Abstract
Our goal was to understand the mechanisms behind the impact of nutrient enrichment at intermediate distances from aquaculture on the interactions of a subtidal macroalgae community with its main grazer, the sea urchin Paracentrotus lividus. We assessed the diversity and cover of the macroalgal community, the abundance and biometrics of the sea urchins, the carbon and nitrogen elemental and isotopic compositions, and their metabolome in two stations, at an intermediate distance (station A) and away (station B) from a fish cage facility in the Aegean Sea (Greece), during the warm and cold seasons. The nutrient input at station A favored a shift to a macroalgal assemblage dominated by turf-forming species, depleted of native-erected species and with a higher abundance of invasive algae. A stable isotope analysis showed fish-farm-associated nitrogen enrichment of the macroalgae and trophic transfer to P. lividus. A decrease in metabolites related to grazing, reproduction, and energy reserves was found in P. lividus at station A. Furthermore, the metabolomic analysis was able to pinpoint stress in P. lividus at an intermediate distance from aquaculture. The chosen combination of traditional ecology with omics technology could be used to uncover not only the sublethal effects of nutrient loading but also the pathways for species interactions.
1. Introduction
The environmental effects of aquaculture have been widely studied on several temporal and spatial scales [1,2]. The impact of solid waste is well known and has been shown to cause hypoxia and a shift to opportunistic-dominated macrobenthic assemblages in the area directly beneath or close to the fish cages [2,3,4,5]. On the other hand, the impact of dissolved wastes has been found to reach higher distances evidenced by pronounced effects on water column nutrients and chlorophyll-a concentrations several kilometers from the cages [6,7,8]. Nevertheless, the impact at larger spatial scales is not always straightforward, and studies have reported a lack of significant enrichment at intermediate distances from fish farms [9,10] even though the lack of detectable signs of eutrophication has been attributed to the rapid transfer of organic matter availability up the food web through grazing [11,12,13], suggesting the importance of species interaction in shaping the response of communities to nutrient enrichment. Currently, the mechanism of aquatic organism stress and species interactions under aquaculture nutrient enrichment remains poorly understood.
Even if our current understanding of the effects of fish farming on macroalgae remains limited as the research has been mostly based on seagrasses [14], the alternate state’s theory gives a framework to predict the successional change of the macroalgae community under nutrient stress [15,16]. According to this theory, under nutrient enrichment stress, there is an initial shift in functional groups driving the loss of canopy-forming macroalgae followed by a replacement with fast-growing green filamentous algae e.g., [17,18,19,20]. A similar situation has been recorded in macroalgae assemblages adjacent to aquaculture cages in the Black Sea and Tasmania, with a general increase in algal growth and a shift to an assemblage dominated by filamentous opportunistic macroalgae [21,22,23,24]. This nutrient influence could be expected to vary seasonally depending on the growth cycle of the plants and the seasonality in the release of fish farm effluents, which are known to peak during the warm season [25], albeit to our knowledge, no such information has been reported.
The changes in the macroalgae assemblage under nutrient stress are often translated into an imbalance of the plant–grazer interaction. In several recorded cases, coastal ecosystems under eutrophication stress have experienced blooms of fast-growing macroalgae leading to an abrupt increase in grazer populations (e.g., the sea urchin Paracentrotus lividus) [26]. The uncontrolled population explosion of P. lividus usually results in an increased grazing pressure that surpasses the macroalgae growth rate and creates barren areas or changes in the macroalgae assemblage structure [17,27,28]. The imbalance in grazing from P. lividus is usually enhanced by nutrient enrichment from aquaculture, as the abundance of P. lividus increases near the cages together with the palatability (C\N) of marine plants resulting in increased grazing [29,30,31,32]. An example of this pattern for macroalgae was described in Croatia, where P. lividius and Arbacia lixula populations exploded near marine cages and were observed to overgraze the rocky reef macroalgae leading to barren grounds [33].
The methods that are commonly used to assess the impact of nutrient impact and increased grazing on marine macroalgae are usually focused on measuring the changes in composition, structure, and biometrics of the macroalgae assemblages [34,35]. These methods lack a high resolution and can only detect the obvious change in the macroalgae assemblage on the first 100 m from the pollution source [36,37]. Similarly, the research on the plant–herbivore interaction in sea urchins has relied on traditional methods, such as biometric measurements of the sea urchin individuals, population dynamics, density, and biomass variation [38,39]. When only considering obvious physical changes, such as the structure and composition of macroalgae and those commonly used in sea urchin research, the underlying stressors that are often expressed as subtle changes in the physiology of the organisms at intermediate levels of stress can pass undetected [40]. Metabolomic technology allows the study of macroalgae responses to environmental stress at nonlethal or intermediate levels and has been used to research the effects of biotic interactions on macroalgae in the past [41,42,43]. In related studies with marine plants, a metabolomics analysis combined with traditional, ecological, and biogeochemical tools revealed changes in the seagrass Zostera marina interacting with mussels, and the increased stress of Cymodocea nodosa mediated by aquaculture effluents at an intermediate distance from a fish farm [44,45]. To our knowledge, there is no published research on the use of metabolomics to study the effects of nutrient stress on sea urchins, in particular P. lividus. In this study, we used a set of complementary techniques to assess the effect of nutrient loading from marine fish cages on the subtidal macroalgae assemblage and its interactions with their primary grazer (P. lividus). To achieve this, we assessed the seasonal variation in the macroalgae community structure and the population dynamics of the sea urchin P. lividus at an intermediate distance (station A) and away from a fish farm (station B) in the Aegean Sea (Greece). We first evaluated the diversity and cover of the macroalgae assemblage and the biometry and abundance of P. lividus. Secondly, we measured the physiological variables (elemental and isotopic compositions) of the dominant macroalgae and P. lividus tissue. Finally, we used metabolomics to show metabolic changes in macroalgae and P. lividus in relation to the nutrient enrichment from the fish farm effluents.
2. Materials and Methods
2.1. Sampling Strategy
The aquaculture cages were in Vourlias Bay (Argolikos) in the Peloponnese region, which is a semienclosed embayment with intermediate hydrodynamics. The aquaculture facilities produce mainly sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Two macroalgal assemblages were studied at different distances from the aquaculture facility downstream of the main water currents: Station A was at an intermediate distance from the facility (250 m far from the cages), and station B was at 650 m away from the cages (Figure 1). Several studies assessing the impact of fish farming on the benthic community have shown that a 500 m radius around the aquaculture farms should be considered as the impact area of dissolved nutrient effluents [46,47]. Furthermore, the findings of a study that was performed in parallel to the present one using the same stations, found an increased impact of aquaculture nutrient loading over the seagrass Cymodocea nodosa metabolomic fingerprint at station A compared to station B [45]. The impact of aquaculture in these stations is further confirmed by Tsiaras et al. 2022, who describe the North–West coastal current direction in Vourlias Bay that passes adjacent to our studied fish farm and toward the sampling area and models the nutrient effluent impact from aquaculture in our study location [48]. According to the oceanographic model of the bay presented by Tsiaras et al. (2022), the currents circulate at station A as it is a small bay, while at station B the currents pass and move down the coast to the southeast. Therefore, station A was regarded as an intermediate effect station, and station B served as a control station for our study. To account for the seasonality, sampling was conducted during the warm (June 2017, when the water temperature was 29 °C) and cold (March 2018, when that water temperature was 15 °C) periods.
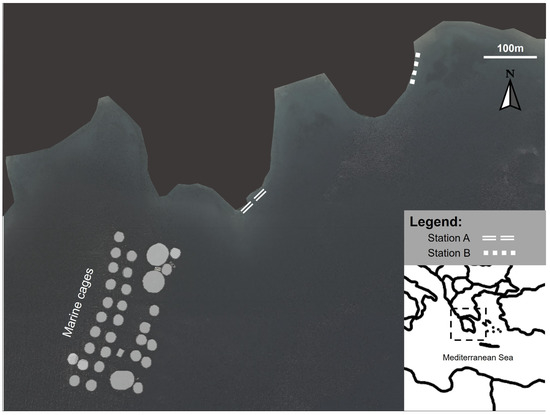
Figure 1.
Sampling site location in the Aegean Sea, showing station A at a 250 m from the cages (37°27′4.03″ N, 23°4′10.37″ E) and station B at 650 m from the cages (37°27′9.49″ N, 23°4′19.79″ E).
At each station, a 50 m transect was deployed toward the inner part of the bay, and 10 high-resolution images of the macroalgae assemblages were taken randomly at 0.2–1 m depth using a PVC quadrat frame with an area of 0.16 m2. To validate the species photo-identification, 5 random replicates of macroalgae were collected at each station using a SCUBA diver-operated suction device attached to a metal frame of 0.2 m2 [49]. The material was sieved and fixed in 90% ethanol for further sorting and identification in the laboratory. The most abundant species of macroalgae (5 replicates each) per station were collected by hand and snap-frozen in liquid nitrogen for the determination of elemental and stable isotopic carbon and nitrogen compositions and metabolomic profile analysis.
The density of adult P. lividus was randomly measured (10 replicates) along the transect using a 0.16 m2 quadrat. Additionally, 30 sea urchins were randomly collected along the same transect at each station, to be used for biometric measurements. The gonads of five sea urchins per station were hand-collected and immediately snap-frozen in liquid nitrogen for elemental and stable isotope analyses and metabolomic profiling in the laboratory. Gonads were selected instead of other tissue as they are the most abundant soft tissue in adult sea urchins and are the tissue regularly used for other biochemical analyses [50,51].
2.2. Laboratory Analysis
The quadrat images taken were imported into the software PhotoQuad v1.4 [52]. For each image, the species that accounted for most of the cover were identified visually, and the identification was validated with the macroscopic observation of the randomly SCUBA-collected specimens and verified by the taxonomy experts in the Hellenic Centre for Marine Research (HCMR). This procedure was followed by the individual measurement of the total cover of each species in the quadrat in each photograph following the methodology in Trygonis and Sini (2012) [52].
P. lividus biometric variables, such as diameter and total dry weight, were measured in the lab. To determine the dry weights, the sea urchins were dried in a convection oven at 60 °C for 48 h or until reaching constant dry weight. Biomass (g DW m−2) was calculated as the relation between density and dry weight for the population of each station, and the condition index (cm g−2) was calculated as the relation between diameter and dry weight for each individual sea urchin.
The tissue of only the most abundant macroalgae (Amphiroa rigida, Dictyota dichotoma, and Jania rubens), along with P. lividus gonads were freeze-dried for 48 h, then homogenized and ground to a fine powder for analysis of total carbon (TC), nitrogen (TN), δ13C, and δ15N using elemental analyzer combustion continuous flow isotope ratio mass spectroscopy. To resolve the metabolome of macroalgae and sea urchin tissue, the analysis was performed following Hasler-Sheetal et al. (2016) [44] by LC-qTOF-MS and GC-qTOF-MS. The extracts from the samples were resuspended for LC-MS or derivatized for GC-MS analysis. Data inspection, data mining, annotation, and interpretation were performed in MassHunter, Profinder, and Mass Profiler Professional (Agilent Technologies, Santa Clara, CA, USA).
2.3. Statistical Analysis
A principal coordinate analysis PCO was performed to visualize possible differences in macroalgae community structure between seasons and stations. Then, an Analysis of Similarities (ANOSIM) was performed to test for the effect of the factors ‘season’ and ‘station’ on assemblage structure [53]. Afterward, a similarity percentages–species contribution test (SIMPER) was performed to identify which taxa contributed more to the similarity among stations [54].
A bifactorial (season x station) two-way Analysis of Variance (ANOVA) was used to check for possible differences in P. lividus abundance and biometry and the elemental and isotopic composition of macroalgae. To account for the missing data of D. dichotoma, the station and period differences in elemental and isotopic compositions were tested with a one-way ANOVA. The Shapiro–Wilk test was used to test normality, and Levene’s test was used to test the homogeneity of variance. When necessary, data transformation prior to the analysis was performed using log2(x + 1).
All the multivariate analyses were performed using the software PRIMER v6 [55], and the univariate statistical tests were performed in PAST v4 [56].
The analysis of the metabolomics data for both sea urchin and macroalgae was performed in MetaboAnalyst 4.0 [57]. First, the missing values in the metabolomics dataset were calculated using the K-nearest neighbor algorithm, discarding variables with over 50% missing values, followed by data normalization using log transformation and auto-scaling (mean-centered and divided by the standard deviation of each variable). Then, to check for visual groupings of the metabolomics fingerprints in terms of season and station for both sea urchins and macroalgae, a principal component analysis (PCA) module was employed.
Afterward, the pattern hunter functionality in MetaboAnalyst that is based on the algorithm developed by Pavlidis and Noble (2001) [58] was used to identify the correlation using the Spearman coefficient of the concentration changes of metabolites that follow a predefined pattern. We applied this method to identify the patterns of metabolite concentration change for all metabolites covering the aquaculture nutrient impact direction from station A to station B in the two seasons. The method produced a graphical representation of the linear correlation among the top 25 metabolite concentrations and the predefined pattern or gradient. In the graphic, the metabolites that increase linearly with the gradient are represented in light pink with a positive correlation coefficient, and the ones that decrease are represented in light blue with a negative correlation coefficient.
3. Results
In the photo quadrat analysis, we found six algae species that accounted for the overall cover in the two stations and seasons (Table 1). These species were D. dichotoma, J. rubens, A. rigida, Cystoseira sp., Stypopodium schimperi, and Padina pavonica. Cystoseira sp. was found to be dominant in station B in the cold period as it was only present in station A in one of the picture replicates with only 0.4% of the total cover. The S. schimperi cover was higher in station A compared to station B, regardless of the sampling period. Similarly, A. rigida presented a higher cover in station A for both the cold and warm periods. It is important to note here that the total cover of each station in the different periods does not add to 100% because the macroalgae simply did not cover the entire quadrat. For example, the sum of all the macroalgae species cover for station A in the warm period is 71.99%, and the difference of this (28.01%) is accounted for barren cover.

Table 1.
Average values of quadrat cover percentage per macroalgae species found in the two stations over the cold and warm periods. Values are ± SE (n = 10).
Two principal components explained 81% of the total variability in the cover among stations and seasons (Figure 2). A spatial organization was evidenced indicating a significant difference between stations A and B (ANOSIM P = 0.01, R = 0.143), while no significant differences between seasons were found (ANOSIM P = 0.33, R = 0.016). The SIMPER (Table 2) showed that 60% of the dissimilarity between stations A and B was due to the higher cover of A. rigida and S. schimperi and the lower cover of J. rubens and D. dichotoma at station A.
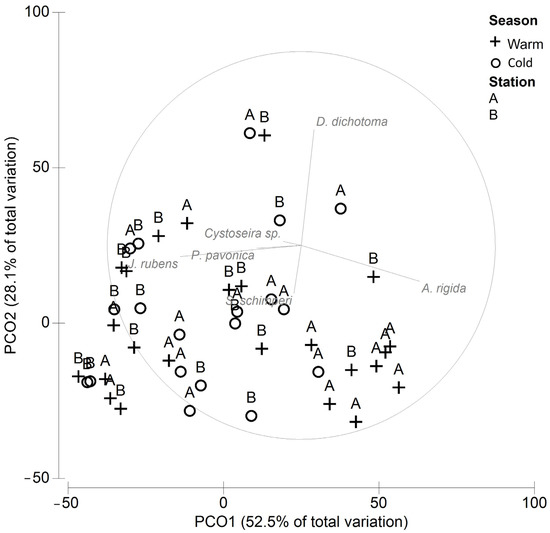
Figure 2.
PCO of the macroalgae cover at stations A and B.

Table 2.
SIMPER results for macroalgae cover at each station, showing the species that contributed to the dissimilarity among groups.
The two-way ANOVA on P. lividus density and biomass showed no significant differences between stations A and B; the only significant variation was found between seasons (p ≤ 0.05). However, a significant variation was found in the condition index between the two stations, with the index being higher at station A (p ≤ 0.05) (Table 3).

Table 3.
Average values of P. lividus biometric measurements for the two stations over the cold and warm periods. Values are ± SE (n = 30). The significant differences (p ≤ 0.05) between stations are represented by capital letters and between seasons by small letters.
D. dichotoma had significantly higher concentrations of TN and δ15N and a lower signal of δ13C at station A (Table 4). J. rubens showed an overall higher signal for all variables at station A, and it was most marked for δ15N, which was significantly higher at station A independent of the season. In the case of A. rigida, there was a seasonal effect on the variability of the physiological variables TN and δ13C; TN was the only one significantly higher in station A independent of the season. P. lividus presented a significant seasonal variation for δ13C and a significantly higher δ15N signature at station A. The detailed ANOVA analysis results for both Table 3 and Table 4 can be found in file S1.

Table 4.
Average values of physiological variables for P. lividus, D. dichotoma, J. rubens, and A. rigida for the two stations in the cold and warm periods. Values are ± SE (n = 5). The significant differences (p ≤ 0.05) between stations are represented by capital letters and between seasons by small letters. * Indicates the interaction effect between station and season.
In general, P. lividus had a closer isotopic signal to the J. rubens and D. dichotoma algae with closer δ13C and δ15N values to these species than to that of A. rigida (Figure 3). A. rigida presented a higher signal of carbon in relation to nitrogen, compared to the other macroalgae.
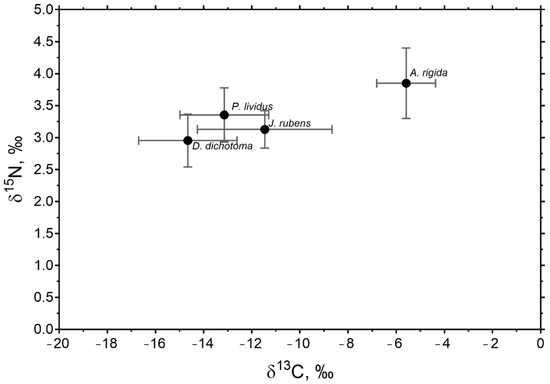
Figure 3.
Average values of δ13C and δ15N for P. lividus and macroalgae collected at all sites and in both seasons in the study area. Values are ± SE. Amphiroa rigida and Jania rubens are of the order Rhodophyta. Dictyota dichotoma is of the order Phaeophyta.
The annotated list of the 202 metabolites detected by mass spectrometry for P. lividus and the macroalgae is given in Table S1. The metabolic profiles of macroalgae did not form any apparent grouping between stations or seasons in the PCA. For A. rigida, 28.2% of the variation was explained in PC1 and 13.2 in PC2. For D. dichotoma, 32.7% of the variation was explained in PC1 and 15.7 in PC2. For J. rubens, 28.7% of the variation was explained in PC1 and 14.2 in PC2. The metabolic profiles of P. lividus were apparently more related to the season than to the stations, as seen in the groups formed in the PCA (Figure 4). For P. lividus, 39.1% of the variation was explained in PC1 and 10.5% in the PC2.
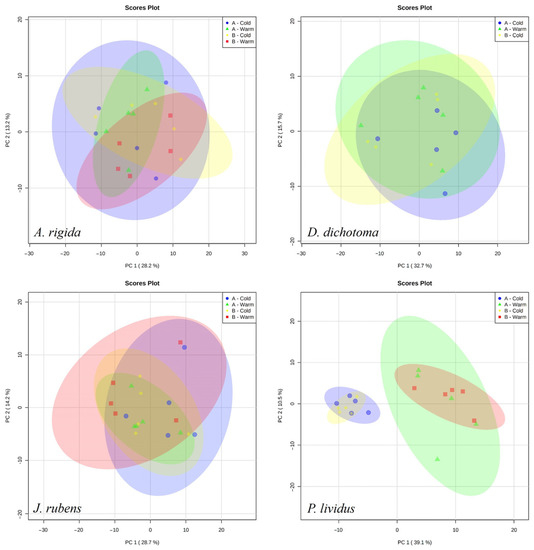
Figure 4.
PCA scores plot for the metabolite fingerprint in the three main species of macroalgae and P. lividus at the different stations and seasons of the study.
Even though, the PCA suggested a visual grouping related to the season, the pattern hunter analysis for the P. lividus metabolome revealed the top 25 metabolites related to distance from the aquaculture facility. Only one metabolite concentration (creatinine) had a positive correlation with the station close to the aquaculture (A), while the rest of the 24 metabolites had a negative correlation. In other words, an increase in creatinine concentration was positively correlated with station A along with a concentration decrease in metabolites, such as 4-hydroxybenzoic acid, Acacetin, 3, 4-dihydroxybenzoic acid, and several others in the direction of station B (Figure 5).
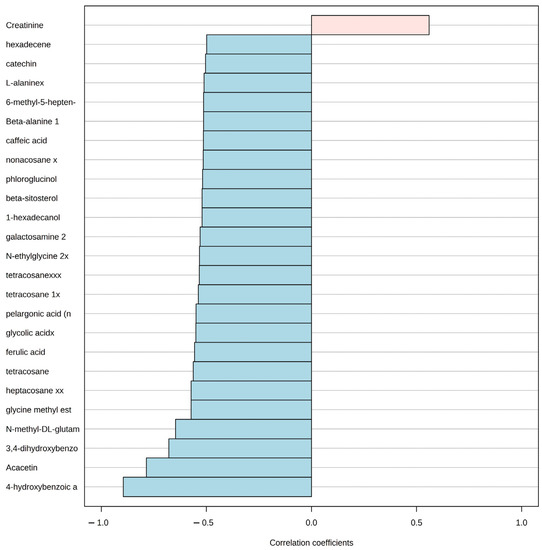
Figure 5.
Pattern hunter analysis for P. lividus, showing the top 25 metabolite spectra bins related to the distance of aquaculture nutrient enrichment in the direction from station A to B. Light red indicates a positive relationship with proximity to fish farm, while blue indicates a negative one. Correlation coefficient: nonparametric Spearman correlation.
4. Discussion
Our analysis revealed that nutrient loading from fish farm activity affected the structure of macroalgae assemblage and the plant–grazer interaction with P. lividus at an intermediate distance from the cages. With our cover analysis, we found that the influence of the fish farm at station A led to a macroalgae assemblage depleted of erected macroalgae, such as D. dichotoma, and dominated by A. rigida. The erected macroalgae are known to provide high primary production and sources of food, substrate for settlement, protection from predators, and shelter from disturbance [59,60]. Previous studies have also reported the grazing preference of P. lividus for erected macroalgae, which has led to a less stratified community dominated by crustose and turf-forming algae (e.g., A. rigida) [61,62,63]. In another coastal embayment in the Aegean Sea with similar physical conditions, Tsirintanis et al. (2018) [64] found that macroalgae assemblages in the presence of P. lividus were almost depleted of erected algae, while turf-forming algae, such as A. rigida, were a major component of the assemblage. This is relevant, as macroalgae assemblage dominated by turf algae is usually associated with low-complexity habitats and disturbed environmental conditions [65,66]. Furthermore, Piazzi and Ceccherelli (2017) [67] found that the recovery of erected macroalgae was limited by the enhanced grazing pressure from P. lividus in a nutrient-enriched environment. Additionally, the isotopic signals showed that P. lividus grazing activity was directed to J. rubens and D. dichotoma, explaining their significantly lower cover at station A. The closeness of δ13C and δ15N signature values between P. lividus and the macroalgae J. rubens and D. dichotoma might be related to their low C/N ratio (high palatability) and higher relative abundance in the transects. In contrast, A. rigida presented the lowest palatability (high C/N ratio) and a different isotopic signature from that of P. lividus, which explains its higher cover at station A. This is a pattern that has been previously recorded in other Mediterranean macroalgae communities, where P. lividus showed a grazing preference over the most abundant and palatable macrophytes [68,69].
The spatial variation in the assemblage structure was also driven by the non-native species S. schimperi. One of the hundreds of species that have entered the Mediterranean through the Suez Canal [70], the macroalgae S. schimperi are considered to be invasive, affecting ecosystem dynamics, taking the place of key species, or being economically harmful [71]. There are records of S. schimperi in the Eastern Mediterranean along the Levantine Coast [72] and in the Aegean Sea [73,74]. In particular, Rilov et al. (2018) [72] found that the southeastern Mediterranean sites disturbed by overfishing, overgrazing, and climate change were dominated by invasive species, such as S. schimperi. In the Aegean, Bianchi et al. (2014) [74] found that the dramatic replacement of the native algal forest by invasive species, such as S. schimperi, was influenced by overgrazing, seawater warming, and human disturbance. The results from both previous studies are in line with the hypothesis of Jauni et al. (2015) [75], which states that an increased abundance and diversity of non-native plant species are often related to sites with elevated grazing pressure and anthropogenic disturbance. Furthermore, in a controlled feeding experimental setting, the sea urchin P. lividus has been found to actively avoid grazing on invasive macroalgae species in the Mediterranean [76]. Thus, the higher abundance of S. schimperi at station A may be the result of a synergy between the fish farm-derived nutrient enrichment and the increasing preferential grazing pressure from P. lividus directed to other macroalgal species.
The higher condition index of P. lividus at station A might be the result of the interaction between nutrient input from fish farming and the increasing grazing activity. The condition index is often used as a proxy for the physiological and health states of organisms in the population [77]. Usually, a positive linear relationship is found between food availability and sea urchin condition indices, finding high condition indices in habitats with high food availability [78,79]. Taking into account that the macroalgae biomass has been found to increase under sources of anthropogenic nutrient inputs [80], we expected a higher condition index of sea urchins near fish farms, as the food availability increases. The Eastern Mediterranean is a special case in terms of nutrient bioavailability as an oligotrophic and especially phosphorous-limited environment that renders the dependence on external sources of nutrients necessary [81]. In an experimental setting in the Mediterranean, Dalsgaard and Krause-Jensen (2006) [82] found that the macroalgae biomass increased even at long distances due to fish farm effluents. This increase in macroalgae abundance mediated by fish farm nutrients, in turn, affects the herbivore dynamics [83], even though, when under nutrient enrichment, the algal assemblage increases its growth rate and can sustain a higher grazer pressure [84]. Eventually, overgrazing can surpass the macroalgae growth rate changing the assemblage structure drastically and ultimately turning it into a barren area [26]. Thus, we hypothesize that this was the case in our study, where station A turned gradually into a less stratified macroalgae assemblage. Consequently, it was transformed into an assemblage dominated by S. schimperi and A. rigida driven by the influence of aquaculture, showing early signs of transformation into a barren ground by the overgrazing of P. lividus. Although the periodicity of our sampling was not sufficient to distinguish the timing of this change, the higher biomass and condition of the urchins suggest an increased grazing pressure that led to the depletion of erected macroalgae and changed the macroalgae structure.
The stable isotope δ15N for macroalgae showed evidence of nutrient enrichment from aquaculture. Macroalgae have been shown to assimilate dissolved inorganic nitrogen (DIN) from aquaculture waste in other studies. For example, Wang et al. (2014) [85] found that growth and δ15N of macroalgae tissue increased near the farm and had a similar δ15N signature to DIN coming from fish urine. A similar pattern was found in another study regarding aquaculture waste impact in the Mediterranean Sea, where benthic organisms, such as P. lividus and the macroalgae Padina pavonica, showed more enriched δ15N signatures in the impact area that was around 500 m from the pollution source [86]. This result is in line with our findings for J. rubens and P. lividus at the station closer to the aquaculture, where both showed increased δ15N signatures. This has been previously recorded in other aquaculture settings in the Mediterranean Sea, where fish waste was found to have an influence on δ15N signatures of macroalgae at distances as high as 700 m from the source [87]. The three more abundant macroalgae (D. dichotoma, J. rubens, and A. rigida) presented a high interspecies variation in physiological variables that can be explained due to species-specific characteristics, such as nutrient uptake and growth rates, responsible for the different sensitivity of macroalgal species to altered nutrient availability and isotopic composition e.g., [88,89]. However, regardless of this variation, the increased signal of TN during the summer in D. dichotoma and A. rigida is an indicator of sensitivity to nutrient enrichment, as is the increase in δ15N of J. rubens at the station closer to the aquaculture. In our case, the aquaculture effluents affected mostly the δ15N values compared to the δ13C. This pattern was noted also by Vizzini et al. (2005) [86] and explained by the fact that the δ13C is accumulated in the feces in the area directly beneath the cages. Conversely, the δ15N-enriched urine effects reach longer distances entering the food web through the primary consumers.
The metabolomic fingerprint of the macroalgae did not change between the two stations (i.e., in relation to the distance from the aquaculture) nor between seasons. We attribute this to the fact that station A is located at an intermediate distance from aquaculture, and therefore the nutrient effect is not directly stressful for the macroalgae. As discussed above, in the nutrient-deprived Mediterranean Sea, intermediate levels of nutrient enrichment are rather beneficial to the macroalgae assemblages [85]. Furthermore, the five individuals sampled for each macroalgae species had no physical evidence of being directly grazed by P. lividus at the moment of collection. Therefore, as a metabolomic analysis serves as a “snapshot” of the specimen physiology at the moment of collection, the metabolomic profile failed to show any pattern regarding metabolite markers of grazing. However, our results highlight the opportunity of performing metabolomic studies with macroalgae under different levels of nutrients to clarify its physiological effect and thresholds, such as those performed in microalgae e.g., [90,91]. In our study, the metabolomic profile of macroalgae apparently was less sensitive to nutrient stress from aquaculture compared to the seagrass meadows sampled in the same station and at the same times and reported by de Kock et al. (2020) [45], which showed clear effects of nutrient stress. The effects of aquaculture nutrients over the macroalgae assemblage are rather evidenced by metabolomics in the grazing activity imbalance of its main grazer P. lividus as described below.
We found a decrease in the accumulation of a set of metabolites in P. lividus that is evidence of the ongoing reduction in macroalgae grazing activity closer to the aquaculture site. Regarding P. lividus, Acacetin, which is one of the major constituents of flavonoids in various Phaeophyta (e.g., Cystoseira sp. and D. dichotoma) species, was found to have a lower concentration in station A [92,93]. Increasing concentrations in the level of Acacetin and related flavonoids have shown to be reliable biomarkers for environmental stress in plants and increasing levels of herbivory [94,95,96,97]. Similarly, the Phlorotannins (polymers of phloroglucinol) were also low in station A. These metabolites are only found in Phaeophyta and have been observed as echinoid herbivore deterrents [98,99,100]. Another group of metabolites that are found only in plants was found to have a lower concentration in P. lividus at station A than B, such as Caffeic acid, 3,4-dihydroxybenzoic acid, and Ferulic acid. Caffeic acid is synthesized by plants as a common deterrent under increasing grazing pressure [101,102]. 3,4-dihydroxybenzoic acid is a common plant-derived phenolic compound [103] and is produced as a metabolite of various natural and synthetic aromatic compounds in the environment that has been found as an algal growth stimulant [104]. Ferulic acid is a natural antioxidant present in plant cell walls and has high anti-inflammatory and antioxidant properties [105]. The trophic transfer and accumulation of metabolites from plants and macroalgae to the grazer tissues that we describe here have been described in several plant–grazer models [106,107]. Hence, the metabolomics analysis can reveal that P. lividus is facing stress in station A by the overgrazing and subsequent depletion of macroalgae evidenced with the traditional methods as it is showing a lower concentration of metabolomic markers of grazing that are unique to the macroalgae with lower cover in station A.
In addition, we found a set of sea urchin metabolites related to a decrease in markers of growth and reproduction in station A. We first identified a marked seasonality of the P. lividus metabolic fingerprint in the PCA with no apparent influence of the distance from aquaculture. This pattern has been previously recorded in other settings in the Mediterranean Sea, where lipid and biochemical profiles of sea urchin gonads were more related to the season than to spatial distribution [108,109]. However, with the pattern hunter analysis, we were able to identify a subset of metabolites related to the pathways of reproduction that decreased in station A. One of these was beta-alanine, which has been recorded to be abundant in mature sea urchin gonads [110]. Beta-alanine is a nonproteinogenic beta-amino acid, thought to be used by the body in the building of proteins. Glutamic acid was also lower at station A, and it is one of the most abundant amino acids in the sea urchin P. lividus egg and is known to act as a fast energy source to the body [111]. Ketones, such as 6-Methyl-5-hepten-2-one, were lower at station A and have been also previously recorded in urchin gonads and could result from the oxidative cleavage of carotenoids [112]. Sea urchin energy reserve metabolites that are regularly attributed to being produced by algae [113] and are accumulated by herbivores through grazing were also lower at station A. For instance, energy-rich saturated hydrocarbons, such as heptacosane, tetracosane, nonacosane, fatty alcohol hexadecanol, and phenols, such as catechin, that were lower at station A have been identified as plant growth promoters and regulators [114,115,116]. Thus, even when the traditional ecological methods, such as the density or condition indexes, suggested that the increasing aquaculture effluents had either a neutral or positive effect on the growth and reproduction of P. lividus, the metabolomic analysis revealed that at an intermediate distance from the aquaculture, the sea urchin population is undergoing a decrease in metabolites related to growth and reproduction.
Our study demonstrates that increased nutrient input from aquaculture acts as a driver of change in the assemblage structure and grazing dynamics of Mediterranean Sea hard-bottom macroalgae ecosystems. The nutrient input leads to an assemblage dominated by turf-forming algae, depleted of native-erected palatable species and with a higher abundance of the invasive species (S. schimperi). The change in plant–herbivore interaction mediated by nutrient enrichment was confirmed by the alteration of metabolic biomarkers of the grazer P. lividus and both macroalgae and sea urchin N stable isotopic analysis. Furthermore, we found a decrease in metabolite makers related to reproduction and energy reserves of P. lividus that are evidence of increasing grazing and the onset of stress at station A. Even though the condition index of P. lividus was higher at station A, implying a positive state of the population, the metabolomic analysis was able to pinpoint underlying stress in P. lividus related to the overgrazing and the change in the macroalgae assemblage influenced by aquaculture nutrients. Taken together, the chosen combination of macroalgae cover, biometrics, physiology, and metabolomic analysis demonstrated the potential to be an efficient method to uncover not only the sublethal effects of aquaculture nutrient loading on a high spatial resolution but also the metabolic pathways and markers for species interactions. However, further testing on a broader scale encompassing different settings around the Mediterranean, with more sample sites in the nutrient-loading gradient is recommended to further validate and calibrate the method.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15010031/s1, Table S1. Annotated list of the metabolites for Paracentroutus lividus, Amphiroa rigida, Jania rubens, Dyctiota dichotoma, Stypopodium schimperi, Laurencia sp. and Cystoceira sp.; File S1: Detailed results for the ANOVA analysis performed in Table 3 and Table 4.
Author Contributions
E.T.A. conceived the research idea and designed the sampling. E.T.A., C.E.-S., W.d.K. and G.C. conducted fieldwork. C.E.-S., H.H.-S., M.H., W.d.K. and G.C. performed the laboratory analyses. C.E.-S., H.H.-S. and E.T.A. analyzed the data. C.E.-S. wrote the first draft of the manuscript. C.E.-S., H.H.-S., M.H., W.d.K., M.T. and G.C. were involved in the Writing—review & editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the ACES Erasmus Mundus JMD (Grant No. 553648-EPP-1-2014-1-UK-EPPKA1-JMD-MOB) and the EU H2020 project TAPAS (Grant Agreement No. 678396).
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the sole use of invertebrate and plant models.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset generated for this study will be made available upon request to E.T.A.
Acknowledgments
We thank I. Glampedakis for assistance with sampling and Katrine Clement Kirkegaard, E. Dafnomili, S. Zivanovic, W. Plaiti, and X. Vlata for helping with the laboratory analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barrett, L.T.; Swearer, S.; Dempster, T. Impacts of marine and freshwater aquaculture on wildlife: A global meta-analysis. Rev. Aquac. 2019, 11, 1022–1044. [Google Scholar] [CrossRef]
- Tičina, V.; Katavić, I.; Grubišić, L. Marine Aquaculture Impacts on Marine Biota in Oligotrophic Environments of the Mediterranean Sea—A Review. Front. Mar. Sci. 2020, 7, 217. [Google Scholar] [CrossRef]
- Karakassis, I.; Tsapakis, M.; Hatziyanni, E.; Papadopoulou, K.-N.; Plaiti, W. Impact of cage farming of fish on the seabed in three Mediterranean coastal areas. ICES J. Mar. Sci. 2000, 57, 1462–1471. [Google Scholar] [CrossRef]
- Hargrave, B. Empirical relationships describing benthic impacts of salmon aquaculture. Aquac. Environ. Interact. 2010, 1, 33–46. [Google Scholar] [CrossRef]
- Grigorakis, K.; Rigos, G. Aquaculture effects on environmental and public welfare—The case of Mediterranean mariculture. Chemosphere 2011, 85, 899–919. [Google Scholar] [CrossRef]
- Sarà, G.; Martire, M.L.; Sanfilippo, M.; Pulicanò, G.; Cortese, G.; Mazzola, A.; Manganaro, A.; Pusceddu, A. Impacts of marine aquaculture at large spatial scales: Evidences from N and P catchment loading and phytoplankton biomass. Mar. Environ. Res. 2011, 71, 317–324. [Google Scholar] [CrossRef]
- Tsagaraki, T.; Pitta, P.; Frangoulis, C.; Petihakis, G.; Karakassis, I. Plankton response to nutrient enrichment is maximized at intermediate distances from fish farms. Mar. Ecol. Prog. Ser. 2013, 493, 31–42. [Google Scholar] [CrossRef][Green Version]
- Jansen, H.; Broch, O.; Bannister, R.; Cranford, P.; Handå, A.; Husa, V.; Jiang, Z.; Strohmeier, T.; Strand, Ø. Spatio-temporal dynamics in the dissolved nutrient waste plume from Norwegian salmon cage aquaculture. Aquac. Environ. Interact. 2018, 10, 385–399. [Google Scholar] [CrossRef]
- Pitta, P.; Apostolaki, E.; Giannoulaki, M.; Karakassis, I. Mesoscale changes in the water column in response to fish farming zones in three coastal areas in the Eastern Mediterranean Sea. Estuar. Coast. Shelf Sci. 2005, 65, 501–512. [Google Scholar] [CrossRef]
- Pitta, P.; Apostolaki, E.; Tsagaraki, T.; Tsapakis, M.; Karakassis, I. Fish Farming Effects on Chemical and Microbial Variables of the Water Column: A Spatio-temporal Study Along the Mediterranean Sea. Hydrobiologia 2006, 563, 99–108. [Google Scholar] [CrossRef]
- Machias, A.; Karakassis, I.; Giannoulaki, M.; Papadopoulou, K.; Smith, C.; Somarakis, S. Response of demersal fish communities to the presence of fish farms. Mar. Ecol. Prog. Ser. 2005, 288, 241–250. [Google Scholar] [CrossRef]
- Machias, A.; Karakassis, I.; Labropoulou, M.; Somarakis, S.; Papadopoulou, K.; Papaconstantinou, C. Changes in wild fish assemblages after the establishment of a fish farming zone in an oligotrophic marine ecosystem. Estuar. Coast. Shelf Sci. 2004, 60, 771–779. [Google Scholar] [CrossRef]
- Pitta, P.; Tsapakis, M.; Apostolaki, E.; Tsagaraki, T.; Holmer, M.; Karakassis, I. ‘Ghost nutrients’ from fish farms are transferred up the food web by phytoplankton grazers. Mar. Ecol. Prog. Ser. 2009, 374, 1–6. [Google Scholar] [CrossRef]
- Boudouresque, C.-F.; Blanfuné, A.; Pergent, G.; Pergent-Martini, C.; Perret-Boudouresque, M.; Thibaut, T. Impacts of Marine and Lagoon Aquaculture on Macrophytes in Mediterranean Benthic Ecosystems. Front. Mar. Sci. 2020, 7, 218. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and Stability of Ecological Systems. Annu. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef]
- May, R.M. Thresholds and breakpoints in ecosystems with a multiplicity of stable states. Nature 1977, 269, 471–477. [Google Scholar] [CrossRef]
- Piazzi, L.; Bulleri, F.; Ceccherelli, G. Limpets compensate sea urchin decline and enhance the stability of rocky subtidal barrens. Mar. Environ. Res. 2016, 115, 49–55. [Google Scholar] [CrossRef]
- Terlizzi, A.; Fraschetti, S.; Guidetti, P.; Boero, F. The effects of sewage discharge on shallow hard substrate sessile assemblages. Mar. Pollut. Bull. 2002, 44, 544–550. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K. Effects of eutrophication, grazing, and algal blooms on rocky shores. Limnol. Oceanogr. 2006, 51, 569–579. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Boström, C.; Engkvist, R.; Labanauskas, V.; Sommer, U. Marine diversity shift linked to interactions among grazers, nutrients and propagule banks. Mar. Ecol. Prog. Ser. 1999, 185, 309–314. [Google Scholar] [CrossRef]
- Vadas, R.L.; Beal, B.F.; Wright, W.A.; Nickl, S.; Emerson, S. Biomass and productivity of red and green algae in Cobscook Bay, Maine. Northeast. Nat. 2004, 11, 163–196. [Google Scholar] [CrossRef]
- Hemmi, A.; Mäkinen, A.; Jormalainen, V.; Honkanen, T. Responses of growth and phlorotannins in Fucus vesiculosus to nutrient enrichment and herbivory. Aquat. Ecol. 2005, 39, 201–211. [Google Scholar] [CrossRef]
- Oh, E.; Edgar, G.; Kirkpatrick, J.; Stuart-Smith, R.; Barrett, N. Broad-scale impacts of salmon farms on temperate macroalgal assemblages on rocky reefs. Mar. Pollut. Bull. 2015, 98, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Fowles, A.E.; Stuart-Smith, R.D.; Hill, N.A.; Thomson, R.J.; Strain, E.M.; Alexander, T.J.; Kirkpatrick, J.; Edgar, G.J. Interactive responses of primary producers and grazers to pollution on temperate rocky reefs. Environ. Pollut. 2018, 237, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Karakassis, I.; Tsapakis, M.; Hatziyanni, E. Seasonal variability in sediment profiles beneath fish farm cages in the Mediterranean. Mar. Ecol. Prog. Ser. 1998, 162, 243–252. [Google Scholar] [CrossRef]
- Pinna, S.; Piazzi, L.; Ceccherelli, G.; Castelli, A.; Costa, G.; Curini-Galletti, M.; Gianguzza, P.; Langeneck, J.; Manconi, R.; Montefalcone, M.; et al. Macroalgal forest vs sea urchin barren: Patterns of macro-zoobenthic diversity in a large-scale Mediterranean study. Mar. Environ. Res. 2020, 159, 104955. [Google Scholar] [CrossRef] [PubMed]
- Pinnegar, J.; Polunin, N.; Francour, P.; Badalamenti, F.; Chemello, R.; Harmelin-Vivien, M.-L.; Hereu, B.; Milazzo, M.; Zabala, M.; D’Anna, G.; et al. Trophic cascades in benthic marine ecosystems: Lessons for fisheries and protected-area management. Environ. Conserv. 2000, 27, 179–200. [Google Scholar] [CrossRef]
- Filbee-Dexter, K.; Scheibling, R. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 2014, 495, 1–25. [Google Scholar] [CrossRef]
- Ruiz, J.; Romero, J. Effects of in situ experimental shading on the Mediterranean seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2001, 215, 107–120. [Google Scholar] [CrossRef]
- Prado, P.; Alcoverro, T.; Romero, J. Seasonal response of Posidonia oceanica epiphyte assemblages to nutrient increase. Mar. Ecol. Prog. Ser. 2008, 359, 89–98. [Google Scholar] [CrossRef]
- Balata, D.; Piazzi, L.; Nesti, U.; Bulleri, F.; Bertocci, I. Effects of enhanced loads of nutrients on epiphytes on leaves and rhizomes of Posidonia oceanica. J. Sea Res. 2010, 63, 173–179. [Google Scholar] [CrossRef]
- Rountos, K.J.; Peterson, B.J.; Karakassis, I. Indirect effects of fish cage aquaculture on shallow Posidonia oceanica seagrass patches in coastal Greek waters. Aquac. Environ. Interact. 2012, 2, 105–115. [Google Scholar] [CrossRef]
- Kušpilić, G.; Tičina, V.; Matijević, S.; Skejić, S.; Antolić, B.; Grubelić, I.; Tudor, M. Impact of fish farming on marine ecosystems-Croatian experiences. In Impact of Mariculture on Coastal Ecosystems: CIESM Workshop Monographs; CIESM: Lisboa, Portugal, 2007; Volume 32, pp. 29–34. [Google Scholar]
- Ballesteros, E.; Torras, X.; Pinedo, S.; García, M.; Mangialajo, L.; de Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Panayotidis, P.; Montesanto, B.; Orfanidis, S. Use of low-budget monitoring of macroalgae to implement the European Water Framework Directive. J. Appl. Phycol. 2004, 16, 49–59. [Google Scholar] [CrossRef]
- Arévalo, R.; Pinedo, S.; Ballesteros, E. Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: Descriptive study and test of proposed methods to assess water quality regarding macroalgae. Mar. Pollut. Bull. 2007, 55, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Pinedo, S.; Arévalo, R.; Ballesteros, E. Seasonal dynamics of upper sublittoral assemblages on Mediterranean rocky shores along a eutrophication gradient. Estuar. Coast. Shelf Sci. 2015, 161, 93–101. [Google Scholar] [CrossRef]
- Eklöf, J.; de la Torre-Castro, M.; Gullström, M.; Uku, J.; Muthiga, N.; Lyimo, T.; Bandeira, S. Sea urchin overgrazing of seagrasses: A review of current knowledge on causes, consequences, and management. Estuar. Coast. Shelf Sci. 2008, 79, 569–580. [Google Scholar] [CrossRef]
- Suskiewicz, T.S.; Johnson, L.E. Consumption rates of a key marine herbivore: A review of the extrinsic and intrinsic control of feeding in the green sea urchin. Mar. Biol. 2017, 164, 131. [Google Scholar] [CrossRef]
- Romero, L. Physiological stress in ecology: Lessons from biomedical research. Trends Ecol. Evol. 2004, 19, 249–255. [Google Scholar] [CrossRef]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic Assessment of Induced and Activated Chemical Defence in the Invasive Red Alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, e29359. [Google Scholar] [CrossRef]
- Rempt, M.; Weinberger, F.; Grosser, K.; Pohnert, G. Conserved and species-specific oxylipin pathways in the wound-activated chemical defense of the noninvasive red alga Gracilaria chilensis and the invasive Gracilaria vermiculophylla. Beilstein J. Org. Chem. 2012, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Greff, S.; Aires, T.; Serrão, E.A.; Engelen, A.H.; Thomas, O.P.; Pérez, T. The interaction between the proliferating macroalga Asparagopsis taxiformis and the coral Astroides calycularis induces changes in microbiome and metabolomic fingerprints. Sci. Rep. 2017, 7, srep42625. [Google Scholar] [CrossRef] [PubMed]
- Hasler-Sheetal, H.; Castorani, M.C.N.; Glud, R.N.; Canfield, D.E.; Holmer, M. Metabolomics Reveals Cryptic Interactive Effects of Species Interactions and Environmental Stress on Nitrogen and Sulfur Metabolism in Seagrass. Environ. Sci. Technol. 2016, 50, 11602–11609. [Google Scholar] [CrossRef] [PubMed]
- de Kock, W.; Hasler-Sheetal, H.; Holmer, M.; Tsapakis, M.; Apostolaki, E.T. Metabolomics and traditional indicators unveil stress of a seagrass (Cymodocea nodosa) meadow at intermediate distance from a fish farm. Ecol. Indic. 2020, 109, 105765. [Google Scholar] [CrossRef]
- Neofitou, N.; Klaoudatos, S. Effect of fish farming on the water column nutrient concentration in a semi-enclosed gulf of the Eastern Mediterranean. Aquac. Res. 2008, 39, 482–490. [Google Scholar] [CrossRef]
- Tomassetti, P.; Gennaro, P.; Lattanzi, L.; Mercatali, I.; Persia, E.; Vani, D.; Porrello, S. Benthic community response to sediment organic enrichment by Mediterranean fish farms: Case studies. Aquaculture 2016, 450, 262–272. [Google Scholar] [CrossRef]
- Tsiaras, K.; Tsapakis, M.; Gkanassos, A.; Kalantzi, I.; Petihakis, G.; Triantafyllou, G. Modelling the impact of finfish aquaculture waste on the environmental status in an Eastern Mediterranean Allocated Ζone for Aquaculture. Cont. Shelf Res. 2022, 234, 104647. [Google Scholar] [CrossRef]
- Chatzigeorgiou, G.; Dailianis, T.; Faulwetter, S.; Pettas, M.; Arvanitidis, C. MANOSS—A manually operated suction sampler for hard bottom benthos. Transit. Waters Bull. 2013, 6, 42–49. [Google Scholar]
- Duran, S.; Palacín, C.; Becerro, M.A.; Turon, X.; Giribet, G. Genetic diversity and population structure of the commercially harvested sea urchin Paracentrotus lividus (Echinodermata, Echinoidea). Mol. Ecol. 2004, 13, 3317–3328. [Google Scholar] [CrossRef]
- Calderon, I.L.; Pita, L.; Brusciotti, S.; Palacín, C.; Turon, X. Time and space: Genetic structure of the cohorts of the common sea urchin Paracentrotus lividus in Western Mediterranean. Mar. Biol. 2012, 159, 187–197. [Google Scholar] [CrossRef]
- Trygonis, V.; Sini, M. photoQuad: A dedicated seabed image processing software, and a comparative error analysis of four photoquadrat methods. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 99–108. [Google Scholar] [CrossRef]
- Clarke, K.; Green, R. Statistical design and analysis for a ‘biological effects’ study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E, Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST-Palaeontological Statistics; University of Oslo: Oslo, Norway, 2001; Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 26 December 2022).
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Noble, W.S. Analysis of strain and regional variation in gene expression in mouse brain. Genome Biol. 2001, 2, RESEARCH0042. [Google Scholar] [CrossRef] [PubMed]
- Verges, A.; Alcoverro, T.; Ballesteros, E. Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2009, 375, 1–11. [Google Scholar] [CrossRef]
- Gianni, F.; Bartolini, F.; Airoldi, L.; Ballesteros, E.; Francour, P.; Guidetti, P.; Meinesz, A.; Thibaut, T.; Mangialajo, L. Conservation and restoration of marine forests in the Mediterranean Sea and the potential role of Marine Protected Areas. Adv. Oceanogr. Limnol. 2013, 4, 83–101. [Google Scholar] [CrossRef]
- Shears, N.T.; Babcock, R. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 2002, 132, 131–142. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic Downgrading of Planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef]
- Agnetta, D.; Badalamenti, F.; Ceccherelli, G.; Di Trapani, F.; Bonaviri, C.; Gianguzza, P. Role of two co-occurring Mediterranean sea urchins in the formation of barren from Cystoseira canopy. Estuar. Coast. Shelf Sci. 2015, 152, 73–77. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Sini, M.; Doumas, O.; Trygonis, V.; Katsanevakis, S. Assessment of grazing effects on phytobenthic community structure at shallow rocky reefs: An experimental field study in the North Aegean Sea. J. Exp. Mar. Biol. Ecol. 2018, 503, 31–40. [Google Scholar] [CrossRef]
- Airoldi, L.; Virgilio, M. Responses of turf-forming algae to spatial variations in the deposition of sediments. Mar. Ecol. Prog. Ser. 1998, 165, 271–282. [Google Scholar] [CrossRef]
- Balata, D.; Piazzi, L.; Bulleri, F. Sediment deposition dampens positive effects of substratum complexity on the diversity of macroalgal assemblages. J. Exp. Mar. Biol. Ecol. 2015, 467, 45–51. [Google Scholar] [CrossRef]
- Piazzi, L.; Ceccherelli, G. Concomitance of oligotrophy and low grazing pressure is essential for the resilience of Mediterranean subtidal forests. Mar. Pollut. Bull. 2017, 123, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ramos, R.; Egea, L.G.; Vergara, J.J.; Brun, F.G. Nutrient load and epiphytes are drivers of increased herbivory in seagrass communities. Mar. Ecol. Prog. Ser. 2018, 599, 49–64. [Google Scholar] [CrossRef]
- Camps-Castellà, J.; Romero, J.; Prado, P. Trophic plasticity in the sea urchin Paracentrotus lividus, as a function of resource availability and habitat features. Mar. Ecol. Prog. Ser. 2020, 637, 71–85. [Google Scholar] [CrossRef]
- Galil, B.; Marchini, A.; Occhipinti-Ambrogi, A.; Ojaveer, H. The enlargement of the Suez Canal—Erythraean introductions and management challenges. Manag. Biol. Invasions 2017, 8, 141–152. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Verlaque, M. Biological pollution in the Mediterranean Sea: Invasive versus introduced macrophytes. Mar. Pollut. Bull. 2002, 44, 32–38. [Google Scholar] [CrossRef]
- Rilov, G.; Peleg, O.; Yeruham, E.; Garval, T.V.; Vichik, A.; Raveh, O. Alien turf: Overfishing, overgrazing and invader domination in south-eastern Levant reef ecosystems. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 351–369. [Google Scholar] [CrossRef]
- Evagelopoulos, A.; Poursanidis, D.; Papazisi, E.; Gerovasileiou, V.; Katsiaras, N.; Koutsoubas, D. Records of alien marine species of Indo-Pacific origin at Sigri Bay (Lesvos Island, north-eastern Aegean Sea). Mar. Biodivers. Rec. 2015, 8, E35. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Corsini-Foka, M.; Morri, C.; Zenetos, A. Thirty years after—Dramatic change in the coastal marine habitats of Kos Island (Greece), 1981–2013. Mediterr. Mar. Sci. 2014, 15, 482. [Google Scholar] [CrossRef]
- Jauni, M.; Gripenberg, S.; Ramula, S. Non-native plant species benefit from disturbance: A meta-analysis. Oikos 2015, 124, 122–129. [Google Scholar] [CrossRef]
- Tomas, F.; Box, A.; Terrados, J. Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol. Invasions 2011, 13, 1559–1570. [Google Scholar] [CrossRef]
- Green, A.J. Mass/length residuals: Measures of body condition or generators of spurious results? Ecology 2001, 82, 1473–1483. [Google Scholar] [CrossRef]
- Harrold, C.; Reed, D.C. Food Availability, Sea Urchin Grazing, and Kelp Forest Community Structure. Ecology 1985, 66, 1160–1169. [Google Scholar] [CrossRef]
- Vadas, R.; Beal, B.; Dowling, T.; Fegley, J. Experimental field tests of natural algal diets on gonad index and quality in the green sea urchin, Strongylocentrotus droebachiensis: A case for rapid summer production in post-spawned animals. Aquaculture 2000, 182, 115–135. [Google Scholar] [CrossRef]
- Liu, D.; Keesing, J.K.; Xing, Q.; Shi, P. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China. Mar. Pollut. Bull. 2009, 58, 888–895. [Google Scholar] [CrossRef]
- Powley, H.R.; Krom, M.D.; Van Cappellen, P. Understanding the unique biogeochemistry of the Mediterranean Sea: Insights from a coupled phosphorus and nitrogen model. Glob. Biogeochem. Cycles 2017, 31, 1010–1031. [Google Scholar] [CrossRef]
- Dalsgaard, T.; Krause-Jensen, D. Monitoring nutrient release from fish farms with macroalgal and phytoplankton bioassays. Aquaculture 2006, 256, 302–310. [Google Scholar] [CrossRef]
- Ruíz, J.M.; Pérez, M.; Romero, J.; Tomas, F. The importance of herbivory in the decline of a seagrass (Posidonia oceanica) meadow near a fish farm: An experimental approach. Bot. Mar. 2009, 52, 449–458. [Google Scholar] [CrossRef]
- Boada, J.; Arthur, R.; Alonso, D.; Pagès, J.F.; Pessarrodona, A.; Oliva, S.; Ceccherelli, G.; Piazzi, L.; Romero, J.; Alcoverro, T. Immanent conditions determine imminent collapses: Nutrient regimes define the resilience of macroalgal communities. Proc. R. Soc. B Boil. Sci. 2017, 284, 20162814. [Google Scholar] [CrossRef]
- Wang, X.; Broch, O.J.; Forbord, S.; Handå, A.; Skjermo, J.; Reitan, K.I.; Vadstein, O.; Olsen, Y. Assimilation of inorganic nutrients from salmon (Salmo salar) farming by the macroalgae (Saccharina latissima) in an exposed coastal environment: Implications for integrated multi-trophic aquaculture. J. Appl. Phycol. 2014, 26, 1869–1878. [Google Scholar] [CrossRef]
- Vizzini, S.; Savona, B.; Caruso, M.; Savona, A.; Mazzola, A. Analysis of stable carbon and nitrogen isotopes as a tool for assessing the environmental impact of aquaculture: A case study from the western Mediterranean. Aquac. Int. 2005, 13, 157–165. [Google Scholar] [CrossRef]
- García-Sanz, T.; Ruiz, J.; Pérez, M.; Ruiz, M. Assessment of dissolved nutrients dispersal derived from offshore fish-farm using nitrogen stable isotope ratios (δ15N) in macroalgal bioassays. Estuar. Coast. Shelf Sci. 2011, 91, 361–370. [Google Scholar] [CrossRef]
- Gartner, A.; Lavery, P.; Smit, A. Use of d15N signatures of different functional forms of macroalgae and filter-feeders to reveal temporal and spatial patterns in sewage dispersal. Mar. Ecol. Prog. Ser. 2002, 235, 63–73. [Google Scholar] [CrossRef]
- Deutsch, B.; Voss, M. Anthropogenic nitrogen input traced by means of δ15N values in macroalgae: Results from in-situ incubation experiments. Sci. Total Environ. 2006, 366, 799–808. [Google Scholar] [CrossRef]
- Bajhaiya, A.K.; Dean, A.P.; Driver, T.; Trivedi, D.K.; Rattray, N.J.W.; Allwood, J.W.; Goodacre, R.; Pittman, J.K. High-throughput metabolic screening of microalgae genetic variation in response to nutrient limitation. Metabolomics 2016, 12, 9. [Google Scholar] [CrossRef]
- Kokabi, K.; Gorelova, O.; Ismagulova, T.; Itkin, M.; Malitsky, S.; Boussiba, S.; Solovchenko, A.; Khozin-Goldberg, I. Metabolomic foundation for differential responses of lipid metabolism to nitrogen and phosphorus deprivation in an arachidonic acid-producing green microalga. Plant Sci. 2019, 283, 95–115. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Abdel-Tawab, H. Cystoseira barbata marine algae have a molluscicidal activity against Biomphalaria alexandrina snails supported by scanning electron microscopy, hematological and histopathological alterations, and larvicidal activity against the infective stages of Schistosoma mansoni. Biologia 2020, 75, 1945–1954. [Google Scholar] [CrossRef]
- Dela, G.; Or, E.; Ovadia, R.; Nissim-Levi, A.; Weiss, D.; Oren-Shamir, M. Changes in anthocyanin concentration and composition in ‘Jaguar’ rose flowers due to transient high-temperature conditions. Plant Sci. 2003, 164, 333–340. [Google Scholar] [CrossRef]
- Robles, C.; Greff, S.; Pasqualini, V.; Garzino, S.; Bousquet-Mélou, A.; Fernandez, C.; Korboulewsky, N.; Bonin, G. Phenols and Flavonoids in Aleppo Pine Needles as Bioindicators of Air Pollution. J. Environ. Qual. 2003, 32, 2265–2271. [Google Scholar] [CrossRef]
- Cannac, M.; Ferrat, L.; Pergent-Martini, C.; Pergent, G.; Pasqualini, V. Effects of fish farming on flavonoids in Posidonia oceanica. Sci. Total Environ. 2006, 370, 91–98. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Zhong, Y.; Yao, L.; Cheng, L.; Wu, T. The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. Plant Mol. Biol. 2015, 89, 35–48. [Google Scholar] [CrossRef]
- Steinberg, P.D. Effects of quantitative and qualitative variation in phenolic compounds on feeding in three species of marine invertebrate herbivores. J. Exp. Mar. Biol. Ecol. 1988, 120, 221–237. [Google Scholar] [CrossRef]
- Hay, M.E.; Fenical, W. MARINE PLANT-HERBIVORE INTERACTIONS: The Ecology of Chemical Defense. Annu. Rev. Ecol. Syst. 1988, 19, 111–145. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Whitman, S.L.; Ehlig, J.M. Differences in herbivore preferences, phlorotannin production, and nutritional quality between juvenile and adult tissues from marine brown algae. Mar. Biol. 2001, 139, 201–210. [Google Scholar] [CrossRef]
- Steele, L.; Valentine, J. Idiosyncratic responses of seagrass phenolic production following sea urchin grazing. Mar. Ecol. Prog. Ser. 2012, 466, 81–92. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; Zaman, Q.U.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective Roles and Mechanisms of Caffeic Acid in Counter Plant Stress: A Mini Review. Pak. J. Agric. Res. 2019, 32, 8–19. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives: Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 8, 1024–1032. [Google Scholar] [CrossRef]
- Kamaya, Y.; Tsuboi, S.; Takada, T.; Suzuki, K. Growth Stimulation and Inhibition Effects of 4-Hydroxybenzoic Acid and Some Related Compounds on the Freshwater Green Alga Pseudokirchneriella subcapitata. Arch. Environ. Contam. Toxicol. 2006, 51, 537–541. [Google Scholar] [CrossRef]
- Picone, P.; Nuzzo, D.; Di Carlo, M. Ferulic Acid: A Natural Antioxidant Against Oxidative Stress Induced by Oligomeric A-beta on Sea Urchin Embryo. Biol. Bull. 2013, 224, 18–28. [Google Scholar] [CrossRef]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Arafa, S.; Chouaibi, M.; Sadok, S.; El Abed, A. The Influence of Season on the Gonad Index and Biochemical Composition of the Sea Urchin Paracentrotus lividus from the Golf of Tunis. Sci. World J. 2012, 2012, 815935. [Google Scholar] [CrossRef]
- Siliani, S.; Melis, R.; Loi, B.; Guala, I.; Baroli, M.; Sanna, R.; Uzzau, S.; Roggio, T.; Addis, M.F.; Anedda, R. Influence of seasonal and environmental patterns on the lipid content and fatty acid profiles in gonads of the edible sea urchin Paracentrotus lividus from Sardinia. Mar. Environ. Res. 2016, 113, 124–133. [Google Scholar] [CrossRef]
- Goel, M.; Mushegian, A. Intermediary metabolism in sea urchin: The first inferences from the genome sequence. Dev. Biol. 2006, 300, 282–292. [Google Scholar] [CrossRef]
- Mol, S.; Baygar, T.; Varlik, C.; Tosun, Ş.Y. Seasonal variations in yield, fatty acids, amino acids and proximate compositions of sea urchin (Paracentrotus lividus) Roe. J. Food Drug Anal. 2008, 16, 5. [Google Scholar] [CrossRef]
- De Quirós, A.R.-B.; López-Hernández, J.; González-Castro, M.; de la Cruz-García, C.; Simal-Lozano, J. Comparison of volatile components in fresh and canned sea urchin ( Paracentrotus lividus, Lamarck ) gonads by GC-MS using dynamic headspace sampling and microwave desorption. Eur. Food Res. Technol. 2001, 212, 643–647. [Google Scholar] [CrossRef]
- Dayananda, C.; Sarada, R.; Kumar, V.; Ravishankar, G.A. Isolation and characterization of hydrocarbon producing green alga Botryococcus braunii from Indian freshwater bodies. Electron. J. Biotechnol. 2007, 10, 78–91. [Google Scholar] [CrossRef]
- Dufourc, E.J. The role of phytosterols in plant adaptation to temperature. Plant Signal. Behav. 2008, 3, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Kumar Vats, S.; Sharma, M.; Kumar, S. Catechin promotes growth of Arabidopsis thaliana with con-comitant changes in vascular system, photosynthesis and hormone content. Biol. Plant. 2011, 55, 779–782. [Google Scholar] [CrossRef]
- Jishma, P.; Hussain, N.; Chellappan, R.; Rajendran, R.; Mathew, J.; Radhakrishnan, E.K. Strain-specific variation in plant growth promoting volatile organic compounds production by five different Pseudomonas spp. as confirmed by response of Vigna radiata seedlings. J. Appl. Microbiol. 2017, 123, 204–216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).