Global Overview of Modern Research Based on Ellenberg Indicator Values
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Selecting Studies to Include in a Systematic Review
2.3. Data Extraction, Management, and Analysis
2.4. Study Limitations
3. Results
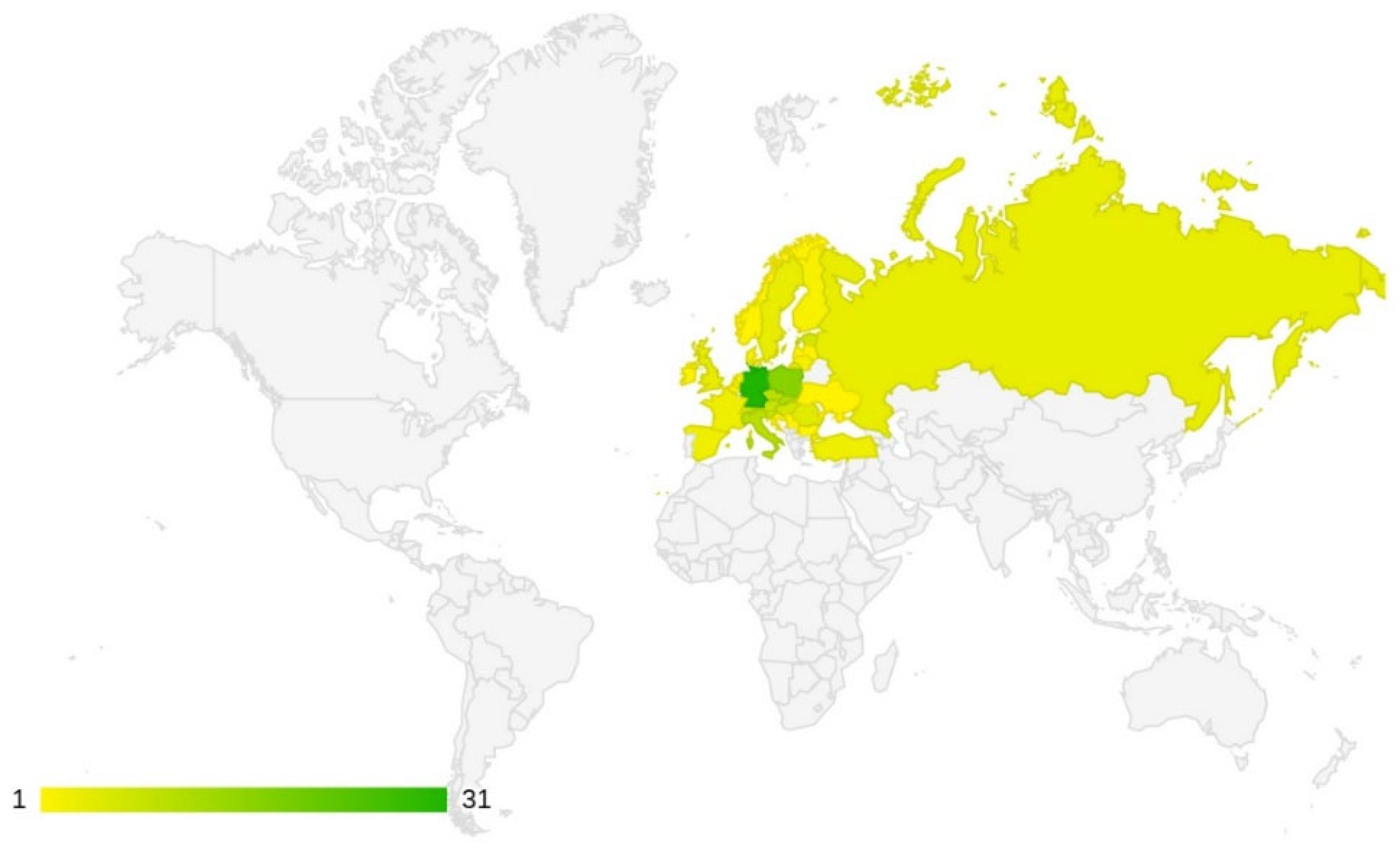
3.1. Frequency of Studies by Country
3.2. Type of Plant Community under Research
3.3. Characterization of Research Topics
3.4. Characterization of Citing
4. Discussion
4.1. Frequency of Studies by Country
4.2. Type of Plant Community under Research
4.3. Characterization of Research Topics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ri, X.; Yang, J.; Zhao, L.-Q.; Qing, H.; Latanzhula, A.; Yao, Z.-Y.; Zhu, L.; Wu, Y.-H.; Tian, J.-B.; Cao, X.-P.; et al. Establishment, development, and decline of Salix psammophila communities: Changes in soil conditions and floristic composition in dune slacks. Glob. Ecol. Conserv. 2020, 22, e00967. [Google Scholar] [CrossRef]
- Ivanova, N.S.; Zolotova, E.S.; Li, G. Influence of soil moisture regime on the species diversity and biomass of the herb layer of pine forests in the Ural Mountains. Ecol. Quest. 2021, 32, 27–38. [Google Scholar] [CrossRef]
- Mumshad, M.; Ahmad, I.; Khan, S.M.; Rehman, K.; Islam, M.; Sakhi, S.; Khan, S.U.; Afridi, S.G.; Shams, S.; Azam, S.; et al. Phyto-ecological studies and distribution pattern of plant species and communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. PLoS ONE 2021, 16, e0257493. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, D.; Guisan, A. Ecological indicator values reveal missing predictors of species distributions. Sci. Rep. 2019, 9, 3061. [Google Scholar] [CrossRef]
- Dítě, Z.; Šuvada, R.; Tóth, T.; Jun, P.; Píš, V.; Dítě, D. Current Condition of Pannonic Salt Steppes at Their Distribution Limit: What Do Indicator Species Reveal about Habitat Quality? Plants 2021, 10, 530. [Google Scholar] [CrossRef]
- Zhivotovsky, L.A.; Osmanova, G.O. Phyto-Indicator of Variation in Environmental Conditions. Biol. Bull. 2021, 48, 207–213. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Sucre, B.; Mileham, K.; Sánchez-Mercado, A.; De Andrade, N.; Bezeng, S.B.; Croukamp, C.; Falcato, J.; García-Borboroglu, P.; González, S.; et al. Addressing the Biodiversity Paradox: Mismatch between the Co-Occurrence of Biological Diversity and the Human, Financial and Institutional Resources to Address Its Decline. Diversity 2022, 14, 708. [Google Scholar] [CrossRef]
- Ellenberg, H. Zeigerwerte der Gefässpflanzen Mitteleuropas. Scr. Geobot 1974, 9, 1–166. [Google Scholar]
- Landolt, E. Okologische Zeigerwerts zur Sweizer Flora. Veroff. Geobot. Inst. ETH. Zurich 1977, 64, 1–208. [Google Scholar]
- Ramensky, L.G.; Tsatsenkin, I.A.; Chizhikov, O.N.; Antipin, N.A. Ecological Assessment of Fodder Lands by Vegetation Cover; Sel’khozhiz: Moscow, Russia, 1956; pp. 1–472. [Google Scholar]
- Tsyganov, D.N. Phytoindication of Ecological Regimes in The Subzone of Coniferous-Deciduous Forests; Nauka: Moskow, Russia, 1983; 196p. [Google Scholar]
- Bartelheimer, M.; Poschlod, P. Functional characterizations of Ellenberg indicator values–A review on ecophysiological determinants. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Descombes, P.; Walthert, L.; Baltensweiler, A.; Meuli, R.G.; Karger, D.N.; Ginzler, C.; Zurell, D.; Zimmermann, N.E. Spatial modelling of ecological indicator values improves predictions of plant distributions in complex landscapes. Ecography 2020, 43, 1448–1463. [Google Scholar] [CrossRef]
- Egorova, N.Y. Influence of Ecological Factors on the Population-Ontogenetic Parameters of Vaccinium vitis-idaea L. in Forest Ecosystems of the European Northeast of Russia. Contemp. Probl. Ecol. 2020, 13, 656–662. [Google Scholar] [CrossRef]
- Hellegers, M.; Ozinga, W.A.; Hinsberg, A.; Huijbregts, M.A.J.; Hennekens, S.M.; Schaminée, J.H.J.; Dengler, J.; Schipper, A.M. Evaluating the ecological realism of plant species distribution models with ecological indicator values. Ecography 2020, 43, 161–170. [Google Scholar] [CrossRef]
- Hájek, M.; Dítě, D.; Horsáková, V.; Mikulášková, E.; Peterka, T.; Navrátilová, J.; Jiménez-Alfaro, B.; Hájková, P.; Tichý, L.; Horsák, M. Towards the pan-European bioindication system: Assessing and testing updated hydrological indicator values for vascular plants and bryophytes in mires. Ecol. Indic. 2020, 116, 106527. [Google Scholar] [CrossRef]
- Saatkamp, A.; Falzon, N.; Argagnon, O.; Noble, V.; Dutoit, T.; Meineri, E. Calibrating ecological indicator values and niche width for a Mediterranean flora. Plant Biosyst. Int. J. Deal. all Asp. Plant Biol. 2022, 156, 1–11. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Dull, R.; Wirth, V.; Werner, W.; Paulußen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1991, 18, 1–248. [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Duell, R.; Wirth, V.; Werner, W. Zeigerwerte der Gefasspflanzen Mitteleuropas (3 Aufl). Scr. Geobot. 2001, 18, 1–262. [Google Scholar]
- Diekmann, M. Species indicator values as an important tool in applied plant ecology–A review. Basic Appl. Ecol. 2003, 4, 493–506. [Google Scholar] [CrossRef]
- Carroll, T.; Gillingham, P.K.; Stafford, R.; Bullock, J.M.; Diaz, A. Improving estimates of environmental change using multilevel regression models of Ellenberg indicator values. Ecol. Evol. 2018, 8, 9739–9750. [Google Scholar] [CrossRef]
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, a comprehensive data base management system for vegetation data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Tichý, L. JUICE, software for vegetation classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar] [CrossRef]
- Zubkova, E.V.; Khanina, L.G.; Grokhlina, T.I.; Dorogova, J.A. Computer Processing of Geobotanical Descriptions on Ecological Scales Using the Program EcoScaleWin Software; Publishing house of Morgan State University: Yoshkar Ola, Russia, 2008; pp. 1–96. (In Russian) [Google Scholar]
- Ewald, J. The sensitivity of Ellenberg indicator values to the completeness of vegetation relevés. Basic Appl. Ecol. 2003, 4, 507–513. [Google Scholar] [CrossRef]
- Smart, S.M.; Scott, W.A. Bias in Ellenberg indicator values—problems with detection of the effect of vegetation type. J. Veg. Sci. 2004, 15, 843–846. [Google Scholar] [CrossRef]
- Otýpková, Z. The influence of sample plot size on evaluations with Ellenberg indicator values. Biologia 2009, 64, 1123–1128. [Google Scholar] [CrossRef]
- Berg, C.; Welk, E.; Jäger, E.J. Revising Ellenberg’s indicator values for continentality based on global vascular plant species distribution. Appl. Veg. Sci. 2017, 20, 482–493. [Google Scholar] [CrossRef]
- Schaffers, A.P.; Sýkora, K.V. Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: A comparison with field measurements. J. Veg. Sci. 2000, 11, 225–244. [Google Scholar] [CrossRef]
- Diekmann, M. Use and improvement of Ellenberg’s indicator values in deciduous forests of the Boreo-nemoral zone in Sweden. Ecography 1995, 18, 178–189. [Google Scholar] [CrossRef]
- Szymura, T.H.; Szymura, M.; Macioł, A. Bioindication with Ellenberg’s indicator values: A comparison with measured parameters in Central European oak forests. Ecol. Indic. 2014, 46, 495–503. [Google Scholar] [CrossRef]
- Hill, M.O.; Mountford, J.O.; Roy, D.B.; Bunce, R.G.H. Ellenberg’s Indicator Values for British Plants; Institute of Terrestrial Ecology: Huntington, UK, 1999. [Google Scholar]
- Bohling, N.; Greuter, W.; Raus, T. Indicator values for vascular plants in the Southern Aegean (Greece). Braun Blanquetia 2002, 32, 1–106. [Google Scholar]
- Marcenò, C.; Guarino, R. A test on Ellenberg indicator values in the Mediterranean evergreen woods (Quercetea ilicis). Rend. Lincei 2015, 26, 345–356. [Google Scholar] [CrossRef][Green Version]
- Lawesson, J.E.; Fosaa, A.M.; Olsen, E. Calibration of Ellenberg indicator values for the Faroe Islands. Appl. Veg. Sci. 2003, 6, 53–62. [Google Scholar] [CrossRef]
- LaPaix, R.; Freedman, B.; Patriquin, D. Ground vegetation as an indicator of ecological integrity. Environ. Rev. 2009, 17, 249–265. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Hahs, A.K.; Vesk, P.A. Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Mengist, W.; Soromessa, T.; Legese, G. Method for conducting systematic literature review and meta-analysis for environmental science research. Methodsx 2020, 7, 100777. [Google Scholar] [CrossRef] [PubMed]
- GeoCharts Quick Start. Available online: https://developers.google.com/chart/interactive/docs/gallery/geochart (accessed on 1 October 2022).
- Stefanov, S. React: Up & Running: Building Web Applications, 1st ed.; O’Reilly Media: Sebastopol, CA, USA, 2016; 222p. [Google Scholar]
- Sun, S.; Jung, E.Y.; Gaviria, J.; Engelbrecht, B.M.J. Drought survival is positively associated with high turgor loss points in temperate perennial grassland species. Funct. Ecol. 2020, 34, 788–798. [Google Scholar] [CrossRef]
- Rehling, F.; Sandner, T.M.; Matthies, D. Biomass partitioning in response to intraspecific competition depends on nutrients and species characteristics: A study of 43 plant species. J. Ecol. 2021, 109, 2219–2233. [Google Scholar] [CrossRef]
- Bartušková, A.; Filartiga, A.L.; Herben, T.; Qian, J.; Klimešová, J. Comparative analysis of root sprouting and its vigour in temperate herbs: Anatomical correlates and environmental predictors. Ann. Bot. 2021, 127, 931–941. [Google Scholar] [CrossRef]
- Roth, M.; Michiels, H.-G.; Puhlmann, H.; Sucker, C.; Winter, M.-B.; Hauck, M. Responses of Temperate Forests to Nitrogen Deposition: Testing the Explanatory Power of Modeled Deposition Datasets for Vegetation Gradients. Ecosystems 2021, 24, 1222–1238. [Google Scholar] [CrossRef]
- Roth, M.; Michiels, H.G.; Puhlmann, H.; Sucker, C.; Hauck, M. Multiple soil factors explain eutrophication signals in the understorey vegetation of temperate forests. J. Veg. Sci. 2021, 32, e13063. [Google Scholar] [CrossRef]
- Roth, M.; Müller-Meißner, A.; Michiels, H.-G.; Hauck, M. Vegetation changes in the understory of nitrogen-sensitive temperate forests over the past 70 years. For. Ecol. Manag. 2022, 503, 119754. [Google Scholar] [CrossRef]
- Günther, K.; Schmidt, M.; Quitt, H.; Heinken, T. Vegetation change in the forests between the Elbe and Havel rivers (NE Germany) from 1960 to 2015. Tuexenia 2021, 41, 53–85. [Google Scholar] [CrossRef]
- Jenssen, M.; Nickel, S.; Schütze, G.; Schröder, W. Reference states of forest ecosystem types and feasibility of biocenotic indication of ecological soil condition as part of ecosystem integrity and services assessment. Environ. Sci. Eur. 2021, 33, 1–18. [Google Scholar] [CrossRef]
- Depauw, L.; Perring, M.P.; Landuyt, D.; Maes, S.L.; Blondeel, H.; De Lombaerde, E.; Brūmelis, G.; Brunet, J.; Closset-Kopp, D.; Decocq, G.; et al. Evaluating structural and compositional canopy characteristics to predict the light-demand signature of the forest understorey in mixed, semi-natural temperate forests. Appl. Veg. Sci. 2021, 24, e12532. [Google Scholar] [CrossRef]
- Travers, E.; Pitz, W.T.; Fichtner, A.; Matthies, D.; Härdtle, W. The role of semi-open habitats as dispersal corridors for plant species of woodlands and open habitats. Appl. Veg. Sci. 2021, 24. [Google Scholar] [CrossRef]
- Řepka, R.; Keclíková, J.; Šebesta, J. Comparison of Forest Species- Diversity and Composition Inside and Outside of the Holedná Game Reserve (The City of Brno, Czech Republic). J. Landsc. Ecol. 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Gafta, D.; Stoica, I.A.; Coldea, G. The ranked species occupancy curves reflect the dominant process of species sorting: Evidence from forest/scrub communities. J. Veg. Sci. 2021, 32. [Google Scholar] [CrossRef]
- Fojcik, B.; Chmura, D. Vertical distribution of epiphytic bryophytes depends on phorophyte type; a case study from windthrows in Kampinoski National Park (Central Poland). Folia Cryptogam. Estonica 2020, 57, 59–71. [Google Scholar] [CrossRef]
- Likhanova, I.A.; Perminova, E.M.; Shushpannikova, G.S.; Zheleznova, G.V.; Pystina, T.N.; Kholopov, Y.V. Dynamics of vegetation after clearcutting bilberry spruce forests (middle taiga subzone of the European North-East of Russia). Rastitel’nost’ Rossii 2021, 40, 108–136. [Google Scholar] [CrossRef]
- Kirillova, I.A.; Dubrovskiy, Y.A.; Degteva, S.V.; Novakovskiy, A.B. Ecological and habitat ranges of Orchids in the northernmost regions of their distribution areas: A case study from Ural Mountains, Russia. Plant Divers. 2022. [Google Scholar] [CrossRef]
- Tunçkol, B.; Aksoy, N.; Çoban, S.; Zengin, H. Diversity and ecology of forest communities in Küre Mountains National Park of Turkey. Bosque Valdivia 2020, 41, 289–305. [Google Scholar] [CrossRef]
- Merle, H.; Garmendia, A.; Hernández, H.; Ferriol, M. Vegetation change over a period of 46 years in a Mediterranean mountain massif (Penyagolosa, Spain). Appl. Veg. Sci. 2020, 23, 495–507. [Google Scholar] [CrossRef]
- Dietz, L.; Collet, C.; Dupouey, J.L.; Lacombe, E.; Laurent, L.; Gégout, J.C. Windstorm-induced canopy openings accelerate temperate forest adaptation to global warming. Glob. Ecol. Biogeogr. 2020, 29, 2067–2077. [Google Scholar] [CrossRef]
- Mayer, M.; Rewald, B.; Matthews, B.; Sandén, H.; Rosinger, C.; Katzensteiner, K.; Gorfer, M.; Berger, H.; Tallian, C.; Berger, T.W.; et al. Soil fertility relates to fungal-mediated decomposition and organic matter turnover in a temperate mountain forest. New Phytol. 2021, 231, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Chmura, D.; Żarnowiec, J.; Staniaszek-Kik, M. Altitude is a better predictor of the habitat requirements of epixylic bryophytes and lichens than the presence of coarse woody debris in mountain forests: A study in Poland. Ann. For. Sci. 2022, 79, 7. [Google Scholar] [CrossRef]
- Sewerniak, P.; Puchałka, R. Topographically induced variation of microclimatic and soil conditions drives ground vegetation diversity in managed Scots pine stands on inland dunes. Agric. For. Meteorol. 2020, 291, 108054. [Google Scholar] [CrossRef]
- Čakšs, R.; Čakša, L.; Desaine, I.; Lībiete, Z.; Elferts, D.; Butlers, A.; Jansons, Ā. Long-Term Influence of Stump-Removal on Components of Hemiboreal Pine Forest Ecosystem. Sustainability 2021, 13, 2095. [Google Scholar] [CrossRef]
- Mandžukovski, D.; Teofilovski, A.; Andreevski, M.; Ćušterevska, R.; Tzonev, R.; Dimitrov, M. Relationships between vegetation of Macedonian pine (Pinus peuce Griseb.) and different types of soils on which it develops. Hacquetia 2022, 21, 89–106. [Google Scholar] [CrossRef]
- Baumann, M.; Dittrich, S.; Körner, M.; von Oheimb, G. Temporal changes in the ground vegetation in spruce forests in the Erzgebirge (Ore Mountains)—bryophytes are better indicators of the impact of liming and of sulphur and nitrogen deposition than the herb layer. Appl. Veg. Sci. 2021, 24. [Google Scholar] [CrossRef]
- Montecchiari, S.; Tesei, G.; Allegrezza, M. Effects of Robinia pseudoacacia coverage on diversity and environmental conditions of central-northern Italian Quercus pubescens sub-Mediterranean forests (habitat code 91Aa*): A threshold assessment. Ann. Bot. 2020, 10, 33–54. [Google Scholar] [CrossRef]
- Prausová, R.; Doležal, J.; Rejmánek, M. Nine decades of major compositional changes in a Central European beech forest protected area. Plant Ecol. 2020, 221, 1005–1016. [Google Scholar] [CrossRef]
- Peppler-Lisbach, C.; Stanik, N.; Könitz, N.; Rosenthal, G. Long-term vegetation changes in Nardus grasslands indicate eutrophication, recovery from acidification, and management change as the main drivers. Appl. Veg. Sci. 2020, 23, 508–521. [Google Scholar] [CrossRef]
- Bauer, M.; Albrecht, H. Vegetation monitoring in a 100-year-old calcareous grassland reserve in Germany. Basic Appl. Ecol. 2020, 42, 15–26. [Google Scholar] [CrossRef]
- Hollmann, V.; Donath, T.W.; Grammel, F.; Himmighofen, T.; Zerahn, U.; Leyer, I. From nutrients to competition processes: Habitat specific threats to Arnica montana L. populations in Hesse, Germany. PLoS ONE 2020, 15, e0233709. [Google Scholar] [CrossRef]
- Mazalla, L.; Ludwig, G.; Peppler-Lisbach, C. Nardus grasslands and wet heaths are affected differently by reintroduction of management and pH recovery. Tuexenia 2021, 41, 227–252. [Google Scholar] [CrossRef]
- Kaulfuß, F.; Rosbakh, S.; Reisch, C. Grassland restoration by local seed mixtures: New evidence from a practical 15-year restoration study. Appl. Veg. Sci. 2022, 25, e12652. [Google Scholar] [CrossRef]
- Cancellieri, L.; Rosati, L.; Brunetti, M.; Mancini, L.D.; Primi, R.; Ronchi, B.; Scoppola, A.; Filibeck, G. The dry grasslands of Abruzzo National Park, the oldest protected area in the Apennines (Central Italy): Overview of vegetation composition, syntaxonomy, ecology and diversity. Tuexenia 2020, 40, 547–571. [Google Scholar] [CrossRef]
- Löfgren, O.; Hall, K.; Schmid, B.C.; Prentice, H.C. Grasslands ancient and modern: Soil nutrients, habitat age and their relation to Ellenberg. N. J. Veg. Sci. 2020, 31, 367–379. [Google Scholar] [CrossRef]
- Kose, M.; Melts, I.; Heinsoo, K. Medicinal Plants in Semi-Natural Grasslands: Impact of Management. Plants 2022, 11, 353. [Google Scholar] [CrossRef]
- Uogintas, D.; Rašomavičius, V. Impact of Short-Term Abandonment on the Structure and Functions of Semi-Natural Dry Grasslands. Botanica 2020, 26, 40–48. [Google Scholar] [CrossRef]
- Mašková, T.; Maternová, J.; Těšitel, J. Shoot: Root ratio of seedlings is associated with species niche on soil moisture gradient. Plant Biol. 2021, 24, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Hegedüšová, K.; Korzeniak, J.; Májeková, J.; Stoica, A.; Coldea, G.; Kuzemko, A.; Budzhak, V.; Tokaryuk, A.; Chorney, I.; Škodová, I. Syntaxonomical revision of the Triseto flavescentis-Polygonion bistortae alliance in the Carpathians. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 155, 16–41. [Google Scholar] [CrossRef]
- Huseyinova, R. Ellenberg Indicator Values (EIVs) in Different Plant Communities in Subalpine Grasslands of the Giresun Mountains (Turkey). Russ. J. Ecol. 2021, 52, 223–229. [Google Scholar] [CrossRef]
- Skálová, H.; Hadincová, V.; Krahulec, F.; Pecháčková, S.; Herben, T. Dynamics of a mountain grassland: Environment predicts long-term trends, while species’ traits predict short-term fluctuations. J. Veg. Sci. 2022, 33, e13138. [Google Scholar] [CrossRef]
- Oroian, S.; Sămărghiţan, M.; Domokos, E. Plant Associations of Petasition officinalis Alliance in the East Carpathians (Călimani and Gurghiu Mountains Romania). In Tools for Landscape-Scale Geobotany and Conservation; Springer: Cham, Switzerland, 2021; pp. 205–229. [Google Scholar] [CrossRef]
- Lengyel, A.; Swacha, G.; Botta-Dukát, Z.; Kącki, Z. Trait-based numerical classification of mesic and wet grasslands in Poland. J. Veg. Sci. 2020, 31, 319–330. [Google Scholar] [CrossRef]
- Łuszczyński, J.; Adamska, E.; Wojciechowska, A.; Czerwik-Marcinkowska, J. Diversity Patterns of Macrofungi in Xerothermic Grasslands from the Nida Basin (Małopolska Upland, Southern Poland): A Case Study. Biology 2022, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, T.; Ziaja, M. Abundance and conservation status of Rosa gallica in Strzyżów Foothills (SE Poland). Ecol. Quest. 2022, 33, 83–90. [Google Scholar] [CrossRef]
- Ahl, L.; Aas, G.; Walentowski, H.; Höltken, A.M.; Feulner, M. Niche differentiation between Malus sylvestris and its hybrid with Malus domestica indicated by plant community, soil and light. J. Veg. Sci. 2021, 32, e13078. [Google Scholar] [CrossRef]
- Cîșlariu, A.G.; Mânzu, C.C.; Baltag, E. Which are the drivers that influence the performance of Ligularia sibirica populations from Romania? Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 394–405. [Google Scholar] [CrossRef]
- Jochner-Oette, S.; Rohrer, T.; Eisen, A.-K.; Tönnes, S.; Stammel, B. Influence of Forest Stand Structure and Competing Understory Vegetation on Ash Regeneration—Potential Effects of Ash Dieback. Forests 2021, 12, 128. [Google Scholar] [CrossRef]
- Hoffmann, M.H.; Gebauer, S.; Lühmann, R. Root anatomy predicts ecological optima in Carex (Cyperaceae) in terms of Ellenberg indicator values. Ecol. Indic. 2021, 129, 107979. [Google Scholar] [CrossRef]
- Novakovskaya, I.V.; Dubrovskiy, Y.A.; Patova, E.N.; Novakovskiy, A.B.; Sterlyagova, I.N. Influence of ecological factors on soil algae in different types of mountain tundra and sparse forests in the Northern Urals. Phycologia 2020, 59, 320–329. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Iberite, M.; Bonari, G.; Nicolella, G.; Rosati, L.; Salerno, G.; Abbate, G. The segetal flora of winter cereals and allied crops in Italy: Species inventory with chorological, structural and ecological features. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 154, 935–946. [Google Scholar] [CrossRef]
- Paal, T.; Zobel, K.; Liira, J. Standardized response signatures of functional traits pinpoint limiting ecological filters during the migration of forest plant species into wooded corridors. Ecol. Indic. 2020, 108, 105688. [Google Scholar] [CrossRef]
- Skrajna, T.; Bogusz, A. Floral diversity of plant communities in field balks. Stud. Quat. 2020, 37, 45–50. [Google Scholar] [CrossRef]
- Glaser, M.; Berg, C.; Buldrini, F.; Buholzer, S.; Bürger, J.; Chiarucci, A.; Chytrý, M.; Dřevojan, P.; Follak, S.; Küzmič, F.; et al. AgriWeedClim database: A repository of vegetation plot data from Central European arable habitats over 100 years. Appl. Veg. Sci. 2022, 25, e12675. [Google Scholar] [CrossRef]
- Fratarcangeli, C.; Fanelli, G.; Testolin, R.; Buffi, F.; Travaglini, A. Floristic changes of vascular flora in the city of Rome through grid-cell census over 23 years. Urban Ecosyst. 2022, 25, 1851–1864. [Google Scholar] [CrossRef]
- Świerkosz, K.; Reczyńska, K. Differentiation of natural scrub communities of the Cotoneastro-Amelanchieretum group in Central Europe. PLoS ONE 2022, 17, e0266868. [Google Scholar] [CrossRef]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Jiménez-Alfaro, B.; Carlón, L.; Fernández-Pascual, E.; Acedo, C.; Alfaro-Saiz, E.; Redondo, R.A.; Cires, E.; Mazuelas, F.D.E.; Del Río, S.; Gonz´alez, T.E.D.; et al. Checklist of the vascular plants of the Cantabrian Mountains. Mediterr. Bot. 2021, 42, e74570. [Google Scholar] [CrossRef]
- Sizonenko, T.A.; Dubrovskiy, Y.A.; Novakovskiy, A.B. Changes in mycorrhizal status and type in plant communities along altitudinal and ecological gradients—A case study from the Northern Urals (Russia). Mycorrhiza 2020, 30, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Bähner, K.W.; Tabarelli, M.; Büdel, B.; Wirth, R. Habitat fragmentation and forest management alter woody plant communities in a Central European beech forest landscape. Biodivers. Conserv. 2020, 29, 2729–2747. [Google Scholar] [CrossRef]
- Klynge, D.; Svenning, J.-C.; Skov, F. Floristic changes in the understory vegetation of a managed forest in Denmark over a period of 23 years–Possible drivers of change and implications for nature and biodiversity conservation. For. Ecol. Manag. 2020, 466, 118128. [Google Scholar] [CrossRef]
- Kowalska, A. Neofityzacja łęgów jesionowo-wiązowych w dolinach polskich rzek = Neophyte-induced degradation of Poland’s riparian hardwood forests. Prz. Geogr. 2020, 92, 327–340. [Google Scholar] [CrossRef]
- Mikulová, K.; Jarolímek, I.; Šibík, J.; Bacigál, T.; Šibíková, M. Long-Term Changes of Softwood Floodplain Forests—Did the Disappearance of Wet Vegetation Accelerate the Invasion Process? Forests 2020, 11, 1218. [Google Scholar] [CrossRef]
- Kaiser, T.; Ahlborn, J. Long-term vegetation monitoring in the floodplain grasslands of the lower Havel Valley (northeastern Germany) and conclusions for sustainable management practices. J. Nat. Conserv. 2021, 63, 126053. [Google Scholar] [CrossRef]
- Rendeková, A.; Miškovic, J.; Mičieta, K.; Michalová, M.; Eliašová, M.; Ballová, D. Changes in the Diversity of Dry Calcareous Grasslands After Abandonment of Traditional Management in Devínska Kobyla National Nature Reserve (Southwestern Slovakia, Central Europe). Ekológia 2020, 39, 101–115. [Google Scholar] [CrossRef]
- Mazalla, L.; Diekmann, M.; Duprè, C. Microclimate shapes vegetation response to drought in calcareous grasslands. Appl. Veg. Sci. 2022, 25, e12672. [Google Scholar] [CrossRef]
- Ziaja, M.; Wójcik, T.; Wrzesień, M. Phytosociological Data in Assessment of Anthropogenic Changes in Vegetation of Rzeszów Reservoir. Sustainability 2021, 13, 9071. [Google Scholar] [CrossRef]
- Smith, P.H.; Lockford, P. Fifteen years of habitat, floristic and vegetation change on a pioneer sand-dune and slack system at Ainsdale, north Merseyside, UK. Br. Ir. Bot. 2021, 3. [Google Scholar] [CrossRef]
- Blaus, A.; Reitalu, T.; Poska, A.; Vassiljev, J.; Veski, S. Mire plant diversity change over the last 10,000 years: Importance of isostatic land uplift, climate and local conditions. J. Ecol. 2021, 109, 3634–3651. [Google Scholar] [CrossRef]
- Hájek, M.; Horsáková, V.; Hájková, P.; Coufal, R.; Dítě, D.; Němec, T.; Horsák, M. Habitat extremity and conservation management stabilise endangered calcareous fens in a changing world. Sci. Total. Environ. 2020, 719, 134693. [Google Scholar] [CrossRef] [PubMed]
- Aikio, S.; Ramula, S.; Muola, A.; Von Numers, M. Island properties dominate species traits in determining plant colonizations in an archipelago system. Ecography 2020, 43, 1041–1051. [Google Scholar] [CrossRef]
- Dudáš, M.; Slezák, M.; Hrivnák, R. Distribution, ecology and vegetation affinity of bog arum (Calla palustris) in Slovakia. Biologia 2021, 76, 2021–2029. [Google Scholar] [CrossRef]
- Wittig, B.; Müller, J.; Quast, R.; Miehlich, H. Arnica montana in Calluna vulgaris-heathlands on the firing range Unterlüß (Lower Saxony). Tuexenia 2020, 40, 131–146. [Google Scholar] [CrossRef]
- Petersson, L.; Nilsson, S.; Holmström, E.; Lindbladh, M.; Felton, A. Forest floor bryophyte and lichen diversity in Scots pine and Norway spruce production forests. For. Ecol. Manag. 2021, 493, 119210. [Google Scholar] [CrossRef]
- Pasta, S.C.; La Rosa, A.; Garfì, G.; Marcenò, C.; Gristina, A.S.; Carimi, F.; Guarino, R. An Updated Checklist of the Sicilian Native Edible Plants: Preserving the Traditional Ecological Knowledge of Century-Old Agro-Pastoral Landscapes. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Kutbay, H.G.; Sürmen, B. Ellenberg ecological indicator values, tolerance values, species niche models for soil nutrient availability, salinity, and pH in coastal dune vegetation along a landward gradient (Euxine, Turkey). Turk. J. Bot. 2022, 46, 346–360. [Google Scholar] [CrossRef]
- Ballelli, S.; Tardella, F.M.; Pennesi, R.; Panichella, T.; Bricca, A.; Vitanzi, A.; Catorci, A. Contribution to the knowledge of the non-calcareous grasslands of the Monti Sibillini National Park (central Italy): Coenological structure, syntaxonomy, ecology, and floristic aspects. Hacquetia 2022, 21, 41–72. [Google Scholar] [CrossRef]
- Dítě, D.; Šuvada, R.; Kliment, J.; Dítě, Z. Vegetation of temperate inland salt marshes on their north-western border (North German Plain). Preslia 2022, 94, 111–141. [Google Scholar] [CrossRef]
- Tomaselli, M.; Carbognani, M.; Foggi, B.; Adorni, M.; Petraglia, A.; Forte, T.G.W.; Segadelli, S.; Rossi, G.; Gennai, M. Scree vegetation in the northern Apennines (N-Italy). Phytocoenologia 2021, 51, 39–94. [Google Scholar] [CrossRef]
- Paal, J.; Jürjendal, I. Diversity of old-drained forests in Estonia. Balt. For. 2020, 26, 40–53. [Google Scholar] [CrossRef]
- Dwyer, C.; Millett, J.; Pakeman, R.J.; Jones, L. Environmental modifiers of the relationship between water table depth and Ellenberg’s indicator of soil moisture. Ecol. Indic. 2021, 132, 108320. [Google Scholar] [CrossRef]
- Simmel, J.; Ahrens, M.; Poschlod, P. Ellenberg N values of bryophytes in Central Europe. J. Veg. Sci. 2021, 32, e12957. [Google Scholar] [CrossRef]
- Lukovičová, M.; Balanac, Z.; David, S. Changes in Habitat Conditions of Invaded Forest Communities in Podunajská Nížina and the Impact of Non-Native Species on Biodiversity (SW Slovakia). Ekológia 2021, 40, 364–378. [Google Scholar] [CrossRef]
- Testi, A.; Fanelli, G.; Nicola, C.D.; D’Angeli, D.N.; Sanctis, M.D. Can ecoindicators be a tool to reduce and understand complexity of plant communities? Flora Mediterr. 2021, 31, 317–337. [Google Scholar] [CrossRef]
- Bruelheide, H.; Jansen, F.; Jandt, U.; Bernhardt-Römermann, M.; Bonn, A.; Bowler, D.; Dengler, J.; Eichenberg, D.; Grescho, V.; Harter, D.; et al. Using incomplete floristic monitoring data from habitat mapping programmes to detect species trends. Divers. Distrib. 2020, 26, 782–794. [Google Scholar] [CrossRef]
- Weldon, J.; Merder, J.; Ferretti, M.; Grandin, U. Nitrogen deposition causes eutrophication in bryophyte communities in central and northern European forests. Ann. For. Sci. 2022, 79, 1–14. [Google Scholar] [CrossRef]
- Šipek, M.; Šajna, N.; Kosić, I.V.; Horvat, E. Presence of alien Prunus serotina and Impatiens parviflora in lowland forest fragments in NE Slovenia. Šumar. List 2022, 146, 215–222. [Google Scholar] [CrossRef]
- Stanojevic, M.; Trailovic, M.; Dubljanin, T.; Krivošej, Z.; Nikolic, M.; Nikolic, N. Sewage Pollution Promotes the Invasion-Related Traits of Impatiens glandulifera in an Oligotrophic Habitat of the Sharr Mountain (Western Balkans). Plants 2021, 10, 2814. [Google Scholar] [CrossRef]
- Gao, J.; Faheem, M.; Yu, X. Global Research on Contaminated Soil Remediation: A Bibliometric Network Analysis. Land 2022, 11, 1581. [Google Scholar] [CrossRef]
- Alavi, S.J.; Veiskarami, R.; Esmailzadeh, O.; Gadow, K.V. Analyzing the Biological and Structural Diversity of Hyrcanian Forests Dominated by Taxus baccata L. Forests 2020, 11, 701. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, P.; Zhao, X.; Fan, C.; Zhang, C.; Yan, Y. Von Gadow, K. Analysing species abundance distribution patterns across sampling scales in three natural forests in Northeastern China. iForest Biogeosci. For. 2020, 13, 482–489. [Google Scholar] [CrossRef]
- Ivanova, N. Forest typological features of herb species abundance distributions of pine forests in the Ural Mountains. In Proceedings of the BIO Web of Conferences: Northern Asia Plant Diversity: Current Trends in Research and Conservation, Novosibirsk, Russia, 6–12 September 2021; Volume 38, p. 00047. [Google Scholar] [CrossRef]
- Ivanova, N.; Petrova, I. Species abundance distributions: Investigation of adaptation mechanisms of plant communities. In E3S Web of Conferences: International Scientific and Practical Conference “Fundamental and Applied Research in Biology and Agriculture: Current Issues, Achievements and Innovations” (FARBA 2021); EDP Sciences: Les Ulis, France, 2021; Volume 254, p. 02003. [Google Scholar] [CrossRef]
- Van Sundert, K.; Radujković, D.; Cools, N.; De Vos, B.; Etzold, S.; Fernández-Martínez, M.; Janssens, I.A.; Merilä, P.; Peñuelas, J.; Sardans, J.; et al. Towards comparable assessment of the soil nutrient status across scales—Review and development of nutrient metrics. Glob. Chang. Biol. 2020, 26, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, D.; Mitchell, C.E.; Pyšek, P.; Jarošík, V. Synergy between pathogen release and resource availability in plant invasion. Proc. Natl. Acad. Sci. 2009, 106, 7899–7904. [Google Scholar] [CrossRef]
- Song, Y.; Yan, E.; Song, K. Synthetic comparison of eight dynamics plots in evergreen broadleaf forests, China. Biodivers. Sci. 2015, 23, 139–148. [Google Scholar] [CrossRef]
- Wittig, R. Shared species of the spontaneous flora of a West African (Burkina Faso) and a Central European country (Germany). Flora Veg. Sudano Sambesica 2015, 18, 21–27. [Google Scholar] [CrossRef]
- Ivanova, N.; Tantsyrev, N.; Li, G. Regeneration of Pinus sibirica Du Tour in the Mountain Tundra of the Northern Urals against the Background of Climate Warming. Atmosphere 2022, 13, 1196. [Google Scholar] [CrossRef]
- Fomin, V.; Mikhailovich, A.; Golikov, D.; Agapitov, E. Reconstruction of the Expansion of Siberian Larch into the Mountain Tundra in the Polar Urals in the 20th—Early 21st Centuries. Forests 2022, 13, 419. [Google Scholar] [CrossRef]
- Moiseev, P.A.; Hagedorn, F.; Balakin, D.S.; Bubnov, M.O.; Devi, N.M.; Kukarskih, V.V.; Mazepa, V.S.; Viyukhin, S.O.; Viyukhina, A.A.; Grigoriev, A.A. Stand Biomass at Treeline Ecotone in Russian Subarctic Mountains Is Primarily Related to Species Composition but Its Dynamics Driven by Improvement of Climatic Conditions. Forests 2022, 13, 254. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Brönnimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Owens, H.L.; Ribeiro, V.; Saupe, E.E.; Cobos, M.E.; Hosner, P.A.; Cooper, J.C.; Samy, A.M.; Barve, V.; Barve, N.; Muñoz-R., C.J.; et al. Acknowledging uncertainty in evolutionary reconstructions of ecological niches. Ecol. Evol. 2020, 10, 6967–6977. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Atwater, Z.D.; Barney, J.N. Climatic Niche Shifts in 815 Introduced Plant Species Affect their Predicted Distributions. Glob. Ecol. Biogeogr. 2021, 30, 1671–1684. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Courchamp, F.; Roura-Pascual, N.; Jeschke, J.M. Biological invasions reveal how niche change affects the transferability of species distribution models. Ecology 2022, 103, e3719. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Eren, Ö.; Hierro, J.L. Community Assembly Theory as a Framework for Biological Invasions. Trends Ecol. Evol. 2018, 33, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Marin, P.; Genitoni, J.; Barloy, D.; Maury, S.; Gibert, P.; Ghalambor, C.K.; Vieira, C. Biological invasion: The influence of the hidden side of the (epi)genome. Funct. Ecol. 2020, 34, 385–400. [Google Scholar] [CrossRef]
- Kusbach, A.; Friedl, M.; Zouhar, V.; Mikita, T.; Šebesta, J. Assessing Forest Classification in a Landscape-Level Framework: An Example from Central European Forests. Forests 2017, 8, 461. [Google Scholar] [CrossRef]
- Muldavin, E.H.; Addicott, E.; Hunter, J.T.; Lewis, D.; Faber-Langendoen, D. Australian vegetation classification and the International Vegetation Classification framework: An overview with case studies. Aust. J. Bot. 2021, 69, 339–356. [Google Scholar] [CrossRef]
- Ivanova, N.; Fomin, V.; Kusbach, A. Experience of Forest Ecological Classification in Assessment of Vegetation Dynamics. Sustainability 2022, 14, 3384. [Google Scholar] [CrossRef]

| Extraction Criteria | Explanatory Information; Research Objective |
|---|---|
| Publication year | From 2020–2022 |
| Country of research site | Across the globe; identify the geographical distribution of researches. |
| Countries of co-authors | Across the globe; analysis of country cooperation network. |
| Type of plant community under research | No restrictions on the plant community type; analysis of distribution by plant communities types and identification of priorities. |
| Research scope of application | Identification of the range of tasks that were solved on the basis of Ellenberg indicator values and analysis of hot research topics. |
| Annotation | In English; quick introduction to the methodology and results. |
| Link to the Internet page | In-depth study of research aim, methods and results. |
| Keywords | In English; identification of the range of tasks that were solved on the basis of Ellenberg indicator values and analysis of hot research topics. |
| Citing by Scopus | Identification of the most significant documents. |
| Research Directions | Number of Studies |
|---|---|
| Assessment of vegetation dynamics, influence of various factors on plants | 28 |
| Analysis of growing conditions, ecological niches | 19 |
| Determination of ecological groups of species, vegetation classification and ordination of plant communities according to environmental factors | 16 |
| Relationship between environmental characteristics and ecological indicators; improving Ellenberg indicator values | 8 |
| Improving ecosystem assessment methodologies | 5 |
| Effect of nitrogen deposition on vegetation | 4 |
| Relationship between plant characteristics and ecological indicators | 3 |
| Monitoring of invasive plant species | 3 |
| Paper Ranker | Number of Citations | Authors, Year | Research Topics of Ellenberg Indicator Values | Journal |
|---|---|---|---|---|
| 1 | 22 | Van Sundert et al., 2020 [133] | Potential of soil-, plant-, and remote sensing-based metrics to compare the nutrient status across space. | Global Change Biology |
| 2 | 15 | Hájek et al., 2020 [109] | Dynamics of plant communities. | Science of the Total Environment |
| 3–4 | 14 | Tyler et al., 2021 [96] | Improvement of Ellenberg indicator values. | Ecological Indicators |
| 3–4 | 14 | Rehling et al., 2021 [43] | Selection of plants with different indicator values for the experiment. | Journal of Ecology |
| 5–6 | 13 | Dietz et al., 2020 [59] | Dynamics of plant communities. | Global Ecology and Biogeography |
| 5–6 | 13 | Descombes et al., 2020 [13] | Spatial modeling of ecological indicator values. | Ecography |
| 7 | 12 | Sun et al., 2020 [42] | Selection of plants with different indicator values for the experiment. | Functional Ecology |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotova, E.; Ivanova, N.; Ivanova, S. Global Overview of Modern Research Based on Ellenberg Indicator Values. Diversity 2023, 15, 14. https://doi.org/10.3390/d15010014
Zolotova E, Ivanova N, Ivanova S. Global Overview of Modern Research Based on Ellenberg Indicator Values. Diversity. 2023; 15(1):14. https://doi.org/10.3390/d15010014
Chicago/Turabian StyleZolotova, Ekaterina, Natalya Ivanova, and Svetlana Ivanova. 2023. "Global Overview of Modern Research Based on Ellenberg Indicator Values" Diversity 15, no. 1: 14. https://doi.org/10.3390/d15010014
APA StyleZolotova, E., Ivanova, N., & Ivanova, S. (2023). Global Overview of Modern Research Based on Ellenberg Indicator Values. Diversity, 15(1), 14. https://doi.org/10.3390/d15010014







