. Wildl. Yearb. 2015, 15, 89–111. [Google Scholar]
- Kumar, S. Attack on elephant by tiger, a choice of food or struggle for survival, ecological study in Corbett Tiger Reserve, Ramnagar and Uttrakhand, India. Int. J. Life. Sci. Scienti. Res. 2016, 2, 506–508. [Google Scholar] [CrossRef]
- Rabinowitz, A.; Zeller, K.A. A range–wide model of landscape connectivity and conservation for the jaguar, Panthera onca. Biol. Conserv. 2010, 143, 939–945. [Google Scholar] [CrossRef]
- Cushman, S.A.; Landguth, E.L.; Flather, C.H. Evaluating population connectivity for species of conservation concern in the American Great Plains. Biodivers. Conserv. 2013, 22, 2583–2605. [Google Scholar] [CrossRef]
- Torrubia, S.; McRae, B.H.; Lawler, J.J.; Hall, S.A.; Halabisky, M.; Langdon, J.; Case, M. Getting the most connectivity per conservation dollar. Front. Ecol. Environ. 2014, 12, 491–497. [Google Scholar] [CrossRef]
- Wade, A.A.; Mckelvey, K.S.; Schwartz, M.K. Resistance–Surface–Based Wildlife Conservation Connectivity Modeling: Summary of Efforts in the United States and Guide for Practitioners; Gen. Tech. Rep. Gen. Tech. Rep. RMRS-GTR-333; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2015; 93p. [Google Scholar]
- Schlaepfer, D.R.; Braschler, B.; Rusterholz, H.-P.; Baur, B. Genetic effects of anthropogenic habitat fragmentation on remnant animal and plant populations: A meta-analysis. Ecosphere 2018, 9, e02488. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Royal Thai Embassy, Washington, D.C. Thailand Taking 4.0 Regional as ASEAN Chair. Available online: https://thaiembdc.org/2019/02/18/thailand-taking-4-0-regional-as-asean-chair/ (accessed on 30 November 2020).
- Thurfjell, H.; Ciuti, S.; Boyce, M.S. Applications of step–selection functions in ecology and conservation. Mov. Ecol. 2014, 2, 4. [Google Scholar] [CrossRef]
- DNP. Eastern Forest Complex in Thailand. Available online: http://www.dnp.go.th/wildlife_it/n_web/the_wild/KPA.php. (accessed on 30 November 2018).
- Suksawang, S.; Temchai, T. Report on Water Yield in Eastern Forest Complex (EFCOM); National Parks Office, Department of National Park, Wildlife and Plant Conservation: Bangkok, Thailand, 2017.
- Pomoim, N.; Trisurat, Y.; Hughes, A.C.; Corlett, R.T. Can Thailand protect 30% of its land area for biodiversity, and will this be enough? Diversity 2022, 14, 344. [Google Scholar] [CrossRef]
- Suksavate, W.; Duengkae, P.; Chaiyes, A. Quantifying landscape connectivity for wild Asian elephant populations among fragmented habitats in Thailand. Global Ecol. Conserv. 2019, 19, e00685. [Google Scholar] [CrossRef]
- Chumsangsri, T. GIS for Analysis of the Elephant Habitat in Taboh–Huai Yai Wildlife Sanctuary, Changwat Phtchabun and Chaiyaphum. Master’s Thesis, Kasetsart University, Bangkok, Thailand, 2002. [Google Scholar]
- Vinitpornsawan, S. Application of GIS to the Analysis of the Distribution of the Elephant (Elephas Maximus Linnaeus, 1758) at Phu Khieo Wildlife Sanctuary, Chaiyaphum Province; Biodiversity Research and Training Program (BRT): Bangkok, Thailand, 2003; pp. 228–234. [Google Scholar]
- Chokcharoen, R.; Sukmasuang, R. Ecology of Asian elephant (Elephas maximus) in Phu Wua Wildlife Sanctuary, Bueng Kan Province. J. Wildl. Thail. 2012, 19, 13–22. [Google Scholar]
- Podchong, S. A Complete Research Project Report: Assessing the Suitable Habitat of Important Wildlife Species in the Phu Khiao–Nam Nao Forest Area; Wildlife Conservation Information Division, Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2015. [Google Scholar]
- Raksapol, P. Habitat Corridors of Wild Elephant (Elephas maximus) at Salakpra Wildlife Sanctuary and Nearby Areas, Kanchanaburi Province; M.S. Kasetsart University: Bangkok, Thailand, 2019. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudı’k, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2016, 29, 129–151. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information theory and statistical mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Rodrigues, E.S.D.C.; Rodrigues, F.A.; Ricardo, L.D.A.; Corrêa, P.L.; Giannini, T.C. Evaluation of different aspects of maximum entropy for niche–based modeling. Procedia Environ. Sci. 2010, 2, 990–1001. [Google Scholar] [CrossRef][Green Version]
- Mullu, D. A review on the effect of habitat fragmentation on ecosystem. J. Nat. Sci. Res. 2016, 6, 1–15. [Google Scholar]
- Beier, P.; Noss, F.R. Do habitat corridors provide connectivity? Conserv. Biol. 1998, 12, 1241–1252. [Google Scholar] [CrossRef]
- Gilbert-Norton, L.; Wilson, R.; Stevens, J.R.; Beard, K.H. A Meta-Analytic Review of Corridor Effectiveness. Conserv. Biol. 2010, 24, 660–668. [Google Scholar] [CrossRef]
- Ford, T.; Rettie, K.; Clevenger, A.P. Fostering ecosystem function through an international public–private partnership: A case study of wildlife mitigation measures along the Trans-Canada Highway in Banff National Park, Alberta, Canada. Int. J. Biodivers. Sci. Manag. 2009, 5, 181–189. [Google Scholar] [CrossRef]
- Beier, P.; Majka, D.R.; Spencer, W.D. Forks in the road: Choices in procedures for designing wildland linkages. Conserv. Biol. 2008, 22, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Majka, D.; Jenness, J.; Beier, P. CorridorDesigner: ArcGIS Tools for Designing and Evaluating Corridors. Available online: http://corridordesign.org (accessed on 20 November 2020).
- Gunawan, G.; Sulistijorini, S.; Chikmawati, T.; Sobir, S. Predicting suitable areas for Baccaurea angulata in Kalimantan, Indonesia using Maxent modeling. Biodiversitas J. Biol. Divers. 2021, 22, 2646–2653. [Google Scholar] [CrossRef]
- Adriaensen, F.; Chardon, J.P.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulinck, H.; Matthysen, E. The application of “least–cost” modelling as a functional landscape model. Landsc. Urban Plan 2003, 64, 233–247. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B.; Mcrae, H. Using circuit theory to model connectivity in ecology. Evol. Conserv. Ecol. 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Carroll, C.; McRae, B.H.; Brookes, A. Use of linkage mapping and centrality analysis across habitat gradients to conserve connectivity of gray wolf populations in western North America. Conserv. Biol. 2012, 26, 78–87. [Google Scholar] [CrossRef]
- Silva, S.D.; Wu, T.; Nyhus, P.; Thieme, A.; Weaver, A.; Johnson, J.; Wadey, J.; Mossbrucker, A.; Vu, T.; Neang, T.; et al. The past, present and future of elephant landscapes in Asia. bioRxiv 2020. [Google Scholar] [CrossRef]
- Sutherland, K.; Ndlovu, M.; Pérez-Rodríguez, A. Use of artificial waterholes by animals in the southern region of the Kruger National Park, South Africa. Afr. J. Wildl. Res. 2018, 48, 023003. [Google Scholar] [CrossRef]
- Kroutnoi, L.; Sriburi, T.; Wijitkosum, S.; Nuanyai, K. Determination of stimulating factors of wild Asian elephant (Elephas maximus) dispersal from the Kaeng Krachan National Park to surrounding land use in Thailand. Walailak J. Sci. Technol. 2020, 17, 392–404. [Google Scholar] [CrossRef]
- Ram, A.K.; Yadav, N.K.; Subedi, N.; Pandav, B.; Mondol, S.; Khanal, B.; Kharal, D.K.; Acharya, H.B.; Dhakal, B.K.; Acharya, K.P.; et al. Landscape predictors of human elephant conflicts in Chure Terai Madhesh Landscape of Nepal. Environ. Chall. 2022, 7, 100458. [Google Scholar] [CrossRef]
- Wato, Y.A.; Heitkönig, I.M.A.; Wieren, V.S.E.; Wahungu, G.; Prins, H.H.T.; Langevelde, V.F. Prolonged drought results in starvation of African elephant (Loxodonta africana). Biol. Conserv. 2016, 203, 89–96. [Google Scholar] [CrossRef]
- Temchai, T.; Kaewkate, C.; Hengswang, D.; Saengswang, M.; Deekaew, P.; Vanmanee, S.; Jitra, N.; Thongsuk, P.; Thongkerd, T.; Khamkran, S.; et al. Population survey of Asian elephant (Elephas maximus) in Kaeng Krachan national park, Thailand. J. Thail. Natl. Park. Res. 2018, 2, 112–121. [Google Scholar]
- Wilson, G.; Gray, R.J.; Radinal, R.; Hasanuddin, H.; Azmi, W.; Sayuti, A.; Muhammad, H.; Abdullah, A.; Nazamuddin, B.S.; Sofyan, H.; et al. Between a rock and a hard place: Rugged terrain features and human disturbance affect behaviour and habitat use of Sumatran elephants in Aceh, Sumatra, Indonesia. Biodiver. Conserv. 2021, 30, 597–618. [Google Scholar] [CrossRef]
- Pan, W.; Lin, L.; Luo, A.; Zhang, L. Corridor use by Asian elephants. Integ. Zool. 2009, 4, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.R.; Liu, X.; Songer, M.; Zahoor, B.; Wickramasinghe, W.M.S.; Mahanta, K.K. Analysis of landscape connectivity among the habitats of Asian elephants in Keonjhar Forest Division, India. Remote Sens. 2021, 13, 4661. [Google Scholar] [CrossRef]
- Wadey, J.; Beyer, H.L.; Saaban, S.; Othman, N.; Leimgruber, P.; Campos-Arceiz, A. Why did the elephant cross the road? The complex response of wild elephants to a major road in Peninsular Malaysia. Biol. Conserv. 2018, 218, 91–98. [Google Scholar] [CrossRef]
- Trisurat, Y.; Bhumpakphan, N.; Reed, D.H.; Kanchanasaka, B. Using species distribution modeling to set management priorities for mammals in northern Thailand. J. Nat. Conserv. 2012, 20, 264–273. [Google Scholar] [CrossRef]
- Sharma, P.S.; Panthi Yadav, S.K.; Bhatta, M.; Karki, A.; Duncan, T.; Poudel, M.; Acharya, K.P. Suitable habitat of wild Asian elephant in Western Terai of Nepal. Ecol. Evol. 2020, 10, 6612–6619. [Google Scholar] [CrossRef]
- Krishnan, V.; Kumar, M.A.; Raghunathan, G.; Vijayakrishnan, S. Distribution and habitat use by Asian elephants (Elephas maximus) in a coffee–dominated landscape of Southern India. Trop. Conserv. Sci. 2019, 12, 1940082918822599. [Google Scholar] [CrossRef]
- Bahar, A.; Kasim, N.H.A.B.; Hambali, K. Home range and movement patterns of Asian elephant (Elephas maximus) in Gua Musang, Kelantan, Malaysia. Malay. Nat. J. 2018, 70, 221–232. [Google Scholar]
- Noe, C. The Dynamics of Land Use Changes and Their Impacts on the Wildlife Corridor between Mt. Kilimanjaro and Amboseli National Park, Tanzania; LUCID Working Paper Number: 31; LUCID Project International Livestock Research Institute: Nairobi, Kenya, 2003. [Google Scholar]
- Sengelela, M. Local People Perceived Benefit and Costs of Trans–Frontier Conservation Areas: The Case of Likuyusekamaganga Village in Selous–Niassa Trans–Frontier Conservation Area. Master’s Thesis, Cultural Geography, Wageningen University, Wageningen, The Netherlands, 2013. [Google Scholar]

| Season | Very High Risk Area | High Risk Area | Moderate Risk Area | Low Risk Area | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| km2 | Presence (Points) | km2 | Presence (Points) | km2 | Presence (Points) | km2 | Presence (Points) | km2 | Points | |
| Dry | 375.8 | 70 | 2060.1 | 48 | 9505.4 | 52 | 24,115.6 | 32 | 36,056.9 | 202 |
| Wet | 225.8 | 25 | 915 | 18 | 3397.7 | 20 | 31,827 | 9 | 36,365.5 | 72 |
| Patches | Habitat Patch | Area (km2) | |
|---|---|---|---|
| Dry Season | Wet Season | ||
| A | Khao Ang Rue Nai Wildlife Sanctuary | 2440.5 | 2082.2 |
| Khao Soi Dao Wildlife Sanctuary | |||
| Khao Sip Ha Chan National Park | |||
| Khao Khitchakut National Park | |||
| B | Khlong Khruea Wai Chaloem Phra Kiat Wildlife Sanctuary | 539.1 | 374 |
| Namtok Khlong Kaeo National Park | |||
| C | Namtok Phlio National Park | 31.7 | 23.1 |
| D | Pang Sida National Park | 1446.4 | 1099.9 |
| Ta Phraya National Park | |||
| Thap Lan National Park | |||
| E | Khao Yai National Park | 229.1 | 60.8 |
| F | Kao Kheow-Kao Chomphu Wildlife Sanctuary | 184.8 | 115.2 |
| G | Khao Yai Da Non-hunting Area (In preparation area) | 54.6 | 19.5 |
| H | Nam Tok Khao Chao-Bo Thong Forest Park | 36.3 | 13.4 |
| Patch Type | Dry Season | Wet Season | ||
|---|---|---|---|---|
| Number of Patches (Patch) | Area (km2) | Number of Patches (Patch) | Area (km2) | |
| Population patch | 253 | 4875 | 223 | 3627.3 |
| Breeding patch | 8 | 68.1 | 33 | 150.5 |
| Other patch | 3291 | 193.9 | 1705 | 73.1 |
| Total | 3552 | 5137 | 1961 | 3850.9 |
| Season | Landscape Corridor | Landcover | Euclidean Distance (Km) | Cost-Weighted Distance (Km) | Least-Cost Path Distance (Km) | Effective Resistance | Core Centrality |
|---|---|---|---|---|---|---|---|
| Dry | A to B | Perennial crop/agriculture/evergreen/deciduous | 1.7 | 1.9 | 0.7 | 1.1 | 12 |
| A to C | Perennial crop/agriculture/evergreen/deciduous | 24.5 | 31.2 | 3.7 | −1 | −1 | |
| A to D | Perennial crop/agriculture/evergreen/deciduous | 52.8 | 68.2 | 9 | 3.6 | 12 | |
| A to E | Perennial crop/agriculture/evergreen/deciduous | 57.9 | 81.6 | 9 | −1 | −1 | |
| A to F | Perennial crop/agriculture/evergreen/deciduous | 49.3 | 151.2 | 9.3 | −1 | −1 | |
| A to G | Plantation/agriculture/ deciduous | 20.5 | 23.6 | 5.9 | 2.9 | 12 | |
| A to H | Perennial crop/agriculture/evergreen/deciduous | 35.9 | 42.6 | 5.5 | 5.5 | 7 | |
| B to C | Plantation/agriculture/ deciduous | 17.4 | 27 | 4 | 2.9 | 7 | |
| B to D | Perennial crop/agriculture/evergreen/deciduous | 119.9 | 146.5 | 16.7 | −1 | −1 | |
| C to H | Perennial crop /agriculture/ deciduous | 80.6 | 118.9 | 12.6 | −1 | −1 | |
| D to E | Perennial crop /agriculture/ deciduous | 0.3 | 0.3 | 0.2 | 0.3 | 7 | |
| E to F | Perennial crop /agriculture/ deciduous | 97.9 | 192.8 | 9.1 | −1 | −1 | |
| E to G | Perennial crop /agriculture/ deciduous | 96.3 | 180.1 | 10.3 | −1 | −1 | |
| F to G | Perennial crop /agriculture/ deciduous | 19.9 | 24.6 | 4.8 | 8.4 | 7 | |
| F to H | Perennial crop /agriculture/ deciduous | 45.3 | 58 | 10.4 | −1 | −1 | |
| G to H | Perennial crop /agriculture/ deciduous | 42.9 | 53.2 | 9.4 | −1 | −1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiyarat, R.; Wettasin, M.; Youngpoy, N.; Cheachean, N. Use of Human Dominated Landscape as Connectivity Corridors among Fragmented Habitats for Wild Asian Elephants (Elephas maximus) in the Eastern Part of Thailand. Diversity 2023, 15, 6. https://doi.org/10.3390/d15010006
Chaiyarat R, Wettasin M, Youngpoy N, Cheachean N. Use of Human Dominated Landscape as Connectivity Corridors among Fragmented Habitats for Wild Asian Elephants (Elephas maximus) in the Eastern Part of Thailand. Diversity. 2023; 15(1):6. https://doi.org/10.3390/d15010006
Chicago/Turabian StyleChaiyarat, Rattanawat, Maneepailin Wettasin, Namphung Youngpoy, and Navee Cheachean. 2023. "Use of Human Dominated Landscape as Connectivity Corridors among Fragmented Habitats for Wild Asian Elephants (Elephas maximus) in the Eastern Part of Thailand" Diversity 15, no. 1: 6. https://doi.org/10.3390/d15010006
APA StyleChaiyarat, R., Wettasin, M., Youngpoy, N., & Cheachean, N. (2023). Use of Human Dominated Landscape as Connectivity Corridors among Fragmented Habitats for Wild Asian Elephants (Elephas maximus) in the Eastern Part of Thailand. Diversity, 15(1), 6. https://doi.org/10.3390/d15010006